Abstract
It has been proposed that the non-saturable component of intestinal glucose absorption, apparent following prolonged exposure to high intraluminal glucose concentrations, is mediated via the low affinity glucose and fructose transporter, GLUT2, upregulated within the small intestinal apical border.
The evidence that the non-saturable transport component is mediated via an apical membrane sugar transporter is that it is inhibited by phloretin, after exposure to phloridzin. Since the other apical membrane sugar transporter, GLUT5, is insensitive to inhibition by either cytochalasin B, or phloretin, GLUT2 was deduced to be the low affinity sugar transport route.
As in its uninhibited state, polarized intestinal glucose absorption depends both on coupled entry of glucose and sodium across the brush border membrane and on the enterocyte cytosolic glucose concentration exceeding that in both luminal and submucosal interstitial fluids, upregulation of GLUT2 within the intestinal brush border will usually stimulate downhill glucose reflux to the intestinal lumen from the enterocytes; thereby reducing, rather than enhancing net glucose absorption across the luminal surface.
These states are simulated with a computer model generating solutions to the differential equations for glucose, Na and water flows between luminal, cell, interstitial and capillary compartments. The model demonstrates that uphill glucose transport via SGLT1 into enterocytes, when short-circuited by any passive glucose carrier in the apical membrane, such as GLUT2, will reduce transcellular glucose absorption and thereby lead to increased paracellular flow. The model also illustrates that apical GLUT2 may usefully act as an osmoregulator to prevent excessive enterocyte volume change with altered luminal glucose concentrations.
Introduction
Intestinal glucose absorption has been studied for more than a century and still remains controversial. During the last fifty years the main research thrust has been to identify and characterize the individual transport components within the intestinal epithelium. This progressively reductivist approach has been very successful: we have a comprehensive knowledge of the nature of the driving forces generating sugar absorption; the specificity range of the sugar transporters involved; their sites of activity within the enterocytes and of how the individual transport processes function at a molecular level 1– 3. Less clear is how the intestine functions as a working ensemble to absorb glucose over the wide range of luminal concentrations occurring within the small intestine and how this process is controlled, both in the short and long-term. These uncertainties arise from the multiplicity and complexity of interactive processes and lack of a comprehensive model permitting an integrated view of intestinal glucose uptake.
The early opinion on intestinal glucose transport was that stereospecific electrogenic active transcellular transport process coexisted with a variable non-specific paracellular diffusive flux 4– 8. Intestinal glucose absorption entails specific sodium-dependent hexose interactions with jejunal and ileal enterocyte glucose transporters in the apical and sodium-independent passive downhill transport via basal-lateral membranes and transit by solvent drag via non-selective paracellular pathways, generated by electro-osmotic flow of Na + and water 7, 9, 10, or by paracellular passive diffusion down the glucose concentration gradient existing between the intestinal lumen and lamina propria 11, 12. This diffusive route permits non-specific transport of L-glucose, D-rhamnose, or mannitol, as well as D-glucose at rates that are correlated with net fluid transport 13. The general consensus was that at around a luminal glucose ≈ 25 mM the active and passive components are about equal and above this passive absorption becomes dominant ( Figure 1).
Figure 1. These diagrams show snapshots of the simulated glucose flows from intestinal lumen loaded with 50 mM in 150 mM NaCl to capillaries perfusing the submucosal spaces, whose afferent arterial concentration contains 150 mM NaCl and 5 mM D glucose.
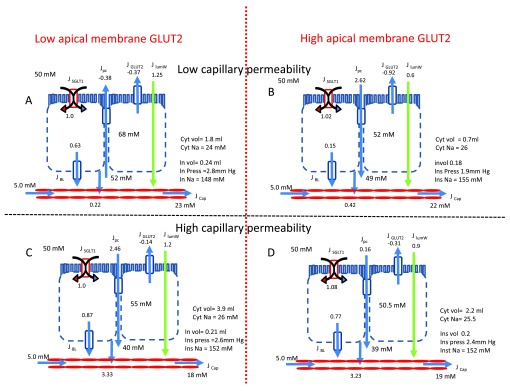
The tissue in panel A has low apical GLUT2 and GLUT5 activity and low capillary permeability and perfusion rates (clearance). In panel B the tissue apical membrane GLUT2 activity is increased by 4-fold above that in panel A, capillary perfusion is unchanged. In panel C, the apical GLUT2 activity is the same as in panel A, but capillary clearance is increased by 10-fold. In panel D, the apical GLUT2 is raised, as in panel B and the capillary clearance raised, as in panel C.
The rates of glucose uptake are normalized relative to the rate of SGLT1 glucose uptake (panel A). Altering either GLUT2, or capillary clearance have negligible effects on glucose inflow via SGLT1. However, after raising the apical GLUT2 activity, the steady state glucose concentration within the cytosol decreases from 68 to 52 mM (c.f. Panels A and C). On raising capillary clearance, the steady state of cytosolic glucose concentration also decreases (c.f. Panel A versus Panel C and Panel B versus Panel D).
Raising capillary glucose clearance increases the rate of glucose inflow from the interstitial to capillary fluid by fourteen fold (c.f. Panel A and C). These changes are accompanied by decreased interstitial fluid glucose from 52 to 40 mM and reductions in the mean capillary glucose from 23 to 18 mM. Reduced interstitial glucose concentrations reverse the direction of the glucose gradient across the paracellular pathway from -2 to + 10 mM. Thus raising the capillary clearance of glucose, reverses the direction of paracellular glucose flow from (-0.38) to (+2.46) and increases the net glucose inflow across the luminal surface from (0.22 to 3.23).
Although raising apical membrane GLUT2 activity by fourfold reduces net glucose influx across the apical border from 0.63 to 0.15, it also indirectly leads to an increase in paracellular glucose flux and thereby causes a slight increase in net glucose flux across the luminal border.
When capillary clearance is raised, either by enhanced perfusion rates, or increased endothelial permeability, increasing apical membrane GLUT2 enhances apical membrane glucose reflux from -0.14 to -0.31. This has no significant effect on glucose flow from the interstitial to capillary fluid. (c.f. panel C and D).
This dual transport model explained why the apparent affinity of total net glucose uptake is much less, K m > 62.3±3.2 mM than the K m obtained for electrogenic glucose transport (K m = 17.9±0.4 mM); and why phloridzin, a blocker of Na-coupled glucose transport via SGLT1 at the luminal surface, affects mainly electrogenic transport, but not transport via the paracellular route 4.
Parsons and colleagues 14, 15 were amongst the first to postulate parallel active and passive absorptive processes in the luminal surface intestinal membrane.
