Abstract
We encountered a case of hypercobalaminemia induced by oral intake of an energy drink after total gastrectomy. The patient was referred to our hospital due to findings suspicious for gastric cancer on screening. A 20 mm type 0-IIc lesion was detected in the gastric subcardia on esophagogastroduodenoscopy. Total gastrectomy followed by Roux-en-Y reconstruction was performed. He was discharged without complications. His basal serum vitamin B12 level was initially maintained with monthly intramuscular injections of vitamin B12. After 9 months, his serum vitamin B12 level suddenly increased up to 36-fold higher than the normal range and persisted there for one year without vitamin B12 injections. The patient ultimately reported consuming half a bottle of an energy drink each day during this time period. This case demonstrates the risk of unexpected hypervitaminemia resulting from self-administration of nutritional supplements.
Keywords: hypercobalaminemia, total gastrectomy, energy drink, supplement, transcobalamin
Introduction
In recent years, supplements and energy drinks have become very popular due to their low cost and wide availability. However, several reports have emerged warning of side effects and even death caused by excessive intake1,2,3).
Here we report a patient with hypercobalaminemia induced by regular intake of an energy drink after total gastrectomy. Vitamin B12 is a water soluble vitamin absorbed in conjunction with intrinsic factor (IF) that is produced by the gastric mucosa4). Excess vitamin B12 is excreted by the kidneys, which accounts for the lack of persistent hypercobalaminemia under normal conditions4). However, recently, there have been several reports of elevated serum vitamin B12 levels associated with multivitamin use in patients after total gastrectomy5, 6) and patients with pernicious anemia7). This case was interesting because extreme hypercobalaminemia was induced by oral intake after total gastrectomy and persisted in a healthy individual after gastrectomy.
Case Report
The patient was a 76-year-old man referred to our clinic with screening results suspicious for gastric cancer. His past medical history consisted of hypertension and benign prostatic hypertrophy. Physical examination and laboratory data were nonspecific except for asymptomatic macroamylasemia. Esophagogastroduodenoscopy showed a 20 mm type 0-IIc lesion in the gastric subcardia, preoperatively diagnosed as cT1, cN0, cM0, cP0, cStage IA. The patient underwent a planned total gastrectomy with D1 lymphadenectomy followed by Roux-en-Y reconstruction with no complications. The postoperative course was unremarkable, and the patient was discharged on postoperative day 10. Pathologic evaluation revealed an 8 mm moderately differentiated tubular adenocarcinoma on the anterior wall. Macroscopically, the tumor was flat and slightly depressed (type 0-IIc). Histologically, it showed an INFβ growth pattern, and the tumor invaded the submucosa (pT1 SM). There was no evidence of lymph node (pN0) or liver (pH0) metastasis or venous (v0) or lymphatic (ly0) invasion. The proximal and distal resection margins were clear. Peritoneal cytology was also negative (pP0, CY0). The final stage was pT1N0M0, Stage IA according to the Japanese classification of gastric carcinoma staging system8).
