Abstract
From 1916 to 2011, an estimated total of 165 050 000 metric tons of titanium dioxide (TiO2) pigment were produced worldwide. Current safety regulations on the usage of the TiO2 pigment as an inactive ingredient additive in human food are based on legislation from 1969 and are arguably outdated. This article compiles new research results to provide fresh data for potential risk reassessment. However, even after 45 years, few scientific research reports have provided truly reliable data. For example, administration of very high doses of TiO2 is not relevant to daily human uptake. Nevertheless, because dose makes the poison, the literature provides a valuable source for understanding potential TiO2 toxicity after oral ingestion. Numerous scientific articles have observed that TiO2 can pass and be absorbed by the mammalian gastrointestinal tract; can bioconcentrate, bioaccumulate, and biomagnify in the tissues of mammals and other vertebrates; has a very limited elimination rate; and can cause histopathological and physiological changes in various organs of animals. Such action is contrary to the 1969 decision to approve the use of TiO2 as an inactive ingredient in human food without an established acceptable daily intake, stating that neither significant absorption nor tissue storage following ingestion of TiO2 was possible. Thus, relevant governmental agencies should reassess the safety of TiO2 as an additive in human food and consider establishing an acceptable maximum daily intake as a precautionary measure. Integr Environ Assess Manag 2015;11:10–20. © 2014 The Author. Integrated Environmental Assessment and Management published by Wiley Periodicals, Inc. on behalf of SETAC.
Keywords: E171 food additive, Oral ingestion, Risk assessment, Titanium dioxide, Toxicology
INTRODUCTION
The first and only risk assessment of titanium dioxide (TiO2) as a food additive was carried out by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1969, who concluded: “Titanium dioxide is a very insoluble compound. The studies in several species, including man, show neither significant absorption nor tissue storage following ingestion of TiO2. Establishment of an acceptable daily intake for man is considered unnecessary” (JECFA 1969). Any subsequent reevaluations have largely cited the initial assessment and added no new or particularly important data to the discussion. The aim of this article is a critical review of the conclusion of the original expert group. This article collects results from independent scientists and research laboratories from the last 45 years to provide fresh data toward potential risk reassessment. Furthermore, this article brings forward the conclusions of governmental expert groups such as the National Cancer Institute and European Commission on the potential toxicology of TiO2 to compare against the more recent conclusions of independent scientists.
Given the rapid pace of industry production and deposition of nano-/micro-TiO2 and the outdated environmental and human health regulations regarding TiO2 discharge and consumption, now is the time to carry out proper tests on its toxicity and mode of action. Such results will provide governments with solid, reliable data to be used in risk assessment. The efficacy of the current United States and European Union government policies on restriction and use of TiO2 is dubious. TiO2 is found in food both in its bulk form and as a nanomaterial; in fact, at least 36% of the TiO2 present in food is in nanoform (Weir et al. 2012). Recently, Justo-Hanani and Dayan (2014) performed a review of governmental regulatory policies for nanomaterial risk based on the claim that the states of the world (including the United States) have only “limited power” in transnational nanotechnology risk regulation, whereas the global private nanotechnology sector has the real power. Examples include private standards on nanoterminology, toxicity guidelines, and voluntary risk management partnerships (ED-DuPont 2007; ISO 2010). Although the final conclusion of Justo-Hanani and Dayan (2014) was that governments have much more regulatory power over nanotechnology rule-making and are not as easy influenced by the private sector as previously thought they are still undergoing large nanotechnology adaptations.
Occurrence and industrial characteristics of TiO2
TiO2 can naturally occur in 4 different mineral forms: anatase, brookite, rutile, and TiO2 (B). Brookite has no commercial value and is not being industrially produced, and TiO2 (B) has an extremely small market and is usually only used in the production of Ti nanowires (Armstrong et al. 2004). Therefore, rutile and anatase are the only important crystal structures of TiO2 used in commercial products (DuPont 2007). The refractive index is 2.56 and 2.49 for anatase and 2.60 to 2.61 and 2.89 to 2.90 for rutile (Phillips and Griffen 1981). Anatase is a much more active UV catalyst then rutile.
The main sources of industrial extraction of TiO2 are mineral and ore deposits. Rutile and anatase mineral deposits may contain up to 95% of TiO2. However, these minerals are difficult to extract from primary rocks and never leach out. They can be extracted only from sands in which they are associated with other minerals, and such deposits are rare. Significant rutile deposits of such quality have been found in Australia and South Africa, whereas anatase is common in Brazil. The majority of the TiO2 pigment used in consumer products is being extracted from ilmenite ore (FeTiO3) and leucoxene ore (TiO2 × xFeO × yH2O), either by sulfate or chloride processing. Under natural conditions, TiO2 is the least soluble common constituent on the planet, and geochemical balances are constructed assuming TiO2 is immobile (E Force, University of Arizona, Phoenix, AZ, personal communication). Thus, under natural conditions, TiO2 is predominantly found in “bound state” and is not readily available to interact with the biota.
In industry terminology, the size of TiO2 particles refers to the primary particle, essentially single crystals bound by crystal planes. Most commercial products contain TiO2 particles with a size range of 200 to 300 nm, and commercial pigments rarely use sizes less than 100 nm. To achieve the most efficient light scattering effect, the TiO2 pigment diameter should be somewhat less than 50% of the wavelength of light to be scattered. The human eye is most sensitive to a wavelength of 0.55 µm; therefore, the ideal particle size of TiO2 is 200 nm (DuPont 2007). Coatings are frequently used to improve dispersion, durability, and gloss of the TiO2 pigment. TiO2 used as a food additive most often does not contain any artificial coatings, but in all other consumer products, it contains 1% to 15% of artificial coatings by weight, most commonly oxyhydrates and oxides of silicone and Al (IARC 2010).
Global production estimate of TiO2 for the period 1916–2012
Nearly 6 million metric tons of TiO2 were consumed worldwide in 2012 as a pigment (USGS 2013). It is unknown what percentage of this quantity can be attributed to nano-TiO2, but Robichaud et al. (2009) estimated that by 2012, close to 5% of all production could be attributed to nanoform amounts. The same report suggested that by 2023, up to 50% TiO2 might be manufactured in nanoform (Robichaud et al. 2009); however, this number might be an overestimate because the most desirable particle size is 200 nm (DuPont 2007). Production of TiO2 was started in 1916 by the Titan Company (Norway) and what is now known as NL Industries (United States). TiO2 was first produced in the United States by the Titanium Pigment Company. In 1925, the National Lead Company purchased a large interest in the Titanium Pigment Company, and production reached 4000 US short tons. Later, the National Lead Company became NL Industries, a predecessor of Kronos. By 1971, there were 8 US producers of TiO2.
This article estimates that from 1916 to 2011, a total of 165 050 000 metric tons of TiO2 were produced worldwide ( Figure 1). These estimates are based on international and national reports, as well as company sources collected on the internet. The United States was the leading producer until 2010, when it was surpassed by China. The United States produced approximately 35% to 40% of the world's TiO2 until 1993, when China began an exponential growth spurt. Before 1955, China produced no TiO2, but by 2010, they had produced 1 475 000 metric tons per year, accounting for 29% of the world market. In 2011, China produced nearly 2 000 000 metric tons, a 35% share of the global market. Figure 1 represents total production of TiO2 (anatase and rutile) in all industrial sectors; it is not known how much TiO2 is used as a food additive or ends up in the environment each year.
Figure 1.
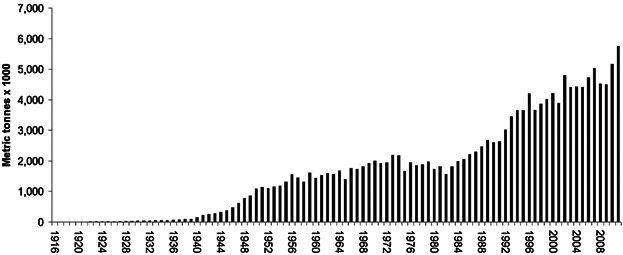
Historical production of titanium dioxide pigment by all countries of the world combined.
