Abstract
A combination of hepatitis B immunoglobulin (HBIG) and nucleoside/nucleotide analogs (NUC) is the current standard of care for controlling hepatitis B recurrence after orthotopic liver transplantation (OLT). However, long-term HBIG administration is associated with several unresolved issues, including limited availability and extremely high cost, and thus several protocols for treatment with low-dose HBIG combined with NUC or HBIG-free regimens have been developed. This article reviews recent advances in post-OLT hepatitis B virus (HBV) control and future methodological directions. New NUC such as entecavir, tenofovir or lamivudine plus adefovir dipivoxil combinations induce a very low frequency of viral resistance. The withdrawal of HBIG after several months of OLT under new NUC continuation also has permissible effects. Even after HBV reactivation, NUC can usually achieve viral control when viral markers are strictly followed up. Another approach is to induce self-producing anti-HBV antibodies via vaccination with a hepatitis B surface antigen vaccine. However, HBV vaccination is not sufficiently effective in patients to treat liver cirrhosis type B after OLT because immune tolerance to the virus has already continued for several decades. Trials of its safety and cost-effectiveness are required. This review advocates a safe and economical approach to controlling post-OLT HBV recurrence.
Keywords: antiviral agent, hepatitis B, hepatitis B immunoglobulin, hepatitis B vaccine, liver transplantation, prophylaxis
Introduction
BEFORE THE INTRODUCTION of effective post-transplantation antiviral prophylaxis, liver transplantation for hepatitis B virus (HBV)-related disease was usually followed by immediate HBV reinfection of the allograft, resulting in fatal hepatitis B recurrence.1 The long-term administration of hepatitis B immunoglobulin (HBIG) is associated with a 36% recurrence rate for HBV, compared with a 75% recurrence rate without or with short-term HBIG administration.2 The first generation nucleoside/nucleotide analog (NUC) lamivudine (LAM) resulted in more than 75% of patients achieving hepatitis B surface antigen (HBsAg) negative status within 1 year,3 but longer follow up has shown more than 50% recurrence rates due to the emergence of HBV DNA mutants.4 The combination of HBIG and LAM results in more than 90% long-term HBsAg negativity,5 and this protocol has proven successful in many institutions. As HBIG is extremely expensive, strategies to decrease the requirement for HBIG have been attempted. The concomitant administration of newer, more powerful NUC such as entecavir (ETV) or tenofovir (TDF) have enabled a decrease in the amount of administrated HBIG. Whether post-orthotopic liver transplantation (OLT) HBIG can be withdrawn after short-term administration is the next question. Low-dose HBIG combined with ETV or TDF is the currently accepted optimal regimen, although patients without detectable HBV DNA at OLT may not require HBIG. Unlike these passive approaches, patients have been actively immunized with HBV vaccine, although the effect was not as beneficial as originally hoped. After achieving more than 90% control of the post-OLT viral burden with HBIG and NUC, studies have become polarized towards designing an appropriate protocol with sufficient safety at minimal cost (Fig. 1).
Figure 1.
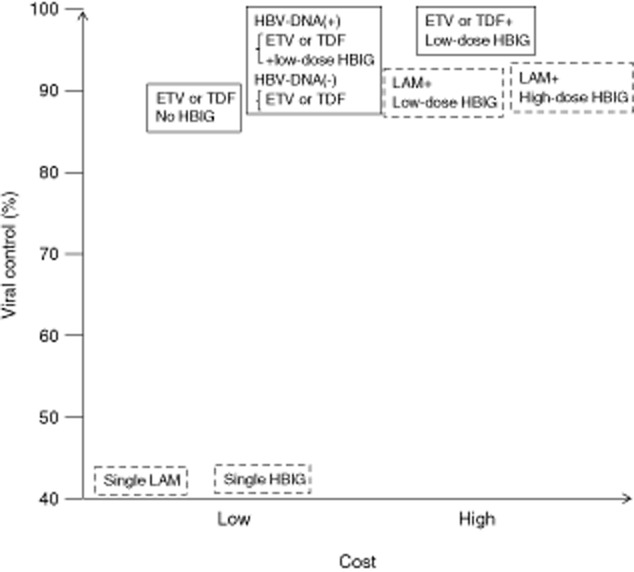
Conceptual diagram of post-orthotopic liver transplantation hepatitis B control effectiveness and cost. Boxes with solid and dashed lines, respectively, indicate present trials and previous methods that are not generally recommended. ETV, entecavir; HBIG, hepatitis B immunoglobulin; HBV, hepatitis B virus; LAM, lamivudine; TDF, tenofovir.
This article reviews recent advances in post-OLT HBV control and explores clinically and economically optimal approaches.
Mechanism of Protection Against HBV Reactivation by HBIG
NUCLEOSIDE/NUCLEOTIDE ANALOGS inhibit pre-genomic RNA reverse transcription resulting in a rapid decrease in serum HBV DNA, but cannot eliminate the covalently closed circular DNA (cccDNA) reservoir in the nucleus of infected cells. The mechanisms of HBIG-induced protection against HBV reinfection or viral control after reinfection remain obscure. HBIG contains high-titer antibodies against HBsAg, which is the major component of the envelope of the HBV virion. The mechanisms through which HBIG prevents HBV transmission may be neutralization of circulating virus by forming immune complexes, protecting naïve hepatocytes against HBV released from extrahepatic sites through blocking putative HBV receptors, or anti-HBs internalized into hepatocytes that interacts with HBsAg and inhibits HBsAg secretion from cells.6
To protect naïve hepatocytes against HBV infection may be difficult, because recent studies have detected intrahepatic HBV DNA in more than 50% of even well-controlled patients post-OLT.7 The release of HBV virions from infected cells could be blocked with anti-HBs. Assays in vitro have shown that internalized antibody induces intracellular viral particle accumulation even after the antibody has been removed from culture supernatants.8 However, an explanation is required for the success of HBIG combined with LAM. The main LAM-resistant associated substitution is located in the reverse transcriptase C-domain of the viral polymerase region. The HBV has overlapping open reading frames including the polymerase gene that overlaps part of the envelope gene that produces HBsAg. Thus, LAM-resistant polymerase gene mutations also change the envelope gene, resulting in a change in the HBV nucleocapsids that bind to or enter hepatocytes. These viruses with mutant envelope HBsAg have a low capacity to secrete expressed HBV Dane particles9 and this may explain why HBV recurrence can be controlled when LAM is combined with HBIG.
