Abstract
Cinnamon is a spice commonly used worldwide to flavor desserts, fruits, cereals, breads, and meats. Numerous health benefits have been attributed to its consumption, including the recent suggestion that it may decrease blood glucose levels in people with diabetes. Insulin signaling is an integral pathway regulating the lifespan of laboratory organisms, such as worms, flies, and mice. We posited that if cinnamon truly improved the clinical signs of diabetes in people that it would also act on insulin signaling in laboratory organisms and increase lifespan. We found that cinnamon did extend lifespan in the fruit fly, Drosophila melanogaster. However, it had no effect on the expression levels of the 3 aging-related Drosophila insulin-like peptides nor did it alter sugar, fat, or soluble protein levels, as would be predicted. In addition, cinnamon exhibited no protective effects in males against oxidative challenges. However, in females it did confer a protective effect against paraquat, but sensitized them to iron. Cinnamon provided no protective effect against desiccation and starvation in females, but sensitized males to both. Interestingly, cinnamon protected both sexes against cold, sensitized both to heat, and elevated HSP70 expression levels. We also found that cinnamon required the insulin receptor substrate to extend lifespan in males, but not females. We conclude that cinnamon does not extend lifespan by improving stress tolerance in general, though it does act, at least in part, through insulin signaling.
Keywords: cinnamon, botanical extract, insulin signaling, lifespan, aging, Drosophila
1. Introduction
Cinnamon is a spice commonly used in various cuisines around the world to flavor desserts, fruits, cereals, breads, and meats, and is derived from the bark of trees in the genus Cinnamomum, most commonly, C. cassia. The extract has been reported to exhibit numerous health benefits, including anti-inflammatory, antimicrobial, antioxidant, and anti-cancer action (Hong et al. 2002; Huss et al. 2002; Kim et al. 2007; Friedman et al. 2002; Lopez et al. 2005; Osawa et al. 1991; Lin et al. 2003; Kim et al. 1995; Jaganathan and Supriyanto 2012; Koppikar et al. 2010). There has been a recent interest in the ability of cinnamon to have a positive effect on diabetes. In particular, several clinical studies on the anti-diabetic properties of cinnamon may create an important role for this botanical in the management of diabetes and prediabetes (Lu et al. 2012; Ranasinghe et al. 2012; Davis and Yokoyama 2011; Kirkham et al. 2009).
The precise molecular action of cinnamon is not known. However, cinnamon and one of its putative active compounds, cinnamaldehyde, have been shown to elevate the expression levels of peroxisome proliferator-activated receptor γ (PPARγ) and activate AMP kinase (AMPK) (Sheng et al. 2008; Huang et al. 2011). These activities mimic the action of the thiazolidinediones and metformin; commonly used antidiabetic drugs (Lee et al. 2011; Scarsi et al. 2007; Zhou et al. 2001; Zhang et al. 2007). Both PPARγ and AMPK elevate metabolism in part by activating mitochondrial biogenesis (Alaynick 2008; Haemmerle et al. 2011; Hardie 2011). Cinnamon has also shown a complex relationship with insulin signaling where it activates insulin-like growth factor 1 (IGF1) signaling in fibroblasts, but down-regulates insulin signaling in adipocytes (Takasao et al. 2012; Cao et al. 2010). Another compound present in cinnamon, β-caryophyllene oxide, has been suggested to activate the mammalian target of rapamycin (mTOR) (Park et al. 2011), which is a complex of proteins that centrally regulates numerous cellular and metabolic processes (Schmelzle and Hall 2000; Wullschleger et al. 2006). In addition, cinnamon, cinnamaldehdye, and eugenol, another constituent compound, have been shown to directly modulate mitochondrial physiology (Usta et al. 2002). Thus, the potential anti-diabetic effects of cinnamon could possibly occur through the elevation of cellular metabolism.
In various model organisms, several pathways such as Insulin and insulin-like growth factor signaling (IIS), mTOR, cyclic-AMP signaling, autophagy, and mitochondrial function have been implicated (Bartke 2011; Giannakou and Partridge 2007; Fontana et al. 2010; Kapahi et al. 2004; Bjedov et al. 2010; Lu et al. 2011; Stenesen et al. 2013; Dai et al. 2009; Esposito et al. 1999; Lee et al. 2010; Morrow et al. 2004; Tong et al. 2007; Eisenberg et al. 2009; Schriner et al. 2005). Since cinnamon appears to modulate all of these pathways, it may be expected to extend lifespan in a short-lived model organism. This was previously shown to be the case in the worm, Caenorhabditis elegans where cinnamon appeared to require several molecular pathways, including IIS, to extend lifespan (Yu et al. 2010). Here, we found that cinnamon is able to extend lifespan the fruit fly, Drosophila melanogaster with an apparent requirement of IIS in males, but not in females. Cinnamon also appears to have little or no effect on stress tolerance, including oxidative stress in males, and is unlikely to act as an antioxidant.
2. Materials and Methods
2.1 Dietary supplements
Cinnamon was obtained from McCormick & Company, Sparks MD. Coumarin and cinnamaldehyde were obtained from Sigma-Aldrich, St. Louis, MO. Cinnamaldehyde, coumarin, cinamic alcohol, and cinnamic acid levels in the cinnamon extract were measured by high-performance liquid chromatography. Two hundred mg of cinnamon powder was suspended in 25 mL of 50% methanol and sonicated for 30 min and then filtered though a 0.2 µm Whatman filter. The samples were run on an ACE C18 column with a mobile phase initially composed of 10% acetonitrile, which was then ramped up to 70% over 30 min. The cinnamon powder used in this study was found to contain 3.8% cinnamaldehyde, 1.4% coumarin, and 0.9% cinnamic acid. No cinnamic alcohol was detected. Cinnamaldehyde and coumarin concentrations were selected based on the highest levels of what is detected in commercial preparations (Woehrlin et al. 2010).
2.2. Fly stocks
The w1118 control (Stock 3605) and chico1 (Stock 10738) flies were obtained from the Bloomington Drosophila Stock Center at Indiana University. The JIV flies used in this study were derived from an Amherst, Massachusetts’s population established by P.T. Ives in 1975 (Rose and Charlesworth 1981, Rose, 2002 #149). This population has been cultured at moderate to large population sizes and controlled densities (50–80 eggs per vial) for more than 700 generations with discrete generations cultured every 2 weeks.
Flies were reared by placing approximately 100 males and 100 females in a plastic cylinder with a screen top, housing at the bottom a 10 cm petri dish filled with banana-molasses food composed of 9% carbohydrate content and 10% yeast content. These flies were allowed to lay eggs for one day. The food around the edge of the dish was then cut into approximately 1-cm2 squares and placed into 8-dram vials. Each of these vials then contained approximately 35 eggs. Two weeks later, approximately 30 flies/vial were recovered after eclosure, which were then used for our lifespan studies or other experiments.
2.3 Feeding and lifespan assays
Flies were fed Cinnamon, coumarin, and cinnamaldehyde on the methods described in Jafari et al. (Jafari et al. 2007). For all experiments, feeding was initiated immediately post-eclosion and, other than lifespan assays, were completed when the flies were 10 days of age. Cinnamon or chemicals were dissolved in a yeast solution (4% yeast in 1% acetic acid), and 75 µL of this mixture was overlaid on a banana-molasses food composed of 9% carbohydrate content and 3.6% yeast content. Flies were maintained at 22 ± 1° C under a 12 h light: 12 h dark cycle for all experiments. For lifespan studies, flies were housed 12 per vial (6 males and 6 females). This density was maintained as long as feasible. Flies were given fresh food every two days and deaths were recorded at these times. Survival analyses were calculated based on the number of deaths recorded and evaluated by the log-rank Mantel-Cox test. No deaths were censored. Flies were also housed 12 per vial (6 males and 6 females) for all other experiments, independent of the total number needed, and transferred to fresh food every other day.
2.4 Measurement of fecundity and locomotion
For the fecundity assays, 40 total pairs per treatment group, collected less than 12 h post eclosion, were placed in 8 dram vials (one male and one female per vial) containing 5 mL of a sugar-charcoal media covered with 75 µL yeast solution (1 g yeast/mL 1% acetic acid). Pairs were permitted to lay eggs for 24 hours and then transferred to new vials every 24 h for 10 ten days. Eggs were counted every 24 hours.
Vertical locomotion (climbing) was assessed by the rapid iterative negative geotaxis (RING) assay (Gargano et al. 2005). For each treatment group, 15 flies were placed in an 8 dram vial. The vial was gently shaken until all flies were displaced to the bottom of the vial. Flies were then permitted to climb for 4 seconds, and then photographed. All four groups (males and females, cinnamon-fed and controls) were assayed simultaneously. The assay was repeated 3 times with independent groups of flies. The distance climbed was measured in Adobe Photoshop and the speed was calculated by distance/4 sec. Horizontal locomotion was measured by the Drosophila activity monitor (DAM) assay (Zordan et al. 2007) by the number of beam breaks over a 24-hour period.
