Abstract
Background
Galactose-deficient O-glycans in the hinge region (HR) of immunoglobulin A1 (IgA1) play a key role in the pathogenesis of IgA nephropathy (IgAN). O-Glycans of circulatory IgA1 consist of N-acetylgalactosamine (GalNAc) with a β1,3-linked galactose; both sugars may be sialylated. In patients with IgAN, α2,6-sialylated GalNAc is a frequent form of the galactose-deficient O-glycans. Prior analyses of IgA1-producing cells had indicated that α2,6-sialyltransferase II (ST6GalNAc-II) is likely responsible for sialylation of GalNAc of galactose-deficient IgA1, but direct evidence is missing.
Methods
We produced a secreted variant of recombinant human ST6GalNAc-II and an IgA1 fragment comprised of Cα1-HR-Cα2. This IgA1 fragment and a synthetic HR peptide with enzymatically attached GalNAc residues served as acceptors. ST6GalNAc-II activity was assessed in vitro and the attachment of sialic acid to these acceptors was detected by lectin blot and mass spectrometry.
Results
ST6GalNAc-II was active with both acceptors. High-resolution mass spectrometry analysis revealed that up to three sialic acid residues were added to the GalNAc residues of the HR glycopeptide.
Conclusions
Our data provide direct evidence that ST6GalNAc-II can sialylate GalNAc of galactose-deficient IgA1. As serum levels of galactose-deficient IgA1 with sialylated glycoforms are increased in IgAN patients, our data explain the corresponding part of the biosynthetic pathway.
Keywords: aberrant O-glycosylation; galactose-deficient IgA1; IgA nephropathy; immunoglobulin A1; α2,6 sialyltransferase ST6GalNAc-II
INTRODUCTION
Immunoglobulin A (IgA) nephropathy (IgAN) is associated with alterations of hinge region (HR) O-glycans of IgA1 [1–3]. O-glycans on circulatory IgA1 are Core 1 glycans consisting of N-acetylgalactosamine (GalNAc) attached to HR serine or threonine (GalNAc-α-Ser/Thr, also called Tn antigen) with a β1,3-linked galactose; both sugars may be sialylated. Galactose-GalNAc-α-Ser/Thr disaccharide (also called T antigen) represents a prevailing glycoform on normal circulatory IgA1 [4–8]. In patients with IgAN, an elevated fraction of IgA1 has some O-glycans without galactose, leaving terminal GalNAc residue(s) (GalNAc-α-Ser/Thr) accessible for recognition by IgG and/or IgA1 autoantibodies. The consequence is formation of nephritogenic immune complexes [9] that may deposit in the glomeruli, activate mesangial cells [3, 10, 11] and induce renal injury. This process culminates in end-stage renal failure within 20 years after diagnosis in 20–40% of patients [12]. The IgA1 HR, located between Cα1 and Cα2, consists of two octapeptide repeats [13] with nine potential O-glycosylation sites (POGSs) of which three to six are glycosylated (PVPST225PPT228PS230PS232T233PPT236PSPSC; POGSs are in bold and the six commonly glycosylated sites are numbered) [14–16].
O-Glycosylation of IgA1 is initiated by attachment of GalNAc to Ser/Thr residues by a polypeptide N-acetylgalactosaminyltransferase (GalNAc-T) [1, 17]. Attachment of galactose by a β1,3-galactosyltransferase (C1GalT1) follows, leading to formation of galactose-GalNAc-α-Ser/Thr disaccharides [18, 19]. IgA1-producing cells from patients with IgAN secrete IgA1 with several galactose-deficient O-glycans. While some glycans have a terminal GalNAc residue, in other glycans the GalNAc residue is capped with sialic acid, forming sialyl-Tn (STn) antigens [20]. The addition of sialic acid is mediated by an α2,6-sialyltransferase (ST6GalNAc) [20–22]. Sialylation of GalNAc-α-Ser/Thr prevents further galactosylation of this structure on IgA1 HR [23]. Therefore, the elevated activity of an ST6GalNAc may play an important role in the pathogenesis of IgAN, by enhancing production of IgA1 with some of the clustered O-glycans deficient in galactose [23, 24].