Historical Advances in Post-OLT HBV Control Reaching More Than 90% Freedom from Recurrence with First Generation NUC
SHORT-TERM HBIG ADMINISTRATION appeared effective in 1978.10 An early multicenter study in Europe in 1993 identified a risk of post-OLT HBV recurrence and the effect of HBIG administration. The risk was low in patients with acute liver failure who were intolerant of HBV. However, the recurrence rate in patients with liver cirrhosis, especially with high serum HBV DNA, was more than 80%.2 The rates of HBV recurrence in patients with liver cirrhosis without prophylaxis and with short-term HBIG administration were 78% and 90%, respectively, whereas long-term HBIG administration resulted in 56% recurrence with significantly lower risk.
Lamivudine was the first NUC to become commercially available. Early reports of LAM monotherapy showed only one of 10 HBV DNA reappearances at 72 weeks post-OLT. Two studies of long-term LAM administration to patients after OLT found 40% and 62.5% prevalences of viral breakthrough at 61 and 52 weeks, respectively, which was similar to the prevalences in non-transplanted patients.11,12 However, the combination of HBIG and LAM resulted in far better control.
The first trial of long-term HBIG combined with LAM proceeded in 1998. Monthly HBIG administration with LAM (150 mg/day) resulted in all patients surviving for 1 year after OLT without serum HBV DNA positivity.5 Subsequent reports also described successful control of HBV recurrence with this combination.13–15 Guidelines proposed by Roche and Samuel in 2004 indicate that HBV DNA negative patients with and without liver cirrhosis need to maintain anti-HBs titers of more than 100 IU/L with and without LAM, respectively.16 Because HBV DNA positive status before OLT is associated with high risk for HBV recurrence, they recommended maintaining anti-HBs titers of more than 500 IU/L with concomitant LAM for patients with positive HBV DNA before OLT. To maintain anti-HBs titers of more than 500 IU/L requires 2000–3000 U of HBIG per month at a monthly cost of $US 800–1200. Reducing the HBIG frequency has been suggested from the standpoint of cost-saving (Table 1a).
Table 1.
Recent post OLT HBV prophylaxis with NUC and/or HBIG
| No. of patients | HBV DNA positivity at OLT | HBV DNA recurrence | Follow up (months) | Reference no. | Year published | |
|---|---|---|---|---|---|---|
| (a) Lamivudine + HBIG | ||||||
| HBIG i.v. 10 000 IU/month | 14 | 7% (1/14) | 0 | 13 | 5 | 1998 |
| HBIG i.m. 4300–6800 IU/month | 7 | 0% (0/7) | 0 | 17 (13–21) | 14 | 1999 |
| HBIG i.v. 5000 IU/month | 26 | 27% (7/26) | 4% | 30 ± 8 | 15 | 2001 |
| HBIG i.m. 1480 IU/month | 10 | 20% (2/10) | 10% | 15 (10–21) | 17 | 1999 |
| HBIG i.m. 400–800 IU/month | 141 | 76% (72/94) | 4% | 62 (11–126) | 18 | 2007 |
| (b) Lamivudine + HBIG (on demand) | ||||||
| HBIG i.v. to maintain HBsAb >200 IU/L | 21 | 38% (8/21) | 9.5% | 21 (2.4–49.1) | 19 | 2001 |
| HBIG to maintain HBsAb >100 IU/L | 254 (64 FH) | 21% (53/254) >5 log copies/mL | 8.2% (DNA <5 or ≥5 log copies/mL: 3.8% and 20.8%, respectively) | 60 | 20 | 2010 |
| HBIG to maintain HBsAb >70 IU/L | 11 | 0% (0/11) | 0 | 16 (9–22) | 21 | 2004 |
| HBIG IV to maintain HBsAb >10 IU/L | 18 | 61% (11/18) | 0 | 30 (7–73) | 22 | 2007 |
| Short course (6 months) HBIG | 51 | 45% (23/51) | 9.8% (DNA+, 22%; DNA−, 0%) | DNA+ 28 (1–82), DNA− 70 (5–104) | 23 | 2004 |
| Short course (1 month) HBIG | 14 | 0% (0/14) | 7% | 18 | 24 | 2003 |
| (c) Lamivudine + adefovir + HBIG | ||||||
| HBIG to maintain HBsAb >100 IU/L + ADV 114 days before OLT | 11 | 82% (9/11) | 0 | 29 (16–64) | 25 | 2005 |
| HBIG + LAM 4.4 years and HBIG replaced by ADV | 16 | 19% (3/16) | 0 | 21 (9.4–35.9) | 26 | 2008 |
| LAM + ADV short course (7 days) HBIG i.m. 800 IU/day | 20 | 68% (13/19) | 0 | 57 (27–83) | 27 | 2013 |
| One year HBIG i.m.; 2000 IU/month Lamivudine + adefovir or tenofovir, tenofovir, entecavir |
16 | 4.5% (2/44) | 0 | 24 (6–40) post-HBIG withdrawal | 28 | 2012 |
| (d) Entecavir + HBIG | ||||||
| HBIG i.m. to maintain HBsAb >100 IU/L | 63 | Average 5.49 × 104 copies/mL | 0 | 41 (33–54) | 29 | 2012 |
| HBIG >200 IU/L + ETV | 26 | 46% (12/26) | 0 | 25 (0.2–58.6) | 30 | 2013 |
| HBIG >100 IU/L + ETV | 84 | 44 cases <105 copies/mL | 0 | 57.1 ± 15.9 | 31 | 2014 |
| HBIG; dose not specified | 61 | All cases <172 IU/mL | 0 | 18 | 32 | 2013 |
| One year HBIG i.v.; 10 000 U/month | 29 | 52% (15/29) | 3.4% | 31 | 33 | 2013 |
| (e) Tenofovir + emtricitabine + perioperative HBIG | ||||||
| HBIG >6 months to maintain HBsAb >100 IU/L replaced with TDF/EMT | 21 | 56% (10/18) | 0 | 31 (15–47) | 34 | 2012 |
| HBIG >6 months; various protocols | 17 | 88% (15/17) | 0 | 26 (4–36) | 35 | 2013 |
| (f) HBIG-free with newer NUC | ||||||
| Entecavir | 80 | 74% (59/80) | 1.2% (22.5% for HBsAg seropositivity) | 26 (5–40) | 36 | 2011 |
| Lamivudine + adefovir (no HBIG when HBV DNA <3 log10 IU/mL) | 28 | 35% (10/28) | 0 | 22 (10–58) | 27 | 2013 |
| Entecavir, lamivudine + adefovir, tenofovir, entecavir + tenofovir (no HBIG when HBV DNA <3.3 log10 IU/mL) | 75 (ENT 42, LAM + ADV 19, TDF 12, ENT + TDF 2) | 31% (18/57) | 8% (5/6 patients withdrawn from NUC therapy) | 21 (1–83) | 37 | 2013 |
ADV, adefovir dipivoxil; EMT, emtricitabine; ENT, entecavir; ETV, entecavir; FH, fulminant hepatic failure; HBIG, hepatitis B immunoglobulin; HBsAb; HBV DNA, hepatitis B virus DNA; HBV, hepatitis B virus; hepatitis B surface antibody; IU, international units; LAM, lamivudine; NUC, nucleoside/nucleotide analog; OLT, orthotopic liver transplantation; TDF, tenofovir.