2.5 Gene expression assays
Approximately 400 Flies were fed 25 mg/mL Cinnamon or an identical control diet for 10 days, and frozen in groups of 25. RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Samples were treated with DNase (New England Biolabs, Ipswich, MA) at 37° C for 10 min to remove contaminating DNA. DNase was heat inactivated by incubation at 75° C for 10 min in the presence of 5 mM EDTA. RNA was then purified by use of the RNeasy kit (Qiagen, Hilden, Germany). RNA quantity and quality was measured by spectrophotometry. One µg of RNA from each sample was converted to DNA by the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Samples were diluted 100-fold. Quantitative PCR was performed on a MiniOpticon real-time PCR system with SYBR green dye (Bio-Rad, Hercules, CA). Relative amplification was calculated by the threshold cycle of each respective gene divided by the threshold cycle of the reference gene, RNA polymerase II. Primer sequences, listed in Table S1, were designed by NCBI/Primer-BLAST. All primers were designed to have a melting temperature of 60° C.
2.6 Stress assays
One hundred twenty flies per sex per treatment were fed for 10 days with 25 mg/mL cinnamon or control diet. For the H2O2, paraquat, and iron toxicity assays, flies were fed each compound at the concentrations indicated in the figure captions diluted in a 5% glucose solution supplied on filter paper. Iron was supplied in the form of iron-nitrilotriacetate (Fe-NTA) (Awai et al. 1979). For the desiccation assay, flies were housed in empty vials and deaths were recorded every 2 h. For the starvation assay, flies were housed in vials containing 2% agarose to provide moisture, but no nutritional value. Deaths were recorded every 4 hours. For the heat assay, flies were housed in empty vials at 37° C, and deaths were recorded every hour. For the cold tolerance assay, flies were housed in empty vials at 4° C for 72 h. They were then returned to 22° C, and the numbers of alive and dead were recorded after 12 hours. Survival for cold tolerance was calculated by Fisher’s exact test. Survival for all other assays was determined by the Mantel-Cox log-rank test.
2.7 Measurement of protein, water and fat content and body weight
Flies were fed for 10 days with 25 mg/mL Cinnamon or a control diet. The flies were then collected with CO2 and weighed. For sugar content, flies were homogenized in ice-cold 500 µL 50 mM Tris-HCl buffer, pH 7.8, and then centrifuged for 10 min at 10,000 × g at 4° C. Ten µL was removed for protein measurement, and the remainder was incubated for 10 min at 95° C. The samples were then incubated overnight at 37° C with 0.05 U/mL trehalase to convert trehalose to glucose. Glucose concentrations were then measured by an increase in absorbance at 340 nm in 50 mM Tris-HCl buffer, pH 7.8 containing 1 mM Mg-acetate, 0.66 mM NAD+, 0.4 mM ATP, and 2 mU/mL each of hexokinase and glucose-6-phosphate dehydrogenase (Carroll et al. 1971). For the soluble protein assay, 50 flies per sample were homogenized in 500 µL 100 mM Potassium phosphate buffer, pH 7.4. The samples were centrifuged for 10 min at 10,000 x g. Protein in the supernatant was measured by reaction with Coomassie Brilliant Blue and correlated to a standard curve generated with bovine serum albumin (Bradford 1976) and normalized to fly weight. For water content, 10 flies per sample were weighed, dried for 48 h at 70° C, and then weighed again. The difference in weights divided by the initial weight was taken to be the water content. To determine fat content, the samples used for water measurement were then incubated at RT for 24 h in diethyl ether. The ether was removed and the samples were allowed to dry, and then weighed. Fat content was taken to be the difference in the weights before and after diethyl ether treatment divided by the initial weight (prior to drying at 70° C).
2.8 Measurement of mitochondrial content and respiration
Flies were fed for 10 days. Fifty flies per sample were homogenized in ice-cold isolation buffer (225 mM mannitol, 75 mM sucrose, 10 mM MOPS, 1 mM EGTA, 0.5% fatty acid free BSA, PH 7.2) using a glass-teflon dounce homogenizer, and then filtered through two layers of cotton gauze. A mitochondrial-enriched pellet was obtained by centrifugation for 10 min at 6000 x g and re-suspended in 150 µL ice-cold isolation buffer. Mitochondrial respiration rates were measured using a Clark-type oxygen electrode and monitoring system (Hansatech Instruments, Norfolk, England). An NADH-linked substrate (pyruvate in combination with malate) was added to isolated mitochondria suspended in 1 mL of respiration buffer (225 mM mannitol, 75 mM sucrose, 10 mM KCl, 10 mM Tris-HCl, 5 mM KH2PO4, pH 7.2) at 30° C. To this, 125 nmol ADP was added to generate state 3 respiration. State 3 respiration represents the maximal ability to generate ATP, and is generally 5–6-fold higher than state 4 respiration which is determined after ADP is consumed. The respiratory control ratio (RCR) is defined as the ratio of state 3 to state 4. Uncoupled (maximal rate of O2 consumption) respiration rates were measured the after addition of the uncoupler FCCP (Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone).
Mitochondrial content was measured by fumarase activity and mtDNA copy number. For fumarase activity, 50 flies were homogenized in 50 mM potassium phosphate buffer, pH 7. 4 and centrifuged for 10 min at 10,000 x g at 4° C. Fumarase activity in the supernatant was measured by the conversion of 50 mM malate (in 50 mM potassium phosphate buffer, pH 7. 4) to fumarate at 240 nm (Kuff 1954). Activities were normalized to soluble protein content. Mitochondrial DNA copy was estimated by the relative amplification of a mitochondrial target (16s ribosomal RNA gene) to that of a nuclear target (ribosomal protein Rp49). Twenty-five flies were homogenized in DNA isolation buffer (50 mM Tris-HCl, pH 8.0, 5 mM EDTA, 100 mM NaCl, 0.5% SDS) and supplemented with proteinase K (0.5 mg/mL final concentration) and digested overnight at 55° C. The samples were then extracted by standard phenol-chloroform procedures and precipitated by ethanol. Quantitaive PCR procedures were identical to those listed above except in this case the reference gene used was RP49.
