Abstract
Therapeutic agents that improve the memory loss of Alzheimer’s disease (AD) may eventually be developed if drug targets are identified that improve memory deficits in appropriate AD animal models. One such target is β-secretase which, in most AD patients, cleaves the wild-type (WT) β-secretase site sequence of the amyloid-β protein precursor (AβPP) to produce neurotoxic amyloid-β (Aβ). Thus, an animal model representing most AD patients for evaluating β-secretase effects on memory deficits is one that expresses human AβPP containing the WT β-secretase site sequence. BACE1 and cathepsin B (CatB) proteases have β-secretase activity, but gene knockout studies have not yet validated that the absence of these proteases improves memory deficits in such an animal model. This study assessed the effects of deleting these protease genes on memory deficits in the AD mouse model expressing human AβPP containing the WT β-secretase site sequence and the London γ-secretase site (AβPPWT/Lon mice). Knockout of the CatB gene in the AβPPWT/Lon mice improved memory deficits and altered the pattern of Aβ-related biomarkers in a manner consistent with CatB having WT β-secretase activity. But deletion of the BACE1 gene had no effect on these parameters in the AβPPWT/Lon mice. These data are the first to show that knockout of a putative β-secretase gene results in improved memory in an AD animal model expressing the WT β-secretase site sequence of AβPP, present in the majority of AD patients. CatB may be an effective drug target for improving memory deficits in most AD patients.
Keywords: Amyoid-β, amyoid-β protein precursor, cathepsin B, gene knockout, protease
INTRODUCTION
The primary deficit in Alzheimer’s disease (AD) is the severe loss of memory. There currently are no therapeutic agents that reverse or significantly retard the memory loss in AD. However, it may be possible to develop such therapeutics if new drug targets can be validated that improve memory deficits in appropriate AD animal models of the human disease.
The majority of AD patients (>90%) have sporadic AD and express normal, wild-type (WT) amyloid-β protein precursor (AβPP) [1–3]. Only a small percentage of patients develop familial AD involving inherited gene mutations including several mutant AβPP forms [1–3]. Expression of mutant human AβPP forms in transgenic mice results in development of memory deficits and brain neuropathology that resemble AD [4–8]. Such AD animal models may be considered for their representation of familial or sporadic forms of AD.
Proteases with β-secretase activity are viewed as potential targets for improving memory deficits in AD patients. The β-secretase in sporadic AD patients cleaves the WT β-secretase site sequence in AβPP, and, therefore, an appropriate animal model to evaluate potential β-secretase targets for improving memory deficits in most AD patients is one which expresses AβPP containing the WT β-secretase site sequence. Such a model is represented by transgenic mice expressing human AβPP containing the WT β-secretase site sequence combined with the London mutant (Lon) γ-secretase site sequence (AβPPWT/Lon mice), which results in memory deficits and neuropathology resembling AD [9]. Because the AβPPWT/Lon mouse model expresses the WT β-secretase site of AβPP expressed in most AD patients, it is an appropriate model for assessing candidate β-secretase genes as possible AD drug targets for improving memory loss.
Our recent studies show that treatment of AβPPWT/Lon mice with compounds that inhibit cathepsin B (CatB) results in improved memory deficits [9, 10]. These compounds reduce the biomarkers of brain amyloid-β peptides (Aβ) and C-terminal β-fragment (CTFβ) derived from AβPP by β-secretase, suggesting that CatB may function as a WT β-secretase [9, 10]. But these data do not prove that CatB is a target for improving memory deficits because the compounds could have off-target effects. Therefore, to directly address the hypothesis that CatB participates in the memory deficits in an AD mouse model, this study investigated the effects of deleting the CatB gene on memory deficits and biomarkers in the AβPPWT/Lon mice.
The suggestion that CatB may have WT β-secretase activity is controversial because the aspartyl protease BACE1 has been thought to be the primary β-secretase [11, 12]. But deletion of the BACE1 gene in mice expressing AβPP containing the WT β-secretase site sequence results in worse memory [13], even though brain Aβ and amyloid plaque are reduced [14]. Therefore, further studies of BACE1 gene knockout effects on memory deficits in transgenic mice expressing AβPP containing the WT β-secretase site sequence are needed to validate a β-secretase target suitable for improving memory deficits in most AD patients.
This study evaluated memory deficits in AβPPWT/Lon mice after deletion of the CatB or BACE1 genes. Knockout of the CatB gene resulted in substantial improvement in memory deficits, and reduced brain amyloid plaque, Aβ, and CTFβ. However, knockout of the BACE1 gene in AβPPWT/Lon mice had no effect on memory deficits or the Aβ-related biomarkers. Since elimination of CatB reduced brain CTFβ and Aβ, CatB may have WT β-secretase activity in AβPPWT/Lon mice. Significantly, these are the first results to show improved memory deficits after knockout of a protease gene, CatB, in an AD mouse model expressing the ‘WT β-secretase site’ of AβPP that is present in most AD patients. These findings validate CatB as a drug target to improve memory deficits in the majority of AD patients.
METHODS
Transgenic AD mice
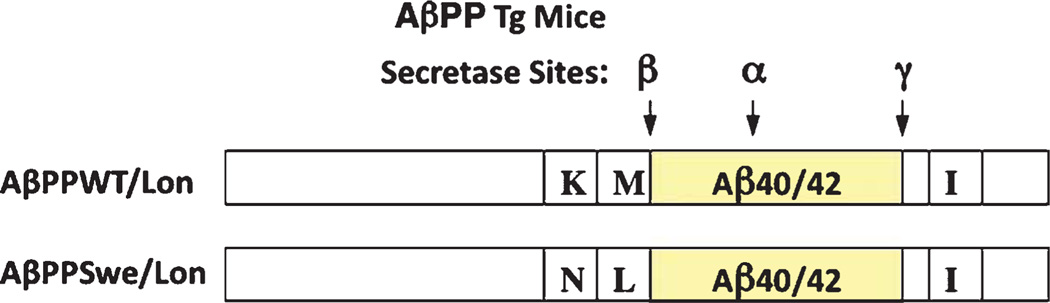
Applied Neurotechnology, Inc. (Charleston, SC) generated the transgenic mice by methods previously described [9, 10, 15–17]. AβPPWT/Lon mice were generated by site-directed mutagenesis to insert the V717I London mutation [18] into human AβPP cDNA (AβPPWT/Lon mice, illustrated in Fig. 1), and the transgenic mouse was created in the C57BL/6 background using the platelet-derived growth factor beta (PDGFβ) promoter containing an SV40 polyadenylation site and expressed the AβPP-695 gene. CatB deficient (CatB KO) mice were obtained from Christoph Peters (Albert Ludwig University, Freiburg, Germany), and BACE1 deficient (BACE1 KO) mice were obtained from Jackson Laboratory (Bar Harbor, ME). All mice were maintained on a C57BL/6 background. The transgenic AβPPWT/Lon mice were crossed with either the CatB KO mice or the BACE1 KO mice and the following transgenic mice were generated: AβPPWT/Lon, AβPPWT/Lon × CatB KO, and AβPPWT/Lon × BACE1 KO.
Fig. 1.
AβPPWT/Lon and AβPPSwe/Lon transgenic (Tg) mice. This figure illustrates the human AβPP forms expressed in transgenic AD mouse models utilized in this study. The AβPPWT/Lon mice express human AβPP containing the WT β-secretase site (normal K–M residues at the N-terminal side of the β-cleavage site) and the London (Lon) mutation near the γ-secretase site sequence with isoleucine (I) substituting for a valine (V) at position 717. Because the AβPPWT/Lon mice express the WT β-secretase site of AβPP, it is a transgenic animal that has WT β-secretase activity. AβPPSwe/Lon mice express human AβPP containing the rare Swe mutation at the β-secretase site sequence consisting of asparagine (N) substituting for lysine (K) at position 670 and a leucine (L) for a methionine (M) at position 671. Cleavage of AβPP by β-secretase generates CTFβ, which in turn is cleaved by γ-secretase to produce Aβ peptides. AβPP is also cleaved by α-secretase, which produces sAβPPα and precludes the production of Aβ.
Transgenic mice expressing the rare Swedish mutation (Swe) at the β-secretase cleavage site (AβPPSwe) and the Lon mutation at the γ-secretase site were also generated (AβPPSwe/Lon mice, illustrated in Fig. 1). The AβPPSwe/Lon mice were a positive control because deletion of the BACE1 gene in transgenic AβPPSwe mice is known to improve memory deficits [19, 20]. The AβPPSwe/Lon mice were generated by site-directed mutagenesis to insert the K670 N/M671 L Swedish mutation into the human AβPP-695 cDNA containing the Lon mutation. Transgenic mice in the C57BL/6 background were crossed with the CatB and BACE1 gene knockout animals as described above, and the following transgenic mice were generated: AβPPSwe/Lon, AβPPSwe/Lon × CatB KO, and AβPPSwe/Lon × BACE1 KO.
PCR analysis was utilized to determine the genotype of the animals, as previously described [17]. All experimental mice were male. Mice were given free access to food and water before and during the experiment.
CatB and BACE1 protease activities in transgenic mice
CatB and BACE1 activities were determined in AβPPWT/Lon and AβPPSwe/Lon mice (at 3 months of age) with knockout of either the CatB or BACE1 genes, to confirm that the protease gene knockouts resulted in the absence of the respective protease activities.
