Abstract
Morquio A syndrome (mucopolysaccharidosis IVA) is a lysosomal storage disorder associated with skeletal and joint abnormalities and significant non-skeletal manifestations including respiratory disease, spinal cord compression, cardiac disease, impaired vision, hearing loss, and dental problems. The clinical presentation, onset, severity and progression rate of clinical manifestations of Morquio A syndrome vary widely between patients. Because of the heterogeneous and progressive nature of the disease, the management of patients with Morquio A syndrome is challenging and requires a multidisciplinary approach, involving an array of specialists. The current paper presents international guidelines for the evaluation, treatment and symptom-based management of Morquio A syndrome. These guidelines were developed during two expert meetings by an international panel of specialists in pediatrics, genetics, orthopedics, pulmonology, cardiology, and anesthesia with extensive experience in managing Morquio A syndrome. © 2014 The Authors. American Journal of Medical Genetics Part A published by Wiley Periodicals, Inc.
Keywords: mucopolysaccharidosis IV, guidelines, symptom assessment, diagnosis, disease management
INTRODUCTION AND METHODS
Morquio A syndrome (mucopolysaccharidosis [MPS] IVA, OMIM #253000) is a lysosomal storage disorder (LSD) inherited in an autosomal recessive fashion. It is caused by a deficiency in the enzyme N-acetylgalactosamine-6-sulfatase (GALNS) due to a mutation in the GALNS gene located on chromosome 16q24.3. This deficiency results in accumulation of the glycosaminoglycans (GAGs) chondroitin-6-sulfate and keratan sulfate (KS) in a variety of tissues [Neufeld and Muenzer, 2001]. The disease is extremely rare, with incidence rates ranging from 1 in 640,000 live births in Western Australia to 1 in 76,000 live births in Northern Ireland [Nelson, 1997; Nelson et al., 2003].
Infants with Morquio A syndrome usually appear normal at birth. However, due to the accumulation of storage material in tissues and organs, leading to cellular dysfunction, they progressively develop profound skeletal and joint abnormalities and an array of non-skeletal manifestations including respiratory disease, spinal cord compression, cardiac disease, impaired vision, hearing loss, dental problems, and to a lesser extent hepatomegaly [Montaño et al., 2007; Harmatz et al., 2013; Hendriksz et al., 2013a]. Morquio A can be distinguished from other types of MPS disorders by the typical short-trunk dwarfism with short neck (Fig. 1). The skeletal manifestations are generally more extensive and severe than in other types of MPS disorders. Hypermobility of joints is very characteristic for Morquio A syndrome and distinguishes this disease from other types of MPS. In contrast to most other types of MPS disorders, Morquio A syndrome has not been associated with cognitive impairment [Davison et al., 2013].
FIG. 1.
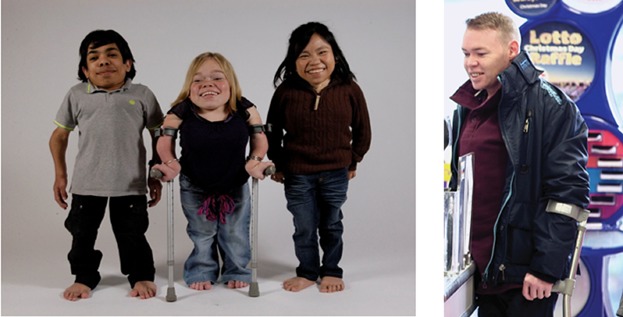
Typical appearance of patients with Morquio A syndrome, showing short stature with short neck and profound skeletal and joint abnormalities (left) and a patient with a non-classical phenotype of Morquio A syndrome showing normal stature (right).
To date, over 220 mutations in the GALNS gene have been identified [Morrone et al., 2014]. The most common gene mutation is present in <9% of the Morquio patients, giving rise to a wide heterogeneity with regard to clinical presentation, severity of disease, and rate of progression [Tomatsu et al., 2011]. Whereas most patients present with the classical phenotype, associated with short stature and severe skeletal and joint abnormalities, some patients do not have this characteristic appearance but may show severe impairment in other domains such as cardiorespiratory disease. The specific disease manifestations depend grossly on the residual GALNS activity and the accumulation rate and location of storage material in tissues. Studies have shown similar clinical manifestations in siblings with Morquio A syndrome, providing some evidence for genotype-phenotype associations [Tylki-Szymańska et al., 1998; Rekka et al., 2012]. The accumulation of GAGs throughout the body ultimately leads to premature death in most instances, with a life expectancy ranging from 10 to 20 years of age to almost normal in some patients [Tomatsu et al., 2011].
Because of the heterogeneous and progressive nature of the disease, the management of patients with Morquio A syndrome is challenging and requires a multidisciplinary approach. However, due to the rarity of the disease, most clinicians are not familiar with the special needs of these patients. Therefore, we developed international guidelines for the management and treatment of Morquio A syndrome based on the outcome of two expert meetings. On August 2–3, 2013, an international panel of 26 specialists in pediatrics, genetics, orthopedics, pulmonology, cardiology, and anesthetics with experience in Morquio A gathered in Amsterdam for an expert meeting sponsored by BioMarin Pharmaceutical Inc. During this meeting, existing literature and clinical data on different aspects of Morquio A syndrome were reviewed and discussed and preliminary management and treatment guidelines were prepared. These were discussed in more detail by a consensus panel of nine experts during a second meeting in Barcelona on September 1, 2013. Multiple observations in Morquio A resulted in the determination that patients cannot be classified into different subgroups based on clinical presentation, severity of disease, and/or rate of progression. These included the observed genotypic and phenotypic heterogeneity, the limited data on natural history and pathophysiology of the disease and the observation that patients may show severe disease in one domain, but not in another (e.g., severe respiratory disease, but normal height). Because patients cannot be classified into different subgroups, management differs depending on the clinical manifestations of the patient.
Diagnosis
Because of the progressive nature of Morquio A syndrome, early diagnosis may be critical to optimize patient outcomes. Patients with the disease generally gain clinical attention due to a cluster of progressive, multisystemic clinical and/or radiographic findings (Table I). Definitive diagnosis is facilitated by referral to a metabolic/genetics center and is dependent upon clinical diagnostic (radiographic imaging) and biochemical testing in a specialized laboratory. A diagnostic testing algorithm for Morquio A syndrome has been published in 2013 (Fig. 2) [Wood et al., 2013]. Although urinary GAG analysis and enzyme activity testing in a dried blood spot (DBS) may raise suspicion of the presence of Morquio A, a definite diagnosis usually entails demonstration of reduced GALNS activity in leukocytes or fibroblasts. Molecular analysis can be performed as a further confirmation of the diagnosis.
TABLE I.
Overview of Common Clinical Manifestations of Morquio A Syndrome and Prevalence of These Manifestations in the MorCAP Study (N = 325) at Baseline [Harmatz et al., 2013]
| Musculoskeletal manifestations | Prevalence in MorCAP (%) |
|---|---|
| Short stature | 93 |
| Short neck | 91 |
| Spinal abnormalities | |
| Kyphoscoliosis | 85 |
| Odontoid dysplasia | 65 |
| Lumbar lordosis | 56 |
| Cervical spinal instability | 49 |
| Spinal disc disease | 23 |
| Hip dysplasia | 71 |
| Genu valgum (knock knees) | 93 |
| Pectus carinatum | 97 |
| Joint abnormalities | |
| Laxity | 87 |
| Stiffness/pain | 83 |
| Contractures | 52 |
| Subluxation | 47 |
| Abnormal gait | 94 |
| Non-skeletal manifestations | |
| Respiratory compromise (upper and lower airway obstruction, respiratory restriction) | 58 |
| Cardiac abnormalities (valve disease, small heart with low stroke volume and increased heart rate) | 43 |
| Nervous system disorders (cervical myelopathy, cervical/thoracolumbar cord compression) | 51 |
| Eye disorders | 79 |
| Corneal clouding | 63 |
| Visual impairment (20/80 or worse) | 22 |
| Ear/labyrinth disorders (most commonly hearing impairment, otitis media) | 77 |
| Dental abnormalities | 69 |
| Abdominal abnormalities | |
| Hepatomegaly | 26 |
| Splenomegaly | 17 |
| Other (e.g., hernias) | 15 |
FIG. 2.
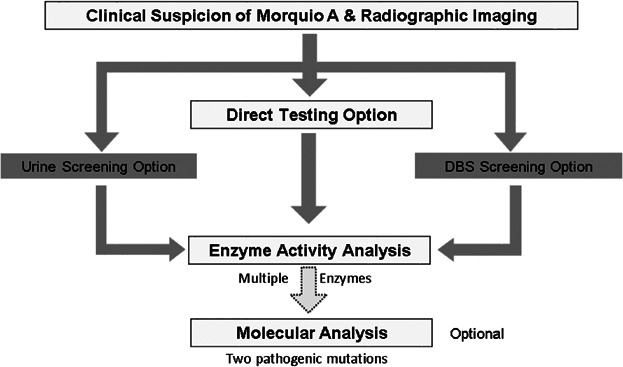
Diagnostic algorithm for Morquio A syndrome. Adapted from Wood TC et al. 2013 [Wood et al., 2013]. DBS: dried blood spot.