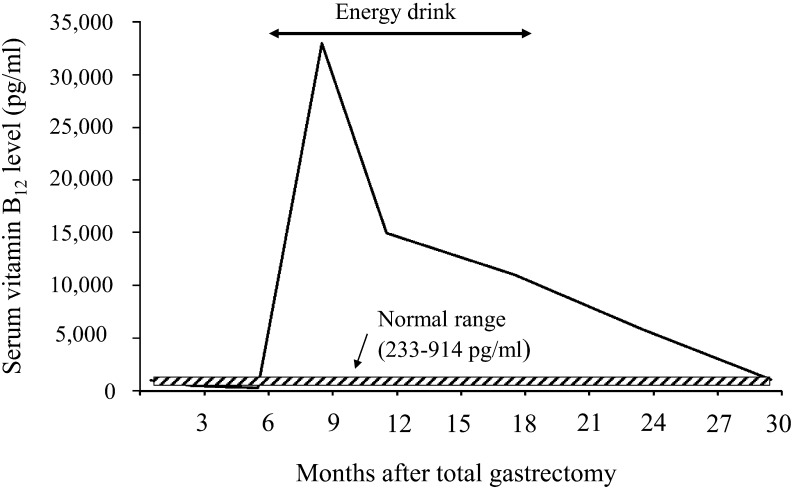
Except for occasional heartburn, a sense of abdominal distension, and elevated serum amylase levels, he had no obvious signs and symptoms during postoperative follow-up. He took nifedipine (Adalat CR®, Bayer Healthcare, Osaka, Japan), theophylline (Theodur®, Mitsubishi Tanabe Pharma, Tokyo, Japan), etizolam (Depas®, Mitsubishi Tanabe Pharma), bifidobacterium (Lac-B Granular Powder®, Kowa Company Ltd., Nagoya, Japan), magnesium hydrate (Yoshida Pharmaceutical, Tokyo, Japan) and eviprostat (Eviprostat®, Nippon Shinyaku, Kyoto, Japan). His basal serum vitamin B12 level was followed monthly and initially maintained within the normal range by intramuscular injections of mecobalamin (Methycobal®, Eisai, Tokyo, Japan). Nine months after surgery, his serum vitamin B12 levels suddenly increased to 33,000 pg/ml, which is approximately 36-fold higher than the normal range (233–914 pg/ml). This level of hypercobalaminemia persisted for approximately one year without mecobalamin injections. The patient’s diet did not contain any obvious excess sources of vitamin B12. Fortunately, he did not show any obvious symptoms or laboratory abnormalities (Table 1). He later reported that he had started drinking half a bottle of an energy drink as a supplement during this time. After he discontinued the energy drink, his serum vitamin B12 levels quickly declined to within the normal range (Figure 1). The ingredients on the energy drink label included caffeine, fructose, β-carotene, vitamins B2, B6 and C, octacosanol, garlic, licorice, ginseng, bracket fungus, Polygonatum falcatum and chlorella extract; there was no mention of vitamin B12. Concentration analysis revealed an undetectable amount of vitamin B12 in the energy drink.
Table 1. Laboratory data after total gastrectomy.
| <Complete blood count> | <Biochemistry> | <Viral markers> | |||||
| WBC | 8,100/µl | Sodium | 140 mEq/l | HBsAg | (–) | ||
| Neutrophils | 47.50% | Potassium | 3.8 mEq/l | HCVAb | (–) | ||
| Lymphocytes | 44.50% | Chloride | 104 mEq/l | HIV | (–) | ||
| Monocytes | 5.00% | Calcium | 9.4 mg/dl | ||||
| Eosinophils | 3.00% | BUN | 15.9 mg/dl | 24 hr Ccr | 72 ml/min | ||
| Basophils | 0.00% | Cre | 0.78 mg/dl | ||||
| Hb | 13.2 g/dl | TP | 6.2 g/dl | ||||
| PLT | 24 × 104/µl | Alb | 3.1 g/dl | ||||
| AST | 20 U/l | ||||||
| <Coagulation test> | ALT | 17 U/l | |||||
| BT | 1 min 30 sec | LDH | 203 U/l | ||||
| APTT | 22.5 sec | ChE | 219 U/l | ||||
| PT-INR | 1.02 | T-Bil | 0.6 mg/dl | ||||
| ALP | 317 U/l | ||||||
| γ-GTP | 18 U/l | ||||||
| AMY | 1205 U/l | ||||||
BT, bleeding time; Ccr, creatinine clearance.
Figure 1.
Serum vitamin B12 levels after total gastrectomy. After total gastrectomy, the basal serum vitamin B12 level was initially maintained within the normal range by intramuscular injections of mecobalamin (Methycobal®). Nine months after surgery, the serum vitamin B12 level suddenly increased to 33,000 pg/ml and remained persistently elevated for approximately one year without injections of mecobalamin. During that time, the patient consumed half a bottle of an energy drink each day as a supplement. After discontinuing the drink, the serum vitamin B12 concentration declined to within the normal range.