We found that IgA1-producing cells from IgAN patients exhibited elevated transcription of the ST6GALNAC2 gene encoding an ST6GalNAc, ST6GalNAc-II [20, 22]. This observation is consistent with elevated enzymatic activity of ST6GalNAc in these cells [20, 22], leading to the hypothesis that ST6GalNAc-II is involved in pathogenesis of IgAN. Recently, we provided indirect evidence for the role of ST6GalNAc-II in the synthesis of sialylated GalNAc-α-Ser/Thr on IgA1 HR by using siRNA knockdown [23]. Here, we provide direct evidence that ST6GalNAc-II can sialylate GalNAc in the IgA1 HR. This process produces a glycoform of IgA1 that is secreted by IgA1-producing cells of IgAN patients of which the serum level is increased. These data further define the pathway for synthesis of galactose-deficient IgA1. This new information may provide leads for development of potential biomarkers and targets for future disease-specific therapy.
MATERIALS AND METHODS
Cell lines and primary cells
We isolated IgA1-producing cell lines by subcloning EBV-immortalized cells derived from peripheral blood mononuclear cells of IgAN patients and healthy controls [20].
Production of secreted recombinant ST6GalNAc-II in mammalian HEK293 cells
Human ST6GALNAC2 cDNA without transmembrane domain (corresponding to 31–374 amino acids, NCBI Acc. No. NM_006456) was amplified by RT-PCR from human colorectal adenocarcinoma cell line HT29 using gene-specific primers (forward-primer 5′-GTGCAGCGGTACCCGGGGCCA-3′; reverse-primer 5′-CGCTGGTACAGCTGAAGGAT-3′). PCR product was in-frame blunt-cloned into mammalian expression plasmid pcDNA3.1 (Thermo Fisher Scientific) ahead of sequence encoding V5 and His tags. This vector was first modified by adding in-frame murine Ig kappa secretion signal-encoding cDNA (corresponding to amino acids METDTLLLWVLLLWVPGSTGDAA) at the 5′-end [25]. Recombinant ST6GalNAc-II protein was isolated from the supernatant of transiently transfected HEK293 FreeStyle suspension cells (293F).
Purification of recombinant ST6GalNAc-II
The recombinant protein was purified by Ni-NTA affinity chromatography under native conditions performed at 4°C, as described for GalNAc-T2 [25], with minor modifications. The culture supernatant was mixed with 1/9 of supernatant volume of binding buffer (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, 10 mM imidazole and 0.1% octyl-β-d-glucopyranoside; OG) and 1/250 supernatant volume of 50% Ni-NTA agarose (Qiagen) and incubated overnight. Ni-NTA agarose was washed with 10 volumes of washing buffer (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, 2 mM imidazole and 0.1% OG). Bound protein was eluted with 6 column-volumes of elution buffer (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, 200 mM imidazole and 0.1% OG) and concentrated on Amicon Ultracell 10K (Millipore) into 50 mM Tris–HCl (pH 7.4), 200 mM NaCl buffer to reach concentration ∼0.5 mg/mL (BCA Assay, Thermo Fisher Scientific). Protein was separated by 10% SDS–PAGE and stained with Silver Stain Kit (Thermo Fisher Scientific). Densitometric evaluation of protein bands was performed with ImageJ software (NIH). Identification of the recombinant protein was confirmed by liquid chromatography (LC)–mass spectrometry (MS), as described for GalNAc-T2 [25].
