Abstract
Purpose
Mycosis fungoides (MF) is a cutaneous T-cell lymphoma (CTCL) characterized by neoplastic skin-homing T cells. To better understand the immunopathogenesis of MF, we analyzed the functional ability of peripheral blood mononuclear cells (PBMC) from early and late MF/CTCL patients to express cytokine genes. In late stage MF/CTCL, patients were separated into those with blood involvement (+B) and without blood involvement (−B).
Experimental Design
We analyzed TH1 (interleukin 2 (IL-2), IFN-γ), TH2 (IL-4, IL-5, IL-10, IL-13), and TH17 (IL-17) cytokine gene expression from activated PBMCs from normal (n = 12), psoriasis (n = 6), early MF/CTCL (n = 11), and late MF/CTCL+B (n = 4) and MF/CTCL-B (n = 3) by quantitative real-time PCR.
Results
PBMCs from early MF/CTCL and psoriasis showed higher induction of IL-2, IL-4, and IFN-γ genes than those from normal and late MF/CTCL-B and MF/CTCL+B (P < 0.05) in descending order. PBMCs from late MF/CTCL-B exhibited generally the highest level of IL-5, IL-10, IL-13, and IL-17 expression compared with the other groups. PBMCs from early MF/CTCL and late MF/CTCL−B had similarly elevated IL-13 and IL-17. Of all groups, PBMCs from late MF/CTCL+B had the lowest levels of IL-2 (P < 0.05), IL-4, IFN-γ, IL-13, and IL-17.
Conclusions
The different pattern of cytokine gene expression suggests a change in immune function in MF/CTCL from early MF/CTCL to late MF/CTCL−B to late MF/CTCL+B. These stages are consistent with localized disease associated with an anti-tumor immune response and late MF/CTCL associated with a loss of immune function mediated by malignant T cells that share regulatory T cell – like properties.
Mycosis fungoides (MF), the most common form of cutaneous T-cell lymphoma (CTCL), is characterized by a malignant proliferation of neoplastic CD4+, CD45RO+ T cells that preferentially traffic to the skin, where they induce clinical lesions manifesting as patches, plaques, tumors, or erythroderma (1–3). Based on the Bunn and Lamberg staging (4), early MF/CTCL (stages I and II) is characterized by the localization of malignant T cells within the skin. As the disease advances, the abnormal CD4+, CD45RO+ T cell becomes the predominant T cell. In late stage MF/CTCL (stages III and IV), the malignant T cell is no longer restricted to the skin and the tumor burden is more extensive. Immune dysfunctions are the sequel of advanced disease and high tumor burden (5) and are characterized by reduced natural killer cell activity, decreased immune responses of T cells to pathogens, and defective immune surveillance (6, 7).
Sézary syndrome is a group of clinical findings marked by erythroderma, generalized lymphadenopathy, and the presence of Sézary cells in the peripheral circulation, which represents extensive “blood involvement” (8). Whereas the Bunn and Lamberg staging and the newly revised International Society for Cutaneous Lymphomas/European Organization for Research and Treatment of Cancer classification designates Sézary syndrome in the late stages of MF/CTCL (4, 9), the WHO/European Organization for Research and Treatment of Cancer staging separates MF and Sézary syndrome as two distinct entities because Sézary syndrome uncommonly results from MF (10, 11). A more unified and clear classification will come from additional studies.
The pathogenesis of MF/CTCL remains unclear. Early data from cytokines in MF/CTCL hinted that the abnormal T cell in MF/CTCL may have TH2-like properties, with increased interleukin 4 (IL-4) and IL-5 (12, 13). As additional T-cell subsets have been identified, recent evidence suggests that the malignant cells have properties shared with regulatory T cells (Treg), defined by the expression of CTLA-4 and Foxp3 (14, 15), but do not completely represent Treg cells (16). Several groups have reported early MF/CTCL to exhibit a TH1 phenotype, evidenced by increased IL-2 and IFN-γ, and late MF/CTCL to have a TH2 phenotype, characterized by increased IL-4, IL-5, IL-10, and IL-13 as the malignancy progresses (17–19). The proliferation of the abnormal malignant T cell may thus be responsible for the increased expression of TH2 cytokines. Another distinctive class of CD4+ T cells, TH17 cells, generates IL-17, which acts on keratinocytes to produce IL-6 and IL-8, and is associated with psoriasis (20, 21). IL-17 has been detected in skin biopsies in MF/CTCL and Sézary syndrome patients, but a distinction in IL-17 levels between these two groups was not detected (22).
In this study, we analyze the peripheral blood mononuclear cells’ (PBMC) immune function and show that PBMCs from MF/CTCL patients exhibit significant defects in the ability to regulate cytokine genes. In early MF/CTCL, where the tumor burden is low, activated PBMCs show an increased TH1-cytokine gene expression at levels similar to those obtained from patients with psoriasis. PBMCs from late MF/CTCL showed variations in cytokine levels and could be subclassified, depending on the extent of blood involvement. Activated PBMCs from late MF/CTCL without blood involvement (−B) had lower levels of IL-2, IL-4, and IFN-γ than those from normal, psoriasis, and early MF/CTCL, except late MF/CTCL with blood involvement (+B). Stimulated PBMCs from late MF/CTCL−B had generally increased expression of IL-5, IL-10, IL-13, and IL-17 upon stimulation compared with those from normal, psoriasis, and late MF/CTCL+B. Most significantly, activated PBMCs from late MF/CTCL+B had the most pronounced decreases in levels of IL-2, IL-4, IFN-γ, IL-13, and IL-17 of all sets of patients, which may reflect consequences from proliferation of malignant T cells with Treg-like properties and the loss of tumor immunity. The profound alterations in the expression of multiple cytokine genes may reflect underlying defects that play a role in the pathogenesis of MF/CTCL.
