Abstract
Cutaneous T-cell lymphomas (CTCL) are a heterogeneous group of malignancies derived from skin-homing T cells. The most common forms of CTCL are Mycosis Fungoides (MF) and Sezary Syndrome (SS). Accurate diagnosis remains a challenge due to the heterogeneity of presentation and the lack of highly characteristic immunophenotypical and genetic markers. Over the past decade molecular studies have improved our understanding of the biology of CTCL. The identification of gene expression differences between normal and malignant T-cells has led to promising new diagnostic and prognostic biomarkers that now need validation to be incorporated into clinical practice. These biomarkers may also provide insight into the mechanism of development of CTCL. Additionally, treatment options have expanded with the approval of new agents, such as histone deacetylase inhibitors. A better understanding of the cell biology, immunology and genetics underlying the development and progression of CTCL will allow the design of more rational treatment strategies for these malignancies. This review summarizes the clinical epidemiology, staging and natural history of MF and SS; discusses the immunopathogenesis of MF and the functional role of the malignant T-cells; and reviews the latest advances in MF and SS treatment.
Keywords: mycosis fungoides, Sezary syndrome, cutaneous T-cell lymphoma, cytokines, interleukin 15
1. Introduction
Cutaneous T-cell lymphomas (CTCL) are a clinically heterogeneous group of lymphoid neoplasms with diverse pathology and clinical course (Hwang, et al 2008) (Olsen, et al 2007a). After a long debate about competing classifications schemes, a joint World Health Organization-European Organization for Research and Treatment of Cancer (WHO-EORTC) effort in 2005 categorized all known types of cutaneous lymphoma, with careful attention to biological profile, clinical presentation, and prognosis (Willemze, et al 2005). Further revisions resulted in consensus criteria that were integrated in the 2008 WHO classification for nodal and extranodal lymphomas (Swerdlow, et al 2008), which captures the full clinical, pathological, and molecular spectrum of these neoplasms, and the features most important in diagnosis, prognosis and treatment.
Advances in the mechanism in T cell development have revealed that T cells have the potential to differentiate into multiple lineages with distinct functions, presenting the opportunity to more precisely classify T-cell lymphomas. This approach provides a conceptual platform to gain insight into the developmental origin of the malignant T cells, identify biological clusters for risk stratification, and improve treatment (Jaffe, et al 2008). For example, a detailed understanding of the normal germinal centre reaction has advanced our knowledge of the development of nodal B-cell lymphomas and defined different risk groups (Klein and Dalla-Favera 2008). Likewise, an ontogenetic model for CTCL, based on normal T-cell differentiation, would facilitate the development and clinical testing of mechanistic hypotheses in T-cell lymphoma. Unfortunately, despite a number of developments described in this review, a cohesive and validated model of CTCL development, grounded in the normal differentiation and function of skin-homing T cells, is lacking.
This review will centre on some of the biological and clinical questions that remain unaddressed in CTCL, with a focus on the putative cell of origin of this malignancy, the memory CD4+CD45RO+ T lymphocyte (Girardi, et al 2004, Hwang, et al 2008). Because the neoplastic cells responsible for the pathology in CTCL are skin-homing T cells, the initial clinical presentation often overlaps with other T-cell mediated dermatoses. Thus, the diagnostic challenges of distinguishing malignant versus reactive T cells in the skin and blood of patients with early stage CTCL will be discussed. Advances from genomics, to cytokine gene expression, and cancer immunosurveillance in CTCL will be presented, with a focus on Mycosis Fungoides (MF) and Sezary Syndrome (SS). Finally, the fundamental principles of systemic therapy for these disorders, and recent advances in drug development will be discussed.
2. Mycosis Fungoides and Sezary Syndrome: clinical epidemiology, staging and natural history
While the term CTCL includes a number of rare disease entities, such as CD30+ primary cutaneous anaplastic large cell lymphoma (pcALCL) and related disorders, the most common subtypes are MF and SS, accounting for approximately 70–75% of all cases (Bradford, et al 2009). The classic presentation of MF was first reported in 1806 by Jean L. Alibert at the Hospital Saint-Louis, in a patient with mushroom-like tumours (Alibert 1806). For a long time the neoplastic cell type in MF/SS was unknown, but with the ability to identify surface markers on the atypical T cells, it became apparent that MF and SS are malignancies of CD4+CD45RO+ skin-homing T-cells. The clinical spectrum is highly polymorphous (Girardi, et al 2004) and ranges from the unilesional form of MF, Worringer-Kolopp, to more generalized skin involvement with extensive tumours and erythroderma (Kim and Hoppe 1999, Medenica and Lorincz 1978, Vonderheid 2006). When peripheral blood is involved, patients have SS, an aggressive variant of CTCL associated with diffuse generalized erythroderma and lymphadenopathy (Willemze, et al 1983).
The age-adjusted incidence rates (IRs) for CTCL in the US have ranged from 4 to 6.4/million person-year, but for 1998–2002 they were up to 9.6/million person-year (Bradford, et al 2009, Criscione and Weinstock 2007). Consequently, the current best estimate for the number of new CTCL cases in the U.S. is about 3,000/year. The reason for the increased IRs is unknown, but improved diagnosis is likely to be a cofactor. Prevalence rates are not available, but with 2,000–3,000 new annual cases, no curative therapy, and median survivals of 12–18 years (Agar, et al 2010; Kim, et al 2003), a gross estimate of the number of patients living with CTCL in the U.S is between 25,000 and 50,000. Specific IRs for SS are more difficult to calculate with confidence because of the rarity of this disorder. Of 2,769 cases of CTCL examined in a Surveillance, Epidemiology and End Results (SEER) database, only 33 (1%) were SS. In another series, SS represented 2.5% of all CTCL cases (Criscione and Weinstock 2007). While MF can be observed in children and early adults (Crowley, et al 1998, Pope, et al 2010), the median age at diagnosis is 50–70 years of age, with an increased incidence in males and African Americans. Conversely, SS is more common in non Hispanic whites (Criscione and Weinstock 2007).
The cause of CTCL is unknown. Although numerous associations with infectious agents have been hypothesized, none have been proven (Mirvish, et al 2011). Case control studies have not identified any consistent occupational, environmental, or iatrogenic factors(Morales Suarez-Varela, et al 2000, Whittemore, et al 1989), and while MF can occur in patients after organ transplantation, it has not been linked to a specific opportunistic infection or immune suppression (Ravat, et al 2006). Other cutaneous lymphoproliferative disorders, such as lymphomatoid papulosis (LyP) and pcALCL, can follow or precede MF (Kunishige, et al 2009). B-cell lymphoid malignancies, such as chronic lymphocytic leukaemia (CLL) and monoclonal gammopathies, can coexist with MF (Hallermann, et al 2007). However, with the exception of cases of MF, LyP and pcALCL that expressed a common T cell receptor (TCR) gene rearrangement (Chott, et al 1996), no markers for multilineage lymphoid involvement in CTCL have been found. Familial clusters of MF have been described (Hodak, et al 2005), and linkages with human leucocyte antigen alleles have been observed (Jackow, et al 1996), but solid leads for genetic loci of familial predisposition in CTCL are lacking. Finally, it has been hypothesized that MF arises from malignant transformation of activated T cells in the setting of persistent antigenic stimulation or chronic inflammation(Burg, et al 2001). However, unlike proliferations of CD4+ and CD8+ T-cell in large granular lymphocytes (LGL), where the TCR repertoire displays hallmarks of chronic antigenic stimulation(Wlodarski, et al 2005), the TCR repertoire in CTCL, while restricted (Yawalkar 2003), does not display a specific antigenic “signature”.
Clinical Features and Outcomes
MF is typically an indolent malignancy and often presents with a premycotic phase. Multiple skin biopsies over many years are needed typically for a diagnosis. Survival correlates with the stage of initial presentation (Kim, et al 2003, Klemke, et al 2005, Morales, et al 2005). The most recent revision of staging is summarized in Table 1 (Olsen, et al 2007a). Patients presenting with skin-restricted patches and plaques that affect less than 10% of the body surface area, have an excellent prognosis, and in analysis of long-term survival, patients with minimal skin disease have survival comparable to the normal age-matched cohort (Agar, et al 2010, Kim, et al 2003, Morales, et al 2005, van Doorn, et al 2000).
Table 1.
Modified ISCL/EORTC revisions of MF/SS (Olsen et al 2007a).
| Stage | T (Skin) | Node* | M (viceral) | Blood* |
|---|---|---|---|---|
| IA | 1 (<10%) | 0 | 0 | 0,1 |
| IB | 2 (>10%) | 0 | 0 | 0,1 |
| IIA | 1,2 | 1,2 | 0 | 0,1 |
| IIB | 3 (tumours) | 0–2 | 0 | 0,1 |
| IIIA | 4 (>80%) | 0–2 | 0 | 0 |
| IIIB | 4 | 0–2 | 0 | 1 |
| IVA1 | 1–4 | 0–2 | 0 | 2 |
| IVA2 | 1–4 | 3 | 0 | 0–2 |
| IVB | 1–4 | 0–3 | 1(involved) | 0–2 |
Nodes: N1=Dutch grade 1, N2= Dutch grade 2, N3= Dutch grade 3–4. Blood : B1:>5% peripheral blood; B2>1 ×109/l Sezary cells
The diagnosis of MF is challenging. No single test is sufficient and a combination of supporting clinical, immunological, and molecular features is necessary to reach a conclusive diagnosis. Loss of the surface T-cell antigens CD5, CD7, CD26 and less frequently CD3, is observed in CTCL (Robson 2010), and may help distinguish normal from malignant T-cells. However, reduced expression of CD5, CD7, and CD26 on T-cells can also occur in reactive and inflammatory skin conditions (Murphy et al 2002), and is not diagnostic of MF. It should be noted that T-cell surface antigen deletion in CTCL is inconsistent and has not been linked to specific mutations or genomic losses. Therefore it cannot be used to identify and monitor the neoplastic clone. Likewise, detection of a clonal TRB or TPΓ gene by polymerase chain reaction (PCR) in a skin biopsy is helpful to support a diagnosis of MF, but negative results do not exclude a diagnosis of MF.
In early MF, the clinical findings can resemble other papulosquamous disorders, such as eczema, contact dermatitis or psoriasis. Diagnosis of early stages of MF based on clinically subtle skin lesions can be challenging where histological features are minimal (Pimpinelli, et al 2005, Tracey, et al 2003). The T-cell infiltrate is heterogeneous, and is composed of both CD4+ and CD8+ T cells(Vonderheid, et al 1987a). An increased ratio of CD4+ to CD8+ T cells and morphological features, such as epidermotropism, exocytosis, and Pautrier’s microabsess support the diagnosis. Loss of CD7 is helpful if present on immunohistochemistry, and TCR clonality can be valuable in confirming malignancy. However in the early stages, CD7 may not be lost and clonality can be negative (Jones and Duvic 2003). When the skin lesions acquire vertical growth with thickened plaques and tumours, the diagnosis is facilitated by the detection of the atypical nuclei of malignant T cells in the epidermis, the formation of Pautrier’s microabscesses (Nickoloff 1988), and by a clonal TCR gene rearrangement. The ratio of CD4+ to CD8+ T cell increases in the skin, and is particularly helpful if found in the epidermis. In tumours, the histopathology shows malignant T cells forming an exuberant infiltrate with few residual CD8+ T cells, a greater degree of atypia, and a frequent loss of CD7 expression.
3. Immunopathogenesis of MF and functional role of the malignant T-cells
The indolent progression of MF has raised a number of hypotheses about malignant T-cell biology. The malignant T cells may not have high, autonomous proliferative potential and may be dependent on the cutaneous microenvironment for growth (Berger, et al 2005). In support of this hypothesis is the observation that T-cells from CTCL patients require stimulation via CD28 for proliferation (McCusker, et al 1997), respond poorly to growth stimuli in vitro and do not proliferate like normal T cells in vitro (Gazdar, et al 1979). A second possible explanation is a role for cancer immunosurveillance in modulating and suppressing disease progression. The histopathology of MF, which shows T-cell infiltration of both malignant CD4+ and reactive CD8+ T cells, and a dominant T helper cell type 1 (Th1) cytokine pattern in early stage, followed by a gradual increase in CD4+ T-cells and Th2 skewing in advanced stages, suggests that loss of a CD8-mediated immune response parallels disease progression. Furthermore, the clinical effectiveness of therapeutic agents that modulate the immune system and reverse Th1 cytokine expression in MF/SS supports an important role of tumour immunity in clinical outcomes.
Clinical lesions manifesting as patches, plaques, tumours, or erythroderma develop from the interactions of the neoplastic T cells with the cutaneous microenvironment (Sterry and Mielke 1989, Vonderheid, et al 2002). A primary determinant of T-cell skin homing in MF/SS is the expression of cutaneous lymphocyte antigen (CLA), the ligand for E-selectin (CD62E) (Yamaguchi, et al 2003). Additional adhesions molecules and skin-homing chemokines, such as CCR4, CCR10, and their respective ligands CCL17/TARC and CCL27/CTACK, play a role in attracting T cells to the skin (Kakinuma, et al 2003, Kallinich, et al 2003). However, the cascade of events that leads to the margination and extravasation of T cells in the cutaneous microvasculature, and drives their migration into the epidermis remains poorly defined. Microscopic and immunophenotypic analysis of Pautrier’s microabscesses reveal that they are intraepidermal collections of malignant lymphocytes in close association with dendritic cells (Langerhans cells, LC)(Edelson 2001). This raises the hypothesis that cytokines produced by LC attract malignant CD4+ T cells into the epidermis, and that LC-T cell cross-talk is important for disease progression. However, in the absence of experimental models to investigate specific mechanisms thought to be operating in vivo, this remains a working hypothesis.
The cytokine profiles in lesional skin (patch vs plaque vs tumour), and in peripheral blood mononuclear cells (PBMC) from patients with various stages of MF have provided clues into the dynamic changes in the microenvironment during the progression of MF (Dummer, et al 1996, Vowels, et al 1994). Analyses of cytokines in the skin have identified specific patterns associated with early and advanced stage. In early stage MF, the skin shows normal to increased expression of IFNG, IL12 and IL2, a Th1 cytokine pattern (Saed, et al 1994)(Figure 1). As the malignancy progresses to late stage MF, there is a loss of Th1 cytokines, evidenced by decreased IL2, IFNG, and IL12, and an increase in Th2 cytokines, such as IL4, IL5, IL10, and IL13. The patterns seen in the skin is also seen in analysis of gene expression from peripheral blood (Chong, et al 2008). Integrating cytokine data from skin and blood, one interpretation is that in early stage MF, the CD8+ T cells contribute to the Th1 skewing, while in advanced stages where the malignant CD4+ T cells are dominant, there is Th2 skewing. Given that CD8+ T cells share a Th1 phenotype and play a role in cell-mediated immunity, their presence in the skin is consistent with an anti-tumour response (Hoppe, et al 1995, Wood, et al 1994). (Figure 1)
Figure 1.
