Abstract
Poaceae plants release 2′-deoxymugineic acid (DMA) and related phytosiderophores to chelate iron (Fe), which often exists as insoluble Fe(III) in the rhizosphere, especially under high pH conditions. Although the molecular mechanisms behind the biosynthesis and secretion of DMA have been studied extensively, little information is known about whether DMA has biological roles other than chelating Fe in vivo. Here, we demonstrate that hydroponic cultures of rice (Oryza sativa) seedlings show almost complete restoration in shoot height and soil-plant analysis development (SPAD) values after treatment with 3–30 μm DMA at high pH (pH 8.0), compared with untreated control seedlings at normal pH (pH 5.8). These changes were accompanied by selective accumulation of Fe over other metals. While this enhanced growth was evident under high pH conditions, DMA application also enhanced seedling growth under normal pH conditions in which Fe was fairly accessible. Microarray and qRT-PCR analyses revealed that exogenous DMA application attenuated the increased expression levels of various genes related to Fe transport and accumulation. Surprisingly, despite the preferential utilization of ammonium over nitrate as a nitrogen source by rice, DMA application also increased nitrate reductase activity and the expression of genes encoding high-affinity nitrate transporters and nitrate reductases, all of which were otherwise considerably lower under high pH conditions. These data suggest that exogenous DMA not only plays an important role in facilitating the uptake of environmental Fe, but also orchestrates Fe and nitrate assimilation for optimal growth under high pH conditions.
Keywords: 2′-deoxymugineic acid, iron, alkali tolerance, Oryza sativa L., plant growth, nitrate transport, nitrate assimilation
Introduction
Iron (Fe) is the fourth most abundant element in soil and is one of the most important microelements for living organisms. In soil with high pH, which accounts for approximately 30% of the cultivable land worldwide (Mori, 1999), the majority of Fe in the soil is in the form of insoluble compounds, making it unavailable for uptake by plants. Under Fe limitation, plants show decreased productivity that is often accompanied by chlorosis (Ma and Nomoto, 1996). Two strategies for improving Fe uptake under Fe-limited conditions have evolved in plants (Curie and Briat, 2003; Conte and Walker, 2011; Kobayashi and Nishizawa, 2012). Strategy I, which functions in most non-graminaceous plants, is dependent on the reduction of Fe(III) to Fe(II), followed by selective uptake of the reduced Fe(II) into the cytoplasm via the Fe transporter IRT1 (Chaney et al., 1972; Römheld and Marschner, 1983; Korshunova et al., 1999; Connolly et al., 2002; Vert et al., 2002). Strategy II functions only in graminaceous species and is characterized by a Fe(III)-chelating mechanism that involves phytosiderophores including 2′-deoxymugineic acid (DMA) and other structurally related mugineic acids (MAs) (Römheld and Marschner, 1986). The Fe–DMA complex is absorbed by, and transported through, specific YELLOW STRIPE 1-LIKE (YSL) transporters localized in the plasma membrane (Curie et al., 2001, 2009; Curie and Briat, 2003; Conte and Walker, 2011; Kobayashi and Nishizawa, 2012).
The molecular basis for the biosynthesis and exudation of MAs has been well characterized. MAs are derived from S-adenosyl-l-methionine (SAM) (Mori and Nishizawa, 1987; Kawai et al., 1988; Shojima et al., 1990; Ma and Nomoto, 1996; Kobayashi et al., 2010). The biosynthesis of MAs is catalysed by nicotianamine synthase (NAS), nicotianamine aminotransferase, deoxymugineic acid synthase, and finally, the dioxygenases IDS2 (for iron deficiency-specific 2) and IDS3, which convert DMA to MA (Nakanishi et al., 1993; Okumura et al., 1994; Higuchi et al., 1999; Takahashi et al., 1999; Bashir et al., 2006). Recently, the DMA efflux transporter of mugineic acid family phytosiderophores (TOM1) has been identified in rice and barley and functionally characterized (Nozoye et al., 2011). Genetically engineered rice lines that overexpress genes related to MA biosynthesis have been shown to have increased tolerance to calcareous soil, highlighting the importance of MAs for absorbing Fe from such soils (Takahashi et al., 2001; Suzuki et al., 2008). Extensive studies on Fe uptake mechanisms that are dependent on phytosiderophores have been performed at the physiological and molecular levels. The uptake and accumulation of Fe are greater in plants grown in medium supplemented with MAs than in plants grown in medium that contains EDTA, as shown by the Fe content in xylem sap (Mino et al., 1983; Takagi et al., 1984; Römheld and Marschner, 1986; Ma et al., 1993; Kawai et al., 2001). These findings and results with transformants harboring MA biosynthesis-related genes (Kobayashi and Nishizawa, 2012) suggest that the increased availability of MAs, as a result of either genetic engineering or exogenous application, induces enhanced Fe uptake under high pH conditions in rice. However, the effects of DMA application on growth and various metabolic processes have not been reported. Therefore, it is still largely unknown whether the exogenous application of MAs would lead to the promotion or restoration of growth under high pH conditions through mechanisms other than the enhancement of Fe uptake. In part, this work could not be undertaken to date because phytosiderophores have not been available at scales large enough for conducting physiological experiments.
To gain further insight into how DMA affects the biology of plant growth at the physiological and molecular levels, we analysed the transcriptome, metal content, and nitrate reductase (NR) activity in rice (Oryza sativa L. cv. Nipponbare) seedlings treated with various concentrations of DMA that was chemically synthesised from tert-butoxycarbonyl-l-allylglycine using a one-pot protocol (Namba et al., 2007). Our results showed that the application of DMA leads to the recovery of rice growth by reinforcing the selective uptake of Fe, triggering prompt translocation of Fe throughout the plant body, and up-regulating NR activity under high pH conditions despite the preference for ammonium over nitrate in rice. DMA application experiments in our study revealed that DMA enhances the nitrate assimilation pathway, thus implicating a role for this compound in promoting plant growth.
Results
DMA application improves rice growth under normal and high pH conditions
To study the physiological effects of exogenous DMA on rice, synthetic DMA was supplied to hydroponic culture media with the sole source of Fe in the form of Fe-DMA complexes, and shoot height and chlorophyll content were evaluated in rice plants. The non-destructive soil-plant analysis development (SPAD) value was used to measure chlorophyll content. Under normal pH conditions (pH 5.8), the addition of 3 μm DMA to the growth medium slightly increased shoot height by 18 ± 3% and SPAD value by 25 ± 3% compared with corresponding values in the no-DMA control (FeCl3 without chelator) (Figure 1). The addition of DMA at concentrations higher than 30 μm did not result in significant increases in shoot height or SPAD value above those induced by 3 μm DMA. Conversely, under high pH conditions (pH 8.0), DMA affected plant growth more evidently. At pH 8.0, compared with no-DMA control, seedlings treated with 3 μm DMA showed a 1.3-fold increase in shoot height and a 1.9-fold increase in SPAD value, while those treated with 30 μm DMA showed a 1.8-fold increase in shoot height and a 4.4-fold increase in SPAD value. The seedlings treated with 30 μm DMA showed almost complete recovery from the decreased shoot height and SPAD value caused by high pH (Figure 1). The growth improvement of the rice seedlings in response to DMA treatment was similar to that observed for the addition of Fe-EDTA in the medium as the sole Fe source (EDTA treatment). At concentrations of 30 μm or higher, EDTA significantly increased the shoot height and SPAD value of rice seedlings compared with controls (Figure S1). Taken together, these results show that supplementation with 3 μm DMA at pH 5.8 or 30 μm at pH 8.0 improves the growth of rice seedlings.
Figure 1.
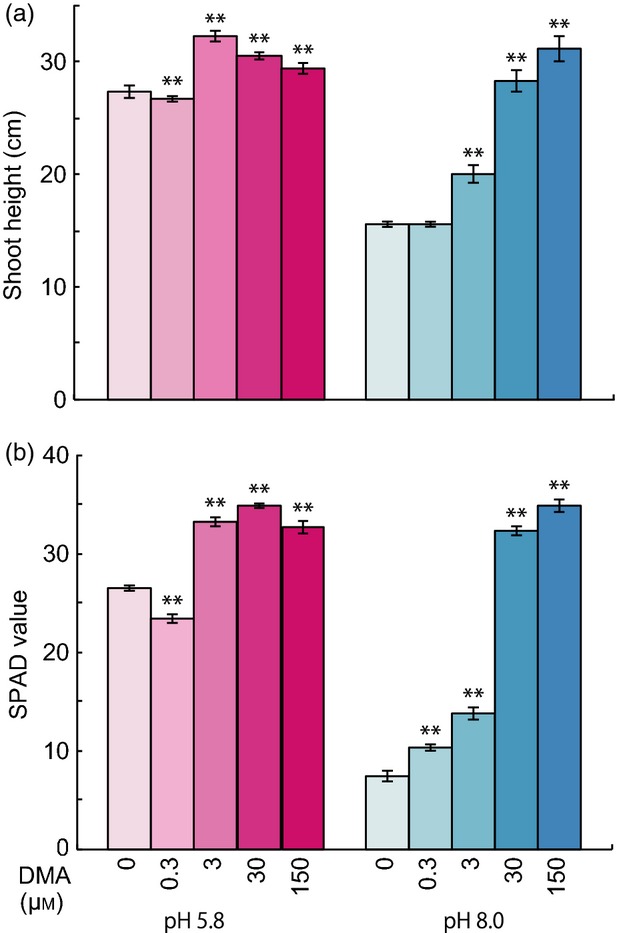
Effects of DMA application and pH on rice seedling growth. Seedlings were germinated in deionized water for 1 week, and grown in nutrient solution containing 0, 0.3, 3, 30, or 150 μm DMA at either pH 5.8 or 8.0 for 2 weeks. All treatments contained equal amounts of Fe.