2.9 Statistical analyses
Data were presented as the mean ± sem except for lifespan curves. Statistical analyses were conducted using Prism software (GraphPad, La Jolla, CA). The tests used and sample sizes for each experiment are indicated in the figure captions and in the methods and results sections.
3. Results
3.1 Cinnamon, but not coumarin or cinamaldehyde, increases Drosophila lifespan
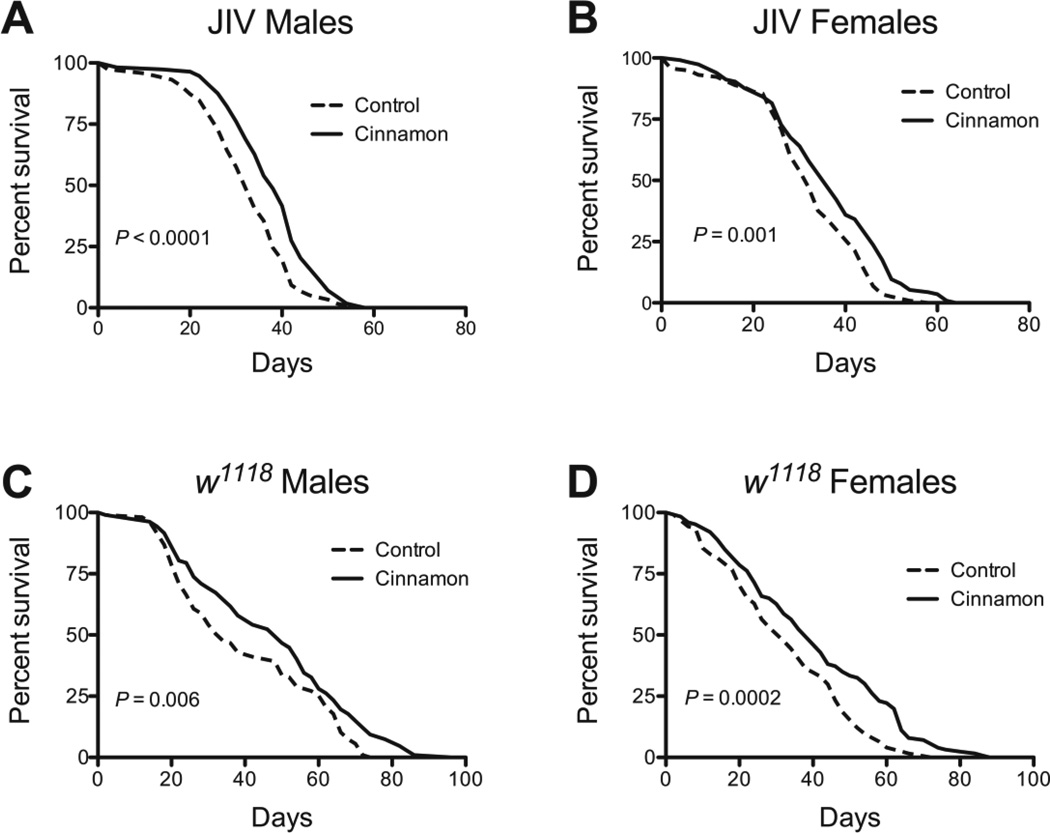
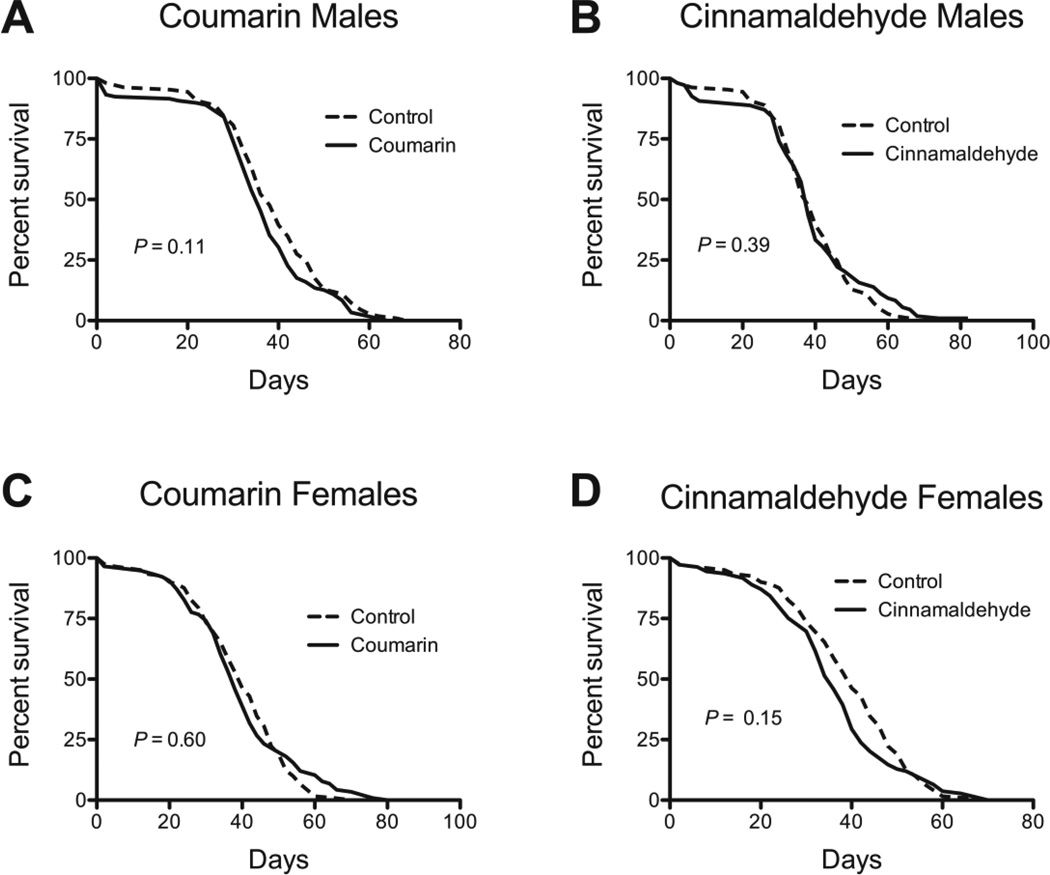
We examined whether cinnamon could extend lifespan in the fly at 3 different doses: 2.5, 25, and 75 mg/mL. The lowest dose had a marginal effect in males and no significant effect in females (Table 1). There was a dose dependent effect in female flies, with 75 mg/mL exhibiting a greater impact on mean lifespan (Table 1). We chose 25 mg/mL as the best dose as it increased lifespan in both sexes. We examined the effect of 25 mg/mL on survival in two types of Drosophila, w1118, a commonly used inbred strain, and JIV, an outbred population derived from one originally collected in 1978 (Rose and Charlesworth 1981; Rose et al. 2002). Cinnamon increased lifespan up to 37% in both of strains indicating its effects are not specific to a single genotype (Fig. 1 and Table 1). The precise active component in cinnamon is not known. However, we tested two of the better-known, and potentially toxic, compounds, coumarin (Cohen 1979; Abraham et al. 2010), and cinnamaldehyde (Bickers et al. 2005). Neither compound was able to extend Drosophila lifespan (Fig. 2). Since cinnamaldehyde and coumarin concentrations were selected based on the highest levels of what is detected in commercial preparations (Woehrlin et al. 2010), the lack of a beneficial effect is unlikely to be explained by an insufficient amount, and may explain the slight toxicity of cinnamaldehyde in female flies (Fig. 2D).
Table 1.
Mean life span values for Cinnamon-fed and control diet flies
| Strain | Sex | Dose (mg/mL) | mean | % increase | P |
|---|---|---|---|---|---|
| w1118 | males | 0 | 32.1 ± 0.9 | ||
| w1118 | males | 2.5 | 34.6 ± 0.9 | 8 | < 0.05 |
| w1118 | females | 0 | 31.8 ± 1.1 | ||
| w1118 | females | 2.5 | 33.9 ± 1.2 | 7 | 0.22 |
| w1118 | males | 0 | 32.1 ± 0.9 | ||
| w1118 | males | 25 | 37.5 ± 0.9 | 17 | < 0.0001 |
| w1118 | females | 0 | 32.6 ± 1.4 | ||
| w1118 | females | 25 | 40.0 ± 1.6 | 23 | < 0.0001 |
| w1118 | males | 0 | 40.7 ± 1.6 | ||
| w1118 | males | 75 | 45.9 ± 2.1 | 13 | 0.07 |
| w1118 | females | 0 | 32.6 ± 1.4 | ||
| w1118 | females | 75 | 44.6 ± 2.3 | 37 | < 0.0001 |
| JIV | males | 0 | 32.1 ± 0.9 | ||
| JIV | males | 25 | 37.5 ± 0.9 | 17 | < 0.0001 |
| JIV | females | 0 | 31.9 ± 1.1 | ||
| JIV | females | 25 | 35.7 ± 1.3 | 12 | < 0.05 |
| chico1 | males | 0 | 50.4 ± 1.6 | ||
| chico1 | males | 25 | 49.7 ± 1.8 | 0 | 0.62 |
| chico1 | females | 0 | 55.3 ± 1.7 | ||
| chico1 | females | 25 | 61.1 ± 1.6 | 9 | < 0.01 |
Values are means ± sem. Units are days. The genotype of chico1. flies are w1118, chico1/chico1. P-values were calculated by Mann-Whitney analysis, control vs. cinnamon.
Fig. 1.
Cinnamon increases survival in the fruit fly, Drosophila melanogaster. Cinnamon increased survival in both (A) male, P <0.0001, and (B) female, P = 0.001, JIV flies, and in (C) male, P = 0.006, and (D) female, P = 0.0002, w1118 flies. The increases in mean lifespan due to cinnamon were 17%, 12%, 18%, and 24%, respectively. P-values were calculated with the Mantel-Cox log-rank test. Sample sizes for the control groups and treated groups respectively were as follows: JIV males: 118, 113; JIV females: 117, 114; w1118 males: 107, 107; w1118 females: 124, 126.
Fig 2.
The effect of coumarin and cinnamaldehdye on w1118 lifespan. Coumarin at a 5 mg/mL had no effect on survival in (A) male or (B) female w1118 flies. Neither did 5 µL /mL cinnamaldehyde in (C) male flies. In (D) females cinnamaldehyde also had no effect on overall survival via the Mantel-Cox log test, but appeared to actually decrease their mean lifespan (P < 0.05). P-values were calculated with the Mantel-Cox Log-Rank test and Man-Whitney nonparametric test. Sample sizes for the control groups and treated groups respectively were as follows: coumarin males: 109, 119; coumarin females: 121, 116; cinnamaldehyde males: 109, 108; cinnamaldehyde females: 121, 109.
3.2 The effect of cinnamon on fecundity and physical activity
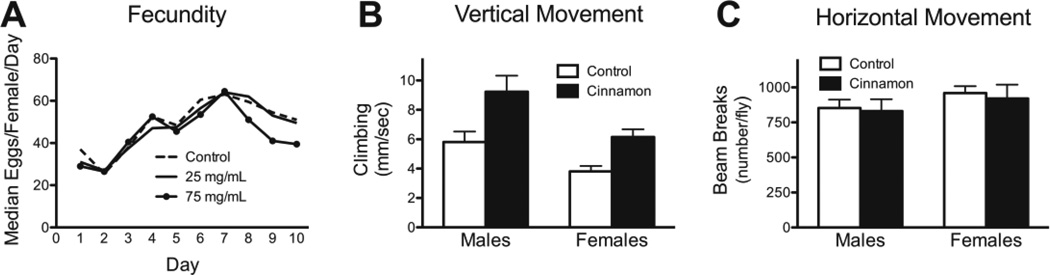
A significant issue with measuring lifespan-extending effect of pharmaceuticals and botanicals is that they may deter the animal from eating due to a repulsive taste or smell. Then if the animal consumed less food, lifespan may be increased due to a calorie restriction effect, rather than a biochemical effect of the drug. This possibility can be indirectly addressed by measuring fecundity (egg laying) as a reduction in caloric or protein intake would result in decreased fecundity. We found that 25 mg/mL of cinnamon had no effect on fecundity, whereas 75 mg/mL did have a moderate negative effect (Fig. 3A). Because of the positive effect on lifespan without a negative effect on fecundity, we used cinnamon at a dose of 25 mg/mL for the remainder the experiments.
Fig. 3.
The effect of cinnamon on reproduction and locomotion in w1118 flies. (A) At 25 mg/mL cinnamon had no effect on fecundity, however, a dose of 75 mg/mL did have a negative effect after day 7. P < 0.05, repeated measures ANOVA. P > 0.05 for control vs. 25 mg/mL, and P < 0.05 for control vs. 75 mg/mL, Dunnett’s multiple comparison test, n= 20 pairs for each group. (B) Cinnamon improved vertical climbing ability in both males and females. P < 0.0001, 2-Way ANOVA for diet, P < 0.01 for each sex, control vs cinnamon, Bonferroni posttest, n = 23 male controls, 26 males fed cinnamon, 39 female controls, 40 females fed cinnamon. (C) Cinnamon had no effect on horizontal locomotion. P = 0.72 for diet, 2-way ANOVA, n = 13 male controls, 19 males fed cinnamon, 13 female controls, 18 females fed cinnamon.
