Abstract
Pancreatic cancers are aggressive because they are highly invasive and highly metastatic; moreover, effective treatments for aggressive pancreatic cancers are lacking. Here, we report that the motor kinesin protein KIF20A promoted the motility and invasiveness of pancreatic cancer cells through transporting the RNA-binding protein IGF2BP3 and IGF2BP3-bound transcripts toward cell protrusions along microtubules. We previously reported that IGF2BP3 and its target transcripts are assembled into cytoplasmic stress granules of pancreatic cancer cells, and that IGF2BP3 promotes the motility and invasiveness of pancreatic cancer cells through regulation of localized translation of IGF2BP3-bound transcripts in cell protrusions. We show that knockdown of KIF20A inhibited accumulation of IGF2BP3-containing stress granules in cell protrusions and suppressed local protein expression from specific IGF2BP3-bound transcripts, ARF6 and ARHGEF4, in the protrusions. Our results provide insight into the link between regulation of KIF20A-mediated trafficking of IGF2BP3-containing stress granules and modulation of the motility and invasiveness in pancreatic cancers.
Introduction
Kinesin superfamily (KIF) members share a highly conserved motor domain, and many motor kinesins have adenosine triphosphatase activity and microtubule-dependent plus-end motion ability [1]. The KIF proteins participate in multiple normal cellular biological activities, including mitosis and intracellular transport of vesicles and organelles [2]. The KIF protein KIF20A has been documented to accumulate in the midzone of the spindle during anaphase and to the cleavage furrow and midbody during telophase [3]. We previously reported that KIF20A is overexpressed in human pancreatic ductal adenocarcinoma (PDAC) based on cDNA microarray analyses and that KIF20A and DLG5, which collaborate in the cytoplasm but not in the midzone of the spindle, are likely to be involved in pancreatic carcinogenesis [4]. KIF20A functions as part of the intracellular trafficking machinery for DLG5, transporting it to membrane sites in PDAC cells in which DLG5 can interact with other proteins including β-catenin [4]. Thus, it is possible that the intracellular trafficking function of KIF20A is highly important for pancreatic carcinogenesis.
RNA-binding proteins are involved in multiple aspects of RNA maturation, RNA turnover, translation, and movement of transcripts throughout the cell. Cytoplasmic RNA granules called stress granules (SGs) contain mRNA, small ribosomal subunit proteins, and stress-dependent RNA-binding proteins that are involved in translation initiation or in mRNA degradation [5]. Several lines of evidence indicate that cytoplasmic SGs are transported along microtubules via kinesin motors [6]. Antisense oligonucleotides that suppress expression of kinesin heavy chains or drugs that disrupt microtubules can inhibit translocation of cytoplasmic SGs that contain myelin basic protein (MBP) mRNA in oligodendrocytes [6]. Kinesin family member 5 (KIF5) transports cytoplasmic SGs containing RNA-binding proteins and mRNAs such as CaMKIIα and Arc along microtubules [7]. It is still unknown whether KIF20A transports SGs containing RNA-binding proteins or mRNAs.
We recently reported that cytoplasmic SGs containing the RNA-binding protein IGF2BP3 and IGF2BP3-bound mRNAs are accumulated in cell protrusions of PDAC cells [8]. Further investigation revealed that IGF2BP3-bound mRNAs such as ADP-ribosylation factor 6 (ARF6) and Rho guanine nucleotide exchange factor 4 (ARHGEF4) are subsequently translated in membrane protrusions; in turn, these locally translated proteins influence the formation of additional membrane protrusions and thereby increase the invasiveness and metastasis of the PDAC cells [8]. Here, we sought to evaluate the role of KIF20A in cell motility and invasion of PDAC cells. In the course of this investigation, we made a surprising observation: specifically, KIF20A transported cytoplasmic SGs containing IGF2BP3-mRNA complexes toward membrane protrusions of PDAC cells and that decreased levels of endogenous KIF20A decreased the expressions of ARF6 and ARHGEF4 localized in cell protrusions, thereby inhibiting the formation of membrane protrusions and the motility and invasiveness of PDAC cells. Our results imply that KIF20A is likely to play a critical role in the trafficking system of IGF2BP3-containing SGs, which is highly important for the motility and invasiveness in PDACs.
Material and Methods
Antibodies
Rabbit anti-IGF2BP3 (2037) and anti-ARHGEF4 (18267) antibodies were purchased from Human Protein Atlas (Stockholm, Sweden). Anti-G3BP monoclonal antibody (611126) was purchased from BD Transduction Laboratory (Palo Alto, CA). Monoclonal antibodies against KIF20A (374508) and c-myc (40) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-ARF6 (77581) antibody and mouse anti-coilin (87913) monoclonal antibody were purchased from Abcam (Cambridge, MA). Rabbit anti-α-tubulin antibody (PM054) was purchased from MBL (Woburn, MA).
Cell Culture and Reagents
The human PDAC cell line S2-013, a subline of SUIT-2, was obtained from Dr. T. Iwamura (Miyazaki Medical College, Miyazaki, Japan) [9]. The human PDAC cell line PANC-1 was purchased from the American Type Culture Collection (Manassas, VA). All cells were grown in Dulbecco’s modified Eagle’s medium (Gibco-BRL, Carlsbad, CA) supplemented with 10% heat-inactivated fetal calf serum (FCS) at 37°C in a humidified atmosphere saturated with 5% CO2. To induce oxidative stress, plated cells were treated for 30 minutes with sodium arsenite (SA) (500 μM; Sigma-Aldrich, St. Louis, MO); to disrupt microtubule networks, plated cells were treated with nocodazol (10 μM; Sigma-Aldrich).
Confocal Immunofluorescence Microscopy
Coverslips were coated with 10 μg/ml fibronectin (Sigma-Aldrich) for 1 hour at room temperature. Cells were seeded on fibronectin-coated glass coverslips and incubated for 5 hours; cells were then fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, covered with blocking solution (3% BSA/PBS), and then incubated with the appropriate primary antibody for 1 hour. Alexa488-, Alexa546-, Alexa594-, or Alexa647-conjugated secondary antibody (Molecular Probes, Carlsbad, CA) was used with or without rhodamine-conjugated phalloidin (Cytoskeleton, Denver, CO). In some experiments, a commercial antibody-labeling technology (Zenon; Life Technologies, Carlsbad, CA) was used according to the manufacturer’s instructions to conjugate green or red fluorophores to primary antibodies. Each specimen was visualized using a Zeiss LSM 510 META microscope (Carl Zeiss, Gottingen, Germany).