(a) Shoot height.
(b) Soil-plant analysis development (SPAD) value.
Asterisks indicate significant difference from control (0 μm DMA) at pH 5.8 or 8.0 (**P < 0.01; Student's t-test). Values shown are mean ± standard deviation (SD) (n = 5).
DMA application selectively restores Fe content in Fe-deficient rice seedlings
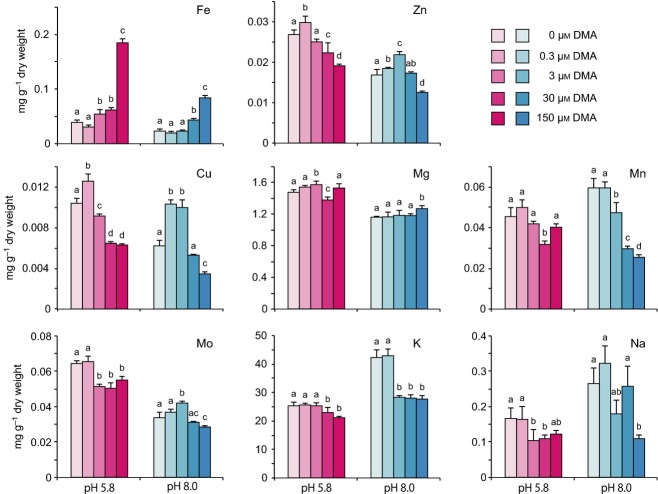
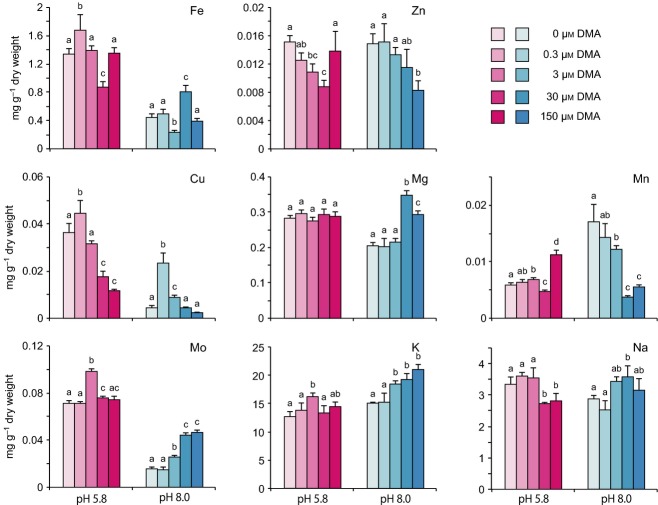
To analyse in detail the physiological changes in rice seedlings during the growth improvement induced by DMA, we measured the concentrations of various metals in shoot and root tissues of DMA-treated seedlings (Figures 2 and 3). In shoot tissues, Fe was basically the only metal to show significant improvement in response to DMA treatment. Fe content increased in a manner dependent on the dose of DMA. The increase in Fe content became significantly higher than that of the respective control at a minimum of 3 μm DMA at pH 5.8 and at 30 μm DMA at pH 8.0 (Figure 2). At pH 5.8, the Fe content in rice seedlings treated with 150 μm DMA was 3.6-fold higher than in the control, indicating that rice tissues are able to accommodate higher levels of Fe with supplementation of DMA. However, increasing amounts of DMA resulted in decreases in the Zn, Cu, Mo, and Na content in shoots of rice seedlings. In particular, the Zn content was markedly decreased by DMA at concentrations higher than 30 μm and the Cu content at concentrations higher than 3 μm, although the seedlings showed slight increases in the levels of these metals in response to 0.3 μm DMA. The Mg, Mn, and K content in rice tissues did not show any clear trend in response to increasing DMA concentration, suggesting that DMA is not directly related to the uptake and/or translocation of these metals in rice seedlings. The Fe content in seedlings grown at pH 8.0 was approximately half of that in seedlings grown at pH 5.8 after treatment at respective concentration of DMA. At both pH 8.0 and pH 5.8, the increase in Fe content in seedlings was proportional to the increase in DMA concentration. Seedlings treated with 150 μm DMA showed 4.9-fold higher Fe content than seedlings in the no-DMA control. The levels of other metals in seedlings were generally consistent between pH 8.0 and pH 5.8. In root tissues, the Fe did not show a clear relationship with DMA treatment level at either pH 5.8 or pH 8.0 (Figure 3). Similarly, the accumulation patterns of other metals (Zn, Mg, K, and Na) did not differ between pH 5.8 and pH 8.0 or different DMA concentrations. However, at pH 8.0, the levels of Cu and Mn markedly decreased as the DMA concentration increased, which is in sharp contrast to patterns observed for other metals. In contrast, Mo accumulation gradually increased in proportion to the amount of DMA added. The levels of Fe, Cu, and Na were approximately 10 times higher in roots than in shoots. The levels of Zn, Mg, and Mn were lower in roots than in shoots. These results reflect the selectivity and complexity of the uptake and transport rates of various metals from the root to the shoot. Together, these results show that exogenous DMA significantly affects the assimilation and translocation of various metals, especially Fe, in rice seedlings.
Figure 2.
Effect of DMA application on concentrations of various metals in rice shoot tissues. Metal content was analysed in rice seedlings grown under various concentrations of DMA at pH 5.8 or 8.0 for 2 weeks. Three seedlings were analysed for each treatment. All treatments contained equal amounts of Fe. Statistical analysis was conducted using Holm's pairwise t-test or Tukey's honest significant difference (HSD) depending on the P-value obtained in the Bartlett test in R program. a,b,cDifferent letters in the same group of bars indicate significant difference (P < 0.05). Values shown are mean ± standard deviation (SD) (n = 5).
Figure 3.
Concentrations of various metals in rice root tissues. Experiment was conducted as described in the legend to Figure 2. a,b,cDifferent letters in the same group of bars indicate significant difference (P < 0.05). Values shown are mean ± standard deviation (SD) (n = 5).
DMA facilitates uptake and mobilization of Fe to the aerial part of rice seedlings
Once assimilated from the rhizosphere, micronutrients must be transported from roots to the above-ground parts of the plant. To investigate the effects of exogenous DMA on Fe uptake and distribution from the root to the shoot, we analysed the transport rate of 55Fe added to the hydroponic solution (Figures 4 and S2). After 4 h of treatment, the amount of 55Fe transported to the aerial part of the seedlings was much higher in DMA-treated seedlings than in EDTA-treated or control seedlings under both pH conditions (Figure 4a,b). In DMA-treated seedlings, radioactivity from 55Fe was detected in the entire leaf blade, including leaf tips. Quantification of the absorbed Fe by phosphorimaging confirmed that DMA-treated seedlings showed much higher rates of Fe uptake and translocation than EDTA-treated or control seedlings under both pH conditions (Figure 4e,f). In clear contrast, EDTA-treated seedlings did not show any significant improvement in Fe absorption by shoot tissues compared with the control, regardless of the pH. This trend continued until 22 h after treatment (Figures 4 and S2).
Figure 4.
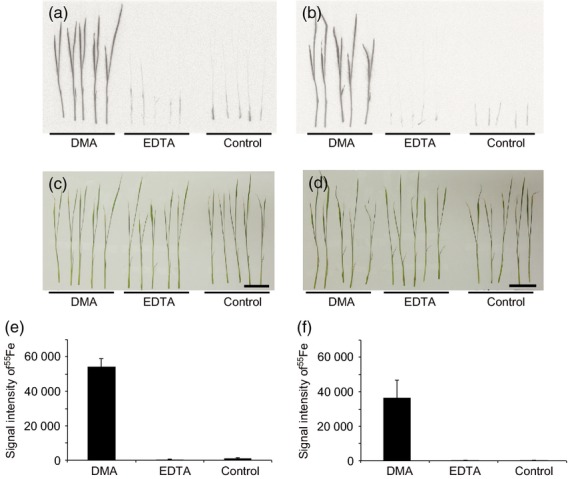
Uptake and distribution of 55Fe in rice seedlings.
Two-week-rice seedlings were treated either with 5μM of 55Fe-DMA or 55Fe-EDTA for 4 h and were subjected to autoradiography. (a, b) Autoradiographs of rice seedlings treated with 55Fe-DMA or 55Fe-EDTA at pH 5.8 (a) and 8.0 (b).
(c, d) Photographs of rice seedlings used for autoradiographic analysis shown in panels (a) and (b), respectively. Scale bars = 4 cm.
(e, f) Quantification of 55Fe signal intensity in rice shoots using the BAS-5000 system at pH 5.8 (e) and pH 8.0 (f). Control, no chelator added. All treatments contained equal amounts of Fe. Values shown are means ± standard deviation (SD) (n = 5).
