Abstract
Background
Chronic systemic inflammation is common in patients with chronic kidney disease on dialysis (CKD5D) and has been considered a key mediator of the increased cardiovascular risk in this patient population. In this study, we tested the hypothesis that supplementation of omega-3 polyunsaturated fatty acids (ω-3 PUFAs) will attenuate the systemic inflammatory process in CKD5D patients.
Methods
The design was a randomized, double-blinded, placebo controlled pilot trial (NCT00655525). Thirty-eight patients were randomly assigned in a 1 : 1 fashion to receive 2.9 g of eicosapentaenoic acid (C20:5, n-3) plus docosahexaenoic acid (C22:6, n-3) versus placebo for 12 weeks. The primary outcome was change in pro-inflammatory chemokines measured by lipopolysaccharide (LPS)-stimulated peripheral blood mononuclear cells (PBMCs). Secondary outcomes were changes in systemic inflammatory markers. Analysis of covariance was used to compare percent change from baseline to 12 weeks.
Results
Thirty-one patients completed 12 weeks and three patients completed 6 weeks of the study. Median age was 52 (interquartile range 45, 60) years, 74% were African-American and 79% were male. Supplementation of ω-3 PUFAs effectively decreased the LPS-induced PBMC expression of RANTES (Regulated upon Activation, Normal T cell Expressed and Secreted) and MCP-1 (Monocyte Chemotactic Protein-1; unadjusted P = 0.04 and 0.06; adjusted for demographics P = 0.02 and 0.05, respectively). There was no significant effect of the intervention on serum inflammatory markers (C-reactive protein, interleukin-6 and procalcitonin).
Conclusions
The results of this pilot study suggest that supplementation of ω-3 PUFAs is beneficial in decreasing the levels of endothelial chemokines, RANTES and MCP-1. Studies of larger sample size and longer duration are required to further evaluate effects of ω-3 PUFAs on systemic markers of inflammation, other metabolic parameters and clinical outcomes, particularly cardiovascular outcomes in CKD5D patients.
Keywords: omega-3, end stage renal disease, inflammation, MCP-1, RANTES
INTRODUCTION
Systemic inflammation is highly prevalent and is associated with a high mortality rate due to cardiovascular causes in patients with chronic kidney disease on dialysis (CKD5D) [1–3]. Epidemiological studies and clinical trials have shown that long-chain omega-3 polyunsaturated fatty acids (ω-3 PUFAs), such as eicosapentaenoic acid (EPA; C20:5, n-3) and docosahexaenoic acid (DHA; C22:6, n-3), have cardioprotective effects in the general population [4], decreasing the risk of myocardial infarction, sudden death and stroke. The anti-inflammatory effects of EPA and DHA have been considered one of the most relevant mechanisms involved in the cardioprotective and beneficial effects of ω-3 PUFAs [5–7]. Studies examining the effects of EPA on cytokine production found a decrease in cytokine production (ranging from 21–90%), mostly using lipopolysaccharide (LPS)-induced cytokine production by peripheral blood mononuclear cells (PBMCs) [5, 8] and some using systemic inflammatory markers [9]. Additionally, asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor, which has been linked to cardiovascular risk and influenced by inflammation [10], and symmetric dimethylarginine (SDMA), which has been shown to be involved in promoting oxidative stress [11], are markedly elevated in patients with CKD5D. However, there is scarce data regarding the effect of n-3 fatty acids on levels of ADMA or SDMA.
Chronic kidney disease (CKD) patients have lower levels of ω-3 PUFAs in plasma and cells compared with patients without CKD, and they have very low consumption of ω-3 PUFAs [7, 12]. Studies in CKD5D patients have shown that fish consumption and ω-3 PUFA supplementation is associated with improved vascular access patency and rates of thrombosis and potentially cardiovascular morbidity and mortality risk and inflammation-associated muscle loss [13–15].
In this study, we hypothesized that EPA and DHA supplementation will ameliorate the systemic inflammatory response observed in CKD5D patients as measured by production of pro-inflammatory cytokines [interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α)], and the early endothelial chemokines [Regulated upon Activation, Normal T cell Expressed and Secreted (RANTES) and Monocyte Chemotactic Protein-1 (MCP-1)] by LPS-stimulated peripheral blood mononuclear cells (PBMCs). Additionally, we examined the effect of EPA and DHA supplementation on systemic markers of inflammation, including plasma concentrations of inflammatorymarkers [pro-inflammatory cytokines, high-sensitivity C-reactive protein (hsCRP) and procalcitonin], insulin sensitivity (insulin sensitivity marker homeostatic model assessment, HOMA-IR) and dimethylarginines (ADMA and SDMA) levels.
MATERIALS AND METHODS
Study participants
The study recruited patients from the Vanderbilt University Medical Center (VUMC) affiliated dialysis units and the Veterans Affairs Tennessee Valley Healthcare System (VATVHS) Nashville campus between September 2008 and June 2011. Inclusion criteria included being on maintenance hemodialysis for >6 months, well-functioning hemodialysis vascular access or permanent dialysis catheter, signs of chronic inflammation (average CRP of ≥5 mg/L over three consecutive measurements) and acceptable dialysis adequacy (Kt/V >1.2). Exclusion criteria included pregnancy; intolerance to study medication; diabetes mellitus on insulin therapy; severe, unstable, active or chronic inflammatory disease (active infection, active connective tissue disorder, active cancer, HIV and liver disease); hospitalization within 1 month prior to study; and receiving steroids (>5 mg/day) and/or need for any immunosuppressive agents. The study was approved by the Institutional Review Boards from both VUMC and VATVHS, and signed informed consent was obtained from all patients.