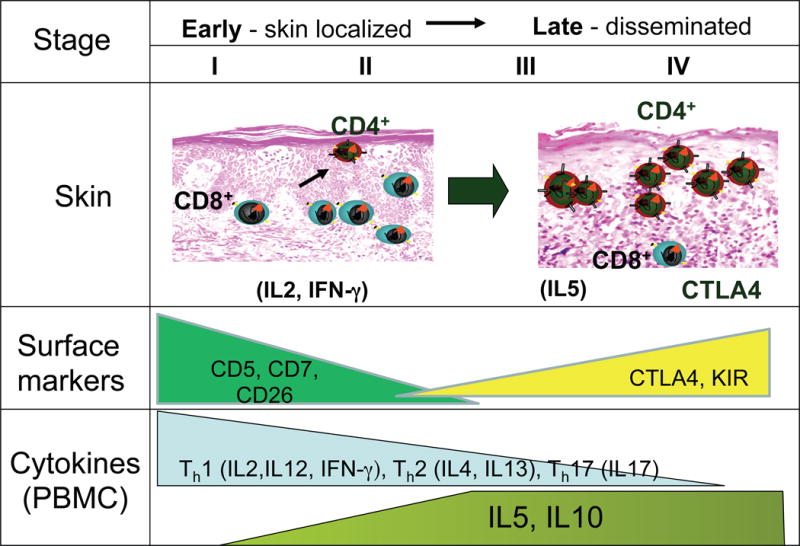
Summary of cytokine expression and cellular changes seen in the early and late stages of MF/SS. The different stages of MF/SS are shown with the corresponding extent of T cell infiltration of the skin by CD4+ and CD8+ T cells. There are more CD8+ T cells relative to the atypical CD4+ tumour cells in the early skin stages of MF/SS. In more advanced disease with tumours, there are fewer CD8+ T cells. The level of cytokine expression for Th1, Th2, and IL17 is shown in the bottom panel. The increase in expression of immune suppressive molecules in more advanced stages of MF/SS is shown.
The “static” cytokine snapshot analysis provided by immunohistochemical studies of the skin in early and advanced stage MF patients has been complemented by dynamic functional studies of cytokine expression by malignant T-cells in response to activation. These studies have been done mostly in SS. While normal memory T cells are known for their ability to more rapidly express cytokines for effector function compared to naïve T cells following stimulation (Ehlers and Smith 1991, Krishnan, et al 2001), expression of IL2 and IFNG in SS T cells in vitro is profoundly decreased. Together with the constitutive expression of CTLA4, the gene profile resembles that seen in regulatory T-cells (Tregs) (Chong, et al 2008). An exception is observed with IL5 and IL10, which are increased compared to normal(Chong, et al 2008). Because IL5 is associated with eosinophil recruitment, this cytokine may be responsible for the pruritus seen in SS. Elevated levels of IL10, an inhibitor of IFNG is also produced in late stage MF, and may contribute to the diminished anti-tumour response(Asadullah, et al 1996). Indeed, Foxp3 has been detected in PBMC from a subset of SS patients (Capriotti, et al 2008). The abnormal expression of CTLA4 and IL10 in SS, together with the expression of Foxp3 in a subset of patients, further suggests a possible differentiation bias toward Tregs (Berger, et al 2005, Wong, et al 2006). However when functional suppressor assays were performed, Treg activity was not detected(Tiemessen, et al 2006).
Other cytokines have recently been implicated in the immunopathogenesis of CTCL. A newly discovered class of CD4+ T cells, Th17, expresses IL17A/F, IL21 and IL22. These Th17-derived cytokines act on keratinocytes to induce IL6 and IL8 production, and recruit circulating T cells and neutrophils to the skin(Teunissen, et al 1998, Weaver, et al 2007). Th17 cells have been implicated in the immune response to extracellular bacteria and fungi, and in autoimmunity (Lowes, et al 2007). IL17A has been detected in approximately 50% of skin biopsies of MF/SS patients (Ciree, et al 2004). However, IL17A is not measurable in the serum(Krejsgaard, et al 2011), suggesting that this cytokine may play a role in MF, but not in SS. This is consistent with our results showing that SS peripheral blood cells do not express IL17A (Chong, et al 2010).
IL15, which is produced by many cell types, including keratinocytes, inhibits T-cell apoptosis, promotes the expansion of CD4+ T cells, and has been directly implicated in the pathogenesis of a number of T-cell lymphoproliferative disorders (Dooms, et al 1998, Fehniger, et al 2001, Malamut, et al 2010). IL15 has been found to be overexpressed in MF, in a stage-dependent way and to induce growth of SS T cells (Asadullah, et al 2000, Dobbeling, et al 1998). This suggests that IL15 may be involved in the initial transformation, survival and expansion of malignant T-cells in CTCL.
Finally, recent data suggest that IL16, a pleiotropic cytokine that functions as chemoattractant for CD4+ T-cells and modulates their activation may be involved in the progression of CTCL (Richmond, et al. 2011). Gradual loss of intracellular IL16 in peripheral blood T-cells is observed from stage IB onwards. This phenomenon correlates closely with loss of surface CD26 and decreased plasma levels of IL16 in SS patients.
The clinical implications of disordered immunity in CTCL are significant. In advanced stage MF and SS, global immune dysfunction results in a high risk of infections, which is the dominant cause of death in these patients (Axelrod, et al 1992, Posner, et al 1981). In addition to cytokine imbalances, decreased immunity may also result from T-cell exhaustion, as evidenced by the profound loss of TCR repertoire (Goronzy and Weyand 2003, Yawalkar, et al 2003), or from direct inhibition by malignant T cells, possibly mediated by CTLA4 (Read, et al 2000, Wong, et al 2006).
MF/SS progression and immunoediting
A role of immune surveillance against cancer was first hypothesized over 50 years ago (Burnet 1957, Thomas 1959). This concept has regained interest from new evidence using transgenic mice that have targeted defects in the innate immune system, and has been updated as the cancer immunoediting hypothesis (Dunn, et al 2002, Shankaran, et al 2001). In MF/SS, the changes in cytokine expression and T-cell subset ratios in disease progression could be explained within the frameworks of the steps in the cancer immunoediting hypothesis: elimination, equilibrium and escape (Dunn, et al 2002, Dunn, et al 2006). In this model, immune interaction with the neoplastic T cells and the genetic instability of the cells are variables that contribute to the progression (Figure 2). In the elimination phase, immunosurveillance is intact and an unrestrained T cell clone proliferates incognito until the local stroma is disrupted, activating the expression of type I IFN from monocytes, which activate CD8+ T-cells and induce a Th1 profile. IFNG plays an important role in the immunoediting concept as loss is associated with increased tumour development (Dighe, et al 1994, Kaplan, et al 1998).
Figure 2.
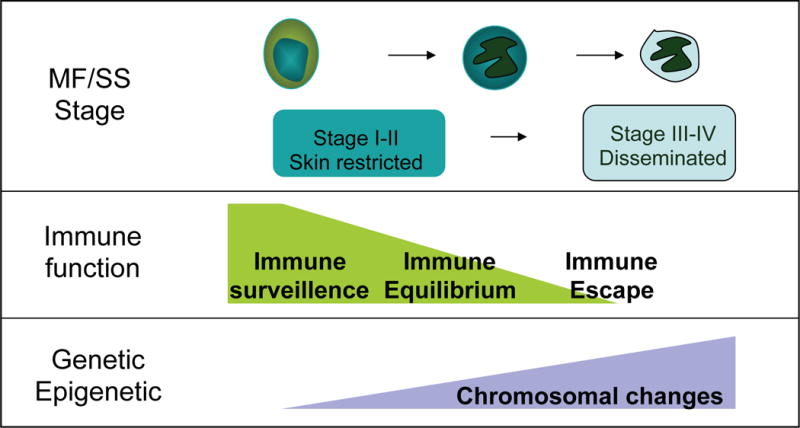
Model for immunoediting in relation to MF/SS stage. The progression of MF/SS is illustrated as impacted by the level of tumour immunity present and underlying genetic alterations that contribute to tumour sculpting to stimulate progression to more advance stages of the disease.
Escape of the malignant T cells leads to the equilibrium phase, where there is a steady state between immunity and neoplastic T cells proliferation. In the skin, the cytokines show a Th1 pattern, and the histology shows a heterogeneous infiltrate of CD8+ T cells and neoplastic CD4+ T cells. This corresponds to CTCL clinical stages I and II. With continued proliferation, the neoplastic cells undergo ‘immune sculpting’ in the background of genetic instability. The tumour antigens undergo alteration and the neoplastic T cells transition to the escape phase. The expression of immunosuppressive molecules seen in MF/SS, such as IL10, Fas ligand, PD1 and CTLA4, contribute to escaping immune recognition (Dummer, et al 1998, Ni, et al 2001, Samimi, et al 2010, Wong, et al 2006). The neoplasm proliferates beyond the local microenvironment into the lymph node and visceral, and tumour immunity is overwhelmed.
Origin and Biomarkers of the Malignant T-cell
Biomarkers can aid diagnosis, and may provide insight into the cellular origin of MF/SS. Specific molecular signatures can be used to identify the malignant T cells, identify their precise lineage, and provide clues into the pathways that led to transformation. In the search for novel biomarkers in MF/SS, one approach has utilized array-based high-throughput methodologies to profile the transcriptome. These robust techniques can elucidate differences in all genes expressed in MF/SS compared to normal individuals. Different groups have identified distinct transcriptional signatures in MF/SS (Table 2). However there is a lack of consensus from these reports, which may result from the inherent heterogeneity of MF/SS, small sample numbers, the platform used, and/or the type of samples. Thus some of the genes identified may be from neoplastic T-cells, or from the reactive microenvironment of the skin. An inherent source of heterogeneity is the fact that some transcriptional analyses have been performed on variably purified blood cell populations from patients with SS, while others have used lesional skin. Currently, the degree of overlap in the transcriptional signatures between MF and SS, and its significance in relation to the biological relatedness of these two types of CTCL, remains to be characterized.
Table 2.
Summary of gene expression analysis of MF/SS from microarray studies.
| Authors | Platform | Source | Findings |
|---|---|---|---|
| Willers et al (2001) | 588 gene Atlas membrane array | SS cells/skin derived T cell culture | Interferon inhibiting cytokine factor |
| Kari et al (2003) | cDNA filter – 4500 genes | SS PBMC | PLS3, GATA3, JUNB, ITGB1, PRG2, RHOB (ARHB), decreased DPP4, STAT4 and IL1R1 |
| Tracey et al (2003) | cDNA oncochip - 6386 | MF skin | 27 genes – BIRC3, BIRC1, TRAF, TNFR apoptosis regulators, STAT4, CD40L |
| Mao et al (2004) | Affy cDNA HuGenFl (12k) | MF/SS | JUNB and BCL2 increased copy number. Affy showed decreased RASA1, STAT3, STAT4, STAT5A |
| Van Doorn (2004) | Affy U95A | SS | Increased: EPHA4 and TWIST1. Decreased: TGFBR2, MXI1, PRDM2, STAT4, BCL11A |
| Mao et al (2006) | FISH, Affy U133A (13.5k genes) | MF/SS | CCND1/BCL1 increased in some. EB1 loss, CCND2, CCND3 increased |
| Mao et al (2008) | FISH, Affy U133A | SS | JUNB and JUND increased. Phosphorylated MAPK1 (ERK). Increase JUNB copy number |
| Hahtola (2006) | Affy U133A | Skin and blood, CD4 | Th2 cytokine increased in SS, loss of T-bet (TBX21) NKG7 and CCL5 (SCYA5) decreased in SS |
| Booken et al (2008) | AffyU133 Plus2(54k genes) | CD4 | 7 genes significant – CDO1, DNM3, NEDD4L, IGFL2, TNFSF11, KLHDC5. TWIST1 affects DNM3 and CDO1 |
| Kennah et al (2009) | Affy 133Plus2 | Hut78 | HCK, AHI1, BIN1 in transformed MF |
| Wozniak (et al 2009) | cDNA oncochip 6386 | MF skin, PUVA-resistant | NF-kB TCR signalling altered in PUVA resistance. Increased NFKB1, and Th2 signalling. Differences seen in tumour microenvironment |
| Shin et al (2007) | AffyU133A | MF skin | 3 clusters- T cell genes in cluster 1, LOR in cluster 2, FOSL1, SERPINB13 in cluster 3. |
| Litvinov et al (2010) | AffyU133A | MF skin | WIF1 favourable prognosis, IL17F poor prognosis |
| Chong et al (2010) | AffyU133 Plus2 | PBMC, SS | 9/14 memory genes decreased in SS |
| Pomerantz et al (2010) | Illumina Sentrix 24k genes | CD4/CD45RO cells | ZBTB16 increased, IRF3 and IFI35 decreased |
SS, Sezary Syndrome; MF, Mycosis Fungoides; PBMC, peripheral blood mononuclear cells; PUVA, psoralen combined with ultraviolet A.
The most recent studies are summarized in Table 2. Genes associated with the tumour necrosis factor (TNF) pathways and with Th2 phenotype, such as GATA3, are increased, whereas genes involved in Th1 lineage, such as STAT4, are decreased. Genes involved in proliferation, such as JUNB, JUND, and other cell cycle genes are increased. Of note is the many-fold upregulation of genes not normally expressed in T cells, such as TWIST1, DNM3 (dynamin 3), NEDD4L, and PLS3 (plastin 3). The functional role and biological significance of these genes in MF/SS are unclear, and adaptation of these genetic biomarkers into clinical testing would benefit from verification in larger sample and multicentre studies. Nevertheless understanding the mechanism for the activation of these genes in MF/SS may provide valuable insight into the pathogenesis.
A subtle but important distinction should be made between biomarkers that are highly overexpressed and thus correlate strongly with the malignant T-cells, regardless of their function, and biomarkers that identify key signalling and survival pathways that are more relevant as molecular targets for therapy. While the two categories undoubtedly overlap, each may be independently useful in the clinic. An ideal diagnostic marker, particularly when clinical or histological features are absent, will be expressed at high levels in the malignant T cells and will be absent or near absent in normal T-cells, providing the highest signal-to-noise ratio in the specific tissue that is being analysed. For example, in SS, the PLS3 gene has been shown by several groups to be expressed at high levels by differential screening (Kari, et al 2003, Su, et al 2003). This gene encodes an actin-binding protein, appears to be unique to SS, and is not expressed in haematopoietic cells, thus representing a potentially useful diagnostic biomarker for SS T-cells in the peripheral blood. While the observation needs to be validated in larger patient samples, we have shown that the PLS3 mRNA expression can be very valuable in monitoring the development of SS and assessing the response to treatment (Tang, et al 2010).