Generation of an S2-013 Cell Line That Stably Expressed Small Interfering RNA (siRNA)
Exponentially growing GP2-293 packaging cells (Clontech, Mountain View, CA) were transiently infected with pGFP-V-RS vectors (OriGene Technologies, Rockville, MD) to generate replication-deficient lentivirus that carried an siRNA expression cassette targeting either a scrambled negative control (TR30013) or KIF20A mRNA (TG311916) into host cells via a replication-deficient lentivirus. Upon transient transfection of the plasmids into the packaging cell line, replication-deficient viruses were obtained and used to infect S2-013 cells; infected S2-013 cells were transferred to flasks 48 hours after infection and then grown in Dulbecco’s modified Eagle’s medium containing 0.5 μg/ml puromycin (Sigma-Aldrich) for 7 days to establish S2-013 cells that stably expressed the appropriate siRNA that targeted KIF20A mRNA. For each experiment, these cells were cultivated until they reached confluence and then for an additional 10 days; medium was refreshed every second day during cell cultivation. Cells were used only when suppression of KIF20A had been validated via Western blot analysis.
KIF20A-Rescue Construct
Reverse transcription polymerase chain reaction was used to amplify the entire coding sequence of the KIF20A cDNA. The resultant polymerase chain reaction product was subsequently inserted into a separate pCMV6-Entry vector (Origene) bearing a C-terminal myc-DDK-tag. X-tremeGENE HP DNA Transfection Reagent (Roche, Penzberg, Germany) was used to transiently transfect target cells with resultant KIF20A-rescue construct.
Transwell Motility Assay
Cells (3.0 × 104) were plated in the upper chamber of BD BioCoat Control Culture Inserts (24-well plates, 8-μm pore size; Becton Dickinson, San Jose, CA). Serum-free culture medium was added to each upper chamber, and medium containing 5% FCS was added to each bottom chamber. Cells were incubated on the membranes for 12 hours. After a 12-hour incubation, three independent visual fields were examined via microscopic observation to count the number of cells that had moved to the bottom chamber.
Matrigel Invasion Assay
A two-chamber invasion assay was used to assess cell invasion (24-well plates, 8-μm pore size membrane coated with a layer of Matrigel extracellular matrix proteins; Becton Dickinson). Cells (4.0 × 104) suspended in serum-free medium were seeded into the upper chamber and allowed to invade toward a 5% FCS chemoattractant in the lower chamber. After a 20-hour incubation, three independent visual fields were examined via microscopic observation, and the number of cells that had moved to the bottom chamber was determined.
Immunoprecipitation
S2-013 cells were incubated on fibronectin for 5 hours, lysed in lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1 mM MgCl2, 0.5% NP-40, and protease inhibitor cocktail tablets (Roche)], and the resulting lysates were immunoprecipitated with 2 μg of anti-KIF20A antibody, anti-IGF2BP3 antibody or IgG isotype control antibody, and Dynabeads Protein G (Dynal). To examine the interaction between endogenous KIF20A and IGF2BP3, immune complexes were analyzed on Western blots.
Microtubule Precipitation Assay
Cells were homogenized in lysis buffer [0.1 M PIPES (pH 6.6), 5 mM EGTA, 1 mM MgSO4, 0.1 M glycerol, 1 mM DTT, and protease inhibitor cocktail tablets (Roche)]. Each homogenate was incubated on ice for 15 minutes to depolymerize microtubules and then centrifuged at 16,000g at 4°C for 30 minutes. The supernatant was further centrifuged at 135,000g at 20°C for 90 minutes. Microtubules in the clarified supernatant were polymerized by the addition of taxol (Abcam) and GTP to 20 μM and 1 mM, respectively. The mixture was incubated at 37°C for 10 minutes, layered on a 15% sucrose cushion prepared in lysis buffer containing 20 μM taxol and 1 mM GTP, and finally centrifuged at 54,000g at 20°C for 30 minutes. The resulting pellet was a standard microtubule pellet that contained microtubule-associated proteins. Western blotting was performed with anti-IGF2BP3 and anti-α-tubulin antibodies.
Immunofluorescence with RNA Fluorescence In Situ Hybridization
The QuantiGene ViewRNA plate-based assay kit (Panomics, Santa Clara, CA) was used according to the manufacturer’s recommendations with some modifications [10] to perform fluorescence in situ hybridization to target RNAs. Fibronectin-stimulated S2-013 cells were fixed in 8% formaldehyde, dehydrated in ethanol (50-70-100%), and held at 4°C overnight. Cells were then rehydrated, permeabilized, and hybridized as recommended. The RNA targets were ARF6 or ARHGEF4 (Panomics), and the reference RNA was ubiquitin C (UBC) (Panomics). After in situ hybridization, sections were washed in PBS, blocked for 1 hour with blocking buffer (4% goat serum in PBS), and incubated for 3 hours at room temperature with anti-KIF20A and anti-IGF2BP3 antibodies in blocking buffer. Secondary antibodies in blocking buffer were applied to the samples for 30 minutes at room temperature, nuclei were stained for 3 minutes with 4′,6-Diamidino-2-Phenylindole (DAPI), and samples were mounted in Aqua Polymount (Polysciences, Warrington, PA). Confocal fluorescence images were captured with a Zeiss LSM 510 META microscope.
Statistical Analysis
GraphPad Prism version 6.0 software (GraphPad Software, Inc., La Jolla, CA) was used for all statistical analyses. The significance of differences between groups was determined using the two-tailed Student’s t test. For all analyses, P < .05 was considered statistically significant.
Results
Subcellular Localization of Migrating KIF20A in PDAC Cells
We used immunocytochemistry to determine the subcellular localization of KIF20A in two types of cultured PDAC cells: moderately differentiated PDAC cells (line S2-013 [9]) and a poorly differentiated PDAC line (PANC-1 [11]). S2-013 is a cloned subline of a PDAC line (SUIT-2) derived from a liver metastasis [9] and was obtained from Dr. T. Iwamura (Miyazaki Medical College). Notably, when S2-013 cells that are initially in suspension attach to an immobilized fibronectin substrate, nascent membrane protrusions (de novo formation of actin patches at the cell periphery) form, and as these protrusions mature, they promote cell motility [12]. To investigate whether KIF20A was localized in cell protrusions, fibronectin-stimulated PDAC cells were used. When S2-013 or PANC-1 cells were cultured on fibronectin, KIF20A was localized in the cytoplasm of the cell bodies and membrane protrusions, which each had many peripheral actin structures (Figure 1A). Z stack panels showed that fibronectin-stimulated S2-013 cells exhibited intracellular expression of KIF20A in cytoplasmic granules that were located in membrane protrusions (Figure 1B).
Figure 1.
