Abstract
Background
There are limited data on the effect of endoscopic mucosal resection (EMR) on changes of histopathologic diagnosis for Barrett’s esophagus (BE) patients undergoing endoscopic eradication therapy (EET); especially those without visible lesions.
Aim
To compare the frequency of changes of diagnosis by EMR compared with pre-EMR biopsy diagnosis for patients with and without visible lesions.
Methods
In this multicenter outcomes project, patients with Barrett’s-related neoplasia undergoing EET at three tertiary-care centers were included. Patients undergoing biopsies followed by EMR within six months were included. The main outcome measures were frequency of overall change of histopathologic diagnosis, change based on pre-EMR biopsy diagnosis, and change based on the presence of visible lesions.
Results
One-hundred and thirty-eight BE patients (low-grade dysplasia (LGD) 15 (10.9 %), high-grade dysplasia (HGD) 87 (63 %), esophageal adenocarcinoma (EAC) 36 (26.1 %)) were included; 114 (82.6 %) patients had visible lesions. EMR resulted in a change of diagnosis for 43 (31.1 %) patients (upgrade 14 (10.1 %); downgrade 29 (21 %)). For HGD patients, EMR downstaged dysplasia grade for 17 (19.5 %) cases and upstaged it to EAC for nine (10.3 %) cases. There was a change of diagnosis for 26 (29.9 %) HGD patients, irrespective of the presence or absence of visible lesions (p = 0.76). For EAC patients, EMR downstaged dysplasia grade in 10 (27.8 %) cases. There was a change of diagnosis for 10 (27.8 %) EAC patients, irrespective of the presence or absence of endoscopically visible lesions (p = 0.48).
Conclusions
EMR results in a change of diagnosis for approximately 30 % of BE patients with early neoplasia (with and without visible lesions) referred for EET.
Keywords: Barrett’s esophagus, Dysplasia, Esophageal adenocarcinoma, Endoscopic mucosal resection
Introduction
Barrett’s esophagus (BE) is the most important risk factor for development of esophageal adenocarcinoma (EAC), a cancer that has increased almost fourfold in the last three decades, making it the cancer with the most rapidly increasing incidence in the Western world [1]. Despite all the recent advances in the diagnosis and management of EAC, overall five-year survival continues to be a dismal 15–20 % [2]. The degree of dysplasia is the most important determinant for management of BE patients, and those with high-grade dysplasia (HGD) are at the highest risk of progression to EAC [3]. It is for this group of patients (and intramucosal cancer) that endoscopic eradication therapy (EET) is now a well-established therapeutic option [4, 5].
With the expanding endoscopic armamentarium for management of BE patients with HGD and/or intramucosal cancer, accurate staging is critical. Patients with neoplasia limited to the mucosa are at minimum risk of lymph node metastasis (0–3 %) and hence are ideal candidates for EET. In contrast, for patients with submucosal infiltration the risk of lymph node involvement increases substantially (20–30 %); such patients should, therefore, be referred for surgical resection [6–8]. Given the limitations of the biopsy-alone strategy (inability to determine depth of invasion and poor interobserver reproducibility among pathologists for biopsy specimens), treatment regimens and decisions based on biopsy specimens may not be stage-appropriate [3, 9, 10]. Although endoscopic ultrasonography (EUS) has been used to estimate cancer depth, several studies have demonstrated that EUS is a suboptimal technique for distinguishing mucosal from submucosal lesions and accurately determining T-stage [2, 11, 12]. Endoscopic mucosal resection (EMR) involves local snare excision of the lesion down to the level of the submucosa and has been increasingly used as both a diagnostic tool and a therapeutic treatment option for management of dysplastic BE. With EMR, the depth of tumor invasion can be established on the basis of histologic criteria (identification of submucosal invasion) that enable clinicians to treat HGD and/or early cancer by use of endoscopic therapy with greater confidence [2, 3]. Inaccurate diagnosis could have substantial deleterious consequences for the patient. A patient with benign disease could be subjected to esophagectomy or patients with submucosal invasion could undergo endoscopic therapy instead of surgery. In addition, interobserver agreement among pathologists is greater for EMR than for biopsy specimens [3, 13].
Although EMR is recommended for visible lesions within Barrett’s segment, evidence-based selection criteria for EMR are sparse. The frequency of change of histologic diagnosis post-EMR is highly variable. In addition, there are limited data on the effect of EMR on changes in dysplasia grade for patients without visible lesions. For a multicenter cohort of patients with BE undergoing EET, the purpose of this study was to:
assess overall change of histopathologic diagnosis with EMR compared with pre-EMR biopsy diagnosis; and
to compare the frequency of change of histopathologic diagnosis by EMR compared with pre-EMR biopsy diagnosis for patients with and without visible lesions.
Methods
Patients
This multi-center outcomes project included three tertiary care referral centers with an interest in BE—the Veterans Affairs Medical Center, Kansas City, Missouri, USA; Washington University School of Medicine, St Louis, Missouri, USA, and Columbia University College of Physicians and Surgeons, New York, New York, USA. The study was approved by the Institutional Review Board at each institution. This outcomes project included patients referred to these tertiary care centers for EET. The objectives of the project were to evaluate the role of diagnostic and/or staging tools and the efficacy of endoscopic eradication therapy for patients with Barrett’s-related neoplasia.