CatB activity in brain was determined using a fluorometric assay kit from Abcam (ab65300) as described by the manufacturer. Briefly, tissues were washed twice in ice-cold PBS and then homogenized in extraction buffer. After 10 min incubation on ice, the extract was centrifuged at 10,0009 g for 5 min and 50 µl of supernatant was mixed with an equal volume of 2× reaction buffer and 2 µl substrate in a 96-well microplate. The plates were kept in the dark at 37°C for 1 h, and the fluorescence was recorded using FLUOstar Optima plate reader (BMG). Protein concentration was determined by the BCA method (Bio-Rad Laboratories). The CatB activity was expressed as fluorescent units/mg protein.
BACE1 activity in brain was determined using a fluorometric assay kit from Abcam (ab65357) as described by the manufacturer. Brain samples were prepared as described for CatB activity analysis.
Age of mice for analysis of memory deficits and biomarkers
Memory function, amyloid plaque and brain biomarkers were evaluated after significant memory deficits developed in the AβPPWT/Lon mice and the AβPPSwe/Lon mice. Because the purpose of this study was to compare the effects of deleting the CatB or BACE1 gene on memory deficits in the two different AD mouse models, it was, therefore, important to evaluate each of these mouse models at ages when they each show similar levels of memory deficits. Similar memory deficits for the AβPPWT/Lon and AβPPSwe/Lon mice occur at ages of 12 months and 9 months, respectively; the AβPPWT/Lon mouse acquires memory deficits at a later age than the AβPPSwe/Lon mouse. Thus, AβPPWT/Lon mice of 12 months and AβPPSwe/Lon mice of 9 months, with equivalent levels of memory deficits, were utilized to assess the effects of deleting either the CatB or BACE1 genes on memory deficits.
Spatial memory deficit
The memory deficit in the animals was measured by the Morris water maze test, as we have described previously [9, 10]. Briefly, the spatial memory capability of each animal was assessed with the Morris water maze test (700-0718-4W SD Instruments) which evaluates memory in a swimming test. Mice were individually trained in a 1.2 m open field water maze in a pool filled with water to a depth of 30 cm and maintained at 25°C. An escape platform (10 cm square) was placed 1 cm below the surface of the water. All animals underwent nonspatial pretraining for 4 consecutive days, which prepared the animals for the final behavioral test to determine the retention of memory to find the platform. Two days following the nonspatial pretraining, the hidden platform was placed in the center of one quadrant of the pool, the animal was released facing the pool wall in a random fashion, the time was recorded (latency period), and the distance traveled to reach the platform was measured using video recording (Smart Video Tracking System; SD Instruments).
On the day after the last training session, the platform was removed and a spatial probe test conducted. Each mouse was allowed to search for the platform for 60 seconds (memory retention) and the percent time spent in quadrant where the platform was located (NE quadrant) and in the outer annular area were determined.
Brain amyloid plaque
Amyloid plaque load was assessed in brain sections (10 from each mouse) as described previously [9, 10], achieved by immunhistochemical staining for Aβ (Aβ antibody 10D5, Elan Pharmaceuticals). Brain tissues were fixed in 4% paraformaldehyde and then in 4% parformaldehyde and 30% sucrose for 24 h each at 4°C. Tissues were washed in buffered saline and transferred to an optimum cutting temperature medium. Cryosections were cut and blocked with normal serum, incubated with anti-Aβ and stained with diaminobenzoic acid (Vector ABC Elite kit, Vector Laboratories). Bright field light microscopy imaged brain areas from which stained amyloid areas were quantitated using image analysis (NIH Image software).
Brain Aβ analysis
Brain Aβ analysis was conducted as previously described for transgenic AD mice [9, 10]. Briefly, animals were sacrificed and brain extracts were homogenized (1 : 3 weight/volume of buffer) in buffer of 5 M guanidine HCl in 50 mM Tris–HCl, pH 7.6, 150 mM NaCl, plus protease inhibitors (Sigma). Homogenates were diluted to 0.5 M guanidine and centrifuged (200,000 g for 20 min), and supernatant and pellet fractions were collected. The pellet from the brain extract procedure was sonicated in 6 M guanidine and centrifuged at 200,000 g for 20 min at 4°C, and the supernatant was diluted to 0.5 M guanidine. The two supernatants were combined, and Aβ40 and Aβ42 (Aβ1–40 and Aβ1–42, respectively) were determined using ELISA kits specific for each peptide (IBL, JP27718 and JP27711). Enzyme-linked immunosorbent assays (ELISAs) measured Aβ peptides by methods previously described [9, 10]. Protein content was determined by the Bradford method.
CTFβ and sAβPPα analyses
CTFβ is generated from AβPP by β-secretase in the amyloidogenic pathway, and soluble AβPP (sAβPPα) is generated from AβPP by α-secretase in the non-amyloidogenic pathway. Western blots measured CTFβ and sAβPPα in brains of transgenic mice, using the same amount of protein per gel lane, performed as previously described [9, 10]. CTFβ was determined in the pellet fraction from the brain extract (antibody 8717, Sigma) and sAβPPα was assessed in the supernatant fraction from the brain extract (antibody 6E10, Signet Laboratories). Relative amounts of CTFβ and sAβPPα were measured by densitometry and results were expressed as percentage of the mean levels of CTFβ and sAβPPα of the control groups (without protease gene knockouts). Control β-actin western blots (anti-β-actin from Cell Signaling Technology) was conducted to monitor equal loading of the same amounts of samples (20 µg protein) in each gel lane.
Animal treatment
Animal studies were conducted according to regulations by the National Institutes of Health and as approved by the IACUC at the Medical University of South Carolina and Ralph H. Johnson VA Medical Center.
Statistical evaluation
Experiments consisted of 10 mice in each group. Each biochemical analysis consisted of two or three replicates. Statistical analyses and data display were conducted utilizing computer software designed for scientific data analysis (Prism 4 GraphPad). Quantitative data are displayed as the mean and standard error of the mean (SEM). Differences between groups were determined by ANOVA analysis and Dunnett’s multiple comparison test used to determine differences between transgenic control mice (AβPPWT/Lon or AβPPSwe/Lon) and the protease gene knockout (KO) mice (AβPPWT/Lon × CatB KO and AβPPWT/Lon × BACE1 KO or AβPPSwe/Lon × CatB KO and AβPPSwe/Lon × BACE1 KO, respectively). These statistical analyses also compared normal control WT mice (non-transgenic mice) and protease deficient mice (CatB KO or BACE1 KO).
RESULTS
AβPPWT/Lon AD mice and protease gene knockouts
Deletion of the CatB gene, but not the BACE1 gene, improves memory deficits in transgenic AβPPWT/Lon mice
The AβPPWT/Lon mice express human AβPP with the WT β-secretase site sequence and the London mutation at the γ-secretase site sequence (Fig. 1) and deletion of the CatB or BACE1 genes resulted in elimination of the respective protease activities (supplementary Figure 1; available online: http://www.j-alz.com/issues/29/vol29-4.html#supplementarydata03). The effects of deleting the CatB or BACE1 genes on memory function in the AβPPWT/Lon mice were then assessed by the Morris water maze test.
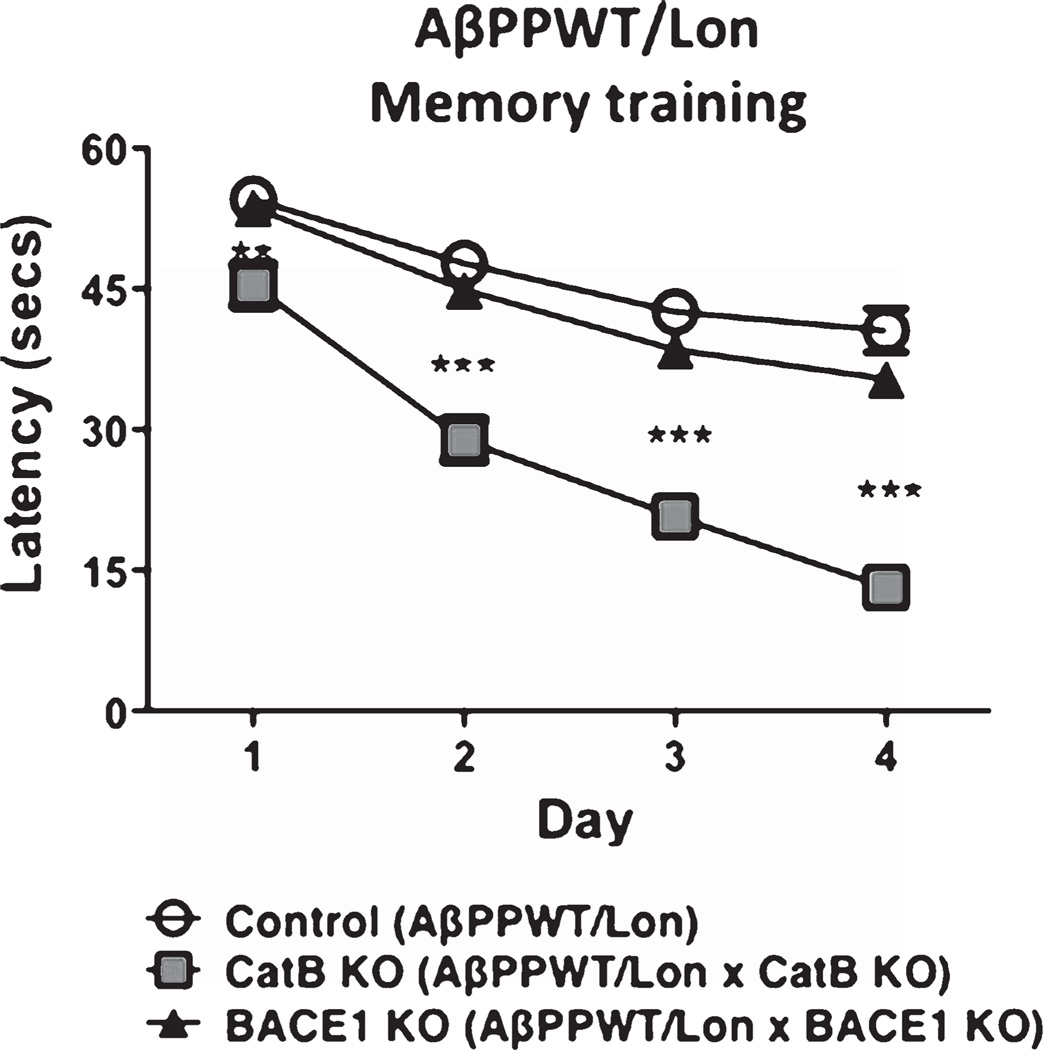
All animals first underwent nonspatial pretraining for four consecutive days to learn the location of the hidden platform. Analyses by the Morris water maze test on each day of the training period (Fig. 2) showed that the mice do learn, indicated by the reduced latency time for the mice to reach the hidden platform during the training period. By the fourth day of training, the AβPPWT/Lon with deletion of CatB showed the shortest latency period, representing enhanced learning, compared to control AβPPWT/Lon mice. Mice with deletion of the BACE1 gene showed similar learning as the control AβPPWT/Lon mice.
