Abstract
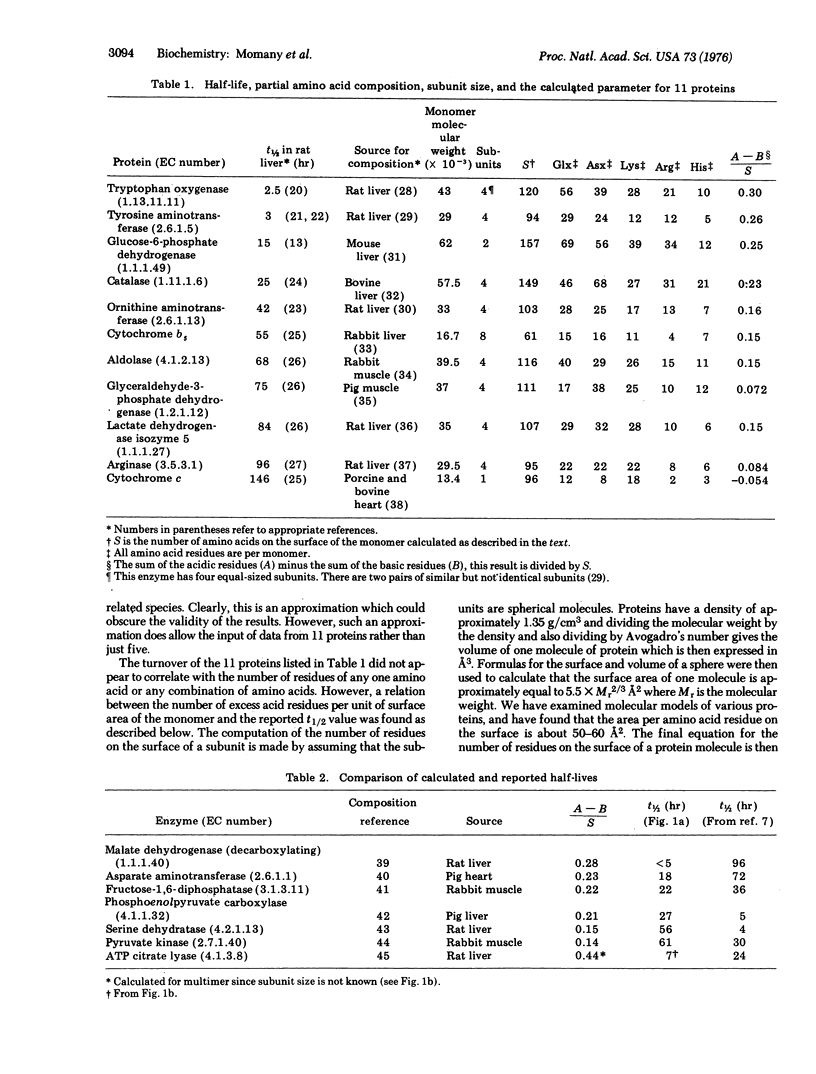
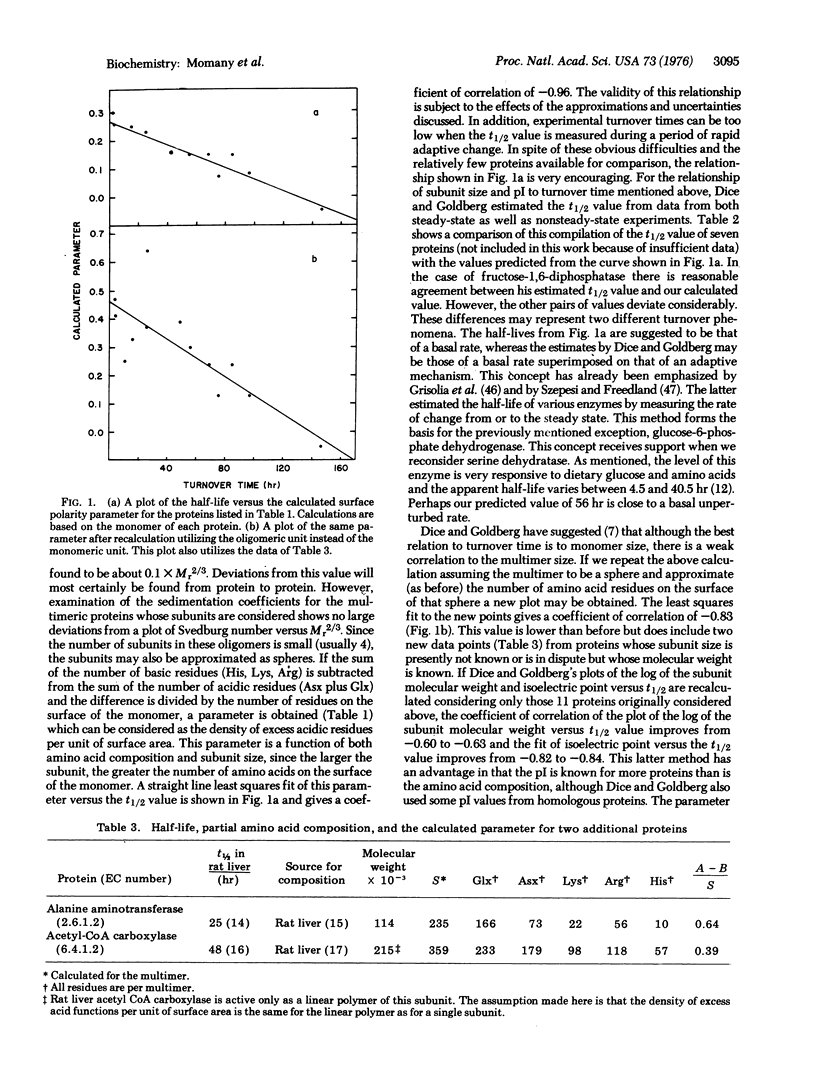
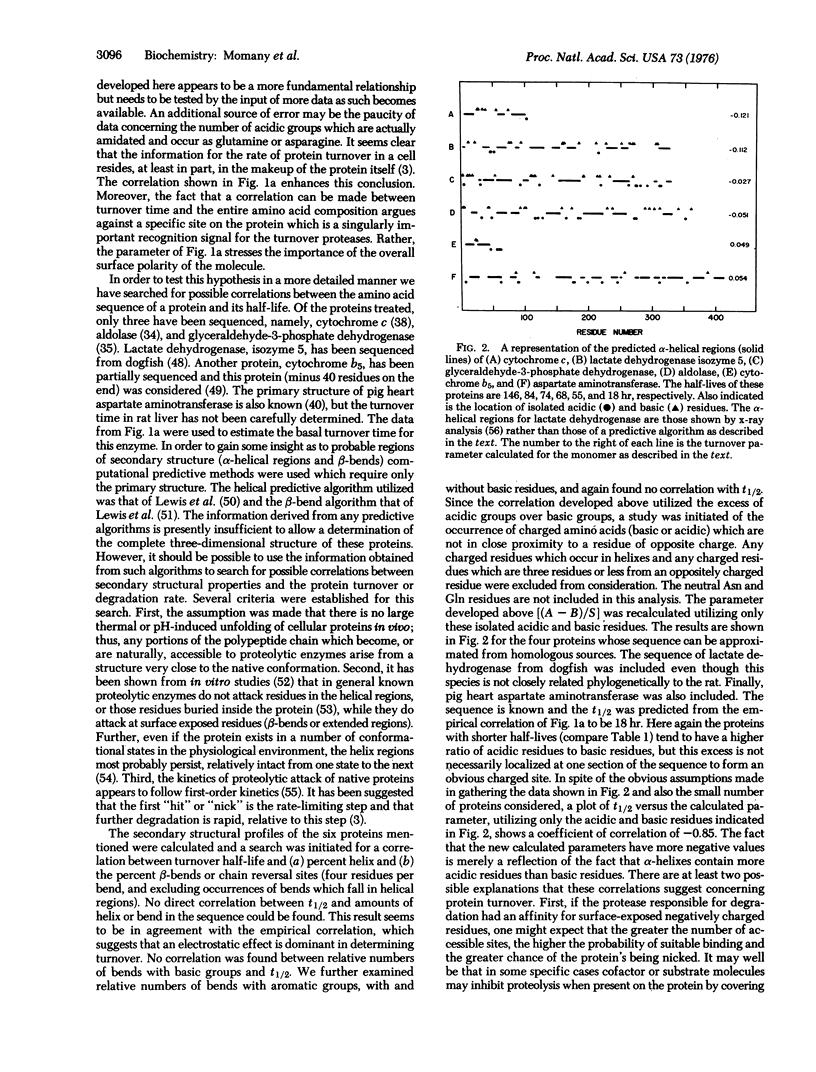
A parameter is developed which relates the amino acid composition and subunit size of a protein to the degradative rate in vivo. This parameter was calculated for 11 rat liver proteins and a plot versus the half-lives of these proteins is linear and has a coefficient of correlation of -0.96. Evidence is presented which suggests that the density of excess acidic amino acids on the surface of the protein is the most important factor in determining differential turnover.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black W. J., Van Tol A., Fernando J., Horecker B. L. Isolation of ahighly active fructose diphosphatase from rabit muscle: its subunit structure and activation by monovalent cations. Arch Biochem Biophys. 1972 Aug;151(2):576–590. doi: 10.1016/0003-9861(72)90535-8. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Scheraga H. A. A hypothesis for the pathway of the thermally-induced unfolding of bovine pancreatic ribonuclease. J Theor Biol. 1975 Sep;53(2):403–420. doi: 10.1016/s0022-5193(75)80012-9. [DOI] [PubMed] [Google Scholar]
- Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966 May 25;241(10):2413–2420. [PubMed] [Google Scholar]
- Chee P. Y., Swick R. W. Effect of dietary protein and tryptophan and the turnover of rat liver ornithine aminotransferase. J Biol Chem. 1976 Feb 25;251(4):1029–1034. [PubMed] [Google Scholar]
- Cottam G. L., Hollenberg P. F., Coon M. J. Subunit structure of rabbit muscle pyruvate kinase. J Biol Chem. 1969 Mar 25;244(6):1481–1486. [PubMed] [Google Scholar]
- Dice J. F., Dehlinger P. J., Schimke R. T. Studies on the correlation between size and relative degradation rate of soluble proteins. J Biol Chem. 1973 Jun 25;248(12):4220–4228. [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. A statistical analysis of the relationship between degradative rates and molecular weights of proteins. Arch Biochem Biophys. 1975 Sep;170(1):213–219. doi: 10.1016/0003-9861(75)90112-5. [DOI] [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. Relationship between in vivo degradative rates and isoelectric points of proteins. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3893–3897. doi: 10.1073/pnas.72.10.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druyan R., DeBernard B., Rabinowitz M. Turnover of cytochromes labeled with delta-aminolevulinic acid-3H in rat liver. J Biol Chem. 1969 Nov 10;244(21):5874–5878. [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Goldberg E. Amino acid composition and properties of crystalline lactate dehydrogenase X from mouse testes. J Biol Chem. 1972 Apr 10;247(7):2044–2048. [PubMed] [Google Scholar]
- Harris J. I., Perham R. N. Glyceraldehyde 3-phosphate dehydrogenase from pig muscle. Nature. 1968 Sep 7;219(5158):1025–1028. doi: 10.1038/2191025a0. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Natori Y. Molecular-size-dependent degradation of liver cytosolic proteins in vitro. J Biochem. 1976 Jan;79(1):221–224. doi: 10.1093/oxfordjournals.jbchem.a131050. [DOI] [PubMed] [Google Scholar]
- Hirsch-Kolb H., Greenberg D. M. Molecular characteristics of rat liver arginase. J Biol Chem. 1968 Dec 10;243(23):6123–6129. [PubMed] [Google Scholar]
- Hizi A., Yagil G. On the mechanism of glucose-6-phosphate dehydrogenase regulation in mouse liver. 2. Purification and properties of the mouse-liver enzyme. Eur J Biochem. 1974 Jun 1;45(1):201–209. doi: 10.1111/j.1432-1033.1974.tb03544.x. [DOI] [PubMed] [Google Scholar]
- Inoue H., Kasper C. B., Pitot H. C. Studies on the induction and repression of enzymes in rat liver. VI. Some properties and the metabolic regulation of two isozymic forms of serine dehydratase. J Biol Chem. 1971 Apr 25;246(8):2626–2632. [PubMed] [Google Scholar]
- Inoue H., Lowenstein J. M. Acetyl coenzyme A carboxylase from rat liver. Purification and demonstration of different subunits. J Biol Chem. 1972 Aug 10;247(15):4825–4832. [PubMed] [Google Scholar]
- Inoue H., Tsunemi T., Suzuki F., Takeda Y. Studies on ATP citrate lyase of rat liver. IV. The role of CoA. J Biochem. 1969 Jun;65(6):889–900. doi: 10.1093/oxfordjournals.jbchem.a129093. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Khairallah E. A., Pitot H. C. Studies on the induction and repression of enzymes in rat liver. V. Regulation of the rate of synthesis and degradation of serine dehydratase by dietary amino acids and glucose. J Biol Chem. 1968 Jun 10;243(11):3057–3066. [PubMed] [Google Scholar]