Preparation of recombinant IgA1 fragment in Escherichia coli
cDNA encoding Cα1-HR-Cα2 (NCBI Acc. No. AY647978.1) was codon-optimized for E. coli expression and synthesized (Generi Biotech, Hradec Kralove, Czech Republic). cDNA was cloned into pET101/D-TOPO vector in-frame with 3′-sequences encoding V5 and His tags (Thermo Fisher Scientific). Cα1-HR-Cα2 was expressed in E. coli BL21 (DE3) grown for 5 h at 30°C after the induction by 1 mM isopropyl-β-d-thiogalactoside and purified from the bacterial pellet lysed with 6 mL of denaturation lysis buffer added per 1 g of cell pellet (100 mM NaH2PO4, 10 mM Tris–HCl, 6 M guanidine–HCl, pH 8.0). After 24 h, the centrifugation-cleared supernatant was mixed at ratio 24 : 1 with 50% Co-NTA agarose (Qiagen), washed with 12 column-volumes of 6 M urea, 50 mM NaH2PO4, 300 mM NaCl buffer, pH 8.0, then eluted with the same buffer adjusted to pH 6.0. Protein was dialyzed against 10 mM Tris, 150 mM NaCl, 0.3 M arginine buffer, pH 7.0 and concentrated by Amicon Ultracell 10K (Millipore) to reach ∼1 mg/mL. The identity of the Cα1-HR-Cα2 protein was confirmed by MALDI-TOF MS, as described [26].
Determination of enzymatic activity of ST6GalNAc-II
IgA1 fragment was GalNAcosylated in vitro with GalNAc-T2 [25]; GalNAc-T2 was inactivated (5 min, 96°C). Six micrograms of GalNAcosylated IgA1 fragment were then sialylated for 36 h at 37°C with 1 µg of recombinant ST6GalNAc-II in the reaction mixture consisting of 50 mM MES (pH 6.0), 100 mM CMP-NeuAc, 2 mM CaCl2, 2 mM MnCl2, 10 mM MgCl2 in a total volume of 25 µL. Recombinant ST6GalNAc-I was used in a control reaction [24]. Three micrograms of sialylated IgA1 fragment were then desialylated (2 U of Arthrobacter ureafaciens sialidase; 37°C, 8 h) [8]. Glycosylated IgA1 fragments were SDS–PAGE western blotted onto PVDF membrane (Millipore), blocked with SuperBlock (Thermo Fisher Scientific) and developed with biotinylated lectin from Helix aspersa (HAA), a lectin specific for terminal GalNAc [9, 20, 22, 27–29] (Sigma-Aldrich) diluted 1 : 1000 in SuperBlock, followed by HRP-conjugated avidin diluted 1:50 000 (Sigma-Aldrich). Protein load of IgA1 fragments was assessed with anti-V5-tag antibody HRP-conjugated (Sigma-Aldrich) diluted 1 : 10 000 in SuperBlock.
Assessment of the activity of ST6GalNAc-II by MS
One microgram of the acceptor peptide VPSTPPTPSPSTPPTPSPSCCHPR was first GalNAcosylated using GalNAc-T2 (8 h, 37°C) in 25 µL of a reaction buffer consisting of 25 mM Tris–HCl (pH 6.64), 5 mM MnCl2 and 250 μM UDP-GalNAc using 0.05 μg of GalNAc-T2. Reaction was terminated by heat inactivation (95°C for 8 min). Sialylation was performed at 37°C for 24 h in 10 μL (∼0.4 μg of GalNAcosylated acceptor) of previous reaction with the addition of 2.5 μL ddH2O, 2.5 μL 500 mM MES buffer (pH 6.0), 2.5 μL 200 mM CMP-NeuAc and 1.25 μg of purified recombinant ST6GalNAc-II protein in 2.5 µL of 50 mM Tris–HCl (pH 7.4), 200 mM NaCl buffer. Five microliters of the sialylation reaction were diluted in 75 µL of 0.1% formic acid. Ten microliters of sample were loaded onto Jupiter reverse-phase C18 beads with a 4-µm particle diameter and 90-Å pore size and reaction products were eluted using solvents A (97.4% water, 0.1% FA and 2.5% ACN) and B (2.5% water, 0.1% FA and 97.4% ACN) to form a non-linear gradient from 8 to 30% B over 40 min. LC was directly coupled to and analyzed by Orbitrap Velos high-resolution mass spectrometer with MS1 full scans (m/z 200–2000) and MS2 collision-induced dissociation activation on the top 3 ions from each MS1 scan. Spectra were manually analyzed in Thermo XcaliberQual Browser software, wherein MS1 peak averages were assigned glycoforms based on m/z data alone.
