Abstract
People with lung cancer experience health-related stigma that is related to poorer psychosocial and quality of life outcomes. The present Phase 1 study applied mixed methods to test the acceptability of an acceptance-focused cognitive behavioural intervention targeting stigma for this patient group. Fourteen lung cancer patients completed a 6-week Psychological Wellness intervention with pre- and post-test outcome measures of psychological and cancer-specific distress, depression, health-related stigma and quality of life. In-depth interviews applying interpretative phenomenological analysis assessed participants' experiences of the intervention. Moderate to large improvements were observed in psychological (ηp2 = 0.182) and cancer-specific distress (ηp2 = 0.056); depression (ηp2 = 0.621); health-related stigma (ηp2 = 0.139). In contrast, quality of life declined (ηp2 = 0.023). The therapeutic relationship; self-management of distress; and relationship support were highly valued aspects of the intervention. Barriers to intervention included avoidance and practical issues. The lung cancer patients who completed the Psychological Wellness intervention reported improvements in psychological outcomes and decreases in stigma in the face of declining quality of life with patients reporting personal benefit from their own perspectives. A randomised controlled trial is warranted to establish the effectiveness of this approach.
Keywords: lung cancer, psychological, quality of life
Introduction
Internationally, lung cancer is the most common cancer and the most common cause of cancer death (International Agency for Research on Cancer, 2014). In Australia lung cancer is the fourth most commonly diagnosed cancer and the leading cause of cancer death with 11 270 new diagnoses in 2012 and 8099 deaths in 2010; and 5-year survival around 13% for men and 17% for women (AIHW & AACR 2012). The most common cause of lung cancer is smoking with 15% of lung cancer patients presenting as non-smokers. Exposure to other carcinogens such as occupational exposures and ionising radiation are also implicated (Cagle et al. 2012). Lung cancer is therefore a major public health concern.
Common physical symptoms for lung cancer include breathlessness, coughing and fatigue and these effects contribute to fear, depression and anxiety for patients. Breathlessness can cause anxiety, panic attacks and fear of impending death (Tanaka et al. 2002); and persistent coughing can lead to sleep disturbances and exhaustion (Vena et al. 2006). Lung cancer patients report higher levels of psychological distress, greater unmet needs and a greater risk of suicide compared with other patient groups. Up to 62% of lung cancer patients report significant psychological distress (Graves et al. 2007); and for many patients this distress does not ameliorate over time, and indeed may worsen (Néron et al. 2007). Patients with lung cancer have a higher risk of suicide compared with those with other cancers such as breast, prostate and colorectal (Misono et al. 2008). Finally, lung cancer patients report higher unmet psychological needs than other cancer patients and more rehabilitative problems of which psychological concerns are central (Li & Girgis 2006). Therefore, evidence-based interventions to enhance psychological adjustment are crucial for lung cancer patients.
Problematically, people with lung cancer, more so than patients with other cancers, feel stigmatised by their disease and this increases distress (Chambers et al. 2012). Stigma occurs when society labels someone as tainted on the basis of an attribute that marks them out as different (Goffman 1963). In lung cancer, health-related stigma results from the association between the disease and smoking and perception of the disease as self-inflicted; the high mortality rates; and perceptions about the type of death that may be experienced (Chambers et al. 2012). Stigma is relationship and context-specific where a specific attribute is associated with a negative evaluation that can lead to negative treatment or discrimination, stereotype activation and identity threat (Major & O'Brien 2005). When internalised these negative evaluations lead to shame or guilt and fear of being discriminated against. Responses to stigma include: increased stress and poor coping leading to negative mental and physical health outcomes and the amplification of psychosocial morbidity (Heijnders & Van Der Meij 2006). A systematic review concluded that lung cancer patients consistently report stigma as part of their cancer experience and report social isolation owing to the perception of lung cancer as a disease that leads to a horrible death; feel stigmatised by the prevailing view that all lung cancer is self-inflicted and smoking-related; and fear that treatment may be denied and/or is futile (Chambers et al. 2012).
Despite this, health-related stigma has not been addressed in supportive care in lung cancer. A Cochrane review (2011) of interventions for improving well-being after lung cancer identified only two trials that applied psychological therapies to reduce psychological distress after lung cancer: neither of these targeted health-related stigma (Rueda et al. 2011). Linn et al. (1982) trialled grief counselling with end stage cancer hospital inpatients (50% lung cancer; all male) and found benefits in depression after 3 months of treatment. However this study only targeted patients approaching the end of life and attrition was high (71% at 3 months). Porter et al. (2011) compared 14 telephone sessions to caregiver–patient dyads delivering either: caregiver assisted coping skills training or education/support. The coping skills training applied a cognitive behavioural approach, while the education/support arm included disease/treatment education with supportive discussion. No differences were found between treatments. More recently a trial of online routine screening for distress in lung cancer patients found that patients who received full screening for distress with personalised triage reported fewer problems with coping and family conflict and were more likely to access services compared with patients who were only screened (Carlson et al. 2013). However, few patients actually accessed supportive care services, even with triage. Hence, while we know lung cancer patients have a high need for psychological support and are at high risk of adverse events (e.g. clinically high distress, suicide), research in this area is scant and no interventions have been reported that targeted health-related stigma.