- KENNEY F. T. Induction of tyrosine-alpha-ketoglutarate transaminase in rat liver. IV. Evidence for an increase in the rate of enzyme synthesis. J Biol Chem. 1962 Nov;237:3495–3498. [PubMed] [Google Scholar]
- Kominami E., Katunuma N. Studies of new intracellular proteases in various organs of rats. Participation of proteases in degradation of ornithine aminotransferase in vitro and in vivo. Eur J Biochem. 1976 Mar 1;62(3):425–430. doi: 10.1111/j.1432-1033.1976.tb10175.x. [DOI] [PubMed] [Google Scholar]
- Kuehl L., Sumsion E. N. Turnover of several glycolytic enzymes in rat liver. J Biol Chem. 1970 Dec 25;245(24):6616–6623. [PubMed] [Google Scholar]
- LIN E. C., KNOX W. E. Specificity of the adaptive response to tyrosine-alpha-ketoglutarate transaminase in the rat. J Biol Chem. 1958 Nov;233(5):1186–1189. [PubMed] [Google Scholar]
- Lai C. Y. Studies on the structure of rabbit muscle aldolase. Determination of the primary structure of the COOH-terminal BrCN peptide; the complete sequence of the subunit polypeptide chain. Arch Biochem Biophys. 1975 Jan;166(1):358–368. doi: 10.1016/0003-9861(75)90398-7. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Go N., Go M., Kotelchuck D., Scheraga H. A. Helix probability profiles of denatured proteins and their correlation with native structures. Proc Natl Acad Sci U S A. 1970 Apr;65(4):810–815. doi: 10.1073/pnas.65.4.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. N., Momany F. A., Scheraga H. A. Folding of polypeptide chains in proteins: a proposed mechanism for folding. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2293–2297. doi: 10.1073/pnas.68.9.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder M. C., Moor J. R., Munro H. N. Subunit heterogeneity in rat liver apoferritin. Differential response of the subunits to iron administration. J Biol Chem. 1974 Dec 10;249(23):7707–7710. [PubMed] [Google Scholar]
- Majerus P. W., Kilburn E. Acetyl coenzyme A carboxylase. The roles of synthesis and degradation in regulation of enzyme levels in rat liver. J Biol Chem. 1969 Nov 25;244(22):6254–6262. [PubMed] [Google Scholar]
