Abstract
Purpose.
To test the hypothesis that in a mouse model of diabetic retinopathy, oxidative stress is linked with impaired light-evoked expansion of choroidal thickness and subretinal space (SRS).
Methods.
We examined nondiabetic mice (wild-type, wt) with and without administration of manganese, nondiabetic mice deficient in rod phototransduction (transducin alpha knockout; GNAT1−/−), and diabetic mice (untreated or treated with the antioxidant α-lipoic acid [LPA]). Magnetic resonance imaging (MRI) was used to measure light-evoked increases in choroidal thickness and the apparent diffusion coefficient (ADC) at 88% to 100% depth into the retina (i.e., the SRS layer).
Results.
Choroidal thickness values were similar (P > 0.05) between all untreated nondiabetic dark-adapted groups and increased significantly (P < 0.05) with light; this expansion was subnormal (P < 0.05) in both diabetic groups. Apparent diffusion coefficient values in the SRS layer robustly increased (P < 0.05) in a light duration-dependent manner, and this effect was independent of the presence of Mn2+. The light-stimulated increase in ADC at the location of the SRS was absent in GNAT1−/− and diabetic mice (P > 0.05). In diabetic mice, the light-dependent increase in SRS ADC was significantly (P < 0.05) restored with LPA.
Conclusions.
Apparent diffusion coefficient MRI is a sensitive method for evaluating choroid thickness and its light-evoked expansion together with phototransduction-dependent changes in the SRS layer in mice in vivo. Because ADC MRI exploits an endogenous contrast mechanism, its translational potential is promising; it can also be performed in concert with manganese-enhanced MRI (MEMRI). Our data support a link between diabetes-related oxidative stress and rod, but not choroidal, pathophysiology.
Keywords: MRI, retina, choroid, diffusion, choroidal thickness, phototransduction, water
We demonstrate that ADC MRI is a sensitive method for evaluating light-evoked expansion of choroid thickness and the subretinal space layer in mice in vivo. Our data support a link between diabetes-related oxidative stress and rod, but not choroidal, pathophysiology.
Introduction
Diabetic retinopathy (DR), a leading cause of vision loss and blindness in patients under the age of 45, is clinically managed based on retinal microcirculation abnormalities. Accumulating evidence has implicated retinal oxidative stress as an important pathogenic factor early in the course of diabetes.1,2 Importantly, the major cell type contributing to this oxidative stress is not the endothelial cell, but dysfunctional rod photoreceptors.3–6 This newly identified pathogenic role of the rod photoreceptor cell in early DR raises questions about a possible role of the choroid, the essential circulation supporting photoreceptor cells and retinal pigment epithelium located posterior to the retinal pigment epithelium (illustrated in the cartoon in Fig. 1D). In fact, there have been reports of choroidal thinning in diabetes, although it remains unclear whether or not this is due to impaired choroidal vasodilation in diabetes, and/or choroidal injury is linked with diabetes-induced rod-dominated retinal oxidative stress.7–18 Few methods are presently available for answering these questions because current imaging methods cannot study both rod function and choroidal anatomy/function at the same time and spatial location in full darkness in a common animal model of DR.
Figure 1.
Choroidal thickness analysis outline and ADC profiles in dark-adapted wt mice. (A) Representative image of a mouse eye showing central retinal regions of interest (white boxes). (B) Representative flattened central retinal region from anatomic (left) and averaged (b100–b990) ADC MRI data (right) illustrating greatly reduced signal intensity in the choroidal region. For simplicity, boundaries are illustrated on the anatomic and ADC images (dotted lines); objectively determined boundaries were used for actual evaluation of thicknesses as described in Methods. Choroidal thickness was objectively measured as the difference in thickness between the anatomic and averaged ADC data (for details see Methods). (C) Summary of dark-adapted choroidal thickness from five separate groups (Grp) of mice (mean ± SEM) analyzed as in (B). (D) Summary of central retinal ADC profiles in five groups of dark-adapted wt mice suggests reasonable topographical precision (i.e., reproducibility of the magnitude and spatial features of the profile). Approximate locations of retinal layers (INL, inner nuclear layer; ONL, outer nuclear layer; IS, inner segments; OS/SRS, outer segments and subretinal space; and choroid) are indicated by dotted vertical blue lines and the cartoon at the top that was modified from a previous study.61 Profiles are spatially normalized to dark-adapted retinal thickness (0%, vitreous/retina border; 100%, retina/choroid border).
In this study, we developed and applied a noninvasive imaging approach for addressing this gap. Our approach was based on high spatial resolution diffusion-weighted magnetic resonance imaging (MRI) measurements of the apparent diffusion coefficient (ADC) within the retina. Apparent diffusion coefficient MRI has been used to measure choroidal thickness in the rat.19 Here, we investigate the potential of ADC MRI for monitoring the expansion of choroidal thickness with light, an effect observed in other species but not yet in the mouse.20–23 Apparent diffusion coefficient MRI has also been reported to be useful for measuring light-stimulated increases in the subretinal space layer (SRS layer) in rats.19 The retina is the tissue bounded by the vitreous anteriorly and choroid posteriorly. Within the retina is the SRS, which contains the extracellular fluid around the photoreceptor outer segments and is located at 88% to 100% depth into the retina posterior to the outer limiting membrane (i.e., the end-feet of the Müller cells) and anterior to the retinal pigment epithelium (illustrated in the cartoon in Fig. 1D). Previous microelectrode studies observed that the SRS volume is substantially smaller in dark than in light as a consequence of light-dependent changes in extracellular ion content and thus represents a biomarker of the rod and retinal pigment epithelium unit function.24–27 Our working hypotheses are that (1) in nondiabetic mice, ADC MRI will be sensitive to light-evoked expansion of the choroidal thickness and SRS layer; (2) in diabetic mice, light-dependent changes in choroidal thickness and SRS layer will be subnormal3,4,28–31; and (3) antioxidant treatment will correct light-stimulated choroidal and SRS pathophysiology associated with diabetes.