In this regard, situational, individual and social factors shape a person's cognitive appraisals of the threat lung cancer poses to their health and future, and their identity; and these appraisals in turn shape the person's psychological outcomes (Chambers et al. 2012). Specifically, social representations of lung cancer and associated situational cues (e.g. being questioned repeatedly about smoking status; viewing anti-tobacco advertisements) represent the context that strongly cues the patient to have negative cognitive appraisals about their lung cancer and themselves. For people with lung cancer their cognitive appraisals about their cancer are crucial intervention targets to ameliorate the negative effects of health-related stigma and to reduce psychological distress. A cognitive behavioural approach with a focus on lessening the effect of negative appraisals and stigma appears relevant then to reduce the psychological distress associated with the experience of lung cancer. Meta-analyses have shown cognitive behavioural therapy (CBT) to reduce depression and anxiety in cancer patients with individualised interventions, including tele-based approaches, more effective than group formats (Osborn et al. 2006). Over the past decade acceptance-based methods have been considered for the enhancement of CBT (Segal et al. 2002). Acceptance strategies target not only the content of presenting concerns; but also the function of and the relationship to these symptoms (i.e. experiential avoidance). Traditional CBT techniques emphasise how realistic or helpful thoughts about cancer may be, whereas acceptance strategies focus on increasing a person's ability to tolerate unpleasant experiences including distressing thoughts and feelings. Preliminary evidence suggests that addressing these ‘second-order’ processes enhances motivation for utilising CBT strategies and supports longer-term maintenance of CBT treatment gains (Forman et al. 2007). Moderate to large effect sizes have been associated with programmes integrating acceptance-based components for the CBT treatment of anxiety and depression (Hoffman et al. 2010). Mindfulness-based cognitive therapy (which is an integration of CBT and a large dose of acceptance-based methods) has been shown to be effective in decreasing distress in a mixed cancer patient sample [effect size (ES) 0.53–0.83] (Foley et al. 2010). To date, this approach has not been tested with lung cancer patients.
Accordingly, the current study describes the development and acceptability testing of a six session telephone delivered Psychological Wellness intervention for lung cancer patients that addresses health-related stigma as a primary therapy target through the integration of acceptance-focused strategies within a cognitive behavioural framework.
Method
Participants and procedure
The Human Research Ethics Committee at Griffith University (PSY/03/13/HREC) granted ethical approval to conduct this study. Eligibility criteria were: previous diagnosis of lung cancer; able to read and speak English; over the age of 18 years; and had no current dementia or psychiatric illness. Recruitment was undertaken from March 2013 to September 2013. Participants were a convenience sample recruited from local cancer support networks and cohorts. Potential participants were mailed a cover letter, information sheet, consent form, self-report survey, and reply-paid envelope. From 56 potential participants, 40 agreed to take part in the study and completed baseline assessments, which consisted of a survey and structured telephone interview. Fifteen participants did not commence the intervention after completing the baseline assessment. Out of the 25 participants who commenced the intervention, 14 completed the 3-month follow-up survey. At the 3-month follow-up, all participants were also invited to take part in a semi-structured qualitative interview regarding the intervention. Participants who completed the intervention were asked questions to assess the acceptability of the intervention (n = 22 interviewed). Participants who did not take part in the intervention were asked why they declined to do so (n = 9 interviewed). See Figure 1 for an overview of the recruitment, baseline and follow-up processes.
Figure 1.
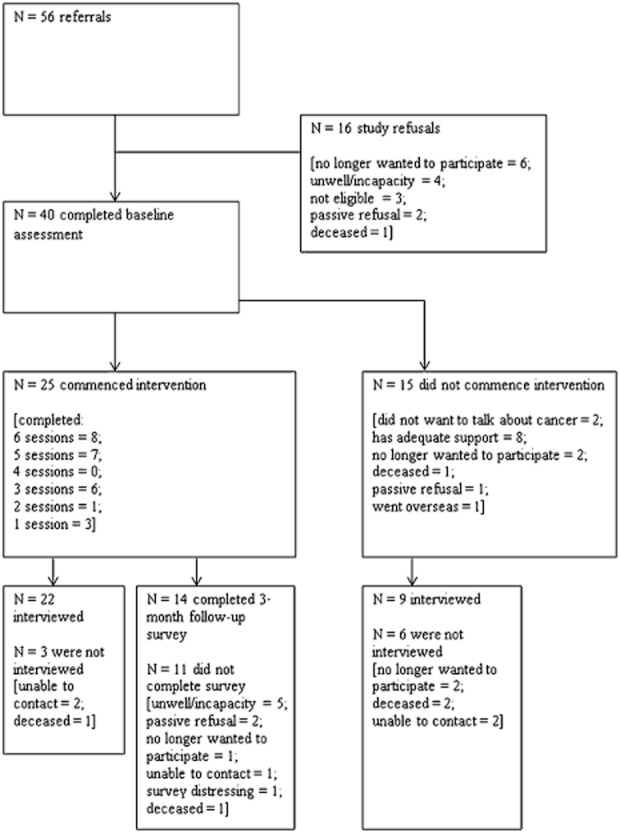
Flowchart of recruitment, participation, data collection and attrition.