Materials and Methods
Patient samples
PBMCs were obtained from 12 normal volunteers, 6 psoriasis patients, and 18 MF/CTCL patients with Bunn and Lamberg stages I-IV (4). Early MF/CTCL (stages I and II) was defined as having skin-restricted lymphoma involvement, and late MF/CTCL (stages III and IV) was defined as having lymphoma spreading beyond the skin with lymph node and blood involvement. Late MF/CTCL patients were subdivided into those with detectable clonal blood involvement (defined as absolute Sézary cell count of >1,000 cells/mm3, which is an International Society for Cutaneous Lymphomas criteria for the diagnosis of Sézary syndrome) and those without blood involvement (defined as absolute Sézary cell count of <1,000 cells/mm3). Characteristics of the MF patients, including stage, total number, age, gender, average percentage, and range of circulating Sézary cells, and number of erythrodermic patients are summarized in Table 1. The study was approved by the Institutional Review Board of Henry Ford Hospital, and informed consent approved under the Institutional Review Board protocol was obtained from each patient.
Table 1.
Summary of characteristics of MF/CTCL patients
| CTCL staging | Stage/TNMB | No. patients | Average age | Male/female | Average no. Sézary cells (K/μL) | Range of no. Sézary cells (K/μL) | No. erythrodermic patients |
|---|---|---|---|---|---|---|---|
| Early MF/CTCL | I–II/T1–T2, N0–N1 | 11 | 66 | 6/5 | 0 | 0 | 0 |
| Late MF/CTCL+B | III–IV/T3–T4, N0–N3, B2 | 4 | 64 | 2/2 | 5.570 | 4.860–6.588 | 4/4 |
| Late MF/CTCL−B | III–IV/T3–T4, N0–N3, B0–B1 | 3 | 64 | 0/3 | 0.130 | 0.048–0.212 | 0/3 |
Cell culture
Normal PBMCs were obtained from pheresis collars from the American Red Cross Pheresis Center and from normal volunteers by phlebotomy. Patients with MF/CTCL or psoriasis had venous blood drawn by phlebotomy after obtaining informed consent. After phlebotomy, the blood samples were diluted with 1× Hank’s buffer and underlayed with Lymphoprep (Axis-Shield). The blood was centrifuged at 500 × g for 30 min, and the lymphocyte interphase was carefully aspirated, collected, and washed with Hank’s buffer. These cells were subsequently resuspended in ACK lysis buffer [150 mmol/L NH4Cl, 10 mmol/L KHCO3, and 0.1 mmol/L EDTA (pH 7.4), sterile filtered] for 5 min. ACK-treated lymphocytes were washed with Hank’s buffer, and the cells were resuspended in RPMI 1640 (with L-glutamine; Invitrogen Corp.) and supplemented with 10% heat-inactivated FCS (Sigma Chemical Co.) and 1% penicillin/streptomycin (5,000 units/mL and 5,000 μg/mL, respectively; Invitrogen Corp.).
PBMCs were cultured overnight at 37°C in a 5% CO2 humidified incubator. PBMCs were set up in duplicate T-25 flasks, stimulated with 50 ng/mL of phorbol 12-myristate 13-acetate (PMA Calbiochem) and 10 μmol/L of the calcium ionophore A23187 (Calbiochem) or 2 μg/mL of anti-CD3 monoclonal antibody (Orthoclone OKT3, Ortho Pharmaceuticals), 2 μg/mL of antihuman CD28 monoclonal antibody (Clone CD28.2, BD PharMingen), and 2 μg/mL of goat anti-mouse IgG (Sigma), and cultured for 2, 4, 6, and 8 h. Controls were treated with 2 μL of DMSO as a mock stimulation. After stimulation, cells were lysed using Trizol (Invitrogen Corp.) as described by the manufacturer.
RNA isolation, reverse transcription, and real-time PCR
RNA isolation via Trizol was carried out according to the manufacturer’s directions. Precipitated RNA was resuspended in 15 to 25 μL of DEPC-treated water and quantified by spectrophotometry. Reverse transcription was carried out using 1 to 3 μg of RNA, oligo dT primers, and 0.5 μL of Superscript RT II according to the manufacturer’s instructions (Invitrogen).
Real-time reverse transcription PCR (RT-PCR) analysis was carried out in 30 μL reactions containing 0.5 μL of cDNA 333 μmol/L forward and reverse primers for the cytokines IL-2, IL-4, IFN-γ, IL-5, IL-10, IL-13, and IL-17 (see Supplementary data for primer sequences), and SYBR Green 1 Master Mix, which includes Taq. The reactions were processed in an ABI Prism 7000 SDS with the following cycling variables: 5 min at 95°C, then 40 cycles of 95°C for 15 s and 60°C for 1 min. Samples that were designated to be analyzed by 2% agarose gels (1 × Tris-borate EDTA buffer) were taken out of the ABI Prism 7000 SDS immediately after the run was finished. Data generated from duplicate quantitative RT-PCR assays are reported as cycle threshold (CT; the point at which the amplification curve of the reaction crosses the threshold) values. The CT values were standardized to the normalizer gene β2-microglobulin (β2M). β2M was run in separate wells on the same 96-well plate, and its expression level (in lymphocytes) was shown to be unaffected by experimental conditions. The CT values were then converted to fold induction over normal at time zero (control) using the 2−ΔΔCT formula (23) and plotted against duration of PMA stimulation. Agarose gels were photographed on a Chem Doc 2000 universal hood (Bio-Rad) to visually depict the results of a RT-PCR run.
Statistical analysis
Data are presented as mean ± SE. The statistical significance of the differences was calculated by the Student’s t test for two samples assuming unequal variances.
Results
IL-2, IL-4, and IFN-γ expression in activated PBMCs is down-regulated in late MF/CTCL but up-regulated in early MF/CTCL
PBMCs from normal and late MF/CTCL were analyzed for the ability to express the cytokines IL-2, IL-4, and IFN-γ after stimulation with PMA/A23187. These genes were selected initially to gain insight into how PBMCs from late MF/CTCL regulate a general T-cell cytokine (IL-2), a TH1 cytokine (IFN-γ), and a TH2 cytokine (IL-4). Total RNA was isolated at different times after stimulation, and expression of the genes was analyzed by RT-PCR. Upon stimulation, normal PBMCs showed a rapid and robust increase in all three cytokines when compared with late MF/CTCL PBMCs (Fig. 1A). PBMCs from late MF/CTCL showed a defect in the ability to up-regulate IL-2, IL-4, and IFN-γ when stimulated with PMA/A23187 compared with normal PBMCs (Fig. 1A).