Obtaining a diagnosis of Morquio A remains a challenge due to the rarity of the disease and similarities with other LSDs and skeletal dysplasias. Specifically, patients that have been diagnosed with, or are undergoing evaluation for spondyloepiphyseal dysplasia, pseudoachondroplasia, multiple epiphyseal dysplasia or bilateral Legg-Calvé-Perthes disease should have Morquio A excluded because of the similarities between these diseases and Morquio A, unless there is a family history of any of these diseases [Mendelsohn et al., 2013; Lachman et al., 2014]. Also, diagnosis of Morquio A syndrome may be delayed in patients that do not exhibit the classical initial signs of the disease such as kyphosis. For example, patients with a more attenuated disease course may first present with atypical signs such as hip stiffness and pain. Furthermore, screening tests for Morquio A disease are subject to false negative results. Total urinary GAG may not be elevated in Morquio A patients, especially as patients grow older, because KS levels decrease naturally with age and can reach levels consistent with the upper range reported in unaffected individuals. Moreover, as not all Morquio A patients have elevated KS levels and as KS may also be elevated in other disorders, KS measurements should not be used as a diagnostic tool for Morquio A syndrome. Therefore, it is optional to perform an enzyme activity assay in DBS in addition to urinary GAG measurement in patients suspected of having the disease. Noteworthy, the outcome of enzyme activity testing in DBS depends on the stability of the enzyme and the storage conditions of the sample [Camelier et al., 2011]. Based on these considerations, it is recommended to proceed to enzyme activity testing in leukocytes or fibroblasts not only when screening is positive or doubtful, but even when it is negative and the clinical-radiological data are suggestive of Morquio A. In fact, if there is strong clinical suspicion, an enzyme activity test could be performed immediately, without prior urinary GAG or DBS screening. When enzyme activity analysis in fibroblasts or leukocytes is inconclusive, the test should be repeated and/or genetic analysis should be performed. In these cases with inconclusive enzyme results, identification of two known pathogenic mutations on separate alleles is required for definitive diagnosis [Wood et al., 2013]. Activity testing of other lysosomal enzymes (including β-galactosidase and another sulfatase) in conjunction with GALNS is recommended to rule out the presence of MPS IVB and other enzyme deficiencies which could have GALNS secondary deficiency, such as mucolipidosis II, III, MPS VI, and multiple sulfatase deficiency [Wood et al., 2013]. Obtaining a correct diagnosis is essential given the current availability of enzyme replacement therapy (ERT) for Morquio A syndrome (see below).
MANAGEMENT GUIDELINES
Treatment Modalities to Provide the Deficient Enzyme
Until recently, treatment of Morquio A syndrome was limited to supportive, symptomatic care, including symptom-based medications, physical therapy, surgery, and rehabilitation. However, in light of the pathophysiology of the disease, with a deficiency in a single enzyme giving rise to an array of clinical manifestations, a therapy that restores or replaces the deficient enzyme is desirable. Current experience with hematopoietic stem cell transplantation (HSCT), which has shown to be valuable in MPS IH (Hurler syndrome), is very limited and not encouraging for patients with Morquio A syndrome [Tomatsu et al., 2011; Algahim and Almassi, 2013]. More data are required to provide evidence of its efficacy in these patients.
ERT with recombinant human GALNS (elosulfase alfa) has recently been approved for Morquio A syndrome, providing a systemic treatment approach. Elosulfase alfa has shown to be effective with a favorable safety profile. A multicenter, phase 1/2, open-label, dose-escalation study including 20 Morquio A patients aged 5–18 years showed sustained improvements in endurance, as measured using a 6-min walk test (6MWT) and a 3-min stair climb test (3MSCT), and respiratory function using elosulfase alfa 1.0 mg/kg/week and 2.0 mg/kg/week, which persisted after approximately 2 years of treatment [Hendriksz et al., 2012]. Urinary KS also decreased with treatment, with the largest decreases observed when dosing at 2.0 mg/kg/week. Based on the outcome of the phase 1/2 study, a multicenter, double-blind, placebo-controlled phase 3 study was performed to assess the efficacy and safety of infusions with elosulfase alfa 2.0 mg/kg every week and every other week (N = 176; aged ≥ 5 years) [Hendriksz et al., 2014a]. The study showed significant improvement in endurance of 22.5 m in 6MWT distance during 24 weeks of treatment with elosulfase alfa at 2.0 mg/kg/week as compared with placebo (P = 0.0174). No significant impact was observed with the every other week dosing regimen. There was no significant effect of any of the dosing regimens on the number of stairs climbed in a 3MSCT. Both dosing regimens led to rapid and sustained reductions in urinary KS, with the greatest effect seen with the weekly dosing regimen. The study also showed numerical improvements over placebo after 24 weeks of treatment with elosulfase alfa 2.0 mg/kg/week for most exploratory endpoints, including maximum voluntary ventilation (MVV; P = 0.094) and forced vital capacity (FVC; P = 0.304). A composite measure developed by Delphi strategy based on an equal weighting of the change from baseline to week 24 of the z-scores of three component measures, i.e., 6MWT, 3MSCT, and MVV, also showed improvement. Both doses of elosulfase alfa had favorable safety profiles, generally similar to that of other ERTs. Most adverse events were mild or moderate infusion-associated reactions (IARs, occurring after infusion onset and within 1 day after infusion end) such as vomiting and pyrexia (fever), which were generally manageable with symptomatic treatment and/or infusion rate modification. The frequency of IARs was higher during the first 12 weeks of treatment and tended to occur less frequently with time. As anaphylactic reactions may occur during infusion, patients should be closely observed during and after administration of elosulfase alfa.
Elosulfase alfa treatment should be implemented as soon as the diagnosis of Morquio A syndrome has been confirmed to replace the deficient GALNs enzyme. The recommended dose of elosulfase alfa is 2.0 mg/kg/week, administered intravenously over approximately 4 hr. The calculated dose should be diluted with saline to a total volume of 100 mL (patients weighing <25 kg) or 250 mL (patients weighing ≥25 kg) at room temperature. Details of reconstitution may be found in the package insert. Supplementary Appendix 1 provides instructions for the infusion of elosulfase alfa. Elosulfase alfa should not be mixed with other infusions aside from saline as compatibility with other products has not been evaluated. Patients should be pre-treated approximately 30–60 min before each drug infusion with an antihistamine with or without antipyretics. Patients with known risk factors for IARs may be pre-treated with additional agents, such as H2 blockers, montelukast sodium, or corticosteroids. Baseline and follow-up assessments should be performed before and after starting ERT (see early assessments).
Multidisciplinary Approach
The diverse spectrum of disease manifestations of Morquio A warrants a multi-disciplinary management approach, involving an array of specialists coordinated by a physician experienced in working with patients with LSDs. The role of this coordinating physician, usually a pediatrician, metabolic physician, or clinical geneticist, is to continuously monitor the evolution of disease, to refer to a specialist as needed, to assist in coordinating the care/recommendations from the multidisciplinary team, and to serve as a medical home for the patient. In addition, this coordinating physician needs to regularly educate other health professionals (other specialists, dentists, and physiotherapists) about the disease and discuss the risks and benefits of interventions and necessary precautions with treatments/evaluations. The coordinating physician should provide guidance to the patient and family and encourage correct follow-up of therapies [Martins et al., 2009]. In order to stimulate continuity of care, it is also important that patients and families are educated about the disease and its possible complications and risks.
It is important that the coordinating physician of Morquio A patients organizes an experienced multidisciplinary team of specialists, concentrating all experience in a limited number of physicians per subspecialty. This requires referral of a critical mass of MPS patients to the same specialists, allowing them to accumulate experience in treating these patients and encourage their education on the disease.
Early Assessments
Early recognition of clinical manifestations of Morquio A syndrome allows timely intervention and may help prevent irreversible damage. Therefore, patients with Morquio A syndrome should undergo a comprehensive multisystem evaluation of physical manifestations of disease, functional ability and disease burden at diagnosis. Table II illustrates the recommended assessments organized by organ system along with a schedule for longitudinal assessment. Each patient should also be referred to appropriate allied health care professionals such as a physiotherapist, occupational therapist, and audiologist depending on the disease burden at diagnosis. More details on these assessments will be discussed below.
TABLE II.
Recommended Schedule of Assessments in Patients With Morquio A Syndrome
| Assessment | At diagnosis | Follow-up frequency | As clinically indicated | Pre-ERTa |
|---|---|---|---|---|
| Medical history | X | Every visit | ||
| Physical examination | X | Every visit | X | |
| Upper limb function | ||||
| Standardized upper extremity function test | X | Annually | X | |
| Hips and lower extremities | ||||
| Hips/pelvis: AP pelvis radiograph | X | X | ||
| Lower extremities: standing AP radiographs | X | X | ||
| Spine/spinal cord compression | ||||
| Plain radiograph spine | X | Every 1–3 years | ||
| MRI spine | X | Annually | ||
| CT neutral region of interest | Xb | |||
| Cardiac function | ||||
| ECGd | X | Every 1–3 yearsc | Xb | |
| Echocardiogram | X | Every 2–3 yearsc | Xb | |
| Heart rate | X | Annuallye | ||
| Respiratory function | ||||
| FVC | X | Annuallyf | X | |
| MVV | X | Annuallyf | X | |
| Respiratory rate | X | Annuallye | X | |
| Oxygen saturationg | X | Annuallye | ||
| Overnight sleep study | X | Annuallyh | ||
| Neurological function | ||||
| Neurological exam | X | Every visit (minimally every 6 months) | X | |
| Ophthalmological function | ||||
| Slit-lamp biomicroscopy of cornea | X | X | ||
| Intraocular pressure | X | X | ||
| Refractive error | X | X | ||
| Examination of posterior segment | X | X | ||
| Scotopic and photopic electroretinogram | X | |||
| Hearing | ||||
| Audiology assessment (multimodal) | X | Annually | ||
| Dental evaluation | ||||
| Evaluation of oral health by dentist | X | Annually | ||
| Endurance | ||||
| 6MWT, T25FWe | X | Annually | Xi | X |
| Growth | ||||
| Height and length | X | Every visit | X | |
| Weight | X | Every visit | X | |
| Head circumference (≤3 years) | X | Every visit | ||
| Pubertal stage (age 9 until mature)j | X | Every visit | X | |
| Disease burden | ||||
| Pain assessment | X | Every 6 months | X | |
| QoL questionnaire | X | Annually | X | |
| Functional testk/ADL questionnaire | X | Annually | X | |
| Evaluation by physiotherapist | X | Annually | Xi | |
6MWT, 6-min walk test; ADL, activities of daily living; AP, anteroposterior; ECG, electrocardiogram; ERT, enzyme-replacement therapy; FVC, forced vital capacity; GAG, glycosaminoglycans; MRI, magnetic resonance imaging; MVV, maximum voluntary ventilation; QoL, quality of life; T25FW, timed 25-foot walk.
If not done within 3-6 months, these assessments should be done before treatment with ERT is started.
For example pre-operative planning.
ECG and echocardiogram at diagnosis and after 1 year. If no signs of cardiac involvement, assessments can be repeated every 3 years, otherwise follow-up in expert centers according to standard of care.