Policy review of TiO2 as a food ingredient and associated risks according to governmental agencies
The Joint FAO/WHO Expert Committee on Food Additives performed the first and only toxicological evaluation of TiO2 safety as a food color and additive in 1969 during a 1 week meeting in Rome (JECFA 1969), concluding that TiO2 did not call for established daily intake levels due to its insolubility, “Titanium dioxide is a very insoluble compound. The studies in several species, including man, show neither significant absorption nor tissue storage following ingestion of TiO2. Establishment of an acceptable daily intake for man is considered unnecessary.” The assessment considered only 5 published references when making its recommendation for approval of the use of TiO2 in human food. All 5 studies reported no significant effects on animals, yet one of these references (Brown and Mastromatteo 1962) had nothing to do with TiO2. The article only investigated the toxicity of Ba, Bi, Ca, and Pb titanate. The other 4 articles explored TiO2 oral exposure, intramuscular injections, or intraperitoneal injections in humans, rats, dogs, rabbits, cats, and guinea pigs. Although studies that clearly showed absorption and accumulation effects as early as 5 min after exposure (Huggins and Froehlich 1966) were available at the time, they were never considered by the expert group. The 2 main features on which the expert group based their opinion were lack of absorption and lack of accumulation in body tissue.
In 1979, with TiO2 already being an official ingredient of human food, the US government carried out the first large scale study trying to link cancer to oral exposure to TiO2. This task was performed by the toxicologists of the National Cancer Institute (National Toxicology Program 1979). Groups of 50 rats of each sex and 50 mice of each sex were given 25 000 ppm or 50 000 ppm of TiO2 in their diets for 103 weeks and then observed for 1 additional week. No tumors occurred in dosed groups at incidences significantly higher than those for corresponding control groups. Thus, it was concluded that TiO2 was not carcinogenic by the oral route for Fischer 344 rats or B6C3F1 mice. However, this study only evaluated the effects of bulk TiO2 and did not consider the effects of nano-TiO2.
The International Agency for Research on Cancer (IARC) performed the last reassessment of TiO2 cancer potential in 2010 (IARC 2010). IARC classified TiO2 as a human carcinogen group 2B, because there was enough evidence that nano-TiO2 may cause lung cancer by exposure through inhalation. That classification states, “The agent (mixture) is possibly carcinogenic to humans. The exposure circumstance entails exposures that are possibly carcinogenic to humans.” This category is used for chemicals for which there is limited evidence of carcinogenicity in humans and less than sufficient evidence of carcinogenicity in experimental animals. It may also be used if there is sufficient evidence of carcinogenicity in experimental animals but inconclusive evidence of carcinogenicity in humans. Oral exposure was debated by IARC, but the final report was inconclusive due to nonexisting standardized procedures for nano-TiO2 risk assessment, as outlined in the journal, NanoEthics (Jacobs et al. 2010).
In the United States, use of TiO2 as a food coloring agent has been permitted since the year 1966 (Federal Register 1966), 3 years before the official assessment by JECFA. The US Food and Drug Administration (USFDA) allows the use of TiO2 as a food additive as long as its weight does not exceed 1% of overall food weight (USFDA 2005). The federal regulation states: “Certification of this color additive is not necessary for the protection of the public health and therefore batches thereof are exempt from the certification requirements of section 721(c) of the act.” Japanese authorities, on the other hand, allow the use of TiO2 as a food coloring agent without any limitations whatsoever (JETRO 2011). Meanwhile, India restricts the use of TiO2 as a food additive to 1% of the food weight in chewing gum and 0.01% in powdered concentrate mixes for the production of beverages (FSS 2011). In countries where there is no limit imposed on the quantity of TiO2 in chewing gum, the average concentration is approximately 2 mg g−1, and over 93% is in nanoform (Chen et al. 2013). The European Union allows TiO2 in its food products in most cases at quantum satis levels under good manufacturing practices, with the exception of a few food products in which it is not allowed at all (European Parliament 1994).
In 2013, the European Commission's Scientific Committee on Consumer Safety (EU-SCCS) endorsed and published its opinion on nanoform TiO2 (EC 2013). Three independent, nonfood scientific committees provided the Commission with advice for policy preparation relating to consumer safety. EU-SCSS provided opinions concerning all types of health and safety risks of nonfood consumer products and services containing nano-TiO2. Regarding oral toxicity, they analyzed only 7 reports. Two of these articles were characterized as flawed and rejected, namely Wang et al. (2007) and the previously mentioned findings of the National Cancer Institute (National Toxicology Program 1979). Wang et al. (2007) was rejected with the following explanation: “The study has a number of flaws, and is therefore of little value to this assessment. Sufficient characterization of the nanomaterials used was not carried out, the administered dose (5 g/kg/bw) was very high, frequent esophageal ruptures were reported that led to animal deaths, translocation of TiO2 from GI tract was measured as Ti with no evidence that it was in nanoparticulate form. It is not clear whether any of the effects observed were due to TiO2 toxicity, or simply overloading the gut at high dose of the particulate material.” For the National Cancer Institute report (1979), EU-SCSS stated: “No information has been provided on the particle size profile of the material tested in this study. The study is therefore of little value in relation to the current assessment for nanoforms of TiO2.” Ultimately, 5 reports were analyzed: 3 nonpeer-reviewed, publicly unavailable internal studies of industrial giants that produce TiO2 (2 by Evonik Degussa, 1 by DuPont) and 2 peer-reviewed articles (Duan et al. 2010; Hu et al. 2010). Based on the findings of Duan et al. (2010) and Hu et al. (2010), EU-SCSS concluded that a lowest observed adverse effect level (LOAEL) of 5 mg kg−1 body weight day−1 may be derived for nano-TiO2. An early study suggested that average daily human consumption of TiO2 in the United Kingdom was 5.4 mg per person (MAFF 1993). Later, a more precise estimate become available with daily estimated human consumption of TiO2 food grade (E171) of 0.2 to 2 mg kg−1 body weight per day (Weir et al. 2012), which is close to this estimated LOAEL value.
METHODS
Methodology of data collection and studies published by independent scientists
A recent review of potential toxicity of nanomaterials to humans through oral exposure revealed only 2 valid scientific studies dealing with TiO2 (Card et al. 2010). The main reason for such a small number was the rather strict focus of a review on nanomaterials. The authors did not consider studies with a particle diameter greater than 100 nm. The current review, on the other hand, did not discriminate between “nano” (<100 nm) and “micro” (>100 nm) TiO2. Thus, all available studies that investigated toxicity of TiO2 after oral administration were collected, and for all cases particle size was reported. The literature search was performed within 5 databases—Web of Science, Scirus, Scopus, Google Scholar, and the University of British Columbia library database—using the following keywords in various combinations: titanium dioxide, TiO2, oral exposure, oral administration, toxicity, administration, distribution, metabolism, and excretion studies (ADME), bioaccumulation, mammals, rats, mice, nanotoxicology, and risk assessment. Abstracts of numerous hits were read, and downloaded articles were evaluated according to 10 characteristics:
Papers must be written in English
The administered dose must be close to the estimated human daily exposure of 0.2 to 2 mg kg−1 body weight per day (Weir et al. 2012).
The crystal structure of TiO2 must be reported (preference was always given to anatase, which is used as food additive in overwhelming majority compared to rutile; only 1 article investigating rutile toxicity was included in the final list).
The primary particle size of TiO2 must be reported
The hydrodynamic diameter of TiO2 must be reported
The volume (liquid) or weight (bolus, gavage, food) of the carrier of the orally administered dose must be reported
The weight of the test animals must be measured and reported
Testing groups must consist of 10 or more animals
The experimental animals must be mammalian, and the experiments must be performed in vivo
The performed study must be chronic (at least 90 days long).
Interestingly, not a single published scientific study satisfied all designated criteria, indicating a deficiency of good laboratory practice in the current literature regarding toxicity of TiO2. Studies matching at least two-thirds of the criteria (score 6/10) were analyzed, and the rest of the studies were discarded. One of the most common missing points was the lack of the volume or weight of the carrier (bolus, gavage, etc.) used to deliver TiO2 orally, which only appeared in 2 studies. Other common instances of nonreported important information included lack of hydrodynamic diameter or lack of crystal phase info. Very often, the TiO2 dose exceeded the potential dose that a human could consume on a daily basis. Furthermore, the duration of the studies was acute in many cases, with some as short as 5 days. Thus, although independent scientific laboratories provided important insight on the potential toxicity of oral consumption of TiO2, good laboratory practice was not strictly followed in the majority of the cases. A total of 16 plausible studies were identified according to the stated criteria (Table 1). Of those, 15 reported toxic effects or bioaccumulation of TiO2, whereas only a single study detected no negative effects. Mice were the model species in 11 studies, rats in 5.
Table 1.