Methods
All animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and Institutional Animal and Care Use Committee authorization. Animals were housed and maintained in 12 hour:12 hour light:dark cycle laboratory lighting, unless otherwise noted.
Groups
We investigated the following groups (summarized in the Table): nondiabetic 3- to 6-month male C57Bl/6J mice (with and without manganese administration) (wild-type [wt]; Jackson Laboratories, Bar Harbor, ME, USA); nondiabetic male or female α-transducin-1 (Tα) knockout mice (GNAT1−/−) (kind gift from Janis Lem, Tufts University)32–34; and 2- to 3-month diabetic male C57Bl/6J mice (untreated or treated with the antioxidant α-lipoic acid [LPA]). α-Lipoic acid (50 mg/kg, subcutaneous) was administered 30 minutes prior to anesthesia and imaging. α-Lipoic acid is most commonly administered chronically in experimental diabetic models with the goal of preventing the development of histopathology.4,35–39 Because we have previously shown that acute single-dose treatment of 11-cis-retinaldehyde (which turns out, unexpectedly, to be an antioxidant [manuscript in preparation]) can specifically impact diabetes-induced rod pathophysiology, in this study we applied LPA acutely (duplicating an experimental design previously used in a sciatic nerve crush injury model) to reduce the oxidative burden.5,40
Table.
Summary of Anatomic, ADC, and Choroidal Thicknesses in Dark and 20 Minutes of Light (μm, Mean ± SEM)
|
Group |
Anatomical Thickness |
ADC Thickness |
Choroidal Thickness |
|||
|
Dark |
Light |
Dark |
Light |
Dark |
Light |
|
| wt, n = 18 | 237 ± 3 | 264 ± 4‡ | 175 ± 3 | 181 ± 4‡ | 62 ± 2 | 86 ± 3‡ |
| wt+Mn2+, n = 6 | 216 ± 4* | 235 ± 8†‡ | 157 ± 3* | 163 ± 2† | 58 ± 3 | 72 ± 8† |
| GNAT1−/−, n = 6 | 224 ± 4 | 257 ± 5‡ | 166 ± 4 | 177 ± 5 | 58 ± 2 | 80 ± 7‡ |
| STZ, n = 16 | 222 ± 2* | 245 ± 5†‡ | 160 ± 5* | 172 ± 5‡ | 62 ± 3 | 73 ± 3†‡ |
| STZ+LPA, n = 6 | 212 ± 4* | 246 ± 5†‡ | 161 ± 3* | 177 ± 5‡ | 52 ± 4 | 70 ± 4†‡ |
STZ, streptozotocin.
P < 0.05 from dark wt.
P < 0.05 from light wt.
P < 0.05 from paired dark/light comparison.
Diabetes was induced in mice at approximately 2 months of age by streptozotocin (60 mg/kg; 10 mM citrate buffer [pH 4.5]) injection IP once a day for 5 consecutive days; mice were maintained diabetic for 2 months. Body weight and blood glucose levels were monitored twice weekly. Insulin (neutral protamine Hagedorn) was administered to mice as needed, based on body weight and blood glucose levels but not more than twice weekly, to allow slow weight gain while maintaining hyperglycemia (blood glucose levels higher than 400 mg/dL). Mice that lost weight and/or had blood glucose levels greater than 600 mg/dL were given up to 0.2 units of insulin (Humulin N, Eli Lilly and Company, Indianapolis, IN, USA). Normal rodent chow (Purina TestDiet 5001; Richmond, IN, USA), which contains 11.2% fat, 26% protein, and 62.7% carbohydrate, and water were provided ad libitum. Glycated hemoglobin was measured from blood collected after each MRI experiment (Glyco-Tek affinity columns, kit 5351; Helena Laboratories, Beaumont, TX, USA). Blood was drawn from the left ventricle, after puncture, into a capillary tube and stored in an Eppendorf tube with a small amount of heparin to prevent coagulation. The blood was kept in the refrigerator until analysis within one week following the MRI experiment.
Light Exposure Protocol
In our initial description of light-dependent ADC changes, we alternated between immediately collecting light and then dark data at each of the different diffusion gradient strengths.19 In this study, we simplified the light exposure protocol by first collecting a full diffusion data set in dark-adapted mice and then another set 15 minutes after turning on a light inside the bore of the magnet. In a subset of mice, we immediately collected a full diffusion data set after turning on the light in addition to collecting the data set after 15 minutes in the light.