- Matsuzawa T., Segal H. L. Rat liver alanine aminotrasferase. Crystallization, composition, and role of sulfhydryl groups. J Biol Chem. 1968 Nov 25;243(22):5929–5934. [PubMed] [Google Scholar]
- OOI T., RUPLEY J. A., SCHERAGA H. A. STRUCTURAL STUDIES OF RIBONUCLEASE. VIII. TRYPTIC HYDROLYSIS OF RIBONUCLEASE A AT ELEVATED TEMPERATURES. Biochemistry. 1963 May-Jun;2:432–437. doi: 10.1021/bi00903a006. [DOI] [PubMed] [Google Scholar]
- Ozols J., Strittmatter P. Correction of the amino acid sequence of calf liver microsomal cytochrome b5. J Biol Chem. 1969 Dec 25;244(24):6617–6618. [PubMed] [Google Scholar]
- PRICE V. E., STERLING W. R., TARANTOLA V. A., HARTLEY R. W., Jr, RECHCIGL M., Jr The kinetics of catalase synthesis and destruction in vivo. J Biol Chem. 1962 Nov;237:3468–3475. [PubMed] [Google Scholar]
- Peraino C., Bunville L. G., Tahmisian T. N. Chemical, physical, and morphological properties of ornithine Aminotransferase from rat liver. J Biol Chem. 1969 May 10;244(9):2241–2249. [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Adams M. J., Buehner M., Ford G. C., Hackert M. L., Lentz P. J., Jr, McPherson A., Jr, Schevitz R. W., Smiley I. E. Structural constraints of possible mechanisms of lactate dehydrogenase as shown by high resolution studies of the apoenzyme and a variety of enzyme complexes. Cold Spring Harb Symp Quant Biol. 1972;36:179–191. doi: 10.1101/sqb.1972.036.01.025. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- SCHIMKE R. T. THE IMPORTANCE OF BOTH SYNTHESIS AND DEGRADATION IN THE CONTROL OF ARGINASE LEVELS IN RAT LIVER. J Biol Chem. 1964 Nov;239:3808–3817. [PubMed] [Google Scholar]
- Salinas M., Wallace R., Grisolia S. Comparative studies in vivo and in vitro of rat-liver enzymes. Eur J Biochem. 1974 May 15;44(2):375–381. doi: 10.1111/j.1432-1033.1974.tb03494.x. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Control of enzyme levels in mammalian tissues. Adv Enzymol Relat Areas Mol Biol. 1973;37:135–187. doi: 10.1002/9780470122822.ch3. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Schroeder W. A., Shelton J. R., Shelton J. B., Robberson B., Apell G. The amino acid sequence of bovine liver catalase: a preliminary report. Arch Biochem Biophys. 1969 May;131(2):653–655. doi: 10.1016/0003-9861(69)90441-x. [DOI] [PubMed] [Google Scholar]