Fig. 1.
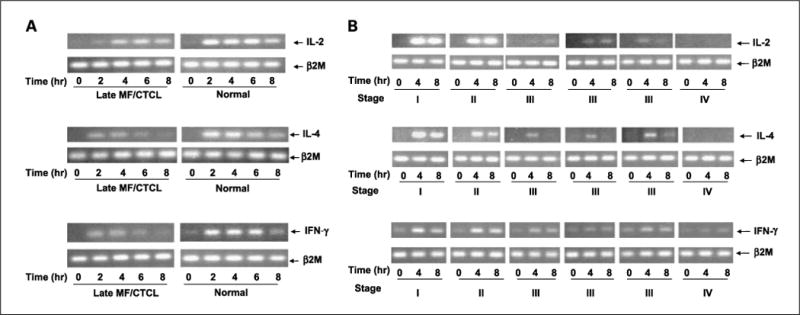
Cytokine gene expression defects in activated PBMCs from normal and MF/CTCL patients in various stages. A, RT-PCR was performed from normal and late MF/CTCL PBMCs that were stimulated for the indicated time and analyzed for IL-2, IL-4, and IFN-γ gene expression. Samples were separated on a 2.0% ethidium – stained agarose gel and visualized on UV transilluminator. B, RT-PCR was performed from early and late MF/CTCL PBMCs that were stimulated for the indicated time and analyzed for IL-2, IL-4, and IFN-γ gene expression. The β2M gene served as control for normalization. Samples were separated on a 2.0% ethidium – stained agarose gel and visualized by UV transilluminaion.
To determine whether the defect in cytokine gene expression was evident in PBMCs from early MF/CTCL when the malignant tumor T cells are restricted to the skin, MF/CTCL patients with varying degrees of clinical disease, as defined by the Bunn and Lamberg stage, were studied (4). When PBMCs from different stages of MF/CTCL were analyzed for the ability to express cytokine genes, the early MF/CTCL cohort showed a greater ability to express the genes for IL-2, IL-4, and IFN-γ when compared with the late MF/CTCL cohort (Fig. 1B).
PMA/A23187 activation of normal and diseased PBMCs revealed cytokine expression defects more efficiently compared with T-cell receptor activation and CD28 costimulation
MF/CTCL cells have numerous surface signaling molecule defects, such as T-cell receptor defects (24, 25), as well as loss of CD7 (26). To confirm this observation and to rule out that the findings were not from altered signaling by PMA/A23187, we compared cytokine gene activation between PMA/A23187 to anti-CD3 and anti-CD28 antibodies. PBMCs from normal, early MF/CTCL, and late MF/CTCL+B patients were stimulated with PMA/A23187 or anti-CD3 and anti-CD28 antibodies. In measuring IL-2 in response to PMA/A23187, by 6 h, normal showed 11,000×, early MF/CTCL showed 40,000×, and late showed MF/CTCL 512× increase from unstimulated controls. For anti-CD3/anti-CD28 stimulation, IL-2 by 6 h for normal showed 51 × increase, early MF/CTCL showed 80 × increase, and late MF/CTCL showed 2× increase from baseline controls. Therefore, PBMCs from all groups stimulated with PMA/A23187 responded with significantly higher levels of IL-2 gene expression than with anti-CD3/anti-CD28 antibodies. Data trends between the groups, with early MF/CTCL having the highest IL-2 levels and late MF/CTCL+B having the lowest levels, were preserved with PMA/A23187 and anti-CD3/anti-CD28 antibody stimulation (Fig. 2A).
Fig. 2.
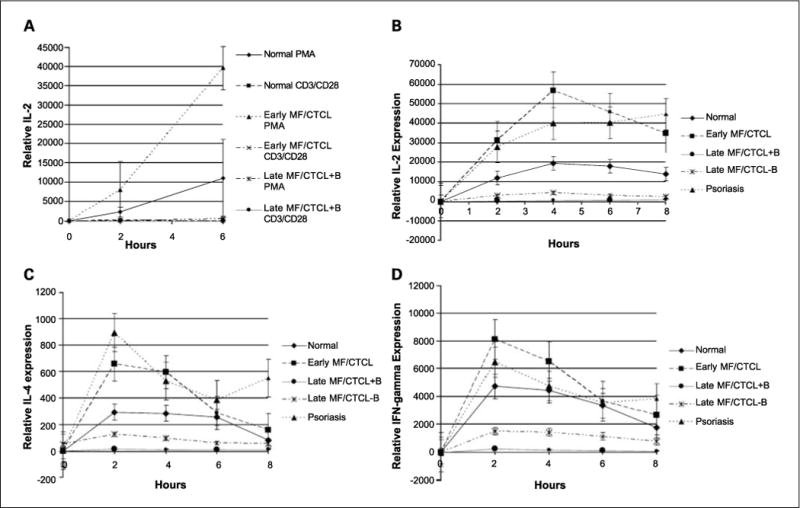
Cytokine gene expression among activated PBMCs. A, IL-2 gene expression levels in PBMCs from normal and MF/CTCL patients stimulated by PMA/A23187 versus anti-CD3/anti-CD28 antibodies. Quantitative RT-PCR was performed on PBMCs from normal (n = 3), early MF/CTCL (n = 2), and late MF/CTCL+B (n = 2) patients that were stimulated by PMA/A23187 or anti-CD3/anti-CD28 antibodies for the indicated time and analyzed for IL-2 gene expression. The relative level of cytokine gene expression was normalized to β2M level of gene expression and plotted to the unstimulated level of gene expression for the respective patient samples for IL-2. Points, mean; bars, SE. B–D, Cytokine gene expression among activated PBMCs from early and late MF/CTCL+B and MF/CTCL−B patients compared with normal and psoriasis patients. (B) Quantitative RT-PCR was performed on PBMCs from normal (n = 8), psoriasis (n = 6), early MF/CTCL (n = 8), and late MF/CTCL+B (n = 4) and late MF/CTCL−B (n = 3) patients that were stimulated for the indicated time and analyzed for (B), IL-2 (C), IL-4 (D) and IFN-γ gene expression. The relative level of cytokine gene expression was normalized to β2M level of gene expression and plotted to the unstimulated level of gene expression for normal PBMCs for IL-2, IL-4, and IFN-γ. Points, mean; bars, SE. Data points that included values that were computed from linear regression analysis are denoted with smaller markers.