Patients with Barrett’s-related neoplasia undergoing EET at these three tertiary referral centers were identified and enrolled in a database at each center. The following information was collected for all patients with BE: demographics (age, gender, and ethnicity), endoscopy results (date of procedure, presence of hiatal hernia, and length of BE), and histologic diagnosis at each endoscopic procedure. Pre-EMR biopsy diagnosis was recorded for all patients. The presence of endoscopically visible lesions was recorded, and the lesions were characterized as either flat and inconspicuous. Mucosal irregularity, nodules, and/or ulceration in Barrett’s segment were recorded. Details of all endoscopic intervention during each procedure were recorded (surveillance biopsies or EET).
To assess the role of diagnostic and/or staging EMR, only patients undergoing biopsies followed by a diagnostic EMR within six months before EET were included in this study. Exclusion criteria were:
patients not undergoing a diagnostic EMR before endoscopic eradication therapy;
pre-EMR biopsies and diagnostic EMR not performed in the 6-month period;
previous history of endoscopic eradication therapy; and
EMR performed to remove all BE.
Histopathologic diagnosis of specimens obtained by EMR was regarded as the reference method for comparison purposes.
Endoscopic Evaluation and Endoscopic Mucosal Resection
All patients were sedated according to standard protocols (intravenous midazolam, demerol, fentanyl, and propofol) at each center and all endoscopic procedures were performed by endoscopists with extensive experience in advanced imaging techniques and endoscopic eradication therapy in Barrett’s-related neoplasia. All endoscopies were performed by use of a high-definition endoscope with narrow band imaging capability (GIF-H180; Olympus, Center Valley, PA, USA; available at all centers). During each endoscopy procedure, the presence and length of BE was measured, and the endoscopist carefully inspected the BE segment for the presence of any visible lesion. Endoscopic evaluation was often supplemented with advanced imaging techniques, for example narrow-band imaging. Use of these techniques to aid management of these patients was at the discretion of the endoscopist and not standardized. In addition, use of pre-EMR staging with CT and/or EUS was not standardized or mandatory for the performance of diagnostic EMR. EMRs of the visible lesions were performed (Figs. 1, 2). For patients with no visible lesions, diagnostic EMR was performed on the basis of results of abnormal narrow band imaging patterns (Figs. 3, 4) [14] or on the basis of the level of abnormal biopsy results. EMR was performed by either the cap technique (K-003, K-008; Olympus, USA) with submucosal injection using normal saline and methylene blue, by the technique described elsewhere [15–18], or by using the multi-band ligator technique with commercially available kits (Duette, Cook Medical, Bloomington, IN, USA), as described elsewhere [16, 18, 19].
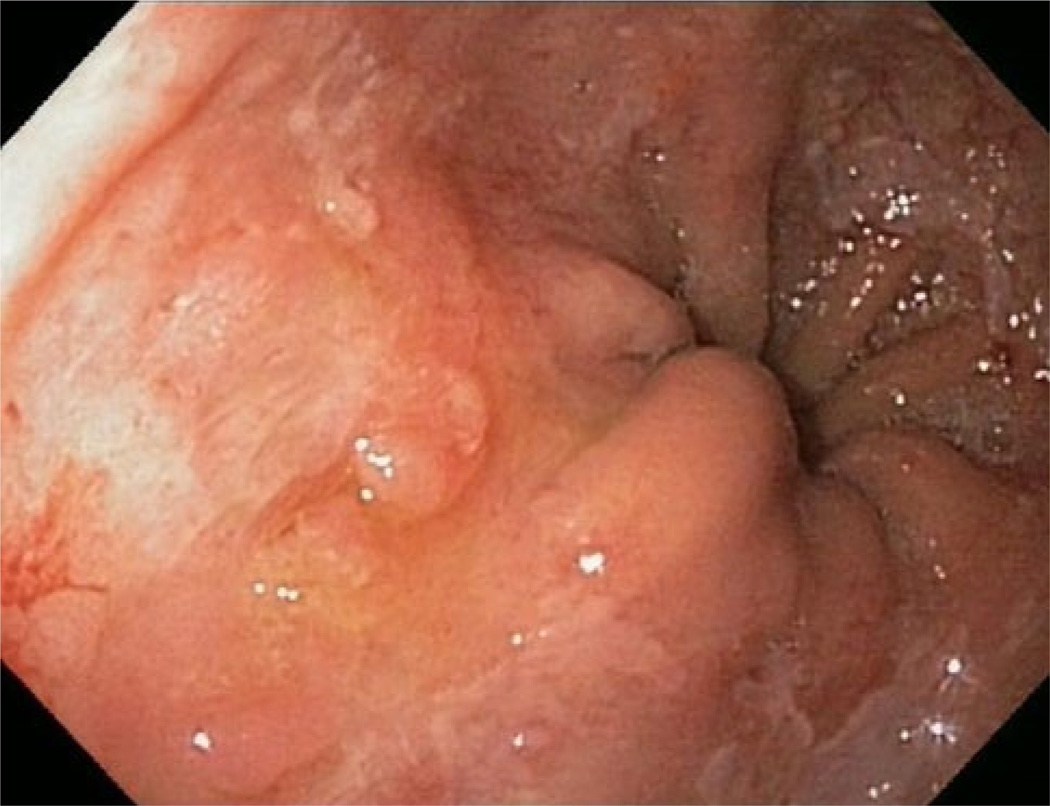
Fig. 1.