An additional surface marker upregulated on some SS cells is the killer immunoreceptor (KIR), KIR3DL2 (p158), which is expressed on natural killer (NK) cells (Poszepczynska-Guigne, et al 2004). In screening SS patients, KIR3DL2 was able to distinguish the malignant clone where multiple dominant clones were present in the peripheral blood(Marie-Cardine, et al 2007). The detection of KIR3DL2 on SS cells has also been reported by other investigators (Bahler, et al 2008). The significance of KIR expression is unclear, but aged T cells and T cells in autoimmune disease have increased KIR expression. The mechanism of increased KIR receptor expression in aged T cells has been suggested to be epigenetic (Li, et al 2009).
Molecular Genetics of CTCL
Distinctive and pathognomonic chromosomal translocations, such as those seen in B-cell lymphomas and myeloid leukaemias, have not been identified in CTCL. Numerous studies show recurrent chromosomal alterations, and genomic gains and losses, affecting chromosomes 1, 6, 7, 8, 9, 10 and 17 (Izykowska and Przybylski 2011, Mohr, et al 1996). However these abnormalities are not consistent, thereby negating their usefulness as diagnostic markers. Rather, there appears to be many chromosomal regions altered, and this hints to an underlying genomic instability in MF/SS. Recent approaches using comparative genomic hybridization (CGH) have identified more detailed chromosomal changes, with the most common types associated with loss of chromosomal regions (1p, 10p, 10q, and 17p) rather than gain of chromosomal regions (8q and 17q) (Table 3). In comparing differences between chromosomal changes and gene expression changes, there is not clear mapping of chromosomal alteration to abnormal gene expression.
Table 3.
Summary of genomics and miRNA studies in MF/SS
| Authors | Platform | Cells | Findings |
|---|---|---|---|
| Mao et al (2003) | Genomic microarray | MF/SS | RAF1 (3p25), CTSB (8p22),PAK1 (11q13) JUNB (19p13) increased in MF. FGFR1 (8p11), PTPN1 (20q13) and BCR (22q11) in SS |
| Van Doorn et al (2005) | Epigenetic CGH | MF/SS | BCL2 hypermethylated, TP73 (p73), CDKN2A (p16), CDKN2B (p15), CHFR, ACS (TMS1) methylated |
| Vermeer et al (2008) | CGH | SS | Gain of MYC, STAT3. Loss of MYC antagonist MXI1 and MNT, ZEB1, DUSP5. |
| Caprini et al (2009) | CGH, SNP | SS | Loss 17, BAG4, BTRC, NKIRAS2, PSMD3, TRAF2 |
| Van Doorn et al (2009) | Array CGH | MF skin | Gain 7q36, 7q21, loss 5q13, 9p21, FASTK and SKAP1 gained. MF differs from SS in chromosomal alteration. 9p21, 8q24, 1q21 associated with poor prognosis. |
| Laharanne et al (2010) | CGH | MF/SS | MF-Gain 1q 7p or 7q. Loss 9p21. SS show 8q and 17q gain. 10p and 17p losses. |
| Ballabio et al (2010) | MicroRNA array | SS | Most MIR decreased. 10 MIR discriminatory. Increased-MIR145, MIR574, MIR200, MIR199A, MIR143. Decreased MIR223, MIR342, MIR150, MIR24-1, MIR186 |
| Narducci et al (2011) | MicroRNA array | SS | MIR21, MIR486 and MIR214 upregulated in SS |
| Van Kester et al (2011) | MicroRNA array | MF | MIR155 and MIR92A increased in tumour. MIR93 highest. Different from MIR in SS. |
SS, Sezary Syndrome; MF, Mycosis Fungoides; CGH, comparative genomic hybridization; SNP, single nucleotide polymorphism
Recently, microRNAs (miRNAs) have been shown to play an important role in the regulation of gene expression in cancer (Garzon, et al 2010). In MF, studies have identified differences in miRNA expression with MIR155 and MIR92A increased in skin lesions (van Kester, et al 2011). In SS, there are significant changes and most miRNA are decreased, but there are several that are increased (Table 3) (Ballabio, et al 2010; Narducci, et al 2011). Further characterization of miRNA functional clusters in MF/SS is in progress.
4. Therapy of MF and SS
Advances in the understanding of MF/SS have been recently complemented by the development of novel therapies. The current treatment of MF/SS is highly varied and there is no simple algorithm. Consensus approaches to the fundamental strategies of therapy for early and late stage MF/SS and in selecting agents within a particular class were recently developed by cooperative networks and organizations in the United States, such as the National Comprehensive Cancer Network (NCCN) (http://www.nccn.org) and the United States Cutaneous Lymphoma Consortium (USCLC) (Olsen, et al 2011). In Europe, similar consensus guidelines were developed by the Cutaneous Lymphoma Task Force of the EORTC, often in collaboration with the International Society of Cutaneous Lymphomas (ISCL) (Trautinger, et al 2006).
In early stage MF, skin-directed therapies offer effective control. When topical therapies fail, systemic therapy is needed. As reviewed in the pathogenesis, immunity plays a role in disease progression, and treatments that maintain or augment the immune response, such as retinoids and interferons, are preferable to those associated with immune suppression, such as cytotoxic chemotherapy. For patients who have failed one or more of the conventional systemic therapies, several new drugs that more specifically target the T cell (monoclonal antibodies) or agents that alter epigenetic control of gene expression (histone deacetylase inhibitors) have been approved or are in the advance phase of development. The impact of the new agents on long-term survival, however, remains to be defined.
Systemic therapies
Biologicals and Immune Enhancing Therapies
Three major types of IFN, IFNα, IFNβ, and IFNγ, induce a spectrum of biological activities and induce cell-mediated immunity (Trinchieri 2010). Numerous studies have shown the clinical efficacy of IFNα as monotherapy from stage IA through stage IV CTCL (Jumbou, et al 1999; Rupoli et al. 2005; McGinnis et al. 2004; Olsen, 2003) and interferons have long been recognized as the most active single agents in CTCL. When combined with oral retinoids, rapid improvements have been reported for erythrodermic and follicular MF (McGinnis, et al 2004). In a multicentre, prospective Phase II clinical study of 89 patients with early-stage IA to IIA MF treated with IFNα-2b (6–18 Miu/wk) and psoralen combined with ultraviolet A (PUVA) (Rupoli, et al 2005), a complete remission (CR) was obtained in 84% with an overall response rate (ORR) of 98% and sustained remissions in 20%. High CD8+ counts were associated with a lower relapse rate. In a retrospective study, subcutaneous IFNα2a (3–6 Miu/day for one month, followed by 3–6 Miu three times a week) resulted in a 41% complete response rate (Jumbou, et al 1999). Recently, pegylated-IFNα-2b was reported to have activity in MF (Yanagi, et al 2006). The major side-effects of IFN therapy are dose-dependent and include flu-like symptoms, myelosuppression, liver function abnormalities and depression.
Retinoids affect cell differentiation and have profound effects on gene expression. Bexarotene is a rexinoid that binds to the retinoid X receptor (RXR) alpha and alters gene expression. RXR activation can induce apoptosis and modulate innate immunity by affecting chemokine expression (Nunez, et al 2010). Results of Phase II and III trials showed ORRs of 54% with doses of 300 mg/m2/day, and 67% with doses above 300 mg/m2/day (Duvic, et al 2001). The most common adverse effects were hypertriglyceridaemia, hypercholesterolaemia, headache, central hypothyroidism, asthenia, and leucopenia. Since its approval, optimal use of bexarotene has included its combination with PUVA or IFNα (Assaf, et al 2006). Monitoring of thyroid hormone levels and fasting lipid levels is important. Other retinoids, such as tretinoin or all-trans retinoid acid (ATRA), have been used for CTCL. In 33 patients with MF/SS treated with ATRA (45 mg/m2) compared to 19 patients treated with oral bexarotene (300 mg/m2), four of 33 patients (12%) achieved a response on ATRA, and 4 of 19 (21%) achieved a response on bexarotene, a difference that was not significant. Mucocutaneous symptoms, such as dryness and redness, were common adverse events with ATRA treatment, as was hyperlipidaemia (Querfeld, et al 2004).
Activating innate immunity by harnessing toll-like receptors (TLR) is another strategy for immunotherapy. TLR2, TLR4, and TLR9 expression is low in atopic dermatitis and psoriasis, but is increased in the epidermis in MF. Imiquimod is a TLR7 agonist that can induce TNFα, IFNγ, and IFNα in vivo (McInturff, et al 2005). Topical imiquimod was evaluated in six patients with stage IA-IIB MF for twelve weeks and showed histological clearance in 50% of the patients (Deeths, et al 2005). Significant improvements were noted for all treated lesions. Importantly, imiquimod may be helpful in treating sanctuary or resistant lesions in patients undergoing PUVA for CTCL (Dummer, et al 2003). CPG 7909 is a new TLR9 agonist administered subcutaneously that has been tested in a Phase I trial in 28 patients with CTCL (IB-IV) and showing clinical activity (3 complete response [CR] and 6 partial response [PR]) (Kim, et al 2010).
Denileukin diftitox is a chimeric fusion protein composed of interleukin-2 (IL2) and the diphtheria toxin A chain. Upon engaging the IL2 receptor on malignant T-cells, the drug is internalized and the diphtheria toxin is cleaved from the fusion protein, released into the cytosol to inhibit protein synthesis, and causes cell death (Foss 2006). Another potential mechanism for denileukin diftitox activity is the depletion of Tregs (Morse, et al 2008), which are typically CD25bright. Denileukin diftitox received US Food and Drug Administration (FDA) approval following completion of a pivotal phase III trial using 9 or 18 μg/kg/d in 71 patients with relapsed or refractory CTCL (Olsen, et al 2001). The ORR was 30% (20% PR, 10% CR) with a median duration of 6.9 months (range 2.7 to 46.1 months) without a statistical difference between the two doses. A follow-up study demonstrated significant improvements in self-rated overall quality of life, skin appearance and pruritus severity(Duvic, et al 2002). The toxicity of denileukin diftitox can be significant and includes fever, rigors, fatigue, peripheral oedema, diarrhoea, anorexia, myalgia, hypoalbuminaemia and hepatic transaminitis. Toxicity can be diminished by premedication with dexamethasone. Serious adverse events include capillary leak syndrome and loss of visual acuity, including loss of colour vision. More recently the FDA expanded approval of denileukin diftitiox for the treatment of persistent or recurrent CD25+ CTCL following confirmation of an improvement in progression-free survival (PFS) and ORR in a placebo-controlled multinational dose-ranging study that enrolled 144 patients with stage IA-III CTCL (Prince et al. 2010). One of the main questions about the clinical use of denileukin diftitox is whether responses depend on the expression of CD25, the high-affinity (α chain) IL2 receptor. In a monotherapy study with 24 patients, clinical response was observed in 78.5% of patients with high CD25+ expression versus 20% with low or undetectable CD25+ expression (Talpur, et al 2006).
Monoclonal antibodies
Targeting tumour cells with antibodies has shown success as a strategy in B cell malignancies with the anti-CD20 monoclonal antibody (mAb) rituximab. In CTCL, several monoclonal antibodies directed against cell surface markers of the neoplastic T cell have shown clinical efficacy and are under development. The approach, although logical, can be associated with risks. For example, siplizumab (MEDI-507), an anti-CD2 mAb, showed response in T-cell malignancies, but was associated with an increased incidence of Epstein-Barr virus-induced B-cell lymphoproliferative disease that resulted in cessation of the trial (O’Mahony, et al 2009).
A human IgG1 mAb, zanolimumab, against CD4 (Fishwild, et al 1996) was tested in CTCL. CD4 is a major histocompatibility complex class II co-receptor expressed on T cells, and less on monocytes, macrophages, and LC. CD4 is retained on the malignant T cells, and is an ideal antigen to target. Zanolimumab showed encouraging findings in both early-stage CTCL and late stage, treatment-refractory CTCL (Kim, et al 2007). Zanolimumab has been shown to act by three distinct pathways(Rider, et al 2007). Firstly, the CD4 tyrosine kinase p56lck is uncoupled from the TCR and transmits inhibitory signals via Dok-1 and SHIP-1 inhibitor molecules. Secondly, it acts to opsonize to enhance cell-mediated killing of the CD4+ cells. Lastly, it down-regulates CD4 from the cell surface via a slow Fc-dependent mechanism. Currently, zanolimumab remains in clinical development in both CTCL and peripheral T-cell lymphoma (PTCL).
Alemtuzumab is an unconjugated humanized IgG1 kappa mAb directed against the CD52 antigen, a glycoprotein highly expressed on most leucocytes (Frampton and Wagstaff 2003). CD52 is also variably expressed on tumour cells in MF/SS(Ginaldi, et al 1998). Numerous studies demonstrate that alemtuzumab is active in CTCL (Bernengo, et al 2007, Dearden and Matutes 2006, Lundin, et al 2003, Zinzani, et al 2005; Alinari et al. 2008). In 22 patients with advanced MF/SS treated with single agent alemtuzumab administered intravenously at 30 mg for up to 12 weeks, there was an ORR of 55% (32% CR and 23% PR). Efficacy was higher in patients with early stage MF and peripheral blood involvement (Lundin, et al 2003). To reduce opportunistic infections, a trial using a lower dose of alemtuzumab (10 mg three times a week for 4 weeks) showed clinical activity in 10 pretreated T-cell lymphomas (6 PTCL-not otherwise specified and 4 MF) with an ORR of 60 % and 20% of CRs (the CR rate was higher (33%) in the PTCL group) (Zinzani, et al 2005). A favourable experience combining the low dose and the subcutaneous (SQ) administration of single agent alemtuzumab has been recently reported in CTCL (Bernengo, et al 2007). The ORR was 85.7% with a 21.4% CR and a disease-free survival (DFS) of 12 months after a median follow-up of 16 months. Furthermore, alemtuzumab was safely administered subcutaneously to five elderly patients, three with SS with durable response (18 to 28+ months) (Alinari, et al 2008).