Study design
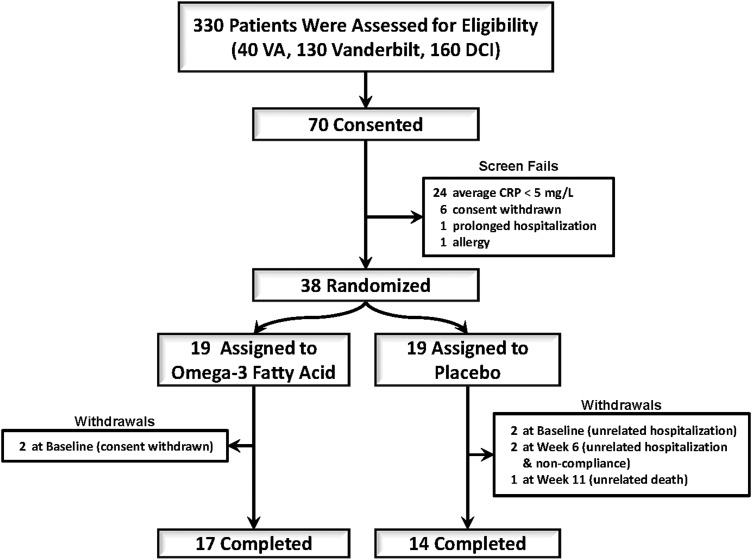
This design was a randomized, placebo-controlled, double-blinded pilot and feasibility study (ClinicalTrials.gov number NCT00655525). Of 330 screened patients, 38 prevalent hemodialysis patients were randomized in a 1 : 1 ratio to receive 2.9 g of EPA/DHA or matching placebo daily for 12 weeks (Figure 1). All laboratory values were measured at baseline, 6 weeks and 12 weeks. In addition to the collection of PBMCs, blood was collected at baseline for biomarkers of inflammation and insulin resistance. The study was overseen by a Data Safety Monitoring Board for safety.
FIGURE 1:
Patient enrollment, randomization and completion flow diagram.
Intervention and randomization
Patients were randomized in a 1 : 1 manner to receive 2.9 g of EPA : DHA (2 : 1 ratio) in capsules or matching placebo daily for 12 weeks (Ocean Nutrition via Yasoo Health, Inc.). The rationale for the dose of EPA/DHA is based on multiple studies showing the effectiveness at this dose [16]. The investigational pharmacies at the participating sites dispensed monthly pill supply to participants. Review of compliance (pill counting) and adverse events were discussed with the participant monthly.
Study end points
The primary end point was the percent changes in pro-inflammatory cytokines (IL-6 and TNF-α) and chemokines (RANTES and MCP-1) that were produced by LPS stimulation of peripheral blood mononuclear cells (PBMCs). Secondary outcomes were the percent changes in systemic inflammatory markers such as IL-6, hsCRP and procalcitonin. Exploratory outcomes included changes in ADMA, SDMA, insulin sensitivity (HOMA-IR) and total free fatty acids (FFA).
Measurements
Blood samples were drawn pre-dialysis into Vacutainer® (Becton Dickinson, Franklin Lakes, NJ) tubes containing ethyldiaminetetraacetic acid for plasma separation. Plasma samples were transported on ice and centrifuged at 4°C at 3000 r.p.m. for 15 min. Supernatants were stored in aliquots at −80°C until measurement day. RANTES, MCP-1, IL-6 and TNF-α concentrations were determined by LPS stimulation of PBMCs using cytometric bead arrays, and two-color flow cytometric analysis was performed using a BD LSR II flow cytometer (Becton Dickinson, San Jose, CA). Peripheral blood mononuclear cells were isolated from whole blood by centrifugation through Ficoll-Hypaque solution. They were then washed twice in RPMI 1640 and re-suspended in culture medium at a concentration of 106/mL. One half milliliter of cell suspension was then added to wells of a 24-well tissue culture plate. Next, 0.5 mL of mitogens at a 2× final concentration in culture medium, or 0.5 mL of additional medium (for the cell control) was added to the wells, yielding a final concentration of 5 × 105 cells/mL. The final concentrations for concanavalin A, pokeweed mitogen, phytohemagglutinin and Staphylococcus aureus Cowen were 5, 5, 10 and 10 µg/mL, respectively. Plates were incubated for 3 days (37°C, 95% air, 5% CO2 and 100% humidity). CRP levels were measured using the high-sensitivity particle-enhanced turbidimetric UniCel Dxl Immunoassay System (Beckman Coulter, Brea, CA). Insulin was measured by using a double-antibody RIA (DA RIA; Millipore, St. Charles, MO). Glucose concentrations were measured by using the glucose oxidase method (Glucose Analyzer 2; Beckman Coulter, Brea, CA). IL-6 concentrations were determined using cytometric bead arrays (Becton Dickinson, San Jose, CA). Free fatty acids were extracted from the plasma using heptane/isopropanol (30 : 70). The fatty acid methyl esters were analyzed by gas chromatography using an Agilent 7890 gas chromatograph equipped with a flame ionization detector and a capillary column (SP2380, 0.25 mm × 30 m, 0.25 µm film, Supelco, Bellefonte, PA). Inclusion of a pentadecanoic acid (15 : 0) internal standard permitted quantitation of the amount of FFA in the sample.