BE with visible lesion
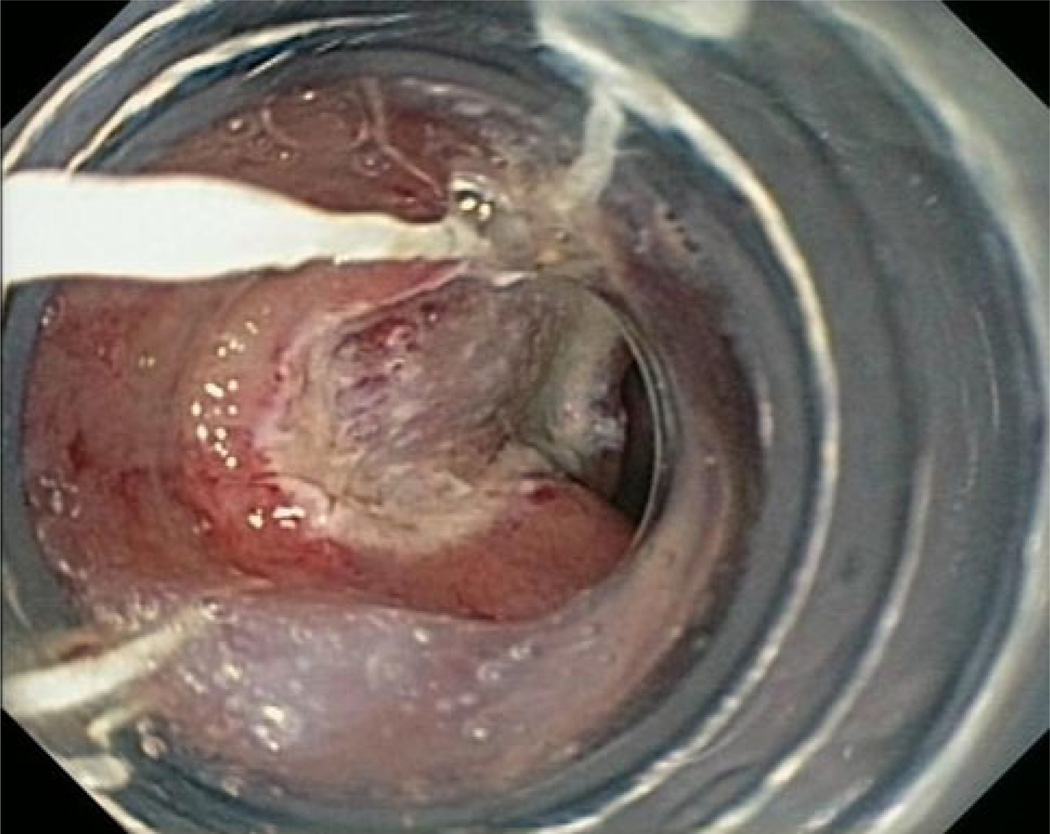
Fig. 2.
Endoscopic mucosal resection of visible lesion
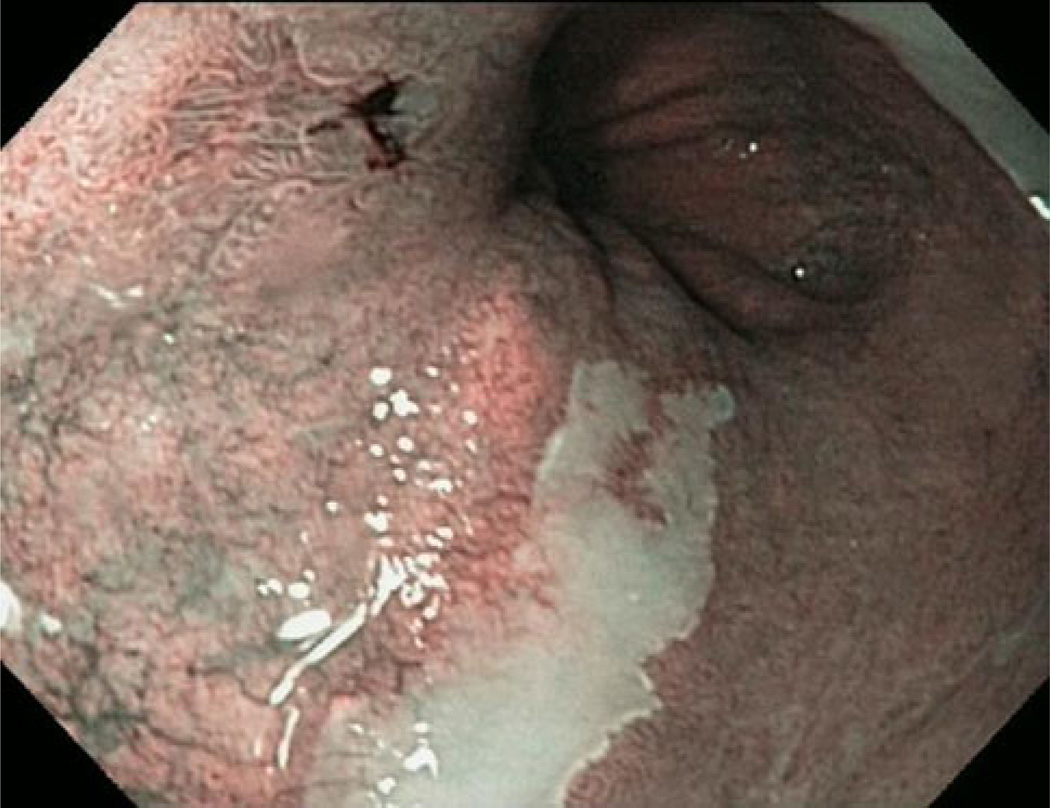
Fig. 3.