Kellett and colleagues 1, 16, 17 later proposed that when luminal glucose is raised above 15 mM, that the non-saturable absorptive component, instead of being via the paracellular route is due to influx via a low affinity glucose transporter, GLUT2, whose presence is regulated within jejunal and ileal enterocytes apical membranes. The salient experimental evidence supporting this view is that the “non-saturable” component of glucose absorption is inhibited by either high phloretin (0.75–1 mM), or high cytochalasin B (0.2 mM) concentrations, both of which inhibit GLUT2 and neither of which inhibit GLUT5.
Using a sigmoid curve fit, Kellett and Helliwell 1 obtained a K m of the phloretin-sensitive component “similar” to that of GLUT2, 56±14 mM; n=1.6±0.4. They argued that GLUT2 is the most likely route for this low affinity transport, since it also transports fructose. Later reports showed that artificial sweeteners e.g. aspartame, sucralose and saccharin in parallel with an increase in intracellular calcium, increase the rate of glucose absorption, by increasing brush border GLUT2 18 and this in turn increased release of several incretins gluco-insulinotropic peptide(GIP); glucagon- like peptide (GLP-1) and peptide tyrosine-tyrosine (PYY) from enteroendocrine cells 19.
Although these arguments seem plausible, there are several reasons to question the assertion that apical membrane GLUT2 mediates the low affinity component of intestinal D-glucose absorption. Many studies have shown that the low affinity glucose absorptive route has low specificity- it can transport sugars e.g. L-glucose or rhamnose, or low molecular weight solutes, such as Cr-EDTA, or mannitol, that are not transported by any GLUTs 13. Thus the explanation that GLUT2 is the sole mediator of the low affinity sugar transport route does not explain transport of these paracellular markers without any affinity for sugar transporters.
The K m of GLUT2 has been measured as approximately 17 mM 20, 21, this value is much lower than the very high K m 56±14 mM observed by Kellett & Helliwell (2000) 1. Additionally, at luminal glucose concentrations > 50 mM absorption linearly correlates with luminal concentration; i.e. is not saturable 8. Thus the high K m of the “phloretin-sensitive” component does not necessarily signify glucose transport via a low affinity glucose transporter.
Furthermore, phloretin- is not uniquely specific as a glucose-transporter inhibitor. Phloretin also blocks chloride, or aquaporin water channels, or urea transporter mediated urea and water transport, probably by intercalating with the lipid membrane and consequently may also inhibit solute and water paracellular transport 22, 23. Hence, a transport process blocked by high concentrations of phloretin or cytochalasin B need not imply that the inhibited flow is mediated via apical membrane GLUT2.
In contrast to Kellett and colleagues’ claims, other studies with GLUT2 knock out (KO) mice have shown that GLUT2 makes no substantial contribution to net glucose absorption and furthermore that D-glucose accumulation in enterocytes is increased in GLUT2 KO mice 20, 24. This increase can in part be ascribed to loss of GLUT2 mediated transport activity from the baso-lateral membranes. Doubts have also been raised as to whether GLUT2 is expressed at all in the intestinal apical membranes 25. Roder et al. 24, were unable to detect significant levels of GLUT2 within the intestinal brush borders of wild type mice. Additionally, in humans there is an absence of any detectible increased response to artificial sweeteners with relation to any increased sugar uptake, or incretin release 26, 27.
However, Kellett 28, has responded to some of these arguments, suggesting that the mice used in these KO studies were not optimally prepared. Starvation leads to loss of both intestinal GLUT2 apical protein and GLUT2 mRNA, whereas re-feeding after a period of starvation leads to a rapid increase in both apical GLUT2 expression and to GLUT2 mRNA expression within the intestine 29.
The later results reported by Brot-Laroche’s group appear to conflict with some earlier data from her laboratory showing that semi-starvation increased the V max and K m of D-glucose uptake into guinea pig jejunal brush border membrane vesicles (BBMV) 30. Starvation was postulated to induce a secondary low affinity glucose transport system. Additional studies revealed that phloretin (0.25 mM) enhanced the initial rate of D-glucose uptake by 15% into guinea-pig BBMV. Application of Student’s two-tailed t–test shows that this increase is significant (P < 0.012). Cytochalasin B (0.1 mM) inhibited D-glucose (10 mM) uptake by 38% (p < 0.0001), but had negligible effects on SGLT1 specific α-methyl-D-glucoside uptake 31. The earlier results imply that phloretin enhances, rather than inhibits, the low affinity D-glucose transport in BBMV, as was later asserted 1, 18.
Recent live imaging studies indicate that GLUT2 is a variable presence within the apical membrane 32; its trafficking being dependent on signals induced by high intracellular glucose concentrations.
Analysis
Although upregulation of apical membrane GLUT2 is a feature of raised luminal D-glucose concentrations, it is far from clear, as contended, that this leads to enhanced net glucose transport 1, 17. Since both active (SGLT1) and passive glucose transporter (GLUTs 2 and 5) elements are present within the brush border membranes, the kinetics of net glucose flow across the brush border ensemble will depend both on the variable glucose concentrations in the adjacent luminal and cytosolic compartments and the relative proportions of active and passive transport components and the area of absorbing intestinal surface exposed to glucose. The steady state cytosolic and interstitial glucose concentrations are also reliant upon the concentration dependence of flows across the baso-lateral membrane into the interstitial fluid and between the luminal fluid and interstitial fluid via the intercellular junctions. GLUT2 (K m ≈ 17 mM) is the main transporter for glucose movement across the basal-lateral membranes 33, 34.
The apparent transport parameters (K m and V max) 4– 6 obtained. In intestinal enterocytes in situ, where flows with varying luminal glucose concentrations are normally measured in steady state, have scant resemblance to those obtained in zero-trans conditions with isolated membrane vesicles or oocytes.
Uphill glucose transport via the apical membrane sodium-glucose cotransporter SGLT1 generates polarized sugar flow, causing the intracellular glucose concentration to increase: eventually the cytosolic and also interstitial glucose concentrations may exceed the luminal concentration 35. Once these conditions are met, glucose will reflux back into the intestinal lumen via passive transporters in the apical membrane, or via the tight junction ( Figure 1A). If the V max of the passive apical membrane glucose transporters is raised, then owing to enhanced glucose reflux via GLUT2, net glucose influx across the apical membrane will be reduced. However, net glucose uptake across the luminal surface, including the paracellular pathways may be augmented. This increase in paracellular glucose flow arises from decreased transcellular flow. The resulting slight decrease in interstitial fluid glucose concentration increases the gradient between the intestinal lumen and interstitial fluid, ( Figure 1B) and Figure 3(A–C).