Statistical analysis
Data are presented as mean ± SD or as median with interquartile range (IQR) depending on their distribution. Baseline characteristics were compared using the Wilcoxon test for continuous variables and χ2 test for categorical variables. Variables included: demographics, dialysis vintage, access type, diabetes type 2 currently on medical treatment, body mass index, and truncal fat mass percent as a marker for visceral adiposity. Analysis of covariance (ANCOVA) was used to compare percent change from baseline to 12 weeks for the primary and secondary outcomes between the groups. Outcome variables were cube-root-transformed to improve normality in residuals, and the transformed baseline value of the outcome variable was adjusted as a covariate. Regression coefficients from the ANCOVA model were exponentiated, which indicates the ratio of percent change from baseline to 12 weeks. ANCOVA assesses change by adjusting baseline as a regression coefficient rather than using change directly as an outcome variable. Due to the small number of participants, we were limited in the number of variables that we could adjust for and we present the unadjusted model, a model adjusted only for demographics, and a model adjusted for demographics and two other variables that were unevenly distributed at baseline, including hsCRP and truncal fat mass percent measured by dual energy X-ray absorptiometry. Additionally, we conducted three exploratory models adjusting for procalcitonin and the dimethylarginines, ADMA and SDMA.
RESULTS
We prescreened 330 prevalent CKD5D patients and consented 70 subjects. Of those, 26 patients screen failed (24 did not meet the hsCRP criteria, 1 had an allergy to fish oil and 1 had a prolonged hospitalization prior to randomization), six patients withdrew consent prior to randomization for personal reasons and 38 patients were randomized. From the randomized patients there were four withdrawals, two due to unrelated hospitalizations and two by personal preferences. Thirty-one completed the trial and another three patients completed to Week 6 (all from the placebo group) for a total of 34 patients having all outcome measurements performed (Figure 1). Baseline characteristics of the study subjects were similar between the study groups with the exceptions noted below (Table 1). Median age was 52 years (IQR 45, 60), 74% were African-American, 79% were male and 91% had a dialysis graft or a fistula. The median time on dialysis was 47 (IQR 14, 113) months and median BMI was 30 (23, 39). The only characteristic that was statistically different between groups was hsCRP [7.8 (4.3, 12.8) in the intervention group versus 15.2 (9.9, 18.3) in the placebo group (P = 0.04)].
Table 1.
Baseline characteristics by the randomization group
| Variables | Omega-3 (n = 17) | Placebo (n = 17) | ALL (n = 34) | P-value |
|---|---|---|---|---|
| General characteristics | ||||
| Age, years | 50 (38, 58) | 53 (45, 65) | 52 (45, 60) | 0.41 |
| Gender, % male (n) | 82 (14) | 77 (13) | 79 (27) | 0.7 |
| Race, % African-Americans (n) | 71 (12) | 77 (13) | 74 (25) | 0.7 |
| Body mass index, kg/m2 | 30 (22, 33) | 32 (26, 44) | 30.2 (23.2, 39) | 0.2 |
| Truncal fat, % | 36 (19, 50) | 46 (37, 52) | 44 (27, 51) | 0.1 |
| Dialysis vintage, months | 50 (21, 111) | 43 (14, 114) | 47 (14, 113) | 0.5 |
| Diabetes type 2, % (n) | 0 (0) | 6 (1) | 3 (1) | 0.5 |
| Access type | ||||
| Dual lumen catheter, % (n) | 12 (2) | 6 (1) | 9 (3) | 0.2 |
| Fistulas, % (n) | 41 (7) | 71 (12) | 56 (19) | |
| Graft, % (n) | 47 (8) | 24 (4) | 35 (12) | |
| Inflammatory markers | ||||
| hsCRP, mg/dL | 7.8 (4.3, 12.8) | 15.2 (9.9, 18.3) | 11.4 (6.4, 17.3) | 0.04* |
| IL-6, pg/mL | 9.06 (4.5, 11.29) | 10.1 (3.3, 23.8) | 9.1 (4.0, 14.3) | 0.5 |
| Procalcitonin, ng/mL | 0.30 (0.25, 0.45) | 0.34 (0.27, 0.44) | 0.33 (0.25, 0.45) | 0.7 |
| ADMA, µmol/L | 0.50 (0.37, 0.66) | 0.57 (0.50, 0.61) | 0.54 (0.41, 0.66) | 0.4 |
| SDMA, µmol/L | 1.94 (1.46, 2.15) | 1.48 (1.13, 1.84) | 1.64 (1.13, 2.02) | 0.1 |
| Nutritional parameters and glucose metabolism | ||||
| Albumin, g/dL | 3.8 (3.4, 4.1) | 3.8 (3.7, 4.0) | 3.8 (3.6, 4.0) | 0.6 |
| Fasting insulin, µU/mL | 12.5 (8.27, 24) | 20 (8.3, 25.1) | 16 (8.3, 25) | 0.4 |
| HOMA-IR | 2.9 (1.7, 5.9) | 3.9 (1.8, 6.2) | 3.1 (1.8, 5.9) | 0.4 |
| Total free fatty acids, µg/mL | 122 (90, 149) | 129 (119, 154) | 126 (90, 154) | 0.6 |
| Cytokines and chemokines | ||||
| aLPS-stimulated PBMC TNF-α | 685 (428, 1314) | 647 (299, 900) | 649 (322, 1159) | 0.3 |
| aLPS-stimulated PBMC IL-6 | 6370 (4657, 14 693) | 12 203 (5353, 22 856) | 7557 (4577, 20 704) | 0.5 |
| aLPS-stimulated PBMC RANTES | 3199 (2134, 6684) | 2394 (1408, 4451) | 2758 (1375, 6232) | 0.4 |
| aLPS-stimulated PBMC MCP-1 | 2911 (1671, 11 473) | 6052 (1438, 13 504) | 3726 (1368, 14 309) | 0.7 |
All values are expressed as median (IQR) unless otherwise specified. Continuous variables were compared using the Mann–Whitney U test and categorical variables were compared using the Pearson's χ2 test. Variables were log-transformed or cubic root-transformed when appropriate.