In symptomatic patients (e.g., suspicious ECG) or post-pubertal patients, prolonged ECG (Holter monitoring for 5-7 days including normal exercising) should be done in expert centers at diagnosis and every 1-3 years.
Heart rate, respiratory rate, and oxygen saturation should be measured before and after each endurance test; choice of endurance measure depends on patient's physical and developmental abilities (for the 6MWT consistently use the same hallway).
Annual follow-up only required until children stop growing or when patient is on treatment. Once growth has stopped, testing frequency can be decreased to every 2-3 years provided that respiratory symptoms remain unchanged.
Oxygen saturation can be determined either by pulse oximetry or by arterial blood gas analysis.
Screening studies should be done in home on an annual basis. Full polysomnography should be performed at diagnosis in an expert center, then every 3 years, unless clinically indicated (or before major surgery). Patients with a positive test and those who need ventilatory support should be evaluated by a sleep expert.
For example pre- and post-operatively.
Pubertal stage can be assessed using two scores: genitalia (male), breast (female), pubic hair (male and female) as described by Marshall and Tanner [Marshall and Tanner, 1969, 1970].
For example 6MWT/T25FW, pinch/grip test and functional dexterity test.
SYMPTOM-BASED MANAGEMENT
Musculoskeletal Manifestations
Overview
Skeletal and joint abnormalities are the most apparent and prevalent disease manifestations of Morquio A syndrome. The majority of patients show short stature with corresponding short trunk and neck, abnormalities in spine, upper extremities, thorax, hips and/or lower extremities, and joint and gait abnormalities [Montaño et al., 2007; Aslam et al., 2013; Dhawale et al., 2013; Harmatz et al., 2013]. Radiographic findings (dysostosis multiplex) suggesting Morquio A syndrome include abnormally shaped vertebral bodies with anterior beaking, posterior scalloping, platyspondyly and dens hypoplasia, thoracolumbar kyphosis, short, broad metacarpals with proximal rounding, irregular carpal bones, rounded iliac wings, acetabular dysplasia, coxa valga, genu valgum, ankle valgus, pectus carinatum, paddle-shaped ribs and short, and thick clavicles (Fig. 3), although only some of these may be present at diagnosis and missed by the non-expert [Hendriksz et al., 2013b].
FIG. 3.
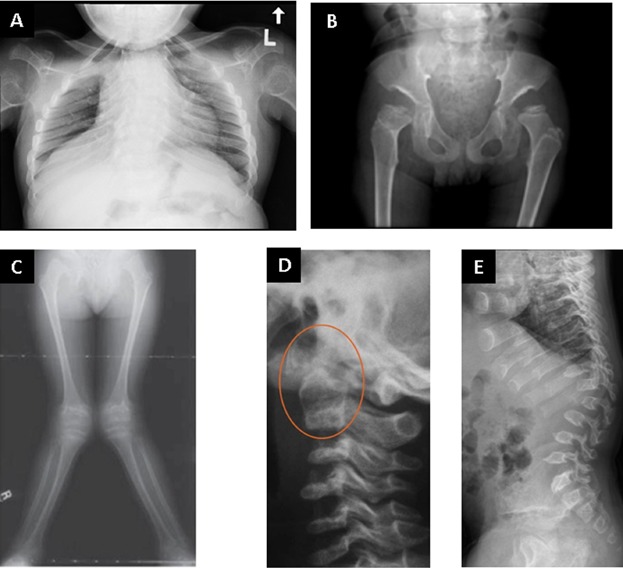
Typical radiographic features (dysostosis multiplex) in patients with Morquio A syndrome: (A) Rib cage from a 12 year-old female Morquio A patient showing paddle shaped ribs. (B) Typical dysostosis multiplex changes in the pelvis and hips of an 8 year-old Morquio A patient showing dysplastic femoral epiphyses and narrowed inferior ilia sloping into the acetabular roofs. (C) Knee valgus in a 7 year-old Morquio A patient. Reproduced from Dhawale et al. [Dhawale et al., 2012] with permission from Lippincott Williams & Wilkins. (D) Cervical spine showing dens hypoplasia, which may be associated with atlantoaxial instability. Reproduced from Solanki et al. [Solanki et al., 2013] with permission from Springer. (E) Thoracolumbar spine changes including platyspondyly, anterior beaking, thoracolumbar kyphosis, and posterior vertebral scalloping. Reproduced from Solanki et al. [Solanki et al., 2013] with permission from Springer.
Typical features of Morquio A not occurring in other types of MPS disorders are joint hypermobility and deformity in the wrists, leading to floppy wrists with weak grip and loss of fine motor skills [Aslam et al., 2013]. Patients may also show subluxation of the hip joints and joint instability in the knees, which can exacerbate genu valgum, patella dislocation, and gait abnormalities. Dens hypoplasia in combination with ligamentous laxity can lead to atlantoaxial instability and subsequently to spinal canal stenosis and spinal cord compression [Solanki et al., 2013].
Evaluation
Each Morquio A patient should be referred to an orthopedic surgeon with experience treating MPS disease at diagnosis. Regular assessments of the hips, lower extremities, and spine are recommended for optimal outcomes (Table II) [Solanki et al., 2013; White et al., 2014]. Radiographic assessments of the hips and lower extremities should focus on the presence of progressive hip dysplasia (shallow acetabulum with subluxation), genu valgum (tibiofemoral angle), and ankle valgus. Information on gait and mobility can be obtained by a simple physical exam and interrogation of patients. The clinical utility of gait analysis has not been established at present.
Evaluation of the spine should focus on the presence of spinal stenosis, instability and cord compression. Detailed instructions for detecting spinal cord compression in patients with Morquio A and indications for surgery have been recently published [Solanki et al., 2013]. Briefly, plain radiography of the cervical spine (anteroposterior, lateral neutral, and flexion extension) and of the thoracolumbar spine (anteroposterior and lateral) should be performed every 1–3 years, depending on clinical history. Magnetic resonance imaging (MRI) of the whole spine (neutral position) should be done annually, focusing on all three potential sites of cord compression, i.e., occipitocervical, cervicothoracic, and thoracolumbar. If available, flexion-extension MRI of the cervical spine is recommended every 1–3 years depending on signs, symptoms and radiographic instability. Computed tomography (CT) of the spine can be valuable for pre-operative planning.
Each patient with Morquio A syndrome also requires regular assessment of upper limb function (fine motor skills). Valuable standardized upper extremity function tests include evaluation of grip strength and pinch strength, the 9-hole peg test and the functional dexterity test [Aaron and Jansen, 2003; Poole et al., 2005]. In order to obtain consistent measurements, it is important to record the position of the wrist and whether the wrist was supported or not during testing. Passive and active assessments of the range of motion of elbows and shoulders can be useful, but generally do not have therapeutic consequences.
Interventions
Interventions for managing hip subluxation, knee valgus, and ankle valgus in patients with Morquio A syndrome have been recently discussed in detail by White et al. [2014]. Briefly, depending on age and severity of the deformity, hip subluxation can be managed using pelvic and femoral osteotomy, shelf acetabuloplasty, or total hip arthroplasty [Tassinari et al., 2008; Pryce Lewis and Gibson, 2010; Dhawale et al., 2012; White, 2012]. Correction of knee valgus using guided growth (8-plate hemiepiphysiodesis) is effective in the growing child and may prevent hip problems and ankle valgus at a later age [Dhawale et al., 2012]. Knee osteotomy or knee arthroplasty can be an option for older patients [Atinga and Hamer, 2008]. Ankle valgus can generally be managed by orthotics, but may sometimes require surgical correction (e.g., guided growth or osteotomy) [Dhawale et al., 2012]. Recurrence of the knee and/or ankle valgus is not uncommon.
Interventions for spinal cord compression in patients with Morquio A syndrome include spinal decompression, fusion or a combination of both [Ransford et al., 1996; White, 2012; Solanki et al., 2013]. Different approaches for managing spinal cord compression at the axial, subaxial, and thoracolumbar spine and the associated risks have been discussed by Solanki et al. [Solanki et al., 2013]. It is important to be aware that instability and stenosis may develop again in the region of previous fusion after surgery [Dede et al., 2013]. Surgical interventions requiring anesthesia warrant pre-operative evaluation of anesthetic risk factors and should be performed by a team experienced in managing these cases. Peri-operative care and anesthesia is discussed in more detail below.
Physical therapy and pain medication can be beneficial to manage musculoskeletal manifestations in some patients. A walking aid or wheelchair can help improve mobility and pain. However, efforts should be made to keep patients independently mobile as long as possible as the quality of life (QoL) drops dramatically when patients become wheelchair dependent [Hendriksz et al., 2014b].
Respiratory Manifestations
Overview
Respiratory impairment is the leading cause of morbidity and mortality in patients with Morquio A syndrome and can be due to obstructive or restrictive disease [Montaño et al., 2007; Berger et al., 2012]. The upper and lower airways of Morquio A patients can be narrowed and tortuous due to a combination of GAG deposition in airway walls (Fig. 4), abnormalities in the skull or spine, tracheal distortion, tracheobrochnomalacia, and thickened secretions. Patients may develop airway occlusion upon neck flexion and adopt a “sniff position” to preserve airway patency. Restrictive disease can develop due to a small and abnormally shaped thoracic cage or impaired diaphragmatic motility [Berger et al., 2012]. An early sign of respiratory impairment is sleep disordered breathing (SDB), i.e., obstructive sleep apnea (OSA) or sustained hypoventilation. Over time, SDB can have cardiovascular consequences, such as pulmonary hypertension, with consecutive development of cor pulmunale, and can lead to cardio-respiratory failure.
FIG. 4.