Glucose influx across the apical membrane remains polarized over a very wide concentration range, due to the very low affinity for glucose at the export site of SGLT1 35, 36. Thus when both the cytosolic and the interstitial glucose concentrations are close to GLUT2 saturation levels; i.e. D-glucose > 30 mM, the resistance to glucose outflow across the baso-lateral membrane will increase. Consequently, cytosolic concentration may increase disproportionally as luminal glucose concentrations rise, since the apical and baso-lateral membranes may act as a double membrane rectifier to glucose flow 37. This promotes non-linear glucose accumulations in the intermediate cytosol between the apical and baso-lateral membranes 11, 38, 39 ( Figure 1A).
Glucose flow from the interstitial fluid into the villus capillaries depends on the glucose diffusion between the interstitial fluid and the mean capillary luminal concentration. The mean capillary luminal glucose concentration is a complex non-linear function of the glucose permeability of the capillary membranes, the systemic arterial glucose concentration and the capillary flow rate 11. The boundary conditions of this flow network determine the steady-state glucose concentrations within all the intermediate compartments.
Raising intestinal luminal glucose above 30 mM results in increased superior mesenteric arterial flow from around 1000–2500 ml min -1 40– 42. Raised capillary glucose clearance will reduce the interstitial glucose concentration, thereby also reducing cytosolic glucose concentration, thus increasing net influx across the luminal surface, whilst reducing glucose reflux both via brush border passive transporters and via the paracellular pathway. The model simulates all these conditions as seen by comparing Figures 1A and 1B with Figures 1C and 1D.
However, even with high rates of vascular perfusion, the interstitial glucose concentration approximates to that of the luminal concentration. Consequently, as luminal glucose concentrations are raised, even although interstitial capillary glucose clearance is increased, the enterocyte cytosolic concentrations continuously rises, ( Figure 2C and 2D) 11.
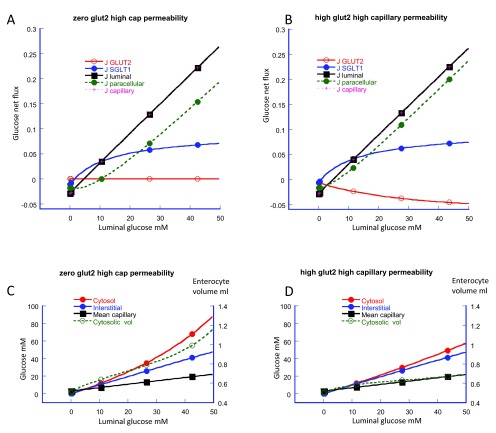
Figure 2. Simulation of altered apical membrane GLUT2 activity on intestinal glucose fluxes from zero (Panel A) to an arbitrary of maximal flux value of 2 (Panel B).
The simulations show the glucose fluxes via apical SGLT1 (blue); apical GLUT2 (red); paracellular pathway (green); the total transluminal membrane, (SGLT1 + GLUT2 + paracellular fluxes), (black) and interstitial to capillary flow (pink crosses) inset on the black square. The main effect of increasing GLUT2 is to cause a negative glucose flux (backflux) via GLUT2 (Panel B). This is accompanied by a increased paracellular flux without any significant change in net transluminal or transepithelial glucose flux. The point at which paracellular glucose flux and SGLT1 flux are equal lies between 20 and 30 mM as has been previously observed 4– 6. This value is used as one of the key registration points for the model.
The cytosolic (red) interstitial (blue) and mean capillary glucose concentrations (black) and enterocyte volume per unit weight of tissue (green) are shown in panel C with zero apical GLUT2 and in Panel D with GLUT2 V max = 2.
Increased apical GLUT2 activity decreases cytosolic glucose concentration (panel D). With rising luminal glucose concentration raised GLUT2 activity prevents the non-linear increase in enterocyte volume seen with zero GLUT2 (Panel C).
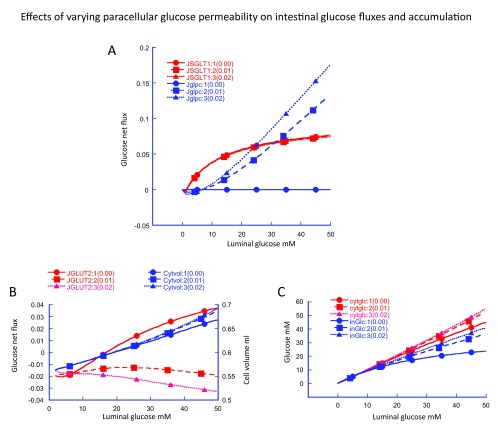
Figure 3. Simulation of effects of varying paracellular glucose permeability on intestinal glucose fluxes and accumulation.
The effects of varying paracellular glucose permeability P glc from 0 to 0.02 cm s -1 are shown in Figure 3A increasing P glc on paracellular glucose flux (Blue) .As P glc is increased from zero the point of equality of paracellular glucose flux Jglpc with glucose flux via SGLT1 decreases from infinity at P glc = 0 to around 20–30 mM luminal glucose when P glc = 0.01–0.02 cm s -1.
Increases P glc raises interstitial glucose concentrations 3C (blue) and in parallel, cysosolic concentrations Figure 3C (red). The reduction in glucose gradient across the basolateral membrane with raised P glc reduces and then reverses glucose flux via GLUT2 ( Figure 3B).
Capillary clearance of glucose is a key factor affecting net intestinal glucose absorption at the intestinal border, as submucosal capillary glucose concentration rapidly equilibrates with that in the interstitial solution 15. Intestinal glucose clearance depends on the local blood flow rate, determined by the superior mesenteric arterial (SMA) pressure and its compliance and also the mean glucose concentration difference between the villus capillaries and the interstitial solution ( Figure 1A–D). Thus, as can be seen by comparing Figures 1A and 1B with Figures 1C and 1D, increasing the capillary clearance reduces the interstitial glucose concentration, thereby increasing the glucose gradients across the baso-lateral membranes and between the luminal and interstitial solutions, thereby enhancing absorptive flux.
With constant high capillary glucose clearance, increasing apical GLUT2 activity, whilst enhancing glucose backflux across the apical membrane, also increases paracellular absorption. This tends to nullify the GLUT2-induced decrease in apical membrane net absorption.
Additional complexity is introduced by glucose-coupled Na + and water flows altering cytosolic and interstitial osmolarities, thereby generating changes in enterocyte cytosolic and interstitial fluid volume and interstitial pressure. The interstitial pressure changes affect fluid and solute flows via the paracellular pathway and via the capillaries and lymphatics 43. The effects on water flows are shown with green arrows in Figures 1A–D. As modelled here, changing the maximal rate of apical GLUT2 or capillary perfusion rates have relatively smaller effects on net water than on glucose flows.