We next analyzed quantitatively the difference in the level of expression of IL-2, IL-4, and IFN-γ in PBMCs derived from early and late MF/CTCL, normal, and psoriasis patients. Psoriasis patients were chosen as disease controls because psoriasis is a cutaneous immune-mediated skin disorder where T cells are involved (27). Quantitative PCR was performed on RNA isolated from stimulated PBMCs from normal, psoriasis, early MF/CTCL, and late MF/CTCL, and the level of expression of each cytokine was measured and normalized to β2M. Figure 2 showed the mean expression patterns of IL-2 (Fig. 2B), IL-4 (Fig. 2C), and IFN-γ (Fig. 2D) from PBMCs from normal, psoriasis, early MF/CTCL, and late MF/CTCL stimulated by PMA/A23187 at various time points. Upon stimulation, the cytokine expression of PBMCs for IL-2, IL-4, and IFN-γ from early MF/CTCL was consistently higher than those from normal and late MF/CTCL. Activated PBMCs from psoriasis patients behaved most similarly to early MF/CTCL PBMCs and produced cytokines at a higher level than normal and late MF/CTCL PBMCs. The level of expression for IL-2 and IFN-γ in activated PBMCs from early MF/CTCL and psoriasis was markedly higher than that for IL-4. Stimulated PBMCs from normal volunteers had consistently higher levels of cytokine expression than late MF/CTCL PBMCs. Furthermore, late MF/CTCL patients could be subclassified into those with blood involvement (+B) and without blood involvement (−B) because of their prominent differences in cytokine expression. Activated PBMCs from late MF/CTCL+B showed the greatest defect in the ability to up-regulate IL-2, IL-4, and IFN-γ compared with PBMCs from normal, early MF/CTCL, psoriasis, and late MF/CTCL-B. Late MF/CTCL-B also had stimulated PBMCs that exhibited deficiencies in expressing IL-2, IL-4, and IFN-γ compared with those from normal, early MF/CTCL, and psoriasis, but were not as profound as those from late MF/CTCL+B. Table 2 summarizes the differences in PBMC expression of IL-2, IL-4, and IFN-γ between normal, psoriasis, early MF/CTCL, and late MF/CTCL+B and MF/CTCL-B at 2 h after stimulation. With the exception of IL-4 expression after 2 h of stimulation, activated PBMCs from early MF/CTCL generally exhibited the highest levels of IL-2, IL-4, and IFN-γ expression, followed by those from psoriasis, normal, late MF/CTCL−B, and late MF/CTCL+B, in descending order. Of note, statistical significance (P < 0.05) was observed in the following: IL-2, IL-4, and IFN-γ expression in activated PBMCs from early MF/CTCL versus late MF/CTCL+B and MF/CTCL-B; IL-2 and IL-4 expression in activated PBMCs from normal versus late MF/CTCL+B and early MF/CTCL; and IL-2 expression in activated PBMCs from psoriasis versus normal and late MF/CTCL+B and MF/CTCL−B and late MF/CTCL+B versus late MF/CTCL−B.
Table 2.
Summary of cytokine expression in PBMCs of normal, psoriasis, early MF/CTCL, and late MF/CTCL+B and MF/CTCL−B after 2 h of stimulation of PMA/A23187
| Cytokine | Normal | Psoriasis | Early MF/CTCL | Late MF/CTCL−B | Late MF/CTCL+B |
|---|---|---|---|---|---|
| IL-2 | 11943.198* | 27887.483† | 31232.621† | 3059.821‡ | 467.042 |
| IL-4 | 295.496§ | 894.575 | 656.166† | 127.036 | 19.571 |
| IFN-γ | 4721.754 | 6469.709 | 8093.450† | 1495.562 | 227.792 |
| IL-5 | 5.679 | 4.944 | 5.385 | 17.933 | 2.267 |
| IL-10 | 9.745‖ | 10.178 | 19.019 | 141.351 | 60.691 |
| IL-13 | 340.519¶ | 288.941 | 479.734 | 510.622 | 207.842 |
| IL-17 | 96.194‖ | 147.602‖ | 358.714‡ | 238.931 | 46.797 |
P < 0.05 compared with early MF/CTCL, psoriasis, late MF/CTCL+B.
P < 0.05 compared with late MF/CTCL−B, late MF/CTCL+B.
P < 0.05 compared with late MF/CTCL+B.
P < 0.05 compared with early MF/CTCL, late MF/CTCL+B.
P < 0.05 compared with early MF/CTCL.
P < 0.05 compared with late MF/CTCL−B.