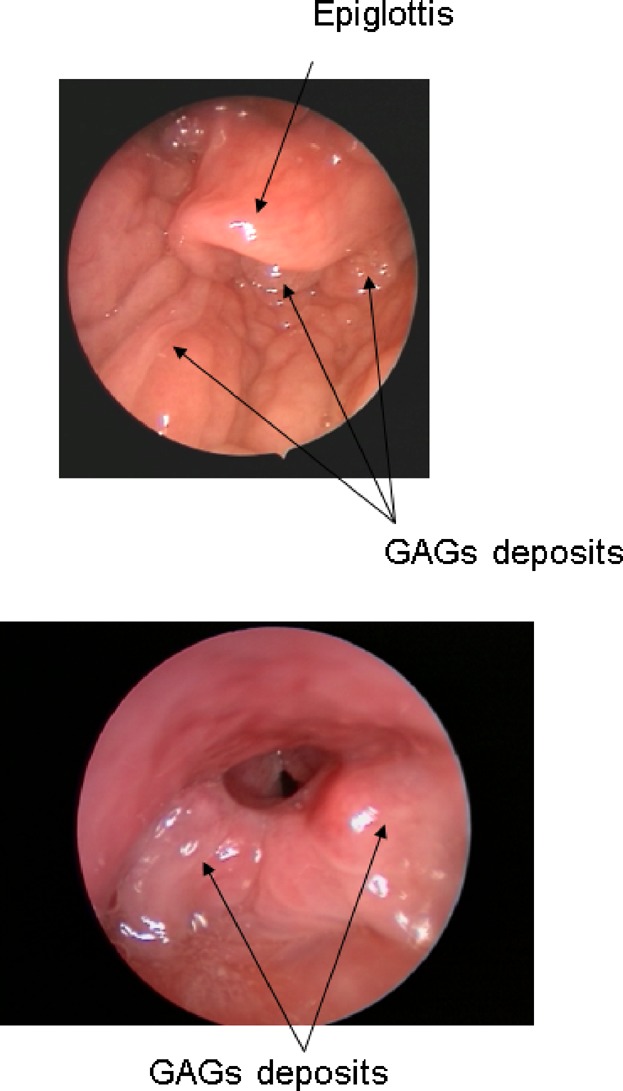
GAG deposits within walls of the upper airway of a patient with Morquio A causing narrowing of the pharynx and larynx.
Evaluation
Forced vital capacity (FVC) and maximum voluntary ventilation (MVV) should be assessed annually until children stop growing. Once growth has stopped, the frequency of testing can be decreased to every 2–3 years provided that respiratory symptoms remain unchanged. Additional testing should be performed if respiratory symptoms change or if intercurrent illnesses occur. In addition, evaluation before and after initiation of ERT is recommended. Because of the growth impairment associated with Morquio A, expressing FVC and MVV as % of normal is of limited value. However, absolute values can be used to monitor respiratory function in patients over time.
In order to detect reduced exercise capacity due to respiratory restriction, it is also recommended to measure respiratory rate and arterial oxygen saturation before and after annual endurance testing (e.g., utilizing the 6MWT). Oxygen saturation can be obtained with a pulse oximeter along with heart rate. Reduction in oxygen saturation during exercise should prompt additional evaluation (e.g., pulmonary function and chest radiograph). Treatment targeted to the etiology for the desaturation may be administered and use of supplemental oxygen can be considered particularly if the oxygen saturation falls below 88–90%. In addition, the presence of inordinate tachycardia during exercise should prompt cardiac evaluation (e.g., assessment of valve and myocardial function and assessment for cardiac arrhythmia).
Routine physical exam can also identify signs of potential respiratory problems such as an enlarged tongue or sniff position. Fiber-optic laryngoscopy may be considered in patients showing obstructive symptoms and prior to surgery. Evaluation of respiratory function is also recommended before any planned air travel to ensure safety during the flight [Ahmedzai et al., 2011; Shrikrishna and Coker, 2011].
In order to timely detect SDB, in-home screening sleep studies that monitor oxygen saturation are recommended on an annual basis. A sleep study with full polysomnography in an expert center is recommended at diagnosis and then every 3 years, unless clinically indicated or before major surgery. Patients with a positive test and those who need ventilatory support should be evaluated by a sleep expert.
Interventions
The management of respiratory manifestations in patients with MPS has been discussed by Berger et al. [Berger et al., 2012]. Briefly, patients may benefit from supportive therapies such as regular influenza and pneumococcus vaccinations, bronchodilators and aggressive and prompt treatment of upper respiratory infections [Berger et al., 2012]. Tonsillectomy and/or adenoidectomy are frequently required in patients with obstructed upper airways. SDB can generally be managed successfully by continuous positive airway pressure (CPAP, for patients with OSA) or non-invasive ventilator support (e.g., bilevel positive airway pressure to treat sustained hypoventilation). Tracheostomy may be required if ventilator support is ineffective or in patients with airway obstruction during wakefulness. However, as many complications may occur during and after tracheostomy placement, this procedure should be performed only in centers with experience in Morquio A disease [Berger et al., 2012].
Cardiovascular Manifestations
Overview
Unlike other MPS disorders [Braunlin et al., 2011], cardiac valve abnormalities in Morquio A patients are generally very mild. Occasionally, older patients may develop clinically important cardiac disease post-pubertally. Detailed evaluation of cardiac data performed in Morquio A patients enrolled in the multicenter, cross-sectional Morquio A Clinical Assessment Program (MorCAP) [Harmatz et al., 2013] demonstrated variable incidence of valvular regurgitation and stenosis. In addition, Morquio A patients typically have a small left ventricular diameter and an abnormally low stroke volume (personal communication Prof. Dr. C. Kampmann). As compensation, these patients may demonstrate an abnormally high heart rate and high myocardial work index. In addition, blood pressure in these patients can increase dramatically if they switch from a supine to a sitting position.
Evaluation
Because cardiac pathology in Morquio A patients is generally mild, electrocardiography (ECG) and echocardiography every 3 years are sufficient, if there are no signs of cardiac involvement (Table II). Heart rate and blood pressure should be measured before and after annual endurance testing. Resting blood pressure and heart rate (before endurance testing) can indicate presence of arterial hypertension or resting tachycardia. Repeat measurement directly after endurance testing provides insight into a patient's cardiovascular reserve and might detect cardiovascular incompetence. The left ventricular diameter generally does not increase after cessation of growth unless significant valve disease is present. In symptomatic patients (e.g., suspicious ECG) or post-pubertal patients, prolonged ECG (Holter monitoring for 5–7 days including normal exercising) should be performed at diagnosis and every 1–3 years to detect cardiac arrhythmias, which may be suspected based on experience in other MPS types.
Interventions
It is important to be aware that an elevated heart rate in Morquio A patients may be needed to compensate for a small cardiac stroke volume. Therefore, treatment of tachycardia with beta blockers should be avoided. Also ACE inhibitors should be used with caution as they may result in a disproportional increase in heart rate, especially in subjects with marked changes in blood pressure between supine and sitting positions.
Valve replacement may be considered for patients that present with or progress to severe aortic or mitral valve disease [Nicolini et al., 2008; Pagel and Almassi, 2009].
Neurological Manifestations
Overview
Morquio A patients can develop neurological symptoms due to myelopathy secondary to spinal cord compression [Harmatz et al., 2013; Solanki et al., 2013]. The MorCAP baseline data (N = 325) showed nervous system disorders or cord compression in 51% of patients, including 30% with cervical myelopathy, 14% with cervical cord compression, and 13% with thoracolumbar cord compression [Harmatz et al., 2013]. The prevalence of these disorders increased with age, i.e., 36% in 0–4 year old patients, 48% in the 5–11 year olds, 58% in 12–18 year olds, and 55% in >18 year olds. Cervical cord compression can lead to unsteady gait, upper and lower extremity weakness, dysesthesias, urinary dysfunction, paralysis and sudden death [Lipson, 1977; White, 2012]. At the thoracolumbar level, cord compression may result in lower back pain, radiating leg pain and paraplegia with insidious onset and associated consequences such as lower limb weakness, sensory anomalies and disturbed bladder function [Dalvie et al., 2001].
Evaluation
In order to identify patients with spinal cord compression in an early stage, neurological examinations at maximum intervals of six months are recommended (Table II). A neurological examination in patients with Morquio A syndrome may reveal hyperreflexia, increased muscle tone, pyramidal tract signs (ankle clonus and Babinski sign) and/or proprioceptive deficits [Solanki et al., 2013]. However, neurological assessment can sometimes be difficult due to lower limb function involvement. Also, neurological signs and symptoms may underestimate the severity of spinal cord compression seen on MRI. Clinical and neurological findings should therefore be correlated with imaging studies of the spine [Solanki et al., 2013]. In patients with multi-segmental myelopathy, it can be difficult to determine the level of compression responsible for the observed neurological deficit. If available, it can be worthwhile to measure somatosensory evoked potentials (SEPPs) and motor evoked potentials (MEPs) on an outpatient basis. However, the value of these studies in the evaluation of spinal cord compression still needs to be established [Solanki et al., 2013].
Interventions
MRI may over or underestimate the risk of cord compression in patients with Morquio A syndrome, therefore a neurosurgeon should be part of the multidisciplinary team to assist with making this assessment. Neurological monitoring during surgical interventions requiring anesthesia is recommended in cases where spinal cord compression is a concern. The treatment of spinal cord compression has been discussed previously (see musculoskeletal manifestations).
Ophthalmological Manifestations
Overview
Diffuse corneal clouding and refractive error problems (astigmatism, myopia, and hyperopia) are very common findings in Morquio A patients and may lead to reduced visual acuity and photosensitivity [Couprie et al., 2010; Summers and Ashworth, 2011; Harmatz et al., 2013; Hendriksz et al., 2013a]. Although corneal clouding worsens with age, it tends to be less severe in Morquio A syndrome than in MPS I and MPS VI [Ashworth et al., 2006]. Cataract, open-angle glaucoma, retinopathy, optic disc swelling and optic nerve atrophy, and pseudoexophthalmos due to shallow orbits have also been reported sporadically in patients with Morquio A syndrome [Dangel and Tsou, 1985; Cahane et al., 1990; Iwamoto et al., 1990; Olsen et al., 1993; Käsmann-Kellner et al., 1999; Ashworth et al., 2010; Couprie et al., 2010; Hendriksz et al., 2013a].
Evaluation
Ophthalmological function should be assessed in each Morquio A patient at diagnosis (Table II) [Fahnehjelm et al., 2012; Hendriksz et al., 2013a]. Afterwards, a basic evaluation of vision/ocular abnormalities should be part of the general physical examination that is recommended at every visit. Referral to an ophthalmologist is only required in case of clinical abnormalities.