This is also illustrated in Figures 2A and B, where increasing apical GLUT2 activity from zero ( Figure 2A) to a high level ( Figure 2B), increases GLUT2 backflux and also enhances glucose influx via the paracellular route. Consequently the net effect of altering apical GLUT2 activity on luminal glucose absorption is almost zero.
Effects of varying paracellular glucose permeability
Increased paracellular glucose diffusion has multiple effects on glucose fluxes and accumulation. Increasing paracellular glucose permeability directly increases paracellular glucose flux ( Figure 3A (blue)). This increases the interstitial glucose concentration ( Figure 3C (blue)). Raising interstitial glucose concentration decreases the glucose concentration gradient across the basolateral membrane, thereby decreasing basolateral glucose flux and raising cytosolic glucose concentration ( Figure 3C (red)). Increasing cytosolic glucose concentration reverses the direction of glucose flow across the apical membrane via GLUT2 ( Figure 3B (red)), but is without significant effect on glucose flux via SGLT1 ( Figure 3A), or cell volume ( Figure 3B (blue)).
Thus, it is evident that paracellular glucose diffusion significantly alters glucose fluxes, both directly via the paracellular and indirectly on the passive glucose fluxes at the apical and baso-lateral membranes. Only when paracellular glucose flux is close to zero is there any significant glucose influx via GLUT2 ( Figure 3B).
Effects of phloridzin and phloretin on intestinal glucose transport
The effect of phloridzin is to block SGLT1 without affecting GLUT2 1. As previously discussed, phloretin inhibits GLUTs 1-IV, but not GLUT5. However, it has additional effects on chloride, urea and water permeability, so also affects paracellular conductivity 22, 23.
Simulation of the temporal effects of phloridzin on intestinal glucose uptake exposed to luminal glucose 30 mM 1 shows that whilst inhibiting glucose influx via SGLT1, net glucose efflux via GLUT2 is abolished as a result of the decreased uphill glucose accumulation in the cytosol. Hence glucose flux across the basolateral membrane is reduced; however, because the interstitial glucose decreases due to diminished, transcellular flow paracellular glucose influx rises. Consequently, the net effect of SGLT1 inhibition by phloridzin on net glucose absorption is negligible, as observed in rabbit ileum pre-incubated with glucose 9. Following phloridzin inhibition of SGLT1, cytosolic glucose falls from ≈ 32 mM to ≈ 17 mM as simulated here ( Figure 4A and 4B).
Figure 4. Simulation of sequential additions of phloridzin then phloretin to the luminal fluid on intestinal glucose fluxes.
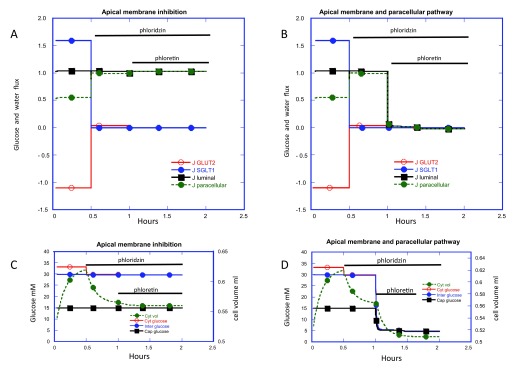
GLUT2 is present in the apical membrane V max 2 and the capillary perfusion rate = 10 is similar to that shown in Figure 2 panels B and D. The luminal glucose is 30 mM and afferent capillary glucose is 5 mM to simulate the conditions used by Kellett & Helliwell 2000 1. In Figure 4, panels A and B inhibition of SGLT1 activity at 0.5h to zero reduces glucose flux via SGLT1 to zero. Simultaneously glucose flux via GLUT2 increases thereby reversing the backflux from -1.1. to 0.05 and also paracellular glucose flux increases from 0.55 to 0.95. In panels C and D the glucose concentration changes in the cytosol (red) interstitial fluid (blue) capillary fluid (black) and cytosolic volume (green). Following phloridzin addition and inhibition of SGLT1, cytosolic glucose falls from 33 to 30 mM; cytosolic volume falls from 0.62 to 0.56 ml at 1 hour without significant changes in capillary or interstitial fluid glucose concentration. This explains both the fall in glucose reflux via GLUT2 and the decrease in basolateral membrane flux is compensated by the rise in paracellular flux thereby nullifying interstitial glucose concentration changes.
Phloretin addition at 1h is simulated by blocking apical GLUT2 (panel A) and by blocking both apical GLUT2 and paracellular glucose and Na permeability (panel B). GLUT2 fluxes fall to zero in both panels A and B and in panel A there is a small increase in paracellular glucose flow but the total transluminal glucose flux is unaffected by addition of phloretin after phloridzin. There is a small decrease in cytosolic volume from 0.56 to ≈ 0.55. the paracellular flux falls to zero as does the transluminal glucose flux in panel B, simulating the effect observed by Kellett and Helliwell 2000 1. This is accompanied by a large decrease in cell volume from 0.56 to 0.51 ml. Since no net glucose transport now occurs from the luminal fluid, capillary glucose concentration also decreases to 5 mM.
Subsequent inhibition of apical GLUT2 by phloretin is accompanied only by a very small decrease in net glucose influx as it falls to zero. However, this decrease in net transcellular glucose influx is supplemented by a reciprocal increase in paracellular flux, so that there is still a negligible change in net luminal glucose absorption. Thus, when glucose fluxes via apical SGLT1 and GLUT2 are completely inhibited, only the paracellular route remains to permit luminal to submucosal glucose flow and this flux rises to compensate for the reduced transcellular flow as a result of reduced interstitial glucose concentration.
The simulation shows that if phloretin inhibits only apical GLUT2, then it exerts no significant effect on luminal glucose uptake Figure 4A. If instead of only inhibiting glucose flux via apical GLUT2, phloretin also inhibits paracellular glucose and electrolyte fluxes, then the observed effect on intestinal glucose absorption ( Figure 4B) is similar to that observed by Kellett and Helliwell 1; namely, reduction in net luminal glucose flux to zero. The cytosolic glucose together with the interstitial glucose concentrations now fall to 5 mM; equal to the sink capillary glucose concentration, since now there is no compensatory rise in paracellular glucose flux occurring when interstitial glucose concentration is reduced.
Effects of GLUT2 on enterocyte volume during glucose loading via SGLT1
GLUT2 functions as an apical glucose shunt, thereby reducing cytosolic glucose accumulation by SGLT1. This shunt functions in two important ways, first by reducing net luminal influx, rather than increasing it as previously deduced 1. It will also redistribute the luminal glucose to more distal intestinal regions, consequently exposing larger intestinal surface areas to luminal glucose.