CCR4 is a chemokine receptor expressed by malignant T cells in CTCL. The increased expression of CCR4 in Th2 and Treg may be especially relevant in the treatment of CTCL. A defucosylated, humanized anti-CCR4 was developed and tested in T-cell lymphoma (Yamamoto, et al 2010). Phase I and II studies have demonstrated efficacy in T-cell lymphoma with an ORR of 31% and one durable response(Yamamoto, et al 2010). Overall the antibody was well tolerated. The additional benefit of reducing Tregs with anti-CCR4 and enhancing tumour immunity may contribute to the long-term efficacy, which has been shown to play a role in an animal model (Ito, et al 2009).
Histone deacetylase (HDAC) inhibitors
HDAC inhibitors affect the regulation of gene transcription by physical alterations of either DNA or the structural components of chromatin (Marks, et al 2001). Blocking histone modifications has been shown to induce apoptosis in tumour cells. There are currently 18 different HDAC that can be divided into four families, depending on the inhibitory spectrum displayed (Table 4). Two of these agents, vorinostat and romidepsin, are approved for use in CTCL.
Table 4.
Summary of histone deactylase inhibitors and specificity to a particular class of HDAC.
| HDAC Class | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | IIA | IIB | IV | |||||||||
| HDACi | 1 | 2 | 3 | 8 | 4 | 5 | 7 | 9 | 6 | 10 | 11 | Diseases studied |
| Vorinostat | + | + | + | + | + | + | + | + | + | + | + | 29.7% RR in CTCL (Olsen et al 2007b) FDA approved in CTCL |
| Panobinostat | + | + | + | + | + | + | + | + | + | + | + | 40% OOR in phase I and 21% phase II in HL (Buglio & Younes 2010). Currently in trials for PTCL |
| Belinostat | + | + | + | + | + | + | + | + | + | + | + | 0 complete response, 5 stable disease in phase I in haematological malignancies (Gimsing et al 2008) Currently phase III PTCL |
| NVP-LAQ824 | + | + | + | + | + | + | + | + | + | + | + | 3/39 stable disease in treated solid tumours (De Bono et al 2008). In trial for multiple myeloma |
| Depsipeptide | + | + | + | + | 34% RR in CTCL (Whittaker et al 2010). Approved CTCL | |||||||
| Entinostat | + | + | Solid tumours 2/27 partial responses (Gore et al 2008). Currently phase II in Hodgkin lymphoma | |||||||||
| Mocetinostat | + | + | + | − | − | − | − | + | Phase I: 3/23 complete responses in leukaemia (Garcia-Manero et al 2008b) | |||
HDAC, histone deactylase; HDACi, histone deactylase inhibitors; CTCL, Cutaneous T-cell lymphoma; PTCL, peripheral T-cell lymphoma;FDA, US Food and Drug Administration.
Vorinostat is an oral pan-histone deacetylase inhibitor (HDACi). Phase I studies showed activity in a variety of haematological malignancies (acute myeloid leukaemia [AML], CLL, myelodysplastic syndrome, acute lymphoblastic leukaemia and chronic myeloid leukaemia)(Garcia-Manero, et al 2008a). In CTCL, a Phase 2 trial of vorinostat in the setting of refractory disease demonstrated a PR in 8 of 33 patients (Duvic, et al 2007). Pruritus relief was observed in almost 50% of patients. A larger Phase IIb trial with 74 advanced stage patients showed an ORR of 29.7% and a time to progression of 4.9 months overall (Olsen, et al 2007b). Toxicities were minimal in this study.
Romidepsin (FK 228, Depsipeptide) is a bicyclic peptide from chromobacterium violaceum. A study in a human T-cell lymphoma cell line showed that depsipeptide caused substantial apoptosis and cell cycle arrest (Blagosklonny, et al 2002; Piekarz, et al 2004). An initial report demonstrated PR in 3 patients with CTCL and a CR in 1 patient with PTCL (Piekarz, et al 2001). These studies demonstrated the sensitivity of T-cell lymphomas to HDACi. In a multi-institutional Phase II trial of depsipeptide (14 mg/m2 on days 1, 8 and 15 of a 28-day cycle) in patients refractory to multiple prior therapies with CTCL and 62 with advanced disease, the ORR was 34% (Piekarz, et al 2009). The median duration of response (DOR) was 13.7 months and the maximum DOR as of data cut-off was greater than 63 months. Adverse events were generally mild, including nausea, fatigue, thrombocytopenia and anaemia.
Panobinostat (LH589) is a novel pan-HDACi under investigation. A Phase I study in 15 patients with primarily AML showed transient improvements in disease with a major dose-limiting toxicity of prolongation of the QT interval (Giles, et al 2006). In a Phase I study in CTCL, panobinostat was well tolerated and 2 of 10 patients showed complete response (Ellis, et al 2008). The most common adverse events include diarrhoea, thrombocytopenia, and fatigue.
Similar to LBH589, PXD101 is an HDACi in the hydroxamate class with greater specificity for HDAC1, HDAC2 and HDAC3. This agent has been studied in CLL (Gimsing, et al 2008). Its role in CTCL will depend on findings from clinical studies evaluating the advantage of HDAC selectivity.
Cytotoxic drugs and antimetabolites
Pralatrexate is a folate analog with enhanced accumulation in cancer cells and greater potency than methotrexate (Sirotnak, et al 1998). Pralatrexate is transported by the reduced folate transporter (RFC-1), with a 14-fold rate compared to methotrexate. A Phase I/II trial of pralatrexate in patients with lymphoma (O’Connor, et al 2009) demonstrated efficacy in aggressive T-cell lymphomas, achieving lasting complete responses (up to 16 months). The PROPEL trial, a prospective phase II open-label, multi-centre study in 115 patients with PTCL, showed a 35% response in the majority with tolerable adverse events: thrombocytopenia (31%), mucositis (22%), anaemia (18%), and neutropenia (22%)(O’Connor, et al 2011). Supplementation with vitamin B12 and folic reduced mucositis.
Gemcitabine is a pyrimidine analog that is incorporated into DNA, causes chain termination and disrupts DNA replication. Multiple Phase II studies have demonstrated its efficacy in PTCL and CTCL. A Phase II trial of 13 patients (8 with PTCL, 5 with CTCL) demonstrated 1 CR and 8 PR using gemcitabine 1200 mg/m2 (Zinzani, et al 1998). A larger prospective Phase II trial in previously treated MF (30 patients) and PTCL (14 patients) demonstrated 5 CR and 26 PR (Zinzani, et al 2000). A Phase II trial as frontline therapy in 32 CTCL patients with MF, PTCL or SS showed response with 7 achieving CR and 17 achieving a PR (total response rate of 75%) with a very modest toxicity(Marchi, et al 2005). Another Phase II trial, employing gemcitabine at 1000 mg/m2 on the same schedule as monotherapy in CTCL, demonstrated a comparable ORR (68%) in 25 patients with CTCL (Duvic, et al 2006a).
Pegylated liposomal doxorubicin (Doxil) at a dose of 20 mg/m2 monthly was first studied in CTCL in a Phase I/II trial with 10 patients, where the best response was CR in 6 patients and a PR in 2 patients with an 80% ORR (Wollina, et al 2001). A subsequent multicentre Phase I/II study at varying doses of Doxil at 20–40 mg/m2 in 34 patients with CTCL showed that 15 patients achieved CR and 15 patients achieved PR for an ORR 88.2% (Wollina, et al 2003). Another recent Phase II trial enrolled 19 patients with advanced/refractory CTCL (Pulini, et al 2007). Adverse events were mild and quickly resolved.
Pentostatin is a purine analog and showed promise in early, smaller studies of CTCL (Greiner, et al 1997). In a Phase I/II trial, pentostatin was given intravenously for 3 days on a 21-day cycle in 24 patients with, on average, 3 prior therapies (Kurzrock, et al 1999); 17 patients had either a PR or CR, with an ORR of 71%. A subsequent Phase I/II trial with 42 patients demonstrated an ORR 54.8% (Tsimberidou, et al 2004). The median duration of response was 4.3 months.
Forodesine (BCX-177; immucillin H) is a purine nucleoside analogue that is a highly selective purine nucleoside phosphorylase (PNP) inhibitor at nanomolar levels. By blocking PNP, forodesine leads to increased levels of deoxyguanosine and deoxyguanosine triphosphate in T cells to inhibit T cell proliferation (Balakrishnan, et al 2006, Balakrishnan, et al 2010). Forodesine has in vitro activity against acute lymphoblastic leukaemic T cells (Gandhi and Balakrishnan 2007). In a Phase I dose-ranging study, 13 CTCL (MF and SS) patients were treated with intravenous forodesine at various doses in a three-week cycle, leading to 3 CR, 1 PR and 6 stable disease (SD) with an excellent safety and tolerability profile. An oral formulation of forodesine was evaluated in a Phase I/II multi-centre dose escalation study in refractory CTCL patients using 80 mg/m2 once daily (Duvic, et al 2006b). The objective response was 53.6% with 2 CR, 13 PR and 22 SD. Forodesine was well tolerated with nausea, dizziness, pruritus, fatigue, peripheral oedema and headache being the most frequent side effects. No opportunistic infections or cytomegalovirus reactivation were noted.
Proteosome inhibitors
Bortezomib is a selective, reversible inhibitor that affects NF-κB activity by preventing the degradation of I-κB (Chaturvedi, et al 2011). Bortezomib has shown marked growth-inhibitory activity, most extensively in multiple myeloma and non-Hodgkin lymphoma (Rajkumar, et al 2005, Strauss, et al 2006), but studies have shown growth inhibition and induction of apoptosis also in malignant T-cells (Tan & Waldmann 2002; Juvekar, et al 2011). A Phase II trial of bortezomib as a single agent in 10 CTCL and 2 PTCL showed an ORR of 67% (Zinzani, et al 2007). 2 CR and 6 PR were observed with relatively modest toxicities. Bortezomib is now being studied in combination with other targeted therapies, such as HDACi and hypomethylating agents. A clinical trial of bortezomib and 5-azacitidine for patients with refractory and relapsed T-cell lymphomas is in progress at the Ohio State University (P. Porcu).
Bone marrow transplantation
In patients with rapidly progressive disease who have failed previous standard treatments, haematopoietic stem cell transplantations have been reported in the treatment of refractory disease MF/SS(Duarte, et al 2008). Relapses of MF/SS developed in autologous transplants, and more recent strategies have focused on allogenic transplant. A recent experience in 60 patients with MF/SS receiving allogenic haematopoietic transplant showed an overall survival of 54% at 3 years (Duarte, et al 2010).
5. CONCLUSIONS
Considerable advances have been made in characterizing MF/SS from array and genomics studies. However the nature of the malignant T cell remains unclear. Response to immune modifiers and gene expression studies suggest that cancer immunosurveillance plays a role in the pathogenesis of MF/SS. Harnessing our current understanding in treatment strategies, rational therapeutic approaches based on augmenting immunity or targeting pathogenic pathways of MF/SS may be an effective strategy. Previous experience using cytotoxic chemotherapy did not yield outcomes better than conservative treatments for survival at all stages of disease except in the most advanced stages (Kaye, et al 1989). Therapies that have immunomodulating activities, such as interferons (Olsen 2003, Thestrup-Pedersen, et al 1988, Vonderheid, et al 1987b, Yanagi, et al 2006) lead to durable clinical responses. Treatment of early disease may benefit from combining skin-directed approaches with immune-enhancing therapies. In advanced disease, the availability of recent novel systemic agents that have clinical activity in highly refractory CTCL offers new options for therapy. However, the survival of patients with extensive tumour stage and visceral involvement remains extremely poor and the development of more effective modalities remains an urgent necessity.
Acknowledgments
Due to space limitations, we apologize to authors whose works were not included in our citations. We graciously acknowledge comments and suggestions from Metin Gurcan, Gerard Lozanski, Heather Gibson, Derek Chan, Mark Bechtel, Guido Marcucci, and Susan Geyer. Henry Wong, Anjali Mishra, Tim Hake and Pierluigi Porcu contributed to drafting, revising and approving the final version. The work was in part supported by an NIH NCI P30 Grant Supplement to the OSU Comprehensive Cancer Center.