RESULTS
Production of secreted recombinant human ST6GalNAc-II and recombinant Cα1-HR-Cα2 fragment of IgA1
To assess whether ST6GalNAc-II can attach sialic acid to GalNAc-α-Ser/Thr on IgA1 HR, we produced recombinant, secreted version of ST6GalNAc-II in 293F cells and Cα1-HR-Cα2 IgA1 fragment in E. coli. Purified proteins were separated on SDS–PAGE and detected by silver staining (Supplementary data, Figure S1A and B). The bands representing ST6GalNAc-II (panel A) and Cα1-HR-Cα2 (panel B) corresponded to the apparent molecular masses of proteins of 54 and 27 kDa, respectively. Densitometric analysis indicated ∼93% purity of ST6GalNAc-II and Cα1-HR-Cα2. Two potential N-glycosylation sites on ST6GalNAc-II were predicted by NetNGlyc 1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc/). Glycosylation of recombinant ST6GalNAc-II expressed in 293F cells was confirmed by mobility-shift assay using SDS–PAGE immunoblot before and after enzymatic deglycosylation with PNGase F [30]. The results showed a reduction of apparent molecular mass from ∼54 to 51 kDa (Supplementary data, Figure S1C). Identity of both ST6GalNAc-II and Cα1-HR-Cα2 proteins was confirmed by mass spectrometry.
Assessment of ST6GalNAc-II enzymatic activity
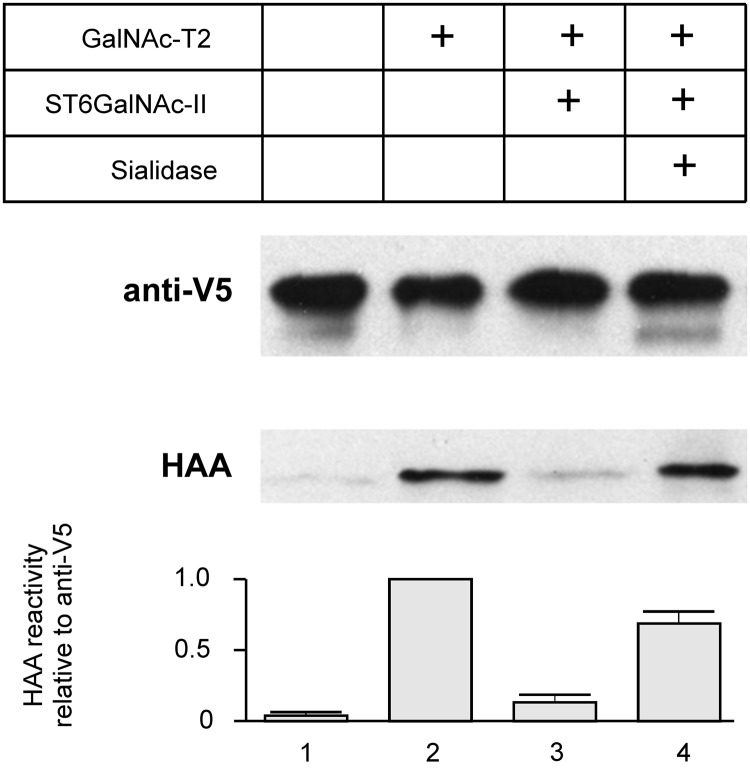
To determine whether ST6GalNAc-II can effectively sialylate GalNAc on IgA1 HR, we used a Cα1-HR-Cα2 fragment of IgA1, produced in E. coli, purified, and subsequently GalNAcosylated with recombinant GalNAc-T2 [1, 25]. GalNAc additions and subsequent sialylation were monitored on western blot with HAA lectin (reacts with GalNAc but not with galactose-GalNAc) [20, 22]. HAA did not react with non-glycosylated Cα1-HR-Cα2 (Figure 1, column 1), but recognized GalNAcosylated Cα1-HR-Cα2 (Figure 1, column 2). Sialylation with recombinant ST6GalNAc-II reduced the reactivity with HAA by ∼80% (Figure 1, column 3); the full reactivity was restored by treatment with sialidase (Figure 1, column 4). Anti-V5 antibody reactivity confirmed equal loads of Cα1-HR-Cα2.