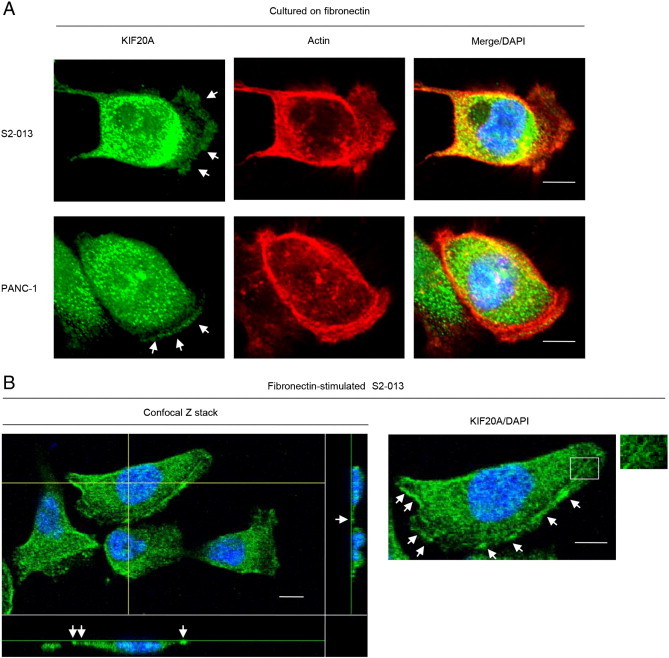
Distribution of KIF20A in PDAC cells.
(A) S2-013 and PANC-1 cells were incubated on fibronectin and immunocytochemically and fluorescently labeled with anti-KIF20A antibody (green) and phalloidin (red). Actin filaments were labeled by phalloidin. Arrows, KIF20A localized in cell protrusions. Bars, 10 μm.
(B) Confocal Z stack shows nuclear DAPI staining (blue) and KIF20A (green) staining associated with granules in spreading S2-013 cells. Arrows, KIF20A localized in cell protrusions. The white box indicates a region in the enlarged image. The lower and right panels in the confocal Z stack show a vertical cross section (yellow lines) through the cells. Bars, 10 μm.
Effects of Stable Knockdown of KIF20A in Cell Motility and Invasion of S2-013 Cells
To investigate whether KIF20A regulates cell motility and invasion, KIF20A expression in S2-013 cells was suppressed by vector-based expression of a KIF20A-specific siRNA. To achieve substantial suppression of KIF20A, we established cell clones subject to RNA interference (RNAi) by expressing a KIF20A-siRNA. KIF20A knockdown was confirmed on immunoblots (Figure 2A). In transwell motility assays, the motility of S2-013 cells was significantly lower in KIF20A-RNAi–expressing cells than in control-RNAi cells (Figure 2B). In two-chamber invasion assays, KIF20A-RNAi S2-013 cells were significantly less invasive than the control-RNAi cells (Figure 2C). We found that transfection of a KIF20A-rescue construct into KIF20A-RNAi S2-013 cells abrogated the changes to cell invasiveness caused by the KIF20A-RNAi (Figure 2D).
Figure 2.
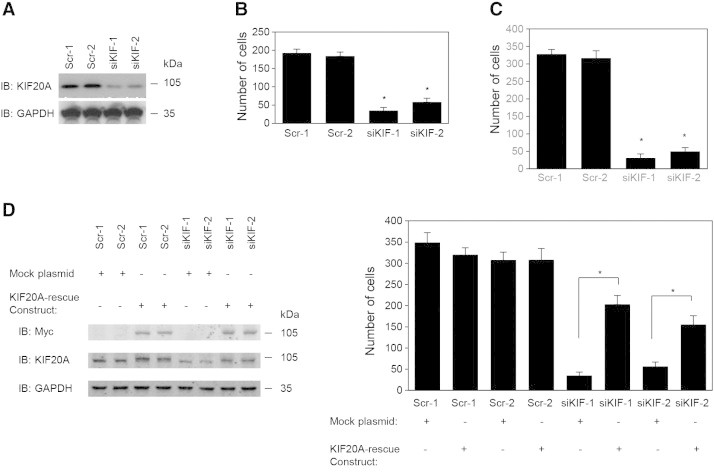
KIF20A promotes cell motility and invasion in PDAC cell culture.
(A) Knockdown effect of KIF20A-siRNA in S2-013 cells. Western blots probed with anti-KIF20A antibody show two S2-013 clones (siKIF-1-2) transfected with siRNA for KIF20A and two scrambled control clones (Scr-1-2).
(B) Scrambled control and KIF20A RNAi S2-013 cells were seeded into transwell motility chambers. Migrating cells in four fields per group were counted. Data were derived from three independent experiments. Columns, mean; bars, SD. *P < .001 compared to Scr-1 or Scr-2 (Student’s t test).
(C) Scrambled control and KIF20A RNAi S2-013 cells were seeded into Matrigel invasion chambers. Invading cells in four fields per group were counted. Data were derived from three independent experiments. Columns, mean; bars, SD. *P < .001 compared to Scr-1 or Scr-2 (Student’s t test).
(D) The mock control vector or myc-tagged KIF20A-rescue construct was transfected into control and KIF20A-RNAi cells; 48 hours later, two-chamber invasion assays were performed. Western blots probed with anti-KIF20A antibody are shown in left panels. Invading cells in four fields per group were counted (right panel). Data were derived from three independent experiments. Columns, mean; bars, SD. *P < .001 compared with corresponding siKIF-1 or siKIF-2 transfected mock vector (Student’s t test).
Association of KIF20A with the Localization of IGF2BP3-Containing SGs in Cell Protrusions
We recently reported that SGs containing the RNA-binding protein IGF2BP3 and IGF2BP3-bound transcripts are transported to cell protrusions of fibronectin-stimulated S2-013 cells [8]. The finding that localization of KIF20A in cell protrusions of fibronectin-stimulated S2-013 cells (Figure 1, A and B) was similar to the IGF2BP3 distribution [8] allowed us to hypothesize that KIF20A could transport IGF2BP3-containing SGs to the protrusions. KIF20A was co-immunoprecipitated with IGF2BP3 from cell lysates of fibronectin-stimulated S2-013 cells (Figure 3A). To investigate whether KIF20A and IGF2BP3 colocalized in cell protrusions, S2-013 cells cultured on fibronectin were double-labeled with anti-KIF20A and anti-IGF2BP3 antibodies (Figure 3B). KIF20A colocalized with IGF2BP3 in cell protrusions. Notably, cytoplasmic KIF20A that localized in the cytoplasm of the cell bodies did not colocalize with IGF2BP3. To investigate whether KIF20A bound IGF2BP3-containing SGs, S2-013 cells cultured on fibronectin were labeled with anti-KIF20A and anti-IGF2BP3 antibodies and an SG marker (anti-G3BP [13]) (Figure 3C). We found that KIF20A colocalized with both IGF2BP3 and G3BP in granules at membrane protrusions. To verify that KIF20A colocalized to SGs, S2-013 cells were subjected to SA-induced oxidative stress and then labeled with anti-KIF20A, anti-IGF2BP3, and anti-G3BP antibodies (Figure 3D). SGs form in the cytoplasm of S2-013 cells when SA is added to complete medium [14]. When S2-013 cells were treated with SA, KIF20A that localized in cytoplasmic granules colocalized with IGF2BP3 and G3BP, but cytoplasmic KIF20A that did not localize in SGs did not colocalize with IGF2BP3 or G3BP (Figure 3D).
Figure 3.