BE with flat dysplasia with an abnormal mucosal and vascular pattern in narrow-band imaging
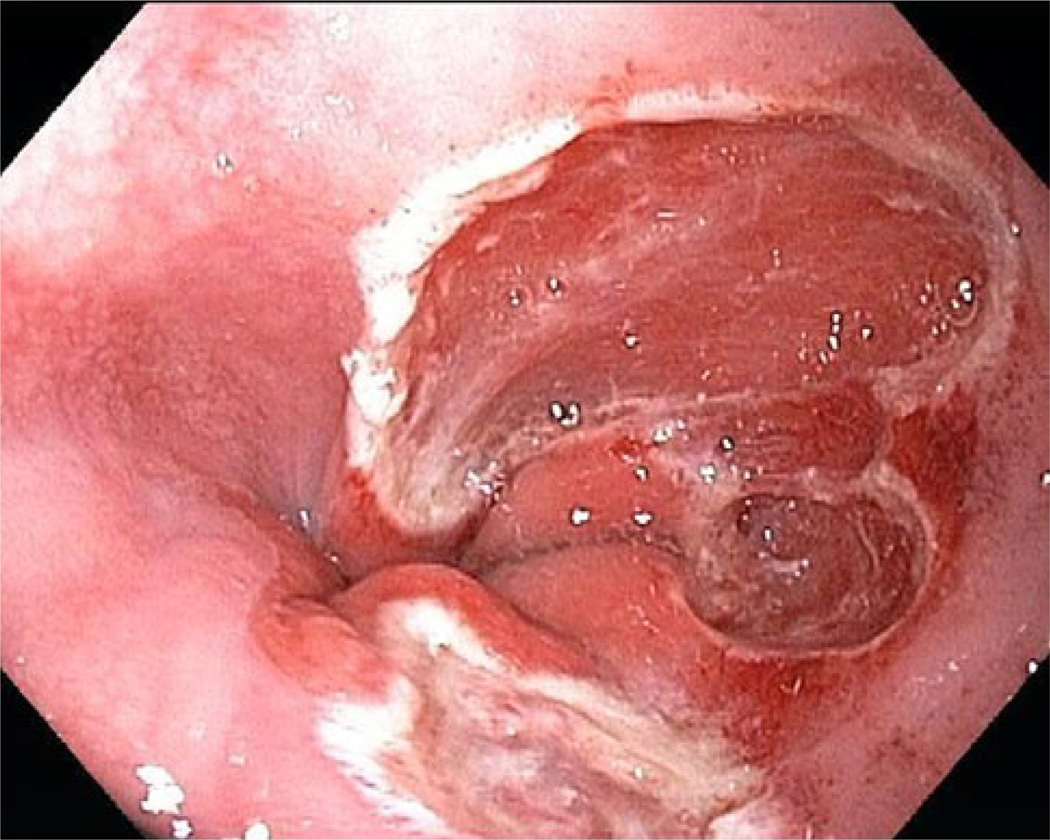
Fig. 4.
Endoscopic mucosal resection of flat dysplasia
Histologic Evaluation of Biopsy and EMR Specimens
Pre-EMR biopsy and EMR specimens were histopathologically assessed for the presence of intestinal metaplasia, and the presence and degree of dysplasia were graded in accordance with the Vienna classification into nondysplastic BE, LGD, HGD, or EAC [20, 21]. Pre-EMR diagnostic biopsy specimens and EMR specimens were compared by use of expert histopathology assessments in all cases. The standardized criteria [3] for each category were:
Non-dysplastic BE: metaplastic columnar epithelium containing goblet cells with uniform glandular architecture, basally located nuclei with smooth membranes, minimal anisonucleosis and preserved polarity, normal nuclear/cytoplasmic (N/C) ratio; greater nuclear alterations and partial mucin depletion were acceptable when associated with evidence of inflammation, erosion, or ulceration.
Low-grade dysplasia (LGD): glandular proliferation and crowding without complex branching, cribriform, or villous architecture, hyperchromatic, enlarged nuclei with mild irregularity of nuclear membrane, and nuclear stratification extending to the surface epithelium.
HGD: complex cribriform or villous architecture, marked nuclear pleomorphism and irregularity of contour, increased N/C ratio, large and irregular nucleoli, full-thickness nuclear stratification, loss of polarity, and prominent mucin depletion.
Adenocarcinoma (EAC): malignant cells, singly or in groups, infiltrating beyond the basement membrane, with or without associated stromal desmoplasia. As previously reported, tumors invading the original muscularis mucosae and the newly formed muscularis mucosae were regarded as intramucosal carcinoma [22, 23].
The worst histologic grade identified was taken as the overall histologic grade for that endoscopy. These were reported by local experienced gastroenterology pathologists at each site. Review of dysplasia and EAC slides by a second local experienced pathologist was performed as part of routine clinical practice at each site.
Outcome Variables
The outcome variables were: frequency of overall change of histopathological diagnosis based on diagnostic and/or staging EMR, frequency of change of diagnosis based on pre-EMR biopsy diagnosis (HGD and cancer), and frequency of change of diagnosis based on the presence of visible lesions during standard white light endoscopy.