We then examined whether cinnamon would have any effect on physical activity in the fly. We found that it dramatically improved vertical climbing ability (Fig. 3B). Since flies have an inherit desire to climb (negative geotaxis), this increased vertical climbing ability demonstrates a positive effect on physical performance. Horizontal locomotion is used to assess whether a drug acts as a stimulant or sedative. We found that cinnamon had no effect on horizontal locomotion (Fig. 3C).
3.3 The action of cinnamon through insulin signaling
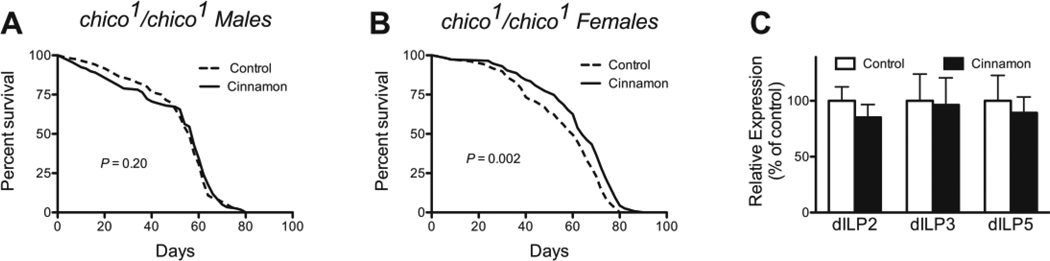
Our original hypothesis was that cinnamon would extend lifespan by acting through insulin and insulin-like signaling (IIS) in the fly. We found that cinnamon required the insulin receptor substrate to extend lifespan in males (Fig. 4A), but the extract was able to extend lifespan in females in its absence (Fig. 4B). Of the 7 Drosophila-like insulin peptides (Dilps) present in flies, 3 are associated with increased lifespan. However, cinnamon had no effect on the expression levels of any of these 3 Dilps (Fig. 4C).
Fig 4.
The dependence of cinnamon on the insulin receptor substrate chico, and its effect on the expression levels of 3 Drosophila-like insulin peptides. Cinnamon was not able to extend lifespan in (A) chico male flies, but could do so in (B) chico female flies. P-values were calculated with the Mantel-Cox Log-Rank test. Sample sizes for the control groups and treated groups respectively were as follows: chico males: 120, 119; chico females: 109, 115. (C) Cinnamon had no effect on the expression levels of the 3 aging-related dilps in male flies. P = 0.54 for diet, 2-way ANOVA, n = 6 groups of 10 flies.
3.4 Cinnamon has sex-specific and variable effects on stress tolerance
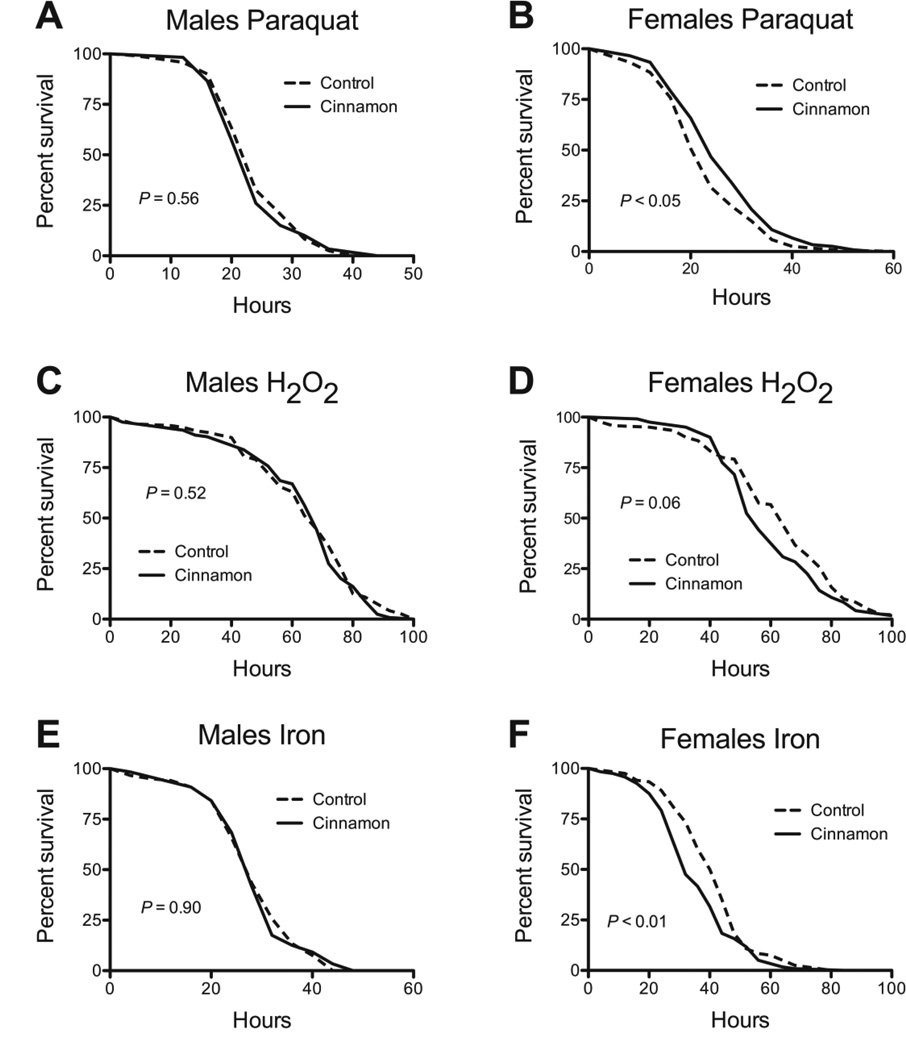
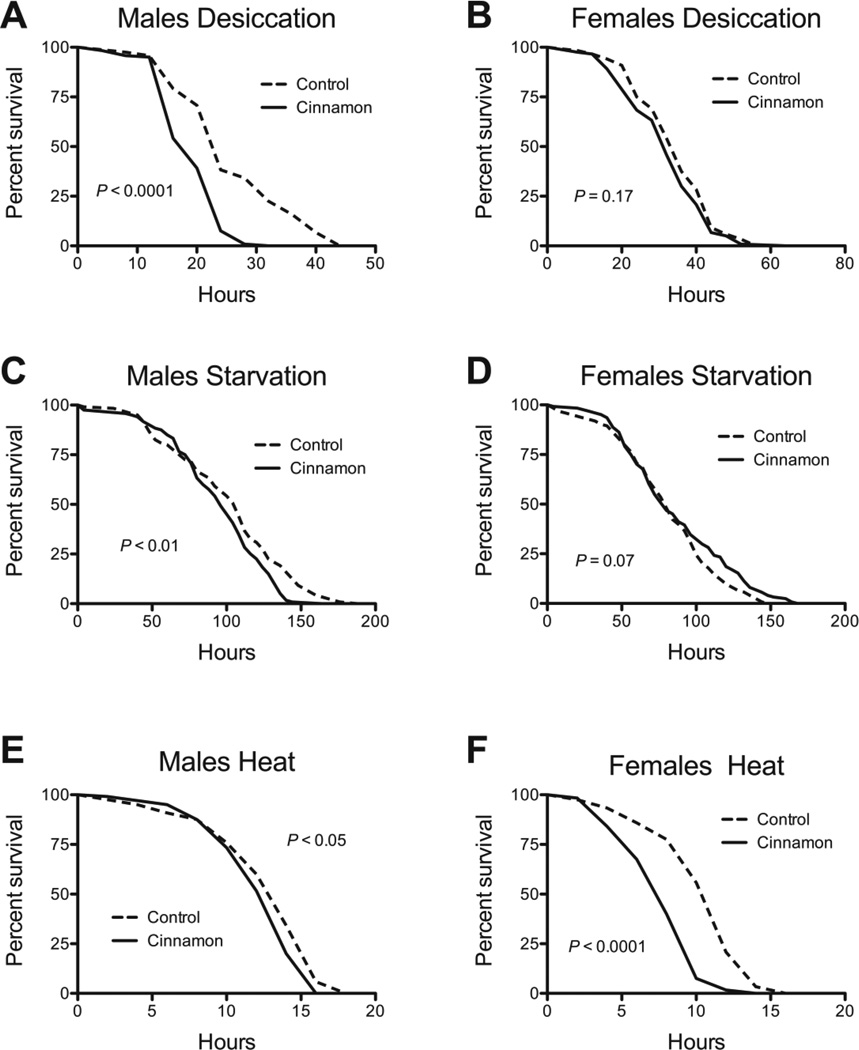
Improved stress tolerance is strongly associated with enhanced lifespan (Kirkwood and Austad 2000; Vermeulen and Loeschcke 2007). We therefore examined whether cinnamon might act by enhanced stress tolerance. We found marked differences between males and females. In males, cinnamon had no protective effect against the superoxide generator paraquat, H2O2, or iron (Fig. 5A, C, E) and sensitized males to desiccation and starvation (Fig. 6A, C). Females fed cinnamon were marginally protected against paraquat (Fig. 5B). However, cinnamon afforded no protective effect in females against H2O2, desiccation, or starvation, and sensitized them to iron (Figs. 5D, F and 6B, D). Two commonalities between the sexes, was that cinnamon protected both against cold (Table 2), and sensitized both to heat (Fig. 6E, F).
