Abstract
This study aims to investigate longitudinal patterns of psychopathology during the antenatal and postnatal periods among women with current (C-ED) and past (P-ED) eating disorders. Women were recruited to a prospective longitudinal study: C-ED (n = 31), P-ED (n = 29) and healthy control (HC; n = 57). Anxiety, depression and ED symptoms were measured at four time points: first/second trimester, third trimester, 8 weeks and 6 months postpartum. Linear mixed effects models were used to test for group differences. Women with C-ED and P-ED, in all diagnostic categories, had significantly higher levels of psychopathology at all time points. ED symptoms decreased in the C-ED group, compared with an overall increase in the other two groups but subsequently increased after pregnancy. Overall, depression and state and trait anxiety scores decreased in the C-ED group compared with the HC group throughout the antenatal and postnatal periods. High levels of psychopathology are common throughout the antenatal and postnatal periods among women with current and past ED, and despite some overall reductions, symptoms remain clinically significant. © 2014 The Authors. European Eating Disorders Review published by John Wiley & Sons, Ltd.
Keywords: eating disorders, psychopathology, pregnancy, perinatal, comorbidity
Introduction
Psychiatric illness during the perinatal period can affect many women (O'Hara and Wisner, 2014); however, very few studies have focused on women with eating disorders (EDs) during this time. Recently, we found that 7.5% of women attending a routine ultrasound scan (USS) met DSM-IV criteria for an ED in early pregnancy (Easter et al., 2013), and as many as a quarter of women in this study experienced weight and shape concerns at a clinical level during the first trimester of pregnancy.
Conflicting findings have been reported regarding the course of ED symptoms during pregnancy. The majority of studies indicate that, in general, ED symptoms improve during pregnancy (Blais et al., 2000; Bonne et al.,1996; Crow, Agras, Crosby, Halmi, & Mitchell, 2008; Lacey & Smith, 1987; Micali, Treasure, & Simonoff, 2007; Morgan, Lacey, & Sedgwick, 1999). It is possible that reduced ED symptoms during pregnancy arise from appetite and hormonal changes experienced during pregnancy. It has also been hypothesised that pregnancy may represent a motivational time for women with ED to discontinue behaviours that may be harmful to their unborn child (Bulik et al, 2007; Micali et al, 2007). However, some studies have shown that ED symptoms worsen or remain stable during pregnancy (Coker, Mitchell-Wong, & Abraham, 2013; Conrad, Schablewski, Schilling, & Liedtke, 2003) or that pregnancy can be a risk factor for a new onset of an ED (Fairburn, Welch, Doll, Davies, & O'Connor, 1997; Nunes, Pinheiro, Hoffmann, & Schmidt, 2014; Tiller & Treasure, 1998).
Nevertheless, women with ED continue to experience high levels of ED symptoms during pregnancy, and a complete absence of symptoms is rare (Blais et al., 2000; Crow et al., 2008). While reductions in ED behaviours, such as binge eating and self-induced vomiting, have frequently been reported, high levels of weight and shape concern are more likely to persist during pregnancy (Easter et al., 2013; Micali et al., 2007). Relapse of symptoms and a return to baseline levels of ED psychopathology in the first 6 to 12 months postpartum is also thought to be typical (Micali et al., 2007, Astrachan-Fletcher, Veldhuis, Lively, Fowler, & Marcks, 2008).
Comorbid psychiatric illnesses are common in women experiencing an ED, particularly depression, anxiety and obsessive compulsive disorder (Hudson, Hiripi, Pope, & Kessler, 2007; Swinbourne et al., 2012). Despite this, few studies have investigated levels of comorbid psychopathology during the perinatal period among women with ED. An early study indicated that women with ED displayed more pregnancy-related anxieties, specifically for the well-being of their unborn child and weight gain during pregnancy (Okun, Stein, Laurie, & Silver, 1996). Carter, McIntosh, Joyce, Frampton, and Bulik (2003) found that 40% of women with ED also had a major depressive episode during the year of childbirth. Similarly, in a twin-based study, 39 and 59% of women with anorexia nervosa (AN) and bulimia nervosa (BN), respectively, experienced depression during pregnancy (Mazzeo et al., 2006).
These findings have more recently been confirmed in a large epidemiological study. Micali, Simonoff, and Treasure (2010) reported that women with a history of ED had increased levels of anxiety and depression during the antenatal and postnatal periods. Women who had ED symptoms during pregnancy and a past history of depression experienced the highest levels of depression and anxiety during pregnancy. However, in this particular study, it is difficult to disentangle how many women had recovered from their ED at the time of assessment.
There remains a paucity of research investigating the trajectory of psychopathology in women with ED during pregnancy and particularly in the postpartum. Very few studies have compared outcomes between women with and without ED or investigated the outcomes in women with a past history of an ED who have recovered at the time of pregnancy. During pregnancy, women can experience dramatic changes in appetite, weight and body shape. Women with a past history of an ED may therefore be at risk of relapse or of high levels of comorbid psychopathology during the pregnancy or in the postnatal period.
There is also a considerable lack of research investigating the distinction between ED diagnostic categories. Despite evidence that approximately 5% of women might have an eating disorder not otherwise specified (EDNOS) in pregnancy (Easter et al., 2013), almost no studies have considered the course of ED symptoms or related psychopathology during the perinatal period in this group of women.
The aims of this study were therefore twofold: (1) to determine the trajectories of anxiety, depression and ED symptoms in women with current and past ED, compared with controls during the antenatal period and in the 6 months following birth and (2) to investigate the effect of lifetime ED diagnosis [AN, BN, EDNOS and binge eating disorder (BED)] on trajectories of anxiety, depression and ED symptoms during the antenatal period and 6 months following birth.
Methods
Design and participants
The Nutrition and Stress in Pregnancy (NEST-p) study is an observational prospective study of pregnant women and their infants. The overall aim of NEST-p is to examine in utero mechanisms and pathways that may account for adverse perinatal and infant outcomes in the infants and children of women with past or active ED.
Three groups of women were recruited for this study during the first or second trimester of pregnancy: women with a current ED (C-ED), women with past ED (P-ED) and a healthy control group.
Women were recruited via three main recruitment methods: (1) Women attending their first or second routine USS at the Harris Birthright Research Centre for Fetal Medicine at King's College Hospital were screened for an ED ((refer to Easter et al., (2013) for further details)), (2) women referred during pregnancy to a specialist psychiatric service for treatment for an ED [i.e. South London and Maudsley NHS Foundation Trust (SLaM) Perinatal Psychiatry services or SLaM ED Inpatient and Outpatient Services] and (3) recruitment posters and online information.