Intervention
The Psychological Wellness intervention was based on a tele-based CBT manualised therapy programme for cancer patients that our group has used extensively in both practice and research that includes: psycho-education, skills in stress reduction, problem-solving, cognitive challenging and enhancing relationship support (Hutchison et al. 2011; Chambers et al. 2014). First, to ensure relevance to the illness experience of this patient group we added evidence-based supportive care components for sleeplessness and breathlessness for lung cancer patients (Bredin et al. 1999). Second, an overarching approach of acceptance, in particular to address health-related stigma in lung cancer, was woven into our CBT framework. Acceptance and interrelated strategies such as defusion, mindfulness and value guided action [consistent with acceptance and commitment therapy (Hayes & Wilson 2003)] were targeted to the challenges associated with a cancer that poses high threat to life and wellness; thoughts and feelings associated with situational cues to stigma (e.g. anti-tobacco media; talking about smoking with family, friends and health professionals); and perceptions of self-blame about the cancer. Examples of strategies included recognising and stepping back from negative thoughts; meditation techniques; acceptance of painful feelings; deliberate self-kindness and self-soothing; identifying and orienting towards valued activities.
The intervention was telephone delivered in six weekly 50–55 min sessions and was delivered by telephone to reduce barriers to access from physical illness and geographic location. Participants were provided with tip sheets matching each weekly session; self-help materials including Jon Kabat Zinn's Full Catastrophe Living (Kabat-Zinn 1990); and a meditation CD.
Measures
A structured telephone interview collected demographic variables, smoking behaviours and cancer-related information at baseline. A self-report survey assessing psychological and cancer-related distress; quality of life; depression and stigma about lung cancer was completed at baseline and 3 months subsequently. At the 3-month follow-up all participants were invited to take part in a semi-structured qualitative interview about how helpful the intervention was; what aspects of the intervention were unhelpful or could have been improved; and for patients who did not commence the intervention the reasons for this were explored.
Psychological distress
The Hospital Anxiety and Depression Scale (HADS) provided a measure of an individual's current distress (Zigmond & Snaith 1983). Items included ‘I have lost interest in my appearance’ and ‘I feel restless as if I have to be on the move’. Fourteen items were responded to on a four-point Likert scale. As in previous studies with cancer survivors, the total HADS score was used to represent levels of psychological distress (Smith et al. 2006). In the present study internal consistency for total HADS scores was high (α = 0.87).
Cancer-related distress
The Impact of Events Scale (IES) (Horowitz et al. 1979) measured distress related to lung cancer. Participants were asked to reflect upon how distressing each item has been for them in relation to their cancer experience on a four-point Likert scale from 0 (not at all), 1 (rarely), 3 (sometimes) to 5 (often). Items included ‘I thought about it when I didn't mean to’ and ‘I tried to remove it from my memory’. Higher total scores indicate greater levels of distress. Internal consistency was high (α = 0.90).
Depression
The Centre for Epidemiological Studies Depression Scale (CESD) (Radloff 1977) assessed depression, with items including ‘My sleep was restless’ and ‘I felt that everything I did was an effort’. The CESD consisted of 20 items rated on a scale from 0 (rarely or none of the times – less than 1 day) to 3 (most or all of the time – 5–7 days). High total scores are indicative of greater depressive symptoms. Internal consistency was high (α = 0.85).
Lung cancer-related stigma
The Cataldo Lung Cancer Stigma Scale (CLCSS) (Cataldo et al. 2011) assessed the impact of health-related stigma on people with lung cancer. The scale contained 30 items loading onto four subscales: stigma and shame; social isolation; discrimination; and smoking. Items included ‘I feel guilt because I have lung cancer’ and ‘I was hurt by how people reacted to learning I have lung cancer’. Participants rated each item on a four-point Likert scale ranging from 1 (strongly disagree) to 4 (strongly agree) with higher scores indicating a greater level of perceived stigma. Internal consistencies for the overall measure (α = 0.94) and subscales (α = 0.76 to 0.96) were high.
Quality of life
The Functional Assessment of Cancer Therapy – Lung (FACT-L) (Cella et al. 1993) assessed perceived global quality of life across five domains: physical, social/family, emotional, functional well-being and lung cancer-specific concerns. The FACT-L consists of 36 items rated on a five-point Likert scale ranging from 0 (not at all) to 4 (very much). Items included ‘I get emotional support from my family’ and ‘I am satisfied with how I am coping with my illness’. Higher total scores indicated greater overall well-being. Internal consistency was high (α = 0.87).
Data analysis
Differences between participants who commenced the intervention and those who did not take part in any sessions were examined for demographic, cancer-related and smoking-related variables via t-tests (normally distributed continuous variables), Mann–Whitney tests (skewed continuous variables) and chi-square analyses (categorical variables). The post-intervention CESD variable contained three scores that were two standard deviations above the mean, and these outliers were removed when analysing differences in CESD scores over time. Differences were also assessed via t-tests on baseline psychosocial variables between participants who commenced the intervention and those who did not.
Effect sizes were used to assess efficacy of the intervention as significance tests are often uninformative in the social sciences with small sample sizes (Schmidt 1996). Effect sizes were estimated using partial Eta squared, which assesses variance in outcome variables due to the intervention. Effect sizes were assessed against Cohen's (Cohen 1988) criteria of ηp2 = 0.01 (small), ηp2 = 0.059 (medium) and ηp2 = 0.138 (large).