Discussion
Vitamin B12 is one of the vitamins not produced by the human body; therefore, it is essential to ingest it through food such as meat, fish and shellfish. Recently, it was clarified that there are two pathways for vitamin B12 absorption, i.e., a conventional IF-dependent pathway and an IF-independent pathway9). In the IF-dependent pathway, hydrochloric acid produced by the gastric mucosa cleaves vitamin B12, which is bound to protein in food. The released vitamin B12 attaches to R-protein and passes into the duodenum, where R-protein is removed and free vitamin B12 binds to IF4). The IF–vitamin B12 complex binds to a receptor located on the luminal membrane of the distal ileum. The IF-independent pathway involves passive diffusion in the ileum that is dependent on the vitamin B12 concentration4). In healthy individuals, the IF-independent pathway accounts for only 2–5% of vitamin B12 absorption10). Therefore, the IF-independent pathway is usually not sufficient to meet daily requirements. However, previous reports indicate that high oral intake can result in sufficient serum levels of vitamin B12 in patients who have a defect in the normal absorption pathway5,6,7, 11). Kim et al.6) reported that patients who received total gastrectomy were successfully maintained within the normal range by oral intake of 1500 μg of vitamin B12 daily. In our preliminary study, 15 patients who took 750 μg/day of oral multivitamins (Vitamedin®, Daiichi-Sankyo, Tokyo, Japan) for 6 months after total gastrectomy showed increases in serum vitamin B12 levels from 192 ± 26 pg/ml to 571 ± 215 pg/ml (p<0.001, unpublished data). However, the elevation in the serum vitamin B12 level by the IF independent pathway is usually at most to the upper limit of the normal range. In the present patient, before analysis of the energy drink, we attributed his extreme hypercobalaminemia to the high concentration of vitamin B12 in the energy drink absorbed through the IF-independent pathway. However, since the concentration of vitamin B12 was undetectable in the energy drink, it was unlikely that passive absorption accounted for this hypercobalaminemia.
In the serum, vitamin B12 is bound to proteins known as transcobalamins (TCs)4). Approximately 80% of serum vitamin B12 is transported on inactive TC I. The active transport protein for vitamin B12 is TC II, which carries the remaining 20% in the circulation4). Vitamin B12 in the circulation that exceeds the binding capacity of TCs is rapidly excreted in the urine, which explains why persistent hypercobalaminemia does not occur under normal conditions4). Persistent hypercobalaminemia can occur under pathological conditions such as renal failure, liver disease12), myeloproliferative disease13), infections14), and emergence of autoantibodies to TCs15). It has been hypothesized that inflammation-induced hepatocyte degradation in liver disease causes the release of vitamin B12 into the circulation12). Zittoun et al.13) reported that myeloblasts contain increased levels of TC II, which induces high serum TC II concentrations in patients with myeloproliferative disease. Cheeramakara et al.14) stated that the mononuclear phagocytic system stimulated by scrub typhus infection increased synthesis and release of TC II into the circulation, leading to a serum vitamin B12 concentration as high as 3,000 pg/ml. Carmel et al.15) described that circulating autoantibody to TC II resulted in retention of both TC II and vitamin B12 due to impaired clearance and that the serum vitamin B12 concentration increased up to 22,000 pg/ml. The authors further described that the autoantibody was induced by infections and that the serum vitamin B12 level fell with clinical improvement.
In this case, the patient did not have renal failure, liver disease or myeloproliferative disease. Although we were unable to examine the amount of TCs in this patient, there were two possibilities that could explain the hypercobalaminemia, i.e., stimulation of mononuclear phagocytic systems or production of antibody to TC II. Ginseng and garlic, constituents of this energy drink, were reported to activate macrophage functions through activation of transcription factors such as NF-kB, AP-1, ERK and JNK16, 17). It was possible that macrophages and monocytes were activated by some constituents in the energy drink, which increased synthesis and release of TC II into the circulation and induced hypercobalaminemia. On the other hand, despite no direct evidence that herbal extracts induce production of anti-TC II antibodies, some herbal extracts actually induce production of autoantibodies18, 19). Wada et al.18) described that ginseng induced production of myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA), which subsequently resulted in rapidly progressive MPO-ANCA-associated vasculitis. Yuce et al.19) reported that noni juice from morinda citrifolia stimulated generation of liver-kidney microsomal type 1 antibody, leading to subacute liver failure. In the present case, since the serum concentration of vitamin B12 increased up to 33,000 pg/ml, we speculate that anti-TC II antibody might have been produced by some constituents in the energy drink rather than by stimulation of mononuclear phagocytic systems.