- Schutz G., Feigelson P. Purification and properties of rat liver tryptophan oxygenase. J Biol Chem. 1972 Sep 10;247(17):5327–5332. [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of cytochrome b5 that contains an additional hydrophobic sequence of 40 amino acid residues. Proc Natl Acad Sci U S A. 1971 May;68(5):1042–1046. doi: 10.1073/pnas.68.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick R. W., Rexroth A. K., Stange J. L. The metabolism of mitochondrial proteins. 3. The dynamic state of rat liver mitochondria. J Biol Chem. 1968 Jul 10;243(13):3581–3587. [PubMed] [Google Scholar]
- Szepesi B., Freedland R. A. Alterations in the activities of several rat liver enzymes at various times after initiation of a high protein regimen. J Nutr. 1967 Nov;93(3):301–306. doi: 10.1093/jn/93.3.301. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Wada K., Okazaki T., Numa S. Acetyl-coenzyme-A carboxylase from rat liver. Subunit structure and proteolytic modification. Eur J Biochem. 1975 Sep 1;57(1):15–24. doi: 10.1111/j.1432-1033.1975.tb02272.x. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Richert D. A., Westerfeld W. W. The concurrent induction of hepatic alpha-glycerophosphate dehydrogenase and malate dehydrogenase by thyroid hormone. Biochim Biophys Acta. 1966 Aug 24;124(2):205–309. [PubMed] [Google Scholar]
- Tweto J., Larrabee A. R. The effect of fasting on synthesis and 4'-phosphopantetheine exchange in rat liver fatty acid synthetase. J Biol Chem. 1972 Aug 10;247(15):4900–4904. [PubMed] [Google Scholar]
- Valeriote F. A., Auricchio F., Tomkins G. M., Riley D. Purification and properties of rat liver tyrosine aminotransferase. J Biol Chem. 1969 Jul 10;244(13):3618–3624. [PubMed] [Google Scholar]
- Wada F., Numata N., Eguchi Y., Sakamoto Y. Crystallization and properties of rat liver malate dehydrogenase (decarboxylating) (NADP). Biochim Biophys Acta. 1975 Dec 18;410(2):237–242. doi: 10.1016/0005-2744(75)90225-9. [DOI] [PubMed] [Google Scholar]