Interview data were investigated using thematic analysis, based on an interpretative phenomenological framework that is widely used in health psychology research (Riessman 2008). An interpretative phenomenological framework focuses on capturing the lived experience of the participant (Smith & Osborn 2003). The coding approach involved a continual reviewing process with overarching superordinate themes being identified from the component themes through an iterative process. In addition to data pertaining to the semi-structured questions, the interpretative phenomenological framework also allowed for uncovering novel and discordant themes (Riessman 2008). Consistent with quality guidelines, two researchers (BAM, SKC) reviewed the transcripts independently to identify responses that described the experience of the intervention (i.e. component themes and overarching superordinate themes) with corresponding representative quotes (Elliott et al. 1999). Discrepancies were resolved via discussion with verification against the transcript data with involvement of a third coder (JD).
Results
Baseline characteristics
On average, participants who commenced the intervention were 65.12 (SD = 9.59) years of age; were 16.35 (SD = 2.80) years of age when they started smoking; and for those that were no longer smoking it was a median of 5 years since they had quit smoking [interquartile range (IQR) = 1.04 (first quartile)–19.00 (third quartile) years]. Time since diagnosis was a highly skewed variable and the median time was 14.50 months [IQR = 9.25 (first quartile)–22.75 (third quartile) months]. Table 1 outlines descriptive statistics for participants who commenced the intervention. Participants who did not commence the intervention had lower quality of life (M = 83.86, SD = 18.64, P = 0.025) and smoked more cigarettes per day (M = 28.33, SD = 2.89, P = 0.029) compared with participants who started the intervention (quality of life: M = 97.60, SD = 17.15; smoking: M = 13.33, SD = 9.27).
Table 1.
Descriptive statistics for participants who commenced the intervention (n = 25)
| n | % | |
|---|---|---|
| Gender | ||
| Female | 22 | 88 |
| Male | 3 | 12 |
| Current smoking status | ||
| Everyday | 5 | 20 |
| Some days | 2 | 8 |
| Do not currently smoke at all | 13 | 52 |
| Has never smoked | 5 | 20 |
| Relationship status | ||
| In a relationship | 12 | 48 |
| Not in a relationship | 13 | 52 |
| Private health insurance | ||
| Yes | 12 | 48 |
| No | 13 | 52 |
| Education | ||
| High school or below | 15 | 60 |
| Trade or technical college | 8 | 32 |
| University | 2 | 8 |
| Current work status | ||
| Retired | 16 | 64 |
| Unable to work due to illness | 5 | 20 |
| Employed – casual | 2 | 8 |
| Home duties/home carer | 1 | 4 |
| Unemployed/looking for work | 1 | 4 |
| Work capacity since diagnosis | ||
| Working less hours | 11 | 44 |
| Same | 14 | 56 |
| Income per year* | ||
| Less than $74 760 | 21 | 84 |
| $74 760 or greater | 4 | 16 |
Percentages do not always equal 100% due to missing data.
Categories based on average income for Australian earnings (Australian Bureau of Statistics 2014).
Adjustment outcomes
Table 2 displays descriptive statistics and effect sizes for pre- and post-intervention distress, depression, stigma and quality of life. Scores for psychological distress, cancer-related distress, depression and stigma (total score, stigma and shame subscale, and social isolation subscale) showed improvement over time with medium to large effect sizes. In contrast, quality of life worsened although the effect size was small.
Table 2.
Pre- and post-intervention differences in psychosocial variables for participants who commenced the intervention and completed follow-up assessment (n = 14)
| Pre-intervention |
Post-intervention |
||||
|---|---|---|---|---|---|
| M | SD | M | SD | ES | |
| HADS – total score | 13.93 | 7.46 | 10.93 | 6.58 | 0.182 |
| CESD – total score | 15.80 | 6.12 | 7.90 | 5.04 | 0.621 |
| IES – total score | 27.08 | 15.72 | 23.08 | 14.36 | 0.056 |
| CLCSS – stigma & shame | 18.21 | 5.15 | 16.50 | 4.69 | 0.212 |
| CLCSS – social isolation | 15.00 | 6.42 | 13.50 | 4.82 | 0.163 |
| CLCSS – discrimination | 8.79 | 4.21 | 8.43 | 3.01 | 0.012 |
| CLCSS – smoking | 13.00 | 3.68 | 12.57 | 3.92 | 0.013 |
| CLCSS – total score | 55.00 | 16.16 | 51.00 | 12.23 | 0.139 |
| FACT-L – total score | 94.14 | 19.29 | 90.93 | 24.96 | 0.023 |
CESD, Center for Epidemiological Studies Depression Scale; CLCSS, Cataldo Lung Cancer Stigma Scale; FACT-L, Functional Assessment of Cancer Therapy – Lung; HADS, Hospital Anxiety Depression Scale; IES, Impact of Events Scale. ES, effect size partial eta-squared (small = 0.01, medium = 0.059, large = 0.138).
Qualitative interviews
Benefits of intervention
Three superordinate themes were identified: the Therapeutic Relationship; Self-management of Distress; Family Relationships.
Within the Therapeutic Relationship, participants described the value of talking to someone who was knowledgeable about the situation they were facing and who was independent of their family and personal support network. From this and within an empathic and supportive interaction participants felt less alone. For example:
It was good knowing you were talking to someone who was aware of the needs of someone like myself and understands the background to it all.
I think independent. I've got lots of friends and family, but it's just to have someone independent to talk to … it's just good to talk it through with somebody totally independent.