Fig. 2.
Knockout of the CatB gene, but not BACE1, in AβPPWT/Lon mice results in improved memory acquisition. AβPPWT/Lon mice (Control), AβPPWT/Lon × CatB KO mice (CatB KO mice), and AβPPWT/Lon × BACE1 KO mice (BACE1 KO mice) were trained in the Morris water maze test on each of 4 consecutive days to learn the location of a submerged, invisible platform in a pool of water. The time that it took the mice to swim to the platform was recorded each day, measured as the latency period (seconds), with shorter latency times indicating better memory acquisition. Latency (secs) is shown as x ± s.e.m. (**, ***Statistically significant, p < 0.05, n = 10 per group).
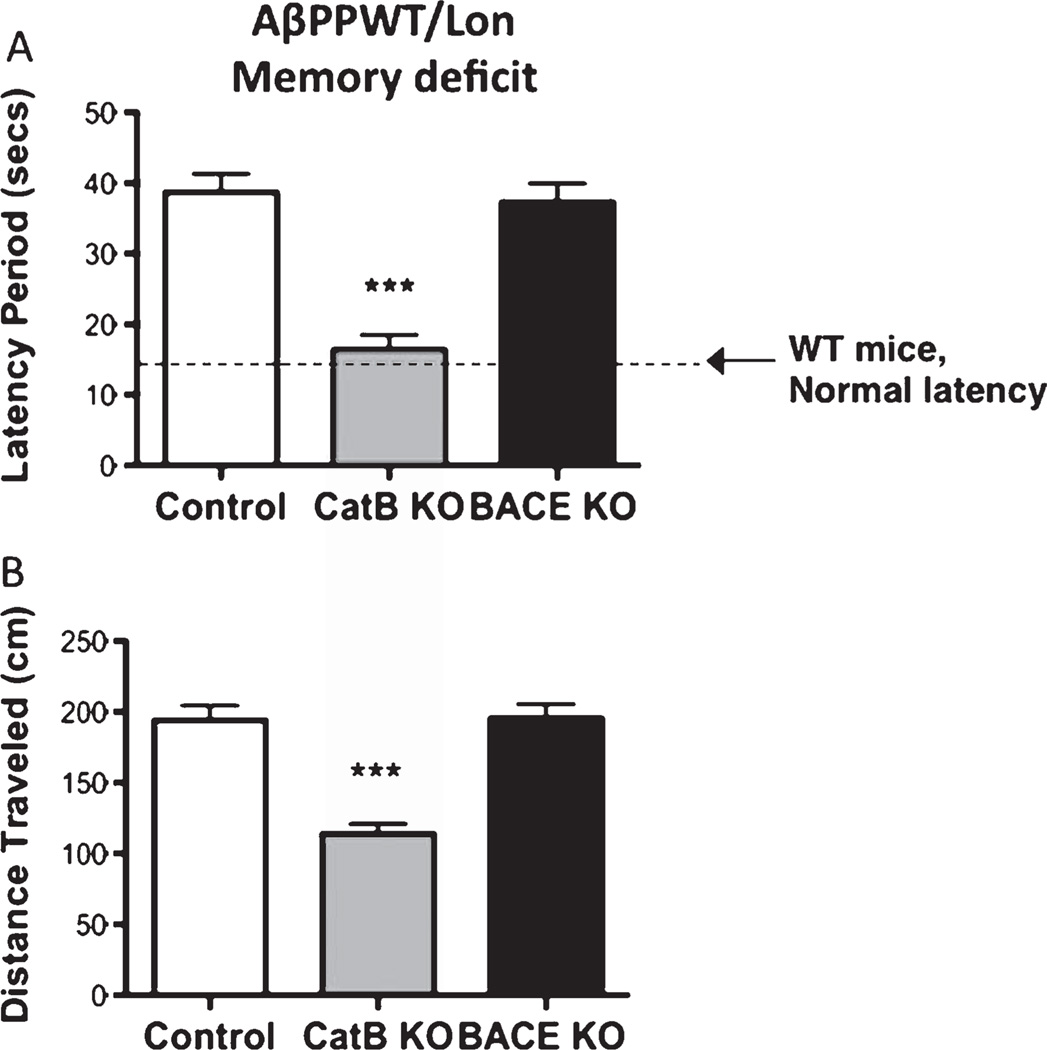
Two days following training, mice were subjected to the final behavioral Morris water maze test to determine the memory deficits. Deletion of the CatB gene in the AβPPWT/Lon mice resulted in substantial improvement in memory deficits, assessed by the latency period and distance traveled, which is the time and distance, respectively, that it took the animal to swim to the submerged platform (Fig. 3). The shorter time and distance traveled indicates better memory. The CatB gene deletion resulted in a 57% and 41% reduction in the latency period and distance traveled, respectively (Fig. 3A, B). The 16 second latency period for the AβPPWT/Lon × CatB KO mice is near that of normal mice (non-transgenic, same strain as AβPPWT/Lon mice) which show a 14 second latency period (Fig. 3A, dotted line).
Fig. 3.
Knockout of the CatB gene, but not BACE1, in AβPPWT/Lon mice improves memory deficits. Memory deficits of AβPPWT/Lon mice were assessed 2 days after completion of the training in the Morris water maze test by measuring the latency period (Panel A) and distance traveled (Panel B) for animals to swim to the submerged, invisible platform. The shorter latency periods and shorter distances traveled indicate improved memory. AβPPWT/Lon (control), AβPPWT/Lon × CatB KO (CatB KO), and AβPPWT/Lon × BACE1 KO (BACE1 KO) mice had mean latency periods of 39, 16, and 37 seconds, respectively (Panel A), and mean distances traveled of 194, 114, and 195 cm, respectively (Panel B). Memory function of normal non-transgenic wild-type mice of the same strain and age is shown by the dotted line, of 14 seconds latency (Panel A) as reported previously [7]. Values are expressed as x ± s.e.m., and n = 10 per group. ***Statistically significant (p < 0.05).