The market value of nutritional supplements and energy drinks is continually growing, and annual consumption is increasing20). The known and unknown pharmacology of these products raises concern for potentially serious adverse effects. Barton et al.1) reported iron overload due to daily intake of iron supplements, manifested as hemochromatosis, arthritis, pigmentation and atherothrombosis. Mursu et al.21) reported that daily oral intake of multivitamins, folic acid, magnesium, zinc or copper was associated with increased risk of overall mortality in older women. Further, four documented cases of caffeine-associated death3, 22), four separate cases of seizures23) and one case of mania24) were associated with the consumption of energy drinks. Worthley et al.25) reported that energy drink consumption acutely increases platelet aggregation and blood pressure, impairing endothelial function and increasing the risk of myocardial infarction in healthy young adults. Most energy drinks contain natural products such as guarana, ginseng and taurine; however, no reports of associated negative effects were found, since their concentrations in many energy drinks are usually below the level expected to deliver either therapeutic benefits or adverse events26). The most commonly reported adverse effects seem to be related to caffeine26). No known symptoms have been reported in hypercobalaminemia alone4), since hypercobalaminemia is usually accompanied by specific health conditions such as renal failure, liver disease and infections. No previous reports have described a healthy person with such a high serum vitamin B12 level as in this case. Except for occasional heartburn and a sense of abdominal fullness, which seemed to be related more with postoperative symptoms, the patient complained of no other symptoms. However, there is no guarantee that he would not show serious symptoms if he kept on taking the energy drink.
Conclusion
We encountered a patient with hypercobalaminemia associated with daily intake of an energy drink after total gastrectomy. Nutritional supplements and energy drinks have exploded in popularity in the past several years; however, their use is not without risk. This case report demonstrates the risk of unexpected hypervitaminemia when patients self-administer such products.
Acknowledgments
We thank Dr. Yoshimichi Nakajima for his extensive assistance and review of this case report. The authors who took part in this study declare that they do not have anything to disclose regarding funding or conflicts of interest with respect to this manuscript.
References
- 1.Barton JC, Lee PL, West C, et al. Iron overload and prolonged ingestion of iron supplements: clinical features and mutation analysis of hemochromatosis-associated genes in four cases. Am J Hematol 2006; 81: 760–767. doi: 10.1002/ajh.20714 [DOI] [PubMed] [Google Scholar]
- 2.Ortega RM, Rodríguez-Rodríguez E, López-Sobaler AM. Effects of omega 3 fatty acids supplementation in behavior and non-neurodegenerative neuropsychiatric disorders. Br J Nutr 2012; 107 (Suppl 2): S261–S270. doi: 10.1017/S000711451200164X [DOI] [PubMed] [Google Scholar]
- 3.Hurlock L, Lee MG. Potential health problems with the use of energy drinks. West Indian Med J 2012; 61: 1–2. doi: 10.7727/wimj.2012.106 [DOI] [PubMed] [Google Scholar]
- 4.O’Leary F, Samman S. Vitamin B12 in health and disease. Nutrients 2010; 2: 299–316. doi: 10.3390/nu2030299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adachi S, Kawamoto T, Otsuka M, et al. Enteral vitamin B12 supplements reverse postgastrectomy B12 deficiency. Ann Surg 2000; 232: 199–201. doi: 10.1097/00000658-200008000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HI, Hyung WJ, Song KJ, et al. Oral vitamin B12 replacement: an effective treatment for vitamin B12 deficiency after total gastrectomy in gastric cancer patients. Ann Surg Oncol 2011; 18: 3711–3717. doi: 10.1245/s10434-011-1764-6 [DOI] [PubMed] [Google Scholar]