MRI Procedures
The general mouse preparation for high-resolution MRI is well established in our laboratory.5 All animals were maintained in darkness for at least 16 hours before and during the dark phase of the MRI examination. In some mouse groups, MnCl2 was administered, under dim red light or in darkness, as an intraperitoneal injection (66 mg MnCl2·4H2O/kg) on the right side of awake mice.3,41 After this injection, mice were maintained in the dark for another 3.5 to 4 hours. High-resolution anatomic and ADC data were acquired on a 7 T system (Bruker ClinScan; Billerica, MA, USA) using a receive-only surface coil (1.0 cm diameter) centered on the left eye. The end of a fiber optic bundle was attached to a light source (Mark II Light Source; Prescott's, Inc., Monument, CO, USA) placed caudal to the eye, projecting at a white screen ~1 cm from the eye, similar to that previously described.19 We exposed the eye to 0 (i.e., dark) or ~500 lux (confirmed outside the magnet using a Traceable Dual-Range Light Meter [Control Company, Friendswood, TX, USA]) placed against a 1-cm-diameter aperture; measured this way, room lighting is ~300 lux). Aside from the fiber optic light source, all lights in the MRI room were turned off. In all groups, immediately before the MRI experiment, animals were anesthetized with urethane (36% solution intraperitoneally; 0.083 mL/20 g animal weight, prepared fresh daily; Sigma-Aldrich Corp., St. Louis, MO, USA) and treated topically with 1% atropine to ensure dilation of the iris during light exposure, followed by 2% lidocaine to reduce eye motion. Anatomic and ADC (parallel to the optic nerve, the most sensitive direction for detecting changes at the location of the SRS19) MRI data sets were collected, first in the dark and then again 15 minutes after turning on the light; since each ADC data set takes 10 minutes to collect, we refer to the midpoint in the ADC collection as 20 minutes of light exposure. The subset of nondiabetic wt mice in which an ADC data set was collected immediately after turning on the light was called the 5 minutes of light exposure time point group. Anatomic images were acquired using a spin echo sequence (slice thickness 600 μm, TR 1000 ms, TE 11 ms, matrix size 192 × 320, field of view 8 × 8 mm2, NA 2, axial resolution for central retina 25 μm); images sensitized to water diffusion were collected (TR 1000 ms, slice thickness 600 μm, TE 33 ms, matrix size 174 × 288, field of view 8 × 8 mm2, axial resolution for central retina 27.8 μm; b = 0, 100, 250, 500, 600, 750, 990 s/mm2 [collected in pseudorandom order, NA 1 per b value]), registered to the anatomic image, and analyzed (using in-house code) to generate ADC profiles from the central retina. The present resolution in the central retina is sufficient for extracting meaningful layer-specific anatomic and functional data, as previously discussed.42 For example, given the present whole-retinal thicknesses of ~238 μm (the average thickness across controls in the Table) and the pixel size (~26 μm [mean of 25 and 27.8 μm]), each pixel axially spanned approximately 11% thickness or ~9 μm. We also note that the uncertainty in a pixel's thickness can be estimated to be ~½ pixel thick. The data support this estimate because converting all of the SEM back to SD from the Table and averaging gives a variance of ~13.5 μm, which is in reasonable agreement with the ½ pixel values of 12.5 to 13.9 μm (from anatomic to ADC images). The data also support our ability to distinguish changes in central retinal thickness on the anatomic images and significant ADC changes at 88% to 100% depth. In all cases, anesthetized animals were humanely euthanized by cervical dislocation followed by bilateral pneumothorax for assurance of death per our DLAR-approved protocol.
Data Analysis
In each animal, we confirmed ocular dilation based on the iris position on the anatomic MRI data; if eyelid position was closed to a degree likely to impeded the light path, only the dark data from that animal were used.42 All images for each animal per lighting condition were registered (rigid body) to the anatomic image. We had previously demonstrated that a simplified estimate of ADC, as the slope describing progressive losses in (log-transformed) signal intensity at progressively higher diffusion weightings (b values), was sufficient for observing dark/light changes in the SRS ADC.42 Thus, in this study we calculated ADC as previously described.42 In all cases, the same central retinal regions of interest (±0.4–1 mm from the optic nerve head) were analyzed; thickness and ADC values from the superior and inferior retina were respectively averaged.
In each mouse, thicknesses (μm) from the anatomic and ADC images were objectively determined using the “half-height method” wherein a border is determined via a computer algorithm based on the crossing point at the midpoint between the local minimum and maximum, as detailed elsewhere.42,43 The distance between two neighboring crossing points thus represents an objectively defined thickness.
From the anatomic image, thickness were normalized with 0% depth at the presumptive vitreoretinal border and 100% depth at the presumptive retina/choroid border. Note that because of partial volume averaging there are slight contributions from nonretinal tissue anteriorly at the vitreoretinal border and posteriorly at the retina/choroid border. The latter contribution was previously demonstrated with gadolinium-based measurements.44 We typically exclude the anterior (0%–8% depth) and posterior (88%–100% depth) regions on the basis of this partial volume averaging argument.42,45 However, the SRS exists at the 88% to 100% depth. We reason that light-evoked changes in the SRS ADC will be largely isolated from (i.e., not contaminated by) ADC and thickness changes in the choroid because the fast-flowing blood in the choroid will be suppressed to a greater degree by the diffusion gradients than in the SRS. In support of this assumption we note that GNAT1−/− mice clearly show a lack of light-dependent changes in SRS ADC but normal choroidal expansion between dark and light (see Results); a similar disconnect between SRS and choroid was noted in an initial study using a diffusion gradient in the direction perpendicular to the optic nerve (data not shown), similar to that previously reported.19
Choroidal thickness in each mouse was estimated as follows. We take advantage of the fact that ADC images show a suppressed signal from the vertical vessels between the horizontal segments of the choroid and the choroidal capillaries (i.e., the direction parallel to the optic nerve) and the vitreous (which has higher ADC than retina due to the lack of cellular barriers46); such diffusion-induced suppression of the choroid is evident in the images in Figure 1. We then used the same computer algorithm as above to objectively determine the thickness from the portion of retina that was not suppressed in the averaged (to improve signal-to-noise) b100 to b990 diffusion images (Fig. 1); herein this is referred to as the ADC thickness. The thickness difference between that generated from the anatomic image (with, e.g., a choroid contribution) and that generated from the ADC image (with suppressed choroidal contribution) was considered an estimate of the choroid thickness for each mouse (Fig. 1). In this manner, choroidal thicknesses were estimated for dark- and light-exposed conditions for each mouse; the accuracy of this choroidal thickness estimate appears to be reasonable (see Discussion).
In our earlier studies in mice, longer (on the order of hours) dark and light adaptation time did not produce significant differences in the retinal thickness from anatomic images.41,47,48 However, in this study, the dark-to-light transition clearly produced a significant increase in anatomic image-derived retinal thickness (Fig. 2; Table). It was clear that this light-evoked thickening was largely due to increases in choroidal thickness (Table).20,21,23 Thus, to allow for comparisons between groups and conditions, the ADC profiles in dark and light in each mouse were spatially normalized to the anatomic thickness value in the dark (since our data indicated that the “extra” thickness in the light was primarily via expansion of the choroid in the light).
Figure 2.