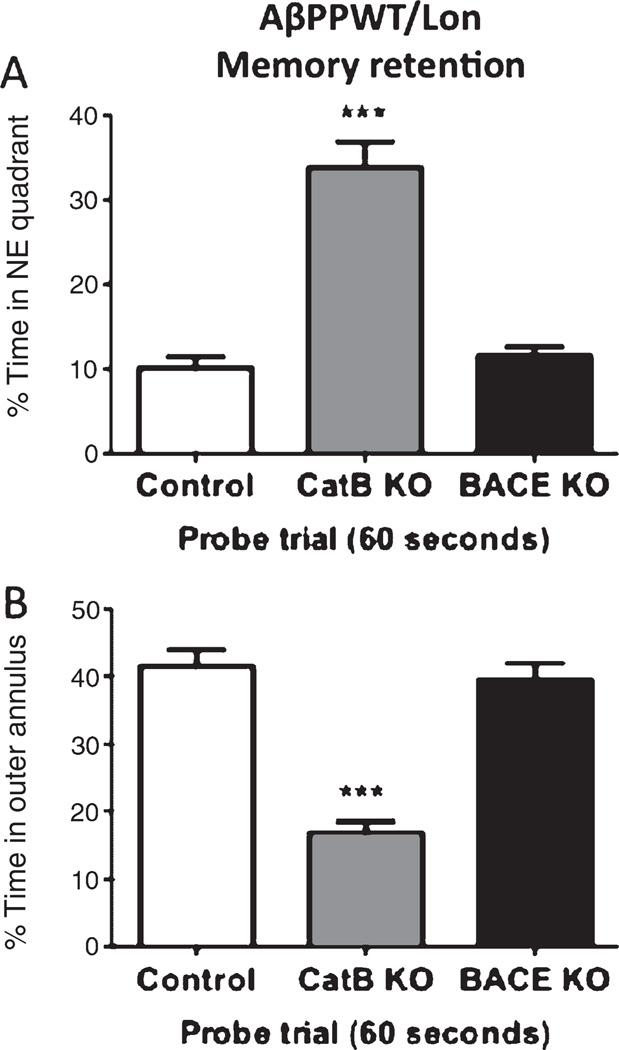
Deletion of the CatB gene also resulted in substantial memory retention in the AβPPWT/Lon mice as illustrated by the higher percent time spent in the northeast (NE) quadrant (from which the submerged platform was removed), and the lower percent time spent in the outer annulus, compared to control AβPPWT/Lon mice (Fig. 4). The CatB deletion resulted in a 230% increase in the percent time spent in the NE quadrant and a 60% decrease in the percent time spent in the annulus. The AβPPWT/Lon mice (control) and AβPPWT/Lon × CatB KO (CatB knockout) mice did not have a different swimming speed (data not shown). Thus, by the four parameters measured in the Morris water maze test, the deletion of the CatB gene improved the memory deficits that develop in the transgenic AβPPWT/Lon mice.
Fig. 4.
Knockout of the CatB gene, but not BACE1, in AβPPWT/Lon mice improves memory retention. The day after the last training session, the submerged platform was removed and the mice were allowed to swim in the pool for 60 s. The percent time each animal swam in the quadrant from which the platform had been removed (Northeast (NE) quadrant, (Panel A) and the percent time an animal swam in the annulus of the pool were recorded (Panel B). Greater memory retention is reflected in a higher percent time in the NE quadrant and lower percent time in the annulus. AβPPWT/Lon (control), AβPPWT/Lon × CatB KO (CatB KO), and AβPPWT/Lon × BACE1 KO (BACE1 KO) mice had percent times in the quadrant of 10%, 33%, and 11% (Panel A) and in the annulus of 42%, 17%, and 39% (Panel B). Values are expressed as the mean ± s.e.m., and n = 10 per group. ***Statistically significant with p < 0.05.
However, deletion of the BACE1 gene in the AβPPWT/Lon mice had no effect on memory deficits in the AβPPWT/Lon mice, assessed by the Morris water maze measurements of latency period and distance traveled (Fig. 3A, B, respectively), as well as percent time spent in the NE quadrant or annulus (Fig. 4A, B, respectively). The AβPPWT/Lon mice (control) and AβPPWT/Lon × BACE1 KO mice had the same swimming speed (data not shown). Thus, the BACE1 gene deletion had no effect on memory deficits in the AβPPWT/Lon mice.
Deletion of the CatB gene, but not the BACE1 gene, reduces brain amyloid plaque load in transgenic AβPPWT/Lon mice
Aβ immunohistochemistry of brain sections showed that deletion of the CatB gene reduced brain amyloid plaque in the AβPPWT/Lon mice (Fig. 5A, B). But deletion of the BACE1 gene had no effect on amyloid plaque load in AβPPWT/Lon mice (Fig. 5A, C). Quantitative image analysis of the Aβ immunohistochemistry showed that the CatB deletion resulted in a significant 78% reduction in brain amyloid plaque load relative to control AβPPWT/Lon animals (Fig. 5D). In contrast, the quantitative analysis showed that the BACE1 gene deletion had no effect on amyloid plaque load in AβPPWT/Lon animals (Fig. 5D). These data show that knockout of the CatB gene, but not the BACE1 gene, reduces brain amyloid plaque load in the AβPPWT/Lon mice.
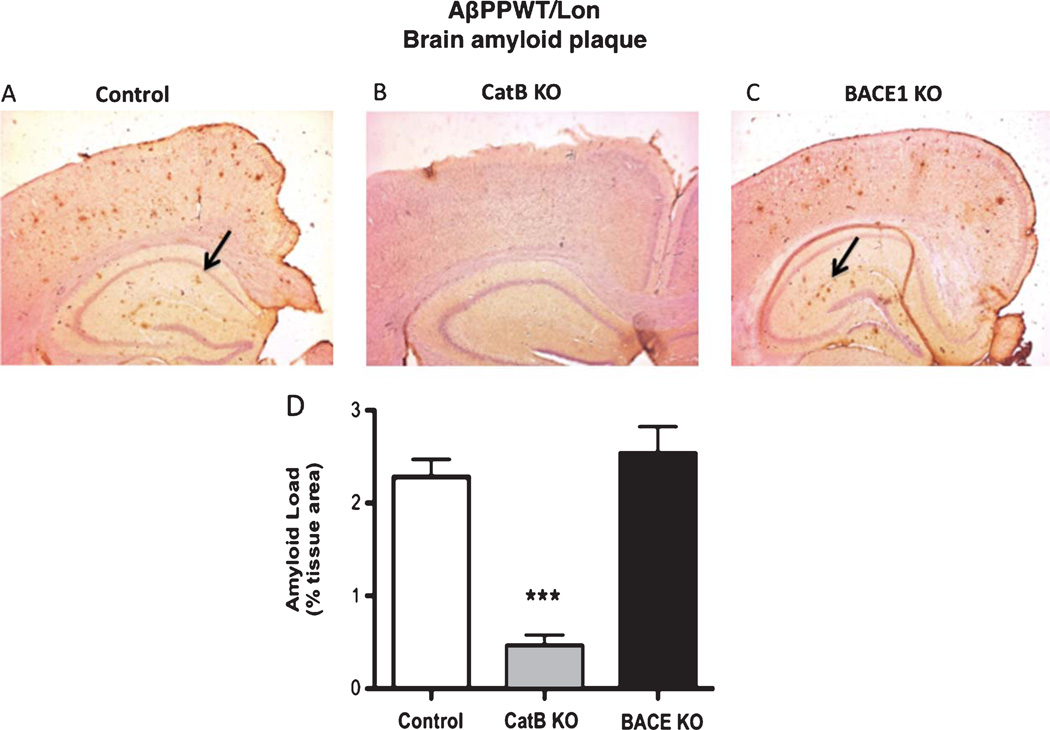
Fig. 5.
Knockout of the CatB gene, but not BACE1, in AβPPWT/Lon mice reduces brain amyloid plaque load. Amyloid plaque load was determined by immunohistochemisry and image analysis of brain sections from AβPPWT/Lon (control), AβPPWT/Lon × CatB KO (CatB KO), and AβPPWT/Lon × BACE1 KO (BACE1 KO) mice as shown in Panels A, B, and C, respectively. Arrows indicate amyloid plaque deposits (Panels A and C). Quantiation showed that AβPPWT/Lon, AβPPWT/Lon × CatB KO, and AβPPWT/Lon × BACE1 KO mice had mean percent amyloid plaque loads of 2.3%, 0.5%, and 2.5% of brain area, respectively (Panel D). (n = 10 per group, Values are expressed as x ± s.e.m., ***statistically significant with p < 0.05).
Deletion of the CatB gene, but not the BACE1 gene, alters brain biomarkers in a manner characteristic of inhibiting WT β-secretase in transgenic AβPPWT/Lon mice
The pattern of AβPP-derived Aβ peptides and AβPP-derived cleavage products resulting from amyloidogenic and non-amyloidogenic processing of AβPP was evaluated. Amyloidogenic processing of AβPP by β-secretase produces the CTFβ fragment and Aβ peptides, and non-amyloidogenic processing of AβPP by α-secretase results in the sAβPPα fragment (secretase sites of AβPP are illustrated in Fig. 1).
Knockout of the CatB gene in the transgenic AβPPWT/Lon mice reduced both brain Aβ40 and Aβ42 by 81% compared to control AβPPWT/Lon mice (Fig. 6A, B), but knockout of the BACE1 gene in these mice did not alter Aβ40 or Aβ42 levels relative to control AβPPWT/Lon mice (Fig. 6A, B). The CatB gene deletion caused a 50% reduction in brain CTFβ relative to controls (Fig. 6C, D), but the BACE1 gene deletion had no effect (Fig. 6C, D) in AβPPWT/Lon mice. The CatB gene deletion increased sAβPPα by 49% relative to controls (Fig. 6E, F), but the BACE1 gene knockout had no effect on sAβPPα in the AβPPWT/Lon mice (Fig. 6E, F).