Fig 5.
The effect of cinnamon on the tolerance of w1118 flies to oxidative stress and iron toxicity. Cinnamon had no ability to improve tolerance to (A) 12.5 mM paraquat, (C) 5% H2O2, or (E) or 80 mM Fe-NTA in male flies. In females, cinnamon had a marginal protective effect against (B) paraquat, had a borderline negative effect against (D) H2O2, and sensitized females to (F) iron. P-values were calculated with the Mantel-Cox Log-Rank test. For all groups, n = 120.
Fig 6.
The effect of cinnamon on the tolerance of w1118 flies to desiccation, starvation, and heat. Cinnamon sensitized male flies to (A) desiccation, (C) starvation, and (E) heat. In females, cinnamon had no effect on (B) desiccation, or (D) starvation, but sensitized females to (F) heat. P-values were calculated with the Mantel-Cox Log-Rank test. For all groups, n = 120.
Table 2.
Protective effect of cinnamon against cold
| Control | Cinnamon-fed | |
|---|---|---|
| Males alive | 89 | 104 |
| Males dead | 24 | 3 |
| Females alive | 77 | 105 |
| Females dead | 35 | 13 |
P < 0.0001 for males, P = 0.0002 for females, Fisher’s exact test.
3.5 The effect of cinnamon on sugar, fat, and protein contents and on body weight
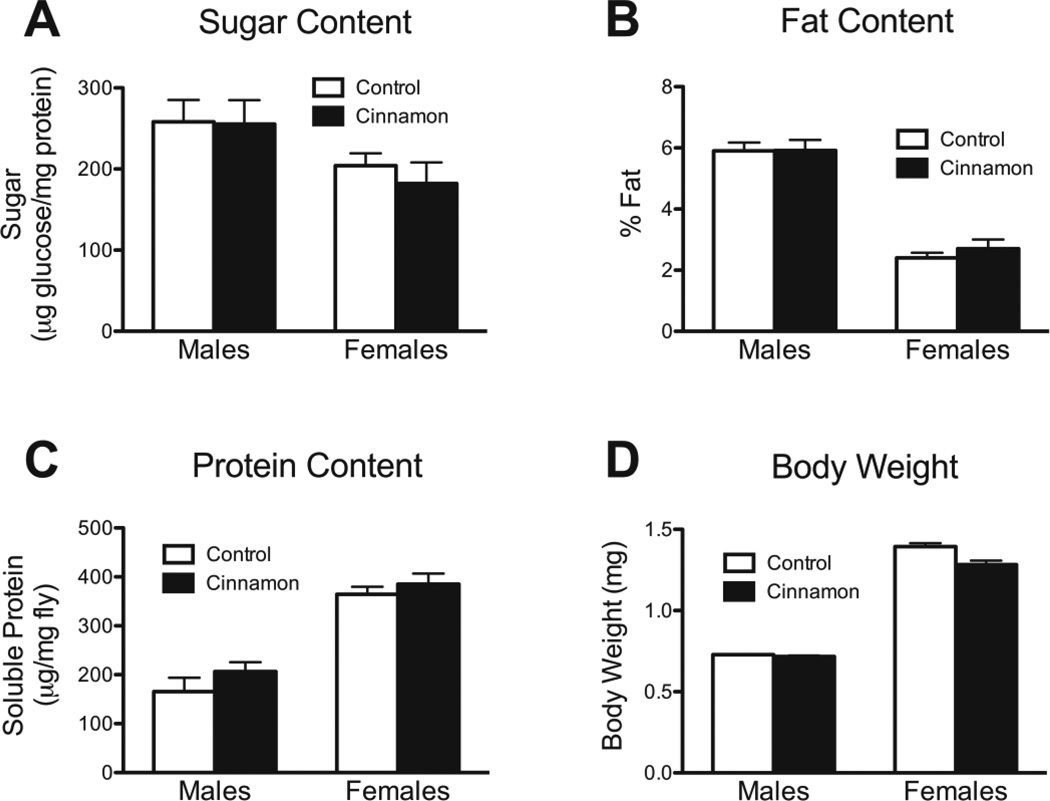
Insulin signaling and dietary restriction are known to regulate total body sugar, fat, and soluble protein content (Hwangbo et al. 2004; Magwere et al. 2006; Bai et al. 2012; Schriner et al. 2013). We found that cinnamon had no effect on these parameters in either sex (Fig. 7A, B, C). However, Cinnamon did decrease body weight in females, but not males (Fig. 7D).
Fig 7.
The effect of cinnamon on sugar, fat, and protein content, and on weight in w1118 flies. Cinnamon had no effect on (A) sugar content, P = 0.62, (B) fat content, P = 0.58, or (C) soluble protein content, P = 0.17, in either males or females. P-values were were calculated by 2-way ANOVA, n = 6. (D) Cinnamon also had no effect on male body weights, but it decreased female body weights, P = 0.0004, 2-way ANOVA, P > 0.05 for males, and P < 0.001 for females, Bonferroni posttest, n = 18.
3.6 Cinnamon has no effect on mitochondrial function, but up-regulates HSP70
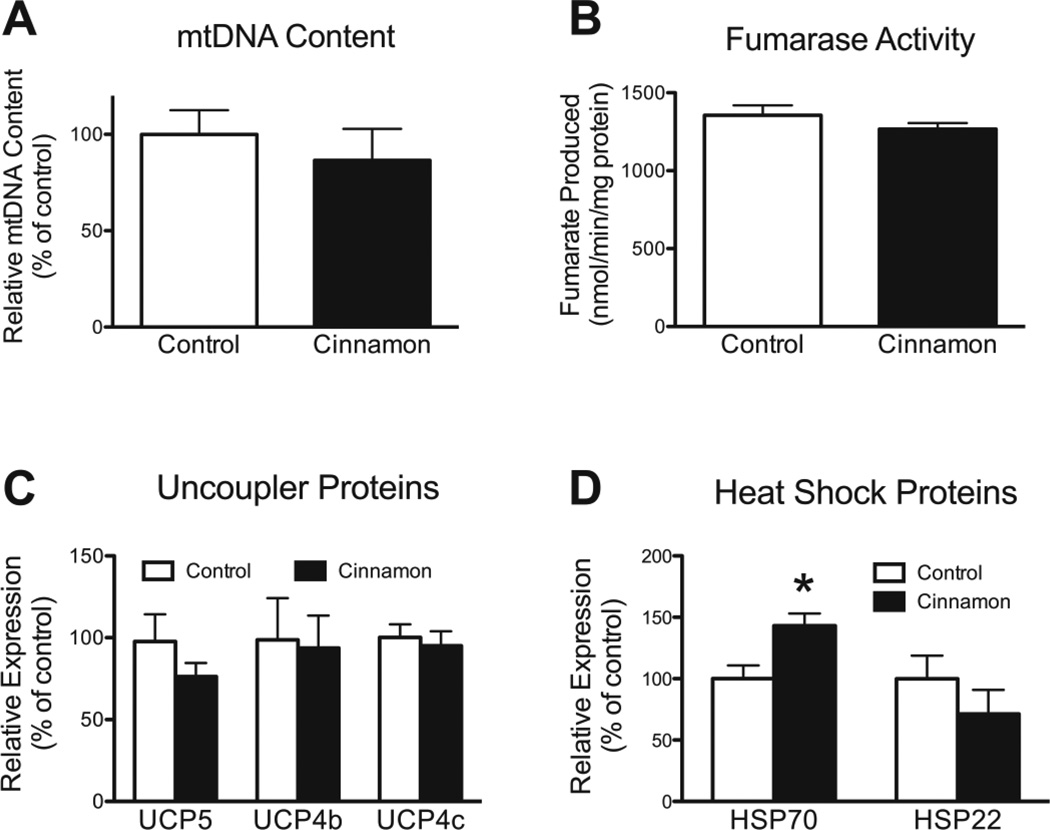
Since cinnamon increased physical performance and protected flies against cold and sensitized them to heat, we hypothesized that cinnamon might modulate mitochondrial function or content. The extract could also increase the expression levels of uncoupling proteins (UCPs). However, we found that cinnamon had no effect on total mitochondrial content (Fig. 8A, B) or respiration rates (Table 3). The extract had no effect on UCP expression (Fig 8C), nor did it alter the expression levels of the mitochondrial HSP22 (Fig. 8D). However, cinnamon did notably increase the expression levels of HSP70 (Fig. 8D).
Fig 8.
The effect of cinnamon on mitochondrial content and expression of heat and uncoupler proteins in w1118 flies. Cinnamon had no effect on mitochondrial content by measures of either (A) mtDNA copy number, P = 0.51, or (B) fumarase activity, P = 0.27, unpaired t test, n = 10 and 6, respectively. (C) Cinnamon had no effect on the expression levels of three uncoupler proteins. P > 0.05 for each UCP, unpaired t test, n = 5 or 6. (D) Cinnamon increased the expression levels of HSP70, *P < 0.05, but had no effect on HSP22, P = 0.32, unpaired t test, n = 5 or 6.