Summary of objectively determined choroidal thickness (see Fig. 1 and Methods for more details) measured from (A) dark and (B) light conditions for wt control mice (“C”), GNAT1−/−, untreated diabetic (“D”), and LPA-treated diabetic (D+LPA) mice. Red *, significant difference (P < 0.05) from light wt controls. Error bars: SEM.
Evaluations of choroid and SRS were done objectively, based strictly on dark-adapted anatomic thickness values (as described above), and thus data were not masked.
Statistical Analysis
All thicknesses in each group were evaluated for a normal distribution and were compared using a one-way ANOVA test. Due to insufficiently opened eyelids, we could not always collect both dark and light data from all animals. Also, the dark baseline ADC data were averaged from all wt groups regardless of subsequent light exposure. Choroid and SRS layer ADC data from untreated and vehicle-treated diabetic groups were not different, so data sets were respectively combined. Comparison of ADC profile data between groups was first performed using a one-tailed unpaired t-test at different locations of the intraretinal profiles to objectively identify regions of interest. Then, a generalized estimating equation (GEE) approach was used to compare selected location ranges, identified from the t-tests as significant.47,49 The GEE method is a more powerful two-tailed method that performs a general linear regression analysis using contiguous locations in each subject and accounts for the within-subject correlation between contiguous locations. When the initial t-test identified a location range as likely significant (P ≤ 0.05, i.e., P less than or equal to 0.05) at the one-tailed level, GEE was performed on the data in that range; regions marked as statistically significant had P ≤ 0.05 on GEE. Data are presented as mean ± standard error of the mean (SEM).
Results
Group Summary
Control mouse body weights were 28.8 ± 0.4 g (n = 18, ages 3–6 months, mean ± SEM); in the age-matched controls for the diabetic group, the glycated hemoglobin levels were 5.6 ± 0.2% (n = 9). In manganese-treated control mice, body weights were 26.7 ± 0.4 g (n = 6); glycated hemoglobin levels were not evaluated. GNAT1−/− mice had body weights of 21.2 ± 0.7 g (n = 6); glycated hemoglobin levels were not evaluated. Two-month diabetic mice had body weights of 24.0 ± 0.7 g (n = 16) and glycated hemoglobin levels of 11.3 ± 0.3%. Three month diabetic mice acutely treated with LPA had body weights of 23.5 ± 1 g (n = 6) and glycated hemoglobin levels of 11.8 ± 0.3%. Body weight in nondiabetic mice given manganese was not different (P > 0.05) than in wt mice, but was lower in GNAT1−/− and both diabetic mouse groups (P < 0.05). As expected, control mice had lower (P < 0.05) glycated hemoglobin levels than both groups of diabetic mice; LPA treatment did not affect glycated hemoglobin levels between diabetic groups (P > 0.05).
Thickness
Reproducibility.
Thickness values within different dark-adapted nondiabetic wt mouse groups not given manganese were compared, as this afforded the largest number of comparisons; dark adaptation was a common condition to all wt groups regardless of subsequent light exposure protocol (Table; Fig. 1).
Choroid.
Choroidal thickness values in the dark were not different between any of the groups studied (Table). All groups demonstrated significantly increased whole-retina thickness over dark values after 20 minutes of light (Table) that was largely due to expansion of the choroid (Fig. 2; Table): wt (38%), GNAT1−/− (+38%), diabetic (+17%), diabetic+LPA (+35%). Smaller but still significant increases in choroidal thickness were noted after 5 minutes of light (data not shown). Notably, in the light but not in the dark, both untreated and LPA-treated diabetic groups had smaller (P < 0.05) choroidal thickness than wt mice (Fig. 2; Table).
Retina.
As summarized in the Table, in the dark, ADC thicknesses in diabetic mice were smaller (P < 0.05, one-way ANOVA) than in nondiabetic wt and GNAT1−/− mice. Apparent diffusion coefficient thickness appeared to increase with 20 minutes of light exposure in the wt (+6%), GNAT1−/− (+7%), diabetic (+8%), and diabetic+LPA (+10%) groups. However, these retinal changes are roughly an order of magnitude smaller than for the choroid (see above); in the absence of a physiologic explanation for retinal expansion, these changes likely represent some residual choroidal contribution using the present MRI resolution.
Effect of Systemic Manganese.
Wild-type mice given manganese had a significant light-evoked expansion of anatomic thickness but not in ADC or choroidal thicknesses (Table); the reason for this is not yet clear.
Apparent Diffusion Coefficient
Reproducibility.
All untreated dark-adapted wt mouse groups exhibited similar topography across the retina (i.e., similar magnitudes of spatial features of the profile, Fig. 1) and notable reproducibility (P > 0.05) at the SRS layer (88%–100% depth), the region tested for our working hypothesis. All dark-adapted profiles were combined for further comparisons of ADC changes at the SRS layer.
Time Course.
Next, ADC profiles across the retina were compared at 5 and 20 minutes of light exposure (Fig. 3). At both time points, light evoked a significant (P < 0.05) increase in ADC at the SRS layer from the all-groups mean dark profile. However, the light-dependent change in ADC values at 88% to 100% depth was lower (P < 0.05) at 5 minutes of light relative to that at 20 minutes.
Figure 3.
Summary of central retinal ADC as a function of retinal depth during dark (closed symbols) and either (A) 5 minutes or (B) 20 minutes of ~500 lux light (open symbols) in untreated nondiabetic wt mice. Data are presented using the conventions in Figure 1D. Red line: region with significant differences (P < 0.05) between profiles.
Effect of Intracellular Manganese.