Interventions
Patients with impaired vision may benefit from refractive correction or low vision aids; filtering glasses and hats can be used to manage photosensitivity [Tomatsu et al., 2011]. Corneal clouding can be managed surgically by corneal transplantation [Leslie et al., 2005; Tomatsu et al., 2011], but reopacification of corneal grafts may occur similar to experience in other MPS disorders [Käsmann-Kellner et al., 1999]. Concomitant retinopathy, glaucoma, or optic nerve atrophy can also limit restoration of vision with corneal transplantation. Patients with cataracts may benefit from cataract surgery.
Audiological Manifestations
Overview
Sensorineural or mixed conductive and sensorineural hearing loss commonly develop in in Morquio A patients in the first decade of life [Bredenkamp et al., 1992; Riedner and Levin, 1977]. Hearing loss may result from recurrent respiratory tract infections or otitis media, deformity of the ossicles, and/or abnormalities of the inner ear [Bredenkamp et al., 1992; Riedner and Levin, 1977; Tomatsu et al., 2011; Hendriksz et al., 2013a]. A study including 18 patients with Morquio A syndrome showed conductive hearing loss in all three patients <8 years and mixed or sensorineural hearing loss in 14 out of 15 patients ≥8 years [Riedner and Levin., 1977]. Ten patients required a hearing aid. In the MorCAP study, ear and labyrinth disorders (mostly hearing impairment and otitis media) were reported by 77% of patients [Harmatz et al., 2013].
Evaluation
Hearing impairment is an underestimated issue in Morquio A patients. Therefore, age-adjusted audiology assessments should be done at diagnosis and on an annual basis thereafter (Table II) (e.g., acoustic emissions in neonates, visual reinforcement audiometry in children from 8 months up to 3 years of age, conventional ear checks for patients of 3 years or older) [Hendriksz et al., 2013a].
Interventions
Conductive hearing loss due to retained middle ear fluid can be treated using ventilation tubes [Hendriksz et al., 2013a]. Long-lasting types of tympanostomy tubes are preferable to use on the first occasion considering the anesthetic risks and the risk of the reoccurrence of the middle ear fluid [Hendriksz et al., 2013a]. Post-aural hearing aids may be most appropriate if a progressive neurosensory element to hearing loss is present.
Abdominal Manifestations
Overview
Abdominal manifestations of Morquio A include umbilical, inguinal or bilateral diaphragmatic hernias, hepatomegaly, splenomegaly (less common), and other gastro-intestinal disorders (e.g., chronic constipation, diarrhea) [Nursal et al., 2000; Harmatz et al., 2013; Hendriksz et al., 2013a]. However, gastro-intestinal manifestations tend to be less prominent than in other types of MPS disorders.
Evaluation
Assessment of gastro-intestinal problems in Morquio A patients should be part of routine clinical evaluation.
Interventions
Hernias can be surgically repaired by herniorraphy and frequently recur.
Dental Abnormalities
Overview
Patients with Morquio A syndrome tend to have small, widely spaced teeth, often with thin, structurally weak enamel and small pointed cusps, spade-shaped incisors, pitted buccal surfaces, and other developmental abnormalities of primary and permanent dentition (Fig. 5) [Kinirons and Nelson, 1990; Rølling et al., 1999; James et al., 2012]. Therefore, they are vulnerable to caries formation [James et al., 2012].
FIG. 5.
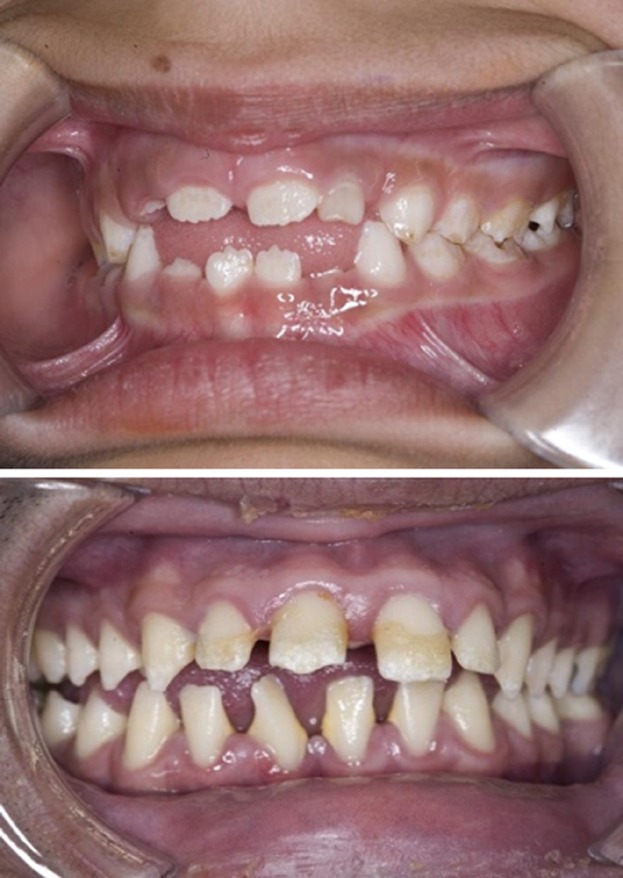
Pediatric (top) and adult (bottom) teeth of patients with Morquio A showing widely spaced teeth, pointed cusps and spade-shaped incisors.
Evaluation
Patients should be referred to a dentist at diagnosis. Close monitoring of dental development (at least annually) and regular dental care is important to prevent caries and attrition of the teeth [Tomatsu et al., 2011; Hendriksz et al., 2013a].
Interventions
In order to prevent caries, Morquio A patients should receive fluoride supplementation; fissure sealing of dentition may be considered in some cases.
Overall Assessments
Endurance
Patients with Morquio A syndrome may show reduced endurance due to impaired cardiac, respiratory, musculoskeletal, and/or neurological function, which may impact significantly on functional status/mobility and QoL [Harmatz et al., 2013]. Endurance testing at diagnosis and annually thereafter is recommended to follow up overall disease progression (Table II). Information on endurance can be obtained by routinely asking endurance questions during clinical exam (e.g., How far can you walk? How many stairs can you climb?) or by using an endurance test.
The 6-min walk test (6MWT) is considered the most appropriate endurance test for patients with Morquio A syndrome, as it is readily available and is a combined assessment of musculoskeletal and cardiopulmonary capacity. This standardized test measures how far a person can walk on a hard, flat surface in 6 min and has been used to assess endurance in several MPS (including Morquio A) clinical trials [American Thoracic Society, 2002; Wraith et al., 2004; Harmatz et al., 2005; Harmatz et al., 2006; Muenzer et al., 2006; Hendriksz et al., 2012]. Detailed instructions for the 6MWT have been published by the American Thoracic Society [American Thoracic Society, 2002]. In order to allow comparison of test outcomes, the 6MWT should always be performed at the same location. Walking aids used during the 6MWT should be documented and should be consistent over time (i.e., do not change type of aid or perform with/without aid over time). In patients with limited ambulation who are unable to do the 6MWT, endurance can be assessed using a timed 25-foot walk test (T25FW). The T25FW, which has originally been developed as a component of the Multiple Sclerosis Functional Composite (MSFC), measures the time needed to cover 25 feet [Polman and Rudick, 2010]. For Morquio A patients, the test has been adapted in order to allow patients to cover the 25 feet distance by crawling or rolling if walking is impossible. Detailed instructions for the T25FW for Morquio A patients, based on the instructions developed for multiple sclerosis patients, are given in Supplementary Appendix 2. Blood saturation, heart rate and respiratory rate should be measured immediately before each endurance test, upon test completion, and 2 min after test completion. Compromised vision and hearing should be taken into consideration when implementing these tests in Morquio A patients. Endurance testing is also recommended before and regularly after initiation of ERT as a measure of treatment efficacy.
Growth
The majority of adult patients with Morquio A syndrome are below the 3rd centile in height on the Center for Disease Control growth charts and shorter than 120 cm [Harmatz et al., 2013]. They also tend to have a higher body mass index than normal (>85th centile) [Montaño et al., 2008]. Growth velocity generally starts to fall below normal levels after the age of 1 year [Montaño et al., 2008]. Growth charts for patients with Morquio A syndrome have been published in 2008 [Montaño et al., 2008].
Standing height (sitting height if the patient is unable to stand), length (supine) and weight, head circumference (up to 3 years of age), and stage of puberty (from age 9 years until maturity) [Marshall and Tanner, 1969, 1970] should be documented at diagnosis and at each visit to follow the relative change in individuals over time (Table II). Height and weight should also be measured before and regularly after initiation of ERT to evaluate the impact of treatment. One should be aware that height may vary (and even decrease) over time due to skeletal changes such as kyphosis or knee valgus. Because obesity may exacerbate lower limb and other problems, it is important to monitor weight (prevent excessive increase in BMI) in patients with Morquio A syndrome. Assessment of bone density is difficult to interpret in MPS patients given the lack of appropriate normative data for comparisons, and therefore not recommended on a routine basis [Fung et al., 2010].
Pain
Pain is a major, but underreported, symptom of Morquio A syndrome [Harmatz et al., 2013]. It is mainly related to musculoskeletal problems (e.g., joint pain and muscular pain) and may occur in many parts of the body, including the spinal area, upper and lower extremities, and the head and neck area. A patient-reported outcomes study in 27 adults and 36 children/adolescents with Morquio A showed that pain can interfere profoundly with activities of daily living (ADL) and can be a driver for wheelchair use [Hendriksz et al., 2014b]. Although increased mobility (less frequent wheelchair use) was found to be associated with more severe and widespread pain, pain interference with ADL was most severe in patients using a wheelchair all the time.
Pain severity should be assessed at each clinical visit by specifically asking patients about their analgesic use, or about recent improvement or worsening of pain (Table II). A simple, self-completed question for rating pain/discomfort is included in the EQ5D-5L (http://www.euroqol.org). This standardized questionnaire is developed to measure health-related QoL in a wide range of health conditions and currently available in 106 languages. External factors and conditions should be considered when interpreting pain assessment, for example pain might be increased after a long flight.