References
- Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, Robson A, Calonje E, Stefanato CM, Wain EM, Wilkins B, Fields PA, Dean A, Webb K, Scarisbrick J, Morris S, Whittaker SJ. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. Journal of Clinical Oncology. 2010;28:4730–4739. doi: 10.1200/JCO.2009.27.7665. [DOI] [PubMed] [Google Scholar]
- Alibert J. Description des maladies de la peau observées a l’Hôpital Saint-Louis, et exposition des meilleures méthodes suivies pour leur traitement. Barrois l’aine; Paris: 1806. [Google Scholar]
- Alinari L, Geskin L, Grady T, Baiocchi RA, Bechtel MA, Porcu P. Subcutaneous alemtuzumab for Sezary Syndrome in the very elderly. Leukemia Research. 2008;32:1299–1303. doi: 10.1016/j.leukres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Asadullah K, Docke WD, Haeussler A, Sterry W, Volk HD. Progression of mycosis fungoides is associated with increasing cutaneous expression of interleukin-10 mRNA. Journal of Investigative Dermatology. 1996;107:833–837. doi: 10.1111/1523-1747.ep12330869. [DOI] [PubMed] [Google Scholar]
- Asadullah K, Haeussler-Quade A, Gellrich S, Hanneken S, Hansen-Hagge TE, Docke WD, Volk HD, Sterry W. IL-15 and IL-16 overexpression in cutaneous T-cell lymphomas: stage-dependent increase in mycosis fungoides progression. Experimental Dermatology. 2000;9:248–251. doi: 10.1034/j.1600-0625.2000.009004248.x. [DOI] [PubMed] [Google Scholar]
- Assaf C, Bagot M, Dummer R, Duvic M, Gniadecki R, Knobler R, Ranki A, Schwandt P, Whittaker S. Minimizing adverse side-effects of oral bexarotene in cutaneous T-cell lymphoma: an expert opinion. British Journal of Dermatology. 2006;155:261–266. doi: 10.1111/j.1365-2133.2006.07329.x. [DOI] [PubMed] [Google Scholar]
- Axelrod PI, Lorber B, Vonderheid EC. Infections complicating mycosis fungoides and Sezary syndrome. Journal of American Medical Association. 1992;267:1354–1358. [PubMed] [Google Scholar]
- Bahler DW, Hartung L, Hill S, Bowen GM, Vonderheid EC. CD158k/KIR3DL2 is a useful marker for identifying neoplastic T-cells in Sezary syndrome by flow cytometry. Cytometry Part B: Clinical Cytometry. 2008;74:156–162. doi: 10.1002/cyto.b.20395. [DOI] [PubMed] [Google Scholar]
- Balakrishnan K, Nimmanapalli R, Ravandi F, Keating MJ, Gandhi V. Forodesine, an inhibitor of purine nucleoside phosphorylase, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2006;108:2392–2398. doi: 10.1182/blood-2006-03-007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan K, Verma D, O’Brien S, Kilpatrick JM, Chen Y, Tyler BF, Bickel S, Bantia S, Keating MJ, Kantarjian H, Gandhi V, Ravandi F. Phase 2 and pharmacodynamic study of oral forodesine in patients with advanced, fludarabine-treated chronic lymphocytic leukemia. Blood. 2010;116:886–892. doi: 10.1182/blood-2010-02-272039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabio E, Mitchell T, van Kester MS, Taylor S, Dunlop HM, Chi J, Tosi I, Vermeer MH, Tramonti D, Saunders NJ, Boultwood J, Wainscoat JS, Pezzella F, Whittaker SJ, Tensen CP, Hatton CS, Lawrie CH. MicroRNA expression in Sezary syndrome: identification, function, and diagnostic potential. Blood. 2010;116:1105–1113. doi: 10.1182/blood-2009-12-256719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger CL, Tigelaar R, Cohen J, Mariwalla K, Trinh J, Wang N, Edelson RL. Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory cells. Blood. 2005;105:1640–1647. doi: 10.1182/blood-2004-06-2181. [DOI] [PubMed] [Google Scholar]
- Bernengo MG, Quaglino P, Comessatti A, Ortoncelli M, Novelli M, Lisa F, Fierro MT. Low-dose intermittent alemtuzumab in the treatment of Sezary syndrome: clinical and immunologic findings in 14 patients. Haematologica. 2007;92:784–794. doi: 10.3324/haematol.11127. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV, Robey R, Sackett DL, Du L, Traganos F, Darzynkiewicz Z, Fojo T, Bates SE. Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Molecular Cancer Therapeutics. 2002;1:937–941. [PubMed] [Google Scholar]
- Booken N, Gratchev A, Utikal J, Weiss C, Yu X, Qadoumi M, Schmuth M, Sepp N, Nashan D, Rass K, Tuting T, Assaf C, Dippel E, Stadler R, Klemke CD, Goerdt S. Sezary syndrome is a unique cutaneous T-cell lymphoma as identified by an expanded gene signature including diagnostic marker molecules CDO1 and DNM3. Leukemia. 2008;22:393–399. doi: 10.1038/sj.leu.2405044. [DOI] [PubMed] [Google Scholar]
- Bradford PT, Devesa SS, Anderson WF, Toro JR. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064–5073. doi: 10.1182/blood-2008-10-184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buglio D, Younes A. Histone deacetylase inhibitors in Hodgkin lymphoma. Investigational New Drugs. 2010;28(Suppl 1):S21–27. doi: 10.1007/s10637-010-9588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg G, Dummer R, Haeffner A, Kempf W, Kadin M. From inflammation to neoplasia: mycosis fungoides evolves from reactive inflammatory conditions (lymphoid infiltrates) transforming into neoplastic plaques and tumors. Archives of Dermatology. 2001;137:949–952. [PubMed] [Google Scholar]
- Burnet M. Cancer; a biological approach. I. The processes of control. Br Med J. 1957;1:779–786. doi: 10.1136/bmj.1.5022.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprini E, Cristofoletti C, Arcelli D, Fadda P, Citterich M, Sampogna F, Magrelli A, Censi F, Torreri P, Frontani M, Scala E, Picchio MC, Temperani P, Monopoli A, Lombardo GA, Taruscio D, Narducci MG. Identification of key regions and genes important in the pathogenesis of sezary syndrome by combining genomic and expression microarrays. Cancer Research. 2009;68:137–146. doi: 10.1158/0008-5472.CAN-09-2367. [DOI] [PubMed] [Google Scholar]
- Capriotti E, Vonderheid EC, Thoburn CJ, Wasik MA, Bahler DW, Hess AD. Expression of T-plastin, FoxP3 and other tumor-associated markers by leukemic T-cells of cutaneous T-cell lymphoma. Leukemia Lymphoma. 2008;49:1190–1201. doi: 10.1080/10428190802064917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-kappaB addiction and its role in cancer: ‘one size does not fit all’. Oncogene. 30:1615–1630. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong BF, Wilson AJ, Gibson HM, Hafner MS, Luo Y, Hedgcock CJ, Wong HK. Immune function abnormalities in peripheral blood mononuclear cell cytokine expression differentiates stages of cutaneous T-cell lymphoma/mycosis fungoides. Clinical Cancer Research. 2008;14:646–653. doi: 10.1158/1078-0432.CCR-07-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong BF, Dantzer P, Germeroth T, Hafner M, Wilson AJ, Xiao G, Wong HK. Induced Sezary syndrome PBMCs poorly express immune response genes up-regulated in stimulated memory T cells. Journal of Dermatologic Science. 2010;60:8–20. doi: 10.1016/j.jdermsci.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chott A, Vonderheid EC, Olbricht S, Miao NN, Balk SP, Kadin ME. The dominant T cell clone is present in multiple regressing skin lesions and associated T cell lymphomas of patients with lymphomatoid papulosis. Journal Investigative Dermatology. 1996;106:696–700. doi: 10.1111/1523-1747.ep12345532. [DOI] [PubMed] [Google Scholar]
- Ciree A, Michel L, Camilleri-Broet S, Jean Louis F, Oster M, Flageul B, Senet P, Fossiez F, Fridman WH, Bachelez H, Tartour E. Expression and activity of IL-17 in cutaneous T-cell lymphomas (mycosis fungoides and Sezary syndrome) International Journal of Cancer. 2004;112:113–120. doi: 10.1002/ijc.20373. [DOI] [PubMed] [Google Scholar]
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Archives of Dermatology. 2007;143:854–859. doi: 10.1001/archderm.143.7.854. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Nikko A, Varghese A, Hoppe RT, Kim YH. Mycosis fungoides in young patients: clinical characteristics and outcome. Journal of American Academy of Dermatology. 1998;38:696–701. doi: 10.1016/s0190-9622(98)70198-7. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Kristeleit R, Tolcher A, Fong P, Pacey S, Karavasilis V, Mita M, Shaw H, Workman P, Kaye S, Rowinsky EK, Aherne W, Atadja P, Scott JW, Patnaik A. Phase I pharmacokinetic and pharmacodynamic study of LAQ824, a hydroxamate histone deacetylase inhibitor with a heat shock protein-90 inhibitory profile, in patients with advanced solid tumors. Clinical Cancer Research. 2008;14:6663–6673. doi: 10.1158/1078-0432.CCR-08-0376. [DOI] [PubMed] [Google Scholar]
- Dearden CE, Matutes E. Alemtuzumab in T-cell lymphoproliferative disorders. Best practice & research. Clinical haematology. 2006;19:795–810. doi: 10.1016/j.beha.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Deeths MJ, Chapman JT, Dellavalle RP, Zeng C, Aeling JL. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. Journal of the American Academy of Dermatology. 2005;52:275–280. doi: 10.1016/j.jaad.2004.04.049. [DOI] [PubMed] [Google Scholar]
- Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Dobbeling U, Dummer R, Laine E, Potoczna N, Qin JZ, Burg G. Interleukin-15 is an autocrine/paracrine viability factor for cutaneous T-cell lymphoma cells. Blood. 1998;92:252–258. [PubMed] [Google Scholar]
- Dooms H, Desmedt M, Vancaeneghem S, Rottiers P, Goossens V, Fiers W, Grooten J. Quiescence-inducing and antiapoptotic activities of IL-15 enhance secondary CD4+ T cell responsiveness to antigen. Journal of Immunology. 1998;161:2141–2150. [PubMed] [Google Scholar]
- Duarte RF, Canals C, Onida F, Gabriel IH, Arranz R, Arcese W, Ferrant A, Kobbe G, Narni F, Deliliers GL, Olavarria E, Schmitz N, Sureda A. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sezary syndrome: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Journal of Clinical Oncology. 28:4492–4499. doi: 10.1200/JCO.2010.29.3241. [DOI] [PubMed] [Google Scholar]
- Duarte RF, Schmitz N, Servitje O, Sureda A. Haematopoietic stem cell transplantation for patients with primary cutaneous T-cell lymphoma. Bone Marrow Transplantation. 2008;41:597–604. doi: 10.1038/sj.bmt.1705968. [DOI] [PubMed] [Google Scholar]
- Dummer R, Heald PW, Nestle FO, Ludwig E, Laine E, Hemmi S, Burg G. Sezary syndrome T-cell clones display T-helper 2 cytokines and express the accessory factor-1 (interferon-gamma receptor beta-chain) Blood. 1996;88:1383–1389. [PubMed] [Google Scholar]
- Dummer R, Geertsen R, Ludwig E, Niederer E, Burg G. Sezary syndrome, T-helper 2 cytokines and accessory factor-1 (AF-1) Leukemia and Lymphoma. 1998;28:515–522. doi: 10.3109/10428199809058359. [DOI] [PubMed] [Google Scholar]
- Dummer R, Urosevic M, Kempf W, Kazakov D, Burg G. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116–118. doi: 10.1159/000070962. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunology. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nature Review Immunology. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- Duvic M, Martin AG, Kim Y, Olsen E, Wood GS, Crowley CA, Yocum RC, Worldwide Bexarotene Study, G Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Archives of Dermatology. 2001;137:581–593. [PubMed] [Google Scholar]
- Duvic M, Kuzel TM, Olsen EA, Martin AG, Foss FM, Kim YH, Heald PW, Bacha P, Nichols J, Liepa A. Quality-of-life improvements in cutaneous T-cell lymphoma patients treated with denileukin diftitox (ONTAK) Clinical Lymphoma and Myeloma. 2002;2:222–228. doi: 10.3816/clm.2002.n.003. [DOI] [PubMed] [Google Scholar]
- Duvic M, Talpur R, Wen S, Kurzrock R, David CL, Apisarnthanarax N. Phase II evaluation of gemcitabine monotherapy for cutaneous T-cell lymphoma. Clinical lymphoma & myeloma. 2006a;7:51–58. doi: 10.3816/CLM.2006.n.039. [DOI] [PubMed] [Google Scholar]
- Duvic M, Forero A, Foss F, Olsen E, Kim Y. Oral Forodesine (Bcx-1777) is clinically active in refractory cutaneous T-cell lymphoma: Results of a phase I/II study. Blood. 2006b;108:2467. [Google Scholar]
- Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM, Frankel SR. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson RL. Cutaneous T cell lymphoma: the helping hand of dendritic cells. Annals of the New York Academy of Science. 2001;941:1–11. [PubMed] [Google Scholar]
- Ehlers S, Smith KA. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. Journal of Experimental Medicine. 1991;173:25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L, Pan Y, Smyth GK, George DJ, McCormack C, Williams-Truax R, Mita M, Beck J, Burris H, Ryan G, Atadja P, Butterfoss D, Dugan M, Culver K, Johnstone RW, Prince HM. Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clinical Cancer Research. 2008;14:4500–4510. doi: 10.1158/1078-0432.CCR-07-4262. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Suzuki K, Ponnappan A, VanDeusen JB, Cooper MA, Florea SM, Freud AG, Robinson ML, Durbin J, Caligiuri MA. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. Journal Experimental Medicine. 2001;193:219–231. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishwild DM, O’Donnell SL, Bengoechea T, Hudson DV, Harding F, Bernhard SL, Jones D, Kay RM, Higgins KM, Schramm SR, Lonberg N. High-avidity human IgG kappa monoclonal antibodies from a novel strain of minilocus transgenic mice. Nature Biotechnology. 1996;14:845–851. doi: 10.1038/nbt0796-845. [DOI] [PubMed] [Google Scholar]
- Foss F. Clinical experience with denileukin diftitox (ONTAK) Seminars in oncology. 2006;33:S11–16. doi: 10.1053/j.seminoncol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Frampton JE, Wagstaff AJ. Alemtuzumab. Drugs. 2003;63:1229–1243. doi: 10.2165/00003495-200363120-00003. discussion 1245–1226. [DOI] [PubMed] [Google Scholar]