We selected the references listed in Table 1(a,b) from 299 results of a published work search of PubMed between 1998 and March 2014 using the terms “HBIG”, “lamivudine”, “liver” and “transplantation”. Among them, 44 studies described the effects of LAM and HBIG on HBV DNA recurrence rates. We selected one milestone article for similar protocols from these reports. Early studies around 2000 administrated several thousand units of HBIG,5,17 but this dose requirement decreased as clinical data accumulated.18–21,23 Thereafter, HBIG was administrated upon demand only when the anti-HBs titer fell below target levels that have since decreased to a point where they now serve only to maintain a positive titer that is sufficient to control hepatitis B recurrence.22 Some reports indicate that only a short duration of HBIG administration is required and that it could be withdrawn several months after OLT.24 Presently, HBIG can be withdrawn after several months post-OLT if HBV DNA is negative at the time of OLT, or if patients with acute liver failure became infected with the virus shortly before hepatitis developed. Of course, HBV DNA and HBsAg titers should be strictly monitored throughout life.
Replacement of HBIG + LAM Combination with Newer NUC
SEVERAL NEW NUC such as adefovir dipivoxil (ADV), ETV, telbivudine (LdT) and TDF have become commercially available.38 Because of the risk of developing resistance, LAM is no longer recommended as a first-line treatment for hepatitis B. The currently recommended first-line agents are ETV and TDF, which have resulted in very low rates of emergent resistance (5- and 6-year resistance rates for ETV and TDF: 1.2% and 0%, respectively).39,40 A single administration of ADV or LdT is less effective and it engenders higher rates of resistance. Add-on ADV or a switch to TDF has been recommended to treat LAM-resistant virus, because such viruses are also cross-resistant to other NUC.38
The newer NUC are very effective when combined with HBIG even for short-duration, post-OLT HBV control.25–35 The published work in Table 1(c) was selected from 89 PubMed search results between 2000 and March 2014 using the terms “HBIG”, “adefovir”, “lamivudine”, “liver” and “transplantation”. Studies of the effects of add-on ADV after LAM-resistant virus appeared were excluded. Among the six studies that described the effects, we selected four milestone articles. The LAM + ADV combination resulted in very powerful effects indicating that the inclusion of HBIG may only be required for short periods.
The published work in Table 1(d) was selected from 134 PubMed search results between 2000 and March 2014 using the terms “liver transplantation” and “entecavir”. Among six studies that described the effect, five studies in the table included the issues that we needed to assess. The published work in Table 1(e) was selected from 97 PubMed search results using the terms “liver transplantation” and “tenofovir”. Three reports described the effects, and two of them included the issues that we needed to assess. Because of low resistance and the powerful antiviral response evoked by ETV and TDF or a combination of NUC, several institutions have developed successful HBIG-free protocols for patients with appropriately low HBV DNA titers at the time of OLT.27,37 Such protocols are listed in Table 1(f), which we found by collating the data shown in Table 1(a–e). A complete regimen without HBIG that included 74% of detectable HBV DNA resulted in a HBV DNA recurrence rate of 1.2%, with 22.5% being HBsAg positive at 26 months after OLT.36 Patients with HBV DNA of less than 5 logs and HBsAg of less than 3 logs at the time of OLT achieved 100% seroclearance of HBsAg, although the number of patients at risk was not high. This complete HBIG-free protocol may impose a risk for patients with high levels of HBV DNA at the time of OLT. As other protocols with short-term HBIG in combination resulted in almost 100% control of HBV DNA recurrence, even among HBV DNA positive patients at the time of OLT, such protocols may be safe and cost-effective. We continue to recommend at least short-term HBIG for patients who are HBV DNA positive at the time of OLT, even with newer NUC such as ETV, TDF or LAM + ADV. A completely HBIG-free protocol may be adopted for patients who are HBV DNA negative at the time of OLT.
