Abstract
Introduction
Cystic Fibrosis (CF), Ulcerative Colitis (UC), and Crohn’s Disease (CD) manifest as various, multiple symptoms from malfunctioning and/or damaged gastrointestinal tract, which plague the patients. These symptoms result from the dysfunctional expression products of the specific mutations of the genes, which either manifest upon birth (CF) or later in life in immuno-genetically susceptible individuals as inflammatory bowel diseases (IBD). They all may potentially lead to malnutrition of the patients. Since only correcting the mutated genes, may cure these diseases permanently, the works on the future safe gene therapies continue vigorously. However, provision of the necessary nutrients to the suffering patients is the requirement for an effective, supportive care at present. In this realm, we have developed a model of the diseased gastrointestinal tract aimed to guide designing and testing various nutritional therapies.
Materials and Methods
It is well known that inflammatory bowel diseases induce crypts within the patients’ gastrointestinal tract. Therefore, we have bioengineered, a novel, three-dimensional model of the gastrointestinal tract to evaluate the rheology of different types of nutrients. The model was assembled out of the bio-inert polymer tube with openings leading to vials of different shapes and sizes, as the simulation of the gastrointestinal tract altered by the diseases to contain multiple crypts.
Results and Conclusions
The newly developed three-dimensional model effectively simulates the structure and functions of the gastrointestinal tract of the patients with mild and severe Ulcerative Colitis, Crohn’s Disease, and Cystic Fibrosis. This model should allow us to design and test different nutritional supplements, with properties complementing the pathologically altered by the diseases functionalities of the patients’ gastrointestinal tracts. Therefore, it should help us to design the effective supportive therapies; thus to prevent the patients’ malnutrition.
Keywords: Cystic Fibrosis, Crohn`s Disease, Ulcerative Colitis, nutrition model, bioengineering, biotechnology, inflammatory bowel disease (IBD), gene therapy, malnutrition
Introduction
Cystic Fibrosis, Ulcerative Colitis, and Crohn’s Disease originate with mutations of the genes and manifest with the symptoms within the gastrointestinal tract.
Specifically, Cystic Fibrosis is an autosomal recessive disorder caused by a mutation in the gene on chromosome 7, which is coding for the protein: Cystic Fibrosis Transmembrane Conductance Regulator (CFTR). The CFTR is responsible for the transport of chloride and sodium ions across epithelial membranes, therefore a defective protein lead to thick and viscous secretions. Mutations at several different locations in the gene can all lead to Cystic Fibrosis manifesting with differing symptoms and with varying prognoses of the disease. Some mutations lead to production of a less functional protein, while others lead to no production at all. While the most common mutation is delta F508, over 1500 different mutations have been reported. The disease primarily affects the lungs, liver, and pancreas. Increased PCO2 and respiratory acidosis is observed in advanced disease. Cystic Fibrosis can be diagnosed before birth. Treatment of Cystic Fibrosis currently consists of bronchodilators, corticosteroids, antibiotics, enzyme replacement, insulin, bisphosphonates and vaccination during influenza exacerbation, and physiotherapy. Lung transplantation is as therapeutic intervention at the diseases’ final stages [12–17]. The vigorous work on gene therapy is rapidly progressing.
Ulcerative Colitis is a chronic inflammatory disease of the gastrointestinal tract. While genetic susceptibility is at the core problem, the factor triggering the disease remains elusive. Dozens of genes have also been linked to Ulcerative Colitis, many of them involved in the protective function of the intestines. In addition, there is an increased incidence of inflammatory bowel disease in people that have a family member with inflammatory bowel disease, suggesting a genetic basis of these diseases. Ulcerative Colitis usually attacks the large intestine. It has exacerbation periods and disease free periods [6], all manifesting by chronic bloody diarrhea. It is mostly treated as an autoimmune disease, although symptoms might diminish spontaneously. Nutritional supplementation certainly improves the patients' well-being. Pregnancy is possible during symptoms-free periods [7–11].