Literature investigating toxic effects of TiO2 after oral administrationa
| Study | Dose (mg kg−1 body weight) | Crystal structure | Primary particle diameter (nm) | Hydrodynamic diameter (nm) | Duration of study (d) | Model species used | Main effect observed | Score (max. 10) |
|---|---|---|---|---|---|---|---|---|
| Cho et al. 2013 | 260–1041 daily | Anatase/rutile mixture (80:20) | 30 | 38 | 91 | Rat, 11/group | No toxic effect observed | 8 |
| Cui et al. 2010 | 5–50 every 2nd day | Anatase | 7 | Not reported | 60 | Mouse, 20/group | Liver damage, hepatocyte apoptosis, ROS accumulation in liver | 6 |
| Cui et al. 2011 | 5–50 daily | Anatase | 5 | Not reported | 60 | Mouse, 20/group | Liver toxicity (induction of hepatitis molecular pathway) and bioaccumulation | 6 |
| Duan et al. 2010 b | 62.5–250 every 2nd day | Anatase | 5 | Not reported | 30 | Mouse, 20/group | Liver histopathology, immune suppression, body weight reduction | 6 |
| Gui et al. 2013 | 2.5–10 daily | Anatase | 5 | 294 | 90 | Mouse, 30/group | Nephrotoxicity, inflammatory response, oxidative stress | 9 |
| Hu et al. 2010 b | 5–50 every 2nd day | Anatase | 5 | Not reported | 60 | Mouse, 20/group | Impairment of spatial recognition memory, brain pathology | 6 |
| Jani et al. 1994 | 12.5 daily | Rutile | 475 | Not reported | 10 | Rat, 10/group | Accumulation in the intestines and translocation of liver and spleen | 6 |
| Nogueira et al. 2012 | 100 daily | Anatase | 66 and 260 | Hard to interpret | 10 | Mouse, 12/group | Mucosal epithelium hypertrophy and hyperplasia in small intestine | 6 |
| Qian et al. 2010 | 160–1000 daily | Anatase/rutile mixture | 50 | Not reported | 14 | Rat, 16/group | Energy and amino acid metabolism disturbance | 6 |
| Sang et al. 2012 | 2.5–10 daily | Anatase | 5 | 294 | 90 | Mouse, 20/group | Spleen histopathology, splenocyte apoptosis, immunosuppression | 9 |
| Sang, Li, et al. 2013 | 10 daily | Anatase | 5 | 294 | 90 | Mouse, 20/group | Spleen bioaccumulation, oxidative stress, splenic inflammation and necrosis, reduction in body weight | 8 |
| Sang, Fei, et al. 2013 | 2.5–10 daily | Anatase | 5 | 294 | 90 | Mouse, 30/group | Spleen and thymus bioaccumulation, spleen histopathology and splenocyte apoptosis, increase in levels of inflammatory proteins | 9 |
| Tassinari et al. 2014 | 1–2 daily | Anatase | 25 | 284 | 5 | Rat, 14/group | Spleen and ovaries bioaccumulation, alteration in thyroid function and testosterone levels | 9 |
| Wang et al. 2007 c | 5000 single administration | Not reported; according to personal statement of 1 coauthor in EPA/600/R-09/057F, 25 and 80 nm particles are of rutile structure and 155 nm of anatase | 25, 85, 155 | Not reported | 14 | Mouse, 20/group | Bioaccumulation in liver, spleen, kidney, and lung; histopathology in liver and kidney; myocardial damage. | 6 |
| Wang et al. 2011 | 5–150 daily | Anatase | 7.5 | Not reported | 30 | Mouse, 20/group | Oxidative stress via p38-Nrf-2 signaling pathway, congestion in spleen | 6 |
| Wang et al. 2013 | 10–200 daily | Anatase | 75 | 473 | 30 | Rat, 7/group | Liver edema, heart injuries, mast cells activation | 6 |
Only references that have satisfied at least 6 of 10 assigned requirements are shown.
This study has been endorsed by EU-SCSS.
This study has been rejected by EU-SCSS.
RESULTS
Dietary exposure to TiO2 and associated risks according to scientific community
As a recent review article has already discussed the uptake of TiO2 particles via the oral route, including absorption, barriers, and passage through barriers (Fröhlich et al. 2013), this article will not focus on details of these issues. We will only focus on the size limit of particles that can be absorbed by intestinal cells. A comprehensive review of current toxicological data of TiO2 can be found in Shi et al. (2013) and Iavicoli et al. (2012).
Penetration of food grade TiO2 (E171) into enterocytes of rats has been experimentally confirmed in vivo in the past (Onishchenko et al. 2012). Briefly, TiO2 particles in the intestines are typically found in the anatase crystal form with spherical particle diameter size 100 to 200 nm (Powell et al. 2010). E171 is claimed by manufacturers (if the information is provided at all) to have a primary particle size around 200 nm (Lomer et al. 2000); therefore, no approval for nanomaterial additive is needed. However, the actual average particle diameter of E171 in food products is 110 nm, with at least 36% of particles less than 100 nm and a particle diameter range of 30 to 400 nm (Weir et al. 2012). Five different powder or paste samples of E171 around the world were analyzed for primary particle and hydrodynamic diameter. Results showed that the average primary particle diameter was between 106 nm and 132 nm with at least 17% to 35% particles being less than 100 nm in diameter, whereas average hydrodynamic diameter was 127 nm to 504 nm (Yang et al. 2014). Quantitative evaluation of foods containing TiO2 is difficult. Manufacturers do not need to include it on food labels, as there are exceptions to the legislation stating that food additives must be identified. For example, labeling is not required when additives perform no additive function in or make up less than 25% of the final product (Lomer et al. 2000). In addition, some ready-to-eat products are exempt from labeling (e.g., in-flight packaged meals or individual catering packs such as coffee creamers). Also, because TiO2 does not have any nutritional value, it is in some cases considered a manufacturing aid rather than a food ingredient, making it exempt from labeling.
Before the 21st century, the general belief among food scientists and toxicologists was that food particles with diameters greater than 50 nm would have limited absorption by the mammalian gastrointestinal tract (GIT). This claim was based on the reasoning that insoluble particles encounter a mesh-like mucous barrier secreted by goblet cells and that the diffusion rate is therefore insignificant. However, it has since been discovered that pores within the mucus layer are much larger, suggesting that particles with a diameter of up to 500 nm can pass through (Lai et al. 2007). Experiments with perfused intestines of vertebrates demonstrated TiO2 uptake across the intestine both for nano-TiO2 and its bulk counterpart, with an average particle aggregate diameter of up to 1124 ± 331 nm (Al-Jubory and Handy 2013). Once the nano- or micro-TiO2 diffuse through mucus pores, they are able to enter hepatic circulation via intestinal cell transcytosis (Koeneman et al. 2010). The uptake of particles by the GIT increases as the size of the particle decreases. After oral administration of equal doses, the GITs of laboratory rats were able to absorb 34% of particles with 50 nm diameter, 26% of particles with 100 nm diameter, and 10% of particles with 500 nm diameter; absorption of particles with 1 µm diameter was marginal (Jani et al. 1989, 1990). It was experimentally determined that the particle diameter of E171 in food products is within a 30 to 400 nm range (Weir et al. 2012); thus, a significant number of TiO2 particles in E171 are likely absorbed by the intestinal cells of humans.
Review of oral exposures to TiO2 and toxic effects in the current literature
An absorption study (Böckmann et al. 2000) determined that humans orally ingesting TiO2 in capsules containing 23 or 46 mg showed a 5 to 10 times increase of TiO2 levels in the blood. TiO2 was absorbed by the GIT in a size-dependent manner, where small particles were absorbed at a better rate than larger ones.