- Gandhi V, Balakrishnan K. Pharmacology and mechanism of action of forodesine, a T-cell targeted agent. Seminars in oncology. 2007;34:S8–12. doi: 10.1053/j.seminoncol.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG, Faderl S, Koller C, Morris G, Rosner G, Loboda A, Fantin VR, Randolph SS, Hardwick JS, Reilly JF, Chen C, Ricker JL, Secrist JP, Richon VM, Frankel SR, Kantarjian HM. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008a;111:1060–1066. doi: 10.1182/blood-2007-06-098061. [DOI] [PubMed] [Google Scholar]
- Garcia-Manero G, Assouline S, Cortes J, Estrov Z, Kantarjian H, Yang H, Newsome WM, Miller WH, Jr, Rousseau C, Kalita A, Bonfils C, Dubay M, Patterson TA, Li Z, Besterman JM, Reid G, Laille E, Martell RE, Minden M. Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood. 2008b;112:981–989. doi: 10.1182/blood-2007-10-115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nature Review Drug Discovery. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar AF, Carney DN, Russell EK, Schechter GP, Bunn PA., Jr In vitro growth of cutaneous T-cell lymphomas. Cancer Treatment Reports. 1979;63:587–590. [PubMed] [Google Scholar]
- Giles F, Fischer T, Cortes J, Garcia-Manero G, Beck J, Ravandi F, Masson E, Rae P, Laird G, Sharma S, Kantarjian H, Dugan M, Albitar M, Bhalla K. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clinical Cancer Research. 2006;12:4628–4635. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
- Gimsing P, Hansen M, Knudsen LM, Knoblauch P, Christensen IJ, Ooi CE, Buhl-Jensen P. A phase I clinical trial of the histone deacetylase inhibitor belinostat in patients with advanced hematological neoplasia. European Journal of Haematology. 2008;81:170–176. doi: 10.1111/j.1600-0609.2008.01102.x. [DOI] [PubMed] [Google Scholar]
- Ginaldi L, De Martinis M, Matutes E, Farahat N, Morilla R, Dyer MJ, Catovsky D. Levels of expression of CD52 in normal and leukemic B and T cells: correlation with in vivo therapeutic responses to Campath-1H. Leukemia Research. 1998;22:185–191. doi: 10.1016/s0145-2126(97)00158-6. [DOI] [PubMed] [Google Scholar]
- Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350:1978–1988. doi: 10.1056/NEJMra032810. [DOI] [PubMed] [Google Scholar]
- Gore L, Rothenberg ML, O’Bryant CL, Schultz MK, Sandler AB, Coffin D, McCoy C, Schott A, Scholz C, Eckhardt SG. A phase I and pharmacokinetic study of the oral histone deacetylase inhibitor, MS-275, in patients with refractory solid tumors and lymphomas. Clinical Cancer Research. 2008;14:4517–4525. doi: 10.1158/1078-0432.CCR-07-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis Research and Therapy. 2003;5:225–234. doi: 10.1186/ar974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner D, Olsen EA, Petroni G. Pentostatin (2′-deoxycoformycin) in the treatment of cutaneous T-cell lymphoma. Journal of the American Academy of Dermatology. 1997;36:950–955. doi: 10.1016/s0190-9622(97)80279-4. [DOI] [PubMed] [Google Scholar]
- Hallermann C, Kaune KM, Tiemann M, Kunze E, Griesinger F, Mitteldorf C, Bertsch HP, Neumann C. High frequency of primary cutaneous lymphomas associated with lymphoproliferative disorders of different lineage. Annals of Hematology. 2007;86:509–515. doi: 10.1007/s00277-007-0276-8. [DOI] [PubMed] [Google Scholar]
- Hahtola S, Tuomela S, Elo L, Hakkinen T, Karenko L, Nedoszytko B, Heikkila H, Saarialho-Kere U, Roszkiewicz J, Aittokallio T, Lahesmaa R, Ranki A. Th1 response and cytotoxicity genes are down-regulated in cutaneous T-cell lymphoma. Clinical Cancer Research. 2006;12:4812–4821. doi: 10.1158/1078-0432.CCR-06-0532. [DOI] [PubMed] [Google Scholar]
- Hodak E, Klein T, Gabay B, Ben-Amitai D, Bergman R, Gdalevich M, Feinmesser M, Maron L, David M. Familial mycosis fungoides: report of 6 kindreds and a study of the HLA system. Journal of the American Academy of Dermatology. 2005;52:393–402. doi: 10.1016/j.jaad.2003.12.052. [DOI] [PubMed] [Google Scholar]
- Hoppe RT, Medeiros LJ, Warnke RA, Wood GS. CD8-positive tumor-infiltrating lymphocytes influence the long-term survival of patients with mycosis fungoides. Journal of the American Academy of Dermatology. 1995;32:448–453. doi: 10.1016/0190-9622(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Hwang ST, Janik JE, Jaffe ES, Wilson WH. Mycosis fungoides and Sezary syndrome. Lancet. 2008;371:945–957. doi: 10.1016/S0140-6736(08)60420-1. [DOI] [PubMed] [Google Scholar]
- Ito A, Ishida T, Yano H, Inagaki A, Suzuki S, Sato F, Takino H, Mori F, Ri M, Kusumoto S, Komatsu H, Iida S, Inagaki H, Ueda R. Defucosylated anti-CCR4 monoclonal antibody exercises potent ADCC-mediated antitumor effect in the novel tumor-bearing humanized NOD/Shi-scid, IL-2Rgamma(null) mouse model. Cancer Immunology Immunotherapy. 2009;58:1195–1206. doi: 10.1007/s00262-008-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izykowska K, Przybylski GK. Genetic alterations in Sezary syndrome. Leukemia and Lymphoma. 2011;52:745–753. doi: 10.3109/10428194.2010.551159. [DOI] [PubMed] [Google Scholar]
- Jackow CM, McHam JB, Friss A, Alvear J, Reveille JR, Duvic M. HLA-DR5 and DQB1*03 class II alleles are associated with cutaneous T-cell lymphoma. Journal of Investigative Dermatology. 1996;107:373–376. doi: 10.1111/1523-1747.ep12363352. [DOI] [PubMed] [Google Scholar]
- Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood. 2008;112:4384–4399. doi: 10.1182/blood-2008-07-077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D, Duvic M. The current state and future of clonality studies in mycosis fungoides. Journal of Investigative Dermatology. 2003;121:ix–x. doi: 10.1046/j.1523-1747.2003.12458.x. [DOI] [PubMed] [Google Scholar]
- Jumbou O, N’Guyen JM, Tessier MH, Legoux B, Dreno B. Long-term follow-up in 51 patients with mycosis fungoides and Sezary syndrome treated by interferon-alfa. The British Journal of Dermatology. 1999;140:427–431. doi: 10.1046/j.1365-2133.1999.02704.x. [DOI] [PubMed] [Google Scholar]
- Juvekar A, Manna S, Ramaswami S, Chang TP, Vu HY, Ghosh CC, Celiker MY, Vancurova I. Bortezomib induces nuclear translocation of IκBα resulting in gene-specific suppression of NF-κB–dependent transcription and induction of apoptosis in CTCL. Molecular Cancer Research. 2011;9:183–94. doi: 10.1158/1541-7786.MCR-10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma T, Sugaya M, Nakamura K, Kaneko F, Wakugawa M, Matsushima K, Tamaki K. Thymus and activation-regulated chemokine (TARC/CCL17) in mycosis fungoides: serum TARC levels reflect the disease activity of mycosis fungoides. Journal of the American Academy of Dermatology. 2003;48:23–30. doi: 10.1067/mjd.2003.132. [DOI] [PubMed] [Google Scholar]
- Kallinich T, Muche JM, Qin S, Sterry W, Audring H, Kroczek RA. Chemokine receptor expression on neoplastic and reactive T cells in the skin at different stages of mycosis fungoides. Journal of Investigative Dermatology. 2003;121:1045–1052. doi: 10.1046/j.1523-1747.2003.12555.x. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proceedings of the National Academy of Science U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari L, Loboda A, Nebozhyn M, Rook AH, Vonderheid EC, Nichols C, Virok D, Chang C, Horng WH, Johnston J, Wysocka M, Showe MK, Showe LC. Classification and prediction of survival in patients with the leukemic phase of cutaneous T cell lymphoma. Journal of Experimental Medicine. 2003;197:1477–1488. doi: 10.1084/jem.20021726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye FJ, Bunn PA, Jr, Steinberg SM, Stocker JL, Ihde DC, Fischmann AB, Glatstein EJ, Schechter GP, Phelps RM, Foss FM, et al. A randomized trial comparing combination electron-beam radiation and chemotherapy with topical therapy in the initial treatment of mycosis fungoides. New England Journal of Medicine. 1989;321:1784–1790. doi: 10.1056/NEJM198912283212603. [DOI] [PubMed] [Google Scholar]
- Kennah E, Ringrose A, Zhou LL, Esmailzadeh S, Qian H, Su MW, Zhou Y, Jiang X. Identification of tyrosine kinase, HCK, and tumor suppressor, BIN1, as potential mediators of AHI-1 oncogene in primary and transformed CTCL cells. Blood. 2009;113:4646–4655. doi: 10.1182/blood-2008-08-174037. [DOI] [PubMed] [Google Scholar]
- Kim YH, Hoppe RT. Mycosis fungoides and the Sezary syndrome. Seminars in Oncology. 1999;26:276–289. [PubMed] [Google Scholar]
- Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Archives of Dermatology. 2003;139:857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- Kim YH, Duvic M, Obitz E, Gniadecki R, Iversen L, Osterborg A, Whittaker S, Illidge TM, Schwarz T, Kaufmann R, Cooper K, Knudsen KM, Lisby S, Baadsgaard O, Knox SJ. Clinical efficacy of zanolimumab (HuMax-CD4): two phase 2 studies in refractory cutaneous T-cell lymphoma. Blood. 2007;109:4655–4662. doi: 10.1182/blood-2006-12-062877. [DOI] [PubMed] [Google Scholar]
- Kim YH, Girardi M, Duvic M, Kuzel T, Link BK, Pinter-Brown L, Rook AH. Phase I trial of a Toll-like receptor 9 agonist, PF-3512676 (CPG 7909), in patients with treatment-refractory, cutaneous T-cell lymphoma. Journal of the American Academy of Dermatology. 2010;63:975–983. doi: 10.1016/j.jaad.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nature Review Immunology. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- Klemke CD, Mansmann U, Poenitz N, Dippel E, Goerdt S. Prognostic factors and prediction of prognosis by the CTCL Severity Index in mycosis fungoides and Sezary syndrome. British Journal of Dermatology. 2005;153:118–124. doi: 10.1111/j.1365-2133.2005.06676.x. [DOI] [PubMed] [Google Scholar]
- Krejsgaard T, Ralfkiaer U, Clasen-Linde E, Eriksen KW, Kopp KL, Bonefeld CM, Geisler C, Dabelsteen S, Wasik MA, Ralfkiaer E, Woetmann A, Odum N. Malignant Cutaneous T-Cell Lymphoma Cells Express IL-17 Utilizing the Jak3/Stat3 Signaling Pathway. Journal of Investigative Dermatology. 2011;131:1331–1338. doi: 10.1038/jid.2011.27. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Warke VG, Nambiar MP, Wong HK, Tsokos GC, Farber DL. Generation and biochemical analysis of human effector CD4 T cells: alterations in tyrosine phosphorylation and loss of CD3zeta expression. Blood. 2001;97:3851–3859. doi: 10.1182/blood.v97.12.3851. [DOI] [PubMed] [Google Scholar]
- Kunishige JH, McDonald H, Alvarez G, Johnson M, Prieto V, Duvic M. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clinical Experimental Dermatology. 2009;34:576–581. doi: 10.1111/j.1365-2230.2008.03024.x. [DOI] [PubMed] [Google Scholar]
- Kurzrock R, Pilat S, Duvic M. Pentostatin therapy of T-cell lymphomas with cutaneous manifestations. Journal of Clinical Oncology. 1999;17:3117–3121. doi: 10.1200/JCO.1999.17.10.3117. [DOI] [PubMed] [Google Scholar]
- Laharanne E, Oumouhou N, Bonnet F, Carlotti M, Gentil C, Chevret E, Jouary T, Longy M, Vergier B, Beylot-Barry M, Merlio JP. Genome-wide analysis of cutaneous T-cell lymphomas identifies three clinically relevant classes. Journal of Investigative Dermatology. 2010;130:1707–1718. doi: 10.1038/jid.2010.8. [DOI] [PubMed] [Google Scholar]
- Li G, Yu M, Weyand CM, Goronzy JJ. Epigenetic regulation of killer immunoglobulin-like receptor expression in T cells. Blood. 2009;114:3422–3430. doi: 10.1182/blood-2009-01-200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov IV, Jones DA, Sasseville D, Kupper TS. Transcriptional profiles predict disease outcome in patients with cutaneous T-cell lymphoma. Clinical Cancer Research. 2010;16:2106–2114. doi: 10.1158/1078-0432.CCR-09-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- Lundin J, Hagberg H, Repp R, Cavallin-Stahl E, Freden S, Juliusson G, Rosenblad E, Tjonnfjord G, Wiklund T, Osterborg A. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood. 2003;101:4267–4272. doi: 10.1182/blood-2002-09-2802. [DOI] [PubMed] [Google Scholar]
- Malamut G, El Machhour R, Montcuquet N, Martin-Lanneree S, Dusanter-Fourt I, Verkarre V, Mention JJ, Rahmi G, Kiyono H, Butz EA, Brousse N, Cellier C, Cerf-Bensussan N, Meresse B. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. Journal of Clinical Investigation. 2010;120:2131–2143. doi: 10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi E, Alinari L, Tani M, Stefoni V, Pimpinelli N, Berti E, Pagano L, Bernengo MG, Zaja F, Rupoli S, Pileri S, Baccarani M, Zinzani PL. Gemcitabine as frontline treatment for cutaneous T-cell lymphoma: phase II study of 32 patients. Cancer. 2005;104:2437–2441. doi: 10.1002/cncr.21449. [DOI] [PubMed] [Google Scholar]
- Marie-Cardine A, Huet D, Ortonne N, Remtoula N, Le Gouvello S, Bagot M, Bensussan A. Killer cell Ig-like receptors CD158a and CD158b display a coactivatory function, involving the c-Jun NH2-terminal protein kinase signaling pathway, when expressed on malignant CD4+ T cells from a patient with Sezary syndrome. Blood. 2007;109:5064–5065. doi: 10.1182/blood-2007-02-071993. [DOI] [PubMed] [Google Scholar]
- Mao X, Orchard G, Lillington DM, Russell-Jones R, Young BD, Whittaker SJ. Amplification and overexpression of JUNB is associated with primary cutaneous T-cell lymphomas. Blood. 2003;101:1513–1519. doi: 10.1182/blood-2002-08-2434. [DOI] [PubMed] [Google Scholar]
- Mao X, Orchard G, Lillington DM, Child FJ, Vonderheid EC, Nowell PC, Bagot M, Bensussan A, Russell-Jones R, Young BD, Whittaker SJ. BCL2 and JUNB abnormalities in primary cutaneous lymphomas. Br J Dermatol. 2004;151:546–556. doi: 10.1111/j.1365-2133.2004.06106.x. [DOI] [PubMed] [Google Scholar]
- Mao X, Orchard G, Vonderheid EC, Nowell PC, Bagot M, Bensussan A, Russell-Jones R, Young BD, Whittaker SJ. Heterogeneous abnormalities of CCND1 and RB1 in primary cutaneous T-Cell lymphomas suggesting impaired cell cycle control in disease pathogenesis. Journal of Investigative Dermatology. 2006;126:1388–1395. doi: 10.1038/sj.jid.5700224. [DOI] [PubMed] [Google Scholar]