Participants in the C-ED and P-ED groups were recruited via all three methods; healthy controls were recruited via method one and three only.
Inclusion and exclusion criteria
Inclusion criteria for the index groups were women with an active or past DSM-IV diagnosis of ED, between the ages of 18–45, and within the first or second trimester of pregnancy.
Exclusion criteria were the following: a comorbid psychotic illness and an active psychiatric disorder other than ED among women with a past ED. Women were excluded if they suffered from any chronic medical disorder or were unable to communicate in English.
Inclusion criteria for the healthy control group were the following: no active or past full or partial psychiatric disorder including an ED, between the ages of 18–45 and pregnant within the first or second trimester of pregnancy. Women were excluded if they suffered from any chronic medical disorder or were unable to communicate in English.
Measures
Socio-demographic data
Maternal age, marital status, ethnicity and education were obtained via self-report at recruitment to the study.
Eating disorder diagnosis
The Eating Disorder Diagnostic Scale (EDDS; (Stice, Telch, & Rizvi, 2000)) was used to screen women attending their routine USS prior to recruitment to the NEST-p study. The EDDS is a 22-item self-report diagnostic tool designed to assess DSM-IV AN, BN and BED on the basis of diagnostic criteria. After completing the EDDS, women were asked if they would be interested in participating in the NEST-p study and invited to leave contact details.
ED diagnosis was determined at baseline using the Structured Clinical Interview for Axis I DSM-IV-TR Disorders (SCID-I; First, Gibbon, Spitzer, Williams, & Janet, 2002). The SCID-I is a semi-structured diagnostic interview used to determine Axis-I DSM-IV disorders (American Psychiatric Association, 2000).
ED, depression and anxiety symptoms were assessed at four time points, two during pregnancy (first/second and third trimester) and two after birth (eight weeks and six months) using the following measures:
Eating disorder symptoms
The Eating Disorder Examination Questionnaire (EDE-Q; Fairburn & Bèglin, 1994) was used to assess ED symptoms. The EDE-Q is a 36-item self-reported questionnaire focusing on ED symptoms and behaviours within the last 28 days. In addition to a global EDE-Q score, four subscores can be derived from the scale relating to dietary restraint, eating concerns and concerns about weight and shape.
ED behaviours ((objective binge eating, subjective binge eating, self-induced vomiting, use of laxative and diuretic)) are assessed in terms of their presence and frequency during the past 28 days.
Depression
The Becks Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) was used to assess women's symptoms of depression. The BDI is a 21-item multiple choice self-reported measure of the intensity and severity of depression. Questions relate to how the respondent has felt within the last week.
Anxiety
The Spielberger Stait-Trait Anxiety Inventory (STAI) is a frequently used measure for self-reported anxiety (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). The STAI consists of two separate 20-item scales of anxiety: state anxiety (which is considered to be relatively temporary and transient) and trait anxiety (which is considered to be more long-standing and stable).
Procedures
Participant assessments
Women who expressed an interest in the study were contacted by a member of the research team and given details of the study. An initial assessment appointment was arranged in which written informed consent was obtained. Following this, women were interviewed with the SCID-I, and socio-demographic information and a medical history were obtained.
SCID assessments were carried out by AE and ET; in order to ensure agreement between interviewers, maternal psychiatric diagnosis was discussed on a monthly basis in team consensus meetings with the senior author of this study (NM). From this information, eligibility for the study and group classification were determined.
Eligible mothers were invited to take part in two assessments during pregnancy, in the first/second (approximately between week 9.4 and 27.8) and third trimester (approximately between week 28 and 39.1) and two assessments in the postnatal period at 8 weeks and 6 months postnatal. During these assessments, participants completed the EDE-Q, BDI and STAI.
Recruitment and group classification
Specialist psychiatric service recruitment
There were 90 known referrals for women with ED during pregnancy to specialist psychiatric services. We were unable to contact or arrange an initial appointment with 16 women, 20 women declined to take part, 12 women were excluded from the study after an initial assessment and three women had miscarried prior to being contacted by the research team. Therefore, 39 (43%) women were recruited to the study from specialist psychiatric services. After an initial assessment, including a full diagnostic interview, 25 women were allocated to the C-ED group and 14 to the P-ED group.
Maternity services recruitment
In total, 1027 women completed an EDDS at the routine USS, 863 women who expressed an interest in the study and consented to be contacted. Of these, 465 women were not contactable or left no or wrong contact details, 113 declined to take part in the study, 181 women were excluded after an initial assessment, and 11 women had miscarried prior to being contacted by the research team. Therefore, 93 women were recruited to the study and after an initial assessment 9 women were allocated to the C-ED group, 25 to the P-ED group and 59 healthy controls.
Advertisements
Seven women responded to online or poster advertisements about the study. When given further information, two women declined to take part in the study, and after an initial assessment, five women were recruited to the study (n = 3 C-ED group; n = 2 HC).
Therefore, a total of 137 eligible women were recruited and grouped according to their current ED status: C-ED (n = 37), P-ED (n = 39) and healthy controls (n = 61). Women were only included in the present study if they had data at three of the four time points studied. Therefore, 117 women are included in the analysis below (C-ED n = 31, P-ED n = 29, healthy control n = 57).
The C-ED group included women meeting DSM-IV diagnostic criteria at the time of recruitment or within the 3 months prior to pregnancy. Women were considered to have recovered from their ED if they did not meet criteria for an ED (neither full nor partial syndrome) in the year prior to their current pregnancy (P-ED).
For subsequent analysis, to investigate the secondary aims of the study, women were grouped according to their lifetime ED diagnosis, based on DSM-IV diagnostic classification: (1) AN (n = 16; 13.7%; including restricting and binge-purge subtypes); (2) BN (n = 20, 17.1%); (3) EDNOS (n = 17, 14.5%); and (4) BED (n = 8, 6.8%).
Women with (P-ED) who had multiple ED diagnoses (n = 4) during their lifetime diagnostic classification was made during the monthly meetings on the bases of illness duration and severity, using the following hierarchy to guide classification of lifetime diagnosis: BN, AN, EDNOS.
Statistical analysis
Frequencies with chi square tests and one-way analysis of variance (ANOVA) were employed to test for group differences with respect to key categorical or continuous demographic indicators.
Pairwise Pearson correlation coefficients were calculated for all measurements at each time point and for each measurement across time points. Linear, logistic, and multinomial logistic regression models according to the nature of the variable (i.e. linear, binary, or categorical, respectively) were fitted. Two-tailed tests were employed throughout, and statistical significance was defined as a p-value ≤ 0.05. All data analyses were undertaken in STATA version 12.