FIGURE 1:
Determination of ST6GalNAc-II activity on IgA1 Cα1-HR-Cα2 using lectin blot. Cα1-HR-Cα2 was GalNAcosylated by GalNAc-T2 and then sialylated for 36 h at 37°C with ST6GalNAc-II. Half of the protein was desialylated by sialidase from Arthrobacter ureafaciens. Glycosylated proteins were analyzed by western blot with HAA lectin. Anti-V5 antibody was used as load control. (1) Recombinant Cα1-HR-Cα2, (2) Cα1-HR-Cα2 glycosylated with GalNAc-T2, (3) sample 2 sialylated by ST6GalNAc-II, and (4) sample 3 after desialylation. Bar graph expresses densitometric data as means + SD from two independent experiments.
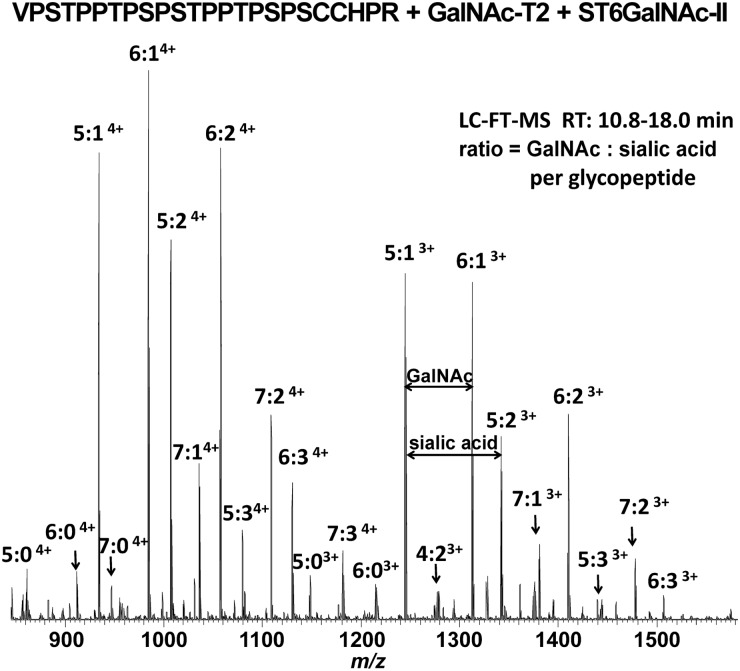
Next, we tested ST6GalNAc-II activity by MS using GalNAcosylated HR peptide as substrate. Figure 2 shows high-resolution Fourier transform (FT) MS analysis of enzyme reaction performed for 24 h. LC-FT-MS spectra revealed most of the GalNAcosylated HR peptide was sialylated, corresponding to glycopeptides with 4–7 GalNAc additions to the synthetic HR peptide in the 3+ and 4+ charge states with additions of one to three sialic acid residues (peaks labeled with 4:X, 5:X, 6:X, and 7:X show number of GalNAc and sialic acid residues; Figure 2, Supplementary data, Table S1). The distance between adjacent ion species reflected the exact masses of GalNAc and/or sialic acid residues. These data directly demonstrated that ST6GalNAc-II can attach sialic acid residues to GalNAc residues of IgA1 HR glycopeptide.
FIGURE 2:
Determination of ST6GalNAc-II activity using mass spectrometry. IgA1 HR peptide VPSTPPTPSPSTPPTPSPSCCHPR was GalNAcosylated with GalNAc-T2 and then sialylated using ST6GalNAc-II. MS1 spectra were averaged over glycopeptide-elution retention times; the glycopeptides were observed in 3+ and 4+ charge states. Both the non-sialylated and sialylated forms of the GalNAcosylated HR glycopeptide were detected, ranging from 4 to 7 additions of GalNAc with 0 to 3 additions of sialic acid. Major glycopeptide ion species are labeled to show the number of GalNAc and sialic acid additions to the acceptor HR peptide.