Oral administration of TiO2 in laboratory mice (5g kg−1 body-weight; 25, 80, and 150 nm primary particle size) has been reported to cause accumulation of particles in liver, spleen, kidney, and lung tissue, with histopathological changes in the liver and kidney, myocardial damage, and changes in serum biochemical parameters (Wang et al. 2007). However, as noted above, this study was rejected by EU-SCSS based on validity grounds (EC 2013). In a similar study, 5 nm (primary particle size) TiO2 anatase was intragastrically administered to mice at 60 to 250 mg kg−1 body weight every second day for 30 days (Duan et al. 2010). At the concentration of 125 mg kg−1 body weight, TiO2 caused reduction in body weight; increase in the hepatosomatic coefficient; histopathological changes in the liver; decreases in white blood cells, thrombocytes, red blood cells, hemoglobin, reticulocytes, lymphocytes, and interleukin-2 activity; and increases in NO levels, platelets, and hematocrit. Furthermore, levels of numerous liver enzymes and cholesterol levels were disrupted by the treatment, suggesting that the liver function damage was likely caused by damage to the hemostatic and immune systems (Duan et al. 2010). On the contrary, a 91 day chronic study (Cho et al. 2013) of daily oral administration of 260 to 1041 mg kg−1 body weight TiO2 (average primary particle diameter 30 nm, average hydrodynamic diameter 38 nm) to rats did not detect accumulation in sampled organs or urine, but a high concentration was detected in feces, suggesting that TiO2 was eliminated from the body. There are several reasons that may explain why this study did not detect accumulation or toxic effects of TiO2. First, this study used a mixture of TiO2 anatase and rutile, whereas the other studies used pure anatase. Second, the particle size in this study was very small compared to other studies. Third, the age of the experimental animals may play a crucial role in the significance of observed effects. Oral administration at 10 to 200 mg kg−1 body weight of anatase TiO2 (average primary particle diameter 75 nm, average hydrodynamic diameter 473 nm) for 30 consecutive days to rats of different ages provided significantly different results (Wang et al. 2013). Severe liver edema, heart injuries, and nonallergic mast cell activation in stomach tissue was found in 3-week-old rats, whereas 8-week-old rats exhibited only slight toxic effects. Young rats treated with TiO2 displayed elevated levels of blood glucose, Low-density lipoprotein cholesterol, ALT/AST ratio, and total bilirubin compared to the control group and to the tested adult rats. On the contrary, levels of α-hydroxybutyrate dehydrogenase and creatine kinase were significantly reduced.
Liver damage and hepatocyte apoptosis linked to TiO2 exposure has also been documented. Exposure of mice via intragastric administration to TiO2 at 5 to 50 mg kg−1 body weight every 2nd day (primary particle diameter 7 nm) caused hepatocyte apoptosis and an increase in reactive oxygen species accumulation in the liver, followed by a decrease in the expression of several key genes involved in detoxification and stress response (Cui et al. 2010). In a separate, similar study conducted by the same researchers, the molecular mechanism of TiO2 toxicity to the liver was deciphered. The results showed that TiO2 accumulated in the liver and caused histopathology changes. Furthermore, it was demonstrated that TiO2 actually induced the hepatitis molecular signaling pathway in the mouse liver by activating TLRs → NIK → IkB kinase → NF-kB → TNF-α → inflammation → apoptosis → liver injury (Cui et al. 2011).
In an acute and chronic study (15–90 days of exposure), mice were daily exposed perorally to anatase TiO2 (average primary particle size 5 nm, average hydrodynamic diameter 294 nm) at 10 mg kg−1 (Sang, Li, et al. 2013). The study clearly showed that TiO2 was deposited in the spleen, where it produced reactive oxygen species and caused time dependent splenic inflammation and necrosis. TiO2 also elevated the expression of several genes in the spleen: COX-2, E2, ERK, AP-1, CRE, Akt, JNK2, MAPKs, P13-K, c-Jun, and c-Fos. In the same study, exposure to TiO2 also caused significant reduction in weight of experimental mice compared to the control group. Oxidative stress caused by TiO2 has been indicated in other studies as well.
In a 30 day study (Wang et al. 2011), mice were exposed to intragastric administration of 5 to 150 mg kg−1 body weight of anatase TiO2 (primary particle size 7.5 nm). Besides congestion and lymph node proliferation in the spleen, TiO2 caused a significant increase in mouse spleen reactive oxygen species accumulation. Further analysis revealed that the mode of action of TiO2 for exerting oxidative stress is via the p38-Nrf-2 signaling pathway (Wang et al. 2011).
In a similar chronic study, mice were exposed via intragastric administration for 90 days to 2.5–10 mg kg−1 body weight of anatase TiO2 (average primary particle size 5 nm, average hydrodynamic diameter 294 nm) (Sang et al. 2012). It was determined that TiO2 caused significant histopathological changes in the spleen and induced splenocyte apoptosis. The TiO2-treated mice's immunoglobulin levels, hemoglobin levels, and numbers of platelets and lymphocytes were significantly reduced compared to control. Furthermore, mice treated with TiO2 exhibited increased expression of genes for NF-kB, TNF-α, macrophage migration inhibitory factor, and interleukins: 2, 4, 6, 8, 10, 18, and 1β, whereas expression of Bcl2 and HSP70 was reduced. The final conclusion of the article was that chronic oral exposure to TiO2 leads to spleen injury and reduction in immune capacity.
Further investigation linking TiO2 and immune “injury” of the spleen was performed with oral gavage of TiO2 in mice for 90 consecutive days (Sang, Fei, et al. 2013). The concentration administered was 2.5–10 mg kg−1 body weight of anatase TiO2 with an average particle hydrodynamic diameter of 294 nm. The conclusion of the study was that exposure to TiO2 caused significant increases in spleen and thymus indices, accumulation of TiO2 in the spleen and thymus, splenocyte apoptosis with histopathology changes, and increased levels of numerous inflammatory proteins. Another acute study (Tassinari et al. 2014) investigated the combined effects of TiO2 on the spleen, reproductive system, and endocrine system. Rats were exposed to anatase at 1 to 2 mg kg−1 body weight daily by oral gavage (average primary particle diameter 25 nm, average hydrodynamic diameter 284 nm) for 5 days. Accumulation of Ti in the spleen tissue and ovaries was documented. In addition, alteration of the thyroid function was observed in male rats, whereas testosterone levels increased in males and decreased in females. The final conclusion was that after exposure to oral dose levels relevant to human exposure, the target tissues for TiO2 toxicity are active endocrine tissues.
An acute oral study (160–1000 mg kg−1 body weight per day) in rats investigated the sublethal effects of TiO2 on metabonomic signature in animals (Qian et al. 2010). Results showed that TiO2 administration caused increases in levels of taurine, citrate, hippurate, histidine, Trimethylamine N-oxide, citrulline, α-ketoglutarate, and phenylacetylglycine in the urine of rats. Moreover, decreases in the levels of lactate, betaine, methionine, threonine, pyruvate, 3-D-HB, choline, and leucine were noted. Rats treated with TiO2 exhibited an increase in TMAO, choline, creatine, phosphocholine, and 3-D-HB and a decrease in glutamine, glutamate, pyruvate, acetoacetate, glutathione, and methionine. Additionally, mitochondrial swelling in the heart tissue was observed, and creatine kinase, AST, and lactate dehydrogenase levels were elevated. The final conclusion of the authors was that oral uptake of TiO2 leads to energy and amino acid metabolism disturbance.
In another chronic study, mice were orally exposed to anatase TiO2 (average hydrodynamic particle diameter 294 nm) at 2.5 to 10 mg kg-−1 body weight daily and screened for nephrotoxicity (Gui et al. 2013). Exposure to TiO2 resulted in a significant reduction of renal glomeruli (by number), apoptosis, necrosis and disorganization of renal tubules, infiltration of inflammatory cells, significant reduction in body weight, and unbalanced element distribution in the kidneys. Microarray analysis verified upregulation of 1246 genes, mostly associated with immune and inflammatory responses, apoptosis, oxidative stress, and metabolic and cell-cycle processes. The occurrence of severe oxidative stress was also experimentally confirmed.
Oral administration of anatase TiO2 (100 mg kg−1 body weight, particle diameter size of 66 and 260 nm) to mice for 10 days by gavage caused increased levels of T CD4+ cells in the duodenum, jejunum, and ileum compared to control and caused hypertrophy and hyperplasia of the mucosal epithelium of the small intestine (Nogueira et al. 2012). The same treatment caused an increase in levels of inflammatory cytokines in the intestines (IL-12, IL-14, IL-23, TNF-α, IFN-γ, and TGF-β), indicating that after oral consumption, TiO2 caused a Th-1 mediated inflammatory response in the small intestine. Rutile TiO2 oral gavage for 10 days at 12.5 mg kg−1 rat body weight (primary particle size 475 nm) also caused accumulation in the intestines and translocation to other organs, such as the liver and spleen (Jani et al. 1994). Although TiO2 accumulates in the intestinal cells, it has been shown that TiO2 in vitro is relatively safe for gastrointestinal cells and does not induce cytotoxicity (Chen et al. 2013). On the other hand, accumulation of inorganic particles (which may or may not include TiO2) in intestinal cells may potentially lead to the pathogenesis of inflammatory bowel disease in humans (Powell et al. 2000, 2010; Lomer et al. 2002, 2004). One particular study showed that all investigated colons of diseased patients (suffering from colon cancer or Crohn's disease) contained significant amounts of nano and micro particles versus healthy cadavers (Gatti 2004). The reduction of nano and microparticles in the diet of the patients suffering from Crohn's disease improved their conditions, with 7 out of 9 treated patients showing signs of remission (Lomer et al. 2001). However, in addition to the absence of nano and microparticles from the diet, reduction of meat-based products might have contributed to the remission (Lomer et al. 2005).