This latter effect may explain why when pigs are exposed to high carbohydrate diets raised SGLT1 protein and mRNA expression is observed in more distal intestinal regions 44. More SGLT1 is also observed in duodenal epithelia of morbidly obese humans 45. Increased density and increased area of intestinal SGLT1 expression implies that the intestine develops the capacity to deal with increased carbohydrate loads by absorbing more carbohydrate in aggregate, although not per unit area ( Figure 5). This will generate higher concentration peaks of carbohydrate in the splanchnic circulation following absorbable carbohydrate ingestion 41, 45 and higher rates of splanchnic blood flow in conscious animals 46– 48.
Figure 5. Diagram showing the predicted effects from the simulation model of loading enterocytes with luminal fluid containing 50 mM glucose and 5 mM glucose in the capillary perfusion fluid in A normal enterocytes expressing apical membrane GLUT2 and in B GLUT2 KO enterocytes.
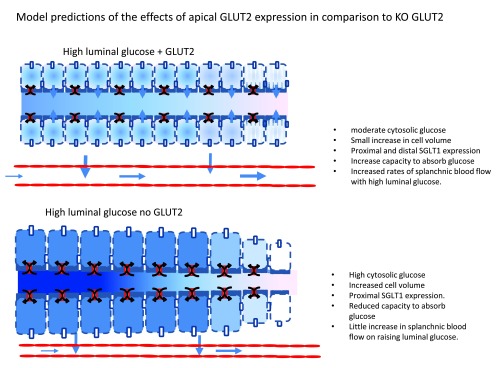
The KO cells have higher enterocyte glucose concentrations in the proximal intestine, but higher paracellular flow and larger cell volumes. The normal enterocytes have lower cytosolic glucose concentrations and SGLT1 is more widely dispersed along the intestinal length with higher rates of glucose permeation in distal regions of the small intestine. Long term exposure may lead to higher maximal glucose absorption rates in normal intestine than with GLUT2 KO.
It would seem more likely that instead of a means of enhancing apical glucose absorption, GLUT2 behaves primarily as an osmoregulator to maintain enterocyte volume in the face of large and rapid changes in the luminal and cytosolic osmotic pressure following ingestion of carbohydrates, or their subsequent dilution upon drinking water.
Since glucose is one of the most variable osmolytes within the intestine and splanchnic circulation, it is likely that a rapid adaptation to hyper or hypo osmotic changes within the intestinal lumen via a GLUT2 shunt pathway in the apical membrane would provide a useful means of regulating enterocyte volume, thereby avoiding excessive membrane stress and cytolysis. The reduced local net influx would also result in redistribution of hypertonic luminal glucose to more distal regions where this excess glucose would be absorbed by SGLT1.
GLUT2 has not previously been considered as an osmoregulator of enterocyte volume. This role has been mainly assigned to potassium and chloride channels 49. Whilst ion channels certainly provide an important role in cell volume regulation, they may not be as well adapted as GLUT2 to fulfilling the enterocytes’ specialized needs for osmotic control due to large changes in sugar dependent osmotic gradients.
Simulation shows that glucose accumulation within the cytosol via SGLT1 is accompanied by an increased cytosolic volume Figures 2C and 2D, Figures 4C and 4D. The effect of increased rates of apical GLUT2 which prevents excessive glucose accumulation at high luminal glucose concentrations, compare Figure 2C with Figure 2D, also reduces enterocyte volume increase.
Summary and conclusions
Kellett & Helliwell (2000) 1 have proposed that the non-saturable component of intestinal glucose absorption, apparent when luminal glucose is raised above 15 mM, is due to enhanced flux via the low affinity glucose transporter GLUT2, which they and others have observed 32 is present within the apical border of the jejunum and ileum following prolonged exposure to high intraluminal glucose or following activation of protein kinase C by phorbol myristate acetate.
Evidence in support of this contention is that this “non-saturable” component is inhibited by high phloretin or high cytochalasin B concentrations – which both can inhibit GLUT2. The K m of the phloretin sensitive component claimed to be similar to that of GLUT2 approximately 56±14; n = 1.6±0.4.
They argue that GLUT2 is the most likely route for this low affinity transport, since it also transports fructose and the only other fructose transporter GLUT5 is insensitive to inhibition by either cytochalasin B or phloretin.
However, it is unclear that upregulation of GLUT2 within the intestinal brush border actually does enhance D-glucose absorption. At raised luminal glucose concentrations the cytosolic concentrations and the submucosal interstitial fluid glucose concentrations will exceed the intestinal luminal glucose concentrations, so GLUT2 will stimulate passive downhill glucose reflux from the enterocyte cytosol, thus reducing net glucose uptake across the luminal surface. This glucose backflux may be augmented by glucose reflux via the paracellular pathway when the interstitial glucose is raised. This will occur when the splanchnic capillary glucose concentration is raised above 10 mM, as occurs during ingestion of high glucose loads, or in hyperglycaemic states.
These states are simulated here with a model of intestinal glucose transport incorporating glucose sodium and water cotransport across the luminal border variable rates of apical GLUT2 and paracellular flows and variable rates of capillary clearance of solutes and water from the submucosal interstitial fluid.
The model demonstrates that apical membrane GLUT2 may usefully function as osmoregulator to prevent excessive enterocyte volume changes during glucose loading, or following sudden decreases in luminal glucose concentration.
Methods
Summary of model equations
The simultaneous flows of glucose Na and water from lumen across the apical membrane to cytosol and across the intercellular junctions from lumen to interstitial space followed by flows across the basolateral membrane of glucose Na and water to the interstitial space and from the interstitial space to the capillary lumen are modelled using Berkeley Madonna version 9.0119 http://www.berkeleymadonna.com/. Water flows generated by the osmotic pressure generated across the membrane boundaries between adjacent compartments generate volume changes in the cytosol and interstitial compartments. These volume changes are controlled by independent apical and baso-lateral hydraulic coefficients. Additionally, Na + and glucose flow via SGLT1 and GLUT2 are assumed to generate a coupled water flow 50, 51 and modelled as an additional component of water flux across both apical and basolateral membranes. The interstitial fluid compartment is assumed to have a non-linear elasticity similar to that observed by Granger 52 so that interstitial pressure rises non-linearly with volume.
The Na glucose and coupled flows and uncoupled flows via SGLT1 are modelled as outlined in 53 glucose flow across both apical and basolateral GLUT2 is modelled according to a simple two site model
i.e J glucose = (G out/(1+G out)-G in/(1+G in).V max;
where G out and G in are the glucose concentration/K m(GLUT2) in the adjoining membrane compartments and K m GLUT2 is the assigned GLUT2 K m = 17 mM. Na flux basal-lateral membranes is assumed to have a similar kinetics between two to saturable sites to that of glucose K m Na = 25 mM.