Microarray analysis reveals a link between DMA-mediated Fe uptake and nitrate assimilation
To evaluate and compare the effects of DMA and EDTA treatments on the transcriptome of rice seedlings under different pH conditions, we conducted a microarray analysis and dissected the gene regulatory network of rice seedlings (GSE56455; Tables S1–S10 and Appendix S1). There were considerable differences in the transcript levels of genes related to Fe homeostasis between DMA-treated and untreated seedlings (Figure S3). In shoot tissues, the transcript levels of IRON-REGULATED TRANSCRIPTION FACTOR 2 (IRO2), IRO3, NAS1, NAS2, TOM1, YSL15, and NATURAL-RESISTANCE-ASSOCIATED MACROPHAGE PROTEIN 1 (Nramp1) were considerably decreased by DMA or EDTA treatment. The fold decrease in the transcript levels of these genes by chelator treatments was larger at pH 5.8 than at pH 8.0. Likewise, the transcription of these genes in root tissues was down-regulated by chelator treatments, although the fold decreases were smaller than those observed in the shoot tissues. These genes are known to be induced under Fe deficiency; therefore, these data suggest that both DMA and EDTA treatments increase the in vivo availability of Fe in rice seedlings. Among the down-regulated genes, IRO2 and Nramp1 were more severely down-regulated by DMA than by EDTA. These results clearly demonstrate that DMA treatment has biological effects on Fe-depleted rice seedlings in a mode distinct from those of EDTA treatment. The transcription of HIGH-AFFINITY NITRATE TRANSPORTER genes (NRT2.1 and NRT2.2), which are induced by nitrate and sucrose (Araki and Hasegawa, 2006; Cai et al., 2008; Feng et al., 2011), was increased approximately 80-fold and 100-fold under normal and high pH conditions, respectively, in response to chelator treatments, but only in root tissues (Tables S3(b) and S7(f) and Figures S4 and S5). This result highlights the clear contrast between shoots and roots in their transcriptional responses to DMA or EDTA treatment.
Expression profiles of Fe and nitrate assimilation genes in response to DMA and EDTA treatments under various pH conditions
To further characterize the physiological effects of DMA or EDTA treatments on rice seedlings, we further analysed the transcriptional patterns of genes involved in Fe homeostasis and nitrate assimilation by qRT-PCR. We analysed the transcript levels of selected genes induced by Fe deficiency (IRT1, OsYSL15, NAS1, and NAS2) and nitrate (NRT2.1, NRT2.2, NRT2.3, NRT2.4, NAR2.1, and the NITRATE REDUCTASE genes NIA1 and NIA2) (Figures 5 and S6). These results were consistent with those obtained in the microarray analysis, in that treatment with DMA or EDTA considerably reduced the transcript levels of genes induced by Fe deficiency and nitrate in shoots in both normal and high pH conditions (Figures 5a and S6). There were no apparent differences in the transcriptional patterns of Fe homeostasis-related genes between the DMA-treated and EDTA-treated seedlings. In root tissues, the transcript level of YSL15 was two-fold higher at pH 8.0 than at pH 5.8 (Figure 5b).
Figure 5.
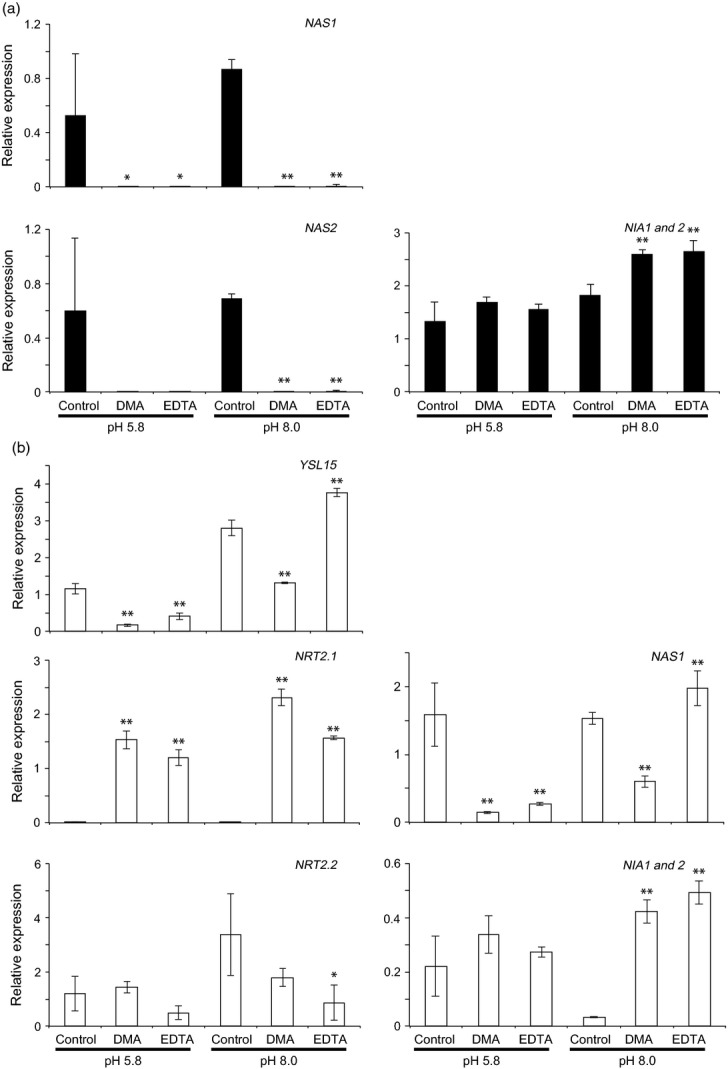
Gene expression analysis of DMA-treated rice seedlings. Seedlings treated with 30 μm DMA, 30 μm EDTA, or no chelator (control), at pH 5.8 and 8.0, were analysed. Transcript levels of various genes in shoots and roots are shown in panel (a) (black bars) and (b) (white bars), respectively. Transcript levels of selected genes related to absorption/assimilation of nitrate and Fe were quantified by qRT-PCR. Primers used to amplify NIA1 may also have amplified NIA2 transcripts because of the high sequence similarity between these genes. Expression profiles of other genes are shown in Figure S6. Asterisks indicate significance difference from transcript level of each gene in control (0 μm DMA) at each pH (*P < 0.05; **P < 0.01; Student's t-test). Values shown are mean ± standard deviation (SD) (n = 3).
In root tissues, the basal level of YSL15 transcripts was seven-fold lower in seedlings treated with DMA and three-fold lower in seedlings treated with EDTA than that in the no-chelator control at pH 5.8. Conversely, at pH 8.0, the transcript level of YSL15 in roots of DMA-treated seedlings was two-fold lower, and in EDTA-treated seedlings it was slightly higher than in roots of control seedlings. These results suggested that, at pH 5.8, the addition of DMA or EDTA in the presence of Fe in a soluble and readily available form results in excess Fe accumulation in plant tissues. The increased accumulation of Fe may have decreased transcription of YSL15 in root tissues. At pH 8.0, however, only DMA, not EDTA, significantly decreased YSL15 transcript levels. Similar transcriptional patterns were observed for NAS1 and NAS2 (Figures 5b and S6). For genes related to nitrate assimilation, both DMA and EDTA drastically induced NRT2.1 to comparable levels under both pH conditions. Similar transcriptional patterns were observed for NAR2.1, NRT2.4 and NIA1/2. Conversely, the transcript level of NRT2.2 was decreased by DMA and by EDTA, while the level of NRT2.3 showed no clear trend.
DMA and EDTA application up-regulates nitrate reductase activity in shoots
To evaluate whether the increase in the transcription of NIAs by DMA or EDTA was linked to enhanced nitrate assimilation, we analysed the enzymatic activity of two classes of NRs: the NADH-dependent NR (NADH-NR) and the NADPH-dependent NR (NADPH-NR). The activities of these enzymes were determined in shoots of rice seedlings treated with various concentrations of DMA or EDTA at pH 5.8 and 8.0. As the transcript levels of NIAs increased, NADH-NR activity was significantly increased by 1.3- and 2-fold in DMA-treated seedlings at pH 5.8 and 8.0, respectively, compared with the respective controls (Figure 6). Likewise, DMA treatment increased NADPH-NR activity by 1.3- and 1.1-fold at pH 5.8 and 8.0, respectively, compared with the respective controls. EDTA application, on the other hand, increased the activity of NADH-NR by 1.5- and 2.8-fold at pH 5.8 and 8.0, respectively, compared with the control, whereas NADPH-NR activity in shoots was increased by 2.4-fold at pH 8.0, while no significant change was observed in NADPH-NR activity in shoots at pH 5.8. In contrast, in root tissues, DMA treatment increased NADH-NR activity by at least 2.5-fold at pH 8.0 but had no significant effect on NADPH-NR activity at pH 5.8 (Figure S7). The activity of NADPH-NR increased approximately 7-fold in response to DMA or EDTA treatment at pH 5.8 but was not significantly affected by either treatment at pH 8.0.
Figure 6.
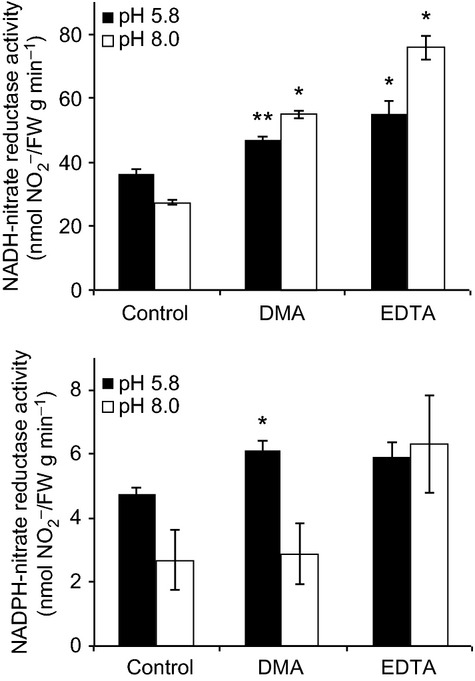
Enzyme activity of NADH- and NADPH-dependent nitrate reductases. Enzyme activities were determined in shoots of seedlings in different treatments. DMA or EDTA was added to medium to a final concentration of 30 μm, no chelators were added to control. Assays were performed separately to measure NADH- and NADPH-dependent NR activities. Asterisks indicate significant difference to control (no DMA) under each pH (*P < 0.05; **P < 0.01; Student's t-test). Values shown are mean ± standard deviation (SD) (n = 3).