Acceptability of Nucleoside/Nucleotide-Free Regimens
NUCLEOSIDE/NUCLEOTIDE-FREE REGIMENS would be the most cost-effective treatment strategy. Administrating newer and more powerful NUC long before OLT and serum HBV DNA negativity at OLT may be required for NUC-free regimens. However, even with the successful outcomes of HBIG-free regimens involving newer NUC, NUC-free regimens must be adopted with discretion, as HBV DNA recurred in all patients in whom NUC were discontinued.37 To administrate these NUC-free regimens, intrahepatic HBV DNA, cccDNA status must be controlled at limits below the positive titers. Lenci et al. described the possibility of NUC-free regimens.41 They screened patients with the following criteria: OLT due to HBV-related liver cirrhosis performed at least 3 years earlier; adherence to anti-HBV prophylaxis with HBIG and NUC since OLT; evidence of undetectable serum HBV DNA at the time of OLT; sustained normal biochemistry and sustained undetectable serum HBV DNA at the time of OLT; and undetectable total and cccDNA in liver tissue before discontinuing NUC. At first, HBIG was withdrawn and then NUC (LAM) was discontinued 6 months later if serum HBV DNA, liver tissue HBV DNA and cccDNA were undetectable. Twenty-five of 30 patients had no signs of HBV recurrence after LAM withdrawal. Only one patient who required further NUC was successfully treated with the newer NUC, TDF. Even with LAM, the criteria were effective enough to select candidate patients who did not require NUC administration. Even if HBV recurs, newer NUC may be powerful enough to control viral activity.
Serum hepatitis B core-related antigen (HBcrAg) correlates with intrahepatic cccDNA42 and intrahepatic cccDNA in the post-OLT setting.7,43 Patients fulfilling Lenci's criteria with serum HBcrAg negativity instead of intrahepatic cccDNA negativity may be good candidates for NUC-free regimens.
Value of Active Immunization with HBV Vaccine
THE ACTIVE IMMUNIZATION of post-OLT recipients with HBV vaccine is emerging. Vaccines containing HBsAg are widely applied and considered 90% or more effective and reasonably safe, although anaphylaxis may occur in yeast-sensitive individuals.44 The US Food and Drug Administration and the Institute of Medicine reviewed deaths after vaccinations reported to the US Vaccine Adverse Event Reporting System (VAERS) between 1990 and 1991 and found no associations with HBV vaccines.45 However, most studies of post-OLT HBV vaccination have found low response rates, even with doubled concentrations or prolonged administration of vaccines (Table 2).46,47
Table 2.
Post OLT HBV-vaccine administration trials
| Methods | No. of patients | Definition of success | Success rate (%) | Reference no. | Year published |
|---|---|---|---|---|---|
| Liver cirrhosis | |||||
| Novel adjuvant MPL/QS2 vaccine for 0, 4, 16, 18 weeks | 16 | HBsAb >500 IU/L without HBIG | 80 | 48 | 2007 |
| Experimental adjuvant vaccine for 0, 1, 2, 6, 12 months | 8 | HBsAb >500 IU/L, 18 months without HBIG | 25 | 49 | 2005 |
| 40 mg for 0, 1, 2, 6, 7, 8 months | 18 | HBsAb >500 IU/L, 12 weeks after last vaccination | 0 | 50 | 2009 |
| 10–20 mg/month with minimal immune suppression | 17 | HBsAb >100 IU/L, without HBIG | 64 | 51 | 2009 |
| 20 mg/month of MPL adjuvant for 12 months | 18 | HBsAb >100 IU/L, 18 months without HBIG | 44.4 | 52 | 2010 |
| 20 mg/month | 22 | HBsAb >100 IU/L, 6 months without HBIG | 40 | 53 | 2012 |
| 20 mg/month | 15 | HBsAb >100 IU/L, 3 months without HBIG | 0 | 47 | 2011 |
| 40 mg 0, 1, 2, 3 months, 20 mg 4, 5, 6 months | 50 | HBsAb >60 IU/L, 3 months without HBIG | 24.6 | 54 | 2013 |
| 40 mg 0, 7, 14, 28 days, 20 mg 2, 3, 4 months | 45 | HBsAb >60 IU/L, 3 months without HBIG | 8.8 | 54 | 2013 |
| 40 mg 0, 1, 6 months | 17 | HBsAb >10 IU/L without HBIG | 82 | 55 | 2000 |
| 40 mg for 0, 1, 2, 3, 4, 5 months | 52 | HBsAb >10 IU/L without HBIG | 7.7 | 56 | 2005 |
| 40 mg for 0, 1, 6 months and additional 0, 1, 2 months if no-response | 14 | HBsAb >10 IU/L, 3 months without HBIG | 7 | 57 | 2005 |
| 20–40 mg for 0, 1, 6 months | 12 | HBsAb >10 IU/L, 16 months without HBIG | 0 | 58 | 2010 |
| 40 mg for 0, 1, 2, 6, 7, 8 months | 7 | HBsAb >10 IU/L without HBIG | 0 | 59 | 2006 |
| Acute liver failure | |||||
| 20 mg/month | 5 | HBsAb >100 IU/L, 6 months without HBIG | 100 | 53 | 2012 |
| 10–20 mg/month with minimal immunosuppression | 3 | HBsAb >100 IU/L without HBIG | 66 | 51 | 2009 |
| Experimental adjuvant vaccine for 0, 1, 2, 6, 12 months | 2 | HBsAb >500 IU/L, 18 months without HBIG | 100 | 49 | 2005 |
HBIG, hepatitis B immunoglobulin; HBsAb, hepatitis B surface antibody; HBV, hepatitis B virus; OLT, orthotopic liver transplantation.
The published work in Table 2 was selected from 139 PubMed search results between 2000 and March 2014 using the terms “HBV”, “liver transplantation” and “vaccine”. Among them, 20 studies showed the effects of vaccination. Follow-up studies with additional vaccine protocols were omitted and the remaining 15 original studies are included in the table. Patients who had not been HBV carriers (such as adult patients with acute liver failure due to sexual transmission and non-chronic HBV carriers with hepatitis B core antibody [anti-HBc antibody] positive donor livers) are good candidates for vaccination.47,60 Patients with acute HBV infection who have received OLT are often positive for anti-HBs even before OLT, and have powerful immune responses. Such patients should respond well to vaccination because they have not developed tolerance to HBV, unlike chronic carriers. However, some chronic HBV carriers do respond to vaccination and thus further studies should clarify differences between good and poor responders.
Whether or not Immune Tolerant Chronic HBV Carriers Can Generate Active Anti-Hbs as Immune Response to Vaccination
THE EFFECTS OF post-OLT vaccination in patients with liver cirrhosis have been disappointing.46,47 Understanding how different cohorts respond to HBV vaccination will be critical to the design of safe, cost-effective and custom-designed prophylactic protocols.
