Abstract
Background/Aim:
Therapeutic hypothermia has become an established therapy in asphyxiated neonates with evidence of moderate/severe hypoxic-ischemic encephalopathy. Herein, we describe our recent experience with total body cooling in asphyxiated neonates, which is the first relevant report in Greece.
Patients and Methods:
The medical records of all asphyxiated newborns treated with therapeutic hypothermia in our center between September 2010 and October 2013 were retrospectively reviewed. We recorded data related to neonatal-perinatal characteristics, whole body cooling and outcome.
Results:
Twelve asphyxiated neonates [median gestational age 38 weeks (36-40)] received whole body cooling (rectal temperature 33.5 ± 0.5 oC for 72 hours) during the study period for moderate (n=3) and severe (n=9) hypoxic-ischemic encephalopathy. Cooling was passive in 4 and active in 8 (66.7%) cases. Therapeutic hypothermia was initiated at the median age of 5 hours (0.5-11) after birth. Seven neonates survived (58.3%) to hospital discharge. On follow-up (7-35 months), neurodevelopment outcome was normal in 1 case, while 3, 1 and 2 subjects had mild, moderate and severe impairment, respectively.
Conclusions:
Our initial experience with whole body cooling supports its beneficial effect in asphyxiated neonates. This treatment should be offered in all centers involved in the care of such neonates using either simple means (passive cooling) or automated cooling devices. Hippokratia 2014; 18 (3): 226-230.
Keywords: neonatal encephalopathy, neonatal care, perinatal asphyxia
Introduction
Hypoxic-ischemic encephalopathy (HIE) is a serious manifestation of perinatal asphyxia. In developed countries, 1-5 term neonates/1000 live births suffer perinatal asphyxia1,2. The incidence of perinatal asphyxia is considerably higher in developing countries accounting for the one-fourth of neonatal deaths, worldwide3. However, even in countries with advanced health care systems, asphyxiated neonates undergoing moderate/severe HIE are at significantly increased risk for severe handicap or death1,4 with important social-economic consequences in survivors5. These poor outcomes are virtually associated with the lack of any effective neuroprotective treatment following perinatal asphyxia, the management of which remained, until recently, supportive.
Brain injury and neuronal damage in acute hypoxia-ischemia is biphasic initially characterized by exhaustion of high energy compounds such as phosphocreatine and adenosine triphosphate (primary energy failure). Reperfusion of the ischemic brain with resuscitation is followed by a latent phase (approximately 6 hours), which may lead to complete recovery or - in severe insults - to secondary energy failure (after 6-15 hours) and late apoptotic cell death 3-10 days after the acute asphyctic event6,7. Nevertheless, as shown in animal studies, there is still a "therapeutic window" - during the latent phase - where secondary neuronal injury could be prevented or reduced by brain cooling6. This was confirmed in clinical trials. Indeed, most recent meta-analyses documented that therapeutic hypothermia - within the first 6 hours of life - in late preterm and term infants with moderate/severe encephalopathy and evidence of intrapartum asphyxia results in a significant reduction in mortality or major neurodevelopmental disability at the age of 18 months in survivors8,9.
Because of the clinical benefits of therapeutic hypothermia, it is nowadays considered the standard of care in many developed countries. In the United States, 50% of the neonatal intensive care units (NICU) were reported (2013) to provide therapeutic hypothermia whereas nearly all (97%) not offering this option transfer eligible neonates to centers where cooling can be applied10. In Europe, therapeutic hypothermia is already implemented in several countries as well, due in part to participation of centers in clinical trials (e.g., TOBY; mainly in the United Kingtom11,12, neo.nEURO; in Germany and in several European countries13) while in others its use is increasing rapidly14,15.
In Greece, our center is the first, and as far as we know the only center until recently (end of 2013), to apply therapeutic hypothermia for the treatment of asphyxiated neonates with HIE. This study aims at describing our recent experience with whole body cooling in neonates with neonatal asphyxia. Moreover, as passive cooling technique was initially used in our center, this study may promote the application of hypothermia in asphyxiated neonates by other NICUs not offering this therapeutic option, yet, using simple cooling methods.
Patients and Methods
We retrospectively reviewed the medical records of all asphyxiated newborns treated with whole body cooling in our level III NICU between September 2010 and October 2013.