DISCUSSION
IgAN is associated with production of IgA1 with galactose-deficient O-glycans recognized by IgG and/or IgA1 autoantibodies, leading to formation of immune complexes that deposit in the glomerular mesangium and incite glomerular injury [31, 32]. Galactose-deficiency of IgA1 clustered O-glycans is associated with elevated levels of sialylated GalNAc, a feature that prevents subsequent galactosylation [20, 23, 24]. The human colon-cancer cell line HCT15 overexpresses ST6GalNAc-I and thereby produces an increased amount of STn [33]. This finding suggests a key role for ST6GalNAc-I in production of sialylated GalNAc-α-Ser/Thr structure on glycoproteins in some types of cancer.
The origin of sialylated GalNAc-α-Ser/Thr in the IgA1 HR in patients with IgAN was puzzling, as IgA1-producing cells from peripheral blood of IgAN patients do not express ST6GALNAC1 [20, 22]. However, abundant transcription of ST6GALNAC2, the gene encoding another sialyltransferase, ST6GalNAc-II, was detected [20, 22]. Based on the overexpression of ST6GALNAC2 in the cells from IgAN patients versus those of healthy controls and the elevated ST6GalNAc enzymatic activity, we suspected that the candidate enzyme responsible for the sialylation of GalNAc-α-Ser/Thr antigens of IgA1 is ST6GalNAc-II [20, 22].
Humans have four additional enzymes with ST6GalNAc activity, ST6GalNAc-III to ST6GalNAc-VI, but their contribution to oversialylation of IgA1 in IgAN is likely negligible. The transcriptional levels of ST6GALNAC4 and ST6GALNAC6 in IgA1-producing cells from IgAN patients versus healthy controls are similar [20, 22]. mRNA for ST6GALNAC3 is present at low levels in IgA1-producing cells from IgAN patients and healthy controls. ST6GALNAC5 mRNA is not detectable in IgA1-producing cells (M. Raska, unpublished observations).
To confirm that ST6GalNAc-II can attach sialic acid to GalNAc-α-Ser/Thr on the IgA1 HR, we produced recombinant human ST6GalNAc-II. Other investigators have shown that ST6GalNAc-II isolated from cells attached sialic acid to terminal GalNAc on a variety of mucins [34, 35]. In cell lines transfected by human ST6GalNAc-I- and ST6GalNAc-II-encoding plasmids, sialylation activity of ST6GalNAc-II toward GalNAc-α-Ser/Thr antigen was less than that of ST6GalNAc-I [34]. Limited activity of ST6GalNAc-II in STn synthesis was shown also for some commonly used breast-cancer cell lines that are STn-negative but express ST6GalNAc-II mRNA [35]. These reports confirmed that human ST6GalNAc-II generally prefers galactose-GalNAc-α-Ser/Thr over GalNAc-α-Ser/Thr and less effectively sialylates GalNAc-α-Ser/Thr than does ST6GalNAc-I [34]. Here, we produced recombinant ST6GalNAc-II in eukaryotic cells and used a recombinant IgA1 fragment expressed in E. coli that was GalNAcosylated by GalNAc-T2 [25]. To test the ability of the recombinant enzyme to sialylate Tn antigen(s) on IgA1, we used HAA lectin western blot, taking advantage of the fact that sialylation of GalNAc blocks HAA binding to GalNAc-containing IgA1 [24]. HAA binding to GalNAcosylated IgA1 fragment was reduced after incubation with ST6GalNAc-II enzyme and restored by enzymatic desialylation. Thus, ST6GalNAc-II enzyme sialylated terminal GalNAc of the IgA1 HR. ST6GalNAc-I, used as a control, also sialylated IgA1, the GalNAcosylated Cα1-HR-Cα2 fragment, and HR peptide [24]. To better characterize the sialylation products of ST6GalNAc-II enzyme, we used mass spectrometry and GalNAcosylated HR peptide as the substrate. ST6GalNAc-II added up to three sialic acid residues to the HR glycopeptide, suggesting that ST6GalNAc-II can effectively sialylate galactose-deficient IgA1 HR glycans. It is to be noted, however, that these experiments used Cα1-HR-Cα2 fragment of IgA1 or HR glycopeptide and, thus, it is not necessarily straightforward to extrapolate an identical function for this enzyme in a complex environment of the Golgi apparatus and the IgA1 molecule as substrate.