Longitudinal data modelling
Crude and adjusted two-level linear mixed effects models (xtmixed Stata command) were employed to test for group differences across the longitudinal outcomes investigated (anxiety, depression, and ED symptoms). Because the model assumes normality of distribution of outcomes, the latter were graphically inspected for normality of distribution and transformed accordingly if failing to meet these assumptions. None of the outcomes were normally distributed; therefore, square root (BDI; EDE-Q) and log (STAI) transformations were performed prior to fitting longitudinal models.
Two different models were fitted according to whether independent predictors were ED history (i.e. no ED, past ED and current ED) or lifetime ED type (i.e. AN, BN, BED, and EDNOS). ED group-by-time-point interactions were also tested.
Models were first fitted using a random intercept and then adding a random slope. All models employed maximum likelihood estimates (mle Stata command). Because likelihood ratio testing (LRT) is not possible on multiply imputed dataset, goodness of fit of the two models were compared using LRT on non-imputed data. The model with a random intercept and random slope proved to best fit the data and was thus retained for analyses on the MI dataset. Multivariate models adjusted for a priori (ethnicity, age) confounders and predictors of missingness (education, and marital status).
Missing data
Missingness on socio-demographic and outcome variables was investigated. A total of 7.6, 8.5, 15.4, and 1.7% women had missing data on ethnicity, marital status, education, and age respectively. Overall, there were no differences between women with missing data on socio-demographic variables and women with complete data. Having a P-ED was associated with having missing data on education. Missing data on socio-demographic variables was therefore assumed to be missing at random (MAR) and imputed using multiple imputations with chained equations, imputing five datasets.
Missing outcome data on two or more time points (n = 33, 28.2%) was predicted by being single (p = 0.03).
Ethical approval
This study was approved by The Joint South London and the Institute of Psychiatry NHS Research Ethics Committee (Ref. 09/H0807/12).
Results
Sample characteristics
The majority of women in the sample were from a white ethnic background (81.2%), were married or cohabiting at the time of recruitment (84.1%), and had a university degree or higher education (76.8%). As shown in Table 1, there was no difference in the ethnicity, education, relationship status or parity between women with C-ED, P-ED and healthy controls. Women in the C-ED group were significantly younger than the P-ED and healthy control groups (refer to Table 1).
Table 1.
Sample characteristics by maternal eating disorder group ED = eating disorder, C-ED = current eating disorder, P-ED = past eating disorder
| No ED | C-ED | P-ED | p-value | |
|---|---|---|---|---|
| Total: N (%) | 57 (48.7) | 29 (24.8) | 31 (26.5) | |
| Ethnicity (n = 108) | ||||
| White | 46 (85.2) | 20 (74.1) | 22 (81.5) | 0.5 |
| Other ethnic background | 8 (14.8) | 7 (25.9) | 5 (18.5) | |
| Marital status (n = 107) | ||||
| Single | 2 (3.8) | 3 (11.1) | 0 (0) | 0.1 |
| Not living w/ partner | 3 (5.7) | 4 (14.8) | 5 (18.5) | |
| Married/cohabiting | 48 (90.6) | 20 (74.1) | 22 (81.5) | |
| Education (n = 99) | ||||
| GCSEs/A levels | 9 (17.7) | 10 (37.0) | 4 (19.1) | 0.1 |
| Higher education | 42 (82.4) | 17 (62.9) | 17 (80.9) | |
| Parity (n = 109) | ||||
| Nulliparous | 29 (52.7) | 19 (79.1) | 19 (67.9) | |
| Primiparous/multiparous | 26 (47.3) | 7 (26.9) | 9 (32.1) | 0.2 |
| Age: mean (SD) | 32.8 (4.6) | 28.1 (5.3) | 32.9 (5.8) | 0.0002 |
Categorical outcomes X2 tests were undertaken for categorical outcomes, and continuous outcomes were tested using ANOVA.
Depression, anxiety, and ED symptoms scores were moderately to highly and significantly correlated at time points 1 and 2 (T1 = r: 0.6–0.9; T2 = r: 0.5–0.9), whereas at time points 3 and 4, high correlations existed between all measures (T3 = r: 0.5–0.9; T4 = r: 0.5–0.9) except between the EDE-Q restraint scale and depression and anxiety scores (T3 = r: 0.2–0.3; T4 = r: 0.2). Each measure showed moderate to high correlation across time points (r: 0.5–0.9) with the exception of the EDE-Q restraint measure at T1 and T4 (r: 0.3).
Trajectories of anxiety, depression, and eating disorder symptoms
Figures 1–3 show the raw mean ED, depression and anxiety scores by maternal ED group. Results of the linear mixed effects model are shown in Table 2. Adjusted coefficients are referred to throughout this results section.
Figure 1.
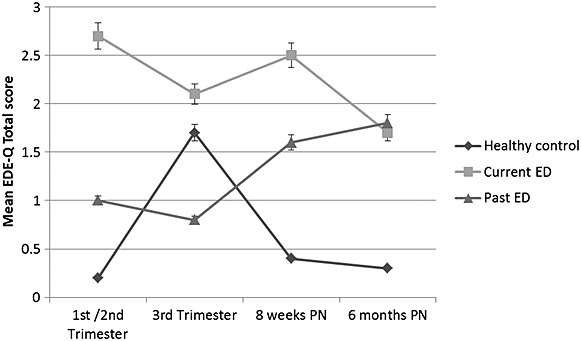
Mean EDE-Q total scores in pregnancy and postpartum assessments according to eating disorder history
Table 2.