I think the listening and understanding, mainly … the listening and the understanding and as far as my family is concerned it's going to go away. Whereas the psychologists helped. She was supportive and it's not gonna go away.
The sessions helped me because there was somebody on the end of the line when you're having a down day. And I mean if you're having a down day you can ring them. You know it's not like you're alone in the world.
Within Self-management of Distress participants described learning a range of new coping skills for managing feelings of distress and physical symptoms as well as ways to manage family relationships, and come to a sense of acceptance about their illness. New coping skills included relaxation, problem solving and managing thoughts that helped them to manage their distress. For example:
I found when she was doing the relaxation, the meditation and explaining all that to me, I found all of that really beneficial. And as I said after each session I really felt uplifted. I really felt ok we can, I can, step forward. I can move forward and deal with what's coming at me, or being thrown at me.
Just again that bit of moral support, a bit of practical suggestions on how to problem solve, and how to stop my brain running off at a million miles an hour. That's the thing, my brain just will not stop.
As well, skills for managing symptoms such as insomnia and breathlessness were acquired with physical and emotional well-being often described as connected. For example:
Well, especially on the breathing she (the therapist) told me what to do and she even sent me some letters to explain, you know the way I gotta do the breathing, and the way when I get depressed like to relax.
Mainly relaxation and sort of talking things through, I've been having trouble sleeping. But that did sort of ease me with you know with my sleeping.
Cause there are things that happen from time to time that you don't know are related to the cancer and that's when you panic a little bit you know. Like I had a symptom the other day that I ended up having to go to the doctor and which I read was a side effect of the treatment. But you immediately think ‘oh dear’, this could be something more serious, you know. … I've learnt to sort of not be so, to procrastinate about things and you know, not let things go and don't self-diagnose.
Managing family relationships was also a described benefit, as below:
The psychologist, she was really good, because she sort of told me [daughter's name] point of view too. That … she wasn't really accepting that I was sick and you know things like that. And she [psychologist] sent me some materials on that side of things. That helped me a lot to look at my daughter's point of view a bit more than what I had. So I thought that was good. And it is really, really good just to have somebody neutral to speak about those sorts of things.
Participants also described acceptance and feeling better able to face death. For example:
I was a bit worried about the last week of my you know, dying, and she talked that over with me and sent me out little booklets on that … I started to look forward to her phone calls, well there you go, it was someone nice to talk to who you could discuss and who knew how you feel.
Stigma
Although in the interviews participants were not probed directly about stigma-related issues, stigma emerged as a superordinate theme with themes apparent about negative emotion (e.g. guilt and hate); being aware of the smoking and lung cancer connection; and the low public profile of lung cancer compared with other cancers. Example quotes are listed below:
I have to be aware to relax and to slow down and not get uptight about issues or things. I find another thing is I need to have a goal or plan my day ahead at least or a few days ahead of what to do otherwise I feel a sense of guilt.
She gave me some ideas on how to cope with the affliction [lung cancer] and my hatred. I was feeling a bit of hate.
Even though I have got quite a few people that I can talk to about it, but being lung cancer … um she told me that people shun people who've got, you know, lung cancer. And she said ‘Were you ashamed?’ and I said ‘no’. I enjoyed it when I was smoking. … I don't know how to explain it. It really satisfied me to talk like that, you know, to somebody. … Open, like really really open. Yes and somebody that understood.
It's just in my opinion, um I have had lung cancer. I've had a tumour taken out. I never went through chemo or all that. But I feel um … no there was not a lot of input talking about lung cancer … When you hear they're all having relays for this, relays for breast cancer, prostate cancer, all the other cancers, and you know, lung cancer there's nothing. They don't have any fundraising for lung cancers and things like that.
Barriers to intervention
A superordinate theme of Avoidance was identified with participants describing not wanting to discuss the cancer; already having adequate support; and being unwilling to change. For example:
I just have a bit of a problem with sort of getting my head around the value of the whole thing. Maybe I have got the wrong attitude or whatever but I have just tried to handle this lung cancer the best way I can … Well to be quite honest I'm not a very good conversationalist. I'm a very private person and don't sort of talk about things much. I don't want to insult you or anything but as I say I'm not a great one at talking about, um, you know sort of the set up with the business with the cancer etcetera. You know it's just something I felt that I had to deal with as best I could.
Cause I've got great family support. Look, I've got a friend that's going through cancer at the moment, I'm talking with her and I've got a friend who survived lung cancer and I talk to her when I wanna talk to someone that's been there.
I haven't changed for 43 years, now why should I change now? I'm not going to change.
Reasons why participants did not access intervention
Nine participants who did not commence the intervention sessions took part in semi-structured interviews with reasons for not commencing including not wanting to discuss the cancer; no need or already having support; and practical issues. Example quotes are listed below:
I just didn't really want to talk about it that much.
I told her I really didn't think I needed her. I would only be wasting my time because I've got no qualms. Well I couldn't see the point in it. … You know it's just another step in life, and I've never worried about it.
I have trouble on the phone, I have dreadful trouble with the mobile. Just mainly because of the complications with the hearing.