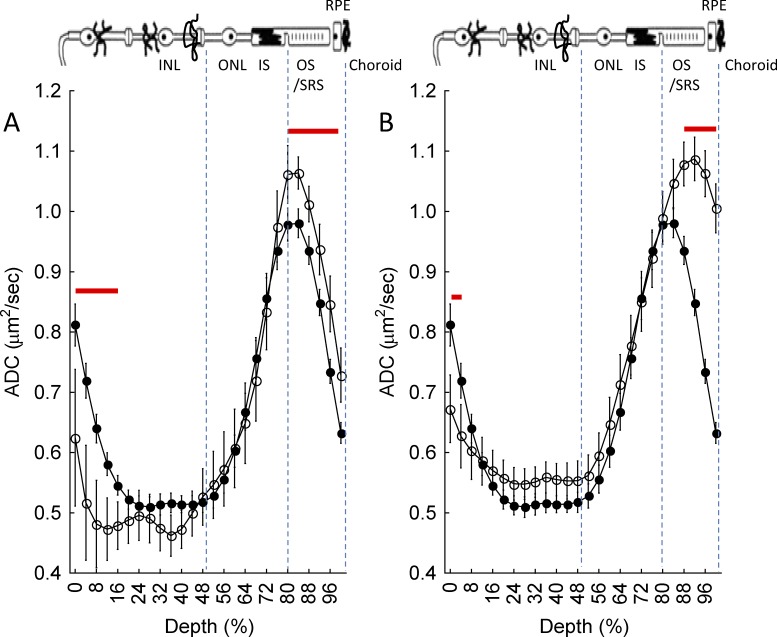
Apparent diffusion coefficient MRI has the potential to evaluate aspects of rod function complementary to that provided by manganese-enhanced MRI (MEMRI), the imaging modality of choice to study retinal calcium channels.50,51 Combining these two methods is expected to provide useful complementary information if the presence of manganese does not somehow alter the ADC MRI measurement at the SRS layer. We thus tested, and confirmed, this possibility. Mn2+-treated wt mice (Fig. 4) were found to have a large light-stimulated change in the ADC at 88% to 100% depth, which was not different (P > 0.05) from that in the untreated mice (Fig. 2).
Figure 4.
Summary of central retinal ADC as a function of retinal depth during dark (closed symbols) and 20 minutes of ~500 lux light (open symbols) in (A) Mn2+-treated wt mice or (B) GNAT1−/− mice. Data are presented using the conventions in Figure 1D. Red line: region with significant differences (P ≤ 0.05) between profiles.
Mice Lacking Rod Phototransduction.
Light-dependent changes in rod extracellular ion content increase the SRS volume compared to that in dark.24–27 Based on this, we hypothesized that in mice null for phototransduction, the ADC at the SRS layer would not change between light and dark. We confirmed this prediction in GNAT1−/− mice (Fig. 4). Consistent with a lack of phototransduction, the ADC values at 88% to 100% depth in GNAT1−/− mice were not different (P > 0.05) from those in dark-adapted wt mice. We did find a significant (P < 0.05) increase in water mobility anterior to the SRS (i.e., 44%–48% depth) in dark- versus light-exposed mice; the interpretation of this change is currently unclear. Nonetheless, these data support the predicted contribution of rod phototransduction to light-stimulated ADC increases at the SRS layer.
Diabetes.
Unlike wt controls, untreated or saline-treated diabetic mice had no significant (P > 0.05) change in ADC at 88% to 100% depth between dark and light conditions (Fig. 5). A significant (P < 0.05) increase in ADC with light was observed at 60% to 68% depth in untreated diabetic mice; the mechanism underlying this change requires additional investigation. The lack of a light-stimulated change in the ADC at 88% to 100% depth likely arises from diabetes-induced changes in rod ion content.3,4,28–31 Antioxidant treatment can correct altered ion regulation in the photoreceptor layer of diabetic rodents.2–4 Consistent with these previous findings,4,41,44–48 systemic treatment with LPA partly corrected light-stimulated increases in ADC at the SRS layer (Fig. 5).4,35–38,52,53 We also note that regions anterior to the SRS layer also demonstrated differences between nondiabetic and diabetic mice, although more work is needed to identify the responsible mechanisms.
Figure 5.
Summary of central retinal ADC as a function of retinal depth during dark (closed symbols) and 20 minutes of ~500 lux light (open symbols) in (A) wt control mice (from Fig. 3B), (B) untreated diabetic mice, (C) saline-treated diabetic mice, and (D) LPA-treated diabetic mice. Data are presented using the conventions in Figure 1D. Red line: region with significant differences (P < 0.05) between profiles.
Discussion
Retinal and Choroid Thickness
Previously, diffusion-weighted MRI was found to be useful for measuring choroid thickness in rats.46 The present data in wt mice further support the precision and accuracy of MRI choroidal thickness measurement.19,44,46 For example, our estimated central retinal choroidal thickness from wt C57BL/6 mice (range, 62 [dark]–86 [light] μm, Table) compares reasonably with published values obtained using other methods from the same mouse strain (52–78 μm46,54,55). In this study, as previously reported, retinal thickness from GNAT1−/− mice was not significantly different from that in control mice.34,41 Here, we note that choroidal thickness is also not different between GNAT1−/− and control mice (Table; Fig. 3).
In diabetic mice, the present data support our previous report of retinal thinning in diabetic mice (Table).3 On the other hand, unlike previous reports showing that the choroid thins in diabetes,7,12–14 we find no evidence that diabetes causes choroidal thinning per se since dark-adapted values are normal (Table). Somewhat surprisingly, our data suggested instead that in early diabetes the extent of light-evoked choroidal expansion was subnormal. These results do not rule out the possibility that choroidal thinning might occur at longer durations of diabetes.7–10,12–14,16
In nondiabetic subjects, the mechanism underlying light-stimulated choroidal thickness expansion remains somewhat unclear, although there is a clear brain-based component.20,21,23 In support of this interpretation, nondiabetic mice with nonfunctioning rods had a normal extent of choroidal thickness increase with light (Table; Fig. 2). One possible mechanism by which light may centrally regulate choroidal thickness is via melanopsin-containing ganglion cells that project back to the same brain structures (e.g., the paraventricular hypothalamus) from which the choroidal innervation arises; more work is needed to test this hypothesis.56,57
In 2-month diabetic mice, an impaired light-stimulated choroidal thickness expansion was observed. The underlying mechanism is not clear, in part because the endothelial cell, nerve, and neuron contributions to the choroidal response to light in nondiabetic mice are not yet known. Nonetheless, we note that oxidative stress plays a pathogenic role in DR, so it seemed reasonable to examine its role in choroid dysfunction. The impaired light-stimulated expansion of the choroid was not corrected by LPA, suggesting that the underlying mechanism does not involve diabetes-induced oxidative stress, although these results do not rule out the possibility that more long-term application of antioxidants might be necessary for the choroid.