Additionally Na is pumped from the cytosol into the interstitial solution, according to the simple saturation equation
J Napump = Na cyt.V m(Napump)/(Na cyt+K m(Napump)).
Flows of glucose and Na between the interstitial fluid and capillary fluid are assume to take the form of the convective diffusion equation
J i = J w(C o+C i)/2 + P i(C o-C i).
Where J i is the solute flux and J w is water flux between interstitial fluid and capillary fluid C o and C i are the external and internal concentrations of solute i and P i is the permeability of solute i.
The water flow across the paracellular pathway Jwc pc is determined by the osmotic and hydraulic pressure difference between the luminal and interstitial solutions hence Jwc pc = L p (2(Na in-Na lum).σ Na + ((G in-G lum).σ Glc - P in) where i in and i lum are the osmotic pressure of solutes I in the luminal and interstitial fluids σ i is the reflection coefficient of solute I and P in the interstitial pressure mm Hg.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
v1; ref status: indexed
Appendix {comments are italicised with curly brackets}
d/dt (Cytgl)= + Japgl – JGlbl { Cytgl = cytosolic glucose mM Japgl = glucose flux across the apical membrane = SGLT1+ GLUT2 flux; JGlbl = basolateral glucose flux.}
INIT Cytgl = 0 /Cytvol { INIT = initial ; cytvol = cytosolic volume /cm 2 }
LIMIT Cytgl >= 0
d/dt (CytN)} = + JNap – JNbl {CytN = cytosol Na mM; JNap = Na flux across apical membrane via SGLT1 coupled and uncoupled; JNbl = Na flux across baso-lateral membrane = Na pump flux and passive Na flux}.
INIT CytN = 1.5/Cytvol
LIMIT CytN >= 0
d/dt (inGlc) = + Jglpc - JcapGl + JGlbl { Jglpc = paracellular glucose flux; JcapGL= glucose flux from interstitial to capillary fluid;}
INIT inGlc = 0/Invol {inGlc = interstitial glucose concentration}
d/dt(InNc) = - JNcap + JNbl + JNpc { JNcap {inNc= interstitial Na concentration; JNcap = Naflux from interstitial to capillary fluid; JNbl Na flux across basolateral membrane}
INIT InNc = 0/{Invol}
LIMIT InNc >= 0
d/dt(Invol)= + Jblw - Jcapw + Jpcw {Invol = interstitial volume; Jblw = water flux across basolateral membrane; Jcapw= water flux between interstitial fluid and capillary fluid; Jpcw = paracellular water flux}
INIT Invol = 0.1 {invol= interstitial volume ml}
LIMIT Invol >= 0
d/dt (Cytvol) = - Jblw + Japw {Cytvol = cytosolic water volume; Jblw water flow across basolateral membrane includes osmotic and sugar coupled flows; Japw= waterflow across apical membrane includes osmotic and sugar coupled flows.}
INIT Cytvol = 0.5
LIMIT Cytvol >= 0
Berkeley Madonna equations
{Reservoirs}
d/dt (Cytgl) = + Japgl - JGlbl
INIT Cytgl = 0 /Cytvol
LIMIT Cytgl >= 0
d/dt (CytN) = + JNap - JNbl
INIT CytN = 1.5/Cytvol
LIMIT CytN >= 0
d/dt (inGlc) = + Jglpc - JcapGl + JGlbl
INIT inGlc = 0/Invol
d/dt (InNc) = - JNcap + JNbl + JNpc
INIT InNc = 0/(Invol)
LIMIT InNc >= 0
d/dt (Invol) = + Jblw - Jcapw + Jpcw
INIT Invol = 0.1
LIMIT Invol >= 0
d/dt (Cytvol) = - Jblw + Japw
INIT Cytvol = 0.5
LIMIT Cytvol >= 0
{Flows}
Japgl = (LumGl1sat-cyGLu1sat)*LGg+Lgn2*(LumNsat-CyNsat)+(LumGl2sat-Cyglu2sat)*VmGlap
JGlbl = (cytglc/(cytglc+KmGbl)-InsGlc/(InsGlc+KmGbl))*VmGlbl
JNap = (LumNsat-CyNsat)*LNn+(LumGl1sat-cyGLu1sat)*Lgn2
JNbl = (CyNsat-InNsat)*LNn+Vpump*CyNsat
JcapGl = Jcapw*(InsGlc+capGlc)/2+(InsGlc-capGlc)*PcapGl/1000
JNcap = Jcapw*(InNc+capNc)/2+PcapN*(InNc-150)/1000
Jglpc = (LumGlc-InsGlc)*Pglpc+Jpcw*(1-sigGl)*(LumGlc+InsGlc)/2
JNpc = (LumN-InNc)*PNpc+Jpcw*(1-SigN)*(LumN+InNc)/2
Jpcw = (insosm2-lumosm2+IntPressure)*(Lppc)/1E6
Jcapw = IntPressure^2*Lpcap/1E6
Japw = (cytosm-Lumosm)*Lpap/1E9+Japgl*Gwcoef/100
Jblw = (Inosm-cytosm)*Lpbl /1E9
{Functions}
LumGl1nor = LumGlc/ KmGl1
CyNnor = CytN/KmNap
LumGlc = if time > 5000 then (time-5000)*1E-5 else 0
LumN = 150
LumNnor = LumN/KmNap
LumGl1sat = LumGl1nor/(LumGl1nor+1)
LumNsat = LumNnor/(LumNnor+1)
LumGlc1nor = cytglc/KmGl1
cyGLu1sat = LumGlc1nor/(LumGlc1nor+1)
CyNsat = CyNnor/(CyNnor+1)
InsGlc = inGlc
Lumgl2nor = LumGlc/KmGl2
LumGl2sat = Lumgl2nor/(Lumgl2nor+1)
Cyglcnor = cytglc/KmGl2
Cyglu2sat = Cyglcnor/(Cyglcnor+1)
InNsat = InNc/KmNap/(InNc/KmNap+1)
Lumosm = LumN*2+LumGlc
Inosm = InNc*2*SigN+InsGlc*sigGl
cytglc = Cytgl
CyNc = CytN
cytosm = fixosm+CyNc*2+cytglc
fixosm = 125/Cytvol
JNlum = JNap+JNpc
Jlumw = Jpcw+Japw
IntPressure = (0.0+289.76*(Invol-0.01)-3553* (Invol-0.1)^2+28191*(Invol-0.01)^3-1.0767e5*(Invol-0.01)^4+1.5528e5*(Invol-0.01)^5)*pccoef
Jtotlumgl = Jglpc+Japgl
Lppc = Lppc*intPressure*pccoef
Lgn2 = LGng*LumNsat*LumGl2sat
pccoef = 1
capGlc = 5+(InsGlc-5)*0.4
capNc = 150+(InNc-150)*0.6
lumosm2 = LumGlc*sigGl+LumN*2*SigN
insosm2 = InNc*2*SigN+InsGlc*sigGl
JGLUT2 = (LumGl2sat-Cyglu2sat)*VmGlap
JSGLT1 = Japgl-JGLUT2
{Globals}
KmGl1=2
KmGl2=15
KmNap=25
KmGbl=5
LGg =1
LGng=0.1
LNn=1
VmGlap=1
VmGlbl=1
Vpump =1
PcapGl=0.1
PcapN=0.1
Pglpc=0.1
PNpc= 0.01
Pprot=0.1
Lppc=1E-5
Lpcap= 1E-6
Lpap=1E-6
Lpbl=1E-6
sigGl= 0.2
SigN=0.5
Gwcoef=500
References
- 1.Kellett GL, Helliwell PA: The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J. 2000;350(Pt 1):155–62. 10.1042/0264-6021:3500155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hummel CS, Lu C, Loo DD, et al. : Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol. 2011;300(1):C14–21. 10.1152/ajpcell.00388.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hummel CS, Lu C, Liu J, et al. : Structural selectivity of human SGLT inhibitors. Am J Physiol Cell Physiol. 2012;302(2):C373–82. 10.1152/ajpcell.00328.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debnam ES, Levin RJ: Effects of fasting and semistarvation on the kinetics of active and passive sugar absorption across the small intestine in vivo. J Physiol. 1975;252(3):681–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IIundain A, Lluch M, Pons F: Kinetics of intestinal sugar transport, in vivo. Rev Esp Fisiol. 1979;35(3):359–66. [PubMed] [Google Scholar]