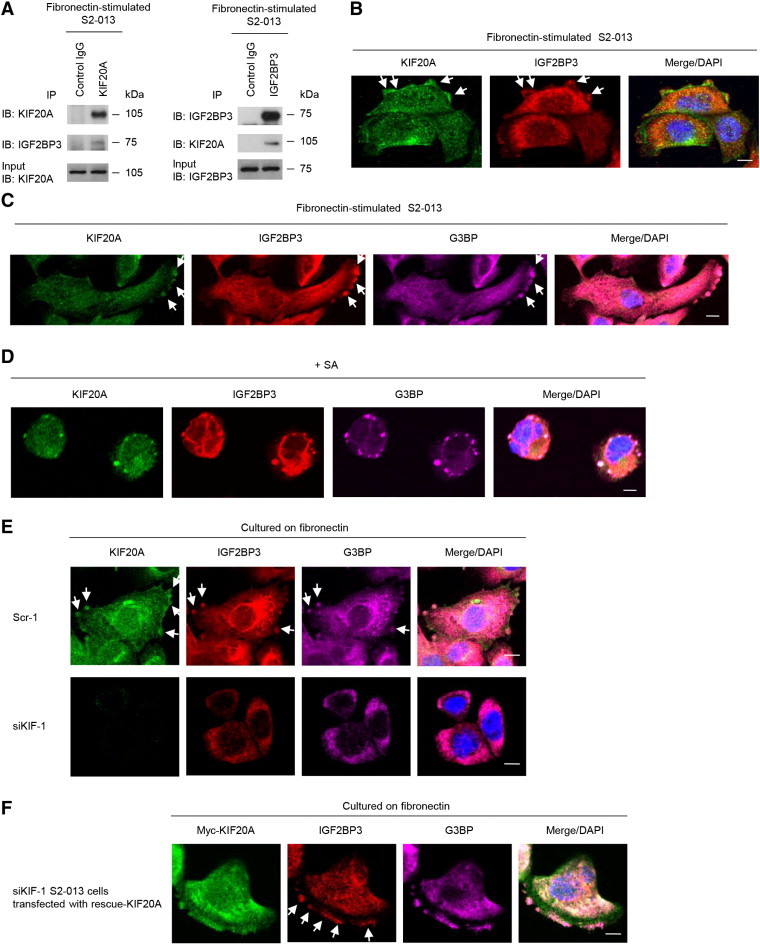
KIF20A colocalizes with IGF2BP3 in cytoplasmic RNA granules assembled in cell protrusions.
(A) Immunoprecipitation of KIF20A or IGF2BP3 from fibronectin-stimulated S2-013 cells. Immunoprecipitants were examined by Western blotting using anti-KIF20A and anti-IGF2BP3 antibodies. Mouse IgG isotype control antibody was used as an isotype control.
(B) Immunocytochemical staining of S2-013 cells cultured on fibronectin; anti-KIF20A (green) and anti-IGF2BP3 (red) antibodies were used to label endogenous proteins. Arrows, KIF20A colocalized with IGF2BP3 in cell protrusions. Blue, DAPI staining. Bar, 10 μm.
(C) S2-013 cells were incubated on fibronectin and immunocytochemically stained using anti-KIF20A (green), anti-IGF2BP3 (red), and anti-G3BP (violet) antibodies. Arrows, KIF20A colocalized with IGF2BP3 and G3BP in cell protrusions. Bar, 10 μm.
(D) S2-013 cells were exposed to 500 μM SA for 30 minutes. Immunocytochemical staining with anti-KIF20A (green), anti-IGF2BP3 (red), and anti-G3BP (violet) antibodies is shown. Blue, DAPI staining. Bar, 10 μm.
(E) Scrambled control (Scr-1) S2-013 cells or KIF20A-knockdown (siKIF-1) S2-013 cells were incubated on fibronectin and immunocytochemically stained with anti-KIF20A (green), anti-IGF2BP3 (red), and anti-G3BP (violet) antibodies. Arrows, KIF20A colocalized with IGF2BP3 and G3BP in cell protrusions. Blue, DAPI staining. Bars, 10 μm.
(F) The myc-tagged KIF20A-rescue construct was transfected into KIF20A-knockdown (siKIF-1) S2-013 cells. Forty-eight hours later, the cells were incubated on fibronectin. The cells were immunocytochemically stained with antibodies against myc (green), IGF2BP3 (red), and G3BP (violet). Arrows, reexpressed IGF2BP3 colocalized with G3BP in cell protrusions. Blue, DAPI staining. Bar, 10 μm.
We used scrambled control-RNAi S2-013 cells (KIF20A-RNAi S2-013) and immunocytochemistry to determine whether KIF20A had a role in the localization of IGF2BP3-containing SGs in cell protrusions. Immunofluorescence indicated that granular colocalization of IGF2BP3 and G3BP in membrane protrusions of fibronectin-stimulated control S2-013 cells was decreased in fibronectin-stimulated KIF20A-RNAi S2-013 cells (Figure 3E). In contrast, KIF20A knockdown did not affect the localization of cytoplasmic IGF2BP3 and G3BP that localized in the cytoplasm of the cell bodies (Figure 3E). Transfection of a KIF20A-rescue construct renewed the granular colocalization of IGF2BP3 and G3BP in membrane protrusions of KIF20A-RNAi S2-013 cells cultured on fibronectin (Figure 3F). These data indicate that KIF20A associated with the localization of IGF2BP3-containing SGs in cell protrusions.
Association of KIF20A with the Localization of IGF2BP3 and IGF2BP3-Bound Transcripts in Cell Protrusions
We recently reported two IGF2BP3-bound mRNAs, ARF6 and ARHGEF4, that assemble with IGF2BP3 in cell protrusions of fibronectin-stimulated S2-013 cells [8]. Immunocytochemistry and RNA fluorescence in situ hybridization were used together to determine whether KIF20A colocalized with IGF2BP3 and each mRNA (ARF6 and ARHGEF4) within cell protrusions of S2-013 cells cultured on fibronectin. Each mRNA colocalized with KIF20A and IGF2BP3 assembled in cell protrusions (Figure 4A). Ubiquitin C mRNA did not colocalize with KIF20A or IGF2BP3 in fibronectin-stimulated S2-013 cells (Figure 4A). In contrast, the mRNAs for ARF6 and ARHGEF4 that accumulated in cell protrusions were decreased in fibronectin-stimulated KIF20A-knockdown S2-013 cells compared to scrambled control S2-013 cells (Figure 4B). These results indicated that KIF20A associated with the localization of IGF2BP3 and IGF2BP3-bound transcripts in cell protrusions.
Figure 4.