Discussion
Exogenous DMA application promotes the growth of rice seedlings under normal and high pH conditions
High pH conditions in soil have a significantly negative effect on plant growth, which is triggered in part by changes in the concentrations of Fe and other micronutrients that are available to plants (Treeby et al., 1989). Here, we showed that exogenous application of a minimum of 30 μm DMA confers tolerance to Fe deficiency at pH 8.0, as highlighted by the restoration of shoot height and SPAD value as well as the Fe content of DMA-treated rice seedlings compared with those of the no-DMA control at pH 5.8 (Figures 1, 2 and 3). Thus, exogenous application of DMA is sufficient to induce not only enhanced Fe uptake but also the recovery of growth of rice seedlings under high pH conditions. This finding corroborates those of previous studies in which transgenic rice plants that overexpress genes involved in MA biosynthesis showed enhanced viability in calcareous conditions (Takahashi et al., 2001; Suzuki et al., 2008). Furthermore, the concentration of DMA (30 μm) used here is equivalent to the estimated concentration of DMA within 1 mm of the root surface (Shi et al., 1988; Kawai et al., 2001). Our data therefore clearly indicate that exogenous application of 30 μm DMA, which is at a physiological level, is sufficient to promote the growth of rice seedlings under normal and high pH conditions.
The data also show that DMA application positively affects rice seedling growth not only at high pH (pH 8.0), but also at normal pH (pH 5.8). Application of 3 μm DMA at pH 5.8 resulted in an 18 ± 3% increase in shoot height and a 25 ± 3% in SPAD value, although application of 30 and 150 μm DMA did not further improve growth (Figure 1). These results indicate that supplying at least 3 μm DMA to the growth medium of hydroponic rice seedlings at pH 5.8 was sufficient to promote growth. Our data also suggest that assimilation of Fe is a rate-limiting factor in the growth of rice seedlings, even under normal pH conditions. Therefore, DMA not only specifically complements Fe deficiency, allowing rice seedlings to grow normally under Fe deficiency induced by high pH, but might also function as a rate-limiting factor for achieving maximum growth under normal pH conditions. Additionally, as exogenous application of DMA exhibited no apparent negative impact on the growth of rice seedlings (Figure 1), the use of chemically synthesised DMA as a ‘natural’ chelator thus becomes quite feasible in promoting Fe uptake and growth in graminaceous plants under calcareous conditions.
Alternative approaches to conferring tolerance to alkali stress in plants by overexpression of MA biosynthetic genes have been reported (Takahashi et al., 2001; Suzuki et al., 2008). These results clearly indicate that the overexpression of MA biosynthetic genes contributes to tolerance to alkali stress. However, as these reports showed the effect of overexpression of transgenes only as growth promoted relative to non-transgenic lines grown under calcareous soil conditions and not to non-transgenic lines under normal conditions, it is unclear whether the ‘recovered’ growth of the transgenic plants is better than that of the respective wild-type plants under normal conditions. In contrast, we found that exogenous application of DMA fully restored the shoot height and SPAD values of rice seedlings under high pH conditions to those of rice seedlings under normal growth conditions (Figures 1 and 2).
Conversely, SAM, which is a substrate for MA biosynthesis, is synthesised from methionine and ATP by SAM synthetase and is used for the synthesis of divergent metabolites (Roje, 2006). Therefore, adequate ectopic expression of one or more MA synthesis genes is important for the reduction of total energy consumption to maintain plant productivity. Recently, modulating polyamine metabolism by overexpression of SAM synthetase demonstrated alkali stress tolerance in tomato (Gong et al., 2014), suggesting that SAM-dependent metabolic pathways other than MA biosynthesis are partly involved in alkali stress tolerance. Alternatively, the repression of those SAM-dependent pathway genes may be triggered by the overexpression of MA synthesis-related genes. Therefore, our quantitative data provide the molecular basis for understanding the level of MA that is required for transgenic plants to become tolerant to alkali stress with the minimum energy consumption.
Fe-sensing mechanism distinguishes Fe-DMA and Fe-EDTA to maintain Fe homeostasis in rice seedlings
The application of either DMA or EDTA resulted in increased shoot height and SPAD value, higher NADH-NR and NADPH-NR activities, and increased expression of various other genes related to nitrate homeostasis in shoots compared with those of the no-chelator control at normal pH (pH 5.8) as well as high pH (pH 8.0) (Figures 1, 5 and 6). In contrast, the decrease in transcript levels of Fe-related genes including YSL15 and NAS1 were more significant in response to DMA treatment than to EDTA treatment at pH 8.0 (Figure 5). These results suggest that rice seedlings distinguish differences between Fe-DMA and Fe-EDTA and therefore have a mechanism to sense the environmental Fe status and coordinate the expression of genes based on that status.
The Fe-sensing mechanisms of plants, however, have not been fully understood. Recently, the two proteins HRZ1 and HRZ2 have been shown to bind to Fe and negatively regulate Fe deficiency response genes in rice shoots (Kobayashi et al., 2013). However, it is still unknown which form of Fe is captured by HRZ1 and HRZ2. Conversely, plants might also monitor the concentration of Fe in phloem sap. Arabidopsis knockout mutant opt3 constitutively exhibits systemic Fe deficiency and expresses IRT1 and FRO2 even under Fe sufficient conditions (Zhai et al., 2014). As OPT3 is localized to phloem cells, OPT3 might be responsible for monitoring the concentration of Fe in phloem in planta. In addition, YSL15 and 18 in rice play an important role in transporting DMA-Fe into the phloem (Aoyama et al., 2009; Inoue et al., 2009), suggesting that Fe chelated by DMA could be directly transported into phloem without ligand exchange. Besides, the uptake efficiency of MA-Fe was shown to be 100- to 1000-fold higher than that of EDTA-Fe (Römheld and Marschner, 1986; Ma et al., 1993). These reports imply that the application of DMA-Fe, compared with EDTA-Fe, contributes considerably to the increase in Fe that is transported to the phloem. Interestingly, the transcript levels of IRO2, which regulates YSL15 and NAS1 expression (Ogo et al., 2007) and thus is thought to be a regulator for the phytosiderophore-Fe uptake system in rice (Walker and Connolly, 2008), were more markedly decreased by DMA than by EDTA treatment (Figure S3). Moreover, when the same concentration of DMA or EDTA was applied, the level of SPAD values was higher in DMA-treated compared with EDTA-treated seedlings (Figures 1 and S1). These data strongly support the hypothesis that the differences in the responses to DMA-Fe and EDTA-Fe in the expression of IRO2, YSL15 and NAS1 essentially arise from the differences in the form of Fe-complex, and that there is a Fe-sensing mechanism during the processes from the uptake of Fe from the rhizosphere to the transport of Fe to the phloem.
Conversely, as both DMA and EDTA equally contributed to the increase in shoot height and NR activities (Figures 1 and 6), the Fe-sensing mechanism linked to those two growth parameters is likely to sense both Fe-DMA and Fe-EDTA equivalently. Alternatively, neither Fe-DMA nor Fe-EDTA is likely to be the major form of Fe that is sensed for those phenotypes to be expressed by rice seedlings.
Various compounds such as citrate, nicotianamine, and DMA function as chelators that mobilize Fe within and outside tissues (Inoue et al., 2003; Ishimaru et al., 2006; Yokosho et al., 2009). Moreover, the Fe transport system involves multiple transporters specific to various Fe complexes that are localized in particular tissues and cells (Curie et al., 2009; Palmer and Guerinot, 2009). In our study, we observed a remarkable example of differences in gene expression profiles between DMA- and EDTA-treated rice seedlings in the expression of genes related to Fe homeostasis; DMA treatment significantly reduced the expression of YSL15 and NAS1 in roots compared with EDTA treatment both under normal pH (pH 5.8) and high pH (pH 8.0) (Figure 5). As the chelator-mediated Fe transport system involves multiple exchanges of various ligands with the help of specific reductases and pH gradients, it is plausible that the differential expression of genes related to Fe homeostasis between DMA- and EDTA-treated rice seedlings reflects the differences in the forms of Fe that are recognised by the mechanisms that regulate Fe homeostasis.