Recorded data
Parameters evaluated included demographic (gestational age, birth, sex) and perinatal-neonatal characteristics (inborn neonates, mode of delivery, acute intrapartum events, Apgar scores at 1, 5 and 10 minutes, neonatal resuscitation needed, blood gases within the 1st hour), severity of HIE as assessed prior to cooling (criteria of Sarnat and Sarnat)16, time of cooling initiation after birth, adverse effects and interventions during cooling and outcome (survival, neurodevelopment outcome). Data on amplitude-integrated electroencephalogram (a-EEG), conventional EEG and magnetic resonance imaging (MRI, conventional T1- and T2-weighted/FLAIR/ Diffusion Weighted Images-ADC) of the brain performed during the hospital stay were also reviewed. Interventions and adverse effects studied included invasive mechanical ventilation, persistent pulmonary hypertension of the neonate necessitating inhaled nitric oxide, arterial hypotension and need for inotropes, sinus bradycardia (heart rate <80 beats/min), acute renal injury (increase of serum creatinine ≥ 0.3 mg/dL from previous value within 48 hours), liver dysfunction (aspartate aminotransferase >200 U/L, alanine aminotransferase >100 U/L), disseminated intravascular coagulation, hyperglycemia treated with insulin infusion, thrombocytopenia (platelet <100 x 109/L) and early-onset sepsis (positive blood culture in the first 72 hours of life).
Selection criteria and whole body cooling method
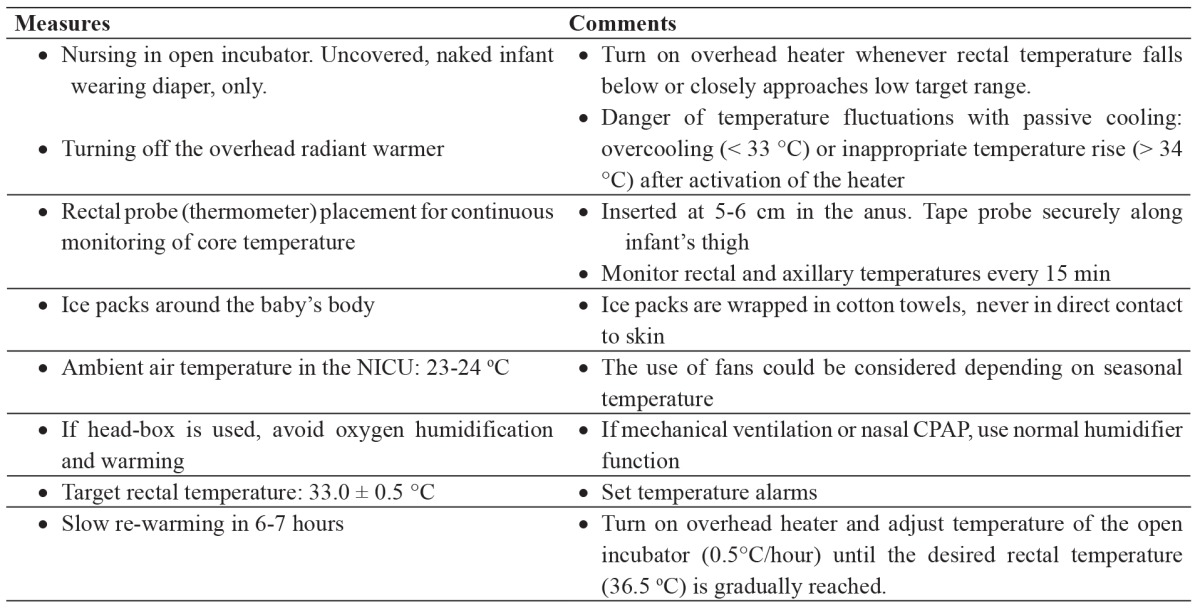
All babies were selected and treated according to our local NICU protocol which was consistent with those used in clinical trials of therapeutic hypothermia. Briefly, newborn infants born at ≥ 36 weeks gestation were eligible for treatment if they had evidence of acute perinatal asphyxia and of moderate/severe HIE according to the Sarnat and Sarnat criteria. An a-EEG assessment for abnormal findings (as proposed by al Naqeeb17) prior to treatment initiation was highly supported, but this was not a strict criterion. An important differentiation of our protocol, however, is the fact that allowed cooling initiation up to 12 hours after birth. Neonates less than 36 weeks gestation or birth weight < 1,800 g, with severe congenital anomalies, known perinatal infection and severe bleeding were precluded from cooling.