aCytokines and chemokines by LPS stimulation are expressed in pg/mL.
*P-value significant for a significance level of <0.05 two-tailed test.
Inflammatory parameters
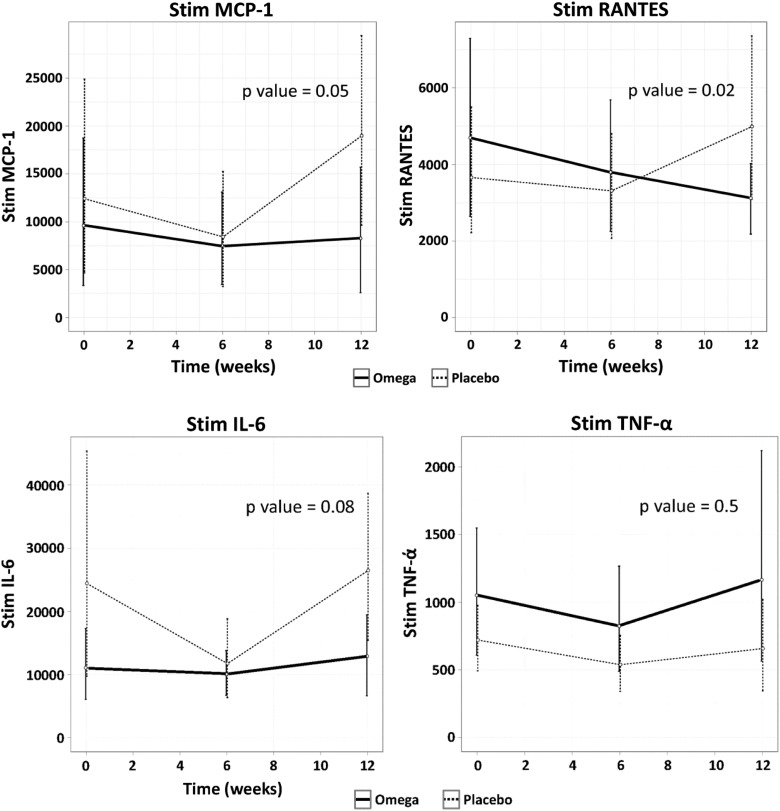
Baseline productions of LPS-induced PBMC chemokines were similar between study groups at baseline (Table 1). Supplementation of ω-3 PUFAs did not reduce the LPS-induced expression of TNF-α or IL-6, nor the serum markers of inflammation: procalcitonin, ADMA or SDMA (Table 2 and Figure 2). Supplementation of ω-3 PUFAs effectively decreased the LPS-induced expression of both RANTES and MCP-1 in the unadjusted model (P = 0.04 and 0.06, respectively; Table 2) and in the model adjusted for demographics (P = 0.02 and 0.05, respectively; see Adjusted Model 1 in Table 3]. We attempted an additional model adjusting for demographics, as well as for hsCRP and truncal fat mass percent given the differences observed at baseline between groups. In this model (see Adjusted Model 2 in Table 3), the effect of the intervention was still significant in decreasing RANTES (P = 0.01) but not for MCP-1 (P = 0.3). We attempted exploratory models adjusting for different biomarkers of inflammation, such as procalcitonin and the dimethylarginines ADMA and SDMA. For these three models, the effect of ω-3 supplementation remained significant for the reduction of MCP-1 and RANTES production by LPS-stimulated PBMCs (Table 3).
Table 2.