Exogenous DMA enhances Fe mobility within rice seedlings
While rice has Strategy II system for the assimilation of Fe(III), rice is also capable of utilising Fe(II) when fed as a sole Fe source (Ishimaru et al., 2006). However, in shoots of rice seedlings, radioactivity from 55Fe that was incorporated within 22 h was significantly higher in plants fed with Fe-DMA than in those fed with Fe-EDTA (Figures 4 and S1), although the shoot height and micronutrient contents were similar in the two treatments after 2 weeks of cultivation. Nevertheless, the role of ferric chelate reductase activity, a rate-limiting factor in Fe assimilation by Strategy I (Römheld and Marschner, 1983), might not be as significant as in paddy fields, where Fe exists essentially in the Fe(II) form as a result of the low redox potential. Most modern and ancestral japonica rice varieties have been adapted to growth in paddies, implying that stronger ferric chelate reductase activity has not been strictly required during japonica rice evolution, which might partly explain why ferric reductase activity is lower in rice than in other Strategy I plants grown under non-paddy conditions (Ishimaru et al., 2006, 2007). In fact, transgenic rice plants overexpressing Arabidopsis or yeast ferric chelate reductase genes show greater Fe uptake and translocation rates to aerial plant parts than control plants (Vasconcelos et al., 2004; Ishimaru et al., 2007). Therefore, under our experimental conditions, the differences in the rates of 55Fe uptake and transport between Fe-DMA and Fe-EDTA might be primarily due to the formation of a Fe3+ complex with DMA that is readily assimilated via YSL transporters such as YSL15 and/or YSL18 but not via ferric chelate reductases (Aoyama et al., 2009; Inoue et al., 2009; Lee et al., 2009). In contrast, Fe-EDTA must be reduced first, possibly by FRO2, before being incorporated to cells via IRT proteins (Römheld and Marschner, 1983), resulting in lower rates of 55Fe transport to the aerial parts within 22 h (Figure 5).
Integral link between Fe and nitrate assimilation processes
Transcriptome analysis showed that the positive effect of DMA application on rice seedling growth was associated with changes in the transcript levels of a variety of genes. As expected, genes involved in Fe assimilation were down-regulated by DMA treatment (Figures 5 and S3–S5), suggesting that DMA treatment triggered a decrease in the Fe assimilation rate to maintain Fe homeostasis. However, at the same time, DMA treatment increased the transcript levels of a range of nitrate assimilation-related genes such as NRT2.1, NRT2.3, NRT2.4, NAR2.1, and NIA1/2, both under normal and high pH conditions. A similar trend in gene expression was observed when seedlings were treated with EDTA; the expression of NRT2 was up-regulated (Figures 5 and S6) and was associated with increased NR activity (Figure 6).
Fe does not seem to have been discussed extensively as a potential inducer of the nitrate assimilation pathway. Most nitrate assimilation-related genes are induced by nitrate, which is known as the primary nitrate response (Krouk et al., 2010). It is known that the expression of NRT2 family genes in rice and Arabidopsis is inducible by nitrate (Cheng et al., 1986; Forde, 2000; Orsel et al., 2002; Okamoto et al., 2003; Araki and Hasegawa, 2006; Miller et al., 2007; Cai et al., 2008; Feng et al., 2011) and sucrose (Lejay et al., 1999; Ono et al., 2000; Krouk et al., 2010; Feng et al., 2011; Yan et al., 2011). Furthermore, NR gene expression and NR activity are regulated by diurnal rhythm, as well as the expression of OsNRT2s (Scheible et al., 1997, 2000; Lillo and Appenroth, 2001; Feng et al., 2011). In our study, the expression of nitrate-inducible genes and the activity of NR were significantly induced by the application of DMA and EDTA in the absence of nitrogen starvation, suggesting that these enhancements were not caused by the primary nitrate response. One possible explanation for the induction of the nitrate assimilation pathway is that a peak in OsNRT2s expression observed 4 h after the onset of the light period under nitrate-sufficient conditions (Feng et al., 2011) is induced by sucrose generated by photosynthesis. This peak coincides with the timing of secretion of phytosiderophores (Takagi et al., 1984; Römheld and Marschner, 1986; Nozoye et al., 2004, 2011). The enhancement of the nitrate assimilation pathway under our experimental conditions coincided with the enhancement of the SPAD values (Figures 1, 5 and 6). These findings strongly suggest that the activity of the nitrate-assimilating machinery can be up-regulated as the concentration of available Fe increases, regardless of pH.
Changes in gene expression in Arabidopsis induced by nitrate affect not only N metabolism, but also C metabolism (Wang et al., 2003, 2004). Nitrate induction has positive effects on plant growth because negative feedback on nitrogen assimilation depends on the C to N ratio (Nunes-Nesi et al., 2010; Xu et al., 2012). In our study, nitrate and ammonium assimilation in DMA-treated rice plants must have cooperated at appropriate rates for optimal growth. In fact, when an Fe chelator, either DMA or EDTA, was present in the medium, the rice seedlings showed healthier growth and higher SPAD values than the no-chelator control (Figure 1), indicating that metabolic processes, including photosynthesis, were operating at sufficient levels to supply enough C metabolites. This is consistent with the pattern of NRT2.1 expression described by Feng et al. (2011); that is, NRT2.1 transcription was repressed by ammonium but enhanced by sucrose. While increased NR activity should contribute to nitrate assimilation, it may also increase ammonium accumulation. Our microarray analysis, however, revealed that DMA treatment did not cause significant changes in the transcript levels of glutamine synthase 1;1 (GS1;1), which encodes an enzyme that plays an important role in ammonium assimilation following nitrate uptake in rice (Kusano et al., 2011). Likewise, a gene encoding NADH-GOGAT1, which is induced by exogenous ammonium treatment (Yamaya et al., 1992; Hirose et al., 1997; Tamura et al., 2010), was only slightly induced by DMA and EDTA at pH 5.8 (Figure S3). These data collectively suggest that the uptake and assimilation of nitrate, but not ammonium, are positively affected by treatment with either DMA or EDTA, so that seedlings are capable of assimilating Fe and nitrate without a negative impact on growth. Interestingly, rice grows better when both nitrate and ammonium are supplied simultaneously (Ta and Ohira, 1981; Kronzucker et al., 1999), although it is adapted to paddies where ammonium is the major source of nitrogen. Therefore, nitrate, which is a potent signal for not only N but also C metabolism (Wang et al., 2004), might also play a role in the promotion of growth under high or normal pH conditions when seedlings are supplied with exogenous Fe chelators. Conversely, transcriptome analyses have revealed that NAS transcript levels are enhanced by nitrate, suggesting that nitrate induces Fe assimilation by activating the synthesis of nicotianamine, which is involved in Fe uptake and transport to maintain Fe homeostasis in plants (Wang et al., 2000, 2003). Enhanced NAS transcription is most likely due to the fact that Fe is an essential cofactor for many enzymes related to nitrate assimilation, including NR (Campbell, 1999), nitrite reductase (Murphy et al., 1974; Swamy et al., 2005), and ferredoxin (Hall et al., 1973; Noodleman et al., 1985). It is noteworthy that nitric oxide (NO) has been implicated not only as a signalling molecule that mediates various biological processes, but a scavenger of deleterious reactive oxygen species (ROS) (Crawford, 2006; Wilson et al., 2008). Since Fe(II) has been shown as a source of ROS (Curie et al., 2009), and the reduction of nitrite by NR has been known as one of the major routes to generate NO (Crawford, 2006; Wilson et al., 2008), there is a possibility that NR plays an indispensable role in detoxifying ROS inevitably generated by Fe that is being transported to the plant tissues. The up-regulation of NR activity by treatment either with DMA or EDTA (Figure 6), as well as previous reports that NO also acts as a potent inducer for Arabidopsis ferritin (Murgia et al., 2002; Giehl et al., 2009) might explain why NR activity needs to be activated upon Fe uptake, and corroborate the previous notion that there might be a functional link between NR activity and Fe uptake in plants and algae (Cohen et al., 2006).
In conclusion, through supplementation with different concentrations of chemically synthesised DMA, we observed a dose-dependent restoration of the growth in hydroponic rice seedlings at high pH based on shoot height and SPAD value after 2 weeks of cultivation. Further analysis revealed that the addition of DMA, but not EDTA, down-regulates the expression of Fe assimilation-related genes YSL15 and nicotianamine synthase, which would otherwise be up-regulated under high pH conditions. Moreover, supplementation of rice seedlings with either DMA or EDTA up-regulated the expression of NR genes and nitrate transporter NRT2.1 regardless of pH. These data demonstrate that DMA links the processes of Fe and nitrate uptake to cope with high pH conditions. In addition, our data indicate that Fe and nitrate assimilation processes require distinct forms of Fe as signals; DMA-Fe for Fe homeostasis and either DMA-Fe or EDTA-Fe for nitrate homeostasis. This suggests that DMA-Fe is not a form of Fe that is recognised by the machinery for nitrate assimilation. The observation that the addition of DMA up-regulates nitrate assimilation-related genes, even at normal pH, strongly suggests that Fe assimilation is a rate-limiting step for achieving the full potential of plant growth. The results, thus, provide strong evidence for the significance of DMA as a Fe chelator as well as an inducer of nitrate assimilation at high pH and further strengthen the feasibility of the practical use of DMA in agriculture.