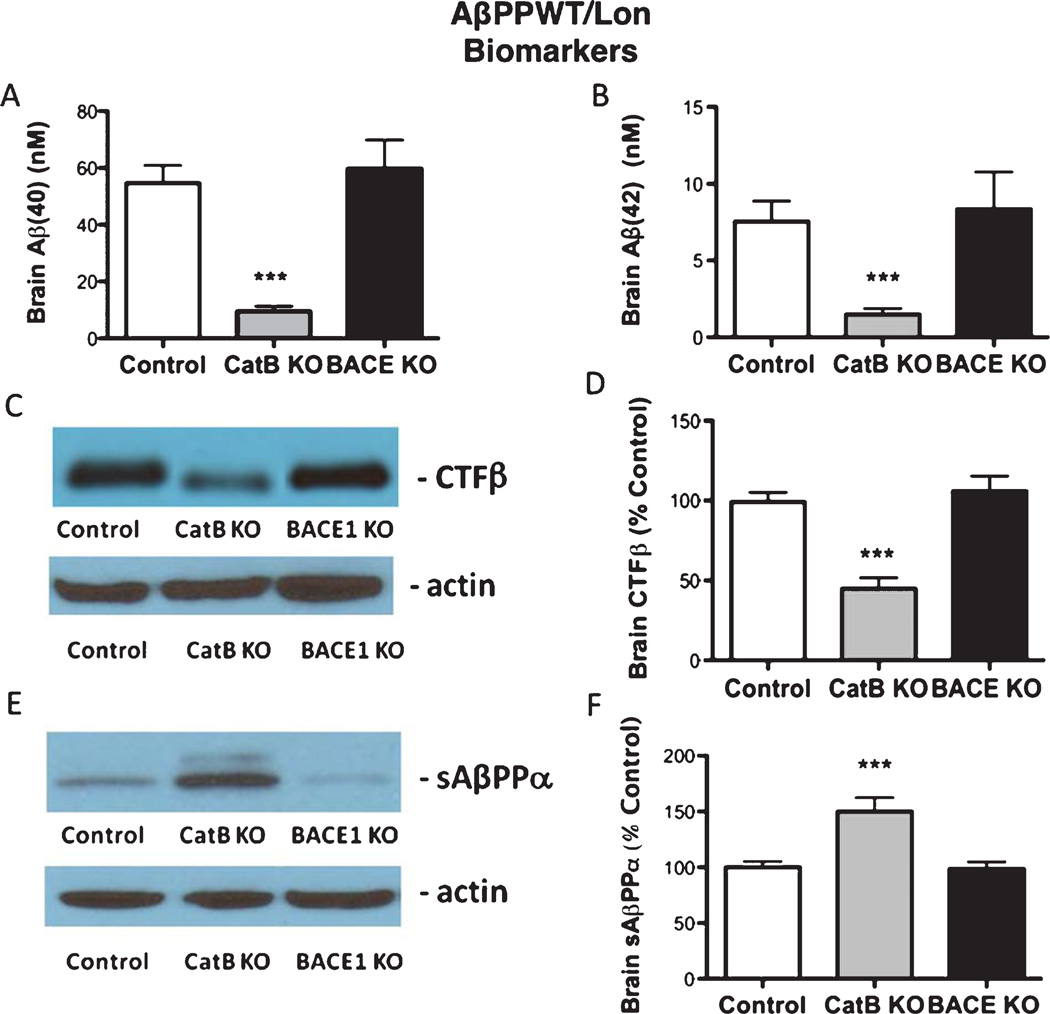
Fig. 6.
Knockout of the CatB gene, but not BACE1, in AβPPWT/Lon mice results in changes in Aβ-related biomarkers. Brain Aβ40 and Aβ42 (Aβ1–40 and Aβ1–42, respectively) levels were determined by ELISA. The AβPPWT/Lon (control), AβPPWT/Lon × CatB KO (CatB KO), and AβPPWT/Lon × BACE1 KO (BACE1 KO) mice had mean brain Aβ40 levels of 54.6, 9.6, and 59.8 nM, respectively (A), and mean Aβ42 levels of 7.5, 1.5, and 8.4 nM, respectively (B). Brain AβPP-derived CTFβ, generated by β-secretase, was assessed by western blot analysis (C, D). Relative quantitation by densitometry showed that the CatB KO and BACE1 KO mice had mean brain CTFβ levels of 50% and 106% compared to control AβPPWT/Lon mice (100%), respectively (D). AβPP-derived sAβPPα was evaluated by western blot analysis (E, F). Quantitation by densitometry showed that the AβPPWT/Lon, AβPPWT/Lon × CatB KO, AβPPWT/Lon × BACE1 KO mice had mean brain sAβPPα levels of 100%, 150%, and 99% of control AβPPWT/Lon mice, respectively (F). (n = 10 per group, values are expressed as x ± s.e.m., ***statistically significant with p < 0.05).
Since CTFβ is a β-secretase cleavage product, the reduction of CTFβ resulting from deletion of the CatB gene in the AβPPWT/Lon mice suggests that CatB has WT β-secretase activity. Reduced production of CTFβ from AβPP in the CatB knockout is likely to result in an increased AβPP available for α-secretase to generate increase in sAβPPα. In contrast, since deletion of the BACE1 gene had no effect on the AβPP-derived peptide patterns, these data illustrate that BACE1 does not have endogenous WT β-secretase activity in AβPPWT/Lon mice.
AβPPSwe/Lon mice and protease gene knockouts
Effects of CatB or BACE1 gene deletion on memory deficits and Aβ-related biomarkers in AβPPSwe/Lon mice
AβPPSwe/Lon mice express the rare Swedish (Swe) mutant β-secretase site and the London mutation near the γ-secretase site (Fig. 1) and deletion of the CatB or BACE1 genes in the AβPPSwe/Lon mice results in the absence of the respective protease activities (supplementary Figure 2). The effects of CatB or BACE1 gene deletion on memory deficits in the AβPPSwe/Lon mice were compared (Fig. 7). During the training period, mice show reduced latencies to find the hidden platform (Fig. 7A), indicating that they learned the location of the platform. After the training period, analyses of memory deficits in the mice showed that knockout of the CatB gene in the AβPPSwe/Lon mice had no effect on memory deficits illustrated by measuring the latency period and the distance traveled (Fig. 7B, C, respectively). Also, CatB gene knockout had no effect on memory retention, measured by the percent time in the northeast quadrant (from which the hidden platform was removed) and the percent time in the outer annulus (Fig. 7D, E, respectively).
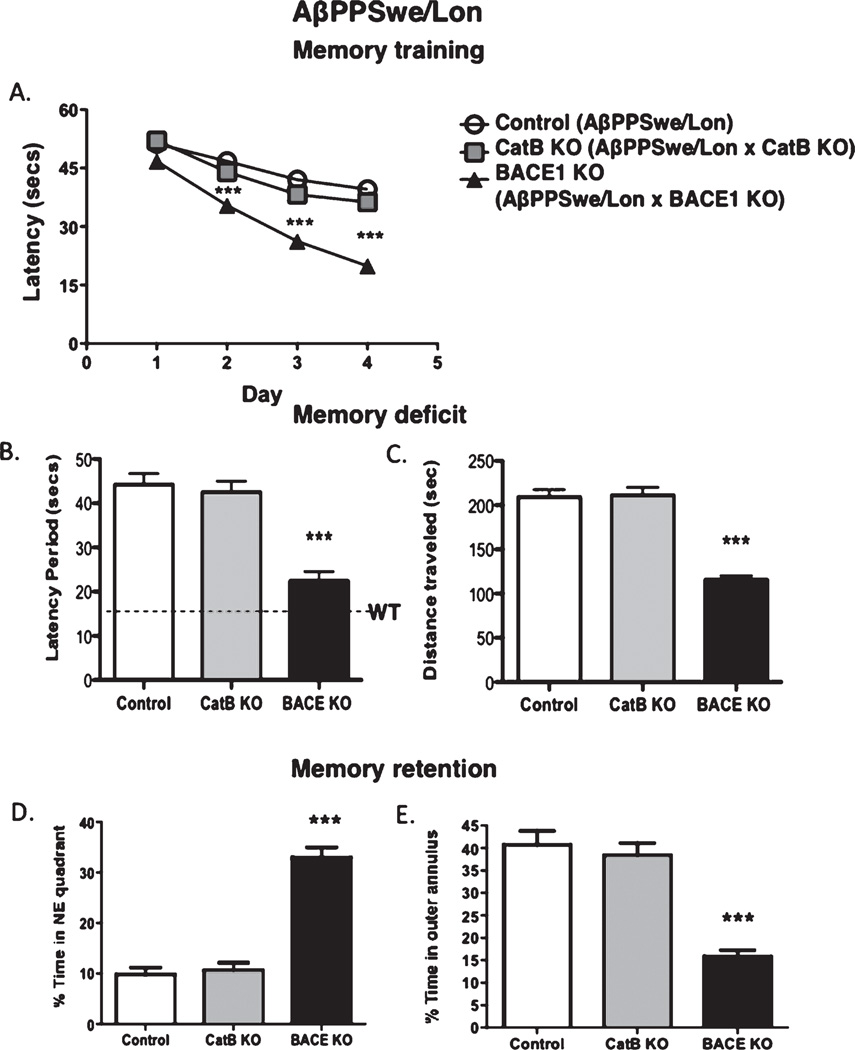
Fig. 7.
Knockout of the BACE1 gene, but not CatB, in AβPPSwe/Lon mice results in improved memory acquisition, memory deficits, and memory retention. Control AβPPSwe/Lon mice, AβPPSwe/Lon × CatB knockout (CatB KO), and AβPPSwe/Lon × BACE1 KO (BACE1 KO) mice were evaluated in the Morris water maze test during each day of the training sessions by measuring the latency period (A). After the training sessions had concluded, memory deficits were determined by measuring the latency period (B) and the distance traveled to the platform (C). Memory retention was assessed by measuring the percent time in the quadrant from which the platform had been removed (Northeast (NE) quadrant) (D) and the percent time in the annulus of the pool (E). Values are expressed as x ± s.e.m., n = 10 per group. ***Statistically significant (p < 0.05).