- Mao X, Orchard G, Mitchell TJ, Oyama N, Russell-Jones R, Vermeer MH, Willemze R, van Doorn R, Tensen CP, Young BD, Whittaker SJ. A genomic and expression study of AP-1 in primary cutaneous T-cell lymphoma: evidence for dysregulated expression of JUNB and JUND in MF and SS. Journal of Cutaneous Patholology. 2008 doi: 10.1111/j.1600-0560.2007.00924.x. [DOI] [PubMed] [Google Scholar]
- Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nature Review Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- McCusker ME, Garifallou M, Bogen SA. Sezary lineage cells can be induced to proliferate via CD28-mediated costimulation. Journal of Immunoogyl. 1997;158:4984–4991. [PubMed] [Google Scholar]
- McGinnis KS, Junkins-Hopkins JM, Crawford G, Shapiro M, Rook AH, Vittorio CC. Low-dose oral bexarotene in combination with low-dose interferon alfa in the treatment of cutaneous T-cell lymphoma: clinical synergism and possible immunologic mechanisms. Journal of the American Academy of Dermatology. 2004;50:375–379. doi: 10.1016/j.jaad.2003.10.669. [DOI] [PubMed] [Google Scholar]
- McInturff JE, Modlin RL, Kim J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. Journal of Investigative Dermatology. 2005;125:1–8. doi: 10.1111/j.0022-202X.2004.23459.x. [DOI] [PubMed] [Google Scholar]
- Medenica M, Lorincz AL. Pagetoid reticulosis (Woringer-Kolopp disease). Histopathologic and ultrastructural observations. Archives of Dermatology. 1978;114:262–268. [PubMed] [Google Scholar]
- Mirvish ED, Pomerantz RG, Geskin LJ. Infectious agents in cutaneous T-cell lymphoma. Journal of American Academy of Dermatology. 2011;64:423–431. doi: 10.1016/j.jaad.2009.11.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr B, Illmer T, Oelschlagel U, Nowak R, Holig K, Paaz U, Kroschinsky F, Schuler U, Ehninger G. Complex cytogenetic and immunophenotypic aberrations in a patient with Sezary syndrome. Cancer Genetics Cytogenetics. 1996;90:33–36. doi: 10.1016/0165-4608(96)00038-6. [DOI] [PubMed] [Google Scholar]
- Morales MM, Putcha V, Evans HS, Olsen J, Llopis A, Moller H. Survival of mycosis fungoides in patients in the Southeast of England. Dermatology. 2005;211:325–329. doi: 10.1159/000088501. [DOI] [PubMed] [Google Scholar]
- Morales Suarez-Varela MM, Llopis Gonzalez A, Marquina Vila A, Bell J. Mycosis fungoides: review of epidemiological observations. Dermatology. 2000;201:21–28. doi: 10.1159/000018423. [DOI] [PubMed] [Google Scholar]
- Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610–618. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Fullen D, Carlson JA. Low CD7 expression in benign and malignant cutaneous lymphocytic infiltrates: experience with an antibody reactive with paraffin-embedded tissue. American Journal of Dermatopathology. 2002;24:6–16. doi: 10.1097/00000372-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Narducci MG, Arcelli D, Picchio MC, Lazzeri C, Pagani E, Sampogna F, Scala E, Fadda P, Cristofoletti C, Facchiano A, Frontani M, Monopoli A, Ferracin M, Negrini M, Lombardo GA, Caprini E, Russo G. MicroRNA profiling reveals that miR-21, miR486 and miR-214 are upregulated and involved in cell survival in Sezary syndrome. Cell Death Disease. 2011;2:e151. doi: 10.1038/cddis.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X, Hazarika P, Zhang C, Talpur R, Duvic M. Fas ligand expression by neoplastic T lymphocytes mediates elimination of CD8+ cytotoxic T lymphocytes in mycosis fungoides: a potential mechanism of tumor immune escape? Clinical Cancer Research. 2001;7:2682–2692. [PubMed] [Google Scholar]
- Nickoloff BJ. Light-microscopic assessment of 100 patients with patch/plaque-stage mycosis fungoides. American Journal of Dermatopathology. 1988;10:469–477. doi: 10.1097/00000372-198812000-00001. [DOI] [PubMed] [Google Scholar]
- Nunez V, Alameda D, Rico D, Mota R, Gonzalo P, Cedenilla M, Fischer T, Bosca L, Glass CK, Arroyo AG, Ricote M. Retinoid X receptor alpha controls innate inflammatory responses through the up-regulation of chemokine expression. Proceedings of the Nattional Academy of Science U S A. 107:10626–10631. doi: 10.1073/pnas.0913545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor OA, Horwitz S, Hamlin P, Portlock C, Moskowitz CH, Sarasohn D, Neylon E, Mastrella J, Hamelers R, Macgregor-Cortelli B, Patterson M, Seshan VE, Sirotnak F, Fleisher M, Mould DR, Saunders M, Zelenetz AD. Phase II-I-II study of two different doses and schedules of pralatrexate, a high-affinity substrate for the reduced folate carrier, in patients with relapsed or refractory lymphoma reveals marked activity in T-cell malignancies. Journal of Clinical Oncology. 2009;27:4357–4364. doi: 10.1200/JCO.2008.20.8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor OA, Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B, Jo Lechowicz M, Savage KJ, Shustov AR, Gisselbrecht C, Jacobsen E, Zinzani PL, Furman R, Goy A, Haioun C, Crump M, Zain JM, Hsi E, Boyd A, Horwitz S. Pralatrexate in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results From the Pivotal PROPEL Study. Journal of Clinical Oncology. 29:1182–1189. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony D, Morris JC, Stetler-Stevenson M, Matthews H, Brown MR, Fleisher T, Pittaluga S, Raffeld M, Albert PS, Reitsma D, Kaucic K, Hammershaimb L, Waldmann TA, Janik JE. EBV-related lymphoproliferative disease complicating therapy with the anti-CD2 monoclonal antibody, siplizumab, in patients with T-cell malignancies. Clinical Cancer Research. 2009;15:2514–2522. doi: 10.1158/1078-0432.CCR-08-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen E, Duvic M, Frankel A, Kim Y, Martin A, Vonderheid E, Jegasothy B, Wood G, Gordon M, Heald P, Oseroff A, Pinter-Brown L, Bowen G, Kuzel T, Fivenson D, Foss F, Glode M, Molina A, Knobler E, Stewart S, Cooper K, Stevens S, Craig F, Reuben J, Bacha P, Nichols J. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. Journal of Clinical Oncology. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, Zackheim H, Duvic M, Estrach T, Lamberg S, Wood G, Dummer R, Ranki A, Burg G, Heald P, Pittelkow M, Bernengo MG, Sterry W, Laroche L, Trautinger F, Whittaker S. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007a;110:1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- Olsen EA. Interferon in the treatment of cutaneous T-cell lymphoma. Dermatologic Therapy. 2003;16:311–321. doi: 10.1111/j.1396-0296.2003.01643.x. [DOI] [PubMed] [Google Scholar]
- Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, Frankel SR, Chen C, Ricker JL, Arduino JM, Duvic M. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. Journal of Clin Oncol. 2007b;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- Olsen EA, Rook AH, Zic J, Kim Y, Porcu P, Querfeld C, Wood G, Demierre MF, Pittelkow M, Wilson LD, Pinter-Brown L, Advani R, Parker S, Kim EJ, Junkins-Hopkins JM, Foss F, Cacchio P, Duvic M. Sezary syndrome: immunopathogenesis, literature review of therapeutic options, and recommendations for therapy by the United States Cutaneous Lymphoma Consortium (USCLC) Journal of the American Academy of Dermatology. 2011;64:352–404. doi: 10.1016/j.jaad.2010.08.037. [DOI] [PubMed] [Google Scholar]
- Piekarz RL, Robey R, Sandor V, Bakke S, Wilson WH, Dahmoush L, Kingma DM, Turner ML, Altemus R, Bates SE. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: a case report. Blood. 2001;98:2865–2868. doi: 10.1182/blood.v98.9.2865. [DOI] [PubMed] [Google Scholar]
- Piekarz RL, Robey RW, Zhan Z, Kayastha G, Sayah A, Abdeldaim AH, Torrico S, Bates SE. T-cell lymphoma as a model for the use of histone deacetylase inhibitors in cancer therapy: impact of depsipeptide on molecular markers, therapeutic targets, and mechanisms of resistance. Blood. 2004;103:4636–4643. doi: 10.1182/blood-2003-09-3068. [DOI] [PubMed] [Google Scholar]
- Piekarz RL, Frye R, Turner M, Wright JJ, Allen SL, Kirschbaum MH, Zain J, Prince HM, Leonard JP, Geskin LJ, Reeder C, Joske D, Figg WD, Gardner ER, Steinberg SM, Jaffe ES, Stetler-Stevenson M, Lade S, Fojo AT, Bates SE. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. Journal of Clinical Oncology. 2009;27:5410–5417. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpinelli N, Olsen EA, Santucci M, Vonderheid E, Haeffner AC, Stevens S, Burg G, Cerroni L, Dreno B, Glusac E, Guitart J, Heald PW, Kempf W, Knobler R, Lessin S, Sander C, Smoller BS, Telang G, Whittaker S, Iwatsuki K, Obitz E, Takigawa M, Turner ML, Wood GS. Defining early mycosis fungoides. Journal of the American Academy of Dermatology. 2005;53:1053–1063. doi: 10.1016/j.jaad.2005.08.057. [DOI] [PubMed] [Google Scholar]
- Pomerantz RG, Mirvish ED, Erdos G, Falo LD, Jr, Geskin LJ. Novel approach to gene expression profiling in Sezary syndrome. British Journal of Dermatology. 2010;163:1090–1094. doi: 10.1111/j.1365-2133.2010.09973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope E, Weitzman S, Ngan B, Walsh S, Morel K, Williams J, Stein S, Garzon M, Knobler E, Lieber C, Turchan K, Wargon O, Tsuchiya A. Mycosis fungoides in the pediatric population: report from an international Childhood Registry of Cutaneous Lymphoma. Journal of Cutaneous Medicine and Surgury. 2010;14:1–6. doi: 10.2310/7750.2009.08091. [DOI] [PubMed] [Google Scholar]
- Posner LE, Fossieck BE, Jr, Eddy JL, Bunn PA., Jr Septicemic complications of the cutaneous T-cell lymphomas. American Journal of Medicine. 1981;71:210–216. doi: 10.1016/0002-9343(81)90107-8. [DOI] [PubMed] [Google Scholar]
- Poszepczynska-Guigne E, Schiavon V, D’Incan M, Echchakir H, Musette P, Ortonne N, Boumsell L, Moretta A, Bensussan A, Bagot M. CD158k/KIR3DL2 is a new phenotypic marker of Sezary cells: relevance for the diagnosis and follow-up of Sezary syndrome. Journal of Investigative Dermatology. 2004;122:820–823. doi: 10.1111/j.0022-202X.2004.22326.x. [DOI] [PubMed] [Google Scholar]
- Prince HM, Duvic M, Martin A, Sterry W, Assaf C, Sun Y, Straus D, Acosta M, Negro-Vilar A. Phase III placebo-controlled trial of denileukin diftitox for patients with cutaneous T-cell lymphoma. Journal of Clinical Oncology. 2010;28:1870–1877. doi: 10.1200/JCO.2009.26.2386. [DOI] [PubMed] [Google Scholar]
- Pulini S, Rupoli S, Goteri G, Pimpinelli N, Alterini R, Tassetti A, Scortechini AR, Offidani M, Mulattieri S, Stronati A, Brandozzi G, Giacchetti A, Mozzicafreddo G, Ricotti G, Filosa G, Bettacchi A, Simonacci M, Novelli N, Leoni P. Pegylated liposomal doxorubicin in the treatment of primary cutaneous T-cell lymphomas. Haematologica. 2007;92:686–689. doi: 10.3324/haematol.10879. [DOI] [PubMed] [Google Scholar]
- Querfeld C, Rosen ST, Guitart J, Rademaker A, Fung BB, Posten W, Kuzel TM. Comparison of selective retinoic acid receptor- and retinoic X receptor-mediated efficacy, tolerance, and survival in cutaneous t-cell lymphoma. Journal of the American Academy of Dermatology. 2004;51:25–32. doi: 10.1016/j.jaad.2003.11.058. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. Journal of Clinical Oncology. 2005;23:630–639. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Ravat FE, Spittle MF, Russell-Jones R. Primary cutaneous T-cell lymphoma occurring after organ transplantation. Journal of the American Academy of Dermatology. 2006;54:668–675. doi: 10.1016/j.jaad.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. Journal of Experimental Medicine. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond J, Tuzova M, Parks A, Adams N, Martin E, Tawa M, Morrison L, Chaney K, Kupper TS, Curiel-Lewandrowski C, Cruikshank W. Interleukin-16 as a marker of Sézary syndrome onset and stage. Journal of Clinical Immunology. 2011;31:39–50. doi: 10.1007/s10875-010-9464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider DA, Havenith CE, de Ridder R, Schuurman J, Favre C, Cooper JC, Walker S, Baadsgaard O, Marschner S, vandeWinkel JG, Cambier J, Parren PW, Alexander DR. A human CD4 monoclonal antibody for the treatment of T-cell lymphoma combines inhibition of T-cell signaling by a dual mechanism with potent Fc-dependent effector activity. Cancer Research. 2007;67:9945–9953. doi: 10.1158/0008-5472.CAN-07-1148. [DOI] [PubMed] [Google Scholar]
- Robson A. Immunocytochemistry and the diagnosis of cutaneous lymphoma. Histopathology. 2010;56:71–90. doi: 10.1111/j.1365-2559.2009.03457.x. [DOI] [PubMed] [Google Scholar]
- Rupoli S, Goteri G, Pulini S, Filosa A, Tassetti A, Offidani M, Filosa G, Mozzicafreddo G, Giacchetti A, Brandozzi G, Cataldi I, Barulli S, Ranaldi R, Scortechini AR, Capretti R, Fabris G, Leoni P. Long-term experience with low-dose interferon-alpha and PUVA in the management of early mycosis fungoides. European J ournal of Haematology. 2005;75:136–145. doi: 10.1111/j.1600-0609.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- Saed G, Fivenson DP, Naidu Y, Nickoloff BJ. Mycosis fungoides exhibits a Th1-type cell-mediated cytokine profile whereas Sezary syndrome expresses a Th2-type profile. Journal of Investigative Dermatology. 1994;103:29–33. doi: 10.1111/1523-1747.ep12388985. [DOI] [PubMed] [Google Scholar]