As aforementioned, initially (September 2010-December 2012), treatment was conducted in our center by passive cooling (Table 1) and afterwards using an automated cooling device (Tecotherm neo®, Inspiration Healthcare Ltd, Leicester, UK). Rectal temperature was maintained with both passive and active cooling at 33.5 ± 0.5 oC for 72 hours while re-warming took place gradually (0.5 oC/h) until the desired rectal temperature (36.5 oC) was reached. All neonates were nursed in an open incubator during treatment, were given continuous fentanyl infusion to eliminate cold stress and received standard neonatal care.
Table 1. Measures taken in our neonatal intensive care unit (NICU) for the application of passive (whole body) cooling in asphyxiated neonates.
Parents or caregivers of the asphyxiated neonates were informed about the importance of offering therapeutic hypothermia to their child with evidence of moderate/severe HIE. However, a written consent was not mandatory for treatment initiation in our institution as cooling is considered the standard of care.
Neurodevelopment in survivors
Developmental outcome in survivors was assessed using the Bayley-III test18. "Normal outcome" was defined as having normal Bayley Scales III (composite scores for cognitive, motor and language ≥ 85) and normal neurologic examination. "Mild impairment" was defined as having Bayley Scales III < 85 and ≥70 with abnormal neurologic examination, while "moderate impairment" as having Bayley Scales III <70 and ≥55 along with abnormal neurologic examination. Patients with Bayley Scales III <55 and severely abnormal neurologic examination were considered as having "severe impairment". In one neonate who had not reached the age of 12 months at the study analysis, assessment corresponded only to the neurological status at evaluation.
Results
Neonatal-perinatal characteristics
During the study period, 12 neonates with median gestational age 38 weeks (36-40) and median birth weight 3,161 g (2,200-4,700) received therapeutic hypothermia for acute perinatal asphyxia and evidence of moderate/severe HIE. The majority of the patients were male, outborn neonates [both 7/12 (58.3%)]. An emergency caesarian section was performed in 50% of the deliveries due to uterine rupture (n=1), abruption of the placenta (n=2) and prolonged labor and a failure to progress (n=3). The remaining neonates were born vaginally but in 4/6 cases an assisted delivery (vacuum extraction) was conducted.
All neonates were depressed upon delivery [median Apgar scores: 1 (0-3), 5 (1-7) and 6 (2-7) at 1, 5 and 10 minutes, respectively]. Eight neonates (66.7%) were intubated in the delivery room and 3 (25%) were given adrenaline. The median standard base deficit (SBD) on the first blood gas performed within 1 hour after birth was -19.8 meq/L (-14.8 to -24.7). Nine (75%) and 3 (25%) neonates had SBD values > 16 meq/L and 10-16 meq/L, respectively.
Severity of HIE and whole body cooling
HIE at assessment for eligibility was found to be moderate and severe in 3 (25%) and 9 (75%) cases, respectively. Abnormal a-EEG recordings were documented prior to cooling in 11 (91.7%) neonates [discontinuous (n=2), burst suppression (n=3), low voltage (n=3), flat trace (n=3)] whereas there was no recording in one neonate.
Cooling was passive in 4 and active in 8 (66.7%) neonates. Therapeutic hypothermia was commenced at the median age of 5 hours (0.5-11) after birth. However, treatment was initiated within the first 6 hours of life in only 8 (66.7%) neonates. In addition, in one case, hypothermia was discontinued at 48 hours (parental request) while in another it was applied for 12 additional hours due to status epilepticus upon re-warming.
Supportive care, complications during cooling and central nervous system manifestations
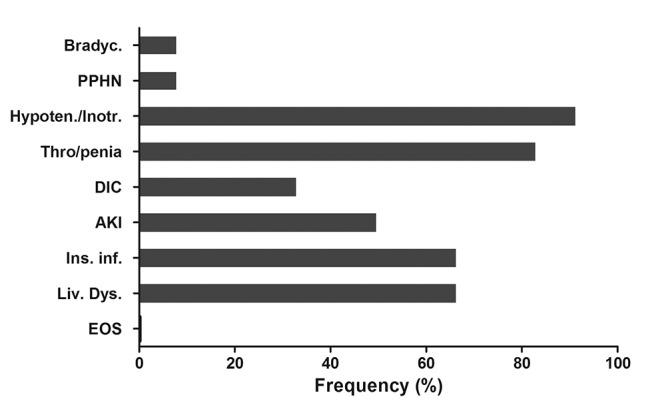
All neonates were mechanically ventilated owing to asphyxia and its consequences and/or opiates (fentanyl) administration. The complications from the various organs and systems observed during the application of therapeutic hypothermia are shown in Figure 1. Clinical and electrical fits (a-EEG) were observed in 9 (75%) and 6 (50%) neonates, respectively. All these neonates received anticonvulsant drugs.