Longitudinal perinatal course of ED symptoms, depression and anxiety in women with current ED, past ED and healthy controls: crude and adjusted b coefficient and 95% confidence intervals from linear mixed effect models with random intercept and random slope
| ED history |
|||
|---|---|---|---|
| C-ED | P-ED | ||
| BDI: N (%) | 37 (46.8) | 21 (26.6) | 21 (26.6) |
| Crude b (95% CI) | Ref | 2.2 (1.7; 2.6)*** | 1.0 (0.5; 1.5)*** |
| Adjusted b (95% CI) | Ref | 2.0 (1.5; 2.6)*** | 0.9 (0.4; 1.4)*** |
| STAI state: N (%) | 40 (47.6) | 21 (25.0) | 23 (27.4) |
| Crude b (95% CI) | Ref | 0.6 (0.4; 0.7)*** | 0.3 (0.2; 0.5)*** |
| Adjusted b (95% CI) | Ref | 0.5 (0.4; 0.7)*** | 0.3 (0.1; 0.4)*** |
| STAI trait: N (%) | 39 (47.0) | 21 (25.3) | 23 (27.7) |
| Crude b (95% CI) | Ref | 0.5 (0.4; 0.6)*** | 0.3 (0.2; 0.4)*** |
| Adjusted b (95% CI) | Ref | 0.5 (0.3; 0.6)*** | 0.2 (0.1; 0.4)*** |
| EDE-Q restraint: N (%) | 40 (48.2) | 20 (24.1) | 23 (27.7) |
| Crude b (95% CI) | Ref | 1.0 (0.7; 1.3)*** | 0.4 (0.1; 0.7)** |
| Adjusted b (95% CI) | Ref | 1.0 (0.6; 1.3)*** | 0.4 (0.1; 0.7)** |
| EDE-Q eating concern: N (%) | 40 (48.2) | 20 (24.1) | 23 (27.7) |
| Crude b (95% CI) | Ref | 1.2 (1.0; 1.5)*** | 0.5 (0.2; 0.7)*** |
| Adjusted b (95% CI) | Ref | 1.2 (1.0; 1.5)*** | 0.4 (0.2; 0.6)*** |
| EDE-Q shape concern: N (%) | 37 (46.8) | 20 (25.3) | 22 (27.9) |
| Crude b (95% CI) | Ref | 1.4 (1.1; 1.7)*** | 0.7 (0.4; 0.9)*** |
| Adjusted b (95% CI) | Ref | 1.5 (1.1; 1.8)*** | 0.7 (0.4; 0.9)*** |
| EDE-Q weight concern: N (%) | 37 (46.8) | 20 (25.3) | 22 (27.8) |
| Crude b (95% CI) | Ref | 1.6(1.3;1.8)*** | 0.8(0.5;1.1)*** |
| Adjusted b (95% CI) | Ref | 1.6 (1.3; 1.9)*** | 0.8 (0.5; 1.0)*** |
| EDE-Q total score: N (%) | 37 (46.8) | 20 (25.3) | 22 (27.8) |
| Crude b (95% CI) | Ref | 1.3 (1.0; 1.5)*** | 0.6 (0.4; 0.8)*** |
| Adjusted b (95% CI) | Ref | 1.3 (1.1; 1.6)*** | 0.6 (0.4; 0.8)*** |
HC, healthy control; ED, eating disorder; C-ED, current eating disorder; P-ED, past eating disorder
*0.05 > p > 0.01
0.01 > p > 0.0001
p < =0.0001
aAdjusted for ethnicity, age, education, marital status
Eating disorder symptoms
Women in the C-ED and P-ED groups had increased levels of all ED symptoms (i.e. eating restraint, eating concern, shape concern, and weight concern) across assessment time points in pregnancy and in the postpartum, compared with the healthy control group; refer to Table 2. Overall, women in the C-ED (b coeff: 1.3, 95% CI: 1.1–1.5) and P-ED groups (b coeff: 0.6, 95% CI: 0.4–0.8) had higher total EDE-Q scores compared with healthy controls
There was a significant effect of time overall for an increase in total EDE-Q scores at 8 weeks postpartum (b coeff: 0.2, 95% CI: 0.1–0.3) and at 6 months postpartum (b coeff: 0.2, 95% CI: 0.1–−0.4). Across all groups, weight and shape concerns increased at 8 weeks and 6 months post-partum. Given the increase ED symptoms in the healthy control group, raw EDE-Q scores when assessed in the third trimester were higher in this group, compared with women in the P-ED group; however, a steep increase in EDE-Q scores was seen in the P-ED group in the postnatal period, Figure 1.
There was a significant group-by-time interaction for total EDE-Q scores in women the C-ED group (b coeff: −0.17, 95% CI: −0.26–−0.08, p < 0.0001). Total EDE-Q scores decreased in the C-ED group, compared with an overall increase in scores in the P-ED group and HC group, refer to Figure 1. At 6 months postpartum, EDE-Q scores in the C-ED and P-ED groups were comparable (1.7 and 1.8 respectively, refer to Figure 1).
The frequency of ED behaviours are shown in Table S1. In the C-ED, the number of women reporting objective binge eating and subjective binge eating was higher compared with the P-ED and HC groups and highest during the first/second trimester than at any other point. Self-induced vomiting was reported by six women (20.7%) in the C-ED during the first/second and third trimester and reduced slightly to 4 (13.8%) and 5 (17.2%) at 8 weeks and 6 months postpartum, respectively. Self-induced vomiting was most commonly reported by women with AN or BN during pregnancy, refer to Table S2.
No women in any of the groups reported misusing laxities during the antenatal or postnatal periods, and only one participant within the C-ED group reported diuretic use at 8 weeks and 6 months postpartum.
Depression
Women with C-ED (b coeff: 2.0, 95% CI: 1.5–2.6) and P-ED (b coeff: 0.9; 95% CI: 0.5–1.4) had higher depression scores at all time points than the control group (Table 2).
As shown in Figure 2, mean depression scores, albeit higher in the C-ED and P-ED groups compared with the control group, tended to decrease or remain relatively stable across pregnancy and the postpartum in all groups, with no significant effect of time in the regression models. The steepest decrease was seen in the C-ED group.
Figure 2.
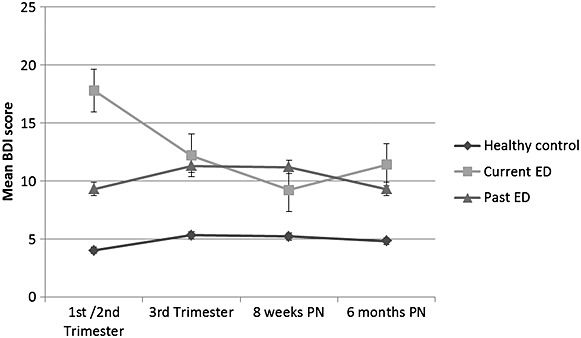
Mean BDI scores in pregnancy and postpartum assessments according to eating disorder history
A significant group-by-time interaction existed in the C-ED group showing an overall decrease in depression (b coeff: −0.37, 95% CI: −0.61–−0.12, p = 0.003), compared with the HC group, refer to Figure 2.
Anxiety
Women with a C-ED and P-ED had higher state (C-ED =b coeff: 0.5, 95% CI: 0.4–0.7; P-ED = b coeff: 0.3, 95% CI: 0.1–0.4) and trait (C-ED = b coeff: 0.5, 95% CI: 0.4–0.6; P-ED = b coeff: 0.2, 95% CI: 0.1–0.4) anxiety scores, compared with healthy controls (Table 2).