Discussion
The present study describes the first report, to our knowledge, of a cognitive behavioural intervention applying acceptance-focused strategies to reduce psychological distress in patients with lung cancer and ameliorate the negative effects of health-related stigma in this vulnerable patient group. Importantly, the intervention was telephone delivered in order to reduce barriers to access from physical illness and geographic location. For those participants who completed final assessments the intervention was accompanied by improvements in psychological outcomes, particularly with regards to depression where large effect sizes were observed. Health-related stigma was also reduced, with shame and stigma about having lung cancer most affected. These improvements occurred in the face of a declining quality of life, and suggest that well targeted psychological care with an acceptance focus may be of value for lung cancer patients.
Qualitative feedback about the intervention also validated the intervention approach. Specifically, patients very eloquently described how learning skills such as problem solving, goal setting, challenging unhelpful thoughts and learning to relax, combined with an empathic therapeutic relationship, improved their ability to cope with their illness. The cognitive behavioural approach applied in this study was linked to practical advice about relieving symptoms such as breathlessness and insomnia, and this appeared well matched to the demand characteristics of lung cancer where symptom distress and psychological adjustment are strongly linked (Tishelman et al. 2005; Lin et al. 2013). Overall, the therapeutic relationship emerged as a crucial and central component of the intervention that allowed the safe discussion of fears and concerns about the self, and the stigmatised self, as well as fears about death and the future; and for these issues to be at least in part resolved. In this regard, the therapeutic relationship is a central part of acceptance-based approaches, where it is used as a metaphor for how participants might adjust their relationship with themselves to be more gentle, non-judgemental and accepting.
Other researchers have noted that at present there are no stigma reduction interventions available for lung cancer patients, and proposed that education about the cancer and its treatment, support groups, and advocacy may be helpful (Brown Johnson et al. 2013). Others have proposed that cognitive behavioural interventions approaches that incorporated self-forgiveness and acceptance may be appropriate (Hamann et al. 2014). In this regard, a recent pilot study of Acceptance Commitment Therapy with 45 patients with mixed cancer types (mostly breast cancer) reported improvements in quality of life, distress and mood at 3-month follow-up with medium to large effect sizes (Feros et al. 2013). These authors suggested that psychological flexibility leading to acceptance of distress appears to be the mechanism of benefit. Our research provides the first proof of concept for applying acceptance approaches to stigma not only in lung cancer, but to cancer more broadly.
In this study we also validate a telephone support approach is acceptable to this patient group and this has important implications for translation in the field and for broad population access. Specifically, a face to face approach that centralises service delivery will likely disadvantage people who do not live near tertiary treatment centres or who are too unwell to travel. As in previous lung cancer patient research (Schofield et al. 2013), we did experience high levels of attrition over the course of the project, due most often to a deterioration of the patients' physical condition. Participants who did not commence the intervention had poorer quality of life and hence may have felt too unwell to participate, a conclusion again supported by the qualitative data. From the qualitative interviews we elicited from some participants a stated unwillingness to even discuss their cancer. As well, they were heavier smokers, and we speculate that in the context of continued smoking after a lung cancer diagnosis, patients may feel more stigmatised by their cancer and this may be a block to not only seeking, but also accepting support. These findings reinforce the challenges of providing psychosocial support to a very unwell patient population, challenges that are magnified in the context of health-related stigma.
The present study comprised a small convenience sample from which generalisations cannot be drawn. However as a first step in the testing of a complex intervention a preliminary study phase to assess feasibility is crucial (Craig et al. 2006). The theoretical basis of the intervention was based on a comprehensive literature review from which the suitability of a cognitive behavioural approach to address stigma was confirmed (Chambers et al. 2012). Further, we were able to build the intervention upon a generic intervention approach that we have found highly acceptable in both the research and practice setting (Chambers et al. 2014). The testing of this Psychological Wellness intervention in a large-scale randomised controlled trial is the next logical step to determine if this intervention is effective in reducing the psychological distress and stigma that is associated with lung cancer.
In conclusion, health-related stigma is part of the lung cancer experience that to date has been neglected in the tobacco control and lung cancer control narrative. If one can envision being diagnosed with a life-threatening illness and facing daily reminders in the form of health warnings about the likely fatal outcome of the disease and assigning personal responsibility for the illness, that stigma results is not surprising. What is now needed is a research and practice response to fully address the psychological needs of these patients and their families. This study is a further step towards such a response.
Acknowledgments
Suzanne Chambers is supported by an Australian Research Council Future Fellowship.