Figure 1. Frequency (%) of the neonatal complications observed during cooling. Bradyc: Bradycardia, PPHN: Persistent pulmonary hypertension of the neonate, Hypoten./Inotr.: Inotropes for arterial hypotension, Thro/penia: Thrombocytopenia, DIC: Disseminated intravascular coagulation, AKI: Acute kidney injury, Ins. Inf.: Insulin infusion for hyperglycemia, Liv. Dys. Liver dysfunction, EOS: Early-onset sepsis.
Survival and neurodevelopmental outcome
Seven neonates survived (58.3%) to hospital discharge. All 5 neonates who died had suffered severe HIE, while deaths occurred after the end of cooling at a median postnatal age of 8.5 (5-15) days. The median hospital stay in survivors was 31 days (21-99). In 3 (42.2%) neonates, MRI performed during the NICU stay was normal as was conventional EEG in 4 (57.2%) neonates. The neurodevelopmental outcome as assessed by Bayley-III in relation to the severity of HIE, time of cooling initiation and MRI findings before hospital discharge is shown in Table 2.
Table 2. Developmental outcome of the studied neonates in relation to the severity of HIE, time of cooling initiation from birth and MRI findings before hospital discharge. Timing of MRI is also shown.
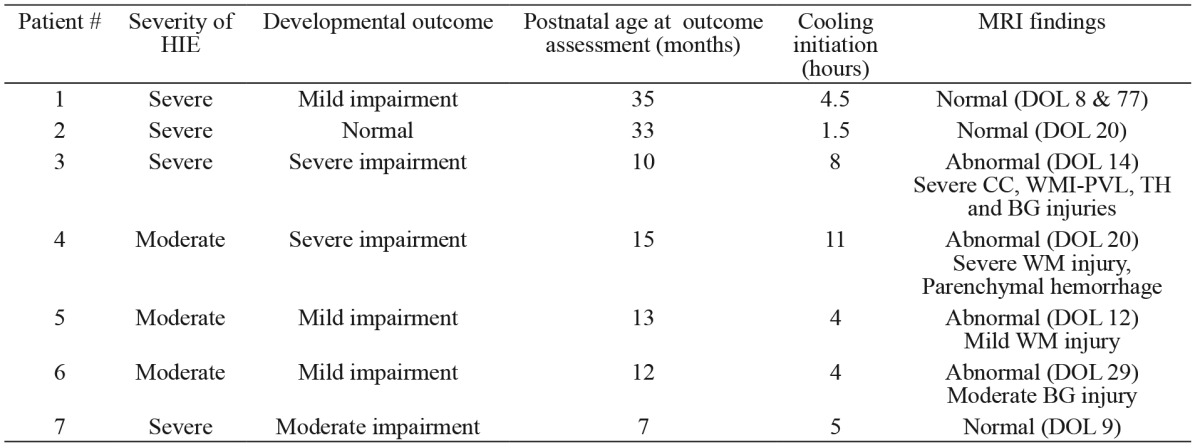
BG: basal ganglia, CC: cerebral cortex, DOL: day of life, HIE: hypoxic-ischemic encephalopathy, MRI: magnetic resonance imaging, WM: white matter, PVL: periventricular leukomalacia, TH: thalamus.
Discussion
In this study, we present our experience with therapeutic hypothermia when preformed for the management of asphyxiated neonates with moderate/severe HIE beyond the framework of clinical trials in a tertiary NICU of Greece. Our results are encouraging with respect to a) the feasibility and safety of passive cooling and b) the beneficial effect of whole body cooling in terms of survival and neurodevelopmental outcome being consistent with the results of large clinical trials.