However, knockout of the BACE1 gene in the AβPPSwe/Lon mice improved memory deficits, observed by the reduced latency period and distance traveled (Fig. 7B, C, respectively), consistent with previous studies [19, 20]. BACE1 gene knockout also improved retention of memory in the AβPPSwe/Lon mice, assessed by the percent time mice spent in the northeast quadrant (from which the hidden platform was removed) and percent time in the annulus area (Fig. 7D, E).
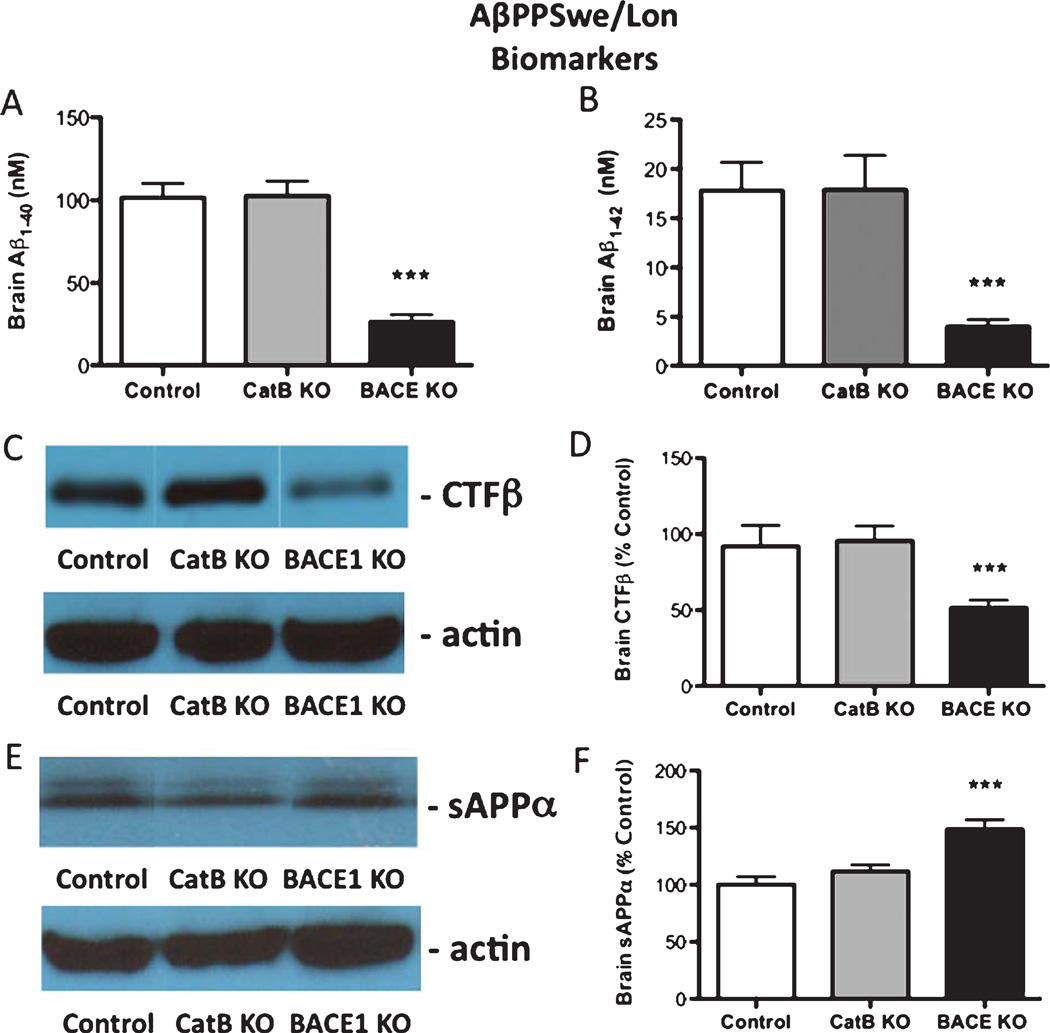
Analyses of biomarkers showed that deletion of the CatB gene in the AβPPSwe/Lon mice had no effect on amyloid plaque load, Aβbrain levels, or AβPP-derived CTFβ and sAβPPα fragments (Figs. 8 and 9). However, deletion of theBACE1 gene in the AβPPSwe/Lon mice reduced brain amyloid plaque, Aβ, and CTFβ, combined with increased sAβPPα (Figs. 8 and 9), consistent with earlier reports [19, 20].
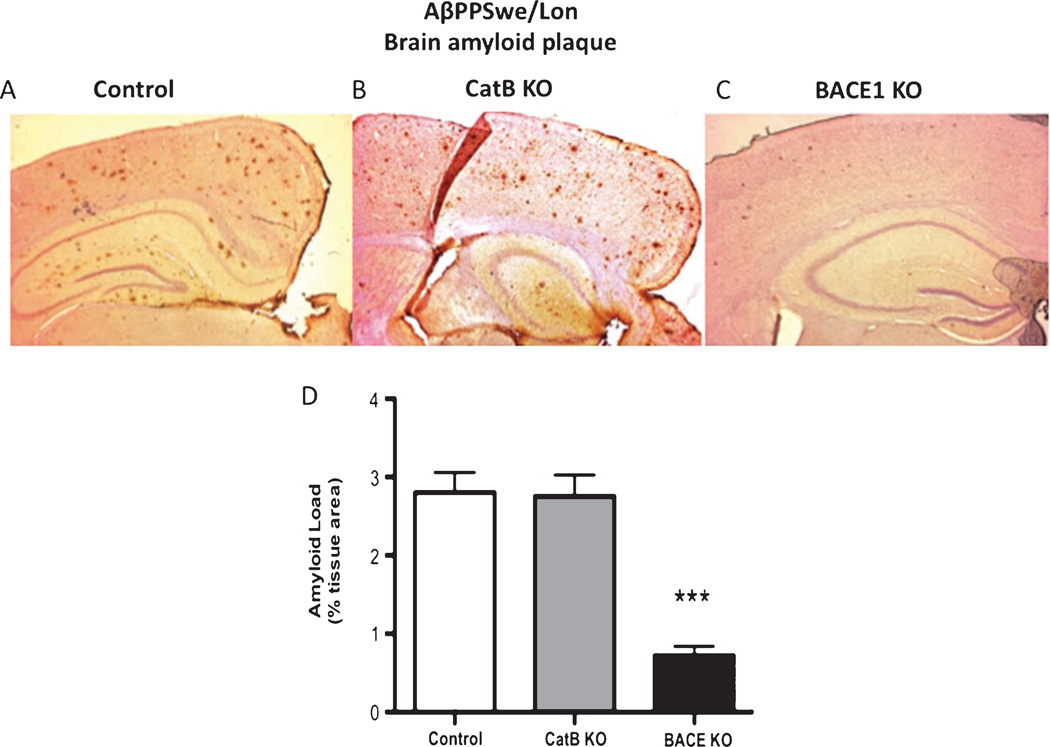
Fig. 8.
Knockout of the BACE1 gene, but not CatB, in AβPPSwe/Lon mice reduces brain amyloid plaque load. Amyloid plaque load was determined by immunohistochemistry and image analysis of brain sections from AβPPSwe/Lon (control), AβPPSwe/Lon × CatB KO (CatB KO), and AβPPSwe/Lon × BACE1 KO (BACE1 KO) as shown in A, B, and C, respectively. Arrows indicate amyloid plaque deposits (A, C). Qualitative analyses of the areas of plaque load were conducted (D). AβPPSwe/Lon, AβPPSwe/Lon × CatB KO, and AβPPSwe/Lon × BACE1 KO mice had mean percent amyloid plaque loads of 2.8%, 2.8%, and 0.7% of brain plaque area, respectively (D). Values in D are expressed as x ± s.e.m., and n = 10. ***Statistically significant (p < 0.05).
Fig. 9.
Knockout of the BACE1 gene, but not CatB, in AβPPSwe/Lon mice alters Aβ-related biomarkers. Brain Aβ40 and Aβ42 brain levels (A and B, respectively) were determined by ELISA in AβPPSwe/Lon (control), AβPPSwe/Lon × CatB KO (CatB KO), and AβPPSwe/Lon × BACE1 KO (BACE1 KO) mice. Relative brain levels of CTFβ were assessed by western blots (C) with actin in control westerns; quantitation of CTFβ western blots for the three groups of mice is shown (D). Relative brain levels of sAβPPα were assessed by western blots (E) with actin in control westerns; quantitation of sAβPPα western blots is shown (F). Values are shown as the x ± s.e.m., and n = 10. ***Statistically significant (p < 0.05).
Comparison of CatB gene deletion in AβPPWT/Lon, AβPPSwe/Lon mice, and normal non-transgenic mice
The data of this study illustrate that the CatB gene knockout specifically improves memory deficits in the AβPPWT/Lon mice, but not in the AβPPSwe/Lon mice. Deletion of the BACE1 gene has no effect in the AβPPWT/Lon mice, but improves memory deficits in the AβPPSwe/Lon mice. The effect of deleting these protease genes in normal mice was also assessed. In normal non-transgenic mice (age-and strain-matched), deletion of either the CatB or BACE1 genes had no effect on normal memory (age and strain-matched, supplementary Figure 3). These data together show that CatB or BACE1 gene knockout improves memory deficits in AβPPWT/Lon or AβPPSwe/Lon mice, respectively; but deletion of these protease genes has no effect on normal memory of non-transgenic mice.