As shown in Figure 3, the course of anxiety differed across groups between the first two and the third trimester of pregnancy. There was a significant effect of time at 8 weeks postpartum (b coeff: 0.1, 95% CI: 0.02–0.2) and at 6 months postpartum (b coeff: 0.1, 95% CI: 0.04–0.2) for an overall increase in state anxiety scores.
Figure 3.
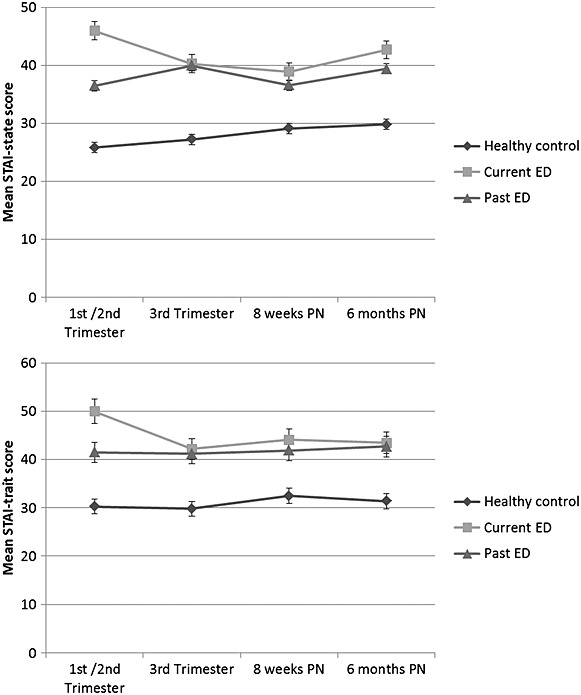
Mean d state and trait anxiety scores in pregnancy and postpartum assessments according to eating disorder history
There was a significant group-by-time interaction in state and trait anxiety for the C-ED group (state = b coeff: −0.07, 95% CI: −0.12–−0.02, p = 0.01/trait = b coeff: −0.06, 95% CI: −0.10–−0.02, p = 0.005), indicating an overall decrease in state and trait anxiety in the women in the C-ED group compared with the other groups.
Lifetime eating disorder diagnosis
Eating disorder symptoms
Results of the linear mixed effects model are shown in Table 3. Raw mean ED, depression and anxiety scores by maternal lifetime diagnosis are displayed as supplementary material Table S3.
Table 3.
Longitudinal perinatal course of ED symptoms, depression and anxiety in women with AN, BN, BED EDNOS, and healthy controls: crude and adjusted b coefficient and 95% confidence intervals from linear mixed effect models with random intercept and random slope
| Outcomes | ED type |
||||
|---|---|---|---|---|---|
| HC | AN | BN | BED | EDNOS | |
| BDI: N (%) | 37 (46.8) | 12 (15.2) | 15 (18.9) | 3 (3.8) | 12 (15.2) |
| Crude b (95% CI) | Ref | 1.9 (1.3; 2.6)*** | 1.5 (0.9; 2.1)*** | 1.6 (0.3; 1.9)** | 1.3 (0.7; 1.9)*** |
| Adjusted b (95% CI) | Ref | 1.8 (1.2; 2.5)*** | 1.3 (0.7; 1.9)*** | 1.4 (0.1; 2.7)* | 1.4 (0.8; 1.9)*** |
| STAI state: N (%) | 40 (47.6) | 12 (14.3) | 16 (19.1) | 3 (3.6) | 13 (15.5) |
| Crude b (95% CI) | Ref | 0.6 (0.4; 0.8)*** | 0.4 (0.2; 0.5)*** | 0.6 (0.3; 1.0)*** | 0.4 (0.2; 0.5)*** |
| Adjusted b (95% CI) | Ref | 0.5 (0.4; 0.7)*** | 0.3 (0.1; 0.5)*** | 0.6 (0.2; 0.9)** | 0.3 (0.2; 0.5)*** |
| STAI trait: N (%) | 39 (46.9) | 12 (14.5) | 16 (19.3) | 3 (3.6) | 13 (15.7) |
| Crude b (95% CI) | Ref | 0.4 (0.3; 0.6)*** | 0.3 (0.2; 0.5)*** | 0.5 (0.2; 0.8)** | 0.4 (0.2; 0.5)*** |
| Adjusted b (95% CI) | Ref | 0.4 (0.2; 0.6)*** | 0.3(0.1;0.4)*** | 0.4(01;0.7)* | 0.4(0.2;0.5)*** |
| EDE-Q restraint: N (%) | 40 (48.2) | 12 (14.5) | 16 (19.3) | 2 (2.4) | 13 (15.7) |
| Crude b (95% CI) | Ref | 0.7 (0.3; 1.0)*** | 0.7 (0.4; 1.0)*** | 1.2 (0.4; 2.0)** | 0.6 (0.2; 0.9)** |
| Adjusted b (95% CI) | Ref | 0.6 (0.2; 1.0)** | 0.7 (0.3; 1.0)*** | 1.3 (0.4; 2.1)** | 0.6 (0.2; 0.9)** |
| EDE-Q eating concern: N (%) | 40 (48.2) | 12 (14.5) | 16 (19.3) | 2 (2.4) | 13 (15.7) |
| Crude b (95% CI) | Ref | 1.0 (0.6; 1.3)*** | 0.8 (0.5; 1.1)*** | 0.7 (−0.0; 1.5)* | 0.7 (0.4; 1.0)*** |
| Adjusted b (95% CI) | Ref | 0.9 (0.5; 1.3)*** | 0.8 (0.4; 1.1)*** | 0.7 (−0.1; 1.5) | 0.7 (0.4; 1.0)*** |
| EDE-Q shape concern: N (%) | 37 (46.8) | 12 (15.2) | 16 (28.3) | 2 (2.5) | 12 (15.2) |
| Crude b (95% CI) | Ref | 1.1 (0.7; 1.5)*** | 1.0 (0.7; 1.4)*** | 1.1 (0.3; 1.9)** | 0.8 (0.5; 1.2)*** |
| Adjusted b (95% CI) | Ref | 1.1 (0.7; 1.5)*** | 1.0 (0.7; 1.4)*** | 1.3 (0.4; 2.1)** | 0.9 (0.6; 1.2)*** |
| EDE-Q weight concern: N (%) | 37 (46.8) | 12 (15.2) | 16 (28.3) | 2 (2.5) | 12 (15.2) |
| Crude b (95% CI) | Ref | 1.3 (0.9; 1.9)*** | 1.2 (0.9; 1.6)*** | 1.1 (0.3; 1.9)** | 1.0 (0.6; 1.4)*** |
| Adjusted b (95% CI) | Ref | 1.3 (0.9; 1.7)*** | 1.3 (0.9; 1.6)*** | 1.3 (0.4; 2.1)** | 1.0 (0.6; 1.3)*** |
| EDE-Q total score: N (%) | 37 (46.8) | 12 (15.2) | 16 (28.3) | 2 (2.5) | 12 (15.2) |
| Crude b (95% CI) | Ref | 1.0 (0.7; 1.3)*** | 0.9 (0.7; 1.2)*** | 1.1 (0.4; 1.7)** | 0.8 (0.5; 1.1)*** |
| Adjusted b (95% CI) | Ref | 1.0 (0.7; 1.3)*** | 0.9 (0.7; 1.2)*** | 1.2 (0.5; 1.9)** | 0.8 (0.5; 1.1)*** |
HC, healthy control; ED, eating disorder; AN, anorexia nervosa; BN, bulimia nervosa; BED, binge eating disorder; EDNOS, eating disorder not otherwise specified
0.05 > p > 0.01
0.01 > p > 0.0001
p ≤ 0.0001
aaAdjusted for ethnicity, age, education, marital status
Women with lifetime AN (b coeff: 1.0, 95% CI: 0.7–1.3), BN (b coeff: 0.9, 95% CI: 0.7–1.2), BED (b coeff: 1.2, 95% CI: 0.5–1.9) and EDNOS (b coeff: 0.8, 95% CI: 0.5–1.1) had higher EDE-Q total scores compared with women without any ED diagnoses, Table 3. In all ED groups, total ED symptoms decreased from the first two pregnancy trimesters to the third but subsequently increased after pregnancy.