Biomarkers at baseline and after intervention by the randomization group
| Variables | 2.9 g EPA : DHA (2 : 1) |
Placebo |
P-value | ||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Stimulated RANTES | |||||
| Baseline | 4696 ± 4326 | 3199 (1696, 6765) | 3660 ± 3554 | 2394 (1243, 4869) | 0.04 |
| 12 weeks | 3121 ± 1783 | 3332 (1649, 4803) | 4457 ± 3813 | 3727 (1548, 5916) | |
| Stimulated MCP1 | |||||
| Baseline | 9607 ± 14 683 | 2911 (1342, 13 329) | 12 413 ± 2126 | 6052 (1297, 15 113) | 0.06 |
| 12 weeks | 8276 ± 12 465 | 3069 (535, 10 699) | 16 537 ± 1773 | 12 361 (527, 29 610) | |
| Stimulated IL-6 | |||||
| Baseline | 11 054 ± 10 674 | 6370 (4577, 17 311) | 24 420 ± 3815 | 12 203 (4780, 24 212) | 0.08 |
| 12 weeks | 12 887 ± 12 056 | 7619 (2282, 25 339) | 23 352 ± 2203 | 14 537 (3942, 38 043) | |
| Stimulated TNF-α | |||||
| Baseline | 1050 ± 860 | 685 (424, 1547) | 720 ± 508 | 647 (295, 973) | 0.7 |
| 12 weeks | 1165 ± 1482 | 992 (272, 1253) | 637 ± 592 | 453 (168, 957) | |
| hsCRP | |||||
| Baseline | 9.4 ± 6.6 | 7.8 (4.3, 12.8) | 15.5 ± 6.9 | 15.2 (10.0, 18.3) | 0.9 |
| 12 weeks | 12.5 ± 12.8 | 7.8 (4.2, 12.6) | 24.3 ± 32.1 | 11.0 (6.6, 28.1) | |
| IL-6 | |||||
| Baseline | 8.5 ± 4.9 | 9.1 (4.5, 11.3) | 25.4 ± 50.7 | 10.1 (3.4, 23.8) | 0.8 |
| 12 weeks | 8.4 ± 8.3 | 7.1 (3.6, 9.8) | 19.8 ± 36.7 | 7.2 (4.4, 13.4) | |
| Albumin | |||||
| Baseline | 3.6 ± 0.8 | 3.8 (3.4, 4.1) | 3.9 ± 0.3 | 3.8 (3.7, 4.0) | 0.9 |
| 12 weeks | 3.9 ± 0.4 | 3.8 (3.6, 4.2) | 3.8 ± 0.4 | 3.7 (3.7, 4.1) | |
| Procalcitonin | |||||
| Baseline | 0.45 ± 0.40 | 0.30 (0.25, 0.45) | 0.36 ± 0.12 | 0.34 (0.27, 0.44) | 0.7 |
| 12 weeks | 0.37 ± 0.20 | 0.33 (0.27, 0.36) | 0.33 ± 0.15 | 0.28 (0.24, 0.31) | |
| ADMA | |||||
| Baseline | 0.53 ± 0.17 | 0.50 (0.37, 0.66) | 0.56 ± 0.12 | 0.57 (0.50, 0.61) | 0.7 |
| 12 weeks | 0.53 ± 0.12 | 0.51 (0.46, 0.58) | 0.55 ± 0.20 | 0.54 (0.43, 0.65) | |
| SDMA | |||||
| Baseline | 1.84 ± 0.65 | 1.94 (1.46, 2.15) | 1.57 ± 0.52 | 1.48 (1.13, 1.84) | 0.7 |
| 12 weeks | 1.77 ± 0.68 | 1.74 (1.12, 2.32) | 1.53 ± 0.57 | 1.40 (1.12, 2.17) | |
| Fasting insulin | |||||
| Baseline | 17.2 ± 12.7 | 12.5 (8.27, 24) | 29.6 ± 50 | 20 (8.3, 25.1) | 0.5 |
| 12 weeks | 22.8 ± 17.3 | 19.3 (7.5, 32.9) | 25.23 ± 19.46 | 20.7 (10.5, 31.8) | |
| HOMA-IR | |||||
| Baseline | 3.9 ± 3.2 | 2.9 (1.7, 5.9) | 8.7 ± 20.4 | 3.9 (1.8, 6.2) | 0.6 |
| 12 weeks | 5.8 ± 5.39 | 4.6 (1.4, 9.1) | 6.1 ± 5.8 | 3.6 (2.5, 8.8) | |
Unadjusted comparison for the percent change from baseline to 12 weeks for each marker between groups using ANCOVA.
FIGURE 2:
Changes of chemokines or cytokines produced by PBMCs in response to LPS stimulation over time by the intervention group. Levels of chemokines and cytokines are in pg/mL. P-values are adjusted for age, gender and race.
Table 3.
Multivariate adjustment for the comparison of the percent changes in biomarkers in response to LPS-stimulated PBMCs from baseline to 12 weeks after randomization to omega-3 versus matching placebo
| Variables | Stimulated RANTES | Stimulated MCP-1 | Stimulated IL-6 | Stimulated TNF-α |
|---|---|---|---|---|
| Adjusted Model 1 | 0.02 | 0.05 | 0.08 | 0.5 |
| Adjusted Model 2 | 0.01 | 0.3 | 0.2 | 0.3 |
| Exploratory models | ||||
| Model a | 0.02 | 0.03 | 0.1 | 0.6 |
| Model b | 0.02 | 0.05 | 0.2 | 0.5 |
| Model c | 0.02 | 0.03 | 0.1 | 0.6 |
ANCOVA was used to compare percent changes from baseline to 12 weeks.
Model 1: adjusted for age, gender and race.
Model 2: adjusted for age, gender, race, truncal fat mass and hsCRP.
Exploratory models a, b and c.
Model a: adjusted for age, gender, race and procalcitonin.
Model b: adjusted for age, gender, race and ADMA.
Model c: adjusted for age, gender, race and SDMA.