Multiple abnormalities in IL-5, IL-10, IL-13, and IL-17 expression in activated PBMCs are observed in localized and late MF/CTCL with and without blood involvement
To determine whether there is a general defect in cytokine expression, additional TH2 cytokines, including IL-5, IL-10, and IL-13, and the TH17 cytokine IL-17 were investigated in early MF/CTCL, late MF/CTCL+B, late MF/CTCL−B, normal, and psoriasis patients. Along with late MF/CTCL−B, activated PBMCs from early MF/CTCL showed elevated levels of IL-13 and IL-17 compared with those from normal, psoriasis, and late MF/CTCL+B. However, PBMCs from early MF/CTCL did not show elevated IL-5 and IL-10 levels upon stimulation. PBMCs from late MF/CTCL+B had the lowest IL-13 and IL-17 levels of all patient groups throughout all time points of stimulation. A deficit in the up-regulation of IL-10 was also noted in activated PBMCs from late MF/CTCL+B compared with those from late MF/CTCL−B. Although depressed at 0 and 2 h, IL-5 expression in activated PBMCs from late MF/CTCL+B increased to levels above those from all other groups at 6 h of stimulation. Except for IL-5 after 6 h of stimulation and IL-17 after 2 h of stimulation, stimulated PBMCs from late MF/CTCL−B showed higher levels of IL-5, IL-10, IL-13, and IL-17 compared with those from all other groups (Fig. 3A–D). Table 2 summarizes the differences in PBMC expression of IL-5, IL-10, IL-13, and IL-17 between normal, psoriasis, early MF/CTCL, and late MF/CTCL+B and MF/CTCL−B at 2 h after stimulation. PBMCs from early MF/CTCL showed increases in IL-13 and IL-17 expression at 2 h after stimulation compared with those from normal, psoriasis, and late MF/CTCL+B, with statistical significance seen in IL-17 compared with normal, psoriasis, and late MF/CTCL+B. Except for IL-17, activated PBMCs from early MF/CTCL had diminished IL-5, IL-10, and IL-13 levels compared with those from late MF/CTCL−B. In comparison to normal PBMCs, activated early MF/CTCL PBMCs had higher levels of IL-10, which showed statistical significance and slightly lower levels of IL-5. Stimulated PBMCs from late MF/CTCL+B showed diminished expression for IL-5, IL-10, IL-13, and IL-17 compared with those from late MF/CTCL−B. Compared with normal, psoriasis, and early MF/CTCL PBMCs, PBMCs from activated late MF/CTCL+B exhibited decreased IL-5, IL-13, and IL-17 levels, with statistical significance seen in IL-17 versus early MF/CTCL patients, and increased IL-10 expression. In addition, except for IL-17 compared with early MF/CTCL patients, activated PBMCs from late MF/CTCL−B exhibited increases in IL-5, IL-10, IL-13, and IL-17 levels compared with those from normal, early MF/CTCL, psoriasis, and late MF/CTCL+B after 2 h of stimulation, with statistical significance seen in IL-13 versus normals.
Fig. 3.
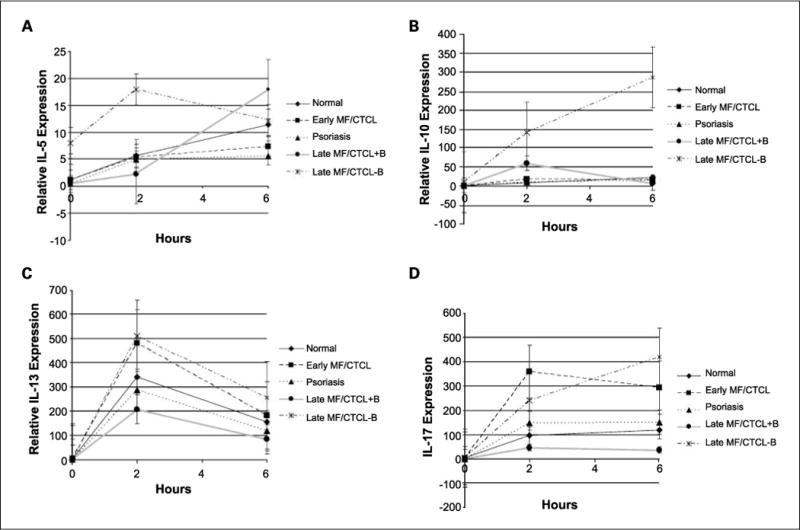
Cytokine gene expression among activated PBMCs from early and late MF/CTCL+B and MF/CTCL−B patients compared with normal and psoriasis patients. A–D, quantitative RT-PCR was performed on PBMCs from normal (n = 8), psoriasis (n = 4), early MF/CTCL (n = 8), and late MF/CTCL+B (n = 4) and late MF/CTCL−B (n = 3) patients that were stimulated for the indicated time and analyzed for IL-5 (A), IL-10 (B), IL-13 (C), and IL-17 (D) gene expression. The relative level of cytokine gene expression was normalized to β2M level of gene expression and plotted to the unstimulated level of gene expression for normal PBMCs for IL-5, IL-10, IL-13, and IL-17. Points, mean; bars, SE. Data points that included values that were computed from linear regression analysis are denoted with smaller markers.
Discussion
Because the atypical malignant T cells in MF/CTCL share surface markers found in memory T cells, one may expect the malignant clone to retain properties of the cell from which it is derived, such as the ability to express high levels of cytokines. In addition, these abnormal cells may gain additional properties during disease progression, and these changes in sum contribute to the clinical phenotype. Our analysis of cytokine gene expression in PBMCs from MF/CTCL patients show for the first time that there are profound changes in PBMC expression of multiple cytokine genes, including IL-2, IL-4, IL-5, IL-10, IL-13, IL-17, and IFN-γ, reflecting functional differences in the immune system in various stages of MF/CTCL. The cytokine pattern indicates that PBMCs from early MF/CTCL has a TH1 pattern, consistent with an antitumor response that is able to limit the malignant T cells to the skin. Whereas late MF/CTCL−B exhibits cytokines with a TH2 and TH17 bias, late MF/CTCL+B shows a global depression in cytokine expression, spanning TH1, TH2, and TH17 cytokines. This widespread decrease in cytokines in late MF/CTCL+B may reflect underlying loss of immune function and the phenotypic origin of the malignant T cells, which may share properties with Treg cells rather than normal memory T cells that express higher levels of cytokines. During disease progression, as the malignant population expands, there is a loss of tumor immunity from a decrease in antitumor T cells and active immunosuppression from Treg cell–like properties, such as the expression of CTLA-4 (15).
As previous studies have shown defective T-cell receptor signaling defects in CTCL, PMA/A23187 was used for stimulation to bypass the T-cell receptor (24, 25). PMA/A23187 permits a more direct measure of cytoplasmic and nuclear signals needed for cytokine gene activation. In PBMCs from normal, early MF/CTCL, and late MF/CTCL patients, the IL-2 gene was more rapidly and intensely activated by PMA/A23187 compared with stimulation with anti-CD3/CD28 and was more sensitive at measuring cytokine gene expression. The slower kinetics of activation seen with anti-CD3/CD28 antibodies is likely due to the cross-linking and membrane signaling necessary for stimulation. The trends in cytokine expression among the different cell populations studied were preserved with PMA/A23187 compared with anti-CD3/anti-CD28 stimulation.