- Samimi S, Benoit B, Evans K, Wherry EJ, Showe L, Wysocka M, Rook AH. Increased programmed death-1 expression on CD4+ T cells in cutaneous T-cell lymphoma: implications for immune suppression. Archives of Dermatology. 146:1382–1388. doi: 10.1001/archdermatol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Shin J, Monti S, Aires DJ, Duvic M, Golub T, Jones DA, Kupper TS. Lesional gene expression profiling in cutaneous T-cell lymphoma reveals natural clusters associated with disease outcome. Blood. 2007;110:3015–3027. doi: 10.1182/blood-2006-12-061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotnak FM, DeGraw JI, Colwell WT, Piper JR. A new analogue of 10-deazaaminopterin with markedly enhanced curative effects against human tumor xenografts in mice. Cancer Chemotherapy and Pharmacology. 1998;42:313–318. doi: 10.1007/s002800050823. [DOI] [PubMed] [Google Scholar]
- Sterry W, Mielke V. CD4+ cutaneous T-cell lymphomas show the phenotype of helper/inducer T cells (CD45RA−, CDw29+) Journal of Investigative Dermatology. 1989;93:413–416. [PubMed] [Google Scholar]
- Strauss SJ, Maharaj L, Hoare S, Johnson PW, Radford JA, Vinnecombe S, Millard L, Rohatiner A, Boral A, Trehu E, Schenkein D, Balkwill F, Joel SP, Lister TA. Bortezomib therapy in patients with relapsed or refractory lymphoma: potential correlation of in vitro sensitivity and tumor necrosis factor alpha response with clinical activity. Journal of Clinical Oncology. 2006;24:2105–2112. doi: 10.1200/JCO.2005.04.6789. [DOI] [PubMed] [Google Scholar]
- Su MW, Dorocicz I, Dragowska WH, Ho V, Li G, Voss N, Gascoyne R, Zhou Y. Aberrant expression of T-plastin in Sezary cells. Cancer Research. 2003;63:7122–7127. [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. 2008. [Google Scholar]
- Talpur R, Jones DM, Alencar AJ, Apisarnthanarax N, Herne KL, Yang Y, Duvic M. CD25 expression is correlated with histological grade and response to denileukin diftitox in cutaneous T-cell lymphoma. Journal of Investigative Dermatology. 2006;126:575–583. doi: 10.1038/sj.jid.5700122. [DOI] [PubMed] [Google Scholar]
- Tan C, Waldmann TA. Proteasome inhibitor PS-341, a potential therapeutic agent for adult T-cell leukemia. Cancer Research. 2002;62:1083–1086. [PubMed] [Google Scholar]
- Tang N, Gibson H, Germeroth T, Porcu P, Lim HW, Wong HK. T-plastin (PLS3) gene expression differentiates Sezary syndrome from mycosis fungoides and inflammatory skin diseases and can serve as a biomarker to monitor disease progression. British Journal of Dermatology. 2010;162:463–466. doi: 10.1111/j.1365-2133.2009.09587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. Journal of Investigative Dermatology. 1998;111:645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- Thestrup-Pedersen K, Hammer R, Kaltoft K, Sogaard H, Zachariae H. Treatment of mycosis fungoides with recombinant interferon-alpha 2a2 alone and in combination with etretinate. British Journal of Dermatology. 1988;118:811–818. doi: 10.1111/j.1365-2133.1988.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Thomas L. Reactions to homologous tissue antigens in relation to hypersensitivity [Discussion] In: Lawrence H, editor. Cellular and Humoral Aspects of the Hypersensitive States. Hoeber-Harper; New York: 1959. pp. 529–532. [Google Scholar]
- Tiemessen MM, Mitchell TJ, Hendry L, Whittaker SJ, Taams LS, John S. Lack of suppressive CD4+CD25+FOXP3+ T cells in advanced stages of primary cutaneous T-cell lymphoma. Journal of Investigative Dermatology. 2006;126:2217–2223. doi: 10.1038/sj.jid.5700371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey L, Villuendas R, Dotor AM, Spiteri I, Ortiz P, Garcia JF, Peralto JL, Lawler M, Piris MA. Mycosis fungoides shows concurrent deregulation of multiple genes involved in the TNF signaling pathway: an expression profile study. Blood. 2003;102:1042–1050. doi: 10.1182/blood-2002-11-3574. [DOI] [PubMed] [Google Scholar]
- Trautinger F, Knobler R, Willemze R, Peris K, Stadler R, Laroche L, D’Incan M, Ranki A, Pimpinelli N, Ortiz-Romero P, Dummer R, Estrach T, Whittaker S. EORTC consensus recommendations for the treatment of mycosis fungoides/Sezary syndrome. European Journal of Cancer. 2006;42:1014–1030. doi: 10.1016/j.ejca.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Type I interferon: friend or foe? Journal of Experimental Medicine. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsimberidou AM, Giles F, Duvic M, Fayad L, Kurzrock R. Phase II study of pentostatin in advanced T-cell lymphoid malignancies: update of an M.D. Anderson Cancer Center series. Cancer. 2004;100:342–349. doi: 10.1002/cncr.11899. [DOI] [PubMed] [Google Scholar]
- van Doorn R, Van Haselen CW, van Voorst Vader PC, Geerts ML, Heule F, de Rie M, Steijlen PM, Dekker SK, van Vloten WA, Willemze R. Mycosis fungoides: disease evolution and prognosis of 309 Dutch patients. Archives of Dermatology. 2000;136:504–510. doi: 10.1001/archderm.136.4.504. [DOI] [PubMed] [Google Scholar]
- van Doorn R, Dijkman R, Vermeer MH, Out-Luiting JJ, van der Raaij-Helmer EM, Willemze R, Tensen CP. Aberrant expression of the tyrosine kinase receptor EphA4 and the transcription factor twist in Sezary syndrome identified by gene expression analysis. Cancer Research. 2004;64:5578–5586. doi: 10.1158/0008-5472.CAN-04-1253. [DOI] [PubMed] [Google Scholar]
- van Doorn R, van Kester MS, Dijkman R, Vermeer MH, Mulder AA, Szuhai K, Knijnenburg J, Boer JM, Willemze R, Tensen CP. Oncogenomic analysis of mycosis fungoides reveals major differences with Sezary syndrome. Blood. 2009;113:127–136. doi: 10.1182/blood-2008-04-153031. [DOI] [PubMed] [Google Scholar]
- van Doorn R, Zoutman WH, Dijkman R, de Menezes RX, Commandeur S, Mulder AA, van der Velden PA, Vermeer MH, Willemze R, Yan PS, Huang TH, Tensen CP. Epigenetic profiling of cutaneous T-cell lymphoma: promoter hypermethylation of multiple tumor suppressor genes including BCL7a, PTPRG, and p73. Journal of Clinical Oncology. 2005;23:3886–3896. doi: 10.1200/JCO.2005.11.353. [DOI] [PubMed] [Google Scholar]
- van Kester MS, Ballabio E, Benner MF, Chen XH, Saunders NJ, van der Fits L, van Doorn R, Vermeer MH, Willemze R, Tensen CP, Lawrie CH. miRNA expression profiling of mycosis fungoides. Molecular Oncology. 2011;5:273–280. doi: 10.1016/j.molonc.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer MH, van Doorn R, Dijkman R, Mao X, Whittaker S, van Voorst Vader PC, Gerritsen MJ, Geerts ML, Gellrich S, Soderberg O, Leuchowius KJ, Landegren U, Out-Luiting JJ, Knijnenburg J, Ijszenga M, Szuhai K, Willemze R, Tensen CP. Novel and highly recurrent chromosomal alterations in Sezary syndrome. Cancer Research. 2008;68:2689–2698. doi: 10.1158/0008-5472.CAN-07-6398. [DOI] [PubMed] [Google Scholar]
- Vonderheid EC. On the diagnosis of erythrodermic cutaneous T-cell lymphoma. Journal of Cutaneous Pathology. 2006;33(Suppl 1):27–42. doi: 10.1111/j.0303-6987.2006.00541.x. [DOI] [PubMed] [Google Scholar]
- Vonderheid EC, Tan E, Sobel EL, Schwab E, Micaily B, Jegasothy BV. Clinical implications of immunologic phenotyping in cutaneous T cell lymphoma. Journal of the American Academy of Dermatology. 1987a;17:40–52. doi: 10.1016/s0190-9622(87)70168-6. [DOI] [PubMed] [Google Scholar]
- Vonderheid EC, Thompson R, Smiles KA, Lattanand A. Recombinant interferon alfa-2b in plaque-phase mycosis fungoides. Intralesional and low-dose intramuscular therapy. Archives of Dermatology. 1987b;123:757–763. [PubMed] [Google Scholar]
- Vonderheid EC, Bernengo MG, Burg G, Duvic M, Heald P, Laroche L, Olsen E, Pittelkow M, Russell-Jones R, Takigawa M, Willemze R. Update on erythrodermic cutaneous T-cell lymphoma: report of the International Society for Cutaneous Lymphomas. Journal of the American Academy of Dermatology. 2002;46:95–106. doi: 10.1067/mjd.2002.118538. [DOI] [PubMed] [Google Scholar]
- Vowels BR, Lessin SR, Cassin M, Jaworsky C, Benoit B, Wolfe JT, Rook AH. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. Journal of Investigative Dermatology. 1994;103:669–673. doi: 10.1111/1523-1747.ep12398454. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual Review of Immunology. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Whittaker SJ, Demierre MF, Kim EJ, Rook AH, Lerner A, Duvic M, Scarisbrick J, Reddy S, Robak T, Becker JC, Samtsov A, McCulloch W, Kim YH. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. Journal of Clinical Oncology. 2010;28:4485–4491. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- Whittemore AS, Holly EA, Lee IM, Abel EA, Adams RM, Nickoloff BJ, Bley L, Peters JM, Gibney C. Mycosis fungoides in relation to environmental exposures and immune response: a case-control study. Journal of the National Cancer Institute. 1989;81:1560–1567. doi: 10.1093/jnci/81.20.1560. [DOI] [PubMed] [Google Scholar]
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S, Meijer CJ. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- Willemze R, van Vloten WA, Hermans J, Damsteeg MJ, Meijer CJ. Diagnostic criteria in Sezary’s syndrome: a multiparameter study of peripheral blood lymphocytes in 32 patients with erythroderma. Journal of Investigative Dermatology. 1983;81:392–397. doi: 10.1111/1523-1747.ep12521991. [DOI] [PubMed] [Google Scholar]
- Willers J, Haffner A, Zepter K, Storz M, Urosevic M, Burg G, Dummer R. The interferon inhibiting cytokine IK is overexpressed in cutaneous T cell lymphoma derived tumor cells that fail to upregulate major histocompatibility complex class II upon interferon-gamma stimulation. Journal of Investigative Dermatolology. 2001;116:874–879. doi: 10.1046/j.1523-1747.2001.01339.x. [DOI] [PubMed] [Google Scholar]
- Wlodarski MW, O’Keefe C, Howe EC, Risitano AM, Rodriguez A, Warshawsky I, Loughran TP, Jr, Maciejewski JP. Pathologic clonal cytotoxic T-cell responses: nonrandom nature of the T-cell-receptor restriction in large granular lymphocyte leukemia. Blood. 2005;106:2769–2780. doi: 10.1182/blood-2004-10-4045. [DOI] [PubMed] [Google Scholar]
- Wollina U, Dummer R, Brockmeyer NH, Konrad H, Busch JO, Kaatz M, Knopf B, Koch HJ, Hauschild A. Multicenter study of pegylated liposomal doxorubicin in patients with cutaneous T-cell lymphoma. Cancer. 2003;98:993–1001. doi: 10.1002/cncr.11593. [DOI] [PubMed] [Google Scholar]
- Wollina U, Graefe T, Kaatz M. Pegylated doxorubicin for primary cutaneous T cell lymphoma: a report on ten patients with follow-up. Annals of the New York Academy of Sciences. 2001;941:214–216. [PubMed] [Google Scholar]
- Wong HK, Wilson AJ, Gibson HM, Hafner MS, Hedgcock CJ, Berger CL, Edelson RL, Lim HW. Increased expression of ctla-4 in malignant T-cells from patients with mycosis fungoides – cutaneous T cell lymphoma. Journal of Investigative Dermatology. 2006;126:212–219. doi: 10.1038/sj.jid.5700029. [DOI] [PubMed] [Google Scholar]
- Wood GS, Edinger A, Hoppe RT, Warnke RA. Mycosis fungoides skin lesions contain CD8+ tumor-infiltrating lymphocytes expressing an activated, MHC-restricted cytotoxic T-lymphocyte phenotype. Journal of Cutaneous Pathology. 1994;21:151–156. doi: 10.1111/j.1600-0560.1994.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Wozniak MB, Tracey L, Ortiz-Romero PL, Montes S, Alvarez M, Fraga J, Fernandez Herrera J, Vidal S, Rodriguez-Peralto JL, Piris MA, Villuendas Deceased R. Psoralen plus ultraviolet A +/− interferon-alpha treatment resistance in mycosis fungoides: the role of tumour microenvironment, nuclear transcription factor-kappaB and T-cell receptor pathways. British Journal of Dermatology. 2009;160:92–102. doi: 10.1111/j.1365-2133.2008.08886.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Ohshima K, Tsuchiya T, Suehuji H, Karube K, Nakayama J, Suzumiya J, Yoshino T, Kikuchi M. The comparison of expression of cutaneous lymphocyte-associated antigen (CLA), and Th1- and Th2-associated antigens in mycosis fungoides and cutaneous lesions of adult T-cell leukemia/lymphoma. European Journal of Dermatology. 2003;13:553–559. [PubMed] [Google Scholar]
- Yamamoto K, Utsunomiya A, Tobinai K, Tsukasaki K, Uike N, Uozumi K, Yamaguchi K, Yamada Y, Hanada S, Tamura K, Nakamura S, Inagaki H, Ohshima K, Kiyoi H, Ishida T, Matsushima K, Akinaga S, Ogura M, Tomonaga M, Ueda R. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. Journal of Clinical Oncology. 28:1591–1598. doi: 10.1200/JCO.2009.25.3575. [DOI] [PubMed] [Google Scholar]
- Yanagi T, Shimizu T, Ujiie H, Ito M, Abe R, Tsuji-Abe Y, Hige S, Shimizu H. Peginterferon alfa-2b for mycosis fungoides. Archives of Dermatology. 2006;142:649–651. doi: 10.1001/archderm.142.5.649. [DOI] [PubMed] [Google Scholar]
- Yawalkar N, Ferenczi K, Jones DA, Yamanaka K, Suh KY, Sadat S, Kupper TS. Profound loss of T-cell receptor repertoire complexity in cutaneous T-cell lymphoma. Blood. 2003;102:4059–4066. doi: 10.1182/blood-2003-04-1044. [DOI] [PubMed] [Google Scholar]
- Zinzani PL, Magagnoli M, Bendandi M, Orcioni GF, Gherlinzoni F, Albertini P, Pileri SA, Tura S. Therapy with gemcitabine in pretreated peripheral T-cell lymphoma patients. Annals of Oncology. 1998;9:1351–1353. doi: 10.1023/a:1008409601731. [DOI] [PubMed] [Google Scholar]
- Zinzani PL, Baliva G, Magagnoli M, Bendandi M, Modugno G, Gherlinzoni F, Orcioni GF, Ascani S, Simoni R, Pileri SA, Tura S. Gemcitabine treatment in pretreated cutaneous T-cell lymphoma: experience in 44 patients. Journal of Clinical Oncology. 2000;18:2603–2606. doi: 10.1200/JCO.2000.18.13.2603. [DOI] [PubMed] [Google Scholar]
- Zinzani PL, Alinari L, Tani M, Fina M, Pileri S, Baccarani M. Preliminary observations of a phase II study of reduced-dose alemtuzumab treatment in patients with pretreated T-cell lymphoma. Haematologica. 2005;90:702–703. [PubMed] [Google Scholar]
- Zinzani PL, Musuraca G, Tani M, Stefoni V, Marchi E, Fina M, Pellegrini C, Alinari L, Derenzini E, de Vivo A, Sabattini E, Pileri S, Baccarani M. Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. Journal of Clinical Oncology. 2007;25:4293–4297. doi: 10.1200/JCO.2007.11.4207. [DOI] [PubMed] [Google Scholar]