Table 3.
Mitochondrial respiratory parameters in Cinnamon-fed and control flies.
| Diet | State 3 | State 4 | RCR | uncoupled |
|---|---|---|---|---|
| Control | 162 ± 11 | 32 ± 2 | 5.1 ± 0.3 | 284 ± 22 |
| Cinnamon | 131 ± 12 | 31 ± 5 | 4.6 ± 0.4 | 235 ± 29 |
| P-values | 0.07 | 0.77 | 0.28 | 0.19 |
Rates are mean ± sem. Units are nmol O2/min/mg protein except for RCR, which is a ratio of state 3/state 4. P-values were calculated by Student’s t test, n = 10.
4. Discussion
Cinnamon has been used to flavor food for thousands of years. Recently, it has been reported that cinnamon may have positive effects on the management of diabetes (Lu et al. 2012; Ranasinghe et al. 2012; Davis and Yokoyama 2011; Kirkham et al. 2009), suggesting that it might affect insulin signaling. Surprisingly, in laboratory animals, perturbation of insulin and insulin-like growth factor signaling (IIS) has been shown to extend lifespan (Clancy et al. 2001; Tu et al. 2002; Fontana et al. 2010). However, not surprisingly, improved insulin sensitivity is also associated with increased lifespan and health (Barzilai et al. 2012). As a result, we hypothesized that if cinnamon did indeed improve insulin signaling in humans, it may also do so in experimental animals, and improve lifespan. This was previously found to be the case in C. elegans, where cinnamon extended lifespan with an apparent dependence on IIS (Yu et al. 2010). Here, we examined the effect of cinnamon on lifespan and its potential mechanism of action in a different invertebrate model, Drosophila melanogaster. We found that the extract increased lifespan in both sexes and in two types of flies, a commonly used inbred strain w1118, and an out-bred population, JIV (Fig 1), demonstrating that its effect on lifespan is not limited to C. elegans, and not specific to any sex or genotype.
In order to determine whether cinnamon works directly through IIS, we used flies that lacked the insulin receptor substrate, chico. Our finding that cinnamon was not able to extend lifespan in chico1/chico1 males, demonstrated a requirement for the insulin receptor substrate, and supports an action through IIS in males. However, in chico1/chico1 females, cinnamon was still able to extend lifespan, though not to the same magnitude as in the control flies (9% increase in chico1/chico1 flies vs. 23% in control flies (Table 1)). This suggests a possible IIS-independent or partial IIS-independent mechanism for cinnamon in females. The lower magnitude of lifespan extension by cinnamon in chico1/chico1 females may be due to a lower feeding rate as has been suggested (Clancy et al. 2001; Wong et al. 2009), and the amount of cinnamon consumed by chico1/chico1 females may be simply less than the w1118 controls. An IIS-independent action of cinnamon in flies would not be too surprising, as it is known that perturbation of IIS has different effects between males and females in flies (Clancy et al. 2001; Tu et al. 2002). For example, chico1/+ heterozygotes are longer lived than wild types in both sexes. Whereas complete loss of chico extends lifespan in females, but has no effect, or can even shorten lifespan in male flies (Clancy et al. 2001; Tu et al. 2002). Thus, it appears that there may be an alternate pathway for IIS present in females that permits them to bypass, to some extent, the loss of chico, though, this may in part be due to the lower feeding rate as suggested above. Nevertheless, our results in males are consistent with the repression of IIS being important in the action of cinnamon. Interestingly, we found no effect of cinnamon on the expression levels of 3 ageing-related Drosophila insulin-like peptides (Dilps). Therefore, cinnamon is unlikely to act on Dilp producing cells, and combined with the chico data, suggests that cinnamon may act directly on the insulin receptor itself. The work of others have shown cinnamon to have a complex relationship with insulin signaling where it activates insulin-like growth factor 1 (IGF1) signaling in fibroblasts, but down-regulates insulin signaling in adipocytes (Takasao et al. 2012; Cao et al. 2010). It seems that cinnamon does have an affect on IIS, thought, its precise mode of action is not clear yet.
A surprising effect of cinnamon in our study was that it had limited and sex-specific effects against stress. Extended lifespans in laboratory organisms are often associated with increased tolerances to various types of stresses (Kirkwood and Austad 2000; Vermeulen and Loeschcke 2007). This observation has even been used as the basis for selecting for genetic variants with increased lifespans (Lin et al. 1998; Rose et al. 1992). In the particular case of botanicals, many are thought to mediate their beneficial effects thought the antioxidant activity of polyphenols (Osawa 1999; Pandey and Rizvi 2009; Landete 2012; Liu 2013). Therefore, it was somewhat curious that cinnamon exhibited no antioxidant effect (Fig. 5A, C, E) in males, and little antioxidant effect in females (Fig. 5B), despite its ability to extend lifespan. In addition, the extract actually sensitized males to desiccation, starvation, and heat. A slightly different picture was present in females, where cinnamon afforded a modest protective effect against paraquat, sensitized them to iron (Fig. 5B, D, F), and had no effect on survival when challenged to starvation and desiccation. The notable commonality between the sexes, though, was that cinnamon sensitized females to heat, as it did in males, and protected both against cold. When we examined the action of cinnamon on the expression levels of HSP22 and HSP70, we found no effect on HSP22, but found HSP70 to be up-regulated. While the relationship of HSPs to aging isn’t exactly clear, increased HSP70 expression levels, and moderate levels of heat stress have resulted in increased lifespans in flies (Tatar et al. 1997; Khazaeli et al. 1997; Le Bourg et al. 2001; Hercus et al. 2003). Therefore, one possible mode of action of cinnamon is that it might effectively mimic heat stress. In popular culture, cinnamon is considered to be a “warming spice” and thought to have the ability to raise body temperature (Inokawa et al. 2006). Our findings here, that cinnamon protects flies against cold, sensitizes them to heat, and elevates HSP70 expression, lends some credibility to this idea.
Cinnamon as a plant extract contains many different compounds, which have been shown to exhibit varied activities. We examined the effect on lifespan of two of the better-known, but potentially toxic, compounds, coumarin and cinamaldehyde (Cohen 1979; Abraham et al. 2010; Bickers et al. 2005). Cinnamaldehyde was particularly interesting, as it has been shown to elevate the expression levels of peroxisome proliferator-activated receptor γ (PPARγ) and activate AMP kinase (AMPK) (Sheng et al. 2008; Huang et al. 2011). Both PPARγ and AMPK can elevate metabolism in part by activating mitochondrial biogenesis (Alaynick 2008; Haemmerle et al. 2011; Hardie 2011). Cinnamon, cinnamaldehdye, and eugenol, another constituent compound, have been shown to directly modulate mitochondrial physiology (Usta et al. 2002). A possible explanation of the “heat” effect of cinnamon could be due to increased mitochondrial content or function. This could also explain the increased physical performance seen in cinnamon flies relative to controls (Fig. 3B). However, we found no effect of cinnamon on mitochondrial content, respirations rates, or on the expression of mitochondrial uncoupling proteins (Fig. 8A, B, C, and Table 3). Thus, it is unlikely that cinnamon modulates mitochondrial function in our flies. Whatever compound in cinnamon is responsible for extending lifespan; it is unlikely to be coumarin or cinnamaldehyde, as neither could extend lifespan alone (Fig. 2). We plan to systematically examine a range of compounds in the near future.
One of the most robust treatments for improving lifespan in laboratory organisms is dietary restriction (DR), which is a decreased caloric intake without malnutrition. One of the dangers of using a natural product, or a drug, is that the treatment may prove distasteful or have an offending odor. In such a situation, an increased lifespan would result, not from a molecular action of our treatment, but simply from the flies eating less food. There are several ways to test for such an effect. In flies, DR has been shown to decrease total body weights, fecundity, and soluble protein levels, and elevate fat content (Hwangbo et al. 2004; Magwere et al. 2006; Bai et al. 2012; Schriner et al. 2013). At the cinnamon dose used for this study, 25 mg/mL, we found no effect on fecundity, or on protein and fat contents in either sex (Fig. 7A, B, C), arguing against any DR effect. We did find that cinnamon decreased female body weights by about 9%, while male weights were unaffected (Fig. 7D). While females would be expected to be more sensitive to a DR effect due to their reproductive systems, we have previously shown that male weights are also decreased in response to DR (Schriner et al. 2013). At the higher dose of 75 mg/mL there was a moderate decrease in fecundity consistent with a DR effect (Fig. 3A). Thus, we can’t completely rule a DR-like effect of cinnamon in females, due to their lowered weights, however, the absence of the elevated fat, decreased soluble protein levels and decreased body weights in males all argue against a simple DR effect.
It is also possible that cinnamon may act by directly inhibiting reproduction. While we found no negative effect on fecundity at the principal dose used in this study, 25 mg/mL, we cannot rule some other effect of cinnamon on reproductive fitness, such as courtship, mating, or sperm production. Any of which could possibly confer a lifespan extension. We have already found such a relationship in green tea, where lifespan extension is associated with decreased fertility in males (Lopez et al. 2014). We are currently examining the effect of cinnamon and other botanicals on these other aspects of reproduction.