Because non-carriers respond well to the HBV vaccination, immune tolerance should play a large role in this process. Analyses of the immunological characteristics of HBsAg positive young carriers and aged patients with active hepatitis have identified comparable peripheral T-cell pro-inflammatory cytokine production capacity and HBV-specific γ-interferon responses.61 These findings indicate that tolerant carriers can react with HBV antigens and can generate active immunity against HBV vaccination. Good responses to newer NUC after OLT cause HBV DNA to decrease even in the liver, and this may recover exhausted HBV-specific T cells to react with HBV.
Sanchez-Fueyo et al. reported an 82% response to HBV vaccination after OLT during 2000.55 They used three cycles of a double-dose recombinant HBsAg vaccine for immunization over 6 months. The cohort included six patients with acute infection and 11 chronic carriers who were HBV DNA negative at OLT. However, recent reports have found that chronic HBV carrier recipients including positive HBV DNA at OLT do not respond well, with response rates being mostly less than 30% (Table 2).49,50,54,56–59,62 Tahara et al. reported 64.7% positive responses to experimentally minimized treatment with immunosuppressants.51 They found that vaccination in patients with a donor-specific mixed lymphocyte reaction hyporesponse was successful. Another protocol of repeated vaccine administration resulted in the successful immunization of 40% of patients with post-OLT liver cirrhosis.53 The donors for good responders were their spouses with high anti-HBs titers before donation. The recipients had probably infected their spouses with HBV after marriage, resulting in the non-tolerant anti-HBs boost. The immune systems of these donors would not have developed tolerance to the virus63 and the adoptive immune transfer of the HBV-specific immune response was achieved.64 The anti-HBs titer of the donors should be high for the successful transfer of immune memory to recipients. Luo et al. have shown that a high anti-HBs titer (>1000 IU/L) in donors is essential for adoptive transfer.65 These results suggest that pre-OLT HBV vaccination for candidate living donors may facilitate improved post-OLT vaccine responses in recipients with liver cirrhosis. The success rates of several experimental adjuvant vaccines is up to 80%.46,48,49,52
The vaccine response depends on immune tolerance to the virus in both recipients and donors. The liver is the largest immune organ in the abdomen; therefore, it plays a critical role in immune responses. Multiple populations of non-hematopoietic liver cells, including sinusoidal endothelial cells, stellate cells located in the sub-endothelial space and liver parenchymal cells, can function as antigen-presenting cells.66 The viral-specific immune competence of the grafted liver may overcome general immunotolerance to the virus in chronic HBV carriers.
Precautions Against Anti-HBS Escape Mutations in the Post-OLT Setting
AS CONTINUOUS HBIG usage is usual in the post-OLT setting, anti-HBs escape mutants should be carefully monitored even after patients with positive anti-HBs become clinically stable. A single administration of HBIG induces anti-HBs escape mutations even in primarily HBV non-infected patients who receive a liver from donors who are positive for anti-HBc.67 The calculated rate of such HBV activation de novo by anti-HBs escape mutations is 12% at 5 years. The frequency of vaccine escape mutants is reportedly increasing, especially among young adults who were vaccinated soon after birth in countries that have implemented universal vaccine programs, such as Taiwan.68 Vaccine-induced anti-HBs titer decreases after entering young adulthood and permits the transmission of HBV via exposure. The administration of anti-HBs containing HBIG and vaccine-induced relatively weak anti-HBs could induce anti-HBs escape mutants in the post-OLT setting. Even after an anti-HBs response is acquired via vaccination, HBV DNA must be strictly followed up to ensure that complementary NUC can be added if necessary.
Conclusion
POST-OLT HEPATITIS B recurrence is now well controlled with a combination of HBIG and NUC. Because HBIG is a blood-based product and extremely expensive, its use will hopefully decline. Several clinical studies have found that the administration of newer NUC precludes the lifetime administration of HBIG, which is applied only to maintain minimal anti-HBs titers or as short-term post-OLT therapy. Active immunization is effective for patients with acute liver failure who are not immune-tolerant to HBV. Vaccination is not sufficiently effective for patients with liver cirrhosis, but it can be attempted because the cost is low and side-effects are minimal.