Very few oral studies have focused on the brain as the target organ with regard to TiO2 toxicity. In the only available study, ICR mice were exposed every second day to anatase TiO2 (5–50 mg kg−1 body weight, primary particle size 5 nm) via intragastric administration, and spatial recognition memory was analyzed (Hu et al. 2010). Results of the Y-maze test indicated that TiO2 exposure significantly impaired spatial recognition behavior. Furthermore, levels of Ca, Mg, Fe, K, Na, and Zn were significantly altered in the brain tissue. Activities of several ATP-ases and the contents of some neurotransmitters decreased, whereas levels of acetylcholine, glutamate, and nitric oxide increased (Hu et al. 2010). Significant accumulation of Ti in the brain after exposure to TiO2 was also noted, causing abnormal pathology of the brain that manifested as calcification of neurocytes, followed by proliferation of ependyma and spongiocyte. Excessive release of nitric oxide, reduction of glutamic acid content, and downregulation of acetylcholinesterase in brain tissue was also confirmed after TiO2 intraperitoneal injections in mice (Ma et al. 2010). Similar effects on the brain were also observed in fish models (Ramsden et al. 2009). After 8 weeks of ingesting 10 to 100 mg kg−1 of TiO2, rainbow trout brains displayed a 50% inhibition in Na+K+-ATPase activity.
Injection route studies
Although oral exposure is more relevant when assessing the risk of food contaminants, ADME are quite often carried out with injection routes as the biomedical mode of exposure. Because insufficient ADME studies are available implementing oral exposure of TiO2, relevant studies using injection delivery are summarized below. Injection studies were also used in JECFA's (1969) assessment.
One early autopsy case study determined that 2 drug addicts, who had been injecting themselves intravenously with crushed Algafan tablets for 7 to 10 years (active ingredient: propoxyphene hydrochloride; inactive ingredient: TiO2, 0.9% weight of the tablet), developed thick, hard nodules of approximately 0.5 cm in diameter in the lungs, liver, and spleen. On further inspection, it was concluded that the nodules were composed of TiO2 and magnesium silicate (Filho et al. 1991).
Intravenous administration of 56 mg kg−1 body weight of 120 nm TiO2 particles caused accumulation in the liver, lung, and spleen with limited clearance after 26 weeks in mice (Umbreit et al. 2012). Other studies, after various injection routes of anatase TiO2 (16 g kg−1 of particles with approximately 1000 nm diameter) on rats, also confirmed that the primary deposition organs were liver and lungs and that TiO2 was disseminated with ease through the blood stream (Olmedo et al. 2003, 2008). Repeated daily IP injections of anatase TiO2 (14 days, primary particle size 5 nm, 5–150 mg kg−1 body weight) outlined TiO2 accumulation in various organs: in decreasing order, liver, kidney, spleen, lung, brain, and heart (Liu et al. 2009). Intraperitoneal injection of anatase TiO2 (324–2592 mg kg−1 body weight, particle size 100 nm) caused the highest accumulation in the spleen, followed by the liver, kidney, and lung (Chen et al. 2009). In addition, the same study found that TiO2 caused loss of appetite, passive behavior, tremor, and lethargy in experimental animals.
A smaller particle diameter of TiO2 (<100 nm, generally 20–30 nm) injected into rats with much lower doses of 5 mg kg−1 displayed the same pattern of accumulation in the liver, lung, spleen, and kidney (Fabian et al. 2008) as the previous studies with higher doses and larger particle diameters. With a dose of 5 mg kg−1, Fabian et al. (2008) did not detect apparent toxic effects; however, the exposure time was relatively short—28 days. During those 28 days, a limited clearance of TiO2 was observed in the spleen, but there was no clearance whatsoever of the TiO2 stored in the liver. In another study, intravenous (IV) injection of TiO2 in mice (1.8 µg per animal, particle size diameter of 15 nm) indicated the liver as the primary storage organ, with a significant increase in TiO2 content as early as 5 min post injection and a limited clearance rate of 30% 1 month after treatment (Sugibayashi et al. 2008). Similarly, IV injection of 0.95 mg kg−1 of nano-TiO2 (average particle diameter 60 nm) in rats resulted in immediate (6 h postinjection time) accumulation of TiO2 in the liver and spleen without any signs of clearance (by urine or feces) until the end of a 30 day experimental trial (Shinohara et al. 2014). A rare, long-term study found that 1 year after a single IV administration of 200 mg kg−1 rat body weight of TiO2 (200–400 nm particle diameter), a significant concentration was still stored in various tissues (predominantly liver, spleen, and lymph nodes), without any apparent clearance since day 1 of the exposure (Huggins and Froehlich 1966). The same study also reported that TiO2 can be deposited in the liver as early as 5 min after initial administration. In a 14 day acute study (Xu et al. 2013), mice were IV injected with 140 to 1387 mg kg−1 body weight with TiO2 (anatase, primary particle size 40 nm). After 2 days of exposure, 75% of the animals in the highest concentration exposure group (1387 mg kg−1 body weight) died. Animals exposed to lower concentrations of TiO2 survived, but a decrease in food and water intake was noted. Swelling of the renal glomerulus was observed in the mice treated with TiO2, and TiO2 also caused increased proliferation of local macrophages in the spleen. The brain and lungs were affected, as well, with brain tissue showing neuronal degeneration and infiltration of inflammatory cells alongside small lesions.
The pharmacokinetics of TiO2 have also been investigated recently (Xie et al. 2011). Rutile TiO2 was labeled with CF680 and 125I and injected into mice or rats at 1 to 10 mg kg−1 body weight (average particle diameter 352 nm). Tissue distribution and excretion were investigated over 30 days. Results indicated that the TiO2 mainly accumulated in the liver and spleen. Excretion of TiO2 was significantly higher in urine compared to feces, indicating that renal excretion is the main pathway for TiO2 (Xie et al. 2011). This finding, however, is contrary to the similar oral study, which indicated that the primary elimination route of TiO2 was through feces (Cho et al. 2013).
DISCUSSION
Based on findings from various studies on mammals, it appears that TiO2 fails to satisfy the 2 conditions on which the JECFA (1969) assessment was based: lack of absorption and lack of accumulation in body tissue. Based on the literature, TiO2 has clear potential for absorption by mammals after ingestion or injection, as well as for storage in various organs (Huggins and Froehlich 1966; Filho et al. 1991; Jani et al. 1994; Böckmann et al. 2000; Olmedo et al. 2003, 2008; Wang et al. 2007; Fabian et al. 2008; Sugibayashi et al. 2008; Liu et al. 2009; Duan et al. 2010; Hu et al. 2010; Cui et al. 2011; Xie et al. 2011; Nogueira et al. 2012; Umbreit et al. 2012; Sang, Fei, et al. 2013; Sang, Li, et al. 2013; Shinohara et al. 2014; Tassinari et al. 2014), where it can cause tissue damage and alter biochemical parameters. These properties, however, are most likely dependent on the concentration and size of TiO2 particles. In addition to mammals, the potential of TiO2 to bioconcentrate, bioaccumalate, and biomagnify in the bodies of nonmammalian animals such as fish is also present (Zhang et al. 2006; Ramsden et al. 2009; Fouqueray et al. 2013). Contaminated fish may provide an additional transfer of TiO2 to other, top food chain consumers, including humans. In addition, there is a valid scientific opinion that accumulation of inorganic particles (including TiO2 particles) in intestinal cells may lead to the pathogenesis of inflammatory bowel disease in humans (Lomer et al. 2000, 2001, 2002, 2004, 2005; Powell et al. 2000, 2010). TiO2 has also been indicated as an immunotoxin in vertebrates (Duan et al. 2010; Jovanović and Palić 2012; Sang et al. 2012; Sang, Fei, et al. 2013). Given these facts, the recommendation of this article is that a toxicological evaluation of TiO2 safety as a food color or additive needs to be reassessed without delay. The assessment performed by JECFA in 1969 may no longer be sufficient, as in the meantime, the scientific community has generated compelling evidence about potential TiO2 toxicity.
Currently, the official regulatory definition of nanoparticles is given by the European Commission. Any natural, incidental, or manufactured particles in an unbound state, aggregate, or agglomerate are considered nanoparticles if they satisfy at least one of the following 3 criteria (EC 2011):
Fifty percent or more of the total number of particles in a given material are in the size range 1–100 nm, in at least 1 dimension.