Retinal ADC Topography
Apparent diffusion coefficient MRI was found to be reasonably precise as a likely result of the “vertical” retina microarchitecture being reliably layered. For example, in dark-adapted wt mice, there was a notable diffusion barrier (i.e., greater ADC values) at the inner limiting membrane (between largely acellular vitreous and structured retina) and at ~80% depth into the retina (presumed location of the outer limiting membrane) (Fig. 1).26 Based on this consideration, it appears that the SRS layer maintains a constant length in dark and light because the distance between the vitreal/retinal border and peak of the presumptive outer limiting membrane remains at ~80% depth (Figs. 3, 4).26 It would seem that the SRS increases its volume in light by increasing its width, probably in concert with adaptations to RPE processes and photoreceptor cells to accommodate this change.26
SRS Layer
Based on experiments in rats, we previously suggested that light-dependent expansion of the SRS layer can be monitored via the light-evoked increase in ADC.19 Here, we found an increase in ADC at the SRS layer with light in wt mice and demonstrate that this effect is regulated at least in part by phototransduction. These data strongly support the use of ADC MRI to monitor light-stimulated expansion of the SRS layer.
In 2-month diabetic mice, we did not find evidence for light-evoked expansion of the SRS layer during the dark-to-light transition based on our ADC measurement. The mechanism underlying normal SRS volume expansion depends on light-dependent changes in extracellular ion content, including hydrogen ions, since acidification dramatically decreases the SRS volume via increased fluid absorption by the retinal pigment epithelium.24–27,58 Intriguingly, diabetes-induced acidification has been observed in acutely hyperglycemic nondiabetic rats and in diabetic rats (Dmitriev AV, et al. IOVS 2014;55:ARVO E-Abstract 1049; Henderson DI, et al., IOVS 2012;53:ARVO E-Abstract 5379). More work is needed to understand the role of different ions in nondiabetic mice. Nonetheless, the impaired light-stimulated ADC increase at the SRS layer was significantly restored by LPA, suggesting involvement of an oxidative stress mechanism.
In conclusion, ADC MRI, even after systemic injection of manganese, appears sensitive to light-evoked expansion of choroidal thickness and SRS layer. In diabetes, no evidence was found for choroidal thinning, and diabetes-induced oxidative stress appeared to impair only light-stimulated expansion in the SRS layer and not the light-dependent choroidal expansion. Future studies will investigate the possibility that additional information about photoreceptor function is achievable from diffusion MRI by including much higher b values, fitting the higher b value data to a biexponential function, and examining the full diffusion tensor. Potential applications of ADC MRI include (1) combined ADC MRI and MEMRI study of experimental retinopathy models; (2) longitudinal studies of age-related retinal degeneration models where impaired rod function and/or abnormal water handling of the retina is considered to be a critical sight-threatening problem but cannot be investigated using existing electrode assays with an extracellular marker59,60; and (3) translational clinical studies.
Acknowledgments
We thank David Bissig for his careful reading of this manuscript and Zhuo-Hua Pan for the use of a light meter.
Supported by the National Institutes of Health Animal Models of Diabetic Complications Consortium and Mouse Metabolic Phenotyping Centers Pilot and Feasibility Programs (BAB), the National Eye Institute (R21 EY021619, BAB), and an unrestricted grant from Research to Prevent Blindness (Kresge Eye Institute).
Disclosure: B.A. Berkowitz, None; E.M. Grady, None; N. Khetarpal, None; A. Patel, None; R. Roberts, None
References
- 1. Kowluru RA, Kern TS, Engerman RL. Abnormalities of retinal metabolism in diabetes or experimental galactosemia. IV. Antioxidant defense system. Free Radic Biol Med. 1997; 22: 587–592. [DOI] [PubMed] [Google Scholar]
- 2. Kowluru RA, Kern TS, Engerman RL, Armstrong D. Abnormalities of retinal metabolism in diabetes or experimental galactosemia. III. Effects of antioxidants. Diabetes. 1996; 45: 1233–1237. [DOI] [PubMed] [Google Scholar]
- 3. Berkowitz BA, Gradianu M, Bissig D, Kern TS, Roberts R. Retinal ion regulation in a mouse model of diabetic retinopathy: natural history and the effect of Cu/Zn superoxide dismutase overexpression. Invest Ophthalmol Vis Sci. 2009; 50: 2351–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berkowitz BA, Roberts R, Stemmler A, Luan H, Gradianu M. Impaired apparent ion demand in experimental diabetic retinopathy: correction by lipoic acid. Invest Ophthalmol Vis Sci. 2007; 48: 4753–4758. [DOI] [PubMed] [Google Scholar]
- 5. Berkowitz BA, Bissig D, Patel P, Bhatia A, Roberts R. Acute systemic 11-cis-retinal intervention improves abnormal outer retinal ion channel closure in diabetic mice. Mol Vis. 2012; 18: 372–376. [PMC free article] [PubMed] [Google Scholar]
- 6. Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci U S A. 2013; 110: 16586–16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cao J, McLeod S, Merges CA, Lutty GA. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol. 1998; 116: 589–597. [DOI] [PubMed] [Google Scholar]