There was a significant effect of time in increasing EDE-Q total scores at 8 weeks postpartum (b coeff: 0.2, 95% CI: 0.1–0.3) and at 6 months postpartum (b coeff: 0.2, 95% CI: 0.1–0.4). A similar effect of time was identified for shape and weight concern at 8 weeks and at 6 months postpartum.
Overall, there was a significant group-by-time interaction in relation to EDE-Q total scores in the third pregnancy trimester for women with BED (b coeff: −0.24, 95% CI: −0.48–−0.003, p < 0.05), indicating a decrease in total EDE-Q scores compared with the other groups.
Depression
Having a lifetime ED diagnosis was associated with higher depression scores across pregnancy and postpartum (refer to Table 3). There was no significant effect of time over depression scores at any of the time points. There was a group-by-time interaction for women with BED, with a decrease over time (b coeff: −0.54, 95% CI: −1.07, −.01, p = 0.047).
Anxiety
As shown in Table 3, women with a lifetime ED diagnoses also had increased state and trait anxiety, compared with the healthy control group. No significant group-by-time-point interactions were found on the state or trait scales of the STAI.
Discussion
Very few studies have investigated longitudinal trajectories of psychopathology among women with ED during the perinatal period, particularly in comparison to women who have recovered from previous ED and those without an ED history. Comparing symptoms in women with current and past ED as well as between ED diagnostic categories can further our understanding of the effect of pregnancy on psychopathology and help identify risk factors for relapse during pregnancy and in the postnatal period, ultimately informing approaches to treatment.
An overall pattern for decreasing levels of psychopathology throughout the antenatal and postnatal periods was found among women with a current ED at the start of pregnancy; however, symptoms of ED, anxiety and depression remained higher at all time points. By comparison, women with a past history of an ED appear to be at risk of increasing psychopathology throughout the pregnancy and in the postnatal period.
As expected, women with current and past ED had higher ED-related symptoms throughout pregnancy and the postpartum, compared with women without ED. This was true for all EDE-Q subscales (i.e. restraint, eating concern, shape concern and weight concern) and seen amongst all diagnostic categories. In line with previous findings, women with a current or past ED history showed a decrease in ED symptoms by the third trimester of pregnancy (Astrachan-Fletcher et al., 2008; Blais et al., 2000; Bonne et al., 1996; Crow et al., 2008; Lacey & Smith, 1987; Micali et al., 2007; Morgan et al., 1999). During the postnatal period, levels of ED symptoms among women with a past history of ED were comparable to women with a current ED diagnosis in pregnancy, indicating that pregnancy may lead to a worsening of ED symptoms in women who had previously recovered.
These findings are consistent with the majority of previous findings, which suggest that for many women, pregnancy may represent a time of ‘natural’ decrease in ED symptoms. It is possible that the improvement in ED symptoms found among women with active ED during pregnancy could result from hormonal and appetite changes that occur during this time. The findings may also be reflective of women attempting to reduce the potentially harmful effects of their ED on their developing foetus. Pregnancy may therefore be an ideal time for women with ED to engage in therapy and an optimal time for psychological intervention (Crow et al., 2008; Micali, 2010).
However, it is important to note that global EDE-Q scores in women with active ED at the start of pregnancy remained comparable to the clinical cut-off, particularly during pregnancy and at 8 weeks postnatal (Mond, Hay, Rodgers, Owen, & Beumont, 2004). A longer follow-up of symptom changes in the next 6 to 12 months postpartum will help elucidate if these changes in symptoms are transient or more long-standing.
Very few studies have investigated ED psychopathology in women with BED during the perinatal period. Bulik and colleagues suggested that BED may be associated with a worsening of symptoms during pregnancy (Bulik et al., 2007). In contrast to these findings, in this study, women with a lifetime history of BED specifically showed an overall decrease in ED symptoms. Replication of these findings is particularly important because the number of women with lifetime BED in this study is very small and a wide variation in psychopathology existed within the group.
Current or past history of ED was also found to be associated with higher levels of depression and anxiety in pregnancy and in the postpartum. Higher levels of depression and anxiety were apparent among women with any lifetime diagnosis of an ED (i.e. AN, BN, BED and EDNOS), compared with women without a history of an ED. To our knowledge, only one previous study has investigated the course of depression and anxiety in women with ED during the perinatal period (Micali et al., 2010). Micali and colleagues also reported high levels of anxiety during pregnancy in women with past and active ED, particularly when combined with a past depressive disorder (Micali et al., 2010).
In the present study, women with a current ED showed a decrease in comorbid depressive and anxiety symptoms during the third trimester of pregnancy that persisted into the postnatal period; whereas, higher levels of depression and anxiety remained stable among women with a past history of an ED. Despite the overall reduction in depressive symptoms found in women with active ED, BDI scores among women with both active and a past history of were approximately twice as high as the scores observed in women without ED at 6 months postpartum.