Nutritional parameters and insulin resistance
At baseline, serum albumin concentration was similar between groups: median 3.8 (IQR 3.4, 4.1) versus 3.8 (3.7, 4.0) for the intervention versus the placebo group, respectively (P = 0.6). Albumin concentration did not change with the intervention (P = 0.9). Insulin sensitivity indices were slightly higher in the placebo group at baseline [3.9 (1.8, 6.2) versus 2.9 (1.7, 5.9)] and, similarly, the levels of total free fatty acids were higher in the placebo group, but both of these differences were not statistically significant (P = 0.4 and 0.6, respectively). HOMA-IR levels or the levels of total free fatty acids in plasma did not change significantly with the intervention. The levels of total free fatty acids in plasma were tightly correlated with HOMA-IR at baseline (P = 0.005).
Adverse events
There were no related serious adverse events during the study (Table 4). In the intervention arm, the serious adverse events included sprained hand due to mechanical fall, fluid overload and ruptured appendix. In the placebo group, the serious adverse events included vascular graft infection, and chest pain followed by cardiac arrest and death. None of the patients discontinued the intervention due to the lack of tolerability based on taste or gastrointestinal side effects.
Table 4.
Adverse events
| Events | Fish oil | Placebo |
|---|---|---|
| Sprained hand due to mechanical fall | 1 | 0 |
| Fluid overload | 1 | 0 |
| Ruptured appendix followed by abdominal infection | 1 | 0 |
| Access infection of vascular graft | 0 | 1 |
| Chest pain and subsequently died from asystolic arrest (unrelated to the study) | 0 | 1 |
DISCUSSION
Epidemiological studies and randomized controlled trials have shown that ω-3 PUFAs have cardioprotective effects and survival benefit in the general population [4, 17, 18]. These beneficial effects have been attributed predominantly to the anti-inflammatory properties of omega-3 fatty acids [6]. CKD5D patients suffer from chronic systemic inflammation, which has been consistently shown to predict cardiovascular and all-cause mortality [2, 3, 19]. The results of this pilot study indicate that short-term ω-3 PUFA supplementation effectively down-regulates the inflammatory response, particularly endothelial chemokines MCP-1 and RANTES, in a selected cohort of systemically inflamed CKD5D patients.
This study was designed as a pilot trial to assess the effects of short-term administration over a 12-week period of 2.9 g of EPA:DHA on markers of inflammation. The rationale for using LPS-induced PBMC production of pro-inflammatory cytokines and chemokines as the primary outcome was multiple. Chemokines are chemo-attractant agents that attract mature immune effector cells, such as T lymphocytes and monocytes, and enhance their activation and adherence to the vascular endothelium. Hence, chemokines are not only early markers of vascular inflammation, but also play a key role in the initiation of the atherosclerotic process [20, 21]. High plasma MCP-1 levels may reflect a higher burden of atherosclerotic disease, may exert prothrombotic effects resulting in recurrent coronary events or may identify patients who mount a more intense cardiac inflammatory reaction following a coronary event. Furthermore, MCP-1 levels have been considered to be valuable independent predictors of CVD mortality [18, 22] and also to have a prognostic value following acute coronary syndrome. Currently chemokines, particularly MCP-1, are being pursued not only as biomarkers for risk stratification, but also as targets for intervention given their role in vascular inflammation [22, 23]. The down-regulation of the early chemokines RANTES and MCP-1 has been previously described in response to ω-3 PUFA supplementation both in animal studies [24, 25] and in a randomized controlled trial in human subjects with insulin resistance [5]. Our results are consistent with these findings and confirm the beneficial anti-inflammatory effects of ω-3 PUFAs in systemically inflamed CKD5D patients.
In our study, we did not observe changes in systemic inflammatory markers, such as hsCRP, IL-6 or procalcitonin. Our study coincides with other studies where no changes in systemic inflammatory biomarkers have been observed (including hsCRP, IL-6 and procalcitonin) despite the changes observed in early vascular chemokines such as MCP-1 [18, 26]. In one particular study, investigators showed that ω-3 fatty acids suppressed the up-regulation of adipocyte MCP-1 expression that occurred when adipocytes from adipose tissue biopsies were co-cultured with macrophages. There was also a significant reduction in adipose tissue macrophages and an increase in adipose tissue capillaries; whereas, there was no change in the plasma levels of other cytokines or of adiponectin, or in insulin sensitivity [5]. The authors suggested that more prolonged treatment may translate into changes in systemic metabolic parameters. Another plausible explanation for this observation is that LPS-induced inflammatory responses in PBMCs can be measured with less variability than the measurement of other inflammatory markers, which results in a smaller number of subjects being needed to evaluate the impact of an intervention compared with the sample size required for other biomarkers of inflammation. Additionally, leukocyte chemotaxis is one of the first steps in the development of atherosclerosis and in the generation of vascular inflammation. Hence, down-regulation of the production of early chemokines is a more sensitive measure of the effects of an intervention.
The cardioprotective effects of ω-3 PUFA supplementation also include the inhibition of platelet activation/adhesion, reduced thrombosis and reduced triglyceride levels. These properties may explain the beneficial effects of fish oil supplementation compared with placebo observed in the rate of loss of graft patency in CKD5D patients observed in a recent randomized controlled trial, a difference that became more pronounced over time [15]. Furthermore, this study by Lok et al. [15], which is consistent with other studies in the general population, also showed improved cardiovascular event-free survival [hazard ratio 0.43 (95% CI 0.19–0.96); P = 0.04] and lower mean systolic blood pressure (−3.61 versus 4.49 mmHg; P = 0.01) with the intervention, findings that are highly relevant despite the small number of cardiovascular events observed in the trial. Other studies of ω-3 PUFA supplementation in the CKD5D population have shown a reduction in the rate of acute myocardial infarction; but not in the overall cardiovascular events [27]. Fish oil may reduce cardiovascular events by multiple mechanisms including anti-inflammatory mechanism, plaque stabilizing, endothelial function, improvement of dyslipidemias and the protective effects against sudden cardiac death which are linked to the antiarrhythmic effects of ω-3 PUFA. A recent nested case–control of incident dialysis patients showed that ω-3 PUFA levels in blood were inversely associated with the odds of sudden cardiac death during the first year of dialysis initiation [28]. Large randomized controlled trials of the role of ω-3 PUFA in cardiovascular morbidity and mortality in CKD5D patients need to be undertaken.