Data Management and Statistical Analysis
The study coordinators at each center collected patient information, and data entry was performed for all the above stated variables. A database of all BE patients undergoing endoscopic eradication therapy was created at each center and each patient was provided with a unique identification number. All patient identifiers were deleted in compliance with Health Information Portability and Accountability Act regulations. Data sets from each center were then merged into the main study database by use of Microsoft Access for Windows 2007 (Microsoft, Redmond, WA, USA). All collected and merged data were compared and reconciled for accuracy. Categorical covariates were summarized as counts and percentages whereas continuous covariates were summarized as means and standard deviations. Fishers’ exact test was used to compare the frequency of change of diagnosis for patients with and without visible lesions. All analysis was performed by use of Stata/IC 10.1 (StataCorp: College Station, TX, USA), and p values <0.05 were considered statistically significant.
Results
Of the 281 patients with BE undergoing endoscopic eradication therapy, 138 patients met the inclusion criteria. The reasons for exclusion were:
-
–
100 patients did not undergo diagnostic EMR;
-
–
11 patients had undergone previous endoscopic eradication therapy;
-
–
31 patients did not have pre-EMR biopsies and diagnostic EMRs performed in the 6-month interval; and
-
–
for one patient the pre-EMR biopsy result was not available.
The mean age of the patients in this cohort was 67.1 years (standard deviation, SD, 10.2) and the vast majority were males (92.8 %) and Caucasians (97.8 %). The mean BE length was 4.8 cm (SD 3.7). Hiatal hernia was present in 83.3 % of the BE patients; the mean size was 3.1 cm (SD 1.5). Visible lesions were described for 114 of 138 (82.6 %) BE patients.
Overall Change of Histopathologic Diagnosis
Baseline histology based on pre-EMR biopsies for this cohort was: LGD 15 (10.9 %), HGD 87 (63 %), and EAC 36 (26.1 %). Post diagnostic EMR diagnosis was: no intestinal metaplasia four (2.9 %), intestinal metaplasia eight (5.8 %), LGD 16 (11.6 %), HGD 74 (53.6 %), and EAC 36 (26.1 %). Overall, EMR resulted in a change of histopathologic diagnosis for 43 (31.1 %) patients, either an upgrade in diagnosis for 14 (10.1 %) or a downgrade for 29 (21 %). For patients with visible lesions, EMR resulted in a change for 35 (30.7 %) patients, either an upgrade (n = 12, 10.5 %) or a downgrade (n = 23, 20.2 %) in diagnosis. Similarly, for patients with no visible lesions, EMR resulted in a change for eight (33.3 %) patients, either an upgrade (8.3 %) or a downgrade (25 %) in diagnosis.
Change of Diagnosis on the Basis of Baseline Histology
For patients diagnosed with HGD on biopsies (n = 87), EMR resulted in downstaging of dysplasia grade for 17 (19.5 %) cases (low grade dysplasia (LGD) seven (8 %), non-dysplastic BE (NDBE) seven (8 %), no BE three (3.4 %)) and upstaging to EAC for nine (10.3 %) cases. Overall, there was a change of diagnosis for 26 (29.9 %) HGD patients. Visible lesions were noted for 72 of 87 (82.7 %) patients with HGD on pre-EMR biopsies. The frequency of change of diagnosis and that based on the presence or absence of endoscopically visible lesions are listed in Table 1. There was no significant difference between frequency of change of diagnosis on the basis of the presence or absence of visible lesions (29.2 vs. 33.3 %, p = 0.76).
Table 1.
Frequency of change of diagnosis by EMR for patients with HGD on biopsy
| HGD (n = 87) |
Change of diagnosis |
Visible lesions present (n = 72) |
Visible lesions absent (n = 15) |
|---|---|---|---|
| Downstaging (n, %) | 17 (19.5) | 12 (16.7) | 5 (33.3) |
| Upstaging (n, %) | 9 (10.3) | 9 (12.5) | 0 (0) |
| No change (n, %) | 61 (70.1) | 51 (70.8) | 10 (66.7) |
For patients diagnosed with EAC on biopsies (n = 36), EMR resulted in downstaging to dysplasia grade for 10 (27.8 %) cases (HGD nine (25 %), and LGD one (2.8 %)). Visible lesions were noted for 34 of 36 (94.4 %) patients with EAC on pre-EMR biopsies. The frequency of change of diagnosis and that based on the presence or absence of endoscopically visible lesions are listed in Table 2. There was no significant difference between frequency of change of diagnosis on the basis of the presence or absence of visible lesions (26.5 vs. 50 %, p = 0.48). Using an endpoint of HGD/EAC (n = 123), EMR resulted in a change of diagnosis for 36 of 123 (29.2 %) patients. There was no difference between frequency of change of diagnosis on the basis of the presence or absence of visible lesions (28.3 vs. 35.2 %, p = NS).
Table 2.
Frequency of change of diagnosis by EMR for patients with EAC on biopsy
| EAC (n = 36) |
Change of diagnosis |
Visible lesions present (n = 34) |
Visible lesions absent (n = 2) |
|---|---|---|---|
| Downstaging (n, %) | 10 (27.8) | 9 (26.5) | 1 (50) |
| No change (n, %) | 26 (72.2) | 25 (73.5) | 1 (50) |
For patients diagnosed with LGD on biopsies (n = 15), EMR resulted in downstaging for two (13.3 %) cases and upstaging for five (33.3 %) cases. Visible lesions were noted for eight (53.3 %) of cases. There was no significant difference between frequency of change of diagnosis on the basis of the presence or absence of visible lesions (p = 0.26).
