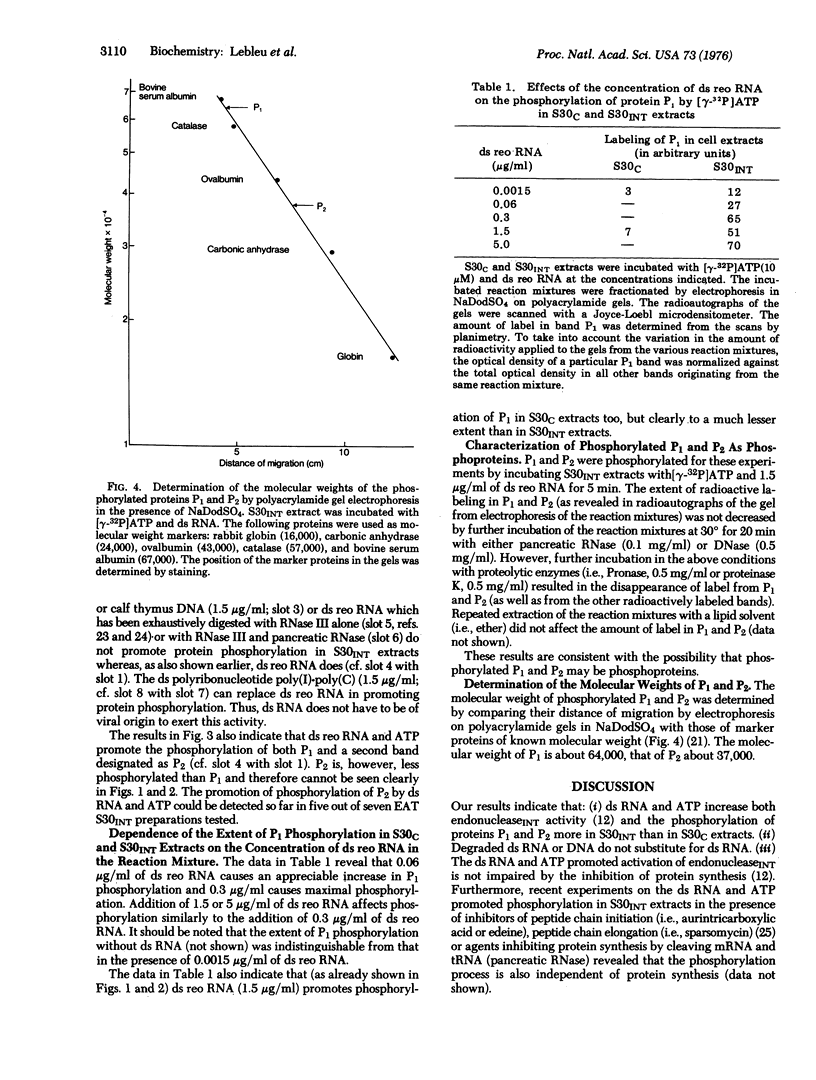
Abstract
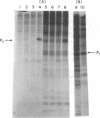
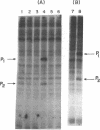
We reported earlier that the addition of double-stranded RNA and ATP increases the endonuclease activity more in an extract of Ehrlich ascites tumor cells which have been treated with an interferon preparation than in a comparable extract from control cells. We report here that the addition of double-stranded RNA to an extract from Ehrlich ascites tumor cells which have been treated with an interferon preparation [or with the interferon inducer poly(I)-poly(C)] promotes the phosphorylation by [gamma-32P]ATP of at least two proteins: P1 (molecular weight of 64,000) and P2 (molecular weight of 37,000). Double-stranded RNA also promotes the phosphorylation of at least one (i.e., P1) of these two proteins in an extract from cells which have not been treated with interferon, but the extent of phosphorylation is much smaller. Double-stranded RNA which has been degraded by RNase III, or DNA, does not promote the phosphorylation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkow K., Hunt T., Jackson R. J. Control of protein synthesis in reticulocyte lysates: the effect of nucleotide triphosphates on formation of the translational repressor. Biochem Biophys Res Commun. 1975 Nov 3;67(1):366–375. doi: 10.1016/0006-291x(75)90325-3. [DOI] [PubMed] [Google Scholar]
- Brown G. E., Lebleu B., Kawakita M., Shaila S., Sen G. C., Lengyel P. Increased endonuclease activity in an extract from mouse Ehrlich ascites tumor cells which had been treated with a partially purified interferon preparation: dependence of double-stranded RNA;. Biochem Biophys Res Commun. 1976 Mar 8;69(1):114–122. doi: 10.1016/s0006-291x(76)80280-x. [DOI] [PubMed] [Google Scholar]
- Crouch R. J. Ribonuclease 3 does not degrade deoxyribonucleic acid-ribonucleic acid hybrids. J Biol Chem. 1974 Feb 25;249(4):1314–1316. [PubMed] [Google Scholar]
- Ernst V., Levin D. H., Ranu R. S., London I. M. Control of protein synthesis in reticulocyte lysates: effects of 3':5'-cyclic AMP, ATP, and GTP on inhibitions induced by hemedeficiency, double-stranded RNA, and a reticulocyte translationa inhibitor. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1112–1116. doi: 10.1073/pnas.73.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcoff E., Falcoff R., Lebleu B., Revel M. Correlation between the antiviral effect of interferon treatment and the inhibition of in vitro mRNA translation in noninfected L cells. J Virol. 1973 Sep;12(3):421–430. doi: 10.1128/jvi.12.3.421-430.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Metz D. H., Esteban R. M., Tovell D. R., Ball L. A., Kerr I. M. Mechanism of interferon action: inhibition of viral messenger ribonucleic acid translation in L-cell extracts. J Virol. 1972 Dec;10(6):1184–1198. doi: 10.1128/jvi.10.6.1184-1198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galster R. L., Lengyel P. Formation and characteristics of reovirus subviral particles in interferon-treated mouse L cells. Nucleic Acids Res. 1976 Mar;3(3):581–598. doi: 10.1093/nar/3.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei W. D., 3rd, Lengyel P. Translation of in vitro synthesized reovirus messenger RNAs into proteins of the size of reovirus capsid proteins in a mouse L cell extract. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1816–1823. doi: 10.1016/0006-291x(72)90056-3. [DOI] [PubMed] [Google Scholar]