References
- 1.Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzl TE. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology. 1991;13:619–626. [PMC free article] [PubMed] [Google Scholar]
- 2.Samuel D, Muller R, Alexander G, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993;329:1842–1847. doi: 10.1056/NEJM199312163292503. [DOI] [PubMed] [Google Scholar]
- 3.Grellier L, Mutimer D, Ahmed M, et al. Lamivudine prophylaxis against reinfection in liver transplantation for hepatitis B cirrhosis. Lancet. 1996;348:1212–1215. doi: 10.1016/s0140-6736(96)04444-3. [DOI] [PubMed] [Google Scholar]
- 4.Perrillo R, Rakela J, Dienstag J, et al. Multicenter study of lamivudine therapy for hepatitis B after liver transplantation. Lamivudine Transplant Group. Hepatology. 1999;29:1581–1586. doi: 10.1002/hep.510290507. [DOI] [PubMed] [Google Scholar]
- 5.Markowitz JS, Martin P, Conrad AJ, et al. Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology. 1998;28:585–589. doi: 10.1002/hep.510280241. [DOI] [PubMed] [Google Scholar]
- 6.Schilling R, Ijaz S, Davidoff M, et al. Endocytosis of hepatitis B immune globulin into hepatocytes inhibits the secretion of hepatitis B virus surface antigen and virions. J Virol. 2003;77:8882–8892. doi: 10.1128/JVI.77.16.8882-8892.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasunaka T, Takaki A, Yagi T, et al. Serum hepatitis B virus DNA before liver transplantation correlates with HBV reinfection rate even under successful low-dose hepatitis B immunoglobulin prophylaxis. Hepatol Int. 2011 doi: 10.1007/s12072-011-9265-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neumann AU, Phillips S, Levine I, et al. Novel mechanism of antibodies to hepatitis B virus in blocking viral particle release from cells. Hepatology. 2010;52:875–885. doi: 10.1002/hep.23778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villet S, Billioud G, Pichoud C, et al. In vitro characterization of viral fitness of therapy-resistant hepatitis B variants. Gastroenterology. 2009;136:168–176. doi: 10.1053/j.gastro.2008.09.068. [DOI] [PubMed] [Google Scholar]
- 10.Johnson PJ, Wansbrough-Jones MH, Portmann B, et al. Familial HBsAg-positive hepatoma: treatment with orthotopic liver transplantation and specific immunoglobulin. Br Med J. 1978;1:216. doi: 10.1136/bmj.1.6107.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Ari Z, Mor E, Shapira Z, Tur-Kaspa R. Long-term experience with lamivudine therapy for hepatitis B virus infection after liver transplantation. Liver Transpl. 2001;7:113–117. doi: 10.1053/jlts.2001.21308. [DOI] [PubMed] [Google Scholar]
- 12.Fontana RJ, Hann HW, Wright T, et al. A multicenter study of lamivudine treatment in 33 patients with hepatitis B after liver transplantation. Liver Transpl. 2001;7:504–510. doi: 10.1053/jlts.2001.24896. [DOI] [PubMed] [Google Scholar]
- 13.Dumortier J, Chevallier P, Scoazec JY, Berger F, Boillot O. Combined lamivudine and hepatitis B immunoglobulin for the prevention of hepatitis B recurrence after liver transplantation: long-term results. Am J Transplant. 2003;3:999–1002. doi: 10.1034/j.1600-6143.2003.00191.x. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida EM, Erb SR, Partovi N, et al. Liver transplantation for chronic hepatitis B infection with the use of combination lamivudine and low-dose hepatitis B immune globulin. Liver Transpl Surg. 1999;5:520–525. doi: 10.1002/lt.500050602. [DOI] [PubMed] [Google Scholar]
- 15.Marzano A, Salizzoni M, Debernardi-Venon W, et al. Prevention of hepatitis B virus recurrence after liver transplantation in cirrhotic patients treated with lamivudine and passive immunoprophylaxis. J Hepatol. 2001;34:903–910. doi: 10.1016/s0168-8278(01)00080-0. [DOI] [PubMed] [Google Scholar]
- 16.Roche B, Samuel D. Evolving strategies to prevent HBV recurrence. Liver Transpl. 2004;10:S74–85. doi: 10.1002/lt.20258. [DOI] [PubMed] [Google Scholar]
- 17.Yao FY, Osorio RW, Roberts JP, et al. Intramuscular hepatitis B immune globulin combined with lamivudine for prophylaxis against hepatitis B recurrence after liver transplantation. Liver Transpl Surg. 1999;5:491–496. doi: 10.1002/lt.500050605. [DOI] [PubMed] [Google Scholar]
- 18.Gane EJ, Angus PW, Strasser S, et al. Lamivudine plus low-dose hepatitis B immunoglobulin to prevent recurrent hepatitis B following liver transplantation. Gastroenterology. 2007;132:931–937. doi: 10.1053/j.gastro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Rosenau J, Bahr MJ, Tillmann HL, et al. Lamivudine and low-dose hepatitis B immune globulin for prophylaxis of hepatitis B reinfection after liver transplantation possible role of mutations in the YMDD motif prior to transplantation as a risk factor for reinfection. J Hepatol. 2001;34:895–902. doi: 10.1016/s0168-8278(01)00089-7. [DOI] [PubMed] [Google Scholar]
- 20.Jiang L, Yan L, Li B, et al. Prophylaxis against hepatitis B recurrence posttransplantation using lamivudine and individualized low-dose hepatitis B immunoglobulin. Am J Transplant. 2010;10:1861–1869. doi: 10.1111/j.1600-6143.2010.03208.x. [DOI] [PubMed] [Google Scholar]
- 21.Di Paolo D, Tisone G, Piccolo P, Lenci I, Zazza S, Angelico M. Low-dose hepatitis B immunoglobulin given “on demand” in combination with lamivudine: a highly cost-effective approach to prevent recurrent hepatitis B virus infection in the long-term follow-up after liver transplantation. Transplantation. 2004;77:1203–1208. doi: 10.1097/01.tp.0000118904.63669.eb. [DOI] [PubMed] [Google Scholar]
- 22.Takaki A, Yagi T, Iwasaki Y, et al. Short-term high-dose followed by long-term low-dose hepatitis B immunoglobulin and lamivudine therapy prevented recurrent hepatitis B after liver transplantation. Transplantation. 2007;83:231–233. doi: 10.1097/01.tp.0000246310.75638.86. [DOI] [PubMed] [Google Scholar]
- 23.Neff GW, O'Brien CB, Nery J, et al. Outcomes in liver transplant recipients with hepatitis B virus: resistance and recurrence patterns from a large transplant center over the last decade. Liver Transpl. 2004;10:1372–1378. doi: 10.1002/lt.20277. [DOI] [PubMed] [Google Scholar]