Studies have demonstrated that ω-3 PUFAs have a peroxisome proliferator-activated receptor gamma-like effect that potentially improves not only inflammation, but also oxidative stress and insulin sensitivity [26]. In our study, we did not observe an effect on HOMA. This is also consistent with the findings of a recent study that examined the effect of omega-3 supplementation (4 g/day) for 12 weeks [15]. This study showed that ω-3 PUFAs reduced MCP-1 expression, but did not improve insulin sensitivity parameters. We did not observe an improvement in the ADMA and SDMA either, which is also consistent with a previous study of omega-3 supplementation on CKD5D with a 12-week duration and a lower dose of EPA:DHA [29].
This study has several strengths. First, our patient population had a documented persistent systemic inflammation (i.e. three consecutive hsCRP measurements >5 mg/L), which improved the likelihood that we would be able to see an anti-inflammatory effect. A careful selection process identified all treatable causes of systemic inflammation prior to inclusion. Secondly, the dose administered is considered sufficient and was selected based on studies that observed an effect in different parameters [16]. Thirdly, the compliance and the side effect profile were acceptable and tolerability was excellent. Fourthly, we used more sensitive and specific measures of inflammatory response that allowed us to use a smaller sample size. Finally, we tested a relatively generalizable anti-inflammatory intervention in contrast to interventions that are more effective but with less applicability. Our study also had certain limitations. First and foremost, this was a relatively short pilot trial examining proximal markers of inflammation, and whether the results can be extrapolated to all systemically inflamed CKD5D patients and whether more distal markers of inflammation will also improve are questions that need to be studied in the future. Furthermore, whether these beneficial effects would translate into improved cardiovascular outcomes cannot be decided based on these results. Clearly, larger scale studies with longer duration are necessary to examine these questions and the study by Lok et al., along with our pilot data, provide strong rationale for such an approach. In this study, we also did not perform dietary interviews and, therefore, do not have data documenting if there was differential consumption of fish between groups. However, it is known that the dialysis population has a very low fish consumption overall and it is highly unlikely that there was a significant overlap between groups regarding ω-3 PUFA levels during the study. Unfortunately, we were unable to measure the omega-3 content in the PBMCs, which would have confirmed compliance; however, the pill counts supported acceptable compliance and suggested that the intervention was indeed the cause of the observed beneficial effects.
In summary, this study shows that supplementation of ω-3 PUFA promotes a more favorable inflammatory profile in prevalent CKD5D patients, particularly down-regulating chemokines such as MCP-1 and RANTES. These specific anti-inflammatory effects are not coupled with any notable improvements in serum concentrations of inflammatory markers, such as hsCRP, IL-6 and procalcitonin. Larger studies of longer duration are needed to evaluate the impact of ω-3 PUFA supplementation on cardiovascular disease risk and overall and cause-specific mortality in systemically inflamed CKD5D patients.
ACKNOWLEDGEMENTS
This study was supported in part by grants R21 AT003844 from the National Institutes of Health/National Center for Complementary and Alternative Medicine (NCCAM), Clinical Translational Science Award UL1 TR000445 from the National Center for Advancing Translational Sciences, K24 DK062849 from the National Institute of Diabetes and Digestive and Kidney Diseases, Vanderbilt Diabetes Research and Training Center (grant P30 DK020593), Vanderbilt Center in Molecular Toxicology (grant P30 ES000267) and Vanderbilt O'Brien Mouse Kidney Center (grant P30 DK079341). A.H.'s work was supported by a Career Development Award (2-031-09S) from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Clinical Sciences Research.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis. 2013;61:A7. doi: 10.1053/j.ajkd.2012.11.031. e1–476. [DOI] [PubMed] [Google Scholar]
- 2.Hung A, Pupim L, Yu C, et al. Determinants of C-reactive protein in chronic hemodialysis patients: relevance of dialysis catheter utilization. Hemodial Int. 2008;12:236–243. doi: 10.1111/j.1542-4758.2008.00260.x. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann J, Herrlinger S, Pruy A, et al. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 4.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 5.Spencer M, Finlin BS, Unal R, et al. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes. 2013;62:1709–1717. doi: 10.2337/db12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saifullah A, Watkins BA, Saha C, et al. Oral fish oil supplementation raises blood omega-3 levels and lowers C-reactive protein in haemodialysis patients—a pilot study. Nephrol Dial Transplant. 2007;22:3561–3567. doi: 10.1093/ndt/gfm422. [DOI] [PubMed] [Google Scholar]