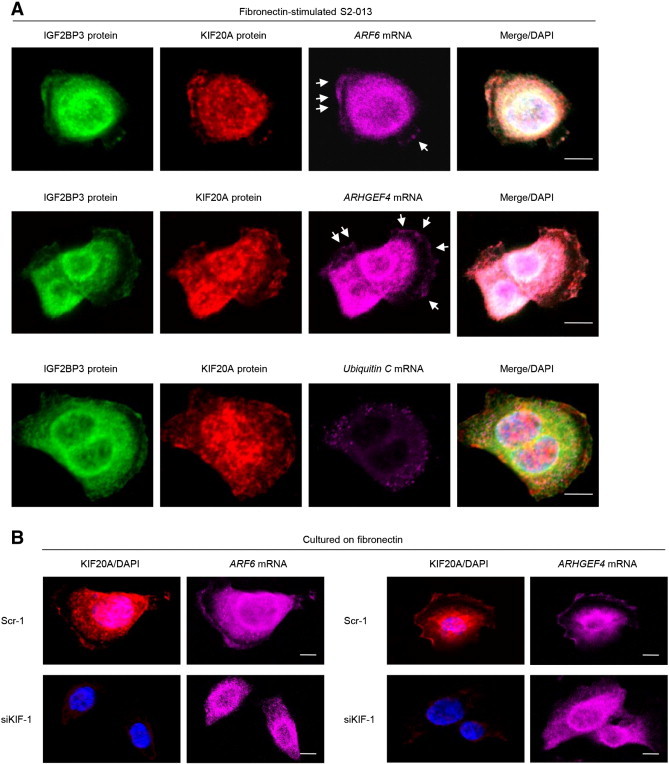
KIF20A colocalizes with IGF2BP3 and mRNAs for ARF6 and ARHGEF4.
(A) Colocalization of KIF20A protein (green), IGF2BP3 protein (red), and ARF6 or ARHGEF4 mRNA (violet) in S2-013 cells cultured on fibronectin. Ubiquitin C mRNA was used as a negative control for colocalization. Arrows, mRNAs colocalized with KIF20A and IGF2BP3 in cell protrusions. Blue, DAPI staining. Bars, 10 μm.
(B) Immunocytochemistry and RNA fluorescence in situ hybridization were performed in fibronectin-stimulated scrambled control (Scr-1) S2-013 cells and KIF20A-knockdown (siKIF-1) S2-013 cells. KIF20A protein (green), IGF2BP3 protein (red), ARF6 or ARHGEF4 mRNA (violet), and DAPI staining (blue). Ubiquitin C mRNA was used as a negative control for colocalization. Bars, 10 μm.
Effects of KIF20A in Decreasing the Protein Expression from IGF2BP3-Bound Transcripts in Cell Protrusions
We previously found that IGF2BP3-bound transcripts, ARF6 and ARHGEF4, are translated in membrane protrusions of spreading S2-013 cells [8]. We hypothesized that KIF20A could transport IGF2BP3-containing SGs to cell protrusions, and therefore knockdown of KIF20A could decrease the protein expression from IGF2BP3-bound mRNAs in cell protrusions via inhibition of trafficking of IGF2BP3, ARF6 mRNA, and ARHGEF4 mRNA. We used scrambled control S2-013 cells and KIF20A-RNAi S2-013 cells to perform an immunocytochemical analysis of ARF6 and ARHGEF4 protein localization; all cells were cultured on fibronectin. ARF6 and ARHGEF4 were expressed in the cytoplasm of the cell bodies and membrane protrusions in control-RNAi cells, but immunofluorescent signals from ARF6 and ARHGEF4 in the protrusions of KIF20A-RNAi cells were decreased relative to those in control-RNAi cells, and in KIF20A-RNAi cells, the ARF6 and ARHGEF4 signals remained in the cytoplasm of the cell bodies (Figure 5, A and B). Transfection of a KIF20A-rescue construct renewed the expressions of ARF6 (Figure 5C) and ARHGEF4 (Figure 5D) in membrane protrusions of KIF20A-RNAi S2-013 cells cultured on fibronectin. These findings indicated that KIF20A associates with protein expression from IGF2BP3-bound transcripts in these membrane protrusions.
Figure 5.
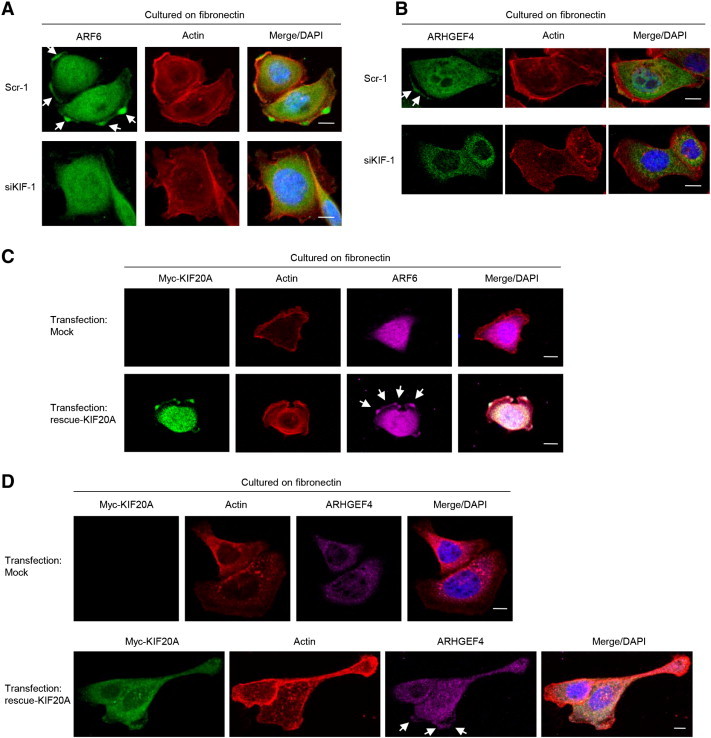
KIF20A associates with local translation of ARF6 and ARHGEF4 in cell protrusions.
(A) Scrambled control (Scr-1) S2-013 cells or KIF20A-knockdown (siKIF-1) S2-013 cells were incubated on fibronectin and immunocytochemically stained with anti-ARF6 antibody (green) and phalloidin (red). Actin filaments were labeled by phalloidin. Arrows, ARF6 localized in cell protrusions. Blue, DAPI staining. Bars, 10 μm.
(B) Scr-1 and siKIF-1 S2-013 cells treated as in A were immunocytochemically stained with anti-ARHGEF4 antibody (green) and phalloidin (red). Arrows, ARHGEF4 localized in cell protrusions. Blue, DAPI staining. Bars, 10 μm.
(C) The mock-plasmid or myc-tagged KIF20A-rescue construct was transfected into KIF20A-knockdown S2-013 cells. Forty-eight hours later, the cells were incubated on fibronectin. The cells were immunocytochemically stained with antibodies against myc (green) and ARF6 (violet) and phalloidin (red). Arrows, ARF6 reexpressed in cell protrusions. Bars, 10 μm.
(D) Scr-1 and aiKIF-1 S2-013 cells treated as in C were immunocytochemically stained with antibodies against myc (green) and ARHGEF4 (violet) and phalloidin (red). Arrows, ARHGEF4 re-expressed in cell protrusions. Bars, 10 μm.