DISCUSSION
The major result of this study is that deletion of the CatB gene improves memory deficits in the AβPPWT/Lon AD mouse model expressing human AβPP containing the WT β-secretase site sequence that is present in most AD patients. This is the first study that we are aware of to demonstrate that deletion of any gene results in improved memory deficits in an AD mouse model expressing the WT β-secretase site sequence that is present in the majority of AD patients. Moreover, mice with deletion of the CatB gene remain healthy [17]. These significant findings validate CatB as a target for developing therapeutic inhibitor compounds to improve memory deficits of AD.
The significant finding of this study is the identification of a new protease target, CatB, which improves memory deficits upon gene deletion in a mouse model expressing the WT β-secretase site sequence of AβPP that is relevant to sporadic AD patients, representing more than 90% of the AD population. Protease activity that cleaves the β-secretase site of AβPP is viewed in the field as a potential target for developing inhibitors to improve memory deficits. Identification of such protease activity involved in memory deficits was accomplished in this study by using the AβPPWT/Lon mouse model of AD, which expresses human AβPP containing the WT β-secretase site sequence combined with the London mutation near the γ-secretase site and results in development of memory deficits [5, 9, 10]. The AβPPWT/Lon AD mouse model is an appropriate for defining proteases functioning as ‘wild-type’ β-secretase activity utilized by the majority of AD patients.
Deletion of the CatB gene in the AβPPWT/Lon mice resulted in an altered biomarker pattern consistent with CatB having WT β-secretase activity. Biomarker analyses showed that knockout of the CatB gene in the AβPPWT/Lon mice reduced brain Aβ and CTFβ derived from AβPP by β-secretase, and increased sAβPPα; these changes represent an altered Aβ-related pattern characteristic of inhibiting the processing of AβPP at the WT β-secretase site. In transgenic mice expressing human wild-type AβPP (no mutations), deletion of the CatB gene alters these biomarkers in the same manner [17] as in the AβPPWT/Lon mice of this study. These data demonstrate that CatB has β-secretase activity in two transgenic mouse models expressing AβPP containing the WT β-secretase site sequence of AβPP. The biomarker data supports the hypothesis that deletion of the CatB gene in the AβPPWT/Lon mice improves memory by reducing CatB WT β-secretase activity. Moreover, the data suggest that the improvement in memory deficits occurring with administration of CatB inhibitors [9, 10] is also likely due to inhibition of CatB WT β-secretase function.
Of interest is the finding from this study that improvement in memory deficits results from CatB gene knockout in the AβPPWT/Lon mice, but not in the AβPPSwe/Lon mice. These data illustrate the specificity of CatB gene deletion in transgenic mice expressing the WT β-secretase site but not the Swedish mutant site of human AβPP. The difference in CatB gene knockout in the AβPPWT/Lon mice compared to the AβPPSwe/Lon mice can be explained by the preference of CatB to readily cleave the WT β-secretase site, rather than the Swe mutant β-secretase site of AβPP [9].
One report speculated that inhibiting CatB may worsen memory deficits based on the finding that CatB degrades Aβ in vitro and over-expression of CatB by lentiviral vector injection into brains of transgenic mice resulted in a reduction of amyloid plaque in the region receiving the injection [21]. However, that study did not evaluate the effects of inhibiting CatB on memory deficits, whereas the data here shows that CatB gene deletion in the AβPPWT/Lon mice resulted in improved memory deficits. Moreover, we previously showed that chemical inhibition of CatB in the AβPPWT/Lon mice also results in improved memory deficits [9, 10]. Chemical inhibition of CatB in the guinea pig, a natural model of normal human WT AβPP processing [22], also results in reduction of brain Aβ [9, 10, 23, 24]. These data show that elimination or inhibition of CatB improves, and does not worsen, memory deficits in these animal models expressing the WT β-secretase site that is present in the majority of AD patients.
As control, our studies of memory function after BACE1 gene knockout found that its deletion in AβPPSwe/Lon mice expressing the Swe mutant β-secretase site (with the London mutation) resulted in improved memory deficits, similar to previous reports [19, 20]. But since the majority of AD patients express the WT β-secretase site of AβPP, we investigated the representative AβPPWT/Lon AD mouse model in BACE1 gene knockout experiments; results showed no effect of BACE1 knockout on memory deficits in the AβPPWT/Lon mice. Two other studies showed that in a mouse model expressing the WT β-secretase site sequence of AβPP, BACE1 gene knockout resulted in worse memory [13, 25]. So far, there is no report of improving memory deficits by BACE1 gene knockout in a mouse model expressing the WT β-secretase site of AβPP, expressed in most AD patients.
Biomarker results of this study show that deletion of the BACE1 gene in the AβPPWT/Lon mice has no effect on brain Aβ, CTFβ, and sAβPPα, which argues that BACE1 is not acting as an endogenous WT β-secretase in this model. Our previous work also showed that knockout of theBACE1 gene in transgenic mice expressing human AβPP (no mutations) had no effect on these biomarkers [17]. But other reports show reduced Aβ after BACE1 gene knockout in transgenic PDGF-AβPP [14, 26] and AβPP51/16 [27] mice expressing the WT β-secretase site. These differences may be explained by the expression of AβPP695 in the AβPPWT/Lon mice, whereas the PDGF-AβPP [14, 26] and AβPP51/16 [27] mice express primarily the AβPP751 and AβPP770 isoforms that contain the Kunitz protease inhibitor domain [28, 29]. Different protease effects of the Kunitz domain, and differences in the distribution of AβPP695 in neurons that secrete Aβ compared to the presence of AβPP751 and AβPP770 in astrocytes [30, 31], may explain the different effects of BACE1 gene knockout in the different transgenic mouse models. Over-expression of BACE1 in AβPPWT/Lon mice results in elevated brain Aβ [32], indicating that BACE1 may have exogenous effects, but does not demonstrate an endogenous role. In normal mice expressing mouse WT AβPP [33], knockout or over-expression of BACE1 results in contradictory results showing reduction [18, 34] or no effect [35] on brain Aβ levels, respectively. Importantly, careful examination of BACE1 knockout studies [11, 12, 36–38] indicate that the results do not exclude CatB as having WT β-secretase activity [39–41].
In conclusion, this study shows that deletion of the CatB gene in AβPPWT/Lon mice results in improved memory deficits and altered brain biomarkers consistent with CatB having WT β-secretase activity in that model. This is the first gene deletion study showing improvement in memory deficits in a transgenic mouse model expressing AβPP containing the WT β-secretase site sequence found in most AD patients. Importantly, these data validate CatB as a target for developing inhibitors to improve memory loss in most AD patients.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants R21 AG0311963, R44 AG030865, and R44 AG032784 from the National Institute on Aging, National Institutes of Health, Bethesda, MD, and grant no. 20100304 from the Alzheimer’s Drug Discovery Foundation, New York City, NY to American Life Science Pharmaceuticals (ALSP), and a grant from the Alzheimer’s Association to Dr. Vivian Hook.
Footnotes
Supplementary data available online: http://www.j-alz.com/issues/29/vol29-4.html#supplementarydata03
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1131).