- 7.Perunicic-Pekovic GB, Rasic ZR, Pljesa SI, et al. Effect of n-3 fatty acids on nutritional status and inflammatory markers in haemodialysis patients. Nephrology (Carlton) 2007;12:331–336. doi: 10.1111/j.1440-1797.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 8.Calder PC. Omega 3 polyunsaturated fatty acids, inflammation and immunity. World Rev Nutr Diet. 2001;88:109–116. doi: 10.1159/000059774. [DOI] [PubMed] [Google Scholar]
- 9.Balk EM, Lichtenstein AH, Chung M, et al. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Zoccali C, Maas R, Cutrupi S, et al. Asymmetric dimethyl-arginine (ADMA) response to inflammation in acute infections. Nephrol Dial Transplant. 2007;22:801–806. doi: 10.1093/ndt/gfl719. [DOI] [PubMed] [Google Scholar]
- 11.Schepers E, Glorieux G, Dhondt A, et al. Role of symmetric dimethylarginine in vascular damage by increasing ROS via store-operated calcium influx in monocytes. Nephrol Dial Transplant. 2009;24:1429–1435. doi: 10.1093/ndt/gfn670. [DOI] [PubMed] [Google Scholar]
- 12.Friedman AN. Omega-3 fatty acid supplementation in advanced kidney disease. Semin Dial. 2010;23:396–400. doi: 10.1111/j.1525-139X.2010.00748.x. [DOI] [PubMed] [Google Scholar]
- 13.Kutner NG, Clow PW, Zhang R, et al. Association of fish intake and survival in a cohort of incident dialysis patients. Am J Kidney Dis. 2002;39:1018–1024. doi: 10.1053/ajkd.2002.32775. [DOI] [PubMed] [Google Scholar]
- 14.Noori N, Dukkipati R, Kovesdy CP, et al. Dietary omega-3 fatty acid, ratio of omega-6 to omega-3 intake, inflammation, and survival in long-term hemodialysis patients. Am J Kidney Dis. 2011;58:248–256. doi: 10.1053/j.ajkd.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lok CE, Moist L, Hemmelgarn BR, et al. Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts: a randomized controlled trial. JAMA. 2012;307:1809–1816. doi: 10.1001/jama.2012.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taccone-Gallucci M, Manca-di-Villahermosa S, Battistini L, et al. N-3 PUFAs reduce oxidative stress in ESRD patients on maintenance HD by inhibiting 5-lipoxygenase activity. Kidney Int. 2006;69:1450–1454. doi: 10.1038/sj.ki.5000291. [DOI] [PubMed] [Google Scholar]
- 17.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 18.Piemonti L, Calori G, Lattuada G, et al. Association between plasma monocyte chemoattractant protein-1 concentration and cardiovascular disease mortality in middle-aged diabetic and nondiabetic individuals. Diabetes Care. 2009;32:2105–2110. doi: 10.2337/dc09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeun JY, Levine RA, Mantadilok V, et al. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35:469–476. doi: 10.1016/s0272-6386(00)70200-9. [DOI] [PubMed] [Google Scholar]
- 20.Gosling J, Slaymaker S, Gu L, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reape TJ, Groot PH. Chemokines and atherosclerosis. Atherosclerosis. 1999;147:213–225. doi: 10.1016/s0021-9150(99)00346-9. [DOI] [PubMed] [Google Scholar]
- 22.Kavsak PA, Ko DT, Newman AM, et al. Risk stratification for heart failure and death in an acute coronary syndrome population using inflammatory cytokines and N-terminal pro-brain natriuretic peptide. Clin Chem. 2007;53:2112–2118. doi: 10.1373/clinchem.2007.090613. [DOI] [PubMed] [Google Scholar]
- 23.de Lemos JA, Morrow DA, Blazing MA, et al. Serial measurement of monocyte chemoattractant protein-1 after acute coronary syndromes: results from the A to Z trial. J Am Coll Cardiol. 2007;50:2117–2124. doi: 10.1016/j.jacc.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 24.Mishra A, Chaudhary A, Sethi S. Oxidized omega-3 fatty acids inhibit NF-kappaB activation via a PPARalpha-dependent pathway. Arterioscler Thromb Vasc Biol. 2004;24:1621–1627. doi: 10.1161/01.ATV.0000137191.02577.86. [DOI] [PubMed] [Google Scholar]
- 25.Venkatraman J, Meksawan K. Effects of dietary omega3 and omega6 lipids and vitamin E on chemokine levels in autoimmune-prone MRL/MpJ-lpr/lpr mice. J Nutr Biochem. 2002;13:479. doi: 10.1016/s0955-2863(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Ruan XZ, Powis SH, et al. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005;67:867–874. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 27.Svensson M, Schmidt EB, Jorgensen KA, et al. N-3 fatty acids as secondary prevention against cardiovascular events in patients who undergo chronic hemodialysis: a randomized, placebo-controlled intervention trial. Clin J Am Soc Nephrol. 2006;1:780–786. doi: 10.2215/CJN.00630206. [DOI] [PubMed] [Google Scholar]
- 28.Friedman AN, Yu Z, Denski C, et al. Fatty acids and other risk factors for sudden cardiac death in patients starting hemodialysis. Am J Nephrol. 2013;38:12–18. doi: 10.1159/000351764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svensson M, Frobert O, Schmidt EB, et al. The effect of n-3 fatty acids on levels of methylarginines in patients with end-stage renal disease. J Nephrol. 2010;23:459–464. [PubMed] [Google Scholar]