- 24.Buti M, Mas A, Prieto M, et al. A randomized study comparing lamivudine monotherapy after a short course of hepatitis B immune globulin (HBIg) and lamivudine with long-term lamivudine plus HBIg in the prevention of hepatitis B virus recurrence after liver transplantation. J Hepatol. 2003;38:811–817. doi: 10.1016/s0168-8278(03)00087-4. [DOI] [PubMed] [Google Scholar]
- 25.Marzano A, Lampertico P, Mazzaferro V, et al. Prophylaxis of hepatitis B virus recurrence after liver transplantation in carriers of lamivudine-resistant mutants. Liver Transpl. 2005;11:532–538. doi: 10.1002/lt.20393. [DOI] [PubMed] [Google Scholar]
- 26.Angus PW, Patterson SJ, Strasser SI, McCaughan GW, Gane E. A randomized study of adefovir dipivoxil in place of HBIG in combination with lamivudine as post-liver transplantation hepatitis B prophylaxis. Hepatology. 2008;48:1460–1466. doi: 10.1002/hep.22524. [DOI] [PubMed] [Google Scholar]
- 27.Gane EJ, Patterson S, Strasser SI, McCaughan GW, Angus PW. Combination of lamivudine and adefovir without hepatitis B immune globulin is safe and effective prophylaxis against hepatitis B virus recurrence in hepatitis B surface antigen-positive liver transplant candidates. Liver Transpl. 2013;19:268–274. doi: 10.1002/lt.23600. [DOI] [PubMed] [Google Scholar]
- 28.Cholongitas E, Vasiliadis T, Antoniadis N, Goulis I, Papanikolaou V, Akriviadis E. Hepatitis B prophylaxis post liver transplantation with newer nucleos(t)ide analogues after hepatitis B immunoglobulin discontinuation. Transpl Infect Dis. 2012;14:479–487. doi: 10.1111/j.1399-3062.2012.00741.x. [DOI] [PubMed] [Google Scholar]
- 29.Cai CJ, Lu MQ, Chen YH, Zhao H, Li MR, Chen GH. Clinical study on prevention of HBV re-infection by entecavir after liver transplantation. Clin Transplant. 2012;26:208–215. doi: 10.1111/j.1399-0012.2011.01448.x. [DOI] [PubMed] [Google Scholar]
- 30.Ueda Y, Marusawa H, Kaido T, et al. Efficacy and safety of prophylaxis with entecavir and hepatitis B immunoglobulin in preventing hepatitis B recurrence after living-donor liver transplantation. Hepatol Res. 2013;43:67–71. doi: 10.1111/j.1872-034X.2012.01020.x. [DOI] [PubMed] [Google Scholar]
- 31.Gao YJ, Zhang M, Jin B, et al. A clinical-pathological analysis of hepatitis B virus recurrence after liver transplantation in Chinese patients. J Gastroenterol Hepatol. 2014;29:554–560. doi: 10.1111/jgh.12404. [DOI] [PubMed] [Google Scholar]
- 32.Perrillo R, Buti M, Durand F, et al. Entecavir and hepatitis B immune globulin in patients undergoing liver transplantation for chronic hepatitis B. Liver Transpl. 2013;19:887–895. doi: 10.1002/lt.23690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi NJ, Choi JY, Suh KS, et al. Post-transplantation sequential entecavir monotherapy following 1-year combination therapy with hepatitis B immunoglobulin. J Gastroenterol. 2013;48:1401–1410. doi: 10.1007/s00535-013-0761-x. [DOI] [PubMed] [Google Scholar]
- 34.Stravitz RT, Shiffman ML, Kimmel M, et al. Substitution of tenofovir/emtricitabine for Hepatitis B immune globulin prevents recurrence of Hepatitis B after liver transplantation. Liver Int. 2012;32:1138–1145. doi: 10.1111/j.1478-3231.2012.02770.x. [DOI] [PubMed] [Google Scholar]
- 35.Wesdorp DJ, Knoester M, Braat AE, et al. Nucleoside plus nucleotide analogs and cessation of hepatitis B immunoglobulin after liver transplantation in chronic hepatitis B is safe and effective. J Clin Virol. 2013;58:67–73. doi: 10.1016/j.jcv.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 36.Fung J, Cheung C, Chan SC, et al. Entecavir monotherapy is effective in suppressing hepatitis B virus after liver transplantation. Gastroenterology. 2011;141:1212–1219. doi: 10.1053/j.gastro.2011.06.083. [DOI] [PubMed] [Google Scholar]
- 37.Wadhawan M, Gupta S, Goyal N, Taneja S, Kumar A. Living related liver transplantation for hepatitis B-related liver disease without hepatitis B immune globulin prophylaxis. Liver Transpl. 2013;19:1030–1035. doi: 10.1002/lt.23692. [DOI] [PubMed] [Google Scholar]
- 38.Chao DC, Hu KQ. Update on rescue therapies in patients with lamivudine-resistant chronic hepatitis B. Drug Des Devel Ther. 2013;7:777–788. doi: 10.2147/DDDT.S33947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitrinos KM, Corsa A, Liu Y, et al. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology. 2014;59:434–442. doi: 10.1002/hep.26686. [DOI] [PubMed] [Google Scholar]
- 40.Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 41.Lenci I, Tisone G, Di Paolo D, et al. Safety of complete and sustained prophylaxis withdrawal in patients liver-transplanted for HBV-related cirrhosis at low risk of HBV recurrence. J Hepatol. 2011;55:587–593. doi: 10.1016/j.jhep.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 42.Kimura T, Rokuhara A, Sakamoto Y, et al. Sensitive enzyme immunoassay for hepatitis B virus core-related antigens and their correlation to virus load. J Clin Microbiol. 2002;40:439–445. doi: 10.1128/JCM.40.2.439-445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuzaki T, Tatsuki I, Otani M, et al. Significance of hepatitis B virus core-related antigen and covalently closed circular DNA levels as markers of hepatitis B virus re-infection after liver transplantation. J Gastroenterol Hepatol. 2013;28:1217–1222. doi: 10.1111/jgh.12182. [DOI] [PubMed] [Google Scholar]
- 44.DiMiceli L, Pool V, Kelso JM, Shadomy SV, Iskander J Team VAERS. Vaccination of yeast sensitive individuals: review of safety data in the US vaccine adverse event reporting system (VAERS) Vaccine. 2006;24:703–707. doi: 10.1016/j.vaccine.2005.07.069. [DOI] [PubMed] [Google Scholar]
- 45.Zhou W, Pool V, Iskander JK, et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS) – United States, 1991-2001. 2003;52:1–24. Morbidity and mortality weekly report Surveillance summaries Jan 24; [PubMed] [Google Scholar]