- 7.Andrès E, Dali-Youcef N, Vogel T, et al. Oral cobalamin (vitamin B(12)) treatment. An update. Int J Lab Hematol 2009; 31: 1–8. doi: 10.1111/j.1751-553X.2008.01115.x [DOI] [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer AssociationJapanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14: 101–112. doi: 10.1007/s10120-011-0041-5 [DOI] [PubMed] [Google Scholar]
- 9.Okuda K. Mucosal adsorption and absorption of vitamin B12 in the intestine of the rat. Proc Soc Exp Biol Med 1962; 111: 320–323. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka N, Yamazaki Y, Yamada H, et al. Fate of cobalamins in humans following oral and intramuscular administration of cyanocobalamin, hydroxocobalamin, adenosyl-cobalamin and methylcobalamin. Vitamins 1981; 55: 155–161(in Japanese, Abstract in English). [Google Scholar]
- 11.Orihata M, Kato S, Takeuchi H, et al. Effect of the oral administration of vitamin B12 to patients under distal and total gastrectomy. Jpn J Gastroenterol Surg 2001; 34: 439–444(in Japanese, Abstract in English). doi: 10.5833/jjgs.34.439 [DOI] [Google Scholar]
- 12.Dou J, Xu W, Ye B, et al. Serum vitamin B12 levels as indicators of disease severity and mortality of patients with acute-on-chronic liver failure. Clin Chim Acta 2012; 413: 1809–1812. doi: 10.1016/j.cca.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 13.Zittoun J, Marquet J, Zittoun R. The intercellular content of the three transcobalamins at various stages of normal and leukaemic myeloid cell development. Br J Haematol 1975; 31: 299–310[PubMed; 1201243]. doi: 10.1111/j.1365-2141.1975.tb00861.x [DOI] [PubMed] [Google Scholar]
- 14.Cheeramakara C, Thanomsak W, Songmeang K, et al. Elevation of serum transcobalamin II in patients with scrub typhus. Southeast Asian J Trop Med Public Health 2005; 36: 113–117. [PubMed] [Google Scholar]
- 15.Carmel R, Tatsis B, Baril L. Circulating antibody to transcobalamin II causing retention of vitamin B12 in the blood. Blood 1977; 49: 987–1000. [PubMed] [Google Scholar]
- 16.Lau BH, Yamasaki T, Gridley DS. Garlic compounds modulate macrophage and T-lymphocyte functions. Mol Biother 1991; 3: 103–107. [PubMed] [Google Scholar]
- 17.Byeon SE, Lee J, Kim JH, et al. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm 2012; 2012: 732860. [DOI] [PMC free article] [PubMed]
- 18.Wada T, Koizumi K, Inoue H, et al. An autopsy case of MPO-ANCA associated vasculitis which develop and progressed rapidly after taking ginseng. Kantou Riumachi 2007; 40: 148–155(in Japanese). [Google Scholar]
- 19.Yuce B, Gulberg V, Diebold J, et al. Hepatitis induced by Noni juice from Morinda citrifolia: a rare cause of hepatotoxicity or the tip of the iceberg? Digestion 2006; 73: 167–170. doi: 10.1159/000094524 [DOI] [PubMed] [Google Scholar]
- 20.Malinauskas BM, Aeby VG, Overton RF, et al. A survey of energy drink consumption patterns among college students. Nutr J 2007; 6: 35. doi: 10.1186/1475-2891-6-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mursu J, Robien K, Harnack LJ, et al. Dietary supplements and mortality rate in older women: the Iowa Women’s Health Study. Arch Intern Med 2011; 171: 1625–1633. doi: 10.1001/archinternmed.2011.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmgren P, Nordén-Pettersson L, Ahlner J. Caffeine fatalities––four case reports. Forensic Sci Int 2004; 139: 71–73. doi: 10.1016/j.forsciint.2003.09.019 [DOI] [PubMed] [Google Scholar]
- 23.Iyadurai SJ, Chung SS. New-onset seizures in adults: possible association with consumption of popular energy drinks. Epilepsy Behav 2007; 10: 504–508. doi: 10.1016/j.yebeh.2007.01.009 [DOI] [PubMed] [Google Scholar]
- 24.Machado-Vieira R, Viale CI, Kapczinski F, et al. Mania associated with an energy drink: the possible role of caffeine, taurine, and inositol. Can J Psychiatry 2001; 46: 454–455. [DOI] [PubMed] [Google Scholar]
- 25.Worthley MI, Prabhu A, De Sciscio P, et al. Detrimental effects of energy drink consumption on platelet and endothelial function. Am J Med 2010; 123: 184–187. doi: 10.1016/j.amjmed.2009.09.013 [DOI] [PubMed] [Google Scholar]
- 26.Clauson KA, Shields KM, McQueen CE, et al. Safety issues associated with commercially available energy drinks. J Am Pharm Assoc 2008; 48: e55–e63. doi: 10.1331/JAPhA.2008.07055 [DOI] [PubMed] [Google Scholar]