Experimental procedures
Growth and experimental conditions
Rice (O. sativa L. cv. Nipponbare) seeds were surface sterilised using 10% H2O2 solution, then immersed in distilled water overnight at 28°C. Imbibed seeds were placed in 96-well plates and cultivated under a 16-h light/8-h dark photoperiod. Rice seedlings were grown in distilled water for 1 week, then transferred to treatment solutions containing various concentrations of DMA (0, 0.3, 3, 30, or 150 μm) at either pH 5.8 or pH 8.0 and grown for up to 2 weeks. EDTA instead of DMA was used as a reference chelator of Fe at the designated concentration in each experiment. Seedlings were analysed for growth, gene transcription, and NR activity. SPAD values were measured using a SPAD-502 chlorophyll meter (Konica Minolta, http://www.konicaminolta.com). DMA was synthesised as described previously (Namba et al., 2007). Standard growth medium was prepared as described previously (Yokosho et al., 2009), except with FeCl3 as the Fe source. Phosphate buffer prepared with 0.5 m K2HPO4 and 0.5 m K2HPO4 was used to adjust the pH to 5.8 or 8.0. All treatments contained equal amounts of Fe (30 μm) and were conducted in the same growth chamber (Panasonic MLR-351H, http://panasonic.net/business/) under a 16-h light/8-h dark photoperiod at 28°C with a light intensity of 5700 lux. Nutrient media in all treatments was completely replaced every 2 or 3 days to maintain consistent experimental conditions. The purity of synthetic DMA was certified by 1H NMR spectrum in which any other peaks of by-products and impurities were not observed. In addition, the purity was further confirmed by HPLC analysis (210 nm) before use (Namba et al., 2007).
Measurement of metal concentrations
Seedlings cultivated for 2 weeks in the treatment medium after a 1-week pre-cultivation were harvested to measure metals accumulated in root and shoot tissues. Metals were removed from root surfaces by washing with 10 mm EDTA containing 5 mm CaSO4 for 5 min before sampling (Vert et al., 2002). To avoid contamination from the culture medium, leaf blades were wiped with wet paper before separating roots and shoots. Samples were dried at 90°C for a minimum of 2 days before weighing. Samples were digested in 2 ml of 11 m HNO3 at 110°C overnight, and further digested by the addition of a mixture of 1.8 ml of 11 m HNO3 and 200 μl of 90% HClO4 with heating at 110°C for 2 days, followed by the drying process at 120°C for overnight. The residue was dissolved in 3 ml of 1% HNO3 900 μl of the sample was diluted 10 times by adding 8.1 ml of deionized water and filtrated with 0.45-μm filter before metal content was measured by inductively coupled plasma atomic emission spectrometry (ICPE-9000, Shimadzu; http://www.shimadzu.com/an/index.html).
Analysis of 55Fe distribution in rice seedlings
Seedlings were pre-cultivated in distilled water for 1 week, and then transferred to a medium containing 30 μM FeCl3 with no chelator, and further cultivated for 1 week. The seedlings were then treated with 55Fe-labelled Fe(III) either with DMA, EDTA, or no chelator for 4, and 22 h, and then washed twice for 5 sec each with 100 ml 10 mm EDTA. The 55Fe-labelled Fe(III)–DMA was prepared as previously described (Araki et al., 2011). We added 100 μl of 1 mm 55Fe-labelled Fe(III)–DMA to 19.9 ml growth medium without Fe. Similarly, 55Fe-labelled Fe(III)–EDTA solution was prepared as described for 55Fe-labelled Fe(III)–DMA except that 1 mm EDTA was used instead of DMA, and the chelating reaction was performed at 90°C for 2 h. All of the seedlings were treated with 55Fe-labelled Fe(III) with either DMA, EDTA or no chelator for 0, 4, and 22 h, and then washed twice for 5 sec with 100 ml of 10 mm EDTA. After removing the washing solution from the seedlings with filter paper (Whatman 3MM, http://www.3m.com), the seedlings were divided into shoots and roots with a razor blade. Radioisotope activity was measured using a FujiFilm Bio-imaging Analyzer System (BAS)-5000 (GE Healthcare Life Sciences, http://www.gelifesciences.com).
Quantitative RT-PCR
RNA was extracted using RNeasy Plant Mini Kit (Qiagen, http://www.qiagen.com) and used for both microarray analysis and qRT-PCR. cDNA was synthesised using the PrimeScript RT reagent kit with gDNA Eraser (Perfect Real Time) (TaKaRa, http://www.takara-bio.com). cDNA samples were diluted 10-fold with EASY Dilution (for real-time PCR) (TaKaRa) before analysis using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Life Technologies Corp., http://www.lifetechnologies.com). Gene-specific primers are shown in Table S9.
Measurement of nitrate reductase activity
Roots and shoots of seedlings treated with 30 μm DMA, 30 μm EDTA, or no chelator for 1 week after a 1-week pre-cultivation were sampled separately. The samples were ground in liquid nitrogen before measuring NR activity as described previously (Hageman and Reed, 1980; Hasegawa, 1996).
Acknowledgments
We thank Ritsuko Motoyama and Dr Yoshiaki Nagamura at the Genome Resource Unit, National Institute of Agrobiological Sciences (NIAS, Japan), for their technical assistance with the microarray analysis, Dr Hiroshi Hasegawa (University of Shiga Prefecture, Japan) for valuable discussions, and Dr Hiroyuki Kimura (Kyoto University, Japan) for technical support with the radioisotope experiments. This work was supported in part by Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research Grant Number 21310148 to YM.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
List of up-regulated genes in shoot at pH 5.8 classified in the Venn diagram shown in Figure S4.
Table S2. List of down-regulated genes in shoot at pH 5.8 classified in the Venn diagram shown in Figure S4.
Table S3. List of up-regulated genes in root at pH 5.8 classified in the Venn diagram shown in Figure S4.
Table S4. List of down-regulated genes in root at pH 5.8 classified in the Venn diagram shown in Figure S4.
Table S5. List of up-regulated genes in shoot at pH 8.0 classified in the Venn diagram shown in Figure S5.
Table S6. List of down-regulated genes in shoot at pH 8.0 classified in the Venn diagram shown in Figure S5.
Table S7. List of up-regulated genes in root at pH 8.0 classified in the Venn diagram shown in Figure S5.
Table S8. List of down-regulated genes in root at pH 8.0 classified in the Venn diagram shown in Figure S5.
Table S9. Primers used in this study.
Table S10. Gene names and codes shown in Supplemental Figure S3.
Effects of EDTA application on rice seedling growth at pH 8.0.
Iron distribution in rice seedlings treated with DMA or EDTA for 22 h.
Expression profile of iron and nitrate (nitrogen) assimilation related genes.
Venn diagrams illustrating unique and overlapping genes showing more than 10-times up- or down-regulated expression compared with that in the no-chelator control at pH 5.8.
Venn diagrams illustrating unique and overlapping genes up- or down-regulated more than 10-fold by indicated treatments, compared with expression in the respective tissues of control (no chelator) at pH 5.8.
Transcription analysis to validate microarray analysis of gene transcription in the roots and shoots.
Effect of chelator treatments on enzyme activity of NADH- and NADPH-dependent nitrate reductases in roots of rice seedlings.
Additional experimental procedure: microarray analysis.
References
- Aoyama T, Kobayashi T, Takahashi M, Nagasaka S, Usuda K, Kakei Y, Ishimaru Y, Nakanishi H, Mori S, Nishizawa NK. OsYSL18 is a rice iron(III)–deoxymugineic acid transporter specifically expressed in repr-oductive organs and phloem of lamina joints. Plant Mol. Biol. 2009;70:681–692. doi: 10.1007/s11103-009-9500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki R, Hasegawa H. Expression of rice (Oryza sativa L.) genes involved in high-affinity nitrate transport during the period of nitrate induction. Breed. Sci. 2006;56:295–302. [Google Scholar]
- Araki R, Murata J, Murata Y. A novel barley yellow stripe 1-like transporter (HvYSL2) localized to the root endodermis transports metal–phytosiderophore complexes. Plant Cell Physiol. 2011;52:1931–1940. doi: 10.1093/pcp/pcr126. [DOI] [PubMed] [Google Scholar]
- Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J. Biol. Chem. 2006;281:32395–32402. doi: 10.1074/jbc.M604133200. [DOI] [PubMed] [Google Scholar]
- Cai C, Wang JY, Zhu YG, Shen QR, Li B, Tong YP, Li ZS. Gene structure and expression of the high-affinity nitrate transport system in rice roots. J. Integr. Plant Biol. 2008;50:443–451. doi: 10.1111/j.1744-7909.2008.00642.x. [DOI] [PubMed] [Google Scholar]
- Campbell WH. Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:277–303. doi: 10.1146/annurev.arplant.50.1.277. [DOI] [PubMed] [Google Scholar]
- Chaney RL, Brown JC, Tiffin LO. Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol. 1972;50:208–213. doi: 10.1104/pp.50.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, Dewdney J, Kleinhofs A, Goodman HM. Cloning and nitrate induction of nitrate reductase mRNA. Proc. Natl Acad. Sci. USA. 1986;83:6825–6828. doi: 10.1073/pnas.83.18.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Mazzola M, Yamasaki H. Nitric oxide research in agriculture: bridging the plant and bacterial realms. In: Rai A, Takabe T, editors. Abiotic Stress Tolerance in Plants. Netherlands: Springer; 2006. pp. 71–90. [Google Scholar]