- 46.Rosenau J, Hooman N, Rifai K, et al. Hepatitis B virus immunization with an adjuvant containing vaccine after liver transplantation for hepatitis B-related disease: failure of humoral and cellular immune response. Transpl Int. 2006;19:828–833. doi: 10.1111/j.1432-2277.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 47.Ishigami M, Kamei H, Nakamura T, et al. Different effect of HBV vaccine after liver transplantation between chronic HBV carriers and non-HBV patients who received HBcAb-positive grafts. J Gastroenterol. 2011;46:367–377. doi: 10.1007/s00535-010-0313-6. [DOI] [PubMed] [Google Scholar]
- 48.Bauer T, Gunther M, Bienzle U, Neuhaus R, Jilg W. Vaccination against hepatitis B in liver transplant recipients: pilot analysis of cellular immune response shows evidence of HBsAg-specific regulatory T cells. Liver Transpl. 2007;13:434–442. doi: 10.1002/lt.21061. [DOI] [PubMed] [Google Scholar]
- 49.Starkel P, Stoffel M, Lerut J, Horsmans Y. Response to an experimental HBV vaccine permits withdrawal of HBIg prophylaxis in fulminant and selected chronic HBV-infected liver graft recipients. Liver Transpl. 2005;11:1228–1234. doi: 10.1002/lt.20464. [DOI] [PubMed] [Google Scholar]
- 50.Yamashiki N, Sugawara Y, Tamura S, et al. Double-dose double-phase use of second generation hepatitis B virus vaccine in patients after living donor liver transplantation: not an effective measure in transplant recipients. Hepatol Res. 2009;39:7–13. doi: 10.1111/j.1872-034X.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- 51.Tahara H, Tanaka Y, Ishiyama K, et al. Successful hepatitis B vaccination in liver transplant recipients with donor-specific hyporesponsiveness. Transpl Int. 2009;22:805–813. doi: 10.1111/j.1432-2277.2009.00864.x. [DOI] [PubMed] [Google Scholar]
- 52.Di Paolo D, Lenci I, Cerocchi C, et al. One-year vaccination against hepatitis B virus with a MPL-vaccine in liver transplant patients for HBV-related cirrhosis. Transpl Int. 2010;23:1105–1112. doi: 10.1111/j.1432-2277.2010.01104.x. [DOI] [PubMed] [Google Scholar]
- 53.Takaki A, Yagi T, Yasunaka T, et al. Which patients respond best to hepatitis B vaccination after a hepatitis B virus-related liver transplantation? J Gastroenterol. 2013;48:1373–1383. doi: 10.1007/s00535-013-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng L, Niu Y, Chen H, et al. Immunogenicity of different hepatitis B virus vaccination schedules in liver transplant recipients. Hepatol Res. 2013;43:495–501. doi: 10.1111/j.1872-034X.2012.01102.x. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez-Fueyo A, Rimola A, Grande L, et al. Hepatitis B immunoglobulin discontinuation followed by hepatitis B virus vaccination: a new strategy in the prophylaxis of hepatitis B virus recurrence after liver transplantation. Hepatology. 2000;31:496–501. doi: 10.1002/hep.510310233. [DOI] [PubMed] [Google Scholar]
- 56.Lo CM, Liu CL, Chan SC, Lau GK, Fan ST. Failure of hepatitis B vaccination in patients receiving lamivudine prophylaxis after liver transplantation for chronic hepatitis B. J Hepatol. 2005;43:283–287. doi: 10.1016/j.jhep.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 57.Karasu Z, Ozacar T, Akarca U, et al. HBV vaccination in liver transplant recipients: not an effective strategy in the prophylaxis of HBV recurrence. J Viral Hepat. 2005;12:212–215. doi: 10.1111/j.1365-2893.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- 58.Weber NK, Forman LM, Trotter JF. HBIg discontinuation with maintenance oral anti-viral therapy and HBV vaccination in liver transplant recipients. Dig Dis Sci. 2010;55:505–509. doi: 10.1007/s10620-009-0999-6. [DOI] [PubMed] [Google Scholar]
- 59.Di Paolo D, Lenci I, Trinito MO, et al. Extended double-dosage HBV vaccination after liver transplantation is ineffective, in the absence of lamivudine and prior wash-out of human Hepatitis B immunoglobulins. Dig Liver Dis. 2006;38:749–754. doi: 10.1016/j.dld.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Zhou L, Zheng SS. Clinical management of hepatitis B virus infection correlated with liver transplantation. Hepatobiliary Pancreat Dis Int. 2010;9:15–21. [PubMed] [Google Scholar]
- 61.Kennedy PT, Sandalova E, Jo J, et al. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology. 2012;143:637–645. doi: 10.1053/j.gastro.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 62.Rosenau J, Hooman N, Hadem J, et al. Failure of hepatitis B vaccination with conventional HBsAg vaccine in patients with continuous HBIG prophylaxis after liver transplantation. Liver Transpl. 2007;13:367–373. doi: 10.1002/lt.21003. [DOI] [PubMed] [Google Scholar]
- 63.Wursthorn K, Wedemeyer H, Manns MP. Managing HBV in patients with impaired immunity. Gut. 2010;59:1430–1445. doi: 10.1136/gut.2009.195834. [DOI] [PubMed] [Google Scholar]
- 64.Schumann A, Lindemann M, Valentin-Gamazo C, et al. Adoptive immune transfer of hepatitis B virus specific immunity from immunized living liver donors to liver recipients. Transplantation. 2009;87:103–111. doi: 10.1097/TP.0b013e31818bfc85. [DOI] [PubMed] [Google Scholar]
- 65.Luo Y, Lo CM, Cheung CK, Lau GK, Fan ST, Wong J. Identification of hepatitis B virus-specific lymphocytes in human liver grafts from HBV-immune donors. Liver Transpl. 2007;13:71–79. doi: 10.1002/lt.20887. [DOI] [PubMed] [Google Scholar]
- 66.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 67.Ueda Y, Marusawa H, Egawa H, et al. De novo activation of HBV with escape mutations from hepatitis B surface antibody after living donor liver transplantation. Antivir Ther. 2011;16:479–487. doi: 10.3851/IMP1771. [DOI] [PubMed] [Google Scholar]
- 68.Lai MW, Lin TY, Tsao KC, et al. Increased seroprevalence of HBV DNA with mutations in the s gene among individuals greater than 18 years old after complete vaccination. Gastroenterology. 2012;143:400–407. doi: 10.1053/j.gastro.2012.05.002. [DOI] [PubMed] [Google Scholar]