Crohn’s Disease has genetic mutations as the causative factor. Perhaps, the most important of these genes is the NOD2 gene. People with a mutated NOD2 have approximately 20 times increased risk of the disease. Nevertheless, mutations in many other genes may contribute to developing fully blown symptoms. Crohn’s Disease might affect any part of the gastrointestinal tract (GIT). Patients may present with a variety of symptoms such as vomiting, weight loss, abdominal pain and diarrhea. Moreover, other symptoms include eye inflammation, anemia, arthritis and skin rash [1]. The manifestation of this disease, in the genetically susceptible people, depends on the interaction of the immune system, environmental factors and bacteria. Nevertheless, Crohn’s Disease does not appear to have an autoimmune background [2–4]. Treatment consists of anti-inflammatory agents, immune system suppressors, antibiotics, surgery, nutritional support and symptomatic relief (anti-diarrheal, laxatives) [5]. Potential of immune gene therapy is intensively explored.
The rapid progress takes place in refining the genomic background for these diseases. This creates the solid foundations for developing strategies for gene therapy. Meanwhile, the treatments are aimed at providing to the patients some relief from symptoms they are suffering from and preventing malnutrition due to pathologically altered gastrointestinal tract, while providing them with all necessary nutrients.
This work was dedicated to compensating the problems resulting from disturbances of the functions of the patients’ gastrointestinal tract. In this realm, we have developed a three-dimensional polymer model for designing of therapeutic nutrition.
Materials and Methods
The rationale for the project was the well known fact, that that the aforementioned diseases of the gastrointestinal tract induce formation of crypts. These crypts have different sizes with different diameters of entrances. The result is that the nutrient may easily enter, but may have difficulties on exit. This phenomenon aggravates the symptoms of the disease. Therefore, we pursued the concept of designing and engineering of a spatial model of tract simulating pathologically altered gastrointestinal tract of the patients. In order to do that we used the Altech® breathing circuit LOT: 6259.1503.12, 120 cm limb Y connector with ports 2lt latex free. We acquired pipettes with two different diameters 5 mm and 7 mm. We cut out most of the suction tube leaving only 5 mm in length. Afterwards we punctured 10 holes in different parts of the circuit and clued the pipettes head and neck in between the beginning and end (Figure 1). We glued the neck of the pipettes with Pattex® silicone that was melted again with Pattex®-Henkel KGaA melting apparatus (Nr: 10172786 Duesseldorf) (Figure 2). We have chosen to use two different pipettes with different ``necks`` in order to simulate two different types of crypts. We named the 7 mm diameter grade I and the 5 mm diameter grade II (Figure 3). Grade II was relevant to the worse, based on the fact that a crypt with a smaller entrance releases the nutritional material more difficult. We designed three different groups of nutritional material: a) Proteins b) Carbohydrates and c) Fat. For each group we designed additionally six subgroups with different pH and viscosity. Viscosity is the most important factor influencing the rheology and deposition of nutrition within the GI tract. We have to comment at this point that all pipettes had the same volume. We are currently designing and engineering a pump simulating the gastrointestinal tract movement, so that more information can be gathered regarding its function, as well as of pharmaco-kinetics of administered nutrients.
Figure 1.

Polymer tube with crypt-like traps.
Figure 2.

Pattex®-Henkel KGaA melting apparatus.
Figure 3.