Specific surface area by volume of the given material is greater than 60 m2 cm−3.
Fullerenes, graphene flakes, and C nanotubes at least 1 external dimension smaller than 1 nm are also considered as nanomaterials.
Some authors believe that the upper regulatory limit of 100 nm was set based on public scientific opinion that particles with a diameter smaller than 100 nm can be endocytosed, are easily spread through the body, and pose higher toxicological risks (Keck and Müller 2013). The European Commission further states that the 100 nm upper limit was used as a general consensus but cautions that no scientific evidence supported the choice (EC 2011). The Science and Technology Committee of the House of Lords of the UK has openly opposed the European Commission definition, stating that it should not be limited to this arbitrary dimension (House of Lords 2010). Other independent scientists have argued that the bar has been set too low and that the threshold should be raised to 200 nm due to the specific properties of particles as food supplements (Chen et al. 2013).
Similarly, colloids are defined as particles having 1 dimension in the range 1 nm to 1000 nm; thus, smaller colloidal particles can also be considered nanoparticles (Walker et al. 2012). Many proteins, such as casein in milk (50–300 nm) or whey proteins (5–100 nm), recrystallized starch (10–20 nm), and triglycerides (10–100 nm), are also in the nano range. Because specific nano casein, nano starch, and nano fibers may already be produced purposely, under the current European Commission definition, this food should be labeled as including nanoparticles. Furthermore, the DNA molecule has a width of 2.4 nm, so any genetically modified food which contains altered DNA structures (even inclusion of the single base) in the final product should carry the label, “This product contains engineered nanoparticles,” which is absurd.
The author of this article disagrees with the upper limit of 100 nm in the current official definition of nanomaterials (especially TiO2) for 2 reasons. During the last several years, it was discovered that the size limit for nanoparticle uptake by gastrointestinal cells is not 100 nm. Particles with a diameter of up to 500 nm can diffuse through mucus pores (Lai et al. 2007), whereas the passage rate of 1 µm is marginal. Second, simply mathematically speaking, a metric unit conversion system (base, milli, micro, nano) is defined by a 1000-times conversion factor; thus, everything less than 1 µm and greater than 1 nm is by default nano. There is no solid justification for nanoparticles to be defined outside the established metric system convention. Therefore, in the interest of public health, the upper nanoparticle limit should correspond to established metric nomenclature in the official definition. Nanoparticles should be defined as any natural, incidental, or manufactured particles in an unbound state, aggregate, or agglomerate where 50% or more of the total number of particles in a given material are in the size range 1 to 1000 nm, across all 3 dimensions. This definition, however, still does not address all concerns, such as how to exclude modified food proteins from the definition.
Revising the size definition of nanoparticles will impact the safety reevaluation of TiO2 as a food ingredient. The actual average particle diameter of TiO2 E171 particle in food is 110 nm, with at least 36% of particles having a diameter less than 100 nm and with a particle diameter range 30 to 400 nm (Weir et al. 2012). Such a formulation still does not officially qualify for nanotechnology-labeled food under the current official definition. Manufacturers will continue to color food with E171 as long as the E171 is registered as an inactive ingredient (JECFA 1969), rather than as a nanomaterial. If, however, E171 gains the status of nanomaterial, it will automatically trigger a safety reevaluation of its use in food products.
In conclusion, there is overwhelming evidence that TiO2 can pass through and be absorbed by the mammalian gastrointestinal tract. TiO2 can bioconcentrate, bioaccumulate, and biomagnify in the tissues of mammals and other vertebrates. Unfortunately, most scientific studies have used doses far beyond daily estimated human consumption without performing all recommended particle characterizations. Results suggest that the spleen, liver, and kidney are the organs at risk for highest TiO2 bioconcentration. TiO2 can cause histopathological and physiological changes in various organs of animals, depending on the dose. Based on the literature, it appears that the spleen and liver are the major target organs of TiO2 toxicity after oral ingestion. In mammals, TiO2 has a limited elimination rate. All of these facts are contrary to the 1969 JECFA approval of TiO2 as an inactive ingredient in human food, which rendered the establishment of an acceptable daily intake for humans unnecessary after citing that neither significant absorption nor tissue storage following ingestion of TiO2 was possible. Some 45 years since the legislation occurred, the majority of results from both independent scientists and research laboratories point toward a different scenario. Therefore, a reassessment on the safety of TiO2 as an additive in human food should be immediately performed by relevant government agencies.
Acknowledgments
This research was supported by a Marie Curie FP7 Career Integration Grant within the 7th European Union Framework Programme, Project PCIG13-GA-2013-618006. The author also wishes to acknowledge the Scientific and Technological Research Council of Turkey (TUBITAK) for the partial support of this research through the author's visiting scientist fellowship programme (TUBITAK 2221).
REFERENCES
- Al-Jubory AR, Handy RD. Uptake of titanium from TiO2 nanoparticle exposure in the isolated perfused intestine of rainbow trout: nystatin, vanadate and novel CO2-sensitive components. Nanotoxicology. 2013;7:1282–1301. doi: 10.3109/17435390.2012.735268. [DOI] [PubMed] [Google Scholar]
- Armstrong AR, Armstrong G, Canales J, Bruce PG. TiO2-B nanowires. Angewandte Chemie Int Ed. 2004;43:2286–2288. doi: 10.1002/anie.200353571. [DOI] [PubMed] [Google Scholar]
- Böckmann J, Lahl H, Eckert T, Unterhalt B. Blood titanium levels before and after oral administration titanium dioxide. (Article in German) Pharmazie. 2000;55:140–143. [PubMed] [Google Scholar]
- Brown JR, Mastromatteo E. Acute oral and parenteral toxicity of four titanate compounds in the rat. Indust Med Surg. 1962;31:302–304. [PubMed] [Google Scholar]
- Card JW, Jonaitis TS, Tafazoli S, Magnuson BA. An appraisal of the published literature on the safety and toxicity of food-related nanomaterials. Crit Rev Toxicol. 2010;41:20–49. doi: 10.3109/10408444.2010.524636. [DOI] [PubMed] [Google Scholar]
- Chen J, Dong X, Zhao J, Tang G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J Appl Toxicol. 2009;29:330–337. doi: 10.1002/jat.1414. [DOI] [PubMed] [Google Scholar]
- Chen X-X, Cheng B, Yang Y-X, Cao A, Liu J-H, Du L-J, Liu Y, Zhao Y, Wang H. Characterization and preliminary toxicity assay of nano-titanium dioxide additive in sugar-coated chewing gum. Small. 2013;9:1765–1774. doi: 10.1002/smll.201201506. [DOI] [PubMed] [Google Scholar]
- Cho W-S, Kang B-C, Lee JK, Jeong J, Che J-H, Seok SH. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part Fibre Toxicol. 2013;10:9. doi: 10.1186/1743-8977-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Gong X, Duan Y, Li N, Hu R, Liu H, Hong M, Zhou M, Wang L, Wang H, et al. Hepatocyte apoptosis and its molecular mechanisms in mice caused by titanium dioxide nanoparticles. J Hazard Mater. 2010;183:874–880. doi: 10.1016/j.jhazmat.2010.07.109. [DOI] [PubMed] [Google Scholar]
- Cui Y, Liu H, Zhou M, Duan Y, Li N, Gong X, Hu R, Hong M, Hong F. Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J Biomed Mater Res A. 2011;96A:221–229. doi: 10.1002/jbm.a.32976. [DOI] [PubMed] [Google Scholar]
- Duan Y, Liu J, Ma L, Li N, Liu H, Wang J, Zheng L, Liu C, Wang X, Zhao X, et al. Toxicological characteristics of nanoparticulate anatase titanium dioxide in mice. Biomaterials. 2010;31:894–899. doi: 10.1016/j.biomaterials.2009.10.003. [DOI] [PubMed] [Google Scholar]
- DuPont. 2007. PuPont™ Ti-Pure® titanium dioxide. Titanium dioxide for coatings. [cited 2014 June 5]. Available from: http://www2.dupont.com/Titanium_Technologies/en_US/tech_info/literature/Coatings/CO_B_H_65969_Coatings_Brochure.pdf.
- [ED-DuPont] Environmental Defense-DuPont. 2007. Nano risk frame-work. [cited 2014 June 5]. Available from: http://www.nanoriskframework.com/files/2011/11/6496_Nano-Risk-Framework.pdf.