- 6.Bolufer J, Delgado MJ, Murillo F, et al. : Galactose transport across rat small intestine in vivo following distal resections of varying extents. Q J Exp Physiol. 1986;71(3):423–31. [DOI] [PubMed] [Google Scholar]
- 7.Pappenheimer JR, Reiss KZ: Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 1987;100(2):123–36. 10.1007/BF02209145 [DOI] [PubMed] [Google Scholar]
- 8.Pappenheimer JR: Paracellular intestinal absorption of glucose, creatinine, and mannitol in normal animals: relation to body size. Am J Physiol. 1990;259(2 Pt 1):G290–9. [DOI] [PubMed] [Google Scholar]
- 9.Naftalin RJ, Tripathi S: The roles of paracellular and transcellular pathways and submucosal space in isotonic water absorption by rabbit ileum. J Physiol. 1986;370:409–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischbarg J: Fluid transport across leaky pithelia: central role of the tight junction and supporting role of aquaporins. Physiol Rev. 2010;90(4):1271–90. 10.1152/physrev.00025.2009 [DOI] [PubMed] [Google Scholar]
- 11.Pappenheimer JR, Michel CC: Role of villus microcirculation in intestinal absorption of glucose: coupling of epithelial with endothelial transport. J Physiol. 2003;553(Pt 2):561–74. 10.1113/jphysiol.2003.043257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granger DN, Mortillaro NA, Taylor AE: Interactions of intestinal lymph flow and secretion. Am J Physiol. 1977;232(1):E13–8. [DOI] [PubMed] [Google Scholar]
- 13.Bijlsma PB, Fihn BM, Sjöqvist A, et al. : Water absorption enhances the uptake of mannitol and decreases Cr-EDTA/mannitol permeability ratios in cat small intestine in situ. Scand J Gastroenterol. 2002;37(7):799–806. 10.1080/gas.37.7.799.806 [DOI] [PubMed] [Google Scholar]
- 14.Boyd CA, Cheeseman CI, Parsons DS: Effects of sodium on solute transport between compartments in intestinal mucosal epithelium. Nature. 1975;256(5520):747–9. 10.1038/256747a0 [DOI] [PubMed] [Google Scholar]
- 15.Boyd CA, Parsons DS: Movements of monosaccharides between blood and tissues of vascularly perfused small intestine. J Physiol. 1979;287:371–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellett GL, Brot-Laroche E: Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes. 2005;54:3056–62. 10.2337/diabetes.54.10.3056 [DOI] [PubMed] [Google Scholar]
- 17.Kellett GL, Brot-Laroche E, Mace OJ, et al. : Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr. 2008;28:35–54. 10.1146/annurev.nutr.28.061807.155518 [DOI] [PubMed] [Google Scholar]
- 18.Mace OJ, Affleck J, Patel N, et al. : Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582(Pt 1):379–92. 10.1113/jphysiol.2007.130906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mace OJ, Schindler M, Patel S: The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol. 2012;590(Pt 12):2917–36. 10.1113/jphysiol.2011.223800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stumpel F, Burcelin R, Jungermann K, et al. : Normal kinetics of intestinal glucose absorption in the absence of GLUT2: evidence for a transport pathway requiring glucose phosphorylation and transfer into the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2001;98(20):11330–5. 10.1073/pnas.211357698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbuckle MI, Kane S, Porter LM, et al. : Structure-function analysis of liver-type (GLUT2) and brain-type (GLUT3) glucose transporters: expression of chimeric transporters in Xenopus oocytes suggests an important role for putative transmembrane helix 7 in determining substrate selectivity. Biochemistry. 1996;35(51):16519–27. 10.1021/bi962210n [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann EK, Simonsen LO, Sjoholm C: Membrane potential, chloride exchange and chloride conductance in Ehrlich mouse ascites tumour cells. J Physiol. 1979;296:61–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esteva-Font C, Phuan PW, Anderson MO, et al. : A small molecule screen identifies selective inhibitors of urea transporter UT-A. Chem Biol. 2013;20(10):1235–44. 10.1016/j.chembiol.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Röder PV, Geillinger KE, Zietek TS, et al. : The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One. 2014;9(2):e89977. 10.1371/journal.pone.0089977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirazi-Beechey SP, Moran AW, Batchelor DJ, et al. : Glucose sensing and signalling; regulation of intestinal glucose transport. Proc Nutr Soc. 2012;70(2):185–93. 10.1017/S0029665111000103 [DOI] [PubMed] [Google Scholar]
- 26.Ma J, Chang J, Checklin HL, et al. : Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br J Nutr. 2010;104(6):803–6. 10.1017/S0007114510001327 [DOI] [PubMed] [Google Scholar]
- 27.Ford HE, Peters V, Martin NM, et al. : Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr. 2011;65(4):508–13. 10.1038/ejcn.2010.291 [DOI] [PubMed] [Google Scholar]