Here we have shown evidence that cinnamon can extend lifespan of the fruit fly. In male flies, cinnamon requires the insulin receptor substrate, CHICO, to extend lifespan. This supports a plausible action on insulin signaling in humans and lends credibility for its suggested anti-diabetic activity. However, in females cinnamon doesn’t required CHICO, suggesting that it extends lifespan through other pathways possibly independent from IIS. Our future work will be to examine the action of cinnamon on additional known ageing pathways, such as the target of rapamycin (TOR) and cyclic-AMP signaling (Kapahi et al. 2004; Fontana et al. 2010; Tong et al. 2007), and to identify the molecule or molecules responsible for cinnamon lifespan-extending property in flies. It is also important to mention that it was recently reported that cinnamon, along with several other natural products, was unable to extend lifespan in laboratory mice (Spindler et al. 2013). However, this result doesn’t rule a positive effect of cinnamon, or the other extracts, on human health. The mice used in this study were F1 hybrids, and were fed a slightly reduced calorie diet. Thus, these animals were genetically and nutritionally healthy. If cinnamon were able to improve health in diabetic animals, for example, we may very well expect no effect of the extract in this study. By contrast, in our study here, we show that cinnamon does extend lifespan, even in animals that are nutritionally and genetically healthy.
Supplementary Material
Highlights.
Cinnamon has been suggested to lower blood glucose levels in diabetics
Cinnamon extended lifespan in the fruit fly, Drosophila melanogaster
Cinnamon required the insulin receptor substrate in male flies, but not in female flies, to extend lifespan.
Cinnamon also protected flies against cold, sensitized them to heat, and elevated HSP70 expression levels
Acknowledgements
This work was supported by a grant from the National Center for Complementary and Alternative Medicine (NCCAM) awarded to M. J. (Grant number R21 AT004987-01A2). We would also like to thank Peter Fuhrer and Benjamin Van Nguyen for technical assistance.
Abbreviations
- IIS
insulin and insulin-like growth factor signaling
- H2O2
hydrogen peroxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests
The authors have no conflicts of interests.
Contributor Information
Samuel E. Schriner, Email: schriner@uci.edu.
Steven Kuramada, Email: skuramad@uci.edu.
Terry E. Lopez, Email: telopez@uci.edu.
Stephanie Truong, Email: stephanie.truong@ucsf.edu.
Andrew Pham, Email: andrewnp@uci.edu.
References
- Abraham K, Wohrlin F, Lindtner O, Heinemeyer G, Lampen A. Toxicology and risk assessment of coumarin: focus on human data. Mol Nutr Food Res. 2010;54(2):228–239. doi: 10.1002/mnfr.200900281. [DOI] [PubMed] [Google Scholar]
- Alaynick WA. Nuclear receptors, mitochondria and lipid metabolism. Mitochondrion. 2008;8(4):329–337. doi: 10.1016/j.mito.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awai M, Narasaki M, Yamanoi Y, Seno S. Induction of diabetes in animals by parenteral administration of ferric nitrilotriacetate. A model of experimental hemochromatosis. Am J Pathol. 1979;95(3):663–673. [PMC free article] [PubMed] [Google Scholar]
- Bai H, Kang P, Tatar M. Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulinlike peptide-2 from the brain. Aging Cell. 2012;11(6):978–985. doi: 10.1111/acel.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A. Single-gene mutations and healthy ageing in mammals. Philos Trans R Soc Lond B Biol Sci. 2011;366(1561):28–34. doi: 10.1098/rstb.2010.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61(6):1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickers D, Calow P, Greim H, Hanifin JM, Rogers AE, Saurat JH, Sipes IG, Smith RL, Tagami H. A toxicologic and dermatologic assessment of cinnamyl alcohol, cinnamaldehyde and cinnamic acid when used as fragrance ingredients. Food Chem Toxicol. 2005;43(6):799–836. doi: 10.1016/j.fct.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11(1):35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cao H, Graves DJ, Anderson RA. Cinnamon extract regulates glucose transporter and insulin-signaling gene expression in mouse adipocytes. Phytomedicine. 2010;17(13):1027–1032. doi: 10.1016/j.phymed.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Carroll JJ, Smith N, Babson AL. A colorimetric serum glucose determination using hexokinase and glucose-6-phosphate dehydrogenase. Biochem Med. 1971;4(2):171–180. doi: 10.1016/0006-2944(70)90093-1. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292(5514):104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Cohen AJ. Critical review of the toxicology of coumarin with special reference to interspecies differences in metabolism and hepatotoxic response and their significance to man. Food Cosmet Toxicol. 1979;17(3):277–289. doi: 10.1016/0015-6264(79)90289-x. [DOI] [PubMed] [Google Scholar]
- Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119(21):2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. J Med Food. 2011;14(9):884–889. doi: 10.1089/jmf.2010.0180. [DOI] [PubMed] [Google Scholar]
- Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11(11):1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci U S A. 1999;96(9):4820–4825. doi: 10.1073/pnas.96.9.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M, Henika PR, Mandrell RE. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot. 2002;65(10):1545–1560. doi: 10.4315/0362-028x-65.10.1545. [DOI] [PubMed] [Google Scholar]
- Gargano JW, Martin I, Bhandari P, Grotewiel MS. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol. 2005;40(5):386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci. 2007;32(4):180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, Schreiber R, Eichmann T, Kolb D, Kotzbeck P, Schweiger M, Kumari M, Eder S, Schoiswohl G, Wongsiriroj N, Pollak NM, Radner FP, Preiss-Landl K, Kolbe T, Rulicke T, Pieske B, Trauner M, Lass A, Zimmermann R, Hoefler G, Cinti S, Kershaw EE, Schrauwen P, Madeo F, Mayer B, Zechner R. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17(9):1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercus MJ, Loeschcke V, Rattan SI. Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology. 2003;4(3):149–156. doi: 10.1023/a:1024197806855. [DOI] [PubMed] [Google Scholar]
- Hong CH, Hur SK, Oh OJ, Kim SS, Nam KA, Lee SK. Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J Ethnopharmacol. 2002;83(1–2):153–159. doi: 10.1016/s0378-8741(02)00205-2. [DOI] [PubMed] [Google Scholar]
- Huang B, Yuan HD, Kim do Y, Quan HY, Chung SH. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-gamma (PPARgamma) and AMP-activated protein kinase (AMPK) pathways. J Agric Food Chem. 2011;59(8):3666–3673. doi: 10.1021/jf104814t. [DOI] [PubMed] [Google Scholar]
- Huss U, Ringbom T, Perera P, Bohlin L, Vasange M. Screening of ubiquitous plant constituents for COX-2 inhibition with a scintillation proximity based assay. J Nat Prod. 2002;65(11):1517–1521. doi: 10.1021/np020023m. [DOI] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429(6991):562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Inokawa M, Iguchi K, Kohda H. Thermographic evaluation of the efficacy of Kampo medicines. Hiroshima J Med Sci. 2006;55(1):1–8. [PubMed] [Google Scholar]
- Jafari M, Felgner JS, Bussel II, Hutchili T, Khodayari B, Rose MR, Vince-Cruz C, Mueller LD. Rhodiola: a promising anti-aging Chinese herb. Rejuvenation Res. 2007;10(4):587–602. doi: 10.1089/rej.2007.0560. [DOI] [PubMed] [Google Scholar]
- Jaganathan SK, Supriyanto E. Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules. 2012;17(6):6290–6304. doi: 10.3390/molecules17066290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14(10):885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaeli AA, Tatar M, Pletcher SD, Curtsinger JW. Heat-induced longevity extension in Drosophila. I. Heat treatment, mortality, and thermotolerance. J Gerontol A Biol Sci Med Sci. 1997;52(1):B48–B52. doi: 10.1093/gerona/52a.1.b48. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim CH, Kim MS, Kim JY, Jung KJ, Chung JH, An WG, Lee JW, Yu BP, Chung HY. Suppression of age-related inflammatory NF-kappaB activation by cinnamaldehyde. Biogerontology. 2007;8(5):545–554. doi: 10.1007/s10522-007-9098-2. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Han D, Moon KD, Rhee JS. Measurement of superoxide dismutase-like activity of natural antioxidants. Biosci Biotechnol Biochem. 1995;59(5):822–826. doi: 10.1271/bbb.59.822. [DOI] [PubMed] [Google Scholar]
- Kirkham S, Akilen R, Sharma S, Tsiami A. The potential of cinnamon to reduce blood glucose levels in patients with type 2 diabetes and insulin resistance. Diabetes Obes Metab. 2009;11(12):1100–1113. doi: 10.1111/j.1463-1326.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Austad SN. Why do we age? Nature. 2000;408(6809):233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Koppikar SJ, Choudhari AS, Suryavanshi SA, Kumari S, Chattopadhyay S, Kaul-Ghanekar R. Aqueous cinnamon extract (ACE-c) from the bark of Cinnamomum cassia causes apoptosis in human cervical cancer cell line (SiHa) through loss of mitochondrial membrane potential. BMC Cancer. 2010;10:210. doi: 10.1186/1471-2407-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff EL. The distribution of fumarase activity in mouse liver homogenates. J Biol Chem. 1954;207(1):361–365. [PubMed] [Google Scholar]