REFERENCES
- 1.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Turner RS. Alzheimer’s disease. Semin Neurol. 2006;26:499–506. doi: 10.1055/s-2006-951622. [DOI] [PubMed] [Google Scholar]
- 3.Brouwers N, Sleegers K, Van Broeckhoven C. Molecular genetics of Alzheimer’s disease: An update. Ann Med. 2008;40:562–583. doi: 10.1080/07853890802186905. [DOI] [PubMed] [Google Scholar]
- 4.Price DL, Sisodia SS. Mutant genes in familial Alzheimer’s disease and transgenic models. Annu Rev Neurosci. 1998;21:479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- 5.Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, Godaux E, Cordell B, Van Leuven F. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem. 1999;274:6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- 6.Masliah E, Rockenstein E. Genetically altered transgenic models of Alzheimer’s disease. J Neural Transm Suppl. 2000;59:175–183. doi: 10.1007/978-3-7091-6781-6_20. [DOI] [PubMed] [Google Scholar]
- 7.Dodart JC, Mathis C, Bales KR, Paul SM. Does my mouse have Alzheimer’s disease? Genes Brain Behav. 2002;1:142–155. doi: 10.1034/j.1601-183x.2002.10302.x. [DOI] [PubMed] [Google Scholar]
- 8.Ashe KH, Zahs KR. Probing the biology of Alzheimer’s disease in mice. Neuron. 2010;66:631–645. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hook VY, Kindy M, Hook G. Inhibitors of cathepsin B improve memory and reduce beta-amyloid in transgenic Alzheimer disease mice expressing the wild-type, but not the Swedish mutant, beta-secretase site of the amyloid precursor protein. J Biol Chem. 2008;283:7745–7753. doi: 10.1074/jbc.M708362200. [DOI] [PubMed] [Google Scholar]
- 10.Hook G, Hook V, Kindy M. The cysteine protease inhibitor, E64d, reduces brain amyloid-beta and improves memory deficits in Alzheimer’s disease animal models by inhibiting cathepsin B, but not BACE1, beta-secretase Activity. J Alzheimers Dis. 2011;26:387–408. doi: 10.3233/JAD-2011-110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vassar R. BACE1: The beta-secretase enzyme in Alzheimer’s disease. J Mol Neurosci. 2004;23:105–114. doi: 10.1385/JMN:23:1-2:105. [DOI] [PubMed] [Google Scholar]
- 12.Cole SL, Vassar R. The Alzheimer’s disease beta-secretase enzyme, BACE1. Mol Neurodegener. 2007;2:22. doi: 10.1186/1750-1326-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi D, Zeller M, Cole T, Buttini M, McConlogue L, Sinha S, Freedman S, Morris RG, Chen KS. BACE1 gene deletion: Impact on behavioral function in a model of Alzheimer’s disease. Neurobiol Aging. 2008;29:861–873. doi: 10.1016/j.neurobiolaging.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 14.McConlogue L, Buttini M, Anderson JP, Brigham EF, Chen KS, Freedman SB, Games D, Johnson-Wood K, Lee M, Zeller M, Liu W, Motter R, Sinha S. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP Transgenic Mice. J Biol Chem. 2007;282:26326–26334. doi: 10.1074/jbc.M611687200. [DOI] [PubMed] [Google Scholar]
- 15.Kimura R, Devi L, Ohno M. Partial reduction of BACE1 improves synaptic plasticity, recent and remote memories in Alzheimer’s disease transgenic mice. J Neurochem. 2010;113:248–261. doi: 10.1111/j.1471-4159.2010.06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Amouri SS, Zhu H, Yu J, Marr R, Verma IM, Kindy MS. Neprilysin: An enzyme candidate to slow the progression of Alzheimer’s disease. Am J Pathol. 2008;172:1342–1354. doi: 10.2353/ajpath.2008.070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hook VY, Kindy M, Reinheckel T, Peters C, Hook G. Genetic cathepsin B deficiency reduces beta-amyloid in transgenic mice expressing human wild-type amyloid precursor protein. Biochem Biophys Res Commun. 2009;386:284–288. doi: 10.1016/j.bbrc.2009.05.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 19.Ohno M, Sametsky EA, Younkin LH, Oakley H, Younkin SG, Citron M, Vassar R, Disterhoft JF. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer’s disease. Neuron. 2004;41:27–33. doi: 10.1016/s0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 20.Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, Borchelt DR, Price DL, Lee HK, Wong PC. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, Yu G, Esposito L, Mucke L, Gan L. Antiamyloidogenic and neuroprotective functions of cathepsin B: Implications for Alzheimer’s disease. Neuron. 2006;51:703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Beck M, Bigl V, Rossner S. Guinea pigs as a non-transgenic model for APP processing in vitro and in vivo. Neurochem Res. 2003;28:637–644. doi: 10.1023/a:1022850113083. [DOI] [PubMed] [Google Scholar]
- 23.Hook G, Hook VY, Kindy M. Cysteine protease inhibitors reduce brain beta-amyloid and beta-secretase activity in vivo and are potential Alzheimer’s disease therapeutics. Biol Chem. 2007;388:979–983. doi: 10.1515/BC.2007.117. [DOI] [PubMed] [Google Scholar]
- 24.Hook V, Kindy M, Hook G. Cysteine protease inhibitors effectively reduce in vivo levels of brain beta-amyloid related to Alzheimer’s disease. Biol Chem. 2007;388:247–252. doi: 10.1515/BC.2007.027. [DOI] [PubMed] [Google Scholar]
- 25.Ma H, Lesne S, Kotilinek L, Steidl-Nichols JV, Sherman M, Younkin L, Younkin S, Forster C, Sergeant N, Delacourte A, Vassar R, Citron M, Kofuji P, Boland LM, Ashe KH. Involvement of beta-site APP cleaving enzyme 1 (BACE1) in amyloid precursor protein-mediated enhancement of memory and activity-dependent synaptic plasticity. Proc Natl Acad Sci U S A. 2007;104:8167–8172. doi: 10.1073/pnas.0609521104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rockenstein EM, McConlogue L, Tan H, Power M, Masliah E, Mucke L. Levels and alternative splicing of amyloid beta protein precursor (APP) transcripts in brains of APP transgenic mice and humans with Alzheimer’s disease. J Biol Chem. 1995;270:28257–28267. doi: 10.1074/jbc.270.47.28257. [DOI] [PubMed] [Google Scholar]
- 27.Rabe S, Reichwald J, Ammaturo D, de Strooper B, Saftig P, Neumann U, Staufenbiel M. The Swedish APP mutation alters the effect of genetically reducedBACE1 expression on the APP processing. J Neurochem. 2011;199:2310239. doi: 10.1111/j.1471-4159.2011.07412.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnson SA, McNeill T, Cordell B, Finch CE. Relation of neuronal APP-751/APP-695 mRNA ratio and neuritic plaque density in Alzheimer’s disease. Science. 1990;248:854–857. doi: 10.1126/science.2111579. [DOI] [PubMed] [Google Scholar]
- 29.Prior R, Monning U, Schreiter-Gasser U, Weidemann A, Blennow K, Gottfries CG, Masters CL, Beyreuther K. Quantitative changes in the amyloid beta A4 precursor protein in Alzheimer cerebrospinal fluid. Neurosci Lett. 1991;124:69–73. doi: 10.1016/0304-3940(91)90824-d. [DOI] [PubMed] [Google Scholar]
- 30.Rohan de Silva HA, Jen A, Wickenden C, Jen LS, Wilkinson SL, Patel AJ. Cell-specific expression of beta-amyloid precursor protein isoform mRNAs and proteins in neurons and astrocytes. Brain Res Mol Brain Res. 1997;47:147–156. doi: 10.1016/s0169-328x(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 31.Kang J, Muller-Hill B. Differential splicing of Alzheimer’s disease amyloid A4 precursor RNA in rat tissues: PreA4(695)mRNAis predominantly produced in rat and human brain. Biochem Biophys Res Commun. 1990;166:1192–1200. doi: 10.1016/0006-291x(90)90992-v. [DOI] [PubMed] [Google Scholar]
- 32.Willem M, Dewachter I, Smyth N, Van Dooren T, Borghgraef P, Haass C, Van Leuven F. beta-site amyloid precursor protein cleaving enzyme 1 increases amyloid deposition in brain parenchyma but reduces cerebrovascular amyloid angiopathy in aging BACE × APP[V717I] double-transgenic mice. Am J Pathol. 2004;165:1621–1631. doi: 10.1016/s0002-9440(10)63419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnstone EM, Chaney MO, Norris FH, Pascual R, Little SP. Conservation of the sequence of the Alzheimer’s disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res. 1991;10:299–305. doi: 10.1016/0169-328x(91)90088-f. [DOI] [PubMed] [Google Scholar]
- 34.Nishitomi K, Sakaguchi G, Horikoshi Y, Gray AJ, Maeda M, Hirata-Fukae C, Becker AG, Hosono M, Sakaguchi I, Minami SS, Nakajima Y, Li HF, Takeyama C, Kihara T, Ota A, Wong PC, Aisen PS, Kato A, Kinoshita N, Matsuoka Y. BACE1 inhibition reduces endogenous Abeta and alters APP processing in wild-type mice. J Neurochem. 2006;99:1555–1563. doi: 10.1111/j.1471-4159.2006.04178.x. [DOI] [PubMed] [Google Scholar]
- 35.Hirata-Fukae C, Sidahmed EH, Gooskens TP, Aisen PS, Dewachter I, Devijver H, Van Leuven F, Matsuoka Y. Beta-site amyloid precursor protein-cleaving enzyme-1 (BACE1)-mediated changes of endogenous amyloid beta in wild-type and transgenic mice in vivo. Neurosci Lett. 2008;435:186–189. doi: 10.1016/j.neulet.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, Freedman SB, Frigon NL, Games D, Hu K, Johnson-Wood K, Kappenman KE, Kawabe TT, Kola I, Kuehn R, Lee M, Liu W, Motter R, Nichols NF, Power M, Robertson DW, Schenk D, Schoor M, Shopp GM, Shuck ME, Sinha S, Svensson KA, Tatsuno G, Tintrup H, Wijsman J, Wright S, McConlogue L. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: Implications for Alzheimer’s disease therapeutics. Hum Mol Genet. 2001;10:1317–1324. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- 37.Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 38.Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 39.Hook V, Hook G, Kindy M. Pharmacogenetic features of cathepsin B inhibitors that improve memory deficit and reduce beta-amyloid related to Alzheimer’s disease. Biol Chem. 2010;391:861–872. doi: 10.1515/BC.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hook V, Toneff T, Bogyo M, Medzihradszky KF, Nevenu J, Lane W, Hook G, Reisine T. Inhibition of cathepsin B reduces β-amyloid production in regulated secretory vesicles of neuronal chromaffin cells: Evidence for cathepsin B as a candidate β-secretase of Alzheimer’s disease. Biol Chem. 2005;386:931–940. doi: 10.1515/BC.2005.108. [DOI] [PubMed] [Google Scholar]
- 41.Klein DM, Felsenstein KM, Brenneman DE. Cathepsins B and L differentially regulate amyloid precursor protein processing. J Pharmacol Exp Ther. 2009;328:813–821. doi: 10.1124/jpet.108.147082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.