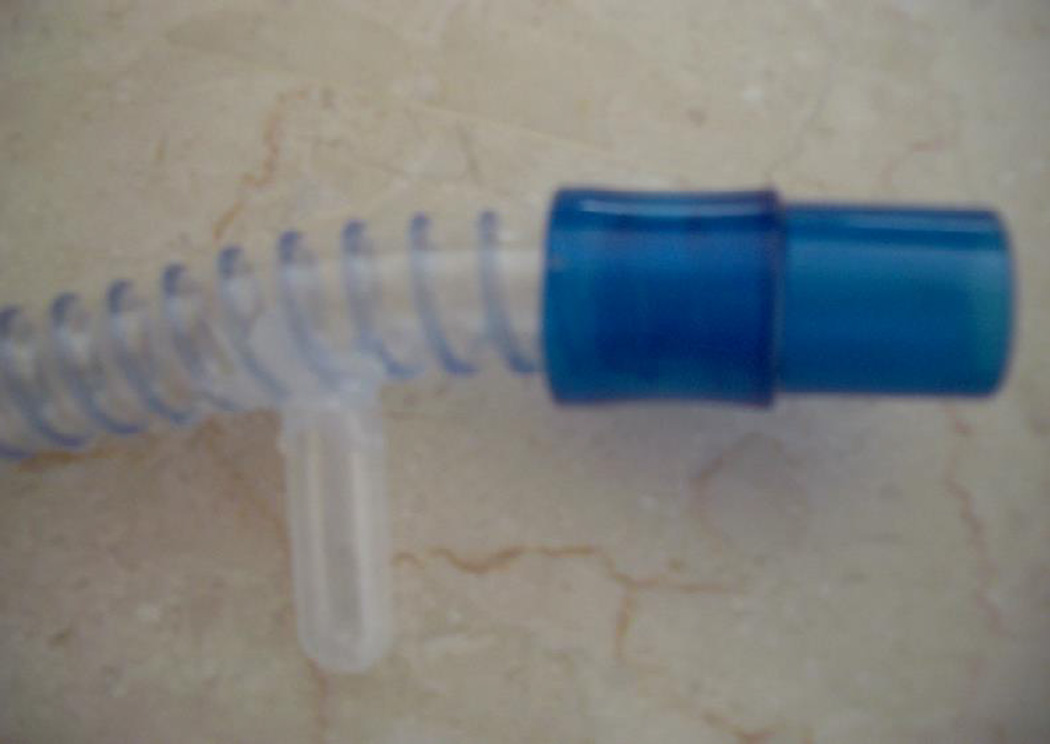
Close picture of a trap.
Results and Conclusions
In all aforementioned diseases, Ulcerative Colitis, Crohn’s Disease, and Cystic Fibrosis, gastrointestinal tract of the patients is pathologically altered. This could potentially lead to the patients’ malnutrition. Therefore, nutrition is the key element of the supportive care.
The newly developed three-dimensional model simulates effectively the structure and functions of the gastrointestinal tract of the patients with mild and severe stages of the aforementioned diseases. Modifications of the different nutrients, with properties complementing functionalities of the patients’ gastrointestinal tracts, which are pathologically altered by the diseases, will help us to design the proper supportive therapies. This should help us in designing and testing effective supportive therapies, thus to prevent the patients from malnutrition. Herein, this has been accomplished by well designed composition and properly adjusted administration of the nutritional therapy, which addresses the spatial changes (crypts) for each of these diseases. While there are already several nutritional supplements on the market that can be used by the patients additionally to their treatment, their selection may benefit from tests run on the model system, which we present herein. We continue experimenting with the different types of nutritional compounds, different viscosities, acid/bases gradients, osmolarity, etc in order to identify the optimal combinations for the particular patient – this becomes an important aspect of their personalized therapy. Optimal rheology of the nutritional supplements could contribute effectively to the supportive treatment for these patients.
In general, spatial models of the pathologically altered gastrointestinal tract are excellent starters for initial experimentation, as we can easily proceed to an in vitro or in vivo experimentation [20]. Further refinement of the model system described herein, should help us in designing optimal composition and properties of the provided nutrients, thus to improve supportive care of our patients.
Acknowledgments
Sources of Funding for the Work
This work was supported by the funds from the National Institutes of Health [grant numbers: P41 RR000570 and P41 RR002301]; and from the Phoenix Biomolecular Engineering Foundation [grant number: 2006070101] to Marek Malecki MD PhD - Principal Investigator. Administrators of the funding institutions and managers of the facilities had no influence on the project design and presented data.
Footnotes
Conflict of Interest Statement
The authors state no conflict of interest.