Quality of Life
Many factors may affect QoL in Morquio A patients, including reduced endurance or mobility, difficulties in ADL, dependence on caregivers, frequent surgical interventions, pain and fatigue [Harmatz et al., 2013; Hendriksz et al., 2013a]. It is important to be aware that although patients using a wheelchair all of the time experience less pain severity and fatigue due to limited functional activity, they tend to have a considerably poorer HRQoL [Hendriksz et al., 2014b]. Regular assessments of QoL and ADL are recommended (Table II). QoL can be assessed using reproducible and age-appropriate questionnaires such as the EQ-5D-5L. There is need for QoL questionnaires for adult patients, covering adult-specific needs such as employment, financial problems, and sexuality and reproduction. Information on the disease impact on ADL can be obtained using previously discussed functional tests (e.g., 6MWT/T25FW, pinch/grip test and functional dexterity test), open-ended questions, and reproducible, age-appropriate ADL questionnaires such as the MPS Health Assessment Questionnaire (MPS HAQ) [Harmatz et al., 2013].
Simple interventions may considerably improve the functional capacity and QoL of patients with Morquio A syndrome. For school children, communication between school staff, physiotherapists and health professionals about the inclusion of the patient in activities at school, specialized equipment, and walking aids is of major importance. It may be valuable to educate schools, including teachers and peers, on the disease and the fact that children may be absent for infusions and may have some limitations. This can enhance understanding, stop the potential for bullying, and stimulate peer tolerance and respect. When mobility is decreasing, adequate advice can be valuable for the patient in order to achieve a good balance between independence and maintaining mobility. Regular assessment of vision and hearing is important to ensure that deficits in these areas are being addressed.
Urinary GAG
In the absence of a well validated, readily available KS assay, measurement of urinary KS has a very limited role in the diagnosis of Morquio A patients and, while it is a robust marker of pharmacodynamic activity of elosulfase alfa, its clinical utility as a surrogate marker for elosulfase alfa efficacy has not been established. In the phase III study, urinary KS demonstrated a pharmacodynamic effect of elosulfase alfa in the weekly 2 mg/kg study population resulting in a 41% decrease in urinary KS at week 24 of the study [Hendriksz et al., 2014a]. However, changes in urinary KS did not correlate with clinical efficacy measures. Urinary KS can be measured prior to and just after starting elosulfase alfa to determine the pharmacodynamic effects of ERT treatment. Annual follow-up assessments are of limited use. Furthermore, the standard dye-based quantitative total GAG tests are predicted to be even less sensitive than the urinary KS test, and there is no current data to suggest that this testing is of any use in clinical management of Morquio A [Wood et al., 2013].
Peri-Operative Care and Anesthesia
Patients with Morquio A syndrome may be at high anesthetic risk due to cervical instability, compromised respiratory function or cardiac problems [Theroux et al., 2012 Walker et al., 2013]. Therefore, any elective surgery requires pre-operative evaluation of anesthetic risk factors and should be performed by an experienced team at centers familiar with MPS disorders. A spectrum of airway management equipment should be available during and after the procedure. The anesthetic problems and detailed instructions for pre-, peri- and post-operative care of patients with MPS in general and for patients with Morquio A have been published in 2013 in two papers by Walker et al and Theroux et al. [Theroux et al., 2012; Walker et al., 2013].
Physiotherapy
As mentioned previously, Morquio A patients require regular evaluation of upper limb function by for example a physiotherapist. Referral to a physiotherapist on a routine basis (Table II) can also be valuable to encourage patients to perform routine exercises to better preserve joint function and fine motor skills [Tomatsu et al., 2011] and to participate in activities such as swimming or hippotherapy (occupational and physical therapy using the movements of a horse). Physiotherapy can be personalized to each patient and directed to address those joints and/or functions that are most impacted for that individual at that time.
Transition From Pediatric to Adult Care
During late adolescence, the relationship of patients with Morquio A syndrome with the medical team changes from a passive (with the parents mostly communicating with the medical team) to an active one (i.e., direct communication between patient and healthcare provider) [Hendriksz, 2013c]. This process, during which patients become increasingly independent from their parents and start managing their own health care, can be difficult, particularly in countries where the organization and facilities of the healthcare system require transition from pediatric to adult services. In order to guide this process and to ensure that the adult providers are knowledgeable in managing these patients and that patients are not lost to follow-up, there is need for a formal, site-specific, transition strategy. For example, joint visits with the pediatrician and the physician coordinating the adult patient for a few years or input of patient information by the pediatrician into a registry accessible for the adult team may guide adult care after transition. Alternatively, patients may carry their own health records. In any case, it is important to involve the patient and his/her family in this transition process.
FUTURE DIRECTIONS
Insights into the natural history and pathophysiology of Morquio A syndrome have grown rapidly over the past decade thanks to large observational studies, mutation analyses, and basic research in the framework of the development of ERT [Tomatsu et al., 2005; Montaño et al., 2007; Tomatsu et al., 2008; Harmatz et al., 2013]. Nevertheless, current knowledge on the disease remains relatively limited. Future studies providing additional insight into genotypic-phenotypic correlations and biomarkers for disease activity are warranted and may allow the development of individualized management strategies according to the clinical manifestations of a patient.
As for other types of MPS disorders, implementations in symptom management and the recent introduction of ERT for Morquio A syndrome may create new challenges for the future management of these patients. As ERT can have a positive impact on mobility and endurance, it may encourage patients to adopt a more active life style. This may also create new expectations for the future and lower the threshold for orthopedic surgery. If the life expectancy of patients increases due to better treatment options, the previously-discussed needs of adult patients will gain even greater importance. A longer life expectancy is likely to be associated with an increase in the number of surgical interventions in patients with Morquio A and with increasing anesthetic challenges when patients grow older.
Another challenge for the future will be improving the convenience of ERT. Weekly visits to the hospital for infusions can be perceived as very bothersome by patients and families, especially if they have to travel far. Creating the opportunity for remote/home infusions may provide an answer to this problem.
Acknowledgments
The authors are grateful to all specialists involved in the creation of these management guidelines. The expert panel involved in the consensus meeting in Barcelona consisted of Christian J Hendriksz, Salford Royal NHS Foundation Trust, Salford, UK; Kenneth I Berger, New York University School of Medicine, NY, USA; Roberto Giugliani, Medical Genetics Service/HCPA, Department of Genetics/UFRGS and INAGEMP, Porto Alegre, Brazil; Paul Harmatz, UCSF Benioff Children's Hospital Oakland, Oakland, CA, USA; Christoph Kampmann, University Children's Hospital, Mainz, Germany; William G Mackenzie, Nemours/Alfred I. duPont Hospital for Children, Wilmington, DE, USA; Julian Raiman, Hospital for Sick Children, Toronto, ON, Canada; Martha Solano Villarreal, Fundacion Cardioinfantil, Barranquilla, Colombia; Ravi Savarirayan, Murdoch Children's Research Institute, Parkville Victoria, Australia. Additional consultant specialists involved in the first expert meeting in Amsterdam included Richard Baker, University of Salford, Salford, UK; Kaustuv Bhattacharya, Children's Hospital at Westmead, Westmead, Australia; Elizabeth A Braunlin, University of Minnesota, Minneapolis, MN, USA; Barbara K Burton, Lurie Children's Hospital, Chicago, IL, USA; Lorne Clarke, University of British Columbia, Vancouver, BC, Canada; Carolina FM De Souza, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil; Simone C Fagondes, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil; Edward W Fong, Children's Hospital & Research Center Oakland and California Pacific Medical Center, Oakland and San Francisco, CA, USA; Andrea AM Jester, Birmingham Children's Hopital, Birmingham, UK; Simon Jones, Manchester Centre for Genomic Medicine, Manchester, UK; Christina Lampe, Villa Metabolica, Mainz, Germany; John Mitchell, McGill University Health Center, Montreal, Québec, Canada; David J Pratt, Birmingham Community Healthcare Trust, Birmingham, UK; Mark E Roberts, Salford Royal NHS Foundation, Salford, UK; Matthias K Schaefer, Stiftungs Klinikum, Koblenz, Germany; Fiona Stewart, Belfast City Hospital, Belfast, UK; Reid V Sutton, Baylor College of Medicine, Houston, TX, USA; Mihir M Thacker, Nemours/Alfred I duPont Hospital for Children, Wilmington, DE, USA; Stephen Waldek, Hope Hospital, Salford, UK; Klane K White, Seattle Children's Hospital, Seattle, WA, USA; Tai-Tong Wong, Cheng Hsin General Hospital, Taipei, China. Both expert meetings were coordinated and funded by BioMarin Pharmaceutical Inc.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher's web-site.
Supplementary Appendix 1.
Supplementary Appendix 2.