Discussion
There is a paucity of literature guiding selection of the most effective therapy for dysplastic BE. However, EMR is now a part of the armamentarium for Barrett’s EET and has evolved into an important diagnostic, staging, and therapeutic tool for management of these patients. According to the literature, numbers of changes in diagnosis after use of diagnostic EMR have varied [17, 24–27]. Results for this multicenter cohort of BE patients undergoing EET reveal that EMR resulted in a change of histopathologic diagnosis in 30 % of cases. Similarly, for patients with HGD or EAC on biopsy (excluding patients with LGD), EMR resulted in a change for approximately 30 % of cases. It should be noted that this cohort study only included patients with Barrett’s-related neoplasia undergoing EET and hence reported estimates of changes in diagnosis may not be accurate, because patients with T1b or higher stage cancer were excluded from the analysis.
Consistent with results of this study, Moss et al. [26] reported the effect of EMR on histologic grade and/or T stage for 75 patients with biopsy-proved HGD or EAC. EMR resulted in a change of diagnosis for 48 % of patients (downstaging for 28 % (23 % with either no dysplasia or low-grade dysplasia) and upstaging for 20 %). For a series of 40 patients undergoing EMR (25 HGD, 15 EAC), Larghi et al. [17] reported that six of 25 (24 %) patients diagnosed initially with HGD were upgraded to mucosal EAC and six of 15 (40 %) patients with mucosal EAC were upgraded to invasive EAC. In a study from the Amsterdam group that compared pre-EMR biopsy diagnosis with EMR diagnosis for 150 EMR resected specimens, there was disagreement between the two diagnoses of 73 (49 %) cases. This led to a relevant change in treatment policy for 45 (30 %) patients. In a single-center US study that included 49 patients, EMR resulted in a change of diagnosis for 22 of 49 (44.8 %) patients (upstaging for 14 % and downstaging for 31 %) compared with pre-EMR biopsy results [27]. In a systematic review it was shown that EMR was superior to mucosal biopsies as a diagnostic tool, resulting in a change of diagnosis for approximately 25 % of patients with HGD or early cancer (either upstaging or downstaging of the lesions) [25].
If EET is to be used, it is recommended that any visible abnormalities should be removed by EMR [5]. Literature reports of the utility of diagnostic EMR for patients with flat dysplasia are limited. This study demonstrates that diagnostic EMR resulted in a change of histopathologic diagnosis for 35 % of cases with flat HGD or EAC (33.3 % HGD, 50 % EAC) on the basis of biopsy results. These results could have significant implications for patients undergoing EET on the basis of biopsy diagnosis alone.
The value of EMR as a diagnostic and/or staging tool depends on use of large, thick tissue specimens, with limited distortion compared with biopsy specimens, enabling accurate assessment of the depth of neoplastic involvement and the adequacy of resection. It has also been demonstrated that there is more interobserver agreement among pathologists in the analysis of EMR samples than for biopsy specimens for diagnosis of dysplasia, because of easier evaluation of dysplastic glands, mucosal features, and improved orientation of EMR specimens compared with superficial biopsy specimens [3, 13]. A recent international study analyzed the histopathologic characteristics of more than 500 EMR and biopsy specimens from BE patients. Submucosa could be examined for most of the EMR specimens (EMR 88 % vs. biopsy 1.5 %, p < 0.0001), a critical aspect of accurate staging of BE-related neoplasia. Finally, interobserver agreement in the diagnosis of dysplasia was significantly greater for EMR specimens than for biopsy specimens [3].
EUS has frequently been used as a staging technique for patients with Barrett’s-related neoplasia to evaluate infiltration depth and the presence of suspicious lymph nodes. However, recent studies have demonstrated that EUS has only modest accuracy in delineating the T-stage of esophageal cancer [11, 12, 28, 29]. Despite better delineation of esophageal wall layers, use of high-frequency ultrasound probes was not associated with improved accuracy when differentiating between mucosal and submucosal lesions, with accuracy ranging from 73 to 80 % [28, 30]. In a recent retrospective study that included 131 patients with early esophageal neoplasia, Pouw et al. [11] reported that EUS had no clinical effect on the work up and management of early esophageal neoplasia. Consistent with these results, Young et al. [12] reported T-stage concordance of 65 % in a systematic review, using EMR or surgical pathology as the reference method. Limited accuracy has been attributed to wall thickening because of peritumoral inflammation, heterogeneous tissue architecture, doubled muscularis mucosae, and anatomical impediments in the distal esophagus and gastric cardia [11, 18, 30]. These results call into question the use of EUS for evaluation of BE patients with early neoplasia, and its overall effect on patient management should be assessed in future prospective trials.