- [EC] European Commission. 2011. Commission recommendation of 18 October 2011 on the definition of nanomaterial. OJEC 2011/696/EU:L 275/238-L275/240.
- [EC] European Commission. 2013. SCCS opinion on titanium dioxide (nano form). Luxembourg: Scientific Committee on Consumer Safety. COLIPA no. S75. SCSS/1516/13.
- European Parliament. European Parliament,Council Directive on Colours, 94/36/EC. OJEC. 1994;237:13–29. [Google Scholar]
- Fabian E, Landsiedel R, Ma-Hock L, Wiench K, Wohlleben W, Ravenzwaay B. Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch Toxicol. 2008;82:151–157. doi: 10.1007/s00204-007-0253-y. [DOI] [PubMed] [Google Scholar]
- Federal Register. 1966. Color additives. Washington (DC), USA: Federal Register. Part 8, Title 21. 31:1065.
- Filho JCC, Moreira RA, Crocker PR, Levison DA, Corrin B. Identification of titanium pigment in drug addicts' tissues. Histopathology. 1991;19:190–192. doi: 10.1111/j.1365-2559.1991.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Fouqueray M, Noury P, Dherret L, Chaurand P, Abbaci K, Labille J, Rose J, Garric J. Exposure of juvenile Danio rerio to aged TiO2 nanomaterial from sunscreen. Environ Sci Pollut Res. 2013;20:3340–3350. doi: 10.1007/s11356-012-1256-7. [DOI] [PubMed] [Google Scholar]
- Fröhlich E, Teubl BJ, Roblegg E. Titanium dioxide nanoparticles and the oral uptake-route. Bio Nano Mat. 2013;14:25–35. [Google Scholar]
- [FSS] Food Safety and Standards. India: Food safety and standards (food product standards and food additives) regulation 2011. Gazette of India: Extraordinary. 2011;4:449–529. [Google Scholar]
- Gatti AM. Biocompatibility of micro- and nano-particles in the colon. Part II. Biomaterials. 2004;25:385–392. doi: 10.1016/s0142-9612(03)00537-4. [DOI] [PubMed] [Google Scholar]
- Gui S, Sang X, Zheng L, Ze Y, Zhao X, Sheng L, Sun Q, Cheng Z, Cheng J, Hu R, et al. Intragastric exposure to titanium dioxide nanoparticles induced nephrotoxicity in mice, assessed by physiological and gene expression modifications. Part Fibre Toxicol. 2013;10:4. doi: 10.1186/1743-8977-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- House of Lords. 2010. Nanotechnologies and food. Vol 1. London, UK: Science & Technology Committee, House of Lords.
- Hu R, Gong X, Duan Y, Li N, Che Y, Cui Y, Zhou M, Liu C, Wang H, Hong F. Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO2 nanoparticles. Biomaterials. 2010;31:8043–8050. doi: 10.1016/j.biomaterials.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Huggins CB, Froehlich JP. High concentration of injected titanium dioxide in abdominal lymph nodes. J Exp Med. 1966;124:1099–1106. doi: 10.1084/jem.124.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [IARC] International Agency for Research on Cancer. 2010. Carbon black, titanium dioxide, and talc. Monographs on the evaluation of carcinogenic risks to humans. Lyon, France: IARC. 93:1–452.
- Iavicoli I, Leso V, Berggamschi A. Toxicological effects of titanium dioxide nanoparticles: A review of in vivo studies. J Nanomater. 2012;2012:36. [PubMed] [Google Scholar]
- [ISO] International Organization for Standardization. 2010. Nanotechnologies: characterization of nanoparticles in inhalation exposure chambers for inhalation toxicity testing. Geneva, Switzerland: ISO. ISO 10808:2010.
- Jacobs JF, Poel Ivd, Osseweijer P. Sunscreens with titanium dioxide (TiO2) nanoparticles: a societal experiment. Nanoethics. 2010;4:103–113. doi: 10.1007/s11569-010-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani P, Halbert GW, Langridge J, Florence AT. The uptake and translocation of latex nanospheres and microspheres after oral administration to rats. J Pharm Pharmacol. 1989;41:809–812. doi: 10.1111/j.2042-7158.1989.tb06377.x. [DOI] [PubMed] [Google Scholar]
- Jani P, Halbert GW, Langridge J, Florence AT. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol. 1990;42:821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- Jani PU, McCarthy DE, Florence AT. Titanium dioxide (rutile) particle uptake from the rat GI tract and translocation to systemic organs after oral administration. Int J Pharm. 1994;105:157–168. [Google Scholar]
- [JECFA]. Joint FAO/WHO Expert Committee on Food Additives. 1969. Thirtieth report of the joint FAO/WHO expert committee on food additives. FAO nutrition meetings report series. WHO technical report series, FAS70.36/NMRS 46A-JECFA 13/55 to titanium dioxide (INS 171)
- [JETRO] Japan External Trade Organization. 2011. Specifications and standards for foods, food additives, etc. under the Food Sanitation Act. Tokyo, Japan: JETRO. [cited 2013 November]. Available from: http://www.jetro.go.jp/en/reports/regulations/pdf/foodext2010e.pdf.
- Jovanović B, Palić D. Immunotoxicology of non-functionalized engineered nanoparticles in aquatic organisms with special emphasis on fish: review of current knowledge, gap identification, and call for further research. Aquat Toxicol. 2012;118–119:141–151. doi: 10.1016/j.aquatox.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Justo-Hanani R, Dayan T. The role of the state in regulatory policy for nanomaterials risk: Analyzing the expansion of state-centric rulemaking in EU and US chemicals policies. Res Policy. 2014;43:169–178. [Google Scholar]
- Keck CM, Müller RH. Nanotoxicological Classification System (NCS): A guide for the risk-benefit assessment of nanoparticulate drug delivery systems. Eur J Pharm Biopharm. 2013;84:445–448. doi: 10.1016/j.ejpb.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Koeneman B, Zhang Y, Westerhoff P, Chen Y, Crittenden J, Capco D. Toxicity and cellular responses of intestinal cells exposed to titanium dioxide. Cell Biol Toxicol. 2010;26:225–238. doi: 10.1007/s10565-009-9132-z. [DOI] [PubMed] [Google Scholar]
- Lai SK, O'Hanlon DE, Harrold S, Man ST, Wang Y-Y, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Nat Acad Sci USA. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ma L, Zhao J, Liu J, Yan J, Ruan J, Hong F. Biochemical toxicity of nano-anatase TiO2 particles in mice. Biol Trace Elem Res. 2009;129:170–180. doi: 10.1007/s12011-008-8285-6. [DOI] [PubMed] [Google Scholar]
- Lomer MCE, Grainger SL, Ede R, Catterall AP, Greenfield SM, Cowan RE, Vicary FR, Jenkins AP, Fidler H, Harvey RS, et al. Lack of efficacy of a reduced microparticle diet in a multi-centred trial of patients with active Crohn's disease. Eur J Gastroenterol Hepatol. 2005;17:377–384. doi: 10.1097/00042737-200503000-00019. [DOI] [PubMed] [Google Scholar]
- Lomer MCE, Harvey RSJ, Evans SM, Thompson RPH, Powell JJ. Efficacy and tolerability of a low microparticle diet in a double blind, randomized, pilot study in Crohn's disease. Eur J Gastroenterol Hepatol. 2001;13:101–106. doi: 10.1097/00042737-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Lomer MCE, Hutchinson C, Volkert S, Greenfield SM, Catterall A, Thompson RPH, Powell JJ. Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn's disease. Br J Nutr. 2004;92:947–955. doi: 10.1079/bjn20041276. [DOI] [PubMed] [Google Scholar]
- Lomer MCE, Thompson RPH, Commisso J, Keen CL, Powell JJ. Determination of titanium dioxide in foods using inductively coupled plasma optical emission spectrometry. Analyst. 2000;125:2339–2343. doi: 10.1039/b006285p. [DOI] [PubMed] [Google Scholar]
- Lomer MCE, Thompson RPH, Powell JJ. Fine and ultrafine particles of the diet: influence on the mucosal immune response and association with Crohn's disease. Proc Nutr Soc. 2002;61:123–130. doi: 10.1079/pns2001134. [DOI] [PubMed] [Google Scholar]