- 28.Kellett GL: Comment on: Gorboulev et al. Na+-D-glucose cotransporter SGLT1 Is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012;61:187–196. Diabetes. 2012:61(6):e4. 10.2337/db11-1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habold C, Foltzer-Jourdainne C, Le Maho Y, et al. : Intestinal gluconeogenesis and glucose transport according to body fuel availability in rats. J Physiol. 2005;566(Pt 2):575–86. 10.1113/jphysiol.2005.085217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brot-Laroche E, Dao MT, Alcaldet AI, et al. : Independent modulation by food supply of two distinct sodium-activated D-glucose transport systems in the guinea pig jejunal brush-border membrane. Proc Natl Acad Sci U S A. 1988;85(17):6370–3. 10.1073/pnas.85.17.6370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brot-Laroche E, Supplisson S, Delhomme B, et al. : Characterization of the D-glucose/Na+ cotransport system in the intestinal brush-border membrane by using the specific substrate, methyl alpha-D-glucopyranoside. Biochim Biophys Acta. 1987;904(1):71–80. 10.1016/0005-2736(87)90088-5 [DOI] [PubMed] [Google Scholar]
- 32.Cohen M, Kitsberg D, Tsytkin S, et al. : Live imaging of GLUT2 glucose-dependent trafficking and its inhibition in polarized epithelial cysts. Open Biol. 2014;4(7). 10.1098/rsob.140091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karasov WH, Debnam ES: Rapid adaptation of intestinal glucose transport: a brush-border or basolateral phenomenon? Am J Physiol. 1987;253(1 Pt 1):G54–61. [DOI] [PubMed] [Google Scholar]
- 34.Cheeseman CI, O’Neill D: Basolateral D-glucose transport activity along the crypt-villus axis in rat jejunum and upregulation induced by gastric inhibitory peptide and glucagon-like peptide-2. Exp Physiol. 1998;83(5):605–16. [DOI] [PubMed] [Google Scholar]
- 35.Holman GD, Naftalin RJ: Transport of 3-O-methyl D-glucose and beta-methyl D-glucoside by rabbit ileum. Biochim Biophys Acta. 1976;433(3):597–614. 10.1016/0005-2736(76)90284-4 [DOI] [PubMed] [Google Scholar]
- 36.Eskandari S, Wright EM, Loo DD: Kinetics of the reverse mode of the Na+/glucose cotransporter. J Membr Biol. 2005;204(1):23–32. 10.1007/s00232-005-0743-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kedem O, Katchalsky A: Permeability of composite membranes. Part 3.—Series array of elements. Trans Faraday Soc. 1963;59:1941–53 10.1039/TF9635901941 [DOI] [Google Scholar]
- 38.Bohlen HG: Intestinal tissue PO 2 and microvascular responses during glucose exposure. Am J Physiol. 1980;238(2):H164–71. [DOI] [PubMed] [Google Scholar]
- 39.Bohlen HG: Na +-induced intestinal interstitial hyperosmolality and vascular responses during absorptive hyperemia. Am J Physiol. 1982;242(5):H785–9. [DOI] [PubMed] [Google Scholar]
- 40.Vanis L, Gentilcore D, Rayner CK, et al. : Effects of small intestinal glucose load on blood pressure, splanchnic blood flow, glycemia, and GLP-1 release in healthy older subjects. Am J Physiol Regul Integr Comp Physiol. 2011;300(6):R1524–31. 10.1152/ajpregu.00378.2010 [DOI] [PubMed] [Google Scholar]
- 41.Qamar MI, Read AE, Mountford R: Increased superior mesenteric artery blood flow after glucose but not lactulose ingestion. Q J Med. 1986;60(233):893–6. [PubMed] [Google Scholar]
- 42.Someya N, Endo MY, Fukuba Y, et al. : Blood flow responses in celiac and superior mesenteric arteries in the initial phase of digestion. Am J Physiol Regul Integr Comp Physiol. 2008;294(6):R1790–6. 10.1152/ajpregu.00553.2007 [DOI] [PubMed] [Google Scholar]
- 43.Granger DN, Kvietys PR, Mailman D, et al. : Intrinsic regulation of functional blood flow and water absorption in canine colon. J Physiol. 1980;307:443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moran AW, Al-Rammahi MA, Arora DK, et al. : Expression of Na+/glucose co-transporter 1 (SGLT1) in the intestine of piglets weaned to different concentrations of dietary carbohydrate. Br J Nutr. 2010;104(5):647–55. 10.1017/S0007114510000954 [DOI] [PubMed] [Google Scholar]
- 45.Nguyen NQ, Debreceni TL, Bambrick JE, et al. : Accelerated intestinal glucose absorption in morbidly obese humans - relationship to glucose transporters, incretin hormones and glycaemia. J Clin Endocrinol Metab. 2014:jc20143144. 10.1210/jc.2014-3144 [DOI] [PubMed] [Google Scholar]
- 46.Anzueto Hernandez L, Kvietys PR, Granger DN: Postprandial hemodynamics in the conscious rat. Am J Physiol. 1986;251(1 Pt 1):G117–23. [DOI] [PubMed] [Google Scholar]
- 47.Granger DN, Kvietys PR, Wilborn WH, et al. : Mechanism of glucagon-induced intestinal secretion. Am J Physiol. 1980;239(1):G30–8. [DOI] [PubMed] [Google Scholar]
- 48.Parker HE, Adriaenssens A, Rogers G, et al. : Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia. 2012;55(9):2445–55. 10.1007/s00125-012-2585-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macleod RJ, Hamilton JR: Volume regulation initiated by Na(+)-nutrient cotransport in isolated mammalian villus enterocytes. Am J Physiol. 1991;260(1 Pt 1):G26–33. [DOI] [PubMed] [Google Scholar]
- 50.Zeuthen T: Water-transporting proteins. J Membr Biol. 2010;234(2):57–73. 10.1007/s00232-009-9216-y [DOI] [PubMed] [Google Scholar]
- 51.Naftalin RJ: Osmotic water transport with glucose in GLUT2 and SGLT. Biophys J. 2008;94(10):3912–23. 10.1529/biophysj.107.122531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Granger DN: Intestinal microcirculation and transmucosal fluid transport. Am J Physiol. 1981;240(5):G343–9. [DOI] [PubMed] [Google Scholar]
- 53.Naftalin RJ: Reassessment of models of facilitated transport and cotransport. J Membr Biol. 2010;234(2):75–112. 10.1007/s00232-010-9228-7 [DOI] [PubMed] [Google Scholar]