Several limitations of this database study merit consideration. All participating centers were tertiary care referral centers with a special interest in BE, which may limit the generalizability of these results. This database does not include patients who were not candidates for endoscopic eradication therapy on the basis of EMR results (patients diagnosed with T1b or higher-stage cancer) and potentially referred for surgery. Hence, this study is unable to evaluate the frequency with which diagnostic and/or staging EMR resulted in a true change in management plans. Future large multicenter studies should evaluate the true effect of EMR and provide accurate estimates of change of histologic grade of dysplasia. The possibility of selection bias cannot be excluded, because all EMRs evaluated in this study were performed for patients believed to be candidates for EET. Use of advanced imaging techniques was not standardized and its effect on lesion detection could not be evaluated. In addition, a standardized classification system (Paris classification) to describe visible lesions, including size of the lesion, was lacking in this study. A central pathology reading (especially for all cases of dysplasia) was not routinely performed and is a major limitation of this study. It would have been ideal to have all cases reviewed by an expert central gastrointestinal pathologist. It is possible, for some patients, that central expert review of slides may have changed the diagnosis in a manner congruent with that after EMR. However, the participating sites were all experienced centers in the field of BE with experienced pathologists; in this setting interobserver variability, even among expert pathologists, is well documented. The small sample size precluded comparison of cap-assisted and multiband ligator EMR. However, a recent randomized controlled trial comparing the two techniques revealed no differences between the maximum thickness of specimens and resected submucosa [31]. The use of EMR as reference method can be questioned, because an ideal reference method would have been the surgical specimen, which is obviously not possible in these cases. This study was also limited by the small sample size of patients with BE-related neoplasia with no visible lesions. The lack of a difference between upstaging and downstaging in EAC with regard to whether visible lesions were present or absent may also be related to the small sample size and, hence, the possibility of a Type II error cannot be excluded.
This study is an attempt to build a clinical consortium for endoscopic research in the field of endoscopic eradication therapy for patients with BE. The largest number of patients with BE undergoing endoscopic eradication therapy in the US is the major strength of this endeavor. Future objectives of this consortium include comprehensive collection of data on the effectiveness of endoscopic eradication therapy and long-term durability of mucosal changes post-eradication.
EET is a viable treatment option for BE patients with HGD and early mucosal EAC. As a result, the endoscopist is now of major importance in the management of BE-related neoplasia. This study demonstrates that EMR results in a change of diagnosis for a significant proportion of patients with Barrett’s-related neoplasia (with visible and flat neoplasia) which could result in a change in disease management, and recognition of the value of EMR as a diagnostic and/or staging tool. EMR should be considered as an important initial step in the diagnostic work up of patients with Barrett’s-related neoplasia before embarking on endoscopic eradication therapy.
Footnotes
Results of this study were presented in part at Digestive Disease Week 2011, Chicago.
Conflict of interest None.
Contributor Information
Sachin Wani, Email: sachinwani10@yahoo.com, Division of Gastroenterology and Hepatology, University of Colorado and Veterans Affairs Medical Center, Denver, CO, USA.
Julian Abrams, Division of Digestive and Liver Diseases, Columbia University College of Physicians and Surgeons, New York, NY, USA.
Steven A. Edmundowicz, Division of Gastroenterology and Hepatology, Washington University School of Medicine, St. Louis, MO, USA
Srinivas Gaddam, Division of Gastroenterology and Hepatology, Washington University School of Medicine, St. Louis, MO, USA.
Christine E. Hovis, Division of Gastroenterology and Hepatology, Washington University School of Medicine, St. Louis, MO, USA
Daniel Green, Division of Digestive and Liver Diseases, Columbia University College of Physicians and Surgeons, New York, NY, USA.
Neil Gupta, Division of Gastroenterology and Hepatology, Department of Veterans Affairs Medical Center and University of Kansas School of Medicine, 4801 E. Linwood Blvd, Kansas City, MO 64128-2295, USA.
April Higbee, Division of Gastroenterology and Hepatology, Department of Veterans Affairs Medical Center and University of Kansas School of Medicine, 4801 E. Linwood Blvd, Kansas City, MO 64128-2295, USA.
Ajay Bansal, Division of Gastroenterology and Hepatology, Department of Veterans Affairs Medical Center and University of Kansas School of Medicine, 4801 E. Linwood Blvd, Kansas City, MO 64128-2295, USA.
Amit Rastogi, Division of Gastroenterology and Hepatology, Department of Veterans Affairs Medical Center and University of Kansas School of Medicine, 4801 E. Linwood Blvd, Kansas City, MO 64128-2295, USA.
Dayna Early, Division of Gastroenterology and Hepatology, Washington University School of Medicine, St. Louis, MO, USA.
Charles J. Lightdale, Division of Digestive and Liver Diseases, Columbia University College of Physicians and Surgeons, New York, NY, USA
Prateek Sharma, Email: psharma@kumc.edu, Division of Gastroenterology and Hepatology, Department of Veterans Affairs Medical Center and University of Kansas School of Medicine, 4801 E. Linwood Blvd, Kansas City, MO 64128-2295, USA.