Our previous data indicated that elevated activity of ST6GalNAc-II in IgA1-producing cells of patients with IgAN enhanced synthesis of IgA1 with some of the clustered O-glycans deficient in galactose [23, 24]. However, it is not clear whether sialylated GalNAc is a precursor for the epitope recognized by the autoantibodies or whether sialylation of some GalNAc residues blocks galactosylation of neighboring GalNAc residue(s) and, thus, directly increases the number of glycans in the IgA1 HR with terminal GalNAc.
In summary, we confirmed that ST6GalNAc-II can sialylate GalNAc in the clustered O-glycans of IgA1. As the enzyme is overexpressed in IgA1-producing cells from IgAN patients, its activity may contribute to production of galactose-deficient IgA1 [23], the key autoantigen in the pathogenesis of IgAN.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
FUNDING
This work was supported by Ministry of School, Youth, and Sport (CZ.1.07/2.3.00/20.0164, LH11046 to M.R.) and Grant Agency of the Ministry of the Health (NT11081 to M.R.) Czech Republic; and by the National Institutes of Health (DK082753, DK078244 and GM098539 to J.N., M.B.R. and B.A.J.) and by a gift from the IGA Nephropathy Foundation of America (to B.A.J. and J.N.).
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1.Stuchlova Horynova M, Raska M, Clausen H, et al. Aberrant O-glycosylation and anti-glycan antibodies in an autoimmune disease IgA nephropathy and breast adenocarcinoma. Cell Mol Life Sci. 2013;70:829–839. doi: 10.1007/s00018-012-1082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mestecky J, Raska M, Julian BA, et al. IgA nephropathy: molecular mechanisms of the disease. Annu Rev Pathol. 2013;8:217–240. doi: 10.1146/annurev-pathol-011110-130216. [DOI] [PubMed] [Google Scholar]
- 3.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 4.Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974;249:7270–7281. [PubMed] [Google Scholar]
- 5.Field MC, Dwek RA, Edge CJ, et al. O-linked oligosaccharides from human serum immunoglobulin A1. Biochem Soc Trans. 1989;17:1034–1035. doi: 10.1042/bst0171034. [DOI] [PubMed] [Google Scholar]
- 6.Mattu TS, Pleass RJ, Willis AC, et al. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc α receptor interactions. J Biol Chem. 1998;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- 7.Wada Y, Dell A, Haslam SM, et al. Comparison of methods for profiling O-glycosylation: human proteome organisation human disease glycomics/proteome initiative multi-institutional study of IgA1. Mol Cell Proteomics. 2010;9:719–727. doi: 10.1074/mcp.M900450-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi K, Wall SB, Suzuki H, et al. Clustered O-glycans of IgA1: defining macro- and micro-heterogeneity by use of electron capture/transfer dissociation. Mol Cell Proteomics. 2010;9:2545–2557. doi: 10.1074/mcp.M110.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomana M, Novak J, Julian BA, et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki H, Fan R, Zhang Z, et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668–1677. doi: 10.1172/JCI38468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki H, Kiryluk K, Novak J, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22:1795–1803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol. 2004;24:179–196. doi: 10.1016/j.semnephrol.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Frangione B, Wolfenstein-Todel C. Partial duplication in the "hinge" region of IgA1 myeloma proteins. Proc Natl Acad Sci USA. 1972;69:3673–3676. doi: 10.1073/pnas.69.12.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi K, Smith AD, Poulsen K, et al. Naturally occurring structural isomers in serum IgA1 O-glycosylation. J Proteome Res. 2012;11:692–702. doi: 10.1021/pr200608q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novak J, Renfrow MB, Gharavi AG, et al. Pathogenesis of immunoglobulin A nephropathy. Curr Opin Nephrol Hypertens. 2013;22:287–294. doi: 10.1097/MNH.0b013e32835fef54. [DOI] [PubMed] [Google Scholar]