- Connolly EL, Fett JP, Guerinot ML. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell. 2002;14:1347–1357. doi: 10.1105/tpc.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte SS, Walker EL. Transporters contributing to iron trafficking in plants. Mol. Plant. 2011;4:464–476. doi: 10.1093/mp/ssr015. [DOI] [PubMed] [Google Scholar]
- Crawford NM. Mechanisms for nitric oxide synthesis in plants. J. Exp. Bot. 2006;57:471–478. doi: 10.1093/jxb/erj050. [DOI] [PubMed] [Google Scholar]
- Curie C, Briat JF. Iron transport and signaling in plants. Annu. Rev. Plant Biol. 2003;54:183–206. doi: 10.1146/annurev.arplant.54.031902.135018. [DOI] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature. 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M, Misson J, Schikora A, Czernic P, Mari S. Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 2009;103:1–11. doi: 10.1093/aob/mcn207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Yan M, Fan X, Li B, Shen Q, Miller AJ, Xu G. Spatial expression and regulation of rice high-affinity nitrate transporters by nitrogen and carbon status. J. Exp. Bot. 2011;62:2319–2332. doi: 10.1093/jxb/erq403. [DOI] [PubMed] [Google Scholar]
- Forde BG. Nitrate transporters in plants: structure, function and regulation. Biochim. Biophys. Acta. 2000;1465:219–235. doi: 10.1016/s0005-2736(00)00140-1. [DOI] [PubMed] [Google Scholar]
- Giehl RFH, Meda AR, von Wirén N. Moving up, down, and everywhere: signaling of micronutrients in plants. Curr. Opin. Plant Biol. 2009;12:320–327. doi: 10.1016/j.pbi.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Gong B, Li X, VandenLangenberg KM, Wen D, Sun S, Wei M, Li Y, Yang F, Shi Q, Wang X. Overexpression of S-adenosyl-l-methionine synthetase increased tomato tolerance to alkali stress through polyamine metabolism. Plant Biotech. J. 2014;12:694–708. doi: 10.1111/pbi.12173. [DOI] [PubMed] [Google Scholar]
- Hageman RH, Reed AJ. Nitrate reductase from higher plants. Methods Enzymol. 1980;69:270–280. [Google Scholar]
- Hall DO, Cammack R, Rao KK. The plant ferredoxins and their relationship to the evolution of ferredoxins from primitive life. Pure Appl. Chem. 1973;34:553–578. [Google Scholar]
- Hasegawa H. Selection for mutants with low nitrate uptake ability in rice (Oryza sativa. Physiol. Plant. 1996;96:199–204. [Google Scholar]
- Higuchi K, Suzuki K, Nakanishi H, Yamaguchi H, Nishizawa NK, Mori S. Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiol. 1999;119:471–480. doi: 10.1104/pp.119.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose N, Hayakawa T, Yamaya T. Inducible accumulation of mRNA for NADH-dependent glutamate synthase in rice roots in response to ammonium ions. Plant Cell Physiol. 1997;38:1295–1297. [Google Scholar]
- Inoue H, Higuchi K, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. Three rice nicotianamine synthase genes, OsNAS1 OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J. 2003;36:366–381. doi: 10.1046/j.1365-313x.2003.01878.x. [DOI] [PubMed] [Google Scholar]
- Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y, Suzuki K, Nakazono M, Nakanishi H, Mori S, Nishizawa NK. Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J. Biol. Chem. 2009;284:3470–3479. doi: 10.1074/jbc.M806042200. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Suzuki M, Tsukamoto T, et al. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+ Plant J. 2006;45:335–346. doi: 10.1111/j.1365-313X.2005.02624.x. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Kim S, Tsukamoto T, et al. Mutational reconstructed ferric chelate reductase confers enhanced tolerance in rice to iron deficiency in calcareous soil. Proc. Natl Acad. Sci. USA. 2007;104:7373–7378. doi: 10.1073/pnas.0610555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S, Takagi SI, Sato Y. Mugineic acid-family phytosiderophores in root-secretions of barley, corn and sorghum varieties. J. Plant Nutr. 1988;11:633–642. [Google Scholar]
- Kawai S, Kamei S, Matsuda Y, Ando R, Kondo S, Ishizawa A, Alam S. Concentrations of iron and phytosiderophores in xylem sap of iron-deficient barley plants. Soil Sci. Plant Nutr. 2001;47:265–272. [Google Scholar]
- Kobayashi T, Nishizawa NK. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012;63:131–152. doi: 10.1146/annurev-arplant-042811-105522. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Nakanishi H, Nishizawa NK. Recent insights into iron homeostasis and their application in graminaceous crops. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010;86:900–913. doi: 10.2183/pjab.86.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Nagasaka S, Senoura T, Itai RN, Nakanishi H, Nishizawa NK. Iron-binding haemerythrin RING ubiquitin ligases regulate plant iron responses and accumulation. Nat. Commun. 2013;4:2792. doi: 10.1038/ncomms3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunova Y, Eide D, Gregg Clark W, Lou Guerinot M, Pakrasi H. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol. Biol. 1999;40:37–44. doi: 10.1023/a:1026438615520. [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM, Kirk GJD. Nitrate-ammonium synergism in rice. A subcellular flux analysis. Plant Physiol. 1999;119:1041–1046. doi: 10.1104/pp.119.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouk G, Crawford NM, Coruzzi GM, Tsay YF. Nitrate signaling: adaptation to fluctuating environments. Curr. Opin. Plant Biol. 2010;13:265–272. doi: 10.1016/j.pbi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Kusano M, Tabuchi M, Fukushima A, et al. Metabolomics data reveal a crucial role of cytosolic glutamine synthetase 1;1 in coordinating metabolic balance in rice. Plant J. 2011;66:456–466. doi: 10.1111/j.1365-313X.2011.04506.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Chiecko JC, Kim SA, Walker EL, Lee Y, Guerinot ML, An G. Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiol. 2009;150:786–800. doi: 10.1104/pp.109.135418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejay L, Tillard P, Lepetit M, Olive FD, Filleur S, Daniel-Vedele F, Gojon A. Molecular and functional regulation of two NO3− uptake systems by N- and C-status of Arabidopsis plants. Plant J. 1999;18:509–519. doi: 10.1046/j.1365-313x.1999.00480.x. [DOI] [PubMed] [Google Scholar]
- Lillo C, Appenroth KJ. Light regulation of nitrate reductase in higher plants: which photoreceptors are involved? Plant Biol. 2001;3:455–465. [Google Scholar]
- Ma JF, Nomoto K. Effective regulation of iron acquisition in graminaceous plants. The role of mugineic acids as phytosiderophores. Physiol. Plant. 1996;97:609–617. [Google Scholar]
- Ma JF, Kusano G, Kimura S, Nomoto K. Specific recognition of mugineic acid-ferric complex by barley roots. Phytochemistry. 1993;34:599–603. [Google Scholar]
- Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM. Nitrate transport and signalling. J. Exp. Bot. 2007;58:2297–2306. doi: 10.1093/jxb/erm066. [DOI] [PubMed] [Google Scholar]
- Mino Y, Ishida T, Ota N, Inoue M, Nomoto K, Takemoto T, Tanaka H, Sugiura Y. Mugineic acid-iron(III) complex and its structurally analogous cobalt(III) complex: characterization and implication for absorption and transport of iron in gramineous plants. J. Am. Chem. Soc. 1983;105:4671–4676. [Google Scholar]
- Mori S. Iron acquisition by plants. Curr. Opin. Plant Biol. 1999;2:250–253. doi: 10.1016/S1369-5266(99)80043-0. [DOI] [PubMed] [Google Scholar]
- Mori S, Nishizawa N. Methionine as a dominant precursor of phytosiderophores in graminaceae plants. Plant Cell Physiol. 1987;28:1081–1092. [Google Scholar]
- Murgia I, Delledonne M, Soave C. Nitric oxide mediates iron-induced ferritin accumulation in Arabidopsis. Plant J. 2002;30:521–528. doi: 10.1046/j.1365-313x.2002.01312.x. [DOI] [PubMed] [Google Scholar]
- Murphy MJ, Siegel LM, Tove SR, Kamin H. Siroheme: a new prosthetic group participating in six-electron reduction reactions catalyzed by both sulfite and nitrite reductases. Proc. Natl Acad. Sci. USA. 1974;71:612–616. doi: 10.1073/pnas.71.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Okumura N, Umehara Y, Nishizawa NK, Chino M, Mori S. Expression of a gene specific for iron deficiency (Ids3) in the roots of Hordeum vulgare. Plant Cell Physiol. 1993;34:401–410. [PubMed] [Google Scholar]
- Namba K, Murata Y, Horikawa M, Iwashita T, Kusumoto S. A practical synthesis of the phytosiderophore 2′-deoxymugineic acid: a key to the mechanistic study of iron acquisition by graminaceous plants. Angew. Chem. Int. Ed. Engl. 2007;46:7060–7063. doi: 10.1002/anie.200702403. [DOI] [PubMed] [Google Scholar]
- Noodleman L, Norman JG, Osborne JH, Aizman A, Case DA. Models for ferredoxins: electronic structures of iron-sulfur clusters with one, two, and four iron atoms. J. Am. Chem. Soc. 1985;107:3418–3426. [Google Scholar]
- Nozoye T, Itai RN, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. Diurnal changes in the expression of genes that participate im phytosiderophore synthesis in rice. Soil Sci. Plant Nutr. 2004;50:1125–1131. [Google Scholar]
- Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J. Biol. Chem. 2011;286:5446–5454. doi: 10.1074/jbc.M110.180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Nesi A, Fernie AR, Stitt M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant. 2010;3:973–996. doi: 10.1093/mp/ssq049. [DOI] [PubMed] [Google Scholar]