- 8. Fryczkowski AW, Hodes BL, Walker J. Diabetic choroidal and iris vasculature scanning electron microscopy findings. Int Ophthalmol. 1989; 13: 269–279. [DOI] [PubMed] [Google Scholar]
- 9. Hidayat AA, Fine BS. Diabetic choroidopathy. Light and electron microscopic observations of seven cases. Ophthalmology. 1985; 92: 512–522. [PubMed] [Google Scholar]
- 10. McLeod DS, Lefer DJ, Merges C, Lutty GA. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol. 1995; 147: 642–653. [PMC free article] [PubMed] [Google Scholar]
- 11. Muir ER, Rentería RC, Duong TQ. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Invest Ophthalmol Vis Sci. 2012; 53: 6488–6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esmaeelpour M, Brunner S, Ansari-Shahrezaei S, et al. Choroidal thinning in diabetes type 1 detected by 3-dimensional 1060 nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 6803–6809. [DOI] [PubMed] [Google Scholar]
- 13. Schocket LS, Brucker AJ, Niknam RM, Grunwald JE, DuPont J, Brucker AJ. Foveolar choroidal hemodynamics in proliferative diabetic retinopathy. Int Ophthalmol. 2004; 25: 89–94. [DOI] [PubMed] [Google Scholar]
- 14. Regatieri CV, Branchini L, Carmody J, Fujimoto JG, Duker JS. Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina. 2012; 32: 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Langham ME, Grebe R, Hopkins S, Marcus S, Sebag M. Choroidal blood flow in diabetic retinopathy. Exp Eye Res. 1991; 52: 167–173. [DOI] [PubMed] [Google Scholar]
- 16. Savage HI, Hendrix JW, Peterson DC, Young H, Wilkinson CP. Differences in pulsatile ocular blood flow among three classifications of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004; 45: 4504–4509. [DOI] [PubMed] [Google Scholar]
- 17. Pemp B, Schmetterer L. Ocular blood flow in diabetes and age-related macular degeneration. Can J Ophthalmol. 2008; 43: 295–301. [DOI] [PubMed] [Google Scholar]
- 18. Braun RD, Wienczewski CA, Abbas A. Erythrocyte flow in choriocapillaris of normal and diabetic rats. Microvasc Res. 2009; 77: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bissig D, Berkowitz BA. Light-dependent changes in outer retinal water diffusion in rats in vivo. Mol Vis. 2012; 18: 2561–xxx. [PMC free article] [PubMed] [Google Scholar]
- 20. Fitzgerald ME, Gamlin PD, Zagvazdin Y, Reiner A. Central neural circuits for the light-mediated reflexive control of choroidal blood flow in the pigeon eye: a laser Doppler study. Vis Neurosci. 1996; 13: 655–669. [DOI] [PubMed] [Google Scholar]
- 21. Longo A, Geiser M, Riva CE. Subfoveal choroidal blood flow in response to light-dark exposure. Invest Ophthalmol Vis Sci. 2000; 41: 2678–2683. [PubMed] [Google Scholar]
- 22. Fuchsjäger-Mayrl G, Malec M, Amoako-Mensah T, Kolodjaschna J, Schmetterer L. Changes in choroidal blood flow during light/dark transitions are not altered by atropine or propranolol in healthy subjects. Vision Res. 2003; 43: 2185–2190. [DOI] [PubMed] [Google Scholar]
- 23. Fuchsjäger-Mayrl G, Polska E, Malec M, Schmetterer L. Unilateral light-dark transitions affect choroidal blood flow in both eyes. Vision Res. 2001; 41: 2919–2924. [DOI] [PubMed] [Google Scholar]
- 24. Huang B, Karwoski CJ. Light-evoked expansion of subretinal space volume in the retina of the frog. J Neurosci. 1992; 12: 4243–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li JD, Govardovskii VI, Steinberg RH. Light-dependent hydration of the space surrounding photoreceptors in the cat retina. Vis Neurosci. 1994; 11: 743–752. [DOI] [PubMed] [Google Scholar]
- 26. Govardovskii VI, Li JD, Dmitriev AV, Steinberg RH. Mathematical model of TMA+ diffusion and prediction of light-dependent subretinal hydration in chick retina. Invest Ophthalmol Vis Sci. 1994; 35: 2712–2724. [PubMed] [Google Scholar]
- 27. Adijanto J, Banzon T, Jalickee S, Wang NS, Miller SS. CO2-induced ion and fluid transport in human retinal pigment epithelium. J Gen Physiol. 2009; 133: 603–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. MacGregor LC, Matschinsky FM. Altered retinal metabolism in diabetes. II. Measurement of sodium-potassium ATPase and total sodium and potassium in individual retinal layers. J Biol Chem. 1986; 261: 4052–4058. [PubMed] [Google Scholar]
- 29. Kern TS, Kowluru RA, Engerman RL. Abnormalities of retinal metabolism in diabetes or galactosemia: ATPases and glutathione. Invest Ophthalmol Vis Sci. 1994; 35: 2962–2967. [PubMed] [Google Scholar]
- 30. Ottlecz A, Garcia CA, Eichberg J, Fox DA. Alterations in retinal Na+, K(+)-ATPase in diabetes: streptozotocin-induced and Zucker diabetic fatty rats. Curr Eye Res. 1993; 12: 1111–1121. [DOI] [PubMed] [Google Scholar]
- 31. Szabo ME, Droy-Lefaix MT, Doly M, Carre C, Braquet P. Ischemia and reperfusion-induced histologic changes in the rat retina. Demonstration of a free radical-mediated mechanism. Invest Ophthalmol Vis Sci. 1991; 32: 1471–1478. [PubMed] [Google Scholar]
- 32. Burns ME, Mendez A, Chen CK, et al. Deactivation of phosphorylated and nonphosphorylated rhodopsin by arrestin splice variants. J Neurosci. 2006; 26: 1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen J, Simon MI, Matthes MT, Yasumura D, LaVail MM. Increased susceptibility to light damage in an arrestin knockout mouse model of Oguchi disease (stationary night blindness). Invest Ophthalmol Vis Sci. 1999; 40: 2978–2982. [PubMed] [Google Scholar]