Translocation of IGF2BP3 and IGF2BP3-Bound Transcripts Toward Membrane Protrusions via KIF20A and a Microtubule Network
We previously reported that KIF20A overlaps with α-tubulin in the cytoplasm of the PDAC cell line PK59 [4]. We used immunocytochemistry to determine the subcellular localization of IGF2BP3 in fibronectin-stimulated S2-013 cells with or without nocodazole treatment that disrupted microtubules. IGF2BP3 was overlapped with that of α-tubulin both in the cytoplasm of the cell bodies and in cell protrusions of nontreated S2-013 cells (Figure 6A). When nocodazole was added to the culture medium of S2-013 cells growing on fibronectin, IGF2BP3 that did not colocalize with disrupted microtubules in the cytoplasm of the cell body was not present at membrane protrusions (Figure 6A). These findings indicated that, like IGF2BP1 [15], translocation of IGF2BP3 to cell protrusions was dependent on microtubule networks. Importantly, IGF2BP3 co-precipitated with taxol-stabilized microtubules in fibronectin-stimulated S2-013 cells, and coilin, which was not expected to associate with these microtubules, did not co-precipitate with them (Figure 6B). Moreover, treatment of fibronectin-stimulated S2-013 cells with nocodazole decreased KIF20A co-immunoprecipitated with IGF2BP3 (Figure 6C). In addition, levels of ARF6 (Figure 6D) and ARHGEF4 (Figure 6E), like IGF2BP3 levels, were lower in membrane protrusions of nocodazole-treated fibronectin-stimulated S2-013 cells than in those of untreated fibronectin-stimulated S2-013 cells; all cells were grown on fibronectin. In contrast, ARF6 and ARHGEF4 that localized in the cytoplasm of the cell bodies remained unaltered upon knockdown of KIF20A (Figure 6, D and E). These results indicated that translational regulation of ARF6 and ARHGEF4 in cell protrusions was dependent on the role of KIF20A in transporting IGF2BP3 and IGF2BP3-bound transcripts to cell protrusions along microtubules.
Figure 6.
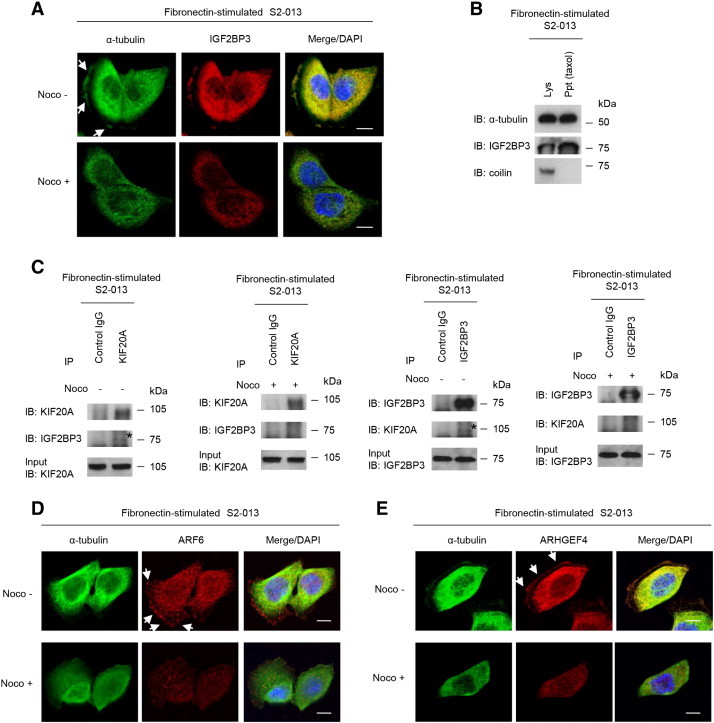
KIF20A and IGF2BP3 associate with microtubules.
(A) S2-013 cells were incubated on fibronectin with or without 10 μM nocodazole (Noco) and were then immunocytochemically stained with antibodies against α-tubulin (green) and IGF2BP3 (red). Arrows, IGF2BP3 localized in cell protrusions. Blue, DAPI staining. Bars, 10 μm.
(B) Association of IGF2BP3 with microtubules. The soluble fraction of fibronectin-stimulated S2-013 lysates (Lys) was treated with 20 μM taxol and 1 mM GTP and then centrifuged to separate the precipitate (Ppt) from the supernatant. Immunoblot analyses were performed with antibodies against α-tubulin, IGF2BP3, and coilin.
(C) Immunoprecipitation by anti-KIF20A or anti-IGF2BP3 antibody was performed from fibronectin-stimulated S2-013 cells with or without 10 μM nocodazole treatment, followed by Western blotting using antibodies against the identified proteins. *, KIF20A or IGF2BP3 immunoprecipitated from fibronectin-stimulated S2-013 cell lysates without nocodazole treatment. Mouse or rabbit IgG isotype control antibody was used as an isotype control.
(D) S2-013 cells treated as in A were immunocytochemically stained with antibodies against α-tubulin (green) and ARF6 (red). Arrows, ARF6 localized in cell protrusions. Blue, DAPI staining. Bars, 10 μm.
(E) S2-013 cells treated as in A were immunocytochemically stained with antibodies against α-tubulin (green) and ARHGEF4 (red). Arrows, ARHGEF4 localized in cell protrusions. Blue, DAPI staining. Bars, 10 μm.
Effects of KIF20A in Forming Cell Protrusions
We found that ARF6 and ARHGEF4 transcripts that are translated in cell protrusions induce further formation of membrane protrusions; consequently, IGF2BP3 promotes cell motility and invasion of PDAC cells [8]. Confocal microscopy was used to examine the three-dimensional configurations of peripheral actin structures and cell protrusions. Peripheral actin structures (Figure 7A) and cell protrusions (Figure 7B) were less abundant in KIF20A-RNAi S2-013 cells than in control-RNAi S2-013 cells. Transfection of a KIF20A-rescue construct renewed peripheral actin structures in KIF20A-RNAi S2-013 cells (Figure 7A). Consequently, cell protrusions were significantly more abundant in KIF20A-RNAi S2-013 cells carrying a KIF20A-rescue construct than in KIF20A-RNAi S2-013 cells lacking this construct (Figure 7B). Whereas control-RNAi S2-013 clones exhibited spindle-shaped cells and fibroblastic morphology, KIF20A-RNAi cells typically displayed a cobblestone-like, epithelial morphology (Figure 7C). These results indicated that KIF20A drove rearrangement of peripheral actin to induce formation of additional membrane protrusions.
Figure 7.