REFERENCES
- Aaron DH, Jansen CW. Development of the Functional Dexterity Test (FDT): Construction, validity, reliability, and normative data. J Hand Ther. 2003;16:12–21. doi: 10.1016/s0894-1130(03)80019-4. [DOI] [PubMed] [Google Scholar]
- Ahmedzai S, Balfour-Lynn IM, Bewick T, Buchdahl R, Coker RK, Cummin AR, Gradwell DP, Howard L, Innes JA, Johnson AO, Lim E, Lim WS, McKinlay KP, Partridge MR, Popplestone M, Pozniak A, Robson A, Shovlin CL, Shrikrishna D, Simonds A, Tait P, Thomas M. Managing passengers with stable respiratory disease planning air travel: British Thoracic Society recommendations. Thorax. 2011;66:i1–i30. doi: 10.1136/thoraxjnl-2011-200295. [DOI] [PubMed] [Google Scholar]
- Algahim MF, Almassi GH. Current and emerging management options for patients with Morquio A syndrome. Ther Clin Risk Manag. 2013;9:45–53. doi: 10.2147/TCRM.S24771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- Ashworth JL, Biswas S, Wraith E, Lloyd IC. Mucopolysaccharidoses and the eye. Surv Ophthalmol. 2006;51:1–17. doi: 10.1016/j.survophthal.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Ashworth JL, Kruse FE, Bachmann B, Tormene AP, Suppiej A, Parini R, Guffon N. Ocular manifestations in the mucopolysaccharidoses—A review. Clin Experiment Ophthalmol. 2010;28:12–22. [Google Scholar]
- Aslam R, van Bommel AC, Hendriksz CJ, Jester A. Subjective and objective assessment of hand function in mucopolysaccharidosis IVa patients. JIMD Rep. 2013;9:59–65. doi: 10.1007/8904_2012_179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atinga M, Hamer AJ. Total knee replacements in a patient with the Morquio syndrome. J Bone Joint Surg Br. 2008;90:1631–1633. doi: 10.1302/0301-620X.90B12.20641. [DOI] [PubMed] [Google Scholar]
- Berger KI, Fagondes SC, Giugliani R, Hardy KA, Lee KS, McArdle C, Scarpa M, Tobin MJ, Ward SA, Rapoport DM. Respiratory and sleep disorders in mucopolysaccharidosis. J Inherit Metab Dis. 2012;36:201–210. doi: 10.1007/s10545-012-9555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunlin EA, Harmatz PR, Scarpa M, Furlanetto B, Kampmann C, Loehr JP, Ponder KP, Roberts WC, Rosenfeld HM, Giugliani R. Cardiac disease in patients with mucopolysaccharidosis: Presentation, diagnosis and management. J Inherit Metab Dis. 2011;34:1183–1197. doi: 10.1007/s10545-011-9359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredenkamp JK, Smith ME, Dudley JP, Williams JC, Crumley RL, Crockett DM. Otolaryngologic manifestations of the mucopolysaccharidoses. Ann Otol Rhinol Laryngol. 1992;101:472–478. doi: 10.1177/000348949210100605. [DOI] [PubMed] [Google Scholar]
- Cahane M, Treister G, Abraham FA, Melamed S. Glaucoma in siblings with Morquio syndrome. Br J Ophthalmol. 1990;74:382–383. doi: 10.1136/bjo.74.6.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camelier MV, Burin MG, De Mari J, Vieira TA, Marasca G, Giugliani R. Practical and reliable enzyme test for the detection of mucopolysaccharidosis IVA (Morquio Syndrome type A) in dried blood samples. Clin Chim Acta. 2011;412:1805–1808. doi: 10.1016/j.cca.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Couprie J, Denis P, Guffon N, Reynes N, Masset H, Beby F. Ocular manifestations in patients affected by Morquio syndrome (MPS IV) J Fr Ophtalmol. 2010;33:617–622. doi: 10.1016/j.jfo.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Dalvie S, Skinner J, Vellodi A, Noorden MHH. Mobile thoracolumbar gibbus in Morquio type A: The cause of paraparesis and its management. J Pediatr Orthop B. 2001;10:328–330. [PubMed] [Google Scholar]
- Dangel ME, Tsou BH. Retinal involvement in Morquio's syndrome (MPS IV) Ann Ophthalmol. 1985;17:349–354. [PubMed] [Google Scholar]
- Davison JE, Kearney S, Horton J, Foster K, Peet AC, Hendriksz CJ. Intellectual and neurological functioning in Morquio syndrome (MPS IVa) J Inherit Metab Dis. 2013;36:323–328. doi: 10.1007/s10545-011-9430-5. [DOI] [PubMed] [Google Scholar]
- Dede O, Thacker MM, Rogers KJ, Oto M, Belthur MV, Baratela W, Mackenzie WG. Upper cervical fusion in children with Morquio syndrome: Intermediate to long-term results. J Bone Joint Surg Am. 2013;95:1228–1234. doi: 10.2106/JBJS.J.01135. [DOI] [PubMed] [Google Scholar]
- Dhawale AA, Church C, Henley J, Holmes L, Jr, Thacker MM, Mackenzie WG, Miller F. Gait pattern and lower extremity alignment in children with Morquio syndrome. J Pediatr Orthop B. 2013;22:59–62. doi: 10.1097/BPB.0b013e32835a0e6d. [DOI] [PubMed] [Google Scholar]
- Dhawale AA, Thacker MM, Belthur MV, Rogers K, Bober MB, Mackenzie WG. The lower extremity in Morquio syndrome. J Pediatr Orthop. 2012;32:534–540. doi: 10.1097/BPO.0b013e318259fe57. [DOI] [PubMed] [Google Scholar]
- Fahnehjelm KT, Ashworth JL, Pitz S, Olsson M, Törnquist AL, Lindahl P, Summers CG. Clinical guidelines for diagnosing and managing ocular manifestations in children with mucopolysaccharidosis. Acta Ophthalmol. 2012;90:595–602. doi: 10.1111/j.1755-3768.2011.02280.x. [DOI] [PubMed] [Google Scholar]
- Fung EB, Johnson JA, Madden J, Kim T, Harmatz P. Bone density assessment in patients with mucopolysaccharidosis: A preliminary report from patients with MPS II and VI. J Pediatr Rehabil Med. 2010;3:13–23. [PMC free article] [PubMed] [Google Scholar]
- Harmatz P, Ketteridge D, Giugliani R, Guffon N, Teles EL, Miranda MC, Yu ZF, Swiedler SJ, Hopwood JJ. Direct comparison of measures of endurance, mobility, and joint function during enzyme-replacement therapy of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): Results after 48 weeks in a phase 2 open-label clinical study of recombinant human N-acetylgalactosamine 4-sulfatase. Pediatrics. 2005;115:e681–e689. doi: 10.1542/peds.2004-1023. [DOI] [PubMed] [Google Scholar]
- Harmatz P, Giugliani R, Schwartz I, Guffon N, Teles EL, SáMiranda MC, Wraith JE, Beck M, Arash L, Scarpa M, Yu ZF, Wittes J, Berger KI, Newman MS, Lowe AM, Kakkis E, Swiedler SJ. Enzyme replacement therapy for mucopolysaccharidosis VI: A phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J Pediatr. 2006;148:533–539. doi: 10.1016/j.jpeds.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Harmatz P, Mengel KE, Giugliani R, Valayannopoulos V, Lin SP, Parini R, Guffon N, Burton BK, Hendriksz CJ, Mitchell J, Martins A, Jones S, Guelbert N, Vellodi A, Hollak C, Slasor P, Decker C. The Morquio A Clinical Assessment Program: Baseline results illustrating progressive, multisystemic clinical impairments in Morquio A subjects. Mol Genet Metab. 2013;109:54–61. doi: 10.1016/j.ymgme.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Hendriksz C, Vellodi A, Jones S, Takkele H, Lee S, Chesler S, Decker C. Long term outcomes of a phase 1/2, multicenter, open-label, dose-escalation study to evaluate the safety, tolerability, and efficacy of BMN 110 in patients with mucopolysaccharidosis IVA (Morquio A syndrome) Mol Genet Metab. 2012;105:S35. [Google Scholar]
- Hendriksz CJ, Al-Jawad M, Berger KI, Hawley SM, Lawrence R, McArdle C, Summers CG, Wright E, Braunlin E. Clinical overview and treatment options for non-skeletal manifestations of mucopolysaccharidosis type IVA. J Inherit Metab Dis. 2013a;36:309–322. doi: 10.1007/s10545-012-9459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksz CJ, Harmatz P, Beck M, Jones S, Wood T, Lachman R, Gravance CG, Orii T, Tomatsu S. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol Genet Metab. 2013b;110:54–64. doi: 10.1016/j.ymgme.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksz CJ. Transition services for adolescents with lysosomal storage disorders. CML-Lysosomal Storage Diseases. 2013c;11:69–76. [Google Scholar]
- Hendriksz CJ, Burton B, Fleming TR, et al. Efficacy and safety of enzyme replacement therapy with BMN 110 for Morquio A Syndrome (mucopolysaccharidosis IVA): A phase 3 randomised placebo-controlled study. J Inherit Metab Dis. 2014a doi: 10.1007/s10545-014-9715-6. doi: 10.1007/s10545-014-9715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksz CJ, Lavery C, Coker M, KalkanUcar S, Jain M, Bell L, Lampe C. Burden of disease in patients with Morquio A syndrome: Results from an international patient-reported outcomes survey. Orphanet J Rare Dis. 2014b;9:32. doi: 10.1186/1750-1172-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M, Nawa Y, Maumenee IH, Young-Ramsaran J, Matalon R, Green WR. Ocular histopathology and ultrastructure of Morquio syndrome (systemic mucopolysaccharidosis IV A) Graefes Arch Clin Exp Ophthalmol. 1990;228:342–349. doi: 10.1007/BF00920060. [DOI] [PubMed] [Google Scholar]
- James A, Hendriksz CJ, Addison O. The oral health needs of children, adolescents and young adults affected by a mucopolysaccharide disorder. JIMD Rep. 2012;2:51–58. doi: 10.1007/8904_2011_46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käsmann-Kellner B, Weindler J, Pfau B, Ruprecht KW. Ocular changes in mucopolysaccharidosis IV A (Morquio A syndrome) and long-term results of perforating keratoplasty. Ophthalmologica. 1999;213:200–205. doi: 10.1159/000027420. [DOI] [PubMed] [Google Scholar]
- Kinirons MJ, Nelson J. Dental findings in mucopolysaccharidosis type IV A (Morquio's disease type A) Oral Surg Oral Med Oral Pathol. 1990;70:176–179. doi: 10.1016/0030-4220(90)90114-8. [DOI] [PubMed] [Google Scholar]