References
- AIHW & AACR. Cancer in Australia: an overview 2012. In: AIHW, editor. Cancer Series. Canberra, Australia: AIHW; 2012. [Google Scholar]
- Australian Bureau of Statistics. 2014. 6302.0 – Average weekly earnings, Australia, Nov 2013. Available at: http://www.abs.gov.au/ausstats/abs@.nsf/Products/6302.0∼Nov+2013∼Main+Features∼Australia?OpenDocument (cited 30 April 2014) [Google Scholar]
- Bredin M, Corner J, Krishnasamy M, Plant H, Bailey C. A'hern R. Multicentre randomised controlled trial of nursing intervention for breathlessness in patients with lung cancer. British Medical Journal. 1999;318:901–904. doi: 10.1136/bmj.318.7188.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown Johnson CG, Brodsky JL. Cataldo JK. Lung cancer stigma, anxiety, depression, and quality of life. Journal of Psychosocial Oncology. 2013;32:59–73. doi: 10.1080/07347332.2013.855963. doi: 10.1080/07347332.2013.855963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle PT, Craig T. Beth M. Molecular Pathology of Lung Cancer. New York: Springer; 2012. [Google Scholar]
- Carlson LE, Waller A, Groff SL. Bultz BD. Screening for distress, the sixth vital sign, in lung cancer patients: effects on pain, fatigue, and common problems – secondary outcomes of a randomized controlled trial. Psycho-Oncology. 2013;22:1880–1888. doi: 10.1002/pon.3223. doi: 10.1002/pon.3223. [DOI] [PubMed] [Google Scholar]
- Cataldo JK, Slaughter R, Jahan TM, Pongquan VL. Hwang WJ. Measuring stigma in people with lung cancer: psychometric testing of the Cataldo Lung Cancer Stigma Scale. Oncology Nursing Forum. 2011;38:46–54. doi: 10.1188/11.ONF.E46-E54. doi: 10.1188/11.ONF.E46-E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P. Brannon J. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Journal of Clinical Oncology. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Chambers S, Girgis A, Occhipinti S, Hutchison S, Turner J, McDowell M, Mihopoluos C. Dunn J. A randomized trial comparing two low-intensity psychological interventions for distressed patients with cancer and their caregivers. Oncology Nursing Forum. 2014;41:E256–E266. doi: 10.1188/14.ONF.E256-E266. [DOI] [PubMed] [Google Scholar]
- Chambers SK, Dunn J, Occhipinti S, Hughes S, Baade P, Sinclair S, Aitken J, Youl P. O'connell D. A systematic review of the impact of stigma and nihilism on lung cancer outcomes. BMC Cancer. 2012;12:184. doi: 10.1186/1471-2407-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DS. Statistical Power Analyses for the Behavioral Sciences. 2nd edn. New Jersey, USA: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I. Petticrew M. Developing and Evaluating Complex Interventions: New Guidance. London, UK: Medical Research Council; 2006. [Google Scholar]
- Elliott R, Fischer CT. Rennie DL. Evolving guidelines for publication of qualitative research studies in psychology and related fields. The British Journal of Clinical Psychology. 1999;38:215–229. doi: 10.1348/014466599162782. [DOI] [PubMed] [Google Scholar]
- Feros DL, Lane L, Ciarrochi J. Blackledge JT. Acceptance and Commitment Therapy (ACT) for improving the lives of cancer patients: a preliminary study. Psycho-Oncology. 2013;22:459–464. doi: 10.1002/pon.2083. doi: 10.1002/pon.2083. [DOI] [PubMed] [Google Scholar]
- Foley E, Baillie A, Huxter M, Price M. Sinclair E. Mindfulness-based cognitive therapy for individuals whose lives have been affected by cancer: a randomized controlled trial. Journal of Consulting and Clinical Psychology. 2010;78:72–79. doi: 10.1037/a0017566. doi: 10.1037/a0017566. [DOI] [PubMed] [Google Scholar]
- Forman EM, Herbert JD, Moitra E, Yeomans PD. Geller PA. A randomized controlled effectiveness trial of acceptance and commitment therapy and cognitive therapy for anxiety and depression. Behavior Modification. 2007;31:772–799. doi: 10.1177/0145445507302202. doi: 10.1177/0145445507302202. [DOI] [PubMed] [Google Scholar]
- Goffman E. Stigma: Notes on the Management of Spoiled Identity. New York, USA: Simon & Schuster; 1963. [Google Scholar]
- Graves KD, Arnold SM, Love CL, Kirsh KL, Moore PG. Passik SD. Distress screening in a multidisciplinary lung cancer clinic: prevalence and predictors of clinically significant distress. Lung Cancer (Amsterdam, Netherlands) 2007;55:215–224. doi: 10.1016/j.lungcan.2006.10.001. doi: 10.1016/j.lungcan.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann HA, Ostroff JS, Marks EG, Gerber DE, Schiller JH. Lee SJC. Stigma among patients with lung cancer: a patient-reported measurement model. Psycho-Oncology. 2014;23:81–92. doi: 10.1002/pon.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SC. Wilson KG. Mindfulness: method and process. Clinical Psychology: Science and Practice. 2003;10:161–165. doi: 10.1093/clipsy.bpg018. [Google Scholar]
- Heijnders M. Van Der Meij S. The fight against stigma: an overview of stigma-reduction strategies and interventions. Psychology, Health and Medicine. 2006;11:353–363. doi: 10.1080/13548500600595327. doi: 10.1080/13548500600595327. [DOI] [PubMed] [Google Scholar]