Previously, the expression of several cytokines has been found to be abnormal in MF/CTCL patients (17, 28) in affected skin (patch versus plaque versus tumor) and PBMCs from patients in various stages of MF/CTCL. The previous reports suggested that, as MF/CTCL advances from early disease to late disease, there is a change in the cytokine pattern detected from TH1 cytokines (i.e., IL-2 and IFN-γ) to TH2 cytokines (i.e., IL-4 and IL-5; refs. 17, 19, 29–32). This observation is partly reflected in the results in this study, with the exception of findings from late MF/CTCL+B.
To determine whether the enhanced cytokine gene expression in early MF/CTCL is related to immune activation, psoriasis PBMCs were examined. The levels of IL-2, IL-4, and IFN-γ gene expression in psoriasis PBMCs showed similarities to those of early MF/CTCL and were higher than those observed for normal and late MF/CTCL+B and MF/CTCL-B. In early MF/CTCL PBMCs, there was an increased expression of all cytokine genes except for IL-5 compared with normal and late MF/CTCL+B. These findings are consistent with early disease being associated with an immune response against the malignant T cells.
On the other hand, late MF/CTCL PBMCs showed a profound defect in the ability to express multiple cytokine genes (i.e., IL-2, IL-4, IFN-γ) compared with those from normal, early MF/CTCL, and psoriasis. In late MF/CTCL patients, particularly those with blood involvement, the malignant cells may constitute a significant tumor burden, and there is a widespread loss of TH1, TH2, and TH17 cytokines. The depressed cytokine levels in late MF/CTCL patients parallels the decline in immunocompetence and may explain why late MF/CTCL patients often succumb to opportunistic infections (23). Moreover, because PMA/A23187 is a strong stimulus, the failure to activate these genes is significant.
The presence of malignant T cells contributing to a suppressed immune response suggests that the malignant cells may have similarities to Treg cells. Treg cells poorly express IL-2, IL-4, IL-10, and IFN-γ in response to T-cell receptor stimulation (33), similar to PBMCs from late MF/CTCL+B. Previously, we have shown that tumor T cells express CTLA-4, a costimulatory molecule present on CD4+ CD25+ Treg cells (34). Specifically, late MF/CTCL patients had increased CTLA-4 RNA and protein expression, and a clonal population of malignant T cells expressed CTLA-4 at high levels (15). An in vitro CTCL model, where CD4+ CTCL cells were activated by dendritic cells loaded with apoptotic CTCL cells, revealed that these CTCL cells resembled Treg cells by up-regulating CTLA-4 expression and suppressing IL-2 and IFN-γ expression by normal T cells (14). Thus, targeting CTLA-4 by using an anti–CTLA-4 monoclonal antibody may improve the immune response. Also, it should be noted that MF/CTCL cells may provide a source of clonal Treg cells for further investigation into the molecular and cellular properties of human Treg cells.
An exception to this trend of decreased cytokines in late MF/CTCL+B was noted in IL-5 and IL-10, which were elevated in PBMCs from both late MF/CTCL cohorts. Because IL-5 is associated with eosinophil recruitment, this cytokine may be associated with pruritus seen in late MF/CTCL. Elevated levels of IL-10, which serves to inhibit cytokine production by TH1 cells (35), in late MF/CTCL may also contribute to the diminished anti-tumor response.
The decline in IL-4 expression seen with the progression of MF/CTCL, at first, may seem inconsistent with findings of several groups that found an increase in IL-4 level in the latter stages of MF/CTCL. The discrepancies, however, may be explained by the use of skin biopsies instead of PBMCs (17, 36). Other studies measured IL-4 protein by ELISA at the latter stages of the disease (12), whereas we measured expression of the IL-4 gene normalized to each cell. In fact, the finding is not inconsistent. Although per tumor cell production of IL-4 may be low, as detected by our analysis, the clonal population of malignant cells is high. In turn, the total production of relatively stable IL-4 from these tumor cells may account for the increased level of IL-4 that is detected by ELISA.
In this study, the sample size of late MF/CTCL+B and MF/CTCL−B patients was small. However, it is worthy to note the statistically significant cytokine expression differences between early and late MF/CTCL patients with and without blood involvement.
Although purification of PBMCs from early MF/CTCL using Vβ monoclonal antibodies may enrich the malignant clone, it is likely that only a small subset of patients have detectable clones. Furthermore, it was previously reported that in 30% of patients, a Vβ clone cannot be detected (37–39). To address this further, when we purified memory cells (CD4+CD45RO+) in PBMCs from early MF/CTCL patients (n = 4) to enrich the malignant clone, clonality by TCR-γ was not identified nor was there any functional differences when compared with the unpurified population (data not shown). Only purified memory PBMCs from late MF/CTCL+B patients (n = 3) showed the presence of clonality in the blood and gene expression defects (data not shown).
From this study, a distinct difference in cytokine expression was revealed between late MF/CTCL cohorts; specifically, late MF/CTCL+B had diminished levels of all cytokines studied compared with late MF/CTCL−B. This finding, combined with the data showing increased IL-2, IL-4, and IFN-γ expression in early MF/CTCL versus late MF/CTCL, supports a possible progression of MF/CTCL, with early MF/CTCL (i.e., stages I and II), advancing to late MF/CTCL−B (i.e., stage IVa) and ending at late MF/CTCL+B (i.e., stage III; Fig. 4). Interestingly, the elevated expression of IL-13 and IL-17 in late MF/CTCL−B patients paralleled that in early MF/CTCL patients. Both of these conditions do not have an elevated presence of tumor cells in the blood, suggesting that the increase in these cytokines reflect a chronic inflammatory response to the malignant T cells in the skin. Moreover, because IL-13 and IL-17 expression in PBMCs from these two groups are higher than those from late MF/CTCL+B, this further support a progression of MF/CTCL as proposed.
Fig. 4.