References
- 1.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 2.Wani S, Sayana H, Sharma P. Endoscopic eradication of Barrett’s esophagus. Gastrointest Endosc. 2010;71:147–166. doi: 10.1016/j.gie.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 3.Wani S, Mathur SC, Curvers WL, et al. Greater interobserver agreement by endoscopic mucosal resection than biopsy samples in Barrett’s dysplasia. Clin Gastroenterol Hepatol. 2010;8:783–788. doi: 10.1016/j.cgh.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 5.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140:e18–e52. doi: 10.1053/j.gastro.2011.01.031. quiz e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566–573. doi: 10.1097/01.sla.0000184211.75970.85. discussion 573–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice TW, Zuccaro G, Jr, Adelstein DJ, et al. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg. 1998;65:787–792. doi: 10.1016/s0003-4975(97)01387-8. [DOI] [PubMed] [Google Scholar]
- 8.Badreddine RJ, Prasad GA, Lewis JT, et al. Depth of submucosal invasion does not predict lymph node metastasis and survival of patients with esophageal carcinoma. Clin Gastroenterol Hepatol. 2010;8:248–253. doi: 10.1016/j.cgh.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downs-Kelly E, Mendelin JE, Bennett AE, et al. Poor interobserver agreement in the distinction of high-grade dysplasia and adenocarcinoma in pretreatment Barrett’s esophagus biopsies. Am J Gastroenterol. 2008;103:2333–2340. doi: 10.1111/j.1572-0241.2008.02020.x. quiz 2341. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–378. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 11.Pouw RE, Heldoorn N, Herrero LA, et al. Do we still need EUS in the workup of patients with early esophageal neoplasia? A retrospective analysis of 131 cases. Gastrointest Endosc. 2011;73:662–668. doi: 10.1016/j.gie.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Young PE, Gentry AB, Acosta RD, et al. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol. 2010;8:1037–1041. doi: 10.1016/j.cgh.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Mino-Kenudson M, Hull MJ, Brown I, et al. EMR for Barrett’s esophagus-related superficial neoplasms offers better diagnostic reproducibility than mucosal biopsy. Gastrointest Endosc. 2007;66:660–666. doi: 10.1016/j.gie.2007.02.063. quiz 767–769. [DOI] [PubMed] [Google Scholar]
- 14.Sharma P, Bansal A, Mathur S, et al. The utility of a novel narrow band imaging endoscopy system in patients with Barrett’s esophagus. Gastrointest Endosc. 2006;64:167–175. doi: 10.1016/j.gie.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 15.Inoue H, Takeshita K, Hori H, et al. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58–62. doi: 10.1016/s0016-5107(93)70012-7. [DOI] [PubMed] [Google Scholar]
- 16.Chandrasekhara V, Ginsberg GG. Endoscopic mucosal resection: not your father’s polypectomy anymore. Gastroenterology. 2011;141:42–49. doi: 10.1053/j.gastro.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Larghi A, Lightdale CJ, Memeo L, et al. EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett’s esophagus. Gastrointest Endosc. 2005;62:16–23. doi: 10.1016/s0016-5107(05)00319-6. [DOI] [PubMed] [Google Scholar]
- 18.Namasivayam V, Wang KK, Prasad GA. Endoscopic mucosal resection in the management of esophageal neoplasia: current status and future directions. Clin Gastroenterol Hepatol. 2010;8:743–754. doi: 10.1016/j.cgh.2010.05.030. quiz e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleischer DE, Wang GQ, Dawsey S, et al. Tissue band ligation followed by snare resection (band and snare): a new technique for tissue acquisition in the esophagus. Gastrointest Endosc. 1996;44:68–72. doi: 10.1016/s0016-5107(96)70233-x. [DOI] [PubMed] [Google Scholar]
- 20.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riddell RH, Goldman H, Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 22.Abraham SC, Krasinskas AM, Correa AM, et al. Duplication of the muscularis mucosae in Barrett esophagus: an underrecognized feature and its implication for staging of adenocarcinoma. Am J Surg Pathol. 2007;31:1719–1725. doi: 10.1097/PAS.0b013e318093e3bf. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JT, Wang KK, Abraham SC. Muscularis mucosae duplication and the musculo-fibrous anomaly in endoscopic mucosal resections for Barrett esophagus: implications for staging of adenocarcinoma. Am J Surg Pathol. 2008;32:566–571. doi: 10.1097/PAS.0b013e31815bf8c7. [DOI] [PubMed] [Google Scholar]
- 24.Peters FP, Brakenhoff KP, Curvers WL, et al. Histologic evaluation of resection specimens obtained at 293 endoscopic resections in Barrett’s esophagus. Gastrointest Endosc. 2008;67:604–609. doi: 10.1016/j.gie.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 25.Sayana H, Wani S, Keighley J, et al. Endoscopic mucosal resection (EMR) as a diagnostic tool in Barrett’s esophagus (BE) patients with high-grade dysplasia (HGD) and early esophageal adenocarcinoma (EAC): a systematic review. Gastroenterology. 2008;132:W1878. [Google Scholar]
- 26.Moss A, Bourke MJ, Hourigan LF, et al. Endoscopic resection for Barrett’s high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010;105:1276–1283. doi: 10.1038/ajg.2010.1. [DOI] [PubMed] [Google Scholar]
- 27.Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma—an American single-center experience. Am J Gastroenterol. 2009;104:2684–2692. doi: 10.1038/ajg.2009.465. [DOI] [PubMed] [Google Scholar]
- 28.May A, Gunter E, Roth F, et al. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut. 2004;53:634–640. doi: 10.1136/gut.2003.029421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pech O, Gunter E, Dusemund F, et al. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: results from a referral center for early esophageal cancer. Endoscopy. 2010;42:456–461. doi: 10.1055/s-0029-1244022. [DOI] [PubMed] [Google Scholar]
- 30.Chemaly M, Scalone O, Durivage G, et al. Miniprobe EUS in the pretherapeutic assessment of early esophageal neoplasia. Endoscopy. 2008;40:2–6. doi: 10.1055/s-2007-966958. [DOI] [PubMed] [Google Scholar]
- 31.Pouw RE, van Vilsteren FG, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett’s neoplasia. Gastrointest Endosc. 2011;74:35–43. doi: 10.1016/j.gie.2011.03.1243. [DOI] [PubMed] [Google Scholar]