- 16.Franc V, Rehulka P, Raus M, et al. Elucidating heterogeneity of IgA1 hinge-region O-glycosylation by use of MALDI-TOF/TOF mass spectrometry: role of cysteine alkylation during sample processing. J Proteom. 2013;92:299–312. doi: 10.1016/j.jprot.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasaki H, Zhang Y, Tachibana K, et al. Initiation of O-glycan synthesis in IgA1 hinge region is determined by a single enzyme, UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 2. J Biol Chem. 2003;278:5613–5621. doi: 10.1074/jbc.M211097200. [DOI] [PubMed] [Google Scholar]
- 18.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β 3-galactosyltransferase. Proc Natl Acad Sci USA. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ju T, Brewer K, D'Souza A, et al. Cloning and expression of human core 1 beta1,3-galactosyltransferase. J Biol Chem. 2002;277:178–186. doi: 10.1074/jbc.M109060200. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H, Moldoveanu Z, Hall S, et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dall'Olio F, Chiricolo M. Sialyltransferases in cancer. Glycoconj J. 2001;18:841–850. doi: 10.1023/a:1022288022969. [DOI] [PubMed] [Google Scholar]
- 22.Raska M, Moldoveanu Z, Suzuki H, et al. Identification and characterization of CMP-NeuAc:GalNAc-IgA1 α2,6-sialyltransferase in IgA1-producing cells. J Mol Biol. 2007;369:69–78. doi: 10.1016/j.jmb.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki H, Raska M, Yamada K, et al. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem. 2014;289:5330–5339. doi: 10.1074/jbc.M113.512277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi K, Raska M, Stuchlova Horynova M, et al. Enzymatic sialylation of IgA1 O-glycans: implications for studies of IgA nephropathy. PLoS ONE. 2014;9:e99026. doi: 10.1371/journal.pone.0099026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horynova M, Takahashi K, Hall S, et al. Production of N-acetylgalactosaminyl-transferase 2 (GalNAc-T2) fused with secretory signal Igkappa in insect cells. Protein Expr Purif. 2012;81:175–180. doi: 10.1016/j.pep.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stosova T, Sebela M, Rehulka P, et al. Evaluation of the possible proteomic application of trypsin from Streptomyces griseus. Anal Biochem. 2008;376:94–102. doi: 10.1016/j.ab.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Tomana M, Matousovic K, Julian BA, et al. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997;52:509–516. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]
- 28.Moldoveanu Z, Wyatt RJ, Lee JY, et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007;71:1148–1154. doi: 10.1038/sj.ki.5002185. [DOI] [PubMed] [Google Scholar]
- 29.Novak J, Julian BA, Tomana M, et al. Progress in molecular and genetic studies of IgA nephropathy. J Clin Immunol. 2001;21:310–327. doi: 10.1023/a:1012284402054. [DOI] [PubMed] [Google Scholar]
- 30.Raska M, Takahashi K, Czernekova L, et al. Glycosylation patterns of HIV-1 gp120 depend on the type of expressing cells and affect antibody recognition. J Biol Chem. 2010;285:20860–20869. doi: 10.1074/jbc.M109.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen AC, Bailey EM, Brenchley PE, et al. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int. 2001;60:969–973. doi: 10.1046/j.1523-1755.2001.060003969.x. [DOI] [PubMed] [Google Scholar]
- 32.Hiki Y, Odani H, Takahashi M, et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59:1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 33.Ikehara Y, Kojima N, Kurosawa N, et al. Cloning and expression of a human gene encoding an N-acetylgalactosamine-α2,6-sialyltransferase (ST6GalNAc I): a candidate for synthesis of cancer-associated sialyl-Tn antigens. Glycobiology. 1999;9:1213–1224. doi: 10.1093/glycob/9.11.1213. [DOI] [PubMed] [Google Scholar]
- 34.Marcos NT, Pinho S, Grandela C, et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004;64:7050–7057. doi: 10.1158/0008-5472.CAN-04-1921. [DOI] [PubMed] [Google Scholar]
- 35.Samyn-Petit B, Krzewinski-Recchi MA, Steelant WF, et al. Molecular cloning and functional expression of human ST6GalNAc II. Molecular expression in various human cultured cells. Biochim Biophys Acta. 2000;1474:201–211. doi: 10.1016/s0304-4165(00)00020-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.