References
- 1.Dessein R, Chamaillard M, Danese S. Innate immunity in Crohn’s disease: the reverse side of the medal. J Clin Gastroenterol. 2008;42(Pt 1) Suppl 3:S144–S147. doi: 10.1097/MCG.0b013e3181662c90. [DOI] [PubMed] [Google Scholar]
- 2.Marks DJ, Rahman FZ, Sewell GW, Segal AW. Crohn’s disease: an immune deficiency state. Clin Rev Allergy Immunol. 2010;38:20–31. doi: 10.1007/s12016-009-8133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lalande JD, Behr MA. Mycobacteria in Crohn’s disease: how innate immune deficiency may result in chronic inflammation. Expert Rev Clin Immunol. 2010;6:633–641. doi: 10.1586/eci.10.29. [DOI] [PubMed] [Google Scholar]
- 4.Cuthbert AP, Fisher SA, Mirza MM, King K, Hampe J, et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology. 2002;122:867–874. doi: 10.1053/gast.2002.32415. [DOI] [PubMed] [Google Scholar]
- 5.Shoda R, Matsueda K, Yamato S, Umeda N. Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am J Clin Nutr. 1996;63:741–745. doi: 10.1093/ajcn/63.5.741. [DOI] [PubMed] [Google Scholar]
- 6.Danese S, Fiocchi C. Ulcerative Colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 7.Hanauer SB, Sandborn W Practice Parameters Committee of the American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol. 2001;96:635–643. doi: 10.1111/j.1572-0241.2001.3671_c.x. [DOI] [PubMed] [Google Scholar]
- 8.Kornbluth A, Sachar DB Practice Parameters Committee of the American College of Gastroenterology. Ulcerative Colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–1385. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- 9.Järnerot G, Järnmark I, Nilsson K. Consumption of refined sugar by patients with Crohn’s disease, Ulcerative Colitis, or irritable bowel syndrome. Scand J Gastroenterol. 1983;18:999–1002. doi: 10.3109/00365528309181832. [DOI] [PubMed] [Google Scholar]
- 10.Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, et al. Influence of dietary factors on the clinical course of Ulcerative Colitis: a prospective cohort study. Gut. 2004;53:1479–1484. doi: 10.1136/gut.2003.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tilg H, Kaser A. Diet and relapsing Ulcerative Colitis: take off the meat? Gut. 2004;53:1399–1401. doi: 10.1136/gut.2003.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yankaskas JR1, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125:1S–39S. doi: 10.1378/chest.125.1_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 13.Flume PA, Mogayzel PJ, Jr, Robinson KA, Rosenblatt RL, Quittell L, et al. Cystic Fibrosis pulmonary guidelines: pulmonary complications: hemoptysis and pneumothorax. Am J Respir Crit Care Med. 2010;182:298–306. doi: 10.1164/rccm.201002-0157OC. [DOI] [PubMed] [Google Scholar]
- 14.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 15.Eggermont E, De Boeck K. Small-intestinal abnormalities in cystic fibrosis patients. Eur J Pediatr. 1991;150:824–828. doi: 10.1007/BF01954999. [DOI] [PubMed] [Google Scholar]
- 16.Alves Cde A, Aguiar RA, Alves AC, Santana MA. Diabetes mellitus in patients with cystic fibrosis. J Bras Pneumol. 2007;33:213–221. doi: 10.1590/s1806-37132007000200017. [DOI] [PubMed] [Google Scholar]
- 17.Haworth CS, Selby PL, Webb AK, Dodd ME, Musson H, et al. Low bone mineral density in adults with cystic fibrosis. Thorax. 1999;54:961–967. doi: 10.1136/thx.54.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and Ulcerative Colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 19.Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. ISME J. 2007;1:403–418. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- 20.Zarogoulidis P, Darwiche K, Walter R, Li Q, Teschler H, et al. Research spotlight: sirolimus-coated stents for airway tracheal stenosis: a future 3D model concept with today’s knowledge. Ther Deliv. 2013;4:1093–1097. doi: 10.4155/tde.13.86. [DOI] [PubMed] [Google Scholar]