- 34. Calvert PD, Krasnoperova NV, Lyubarsky AL, et al. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha-subunit. Proc Natl Acad Sci U S A. 2000; 97: 13913–13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnsen-Soriano S, Garcia-Pous M, Arnal E, et al. Early lipoic acid intake protects retina of diabetic mice. Free Radic Res. 2008; 42: 613–617. [DOI] [PubMed] [Google Scholar]
- 36. Lin J, Bierhaus A, Bugert P, et al. Effect of R-(+)-alpha-lipoic acid on experimental diabetic retinopathy. Diabetologia. 2006; 49: 1089–1096. [DOI] [PubMed] [Google Scholar]
- 37. Roberts R, Luan H, Berkowitz BA. Alpha-lipoic acid corrects late-phase supernormal retinal oxygenation response in experimental diabetic retinopathy. Invest Ophthalmol Vis Sci. 2006; 47: 4077–4082. [DOI] [PubMed] [Google Scholar]
- 38. Kowluru RA, Odenbach S. Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004; 53: 3233–3238. [DOI] [PubMed] [Google Scholar]
- 39. Packer L. Antioxidant properties of lipoic acid and its therapeutic effects in prevention of diabetes complications and cataracts. Ann N Y Acad Sci. 1994; 738: 257–264. [DOI] [PubMed] [Google Scholar]
- 40. Senoglu M, Nacitarhan V, Kurutas EB, et al. Intraperitoneal alpha-lipoic acid to prevent neural damage after crush injury to the rat sciatic nerve. J Brachial Plex Peripher Nerve Inj. 2009; 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berkowitz BA, Grady EM, Roberts R. Confirming a prediction of the calcium hypothesis of photoreceptor aging in mice. Neurobiol Aging. 2014; 35: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 42. Bissig D, Berkowitz BA. Same-session functional assessment of rat retina and brain with manganese-enhanced MRI. NeuroImage. 2011; 58: 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheng H, Nair G, Walker TA, et al. Structural and functional MRI reveals multiple retinal layers. Proc Natl Acad Sci U S A. 2006; 103: 17525–17530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berkowitz BA, Roberts R, Luan H, et al. Manganese-enhanced MRI studies of alterations of intraretinal ion demand in models of ocular injury. Invest Ophthalmol Vis Sci. 2007; 48: 3796–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Berkowitz BA, Bissig D, Ye Y, Valsadia P, Kern TS, Roberts R. Evidence for diffuse central retinal edema in vivo in diabetic male Sprague Dawley rats. PLoS One. 2012; 7: e29619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen J, Wang Q, Zhang H, et al. In vivo quantification of T1, T2, and apparent diffusion coefficient in the mouse retina at 11.74T. Magn Reson Med. 2008; 59: 731–738. [DOI] [PubMed] [Google Scholar]
- 47. Berkowitz BA, Roberts R, Goebel DJ, Luan H. Noninvasive and simultaneous imaging of layer-specific retinal functional adaptation by manganese-enhanced MRI. Invest Ophthalmol Vis Sci. 2006; 47: 2668–2674. [DOI] [PubMed] [Google Scholar]
- 48. Berkowitz BA, Roberts R, Bissig D. Light-dependant intraretinal ion regulation by melanopsin in young awake and free moving mice evaluated with manganese-enhanced MRI. Mol Vis. 2010; 16: 1776–1780. [PMC free article] [PubMed] [Google Scholar]
- 49. Liang Z. Longitudinal data analysis using generalized linear models. Biometrika. 1986; 73: 13–22. [Google Scholar]
- 50. Ramos de Carvalho JE, Verbraak FD, Aalders MC, van Noorden CJ, Schlingemann RO. Recent advances in ophthalmic molecular imaging. Surv Ophthalmol. 2014; 59: 393–413. [DOI] [PubMed] [Google Scholar]
- 51. Berkowitz BA, Bissig D, Dutczak O, Corbett S, North R, Roberts R. MRI biomarkers for evaluation of treatment efficacy in preclinical diabetic retinopathy. Expert Opin Med Diagn. 2013; 7: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sadi G, Yilmaz O, Guray T. Effect of vitamin C and lipoic acid on streptozotocin-induced diabetes gene expression: mRNA and protein expressions of Cu-Zn SOD and catalase. Mol Cell Biochem. 2008; 309: 109–116. [DOI] [PubMed] [Google Scholar]
- 53. Obrosova IG, Fathallah L, Greene DA. Early changes in lipid peroxidation and antioxidative defense in diabetic rat retina: effect of DL-alpha-lipoic acid. Eur J Pharmacol. 2000; 398: 139–146. [DOI] [PubMed] [Google Scholar]
- 54. Muir ER, Duong TQ. MRI of retinal and choroidal blood flow with laminar resolution. NMR Biomed. 2011; 24: 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barber AJ, Antonetti DA, Kern TS, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005; 46: 2210–2218. [DOI] [PubMed] [Google Scholar]
- 56. Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006; 497: 326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li C, Fitzgerald MEC, LeDoux MS, et al. Projections from the hypothalamic paraventricular nucleus and the nucleus of the solitary tract to prechoroidal neurons in the superior salivatory nucleus: pathways controlling rodent choroidal blood flow. Brain Res. 2010; 1358: 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wolfensberger TJ, Dmitriev AV, Govardovskii VI. Inhibition of membrane-bound carbonic anhydrase decreases subretinal pH and volume. Doc Ophthalmol. 1999; 97: 261–271. [DOI] [PubMed] [Google Scholar]
- 59. Moore DJ, Hussain AA, Marshall J. Age-related variation in the hydraulic conductivity of Bruch's membrane. Invest Ophthalmol Vis Sci. 1995; 36: 1290–1297. [PubMed] [Google Scholar]
- 60. Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996; 37: 1236–1249. [PubMed] [Google Scholar]
- 61. Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003; 121: 547–557. [DOI] [PubMed] [Google Scholar]