- Ogo Y, Itai NR, Nakanishi H, Kobayashi T, Takahashi M, Mori S, Nishizawa NK. The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J. 2007;51:366–377. doi: 10.1111/j.1365-313X.2007.03149.x. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Vidmar JJ, Glass ADM. Regulation of NRT1 and NRT2 gene families of Arabidopsis thaliana: responses to nitrate provision. Plant Cell Physiol. 2003;44:304–317. doi: 10.1093/pcp/pcg036. [DOI] [PubMed] [Google Scholar]
- Okumura N, Nishizawa NK, Umehara Y, Ohata T, Nakanishi H, Yamaguchi T, Chino M, Mori S. A dioxygenase gene (Ids2) expressed under iron deficiency conditions in the roots of Hordeum vulgare. Plant Mol. Biol. 1994;25:705–719. doi: 10.1007/BF00029608. [DOI] [PubMed] [Google Scholar]
- Ono F, Frommer WB, von Wirén N. Coordinated diurnal regulation of low- and high-affinity nitrate transporters in tomato. Plant Biol. 2000;2:17–23. [Google Scholar]
- Orsel M, Krapp A, Daniel-Vedele F. Analysis of the NRT2 nitrate transporter family in Arabidopsis. Structure and gene expression. Plant Physiol. 2002;129:886–896. doi: 10.1104/pp.005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CM, Guerinot ML. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat. Chem. Biol. 2009;5:333–340. doi: 10.1038/nchembio.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roje S. S-Adenosyl-l-methionine: beyond the universal methyl group donor. Phytochemistry. 2006;67:1686–1698. doi: 10.1016/j.phytochem.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Römheld V, Marschner H. Mechanism of iron uptake by peanut plants: I. FeIII reduction, chelate splitting, and release of phenolics. Plant Physiol. 1983;71:949–954. doi: 10.1104/pp.71.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V, Marschner H. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol. 1986;80:175–180. doi: 10.1104/pp.80.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, González-Fontes A, Morcuende R, Lauerer M, Geiger M, Glaab J, Gojon A, Schulze ED, Stitt M. Tobacco mutants with a decreased number of functional nia genes compen-sate by modifying the diurnal regulation of transcription, post-translational modification and turnover of nitrate reductase. Planta. 1997;203:304–319. doi: 10.1007/s004250050196. [DOI] [PubMed] [Google Scholar]
- Scheible WR, Krapp A, Stitt M. Reciprocal diurnal changes of phosphoenolpyruvate carboxylase expression and cytosolic pyruvate kinase, citrate synthase and NADP-isocitrate dehydrogenase expression regulate organic acid metabolism during nitrate assimilation in tobacco leaves. Plant, Cell Environ. 2000;23:1155–1167. [Google Scholar]
- Shi WM, Chino M, Youssef RA, Mori S, Takagi S. The occurrence of mugineic acid in the rhizosphere soil of barley plant. Soil Sci. Plant Nutr. 1988;34:585–592. [Google Scholar]
- Shojima S, Nishizawa NK, Fushiya S, Nozoe S, Irifune T, Mori S. Biosynthesis of phytosiderophores: in vitro biosynthesis of 2′-deoxymugineic acid from L-methionine and nicotianamine. Plant Physiol. 1990;93:1497–1503. doi: 10.1104/pp.93.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Morikawa KC, Nakanishi H, Takahashi M, Saigusa M, Mori S, Nishizawa NK. Transgenic rice lines that include barley genes have increased tolerance to low iron availability in a calcareous paddy soil. Soil Sci. Plant Nutr. 2008;54:77–85. [Google Scholar]
- Swamy U, Wang M, Tripathy JN, Kim SK, Hirasawa M, Knaff DB, Allen JP. Structure of spinach nitrite reductase: implications for multi-electron reactions by the iron–sulfur:siroheme cofactor. Biochemistry. 2005;44:16054–16063. doi: 10.1021/bi050981y. [DOI] [PubMed] [Google Scholar]
- Ta TC, Ohira K. Effects of various environmental and medium conditions on the response of Indica and Japonica rice plants to ammonium and nitrate nitrogen. Soil Sci. Plant Nutr. 1981;27:347–355. [Google Scholar]
- Takagi S, Nomoto K, Takemoto T. Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J. Plant Nutr. 1984;7:469–477. [Google Scholar]
- Takahashi M, Yamaguchi H, Nakanishi H, Shioiri T, Nishizawa NK, Mori S. Cloning two genes for nicotianamine aminotransferase, a critical enzyme in iron acquisition (Strategy II) in graminaceous plants. Plant Physiol. 1999;121:947–956. doi: 10.1104/pp.121.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Nakanishi H, Kawasaki S, Nishizawa NK, Mori S. Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nat. Biotech. 2001;19:466–469. doi: 10.1038/88143. [DOI] [PubMed] [Google Scholar]
- Tamura W, Hidaka Y, Tabuchi M, Kojima S, Hayakawa T, Sato T, Obara M, Kojima M, Sakakibara H, Yamaya T. Reverse genetics approach to characterize a function of NADH-glutamate synthase1 in rice plants. Amino Acids. 2010;39:1003–1012. doi: 10.1007/s00726-010-0531-5. [DOI] [PubMed] [Google Scholar]
- Treeby M, Marschner H, Römheld V. Mobilization of iron and other micronutrient cations from a calcareous soil by plant-borne, microbial, and synthetic metal chelators. Plant Soil. 1989;114:217–226. [Google Scholar]
- Vasconcelos M, Musetti V, Li CM, Datta SK, Grusak MA. Functional analysis of transgenic rice (Oryza sativa L.) transformed with an Arabidopsis thaliana ferric reductase (AtFRO2. Soil Sci. Plant Nutr. 2004;50:1151–1157. [Google Scholar]
- Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell. 2002;14:1223–1233. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EL, Connolly EL. Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Curr. Opin. Plant Biol. 2008;11:530–535. doi: 10.1016/j.pbi.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Wang R, Guegler K, LaBrie ST, Crawford NM. Genomic analysis of a nutrient response in Arabidopsis reveals diverse expression patterns and novel metabolic and potential regulatory genes induced by nitrate. Plant Cell. 2000;12:1491–1509. doi: 10.1105/tpc.12.8.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Okamoto M, Xing X, Crawford NM. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1,000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol. 2003;132:556–567. doi: 10.1104/pp.103.021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Tischner R, Gutierrez RA, Hoffman M, Xing X, Chen M, Coruzzi G, Crawford NM. Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol. 2004;136:2512–2522. doi: 10.1104/pp.104.044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ID, Neill SJ, Hancock JT. Nitric oxide synthesis and signalling in plants. Plant, Cell Environ. 2008;31:622–631. doi: 10.1111/j.1365-3040.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- Xu G, Fan X, Miller AJ. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012;63:153–182. doi: 10.1146/annurev-arplant-042811-105532. [DOI] [PubMed] [Google Scholar]
- Yamaya T, Hayakawa T, Tanasawa K, Kamachi K, Mae T, Ojima K. Tissue distribution of glutamate synthase and glutamine synthetase in rice leaves: occurrence of NADH-dependent glutamate synthase protein and activity in the unexpanded, nongreen leaf blades. Plant Physiol. 1992;100:1427–1432. doi: 10.1104/pp.100.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Fan X, Feng H, Miller AJ, Shen Q, Xu G. Rice OsNAR2.1 interacts with OsNRT2.1, OsNRT2.2 and OsNRT2.3a nitrate transporters to provide uptake over high and low concentration ranges. Plant, Cell Environ. 2011;34:1360–1372. doi: 10.1111/j.1365-3040.2011.02335.x. [DOI] [PubMed] [Google Scholar]
- Yokosho K, Yamaji N, Ueno D, Mitani N, Ma JF. OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol. 2009;149:297–305. doi: 10.1104/pp.108.128132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z, Gayomba SR, Jung HI, et al. OPT3 is a phloem-specific iron transporter that is essential for systemic iron signaling and redistribution of iron and cadmium in Arabidopsis. Plant Cell. 2014;26:2249–2264. doi: 10.1105/tpc.114.123737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of up-regulated genes in shoot at pH 5.8 classified in the Venn diagram shown in Figure S4.
Table S2. List of down-regulated genes in shoot at pH 5.8 classified in the Venn diagram shown in Figure S4.
Table S3. List of up-regulated genes in root at pH 5.8 classified in the Venn diagram shown in Figure S4.
Table S4. List of down-regulated genes in root at pH 5.8 classified in the Venn diagram shown in Figure S4.
Table S5. List of up-regulated genes in shoot at pH 8.0 classified in the Venn diagram shown in Figure S5.
Table S6. List of down-regulated genes in shoot at pH 8.0 classified in the Venn diagram shown in Figure S5.
Table S7. List of up-regulated genes in root at pH 8.0 classified in the Venn diagram shown in Figure S5.
Table S8. List of down-regulated genes in root at pH 8.0 classified in the Venn diagram shown in Figure S5.
Table S9. Primers used in this study.
Table S10. Gene names and codes shown in Supplemental Figure S3.
Effects of EDTA application on rice seedling growth at pH 8.0.
Iron distribution in rice seedlings treated with DMA or EDTA for 22 h.
Expression profile of iron and nitrate (nitrogen) assimilation related genes.
Venn diagrams illustrating unique and overlapping genes showing more than 10-times up- or down-regulated expression compared with that in the no-chelator control at pH 5.8.
Venn diagrams illustrating unique and overlapping genes up- or down-regulated more than 10-fold by indicated treatments, compared with expression in the respective tissues of control (no chelator) at pH 5.8.
Transcription analysis to validate microarray analysis of gene transcription in the roots and shoots.
Effect of chelator treatments on enzyme activity of NADH- and NADPH-dependent nitrate reductases in roots of rice seedlings.
Additional experimental procedure: microarray analysis.