- Hoffman SG, Sawyer AT, Witt AA. Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78:169–183. doi: 10.1037/a0018555. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M, Wilner N. Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosomatic Medicine. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Hutchison SD, Sargeant H, Morris BA, Hawkes AL, Clutton S. Chambers SK. A community-based approach to cancer counselling for patients and carers: a preliminary study. Psycho-Oncology. 2011;20:897–901. doi: 10.1002/pon.1786. doi: 10.1002/pon.1786. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. 2014. Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 . Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (cited 2014)
- Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to face Stress, Pain, and Illness. New York, NY, USA: Delacorte; 1990. [Google Scholar]
- Li J. Girgis A. Supportive care needs: are patients with lung cancer a neglected population? Psycho-Oncology. 2006;15:509–516. doi: 10.1002/pon.983. doi: 10.1002/pon.983. [DOI] [PubMed] [Google Scholar]
- Lin S, Chen Y, Yang L. Zhou J. Pain, fatigue, disturbed sleep and distress comprised a symptom cluster that related to quality of life and functional status of lung cancer surgery patients. Journal of Clinical Nursing. 2013;22:1281–1290. doi: 10.1111/jocn.12228. [DOI] [PubMed] [Google Scholar]
- Linn MW, Linn BS. Harris R. Effects of counseling for late stage cancer patients. Cancer. 1982;49:1048–1055. doi: 10.1002/1097-0142(19820301)49:5<1048::aid-cncr2820490534>3.0.co;2-g. doi: 10.1002/1097-0142(19820301)49:5<1048::aid-cncr2820490534>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Major B. O'Brien LT. The social psychology of stigma. Annual Review of Psychology. 2005;56:393–421. doi: 10.1146/annurev.psych.56.091103.070137. doi: 10.1146/annurev.psych.56.091103.070137. [DOI] [PubMed] [Google Scholar]
- Misono S, Weiss NS, Fann JR, Redman M. Yueh B. Incidence of suicide in persons with cancer. Journal of Clinical Oncology. 2008;26:4731–4738. doi: 10.1200/JCO.2007.13.8941. doi: 10.1200/jco.2007.13.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Néron S, Correa J, Dajczman E, Kasymjanova G, Kreisman H. Small D. Screening for depressive symptoms in patients with unresectable lung cancer. Supportive Care in Cancer. 2007;15:1207–1212. doi: 10.1007/s00520-007-0225-z. doi: 10.1007/s00520-007-0225-z. [DOI] [PubMed] [Google Scholar]
- Osborn RL, Demoncada AC. Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: meta-analyses. International Journal of Psychiatry Medicine. 2006;36:13–34. doi: 10.2190/EUFN-RV1K-Y3TR-FK0L. doi: 10.2190/EUFN-RV1K-Y3TR-FK0L. [DOI] [PubMed] [Google Scholar]
- Porter LS, Keefe FJ, Garst J, Baucom DH, McBride CM, McKee DC, Sutton L, Carson K, Knowles V, Rumble M. Scipio C. Caregiver-assisted coping skills training for lung cancer: results of a randomized clinical trial. Journal of Pain and Symptom Management. 2011;41:1–13. doi: 10.1016/j.jpainsymman.2010.04.014. doi: 10.1016/j.jpainsymman.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [Google Scholar]
- Riessman C. Narrative Methods for the Human Sciences. Los Angeles, CA, USA: Sage Publications; 2008. [Google Scholar]
- Rueda J-R, Solà I, Pascual A. Subirana Casacuberta M. Non-invasive interventions for improving well-being and quality of life in patients with lung cancer (Review) Cochrane Database of Systematic Reviews. 2011;(9) doi: 10.1002/14651858.CD004282.pub3. CD004282. doi: 10.1002/14651858.CD004282.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FL. Statistical significance testing and cumulative knowledge in psychology: implications for training of researchers. Psychological Methods. 1996;1:115–129. [Google Scholar]
- Schofield P, Ugalde A, Gough K, Reece J, Krishnasamy M, Carey M, Ball D. Aranda S. A tailored, supportive care intervention using systematic assessment designed for people with inoperable lung cancer: a randomised controlled trial. Psycho-Oncology. 2013;22:2445–2453. doi: 10.1002/pon.3306. doi: 10.1002/pon.3306. [DOI] [PubMed] [Google Scholar]
- Segal Z, Williams J. Teasdale J. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York, NY, USA: Guilford Press; 2002. [Google Scholar]
- Smith AB, Wright EP, Rush R, Stark DP, Velikova G. Selby PJ. Rasch analysis of the dimensional structure of the Hospital Aamxiety and Depression Scale. Psycho-Oncology. 2006;15:817–827. doi: 10.1002/pon.1015. doi: 10.1002/pon.1015. [DOI] [PubMed] [Google Scholar]
- Smith JA, Osborn M. Qualitative psychology: a practical guide to research methods. In: Smith JA, Flowers P, Larkin M, editors. Interpretative Phenomenological Analysis. Thousand Oaks, CA, USA: Sage Publications; 2003. pp. 51–80. [Google Scholar]
- Tanaka K, Akechi T, Okuyama T, Nishiwaki Y. Uchitomi Y. Factors correlated with dyspnea in advanced lung cancer patients: organic causes and what else? Journal of Pain and Symptom Management. 2002;23:490–500. doi: 10.1016/s0885-3924(02)00400-1. doi: 10.1016/s0885-3924(02)00400-1. [DOI] [PubMed] [Google Scholar]
- Tishelman C, Degner LF, Rudman A, Bertilsson K, Bond R, Broberger E, Doukkali E. Levealahti H. Symptoms in patients with lung carcinoma. Cancer. 2005;104:2013–2021. doi: 10.1002/cncr.21398. [DOI] [PubMed] [Google Scholar]
- Vena C, Parker K, Allen R, Bliwise D, Jain S. Kimble L. Sleep-wake disturbances and quality of life in patients with advanced lung cancer. Oncology Nursing Forum. 2006;33:761–769. doi: 10.1188/06.ONF.761-769. [DOI] [PubMed] [Google Scholar]
- Zigmond AS. Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]