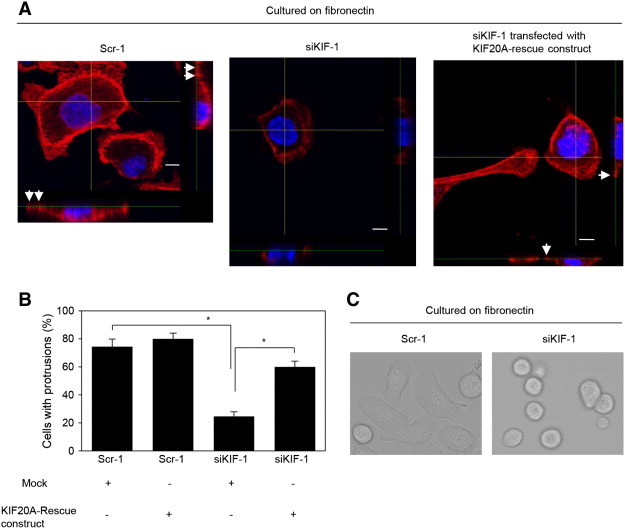
KIF20A associates with forming cell protrusions.
(A) Confocal Z stack shows the peripheral actin structures labeled by phalloidin (red) and nuclear DAPI staining (blue) in fibronectin-stimulated scrambled control (Scr-1) S2-013 cells or KIF20A-knockdown (siKIF-1) S2-013 cells transfected with or without the myc-tagged KIF20A-rescue construct. Arrows, peripheral actin structures in cell protrusions. The lower and right panels in the confocal Z stack show a vertical cross section (yellow lines) through the cells. Bars, 10 μm.
(B) Quantification of data shown in A; the values represent the number of cells with fibronectin-mediated cell protrusions in which peripheral actin structures were increased. All cells in four fields per group were scored. Data were derived from three independent experiments. Columns, mean; bars, SD. *P < .001 compared with Scr-1 or siKIF-1 transfected mock vector (Student’s t test).
(C) Morphology of fibronectin-stimulated scrambled control-RNAi (Scr-1) and KIF20A-RNAi (siKIF-1) S2-013 cells analyzed by phase-contrast microscopy.
Discussion
Here, we describe a newly discovered mechanism of KIF20A in PDAC cells in which KIF20A transports SGs containing IGF2BP3 and IGF2BP3-bound mRNAs to cell protrusions along microtubules. These IGF2BP3-bound mRNAs that were transported by KIF20A are translated in the protrusions, where they probably promote the invasiveness of the PDAC cells.
In neural cells, most proteins destined for dendrites and dendritic spines are conveyed from the cell body; a subset of mRNAs is transported into dendrites to support local protein synthesis [16], [17]. Local translation allows selected synapses to control their strength and efficacy independently among the thousands of synapses in one neuron [18]. Certain mRNAs such as CaMKIIα and Arc are transported to dendrites in SGs that are transported by KIF5, which binds RNA-binding proteins by a recognition motif in its tail domain [7]. With regard to the mechanism of mRNA transport in dendrites, the direct binding proteins for KIF5 in the granules have not yet been identified [19]. In this study, we found that KIF20A directly bound IGF2BP3 localized in SGs and played important roles in transporting IGF2BP3-bound mRNAs such as ARF6 and ARHGEF4 toward membrane protrusions.
The mechanism of mRNA transport and regulation of local protein synthesis in cancers, including PDAC, remains unclear. We previously reported that KIF20A functions as part of the intracellular trafficking machinery for the DLG5 protein, transporting it to membrane sites in PDAC cells [4]. In addition to the protein-trafficking function of KIF20A, we provided a basis for understanding the mRNA transport system of KIF20A to cell protrusions, and these observations suggested the possibility that binding of cargoes such as IGF2BP3-mRNA complexes to KIF20A steers kinesin motors toward the protrusions. Locally translated ARF6 and ARHGEF4 in the protrusions function to induce the generation of cell protrusions and thereby promote the motility and invasiveness of PDAC cells [8]. Similarly with IGF2BP3, KIF20A knockdown also decreased cell protrusions in which peripheral actin structures were abundant and inhibited cell motility and invasion of PDAC cells. The rescued expression of KIF20A renewed the expression of ARF6 and ARHGEF4 only in the protrusions, and induced formation of the protrusions in fibronectin-stimulated KIF20A-RNAi S2-013 cells. These findings indicate that KIF20A can increase cell protrusions through its function in trafficking IGF2BP3-containing SGs and affect local protein synthesis from IGF2BP3-bound transcripts. The molecular mechanisms by which local translation of the particular mRNAs localized in IGF2BP3-containing SGs is initiated are important subjects for future study; nevertheless, our findings that pertain to mRNA transport and local translation in cell protrusions in PDAC cells are probably relevant to those in the dendrites of neural cells.
IGF2BP3 does not contain any tubulin-binding domains that may be involved in the transport of specific mRNAs along microtubules; however, we found that IGF2BP3 was a microtubule-associated protein in PDAC cells. The human double-stranded RNA-binding protein Staufen1 forms SGs [20] and has a role in processes such as microtubule-dependent mRNA transport and localized translation of specific mRNAs in polarized cells [20], [21], [22]. Both Staufen1 and the kinesin protein KIF5C are responsible for dendritic transport of Shank1 mRNA along microtubules [23]. Local protein synthesis from Shank1 mRNA in the dendrites is crucial for postsynaptic density assembly [24] and the formation of dendritic spines [25]. These findings suggest that structures that contain kinesin motor proteins, RNA-binding proteins, and microtubules are necessary for RNA transport and localized translation. Consistent with these previous findings in the dendrites, the association of KIF20A and IGF2BP3 is likely to play important roles in microtubule-dependent RNA transport and localized translation in the protrusions of PDAC cells.
As noted in the introduction, KIF20A is thought to be involved in carcinogenesis in PDAC. The testes and thymus are the only normal tissues that express KIF20A [4], [26]. Therefore, KIF20A could be considered as an ideal cancer-testis antigen, and the KIF20A peptide might be a cancer vaccine for PDAC. A phase I/II clinical trial using a peptide vaccine derived from KIF20A alone (UMIN-CTR; #UMIN000004919) recently revealed that patients with advanced PDAC vaccinated with KIF20A-derived peptide had better prognosis than the control group with the best supportive care and that KIF20A-derived vaccination is significantly effective as an immunotherapy against advanced PDAC [27]. In Japan, a randomized phase III clinical trial using peptide vaccines, including for KIF20A, is currently being carried out for advanced PDAC patients. Concerning other malignant phenotypes of PDAC cells aside from cell proliferation, such as motility, invasiveness, and metastasis, any roles of KIF20A are still unknown. This study is the first to demonstrate the role of KIF20A in the motility and invasiveness of PDAC cells. In addition to the possibility that a KIF20A peptide vaccine may represent a potential novel treatment option for PDAC, the data presented here indicated to us that inhibition of 1) the interaction between IGF2BP3 and KIF20A, 2) the trafficking system that transports IGF2BP3-bound transcripts to membrane protrusions, 3) IGF2BP3, or 4) some combination of the above may be effective for targeted molecular therapy because any such therapy would inhibit local translation in cell protrusions and consequently limit the invasiveness of PDACs.