- Lachman RS, Burton B, Clarke LA, Hoffinger S, Ikegawa S, Jin D-K, Kano H, Kim O-H, Lampe C, Mendelsohn NJ, Shediac R, Tapaiboon P, White KK. Mucopolysaccharidosis IVA (Morquio A syndrome) and VI (Maroteaux–Lamy syndrome): Under-recognized and challenging to diagnose. Skeletal Radiol. 2014;43:359–369. doi: 10.1007/s00256-013-1797-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie T, Siddiqui MAR, Aitken DA, Kirkness CM, Lee WR, Fern AI. Morquio syndrome: Electron microscopic findings. Br J Ophthalmol. 2005;89:925–926. doi: 10.1136/bjo.2004.055400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson SJ. Dysplasia of the odontoid process in Morquio's syndrome causing quadriparesis. J Bone Joint Surg Am. 1977;59:340–344. [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins AM, Dualibi AP, Norato D, Takata ET, Santos ES, Valadares ER, Porta G, de Luca G, Moreira G, Pimentel H, Coelho J, Brum JM, Semionato Filho J, Kerstenetzky MS, Guimarães MR, Rojas MVM, Aranda PC, Pires RF, Faria RGC, Mota RMV, Matte U, Guedes ZC. Guidelines for the management of mucopolysaccharidosis type I. J Pediatr. 2009;155:S32–S46. doi: 10.1016/j.jpeds.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Mendelsohn NJ, Wood T, Olson RA, Temme R, Hale S, Zhang H, Read L, White KK. Spondyloepiphyseal dysplasias and bilateral legg-calve-perthes disease: Diagnostic considerations for mucopolysaccharidoses. JIMD Rep. 2013;11:125–132. doi: 10.1007/8904_2013_231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaño AM, Tomatsu S, Brusius A, Smith M, Orii T. Growth charts for patients affected with Morquio A disease. Am J Med Genet A. 2008;146A:1286–1295. doi: 10.1002/ajmg.a.32281. [DOI] [PubMed] [Google Scholar]
- Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: Clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- Morrone A, Tylee KL, Al-Sayed M, Brusius-Facchin AC, Caciotti A, Church HJ, Coll MJ, Davidson K, Fietz MJ, Gort L, Hegde M, Kubaski F, Lacerda L, Laranjeira F, Leistner-Segal S, Mooney S, Pajares S, Pollard L, Ribeiro I, Wang RY, Miller N. Molecular Testing of 163 Patients with Morquio A (Mucopolysaccharidosis IVA) Identifies 39 Novel GALNS Mutations. Mol Genet Metab. 2014;112:160–170. doi: 10.1016/j.ymgme.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, Vellodi A, Martin R, Ramaswami U, Gucsavas-Calikoglu M, Vijayaraghavan S, Wendt S, Puga AC, Ulbrich B, Shinawi M, Cleary M, Piper D, Conway AM, Kimura A. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum Genet. 1997;101:355–358. doi: 10.1007/s004390050641. [DOI] [PubMed] [Google Scholar]
- Nelson J, Crowhurst J, Carey B, Greed L. Incidence of the mucopolysaccharidoses in Western Australia. Am J Med Genet A. 2003;123A:310–313. doi: 10.1002/ajmg.a.20314. [DOI] [PubMed] [Google Scholar]
- Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL,, Sly WS,, Valle D, editors. The metabolic and molecular bases of inherited disease. 8th edition. New York: McGraw-Hill Medical Publishing; 2001. pp. 3421–3452. [Google Scholar]
- Nicolini F, Corradi D, Bosio S, Gherli T. Aortic valve replacement in a patient with morquio syndrome. Heart Surg Forum. 2008;11:E96–E98. doi: 10.1532/HSF98.20071197. [DOI] [PubMed] [Google Scholar]
- Nursal TZ, Atli M, Kaynaroglu V. Morgagni hernia in a patient with Morquio syndrome. Hernia. 2000;4:37–39. [Google Scholar]
- Olsen H, Baggesen K, Sjolie AK. Cataracts in Morquio syndrome (mucopolysaccharidosis IV A) Ophthalmic Paediatr Genet. 1993;14:87–89. doi: 10.3109/13816819309042908. [DOI] [PubMed] [Google Scholar]
- Pagel PS, Almassi GH. Perioperative implications of Morquio syndrome in a 31-year-old woman undergoing aortic valve replacement. J Cardiothorac Vasc Anesth. 2009;23:855–857. doi: 10.1053/j.jvca.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Polman CH, Rudick RA. The multiple sclerosis functional composite: A clinically meaningful measure of disability. Neurology. 2010;74:S8–S15. doi: 10.1212/WNL.0b013e3181dbb571. [DOI] [PubMed] [Google Scholar]
- Poole JL, Burtner PA, Torres TA, McMullen CK, Markham A, Marcum ML, Anderson JB, Qualls C. Measuring dexterity in children using the Nine-hole Peg Test. J Hand Ther. 2005;18:348–351. doi: 10.1197/j.jht.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Pryce Lewis JR, Gibson PH. Bilateral hip replacement in three patients with lysosomal storage disease: Mucopolysaccharidosis type IV and mucolipidosis type III. J Bone Joint Surg Br. 2010;92:289–292. doi: 10.1302/0301-620X.92B2.23104. [DOI] [PubMed] [Google Scholar]
- Ransford AO, Crockard HA, Stevens JM, Modaghegh S. Occipito-atlanto-axial fusion in Morquio-Brailsford syndrome. A ten-year experience. J Bone Joint Surg Br. 1996;78:307–313. [PubMed] [Google Scholar]
- Rekka P, Rathna PV, Jagadeesh S, Seshadri S. Mucopolysaccharidoses type IV A (Morquio syndrome): A case series of three siblings. J Indian Soc Pedod Prev Dent. 2012;30:66–69. doi: 10.4103/0970-4388.95586. [DOI] [PubMed] [Google Scholar]
- Riedner ED, Levin LS. Hearing patterns in Morquio's syndrome (mucopolysaccharidosis IV) Arch Otolaryngol. 1977;103:518–520. doi: 10.1001/archotol.1977.00780260048003. [DOI] [PubMed] [Google Scholar]
- Rølling I, Clausen N, Nyvad B, Sindet-Pedersen S. Dental findings in three siblings with Morquio's syndrome. Int J Paediatr Dent. 1999;9:219–224. doi: 10.1046/j.1365-263x.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- Shrikrishna D, Coker RK. Air travel working party of the British thoracic society standards of care committee. Managing passengers with stable respiratory disease planning air travel: British thoracic society recommendations. Thorax. 2011;66:831–833. doi: 10.1136/thoraxjnl-2011-200694. [DOI] [PubMed] [Google Scholar]
- Solanki GA, Martin KW, Theroux MC, Lampe C, White KK, Shediac R, Lampe CG, Beck M, Mackenzie WG, Hendriksz CJ, Harmatz PR. Spinal involvement in mucopolysaccharidosis IVA (Morquio-Brailsford or Morquio A syndrome): Presentation, diagnosis, and management. J Inherit Metab Dis. 2013;36:339–355. doi: 10.1007/s10545-013-9586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CG, Ashworth JL. Ocular manifestations as key features for diagnosing mucopolysaccharidoses. Rheumatology (Oxford) 2011;50:v34–v40. doi: 10.1093/rheumatology/ker392. [DOI] [PubMed] [Google Scholar]
- Tassinari E, Boriani L, Traina F, Dallari D, Toni A, Giunti A. Bilateral total hip arthroplasty in Morquio-Brailsford's syndrome: A report of two cases. Chir Organi Mov. 2008;92:123–126. doi: 10.1007/s12306-008-0046-3. [DOI] [PubMed] [Google Scholar]
- Theroux MC, Nerker T, Ditro C, Mackenzie WG. Anesthetic care and perioperative complications of children with Morquio syndrome. Paediatr Anaesth. 2012;22:901–907. doi: 10.1111/j.1460-9592.2012.03904.x. [DOI] [PubMed] [Google Scholar]
- Tylki-Szymańska A, Czartoryska B, Bunge S, van Diggelen OP, Kleijer WJ, Poorthuis BJ, Huijmans JG, Górska D. Clinical, biochemical, and molecular findings in a two-generation Morquio A family. Clin Genet. 1998;53:369–374. doi: 10.1111/j.1399-0004.1998.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Nishioka T, Gutierrez MA, Peña OM, Trandafirescu GG, Lopez P, Yamaguchi S, Noguchi A, Orii T. Mutation and polymorphism spectrum of the GALNS gene in mucopolysaccharidosis IVA (Morquio A) Hum Mutat. 2005;26:500–512. doi: 10.1002/humu.20257. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Ohashi A, Gutierrez MA, Oikawa H, Oguma T, Dung VC, Nishioka T, Orii T, Sly WS. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet. 2008;17:815–824. doi: 10.1093/hmg/ddm353. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Oikawa H, Rowan DJ, Smith M, Barrera L, Chinen Y, Thacker MM, Mackenzie WG, Suzuki Y, Orii T. Mucopolysaccharidosis type IVA (Morquio A disease): Clinical review and current treatment. Curr Pharm Biotechnol. 2011;12:931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- Walker R, Belani KG, Braunlin EA, Bruce IA, Hack H, Harmatz PR, Jones S, Rowe R, Solanki GA, Valdemarsson B. Anaesthesia and airway management in mucopolysaccharidosis. J Inherit Metab Dis. 2013;36:211–219. doi: 10.1007/s10545-012-9563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KK. Orthopaedic surgery for mucopolysaccharidosis. Curr Orthop Pract. 2012;23:394–399. [Google Scholar]
- White KK, Jester A, Bache CE, Harmatz PR, Shediac R, Thacker MM, William G, Mackenzie WG. Orthopedic management of the extremities in patients with Morquio A syndrome. J Child Orthop. 2014;8:295–304. doi: 10.1007/s11832-014-0601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood TC, Harvey K, Beck M, Burin MG, Chien YH, Church HJ, D'Almeida V, van Diggelen OP, Fietz M, Giugliani R, Harmatz P, Hawley SM, Hwu WL, Ketteridge D, Lukacs Z, Miller N, Pasquali M, Schenone A, Thompson JN, Tylee K, Yu C, Hendriksz CJ. Diagnosing mucopolysaccharidosis IVA. J Inherit Metab Dis. 2013;36:293–307. doi: 10.1007/s10545-013-9587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith JE, Clarke LA, Beck M, Kolodny EH, Pastores GM, Muenzer J, Rapoport DM, Berger KI, Swiedler SJ, Kakkis ED, Braakman T, Chadbourne E, Walton-Bowen K, Cox GF. Enzyme replacement therapy for mucopolysaccharidosis I: A randomized, double-blinded, placebo-controlled, multinational study of recombinant human a-L-iduronidase (laronidase) J Pediatr. 2004;144:581–588. doi: 10.1016/j.jpeds.2004.01.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix 1.
Supplementary Appendix 2.