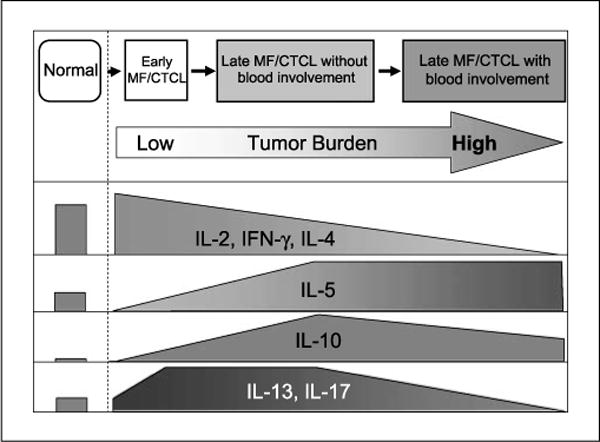
Schematic model for stages of MF/CTCL and summary of PBMC cytokine expression in normal, early MF/CTCL, and late MF/CTCL patients. Based on our cytokine expression results, functional staging of MF begins at early MF/CTCL, progresses to late MF/CTCL−B, and ends with late MF/CTCL +B. IL-2, IL-4, and IFN-γ exhibit a downward trend from early MF/CTCL to late MF/CTCL+B. IL-5 is elevated in late MF/CTCL, whereas IL-10 levels peak at late MF/CTCL−B and then decrease in late MF/CTCL+B. IL-13 and IL-17 levels increase in early MF/CTCL and late MF/CTCL−B but decrease in late MF/CTCL+B.
In summary, we find that the loss of cytokine expression by PBMCs in MF/CTCL patients directly corresponds with the severity of disease, suggesting that there is an inherent defect in the immune response revealed by cytokine gene expression. The cytokine expression reflects immunocompetence in these patients and may serve as a basis for assessing the staging of MF/CTCL patients. The results are consistent with a model where, in early MF/CTCL, the immunosuppressing phenotype of the tumor T cell is restrained and masked by an antitumor immune response. As the burden of malignant T cells increases in late MF/CTCL, the inhibitory properties of the tumor cells induce clinical immunosuppression. Thus, a potential explanation for the changes in cytokine gene expression during disease progression from a skin-localized involvement to extensive systemic involvement is the loss of tumor immunity as the malignant T cells expand. Whether tumor T cells were derived from Treg cells or CD4+ T cells that gain Treg-like functions remains unclear, and the abnormal gene expression pattern suggests that further evaluation of the lineage from which the tumor T cells are derived is necessary to better understand MF/CTCL. Furthermore, the finding of a concerted decrease in cytokine gene expression likely hints to a mechanism that is common in the regulation of these cytokine genes at the promoter or chromosomal level and may underlie the efficacy of histone deacetylase inhibitors in the treatment of MF/CTCL. The findings described indicate that future investigation on the basis for the abnormal regulation of cytokine gene expression will yield further understanding into the pathogenesis of MF/CTCL.
Supplementary Material
Acknowledgments
We thank Dr. Milena Cankovic of Department of Pathology for T-cell gene rearrangement studies and Dr. Eric Vonderheid for critical review of the manuscript.
Grant support: Fund for Henry Ford Hospital, Clarence Livingood Fund, Dermatology Foundation Clinical Career Development Award, and NIH NIAMS K08-47818 and R21-52877 (H.K. Wong).
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Vonderheid EC. Treatment of cutaneous T cell lymphoma: 2001. Recent Results Cancer Res. 2002;160:309–20. doi: 10.1007/978-3-642-59410-6_36. [DOI] [PubMed] [Google Scholar]
- 2.Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350:1978–88. doi: 10.1056/NEJMra032810. [DOI] [PubMed] [Google Scholar]
- 3.Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunn PA, Jr, Lamberg SI. Report of the committee on staging and classification of cutaneous t-cell lymphomas. Canc Treat Rep. 1979;63:725–8. [PubMed] [Google Scholar]
- 5.Axelrod PI, Lorber B, Vonderheid EC. Infections complicating mycosis fungoides and Sezary syndrome. JAMA. 1992;267:1354–8. [PubMed] [Google Scholar]
- 6.Asadullah K, Friedrich M, Docke WD, Jahn S, Volk HD, Sterry W. Enhanced expression of T-cell activation and natural killer cell antigens indicates systemic antitumor response in early primary cutaneous T-cell lymphoma. J Invest Dermatol. 1997;108:743–7. doi: 10.1111/1523-1747.ep12292129. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi S, Yamada Y, Dekio S, Jidoi J. Kaposi’s varicelliform eruption in a patient with mycosis fungoides. Clin Exp Dermatol. 1997;22:41–3. doi: 10.1046/j.1365-2230.1997.1920609.x. [DOI] [PubMed] [Google Scholar]
- 8.Vonderheid EC, Bernengo MG, Burg G, et al. Update on erythrodermic cutaneous T-cell lymphoma: report of the International Society for Cutaneous Lymphomas. J Am Acad Dermatol. 2002;46:95–106. doi: 10.1067/mjd.2002.118538. [DOI] [PubMed] [Google Scholar]
- 9.Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:479–84. doi: 10.1182/blood-2006-10-054601. [DOI] [PubMed] [Google Scholar]
- 10.Willemze R. Cutaneous T-cell lymphoma: epidemiology, etiology, and classification. Leuk Lymphoma. 2003;44(Suppl 3):S49–54. doi: 10.1080/10428190310001623766. [DOI] [PubMed] [Google Scholar]
- 11.Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–22. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 12.Vowels BR, Cassin M, Vonderheid EC, Rook AH. Aberrant cytokine production by Sezary syndrome patients: cytokine secretion pattern resembles murine Th2 cells. J Invest Dermatol. 1992;99:90–4. doi: 10.1111/1523-1747.ep12611877. [DOI] [PubMed] [Google Scholar]
- 13.Dummer R, Geertsen R, Ludwig E, Niederer E, Burg G. Sezary syndrome, T-helper 2 cytokines and accessory factor-1 (AF-1) Leuk Lymphoma. 1998;28:515–22. doi: 10.3109/10428199809058359. [DOI] [PubMed] [Google Scholar]
- 14.Berger CL, Tigelaar R, Cohen J, et al. Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory cells. Blood. 2005;105:1640–7. doi: 10.1182/blood-2004-06-2181. [DOI] [PubMed] [Google Scholar]