Previous studies have suggested that the postnatal period may be a vulnerable time for postnatal depression for women with ED (Carter et al., 2003; Morgan, Lacey, & Chung, 2006). Although the present investigation does not indicate a risk for a resurgence of comorbid psychopathology in the first 6 months following birth, our findings are consistent with previous reports of an increased risk of postnatal depression in this group of women (Morgan et al., 2006).
In the present investigation, state anxiety levels during pregnancy for women with ED (past and current) remained above the clinical cut-off point (39/40) for clinically significant STAI scores (Knight, Waal-Manning, & Spears, 1983). Furthermore, levels of anxiety in women with current and past ED in this sample were found to be higher than levels reported in both community and hospital samples during pregnancy (Gunning et al., 2011).
Given the associations between elevated stress and anxiety during pregnancy and poor birth outcomes (e.g. Kramer et al., 2009), as well as long-term implications for infant development (Glover & O'Connor, 2002; Micali et al., 2011), clinicians should be aware of this elevated risk for affective disorders among women with ED and routinely assess and monitor anxiety in women with a history of an ED during pregnancy.
Strengths and limitations
Very few studies have investigated psychopathology longitudinally among women with both past and current ED during the antenatal and postnatal periods, and this study has several strengths over previous studies that have. The main strength is that DSM-IV diagnoses of ED were made on the basis of an objective and in-depth diagnostic interview, rather than self-report. This allowed for us to distinguish between past and active ED diagnosis and assess the effect of lifetime ED diagnoses on patterns of psychopathology. Furthermore, the women were followed up prospectively, and extensive validated measures of maternal psychopathology were employed.
Given our recruitment strategy, women in this sample were not exclusively recruited via women seeking or referred for treatment; therefore, generalizability of our study is higher than studies using treatment-seeking samples and might include women with a range of ED severity.
The main limitation of this investigation is that the overall sample size and number of women in each lifetime diagnostic category, particularly BED, were small. Therefore, this study may have lacked the power to detect potential differences.
Conclusions and implications
In conclusion, women with both current and past ED had higher depressive and anxiety symptoms during the antenatal and postnatal periods, compared with women without an ED history. Despite some overall reductions in psychopathology observed among women with active ED, ED symptoms and comorbid psychopathology remained elevated, and at a clinically significant level, in women with both active and past ED diagnosis. Consistent with subjective experiences of an improvement in ED psychopathology in pregnancy, a decrease in ED cognitions and behaviours was observed in the third trimester of pregnancy. Further studies should investigate possible biological mechanisms, that is, hormonal changes amongst others, for this improvement.
Women with current ED require close monitoring and consistency of care during pregnancy and in the postnatal period. Women with a past history of ED are at risk for high levels of psychopathology in the perinatal period, and there is a need for increased awareness of ED in antenatal and postnatal care. Women with ED do not readily disclose their ED to health care professionals (Freizinger, Franko, Dacey, Okun, & Domar, 2008); identification of women with ED is therefore a crucial factor in order for appropriate advice and treatment to be provided.
Acknowledgments
We are grateful to all of the mothers and children involved in the Nutrition and Stress in Pregnancy (NEST-p) study for their dedication and time. We would also like to thank the staff and clinicians within the Perinatal Psychiatry and Eating Disorders services at the SLaM NHS Foundation Trust and the Harris Birthright Research Centre for Fetal Medicine for supporting this study. This article presents independent research commissioned by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research scheme (RP-PG-0606-1043). This research was funded by a National Institute of Health Research (NIHR) clinician scientist award (DHCS/08/08/012) to Dr N. Micali. The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the National Health Service (NHS), NIHR or the DH.
Supporting Information
Additional supporting information may be found in the online version of this article at publisher's web site.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Publishing, Inc; 2000. [Google Scholar]
- Astrachan-Fletcher E, Veldhuis C, Lively N, Fowler C. Marcks B. The reciprocal effects of eating disorders and the postpartum period: a review of the literature and recommendations for clinical care. Journal of Women's Health (2002) 2008;17(2):227–239. doi: 10.1089/jwh.2007.0550. , &. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J. Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. , &. [DOI] [PubMed] [Google Scholar]
- Blais MA, Becker AE, Burwell RA, Flores AT, Nussbaum KM, Greenwood DN. Pregnancy: outcome and impact on symptomatology in a cohort of eating-disordered women. International Journal of Eating Disorders. 2000;27(2):140–149. doi: 10.1002/(sici)1098-108x(200003)27:2<140::aid-eat2>3.0.co;2-e. et al. ( [DOI] [PubMed] [Google Scholar]
- Bonne OB, Rubinoff B. Berry EM. Delayed detection of pregnancy in patients with anorexia nervosa: two case reports. International Journal of Eating Disorders. 1996;20(4):423–425. doi: 10.1002/(SICI)1098-108X(199612)20:4<423::AID-EAT10>3.0.CO;2-Z. &. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Von Holle A, Hamer R, Knoph Berg C, Torgersen L, Magnus P. Patterns of remission, continuation and incidence of broadly defined eating disorders during early pregnancy in the Norwegian Mother and Child Cohort Study (MoBa) Psychological Medicine. 2007;37(8):1109–1118. doi: 10.1017/S0033291707000724. et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter FA, McIntosh VV, Joyce PR, Frampton CM. Bulik CM. Bulimia nervosa, childbirth, and psychopathology. Journal of Psychosomatic Research. 2003;55(4):357–361. doi: 10.1016/s0022-3999(02)00641-4. &. [DOI] [PubMed] [Google Scholar]
- Coker EL, Mitchell-Wong LA. Abraham SF. Is pregnancy a trigger for recovery from an eating disorder? Acta Obstetricia et Gynecologica Scandinavica. 2013;92(12):1407–1413. doi: 10.1111/aogs.12256. &. [DOI] [PubMed] [Google Scholar]