- Landete JM. Updated knowledge about polyphenols: functions, bioavailability, metabolism, and health. Crit Rev Food Sci Nutr. 2012;52(10):936–948. doi: 10.1080/10408398.2010.513779. [DOI] [PubMed] [Google Scholar]
- Le Bourg E, Valenti P, Lucchetta P, Payre F. Effects of mild heat shocks at young age on aging and longevity in Drosophila melanogaster. Biogerontology. 2001;2(3):155–164. doi: 10.1023/a:1011561107055. [DOI] [PubMed] [Google Scholar]
- Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ, Zhang D, Woo DK, Shadel GS, Ladiges W, Rabinovitch PS, Santos JH, Petersen KF, Samuel VT, Shulman GI. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12(6):668–674. doi: 10.1016/j.cmet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Ku YH, Kim M, Ahn BY, Chung SS, Park KS. Effects of Sulfonylureas on Peroxisome Proliferator-Activated Receptor gamma Activity and on Glucose Uptake by Thiazolidinediones. Diabetes Metab J. 2011;35(4):340–347. doi: 10.4093/dmj.2011.35.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Wu SJ, Chang CH, Ng LT. Antioxidant activity of Cinnamomum cassia. Phytother Res. 2003;17(7):726–730. doi: 10.1002/ptr.1190. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282(5390):943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4(3):384S–392S. doi: 10.3945/an.112.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P, Sanchez C, Batlle R, Nerin C. Solid- and vapor-phase antimicrobial activities of six essential oils: susceptibility of selected foodborne bacterial and fungal strains. J Agric Food Chem. 2005;53(17):6939–6946. doi: 10.1021/jf050709v. [DOI] [PubMed] [Google Scholar]
- Lopez T, Schriner SE, Okoro M, Lu D, Chiang BT, Huey J, Jafari M. Green Tea Polyphenols Extend the Lifespan of Male Drosophila melanogaster While Impairing Reproductive Fitness. J Med Food. 2014 doi: 10.1089/jmf.2013.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JY, Lin YY, Sheu JC, Wu JT, Lee FJ, Chen Y, Lin MI, Chiang FT, Tai TY, Berger SL, Zhao Y, Tsai KS, Zhu H, Chuang LM, Boeke JD. Acetylation of yeast AMPK controls intrinsic aging independently of caloric restriction. Cell. 2011;146(6):969–979. doi: 10.1016/j.cell.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Sheng H, Wu J, Cheng Y, Zhu J, Chen Y. Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Nutr Res. 2012;32(6):408–412. doi: 10.1016/j.nutres.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Magwere T, Goodall S, Skepper J, Mair W, Brand MD, Partridge L. The effect of dietary restriction on mitochondrial protein density and flight muscle mitochondrial morphology in Drosophila. J Gerontol A Biol Sci Med Sci. 2006;61(1):36–47. doi: 10.1093/gerona/61.1.36. [DOI] [PubMed] [Google Scholar]
- Morrow G, Samson M, Michaud S, Tanguay RM. Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB J. 2004;18(3):598–599. doi: 10.1096/fj.03-0860fje. [DOI] [PubMed] [Google Scholar]
- Osawa K, Matsumoto T, Yasuda H, Kato T, Naito Y, Okuda K. The inhibitory effect of plant extracts on the collagenolytic activity and cytotoxicity of human gingival fibroblasts by Porphyromonas gingivalis crude enzyme. Bull Tokyo Dent Coll. 1991;32(1):1–7. [PubMed] [Google Scholar]
- Osawa T. Protective role of dietary polyphenols in oxidative stress. Mech Ageing Dev. 1999;111(2-3):133–139. doi: 10.1016/s0047-6374(99)00069-x. [DOI] [PubMed] [Google Scholar]
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KR, Nam D, Yun HM, Lee SG, Jang HJ, Sethi G, Cho SK, Ahn KS. beta-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011;312(2):178–188. doi: 10.1016/j.canlet.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Ranasinghe P, Jayawardana R, Galappaththy P, Constantine GR, de Vas Gunawardana N, Katulanda P. Efficacy and safety of ‘true’ cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: a systematic review and meta-analysis. Diabet Med. 2012;29(12):1480–1492. doi: 10.1111/j.1464-5491.2012.03718.x. [DOI] [PubMed] [Google Scholar]
- Rose MR, Charlesworth B. Genetics of life history in Drosophila melanogaster. I. Sib analysis of adult females. Genetics. 1981;97(1):173–186. doi: 10.1093/genetics/97.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MR, Drapeau MD, Yazdi PG, Shah KH, Moise DB, Thakar RR, Rauser CL, Mueller LD. Evolution of late-life mortality in Drosophila melanogaster. Evolution. 2002;56(10):1982–1991. doi: 10.1111/j.0014-3820.2002.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Rose MR, Vu LN, Park SU, Graves JL., Jr Selection on stress resistance increases longevity in Drosophila melanogaster. Exp Gerontol. 1992;27(2):241–250. doi: 10.1016/0531-5565(92)90048-5. [DOI] [PubMed] [Google Scholar]
- Scarsi M, Podvinec M, Roth A, Hug H, Kersten S, Albrecht H, Schwede T, Meyer UA, Rucker C. Sulfonylureas and glinides exhibit peroxisome proliferator-activated receptor gamma activity: a combined virtual screening and biological assay approach. Mol Pharmacol. 2007;71(2):398–406. doi: 10.1124/mol.106.024596. [DOI] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103(2):253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Lee K, Truong S, Salvadora KT, Maler S, Nam A, Lee T, Jafari M. Extension of Drosophila Lifespan by Rhodiola rosea through a mechanism independent from dietary restriction. PLoS One. 2013;8(5):e63886. doi: 10.1371/journal.pone.0063886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308(5730):1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Sheng X, Zhang Y, Gong Z, Huang C, Zang YQ. Improved Insulin Resistance and Lipid Metabolism by Cinnamon Extract through Activation of Peroxisome Proliferator-Activated Receptors. PPAR Res. 2008;2008:581348. doi: 10.1155/2008/581348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler SR, Mote PL, Flegal JM, Teter B. Influence on longevity of blueberry, cinnamon, green and black tea, pomegranate, sesame, curcumin, morin, pycnogenol, quercetin, and taxifolin fed iso-calorically to long-lived, F1 hybrid mice. Rejuvenation Res. 2013;16(2):143–151. doi: 10.1089/rej.2012.1386. [DOI] [PubMed] [Google Scholar]
- Stenesen D, Suh JM, Seo J, Yu K, Lee KS, Kim JS, Min KJ, Graff JM. Adenosine Nucleotide Biosynthesis and AMPK Regulate Adult Life Span and Mediate the Longevity Benefit of Caloric Restriction in Flies. Cell Metab. 2013;17(1):101–112. doi: 10.1016/j.cmet.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasao N, Tsuji-Naito K, Ishikura S, Tamura A, Akagawa M. Cinnamon extract promotes type I collagen biosynthesis via activation of IGF-I signaling in human dermal fibroblasts. J Agric Food Chem. 2012;60(5):1193–1200. doi: 10.1021/jf2043357. [DOI] [PubMed] [Google Scholar]
- Tatar M, Khazaeli AA, Curtsinger JW. Chaperoning extended life. Nature. 1997;390(6655):30. doi: 10.1038/36237. [DOI] [PubMed] [Google Scholar]
- Tong JJ, Schriner SE, McCleary D, Day BJ, Wallace DC. Life extension through neurofibromin mitochondrial regulation and antioxidant therapy for neurofibromatosis-1 in Drosophila melanogaster. Nat Genet. 2007;39(4):476–485. doi: 10.1038/ng2004. [DOI] [PubMed] [Google Scholar]
- Tu MP, Epstein D, Tatar M. The demography of slow aging in male and female Drosophila mutant for the insulin-receptor substrate homologue chico. Aging Cell. 2002;1(1):75–80. doi: 10.1046/j.1474-9728.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- Usta J, Kreydiyyeh S, Bajakian K, Nakkash-Chmaisse H. In vitro effect of eugenol and cinnamaldehyde on membrane potential and respiratory chain complexes in isolated rat liver mitochondria. Food Chem Toxicol. 2002;40(7):935–940. doi: 10.1016/s0278-6915(02)00071-6. [DOI] [PubMed] [Google Scholar]
- Vermeulen CJ, Loeschcke V. Longevity and the stress response in Drosophila. Exp Gerontol. 2007;42(3):153–159. doi: 10.1016/j.exger.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Woehrlin F, Fry H, Abraham K, Preiss-Weigert A. Quantification of flavoring constituents in cinnamon: high variation of coumarin in cassia bark from the German retail market and in authentic samples from indonesia. J Agric Food Chem. 2010;58(19):10568–10575. doi: 10.1021/jf102112p. [DOI] [PubMed] [Google Scholar]
- Wong R, Piper MD, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PLoS One. 2009;4(6):e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yu YB, Dosanjh L, Lao L, Tan M, Shim BS, Luo Y. Cinnamomum cassia bark in two herbal formulas increases life span in Caenorhabditis elegans via insulin signaling and stress response pathways. PLoS One. 2010;5(2):e9339. doi: 10.1371/journal.pone.0009339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, He H, Balschi JA. Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic AMP concentration. Am J Physiol Heart Circ Physiol. 2007;293(1):H457–H466. doi: 10.1152/ajpheart.00002.2007. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordan MA, Benna C, Mazzotta G. Monitoring and analyzing Drosophila circadian locomotor activity. Methods Mol Biol. 2007;362:67–81. doi: 10.1007/978-1-59745-257-1_4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.