Although there were some successful reports in studies in which hypothermia was used to resuscitate newborns after delivery back in the 50s, it has emerged again as a promising therapeutic option in asphyxiated neonates with HIE only during the last two decades6. In the most important randomized clinical trials performed so far, selective head19,20 or total body cooling11,13,21was applied using automated devices designed to maintain target rectal temperature at 34-35oC and of 33-34oC, with the respective cooling modes. No superiority of either modality is supported by the existing evidence9. In this study, one-third of the neonates were cooled passively using simple means shown in Table 1. It is our feeling that passive cooling could be easily applied in centers with no or minimal experience in which an automated machine is lacking, provided that the body temperature is closely monitored so as to remain in the target range. Whole body cooling using low tech methods has been found to be effective in clinical trials (ICE study)22 while, owing to its practicality and low cost, it is used by several centers even in high resource countries14,23. Still, one should be aware of the highest variation of temperature with passive cooling14.
Most of the neonates (75%) in which cooling was performed in the present study had severe HIE. This could be explained by the relative "reluctance", originally, of the medical staff in implementing therapeutic hypothermia in "less severe cases", despite accumulated scientific evidence in 2010. Therefore, as we were in the early phase of the learning curve, most probably there was a biased selection of the severely affected neonates. Interestingly, in other important clinical trials, most of the cooled neonates had moderate HIE as in the NICDH (68%)21, ICE (57.3%)22 and China study group (41%)20studies. Moreover, in the latter two studies, therapeutic hypothermia was performed even in neonates with mild encephalopathy (in 15.5% and 21%, respectively). On the other hand, the severity of perinatal asphyxia and HIE is strongly associated with complications and outcome. This could provide an explanation for the higher percentage of some adverse effects in our study (e.g., use of inotropes for arterial hypotension in around 90% of the studied babies) compared to previous clinical trials. In any case, according to the most recent meta-analysis, sinus bradycardia, thrombocytopenia and leucopenia (in whole body cooling) are the only adverse effects that can be associated with therapeutic hypothermia in neonates9.
In the present study, 58% of the neonates survived to hospital discharge while all deaths involved neonates with severe HIE. Hypothermia has been well documented to decrease mortality in asphyxiated neonates, but we could not know the survival of these babies if this intervention had not been applied (nowadays deemed unethical). On the other hand, the good neurodevelopmental outcome as evaluated at early childhood in 2 of the surviving neonates with severe HIE (#1 and 2) is encouraging. As evidenced by the most recent Cochrane meta-analysis, 8 asphyxiated neonates with moderate/severe encephalopathy would need to be cooled in order to prevent neurodevelopmental disability in 1 survivor9. In addition, it is worth noting that in this cohort of asphyxiated neonates, hypothermia was attempted beyond the "therapeutic window" of the first 6 hours of life, considered to be the optimal time period for neuroprotection6-9. Several factors (late recognition of eligible patients, need for transfer) may delay initiation of treatment. Data analysis on the implementation-conduction of therapeutic hypothermia in the United Kingdom (December 2006-July 2011) showed that 2.2 % of the neonates suffering asphyxial encephalopathy had cooling commenced more than 12 hours after birth12. Furthermore, in a retrospective review of neonates referred to a regional tertiary center in Canada, 44% of the patients had cooling initiated after 6 hours of age24. Nonetheless, delay in cooling initiation may have reduced the efficacy of this intervention possibly explaining the poor developmental outcome of the two neonates with moderate and severe HIE respectively (#3 and 4), in which treatment was attempted at 6-12 hours after delivery. Ongoing studies on this issue (NICDH: Late hypothermia) are expected to provide insights as to whether the later timely application of therapeutic hypothermia is beneficial9. Cooling during transport could be an alternative approach as initiation of effective hypothermia is achieved significantly earlier25, but clinical protocols and devices for cooling in transport are essential to ensure safety and efficacy23.
Disadvantages of the study are the small number of studied neonates and its retrospective nature. However, data presented here are derived from a single center and, therefore, only a limited number of neonates could have been evaluated in a relative short time period, particularly with respect to long-term outcome. On the other hand, the retrospective analysis permits the detection of important clinical parameters which could allow further improvement in the clinical implementation of this novel therapeutic approach.