- Conrad R, Schablewski J, Schilling G. Liedtke R. Worsening of symptoms of bulimia nervosa during pregnancy. Psychosomatics. 2003;44(1):76–78. doi: 10.1176/appi.psy.44.1.76. &. [DOI] [PubMed] [Google Scholar]
- Crow SJ, Agras WS, Crosby R, Halmi K. Mitchell JE. Eating disorder symptoms in pregnancy: a prospective study. International Journal of Eating Disorders. 2008;41(3):277–279. doi: 10.1002/eat.20496. &. [DOI] [PubMed] [Google Scholar]
- Easter A, Bye A, Taborelli E, Corfield F, Ulrike S, Treasure J. Recognising the symptoms: how common are eating disorders in pregnancy?". European Eating Disorders Review. 2013;21(4):340–344. doi: 10.1002/erv.2229. et al. ( [DOI] [PubMed] [Google Scholar]
- Fairburn C. Bèglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? International Journal of Eating Disorders. 1994 &. [PubMed] [Google Scholar]
- Fairburn C, Welch S, Doll H, Davies B. O'Connor M. Risk factors for bulimia nervosa: a community-based case-control study. Archives of General Psychiatry. 1997;54(6):509–517. doi: 10.1001/archpsyc.1997.01830180015003. &. [DOI] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J. Janet B. User's Guide for the Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Research version (SCID-I DSM-IV-TR) New York: Biometrics Research; 2002. &. [Google Scholar]
- Freizinger M, Franko DL, Dacey M, Okun B. Domar AD. The prevalence of eating disorders in infertile women. Fertility and Sterility. 2008;93(1):72–78. doi: 10.1016/j.fertnstert.2008.09.055. &. [DOI] [PubMed] [Google Scholar]
- Glover V. O'Connor TG. Effects of antenatal stress and anxiety. The British Journal of Psychiatry. 2002;180(5):389–391. doi: 10.1192/bjp.180.5.389. &. [DOI] [PubMed] [Google Scholar]
- Gunning M, Denison F, Stockley C, Ho S, Sandhu H. Reynolds R. Assessing maternal anxiety in pregnancy with the State-Trait Anxiety Inventory (STAI): issues of validity, location and participation. Journal of Reproductive and Infant Psychology. 2011;28(3):266–273. &. [Google Scholar]
- Hudson JI, Hiripi E, Pope HG., Jr Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RG, Waal-Manning HJ. Spears G. Some norms and reliability data for the State-Trait Anxiety Inventory and the Zung Self-Rating Depression scale. British Journal of Clinical Psychology. 1983;22(4):245–249. doi: 10.1111/j.2044-8260.1983.tb00610.x. &. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Séguin L, Goulet L, Kahn SR, McNamara H. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. American Journal of Epidemiology. 2009;169(11):1319. doi: 10.1093/aje/kwp061. et al. ( [DOI] [PubMed] [Google Scholar]
- Lacey JH. Smith G. Bulimia nervosa. The impact of pregnancy on mother and baby. British Journal of Psychiatry. 1987;150:777–781. doi: 10.1192/bjp.150.6.777. &. [DOI] [PubMed] [Google Scholar]
- Mazzeo SE, Slof-Op't Landt MC, Jones I, Mitchell K, Kendler KS, Neale MC. Associations among postpartum depression, eating disorders, and perfectionism in a population-based sample of adult women. International Journal of Eating Disorders. 2006;39(3):202–211. doi: 10.1002/eat.20243. et al. ( [DOI] [PubMed] [Google Scholar]
- Micali N. Management of eating disorders during pregnancy. Progress in Neurology and Psychiatry. 2010;14(2):24–26. [Google Scholar]
- Micali N, Simonoff E, Stahl D. Treasure J. Maternal eating disorders and infant feeding difficulties: maternal and child mediators in a longitudinal general population study. Journal of Child Psychology and Psychiatry. 2011;52(7):800–807. doi: 10.1111/j.1469-7610.2010.02341.x. &. [DOI] [PubMed] [Google Scholar]
- Micali N, Simonoff E. Treasure J. Pregnancy and post-partum depression and anxiety in a longitudinal general population cohort: The effect of eating disorders and past depression. Journal of Affective Disorders. 2010;131(1-3):150–157. doi: 10.1016/j.jad.2010.09.034. &. [DOI] [PubMed] [Google Scholar]
- Micali N, Treasure J. Simonoff E. Eating disorders symptoms in pregnancy: a longitudinal study of women with recent and past eating disorders and obesity. Journal of Psychosomatic Research. 2007;63(3):297–303. doi: 10.1016/j.jpsychores.2007.05.003. &. [DOI] [PubMed] [Google Scholar]
- Mond J, Hay P, Rodgers B, Owen C. Beumont P. Validity of the Eating Disorder Examination Questionnaire (EDE-Q) in screening for eating disorders in community samples. Behaviour Research and Therapy. 2004;42(5):551–567. doi: 10.1016/S0005-7967(03)00161-X. &. [DOI] [PubMed] [Google Scholar]
- Morgan JF, Lacey JH. Chung E. Risk of postnatal depression, miscarriage, and preterm birth in bulimia nervosa: retrospective controlled study. Psychosomatic Medicine. 2006;68(3):487–492. doi: 10.1097/01.psy.0000221265.43407.89. &. [DOI] [PubMed] [Google Scholar]
- Morgan JF, Lacey JH. Sedgwick PM. Impact of pregnancy on bulimia nervosa. British Journal of Psychiatry. 1999;174:135–140. doi: 10.1192/bjp.174.2.135. &. [DOI] [PubMed] [Google Scholar]
- Nunes M, Pinheiro A, Hoffmann J. Schmidt M. Eating disorders symptoms in pregnancy and postpartum: A prospective study in a disadvantaged population in Brazil. International Journal of Eating Disorders. 2014;47(4):426–430. doi: 10.1002/eat.22236. &. [DOI] [PubMed] [Google Scholar]
- O'Hara MW. Wisner KL. Perinatal mental illness: Definition, description and aetiology. Best Practice & Research. Clinical Obstetrics & Gynaecology. 2014;28(1):3–12. doi: 10.1016/j.bpobgyn.2013.09.002. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun A, Stein REK, Laurie JB. Silver EJ. Content validity of the psyvhiatric symptom index CES-Depression Scale, and State-Trait Anxiety Inventory from the perspective of DSM-IV. Psychological Reports. 1996;79(3):1059–1069. doi: 10.2466/pr0.1996.79.3.1059. &. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR. Jacobs AG. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto: Inc; 1983. &. [Google Scholar]
- Stice E, Telch CF. Rizvi SL. Development and validation of the Eating Disorder Diagnostic Scale: A brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychological Assessment. 2000;12(2):123–131. doi: 10.1037//1040-3590.12.2.123. &. [DOI] [PubMed] [Google Scholar]
- Swinbourne J, Hunt C, Abbott M, Russell J, St Clare T. Touyz S. The comorbidity between eating disorders and anxiety disorders: Prevalence in an eating disorder sample and anxiety disorder sample. Australian and New Zealand Journal of Psychiatry. 2012;46(2):118–131. doi: 10.1177/0004867411432071. &. [DOI] [PubMed] [Google Scholar]
- Tiller J. Treasure J. Eating disorders precipitated by pregnancy. European Eating Disorders Review. 1998;6(3):178–187. &. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