- Ma L, Liu J, Li N, Wang J, Duan Y, Yan J, Liu H, Wang H, Hong F. Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO2 delivered to the abdominal cavity. Biomaterials. 2010;31:99–105. doi: 10.1016/j.biomaterials.2009.09.028. [DOI] [PubMed] [Google Scholar]
- [MAFF] Ministry of Agriculture, Fisheries, and Food. Dietary intake of food additives in the UK: initial surveillance (food surveillance paper 37) London, UK: MAFF; 1993. [Google Scholar]
- National Toxicology Program. Bioassay of titanium dioxide for possible carcinogenicity. Natl Cancer Inst Carcinog Tech Rep Ser. 1979;97:1–123. [PubMed] [Google Scholar]
- Nogueira CM, de Azevedo WM, Dagli ML, Toma SH, Leite AZ, Lordello ML, Nishitokukado I, Ortiz-Agostinho CL, Duarte MI, Ferreira MA, et al. Titanium dioxide induced inflammation in the small intestine. World J Gastroenterol. 2012;18:4729–4735. doi: 10.3748/wjg.v18.i34.4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo D, Tasat D, Guglielmotti M, Cabrini R. Titanium transport through the blood stream. An experimental study on rats. J Mater Sci Mater Med. 2003;14:1099–1103. doi: 10.1023/b:jmsm.0000004007.26938.67. [DOI] [PubMed] [Google Scholar]
- Olmedo D, Tasat D, Guglielmotti M, Cabrini R. Biodistribution of titanium dioxide from biologic compartments. J Mater Sci Mater Med. 2008;19:3049–3056. doi: 10.1007/s10856-008-3438-x. [DOI] [PubMed] [Google Scholar]
- Onishchenko GE, Erokhina MV, Abramchuk SS, Shaitan KV, Raspopov RV, Smirnova VV, Vasilevskaya LS, Gmoshinski IV, Kirpichnikov MP, Tutelyan VA. Effects of titanium dioxide nanoparticles on small intestinal mucosa in rats. Bull Exp Biol Med. 2012;154:265–270. doi: 10.1007/s10517-012-1928-9. [DOI] [PubMed] [Google Scholar]
- Phillips W, Griffen D. Optical mineralogy: The nonopaque minerals. San Francisco (CA): WH Freeman; 1981. p. 677. [Google Scholar]
- Powell JJ, Faria N, Thomas-McKay E, Pele LC. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J Autoimmun. 2010;34:J226–J233. doi: 10.1016/j.jaut.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Powell JJ, Harvey RSJ, Ashwood P, Wolstencroft R, Gershwin ME, Thompson RPH. Immune potentiation of ultrafine dietary particles in normal subjects and patients with inflammatory bowel disease. J Autoimmun. 2000;14:99–105. doi: 10.1006/jaut.1999.0342. [DOI] [PubMed] [Google Scholar]
- Qian B, Guangyan Y, Pengchi D, Feng P, Hongjun L, Youzhi X, Zhixing C, Tian Z, Aiqin X, Yanli W, et al. NMR-based metabonomic study of the sub-acute toxicity of titanium dioxide nanoparticles in rats after oral administration. Nanotechnology. 2010;21:125105. doi: 10.1088/0957-4484/21/12/125105. [DOI] [PubMed] [Google Scholar]
- Ramsden C, Smith T, Shaw B, Handy R. Dietary exposure to titanium dioxide nanoparticles in rainbow trout (Oncorhynchus mykiss): No effect on growth, but subtle biochemical disturbances in the brain. Ecotoxicology. 2009;18:939–951. doi: 10.1007/s10646-009-0357-7. [DOI] [PubMed] [Google Scholar]
- Robichaud CO, Uyar AE, Darby MR, Zucker LG, Wiesner MR. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ Sci Technol. 2009;43:4227–4233. doi: 10.1021/es8032549. [DOI] [PubMed] [Google Scholar]
- Sang X, Fei M, Sheng L, Zhao X, Yu X, Hong J, Ze Y, Gui S, Sun Q, Ze X, et al. Immunomodulatory effects in the spleen-injured mice following exposure to titanium dioxide nanoparticles. J Biomed Mater Res A. 2013;102:3562–3572. doi: 10.1002/jbm.a.35034. [DOI] [PubMed] [Google Scholar]
- Sang X, Li B, Ze Y, Hong J, Ze X, Gui S, Sun Q, Liu H, Zhao X, Sheng L, et al. Toxicological mechanisms of nanosized titanium dioxide-induced spleen injury in mice after repeated peroral application. J Agricul Food Chem. 2013;61:5590–5599. doi: 10.1021/jf3035989. [DOI] [PubMed] [Google Scholar]
- Sang X, Zheng L, Sun Q, Li N, Cui Y, Hu R, Gao G, Cheng Z, Cheng J, Gui S, et al. The chronic spleen injury of mice following long-term exposure to titanium dioxide nanoparticles. J Biomed Mater Res A. 2012;100A:894–902. doi: 10.1002/jbm.a.34024. [DOI] [PubMed] [Google Scholar]
- Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N, Danno N, Ichinose T, Sasaki T, Fukui H, Honda K, Gamo M. Tissue distribution and clearance of intravenously administered titanium dioxide (TiO2) nanoparticles. Nanotoxicology. 2014;8:132–141. doi: 10.3109/17435390.2012.763001. [DOI] [PubMed] [Google Scholar]
- Sugibayashi K, Todo H, Kimura E. Safety evaluation of titanium dioxide nanoparticles by their absorption and elimination profiles. J Toxicol Sci. 2008;33:293–298. doi: 10.2131/jts.33.293. [DOI] [PubMed] [Google Scholar]
- Tassinari R, Cubadda F, Moracci G, Aureli F, D'Amato M, Valeri M, De Berardis B, Raggi A, Mantovani A, Passeri D, et al. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: Focus on reproductive and endocrine systems and spleen. Nanotoxicology. 2014;8:654–662. doi: 10.3109/17435390.2013.822114. [DOI] [PubMed] [Google Scholar]
- Umbreit TH, Francke-Carroll S, Weaver JL, Miller TJ, Goering PL, Sadrieh N, Stratmeyer ME. Tissue distribution and histopathological effects of titanium dioxide nanoparticles after intravenous or subcutaneous injection in mice. J Appl Toxicol. 2012;32:350–357. doi: 10.1002/jat.1700. [DOI] [PubMed] [Google Scholar]
- [USFDA] US Food and Drug Administration. Titanium dioxide. Washington (DC): USFDA; 2005. Code of Federal Regulations, Title 21, Section 73.575. [cited 2013 November]. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=73.575. [Google Scholar]
- [USGS] United States Geological Survey. 2013. pp. 172–173. Titanium and titanium dioxide. Mineral commodity summaries. [cited 2013 November]. Available from: http://minerals.usgs.gov/minerals/pubs/commodity/titanium/mcs-2013-titan.pdf.
- Walker CH, Sibly RM, Hopkin SP, Peakall DB. Principles of ecotoxicology. 4th ed. Boca Raton (FL): CRC; 2012. p. 360. [Google Scholar]
- Wang J, Li N, Zheng L, Wang S, Wang Y, Zhao X, Duan Y, Cui Y, Zhou M, Cai J, et al. P38-Nrf-2 signaling pathway of oxidative stress in mice caused by nanoparticulate TiO2. Biol Trace Elem Res. 2011;140:186–197. doi: 10.1007/s12011-010-8687-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J, et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007;168:176–185. doi: 10.1016/j.toxlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen Z, Ba T, Pu J, Chen T, Song Y, Gu Y, Qian Q, Xu Y, Xiang K, et al. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small. 2013;9:1742–1752. doi: 10.1002/smll.201201185. [DOI] [PubMed] [Google Scholar]
- Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46:2242–2250. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Wang C, Sun J, Zhong G. Tissue distribution and excretion of intravenously administered titanium dioxide nanoparticles. Toxicol Lett. 2011;205:55–61. doi: 10.1016/j.toxlet.2011.04.034. [DOI] [PubMed] [Google Scholar]
- Xu J, Shi H, Ruth M, Yu H, Lazar L, Zou B, Yang C, Wu A, Zhao J. Acute toxicity of intravenously administered titanium dioxide nanoparticles in mice. PLoS ONE. 2013;8:e70618. doi: 10.1371/journal.pone.0070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Doudrick K, Bi X, Hristovski K, Herckes P, Westerhoff P, Kaegi R. Characterization of titanium dioxide food grade: The presence of nanosized particles. Eviron Sci Technol. 2014;48:6391–6400. doi: 10.1021/es500436x. [DOI] [PubMed] [Google Scholar]
- Zhang XZ, Sun HW, Zhang TZY. Bioaccumulation of titanium dioxide nanoparticles in carp. Huan Jing Ke Xue-Chinese J Environ Sci. 2006;27:1631–1635. [PubMed] [Google Scholar]