In conclusion, given that therapeutic hypothermia is considered to be the optimal care in asphyxiated neonates with evidence of moderate-severe HIE, this treatment should be offered in a timely manner by all centers involved in the care of such neonates. Although active cooling using specific devices is preferable in terms of temperature stability, passive cooling can be safely applied. In all cases, however, local protocols should be developed based on the existing international experience.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Thornberg E, Thiringer K, Odeback A, Milsom I. Birth asphyxia: incidence, clinical course and outcome in a Swedish population. Acta Paediatr. 1995;84:927–932. doi: 10.1111/j.1651-2227.1995.tb13794.x. [DOI] [PubMed] [Google Scholar]
- 2.Snowden JM, Cheng YW, Kontgis CP, Caughey AB. The association between hospital obstetric volume and perinatal outcomes in California. Am J Obstet Gynecol. 2012;207:478. doi: 10.1016/j.ajog.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 4.Robertson CM, Finer NN, Grace MG. School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Pediatr. 1989;114:753–760. doi: 10.1016/s0022-3476(89)80132-5. [DOI] [PubMed] [Google Scholar]
- 5.Odd DE, Gunnell D, Lewis G, Rasmussen F. Long-term impact of poor birth condition on social and economic outcomes in early adulthood. Pediatrics. 2011;127:e1498–e1504. doi: 10.1542/peds.2010-3604. [DOI] [PubMed] [Google Scholar]
- 6.Sahni R, Sanocka UM. Hypothermia for hypoxic-ischemic encephalopathy. Clin Perinatol. 2008;35:717–734. doi: 10.1016/j.clp.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs SE, Tarnow-Mordi WO. Therapeutic hypothermia for newborn infants with hypoxic-ischaemic encephalopathy. J Paediatr Child Health. 2010;46:568–576. doi: 10.1111/j.1440-1754.2010.01880.x. [DOI] [PubMed] [Google Scholar]
- 8.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:558–566. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris MN, Carey WA, Ellsworth MA, Haas LR, Hartman TK, Lang TR, et al. Perceptions and practices of therapeutic hypothermia in American neonatal intensive care units. Am J Perinatol. 2014;31:15–20. doi: 10.1055/s-0033-1334454. [DOI] [PubMed] [Google Scholar]
- 11.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, TOBY Study Group et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 12.Azzopardi D, Strohm B, Linsell L, Hobson A, Juszczak E, Kurinczuk JJ, UK TOBY Cooling Register et al. Implementation and conduct of therapeutic hypothermia for perinatal asphyxial encephalopathy in the UK--analysis of national data. PLoS One. 2012;7:e38504. doi: 10.1371/journal.pone.0038504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simbruner G, Mittal RA, Rohlmann F, Muche R; neo, nEURO.network Trial Participants Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126:e771–e778. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 14.Ramos G, Brotschi B, Latal B, Bernet V, Wagner B, Hagmann C, Swiss Neonatal Network Therapeutic hypothermia in term infants after perinatal encephalopathy: the last 5 years in Switzerland. Early Hum Dev. 2013;89:159–164. doi: 10.1016/j.earlhumdev.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Groenendaal F, Casaer A, Dijkman KP, Gavilanes AW, de Haan TR, ter Horst HJ, et al. Introduction of hypothermia for neonates with perinatal asphyxia in the Netherlands and Flanders. Neonatology. 2013;104:15–21. doi: 10.1159/000348823. [DOI] [PubMed] [Google Scholar]
- 16.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 17.al Naqeeb N, Edwards AD, Cowan FM, Azzopardi D. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics. 1999;103:1263–1271. doi: 10.1542/peds.103.6.1263. [DOI] [PubMed] [Google Scholar]
- 18.Albers CA, Grieve AJ. Review of Bayley Scales of Infant and Toddler Development--Third Edition. Journal of Psychoeducational Assessment. 2007;25:180–190. [Google Scholar]
- 19.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 20.Zhou WH, Cheng GQ, Shao XM, Liu XZ, Shan RB, Zhuang DY, China Study Group et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010;157:367–372. doi: 10.1016/j.jpeds.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 21.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, National Institute of Child Health and Human Development Neonatal Research Network et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, Infant Cooling Evaluation Collaboration et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165:692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 23.Akula VP, Davis AS, Gould JB, Van Meurs K. Therapeutic hypothermia during neonatal transport: current practices in California. Am J Perinatol. 2012;29:319–326. doi: 10.1055/s-0031-1295661. [DOI] [PubMed] [Google Scholar]
- 24.Khurshid F, Lee KS, McNamara PJ, Whyte H, Mak W. Lessons learned during implementation of therapeutic hypothermia for neonatal hypoxic ischemic encephalopathy in a regional transport program in Ontario. Paediatr Child Health. 2011;16:153–156. doi: 10.1093/pch/16.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Reilly D, Labrecque M, O'Melia M, Bacic J, Hansen A, Soul JS. Passive cooling during transport of asphyxiated term newborns. J Perinatol. 2013;33:435–440. doi: 10.1038/jp.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]