Acknowledgments
We thank Makiko Tsuboi, Aki Tanouchi, Chiaki Okura, and Hiroko Oshita for their excellent technical assistance. This study was supported by a Grant-in-Aid for Scientific Research (KAKENHI) (to K.T.), by the Pancreas Research Foundation of Japan (to K.T.), and by the Japanese Foundation for Multidisciplinary Treatment of Cancer (to K.T.).
Footnotes
Conflict of interest: The authors have declared that no competing interests exist.
References
- 1.Diefenbach RJ, Mackay JP, Armati PJ, Cunningham AL. The C-terminal region of the stalk domain of ubiquitous human kinesin heavy chain contains the binding site for kinesin light chain. Biochemistry. 1998;37(47):16663–16670. doi: 10.1021/bi981163r. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein LS, Philp AV. The road less traveled: emerging principles of kinesin motor utilization. Annu Rev Cell Dev Biol. 1999;15:141–183. doi: 10.1146/annurev.cellbio.15.1.141. [DOI] [PubMed] [Google Scholar]
- 3.Hill E, Clarke M, Barr FA. The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO J. 2000;19(21):5711–5719. doi: 10.1093/emboj/19.21.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taniuchi K, Nakagawa H, Nakamura T, Eguchi H, Ohigashi H, Ishikawa O, Katagiri T, Nakamura Y. Down-regulation of RAB6KIFL/KIF20A, a kinesin involved with membrane trafficking of discs large homologue 5, can attenuate growth of pancreatic cancer cell. Cancer Res. 2005;65(1):105–112. [PubMed] [Google Scholar]
- 5.Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans. 2002;30(6):963–969. doi: 10.1042/bst0300963. [DOI] [PubMed] [Google Scholar]
- 6.Carson JH, Worboys K, Ainger K, Barbarese E. Translocation of myelin basic protein mRNA in oligodendrocytes requires microtubules and kinesin. Cell Motil Cytoskeleton. 1997;38(4):318–328. doi: 10.1002/(SICI)1097-0169(1997)38:4<318::AID-CM2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Hirokawa N. mRNA transport in dendrites: RNA granules, motors, and tracks. J Neurosci. 2006;26(27):7139–7142. doi: 10.1523/JNEUROSCI.1821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taniuchi K, Furihata M, Hanazaki K, Saito M, Saibara T. IGF2BP3-mediated translation in cell protrusions promotes cell invasiveness and metastasis of pancreatic cancer. Oncotarget. 2014;5(16):6832–6845. doi: 10.18632/oncotarget.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwamura T, Katsuki T, Ide K. Establishment and characterization of a human pancreatic cancer cell line (SUIT-2) producing carcinoembryonic antigen and carbohydrate antigen 19-9. Jpn J Cancer Res. 1987;78(1):54–62. [PubMed] [Google Scholar]
- 10.Taylor AM, Dieterich DC, Ito HT, Kim SA, Schuman EM. Microfluidic local perfusion chambers for the visualization and manipulation of synapses. Neuron. 2010;66(1):57–68. doi: 10.1016/j.neuron.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deer EL, González-Hernández J, Coursen JD, Shea JE, Ngatia J, Scaife CL, Firpo MA, Mulvihill SJ. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010;39(4):425–435. doi: 10.1097/MPA.0b013e3181c15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taniuchi K, Yokotani K, Saibara T. BART inhibits pancreatic cancer cell invasion by Rac1 inactivation through direct binding to active Rac1. Neoplasia. 2012;14(5):440–450. doi: 10.1593/neo.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kedersha N, Anderson P. Mammalian stress granules and processing bodies. Methods Enzymol. 2007;431:61–81. doi: 10.1016/S0076-6879(07)31005-7. [DOI] [PubMed] [Google Scholar]
- 14.Taniuchi K, Nishimori I, Hollingsworth MA. Intracellular CD24 inhibits cell invasion by posttranscriptional regulation of BART through interaction with G3BP. Cancer Res. 2011;71(3):895–905. doi: 10.1158/0008-5472.CAN-10-2743. [DOI] [PubMed] [Google Scholar]
- 15.Elisha Z, Havin L, Ringel I, Yisraeli JK. Vg1 RNA binding protein mediates the association of Vg1 RNA with microtubules in Xenopus oocytes. EMBO J. 1995;14(20):5109–5114. doi: 10.1002/j.1460-2075.1995.tb00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steward O, Worley P. Localization of mRNAs at synaptic sites on dendrites. Results Probl Cell Differ. 2001;34:1–26. doi: 10.1007/978-3-540-40025-7_1. [DOI] [PubMed] [Google Scholar]
- 17.Pfeiffer BE, Huber KM. Current advances in local protein synthesis and synaptic plasticity. J Neurosci. 2006;26(27):7147–7150. doi: 10.1523/JNEUROSCI.1797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin KC, Kosik KS. Synaptic tagging—who's it? Nat Rev Neurosci. 2002;3(10):813–820. doi: 10.1038/nrn942. [DOI] [PubMed] [Google Scholar]
- 19.Niwa S, Nakajima K, Miki H, Minato Y, Wang D, Hirokawa N. KIF19A is a microtubule-depolymerizing kinesin for ciliary length control. Dev Cell. 2012;23(6):1167–1175. doi: 10.1016/j.devcel.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Krichevsky AM, Kosik KS. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron. 2001;32(4):683–696. doi: 10.1016/s0896-6273(01)00508-6. [DOI] [PubMed] [Google Scholar]
- 21.Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43(4):513–525. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Dugré-Brisson S, Elvira G, Boulay K, Chatel-Chaix L, Mouland AJ, DesGroseillers L. Interaction of Staufen1 with the 5' end of mRNA facilitates translation of these RNAs. Nucleic Acids Res. 2005;33(15):4797–4812. doi: 10.1093/nar/gki794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falley K, Schütt J, Iglauer P, Menke K, Maas C, Kneussel M, Kindler S, Wouters FS, Richter D, Kreienkamp HJ. Shank1 mRNA: dendritic transport by kinesin and translational control by the 5'untranslated region. Traffic. 2009;10(7):844–857. doi: 10.1111/j.1600-0854.2009.00912.x. [DOI] [PubMed] [Google Scholar]
- 24.Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23(3):583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- 25.Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA, Kidd FL, Sung CC, Miyakawa T, Bear MF. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28(7):1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai K, Hirata S, Irie A, Senju S, Ikuta Y, Yokomine K, Harao M, Inoue M, Tomita Y, Tsunoda T. Identification of HLA-A2-restricted CTL epitopes of a novel tumour-associated antigen, KIF20A, overexpressed in pancreatic cancer. Br J Cancer. 2011;104(2):300–307. doi: 10.1038/sj.bjc.6606052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asahara S, Takeda K, Yamao K, Maguchi H, Yamaue H. Phase I/II clinical trial using HLA-A24-restricted peptide vaccine derived from KIF20A for patients with advanced pancreatic cancer. J Transl Med. 2013;11:291. doi: 10.1186/1479-5876-11-291. [DOI] [PMC free article] [PubMed] [Google Scholar]