- Greengard P. Possible role for cyclic nucleotides and phosphorylated membrane proteins in postsynaptic actions of neurotransmitters. Nature. 1976 Mar 11;260(5547):101–108. doi: 10.1038/260101a0. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Graziadei W. D., 3rd, Weideli H., Sopori M. L., Lengyel P. Selective inhibition of viral protein accumulation in interferon-treated cells; nondiscriminate inhibition of the translation of added viral and cellular messenger RNAs in their extracts. Virology. 1974 Jan;57(1):49–63. doi: 10.1016/0042-6822(74)90107-x. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Sopori M. L., Lengyel P. Release of the inhibition of messenger RNA translation in extracts of interferon-treated Ehrlich ascites tumor cells by added transfer RNA. Biochem Biophys Res Commun. 1974 Apr 8;57(3):763–770. doi: 10.1016/0006-291x(74)90612-3. [DOI] [PubMed] [Google Scholar]
- Jordan G. W. Quantitative aspects of interferon-induced plaque reduction: kinetics of interferon action. Virology. 1972 May;48(2):425–432. doi: 10.1016/0042-6822(72)90053-0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Ball L. A. Increased sensitivity of cell-free protein synthesis to double-stranded RNA after interferon treatment. Nature. 1974 Jul 5;250(461):57–59. doi: 10.1038/250057a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin D. H., Ranu R. S., Ernst V., Fifer M. A., London L. M. Association of a cyclic AMP-dependent protein kinase with a purified translational inhibitor isolated from hemin-deficient rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4849–4853. doi: 10.1073/pnas.72.12.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Joklik W. K. A protein synthesizing system from interferon-treated cells that discriminates between cellular and viral messenger RNAs. Virology. 1974 Apr;58(2):476–491. doi: 10.1016/0042-6822(74)90082-8. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Gupta S. L., Brown G. E., Lebleu B., Rebello M. A., Lengyel P. Interferon treatment of Ehrlich ascites tumor cells: effects on exogenous mRNA translation and tRNA inactivation in the cell extract. J Virol. 1975 Jan;17(1):191–203. doi: 10.1128/jvi.17.1.191-203.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. C., Lebleu B., Brown G. E., Rebello M. A., Furuichi Y., Morgan M., Shatkin A. J., Lengyel P. Inhibition of reovirus messenger RNA methylation in extracts of interferon-treated Ehrlich ascites tumor cells. Biochem Biophys Res Commun. 1975 Jul 8;65(1):427–434. doi: 10.1016/s0006-291x(75)80111-2. [DOI] [PubMed] [Google Scholar]
- Vassef A., Beaud G., Paucker K., Lengyel P. Interferon assay based on the inhibition of double-stranded reovirus RNA accumulation in mouse L cells. J Gen Virol. 1973 Apr;19(1):81–87. doi: 10.1099/0022-1317-19-1-81. [DOI] [PubMed] [Google Scholar]
- Vazquez D. Inhibitors of protein synthesis. FEBS Lett. 1974 Mar 23;40(0):suppl–suppl:S84. doi: 10.1016/0014-5793(74)80689-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weber L. A., Feman E. R., Baglioni C. A cell free system from HeLa cells active in initiation of protein synthesis. Biochemistry. 1975 Dec 2;14(24):5315–5321. doi: 10.1021/bi00695a015. [DOI] [PubMed] [Google Scholar]