- 15.Wong HK, Wilson AJ, Gibson HM, et al. Increased expression of ctla-4 in malignant T-cells from patients with mycosis fungoides – cutaneous T cell lymphoma. J Invest Dermatol. 2006;126:212–9. doi: 10.1038/sj.jid.5700029. [DOI] [PubMed] [Google Scholar]
- 16.Tiemessen MM, Mitchell TJ, Hendry L, Whittaker SJ, Taams LS, John S. Lack of suppressive CD4+CD25+ FOXP3+ T cells in advanced stages of primary cutaneous T-cell lymphoma. J Invest Dermatol. 2006;126:2217–23. doi: 10.1038/sj.jid.5700371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vowels BR, Lessin SR, Cassin M, et al. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol. 1994;103:669–73. doi: 10.1111/1523-1747.ep12398454. [DOI] [PubMed] [Google Scholar]
- 18.Asadullah K, Docke WD, Haeussler A, Sterry W, Volk HD. Progression of mycosis fungoides is associated with increasing cutaneous expression of interleukin-10 mRNA. J Invest Dermatol. 1996;107:833–7. doi: 10.1111/1523-1747.ep12330869. [DOI] [PubMed] [Google Scholar]
- 19.Papadavid E, Economidou J, Psarra A, et al. The relevance of peripheral blood T-helper 1 and 2 cytokine pattern in the evaluation of patients with mycosis fungoides and Sezary syndrome. Br J Dermatol. 2003;148:709–18. doi: 10.1046/j.1365-2133.2003.05224.x. [DOI] [PubMed] [Google Scholar]
- 20.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-γ synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–9. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 22.Ciree A, Michel L, Camilleri-Broet S, et al. Expression and activity of IL-17 in cutaneous T-cell lymphomas (mycosis fungoides and Sezary syndrome) Int J Cancer. 2004;112:113–20. doi: 10.1002/ijc.20373. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Fargnoli MC, Edelson RL, Berger CL, et al. Diminished TCR signaling in cutaneous T cell lymphoma is associated with decreased activities of Zap70, Syk and membrane-associated Csk. Leukemia. 1997;11:1338–46. doi: 10.1038/sj.leu.2400745. [DOI] [PubMed] [Google Scholar]
- 25.Hansen ER, Vejlsgaard GL, Cooper KD, et al. Leukemic T cells from patients with cutaneous T-cell lymphoma demonstrate enhanced activation through CDw60, CD2, and CD28 relative to activation through the T-cell antigen receptor complex. J Invest Dermatol. 1993;100:667–73. doi: 10.1111/1523-1747.ep12472333. [DOI] [PubMed] [Google Scholar]
- 26.Wood GS, Hong SR, Sasaki DT, et al. Leu-8/CD7 antigen expression by CD3+ T cells: comparative analysis of skin and blood in mycosis fungoides/Sezary syndrome relative to normal blood values. J Am Acad Dermatol. 1990;22:602–7. doi: 10.1016/0190-9622(90)70080-2. [DOI] [PubMed] [Google Scholar]
- 27.Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J Exp Med. 2004;199:731–6. doi: 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lessin SR, Vowels BR, Rook AH. Th2 cytokine profile in cutaneous T-cell lymphoma. J Invest Dermatol. 1995;105:855–6. doi: 10.1111/1523-1747.ep12326693. [DOI] [PubMed] [Google Scholar]
- 29.Rook AH, Gottlieb SL, Wolfe JT, et al. Pathogenesis of cutaneous T-cell lymphoma: implications for the use of recombinant cytokines and photopheresis. Clin Exp Immunol. 1997;107(Suppl 1):16–20. [PubMed] [Google Scholar]
- 30.Lee BN, Duvic M, Tang CK, Bueso-Ramos C, Estrov Z, Reuben JM. Dysregulated synthesis of intracellular type 1 and type 2 cytokines by T cells of patients with cutaneous T-cell lymphoma. Clin Diagn Lab Immunol. 1999;6:79–84. doi: 10.1128/cdli.6.1.79-84.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo EK, Cassin M, Lessin SR, Rook AH. Complete molecular remission during biologic response modifier therapy for Sezary syndrome is associated with enhanced helper T type 1cytokine production and natural killer cell activity. J Am Acad Dermatol. 2001;45:208–16. doi: 10.1067/mjd.2001.116345. [DOI] [PubMed] [Google Scholar]
- 32.Hahtola S, Tuomela S, Elo L, et al. Th1 response and cytotoxicity genes are down-regulated in cutaneous T-cell lymphoma. Clin Cancer Res. 2006;12:4812–21. doi: 10.1158/1078-0432.CCR-06-0532. [DOI] [PubMed] [Google Scholar]
- 33.Levings MK, Sangregorio R, Sartirana C, et al. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor h, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med. 2002;196:1335–46. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiorentino DF, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 36.Sigurdsson V, Toonstra J, Bihari IC, Bruijnzeel-Koomen CA, van Vloten WA, Thepen T. Interleukin 4 and interferon-g expression of the dermal infiltrate in patients with erythroderma and mycosis fungoides. An immuno-histochemical study. J Cutan Pathol. 2000;27:429–35. doi: 10.1034/j.1600-0560.2000.027009429.x. [DOI] [PubMed] [Google Scholar]
- 37.Fraser-Andrews EA, Russell-Jones R, Woolford AJ, Wolstencroft RA, Dean AJ, Whittaker SJ. Diagnostic and prognostic importance of T-cell receptor gene analysis in patients with Sezary syndrome. Cancer. 2001;92:1745–52. doi: 10.1002/1097-0142(20011001)92:7<1745::aid-cncr1689>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Andrews EF, Woolford A, Jones RR, Whittaker S. A peripheral blood T cell clone is a prognostic marker in mycosis fungoides. J Invest Dermatol. 2001;116:484–5. doi: 10.1046/j.1523-1747.2001.01279-12.x. [DOI] [PubMed] [Google Scholar]
- 39.Potoczna N, Boehncke WH, Nestle FO, et al. T-cell receptor β variable region (Vβ) usage in cutaneous T-cell lymphomas (CTCL) in comparison to normal and eczematous skin. J Cutan Pathol. 1996;23:298–305. doi: 10.1111/j.1600-0560.1996.tb01301.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


