Abstract
Objective
Allogeneic mesenchymal stem cells (MSCs) exhibit therapeutic effects in human autoimmune diseases such as systemic lupus erythematosus (SLE), but the underlying mechanisms remain largely unknown. The aim of this study was to investigate how allogeneic MSCs mediate immunosuppression in lupus patients.
Methods
The effects of allogeneic umbilical cord–derived MSCs (UC-MSCs) on inhibition of T cell proliferation were determined. MSC functional molecules were stimulated with peripheral blood mononuclear cells from healthy controls and SLE patients and examined by real-time polymerase chain reaction. CD4+ and CD8+ T cells were purified using microbeads to stimulate MSCs in order to determine cytokine expression by MSCs and to further determine which cell subset(s) or which molecule(s) is involved in inhibition of MSC–mediated T cell proliferation. The related signaling pathways were assessed. We determined levels of serum cytokines in lupus patients before and after UC-MSC transplantation.
Results
Allogeneic UC-MSCs suppressed T cell proliferation in lupus patients by secreting large amounts of indoleamine 2,3-dioxygenase (IDO). We further found that interferon-γ (IFNγ), which is produced predominantly by lupus CD8+ T cells, is the key factor that enhances IDO activity in allogeneic MSCs and that it is associated with IFNGR1/JAK-2/STAT signaling pathways. Intriguingly, bone marrow–derived MSCs from patients with active lupus demonstrated defective IDO production in response to IFNγ and allogeneic CD8+ T cell stimulation. After allogeneic UC-MSC transplantation, serum IDO activity increased in lupus patients.
Conclusion
We found a previously unrecognized CD8+ T cell/IFNγ/IDO axis that mediates the therapeutic effects of allogeneic MSCs in lupus patients.
Mesenchymal stem cells (MSCs) are non-hematopoietic stem cells (non-HSCs) that can support the function of HSCs in bone marrow (BM). MSCs have been shown to possess regenerative properties and unique immunoregulatory functions that make them an attractive option for cellular therapy in patients with autoimmune diseases and chronic inflammation (1). We have previously shown that allogeneic BM- and umbilical cord (UC)–derived MSC transplantation is a safe and effective treatment of active systemic lupus erythematosus (SLE) (2,3) and other autoimmune diseases, such as systemic sclerosis (4), Sjögren's syndrome (5), and myositis (6). Conversely, autologous MSCs from lupus patients cannot offer therapeutic benefits due to intrinsic abnormal functions (7–9). However, the mechanisms by which allogeneic MSC transplantation ameliorates SLE remain largely unknown.
It is now clear that MSCs exert immunoregulatory properties on various immune cells. This includes suppression of T cell proliferation, regulation of dendritic cell (DC) maturation and function, modulation of B cell proliferation and terminal differentiation, and regulation of natural killer cells and macrophage function (10–12). Many factors are involved in MSC immunomodulation, including but not limited to, production of transforming growth factor β (TGFβ), hepatocyte growth factor (HGF), prostaglandin E2 (PGE2), interleukin-10 (IL-10), indolamine 2,3-dioxygenase (IDO), nitric oxide (NO), heme oxygenase 1 (HO-1), and HLA–G (13–16). IDO, which is mainly produced by DCs and macrophages, is an enzyme that degrades the essential amino acid tryptophan and participates in immune tolerance (17,18). In 2004, a study demonstrated that human MSCs could secrete IDO in vitro in the presence of mixed lymphocyte reaction. The IDO that was secreted by MSCs mediated inhibition of normal T cell proliferation (19). However, other studies have demonstrated that IDO plays a dispensable role in human MSC suppression of T cell proliferation and have instead suggested that HLA–G and IL-10 have a cell-contact–dependent role (20). In animal studies, it has been suggested that NO rather than IDO is involved in immunomodulation by MSCs (21). Importantly, the precise mechanisms responsible for the regulatory effects of MSCs in lupus patients remain unknown.
In this study, we determined that high levels of interferon-γ (IFNγ), produced predominantly by CD8+ T cells in lupus patients, are a key factor involved in the stimulation of allogeneic UC-MSCs to produce IDO, which can then inhibit the proliferation of T cells from lupus patients. Thus, we uncovered a previously unrecognized CD8+ T cell/IFNγ/IDO axis that mediates the therapeutic benefit of allogeneic MSCs in lupus.
Patients and Methods
Lupus patients and healthy subjects
Seventy-nine SLE patients and 89 healthy subjects were included in this study. Informed consent was obtained from each subject for the collection of peripheral blood or BM. Clinical study of UC-MSC transplantation among lupus patients was registered with http://ClinicalTrials.gov (identifier: NCT01741857). Six patients underwent UC-MSC transplantation as previously described (3). This study was approved by the Ethics Committee at The Affiliated Drum Tower Hospital of Nanjing University Medical School and was conducted in accordance with the 1989 Declaration of Helsinki.
Antibodies and reagents
The following antibodies (to humans) were used in this study: fluorescein isothiocyanate (FITC)–conjugated anti-human CD3 (OKT3), anti-CD4 (11830), anti–HLA–DR (L203), phycoerythrin (PE)–conjugated anti-human CD4 (11830), allophycocyanin (APC)–conjugated anti-human CD8 (RPA-T8), CD25 (M-A251), and the respective isotype-matched control antibodies (mouse IgG1 and mouse IgG2a) (all from BD Biosciences); and FITC–conjugated anti-human CD34 (4H11), CD44 (IM7), PE-conjugated anti-human CD45 (HI30), CD29 (TS2/16), CD166 (3A6), CD138 (DL-101), FoxP3 (150D/14), PE–Cy7–conjugated FoxP3 (PCH101), APC-conjugated anti-human CD4 (RPA-T4), CD19 (HIB19), PE–Cy7–conjugated anti-human IFNγ (4S.B3), purified anti-human CD3 (OKT3), CD28 (CD28.2), CD40 (5C3) (no azide and low endotoxin) (all from eBioscience). Recombinant human TGFβ1 and anti-human TGFβ antibody were both from R&D Systems. Recombinant human IL-2, IL-4, IL-10, IL-6, tumor necrosis factor α, IFNγ, IL-1β, IFNα, and IFNβ were from PeproTech. 1-methyl-dl-tryptophan was from Sigma-Aldrich. F(ab′)2 fragment goat anti-human IgM was from Jackson ImmunoResearch. Purified anti-human IFNγ (NIB42) and mouse IgG1 isotype (no azide and low endotoxin) were from BioLegend. Human HGF, total IgG, and IgM enzyme-linked immunosorbent assay (ELISA) kits were from eBioscience. The human TGFβ1 ELISA kit was from BioLegend. Cell isolation kits were from Miltenyi Biotec.
Human MSC isolation and purification
Human MSCs were isolated from the UC and BM of lupus patients and healthy subjects. Information on the purification and identification of MSCs is available upon request from the corresponding author.
Isolation and culture of T cells
Peripheral blood mononuclear cells (PBMCs) were isolated from patients with active lupus and healthy controls. CD4+ and CD8+ T cell subsets were purified by positive isolation using microbeads (Miltenyi Biotec). CD4+CD25− and CD4+CD25+ T cell subsets were purified using a human CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec). CD4+ T cells were purified using negative isolation, then CD25 T cells were purified using positive isolation. The purified CD4+ or CD8+ T cell subsets were cocultured with or without pre-plated allogeneic human MSCs (4:1) in the presence of soluble anti-human CD3 (2 μg/ml) and anti-human CD28 (2 μg/ml) antibodies, and a non-CD4/CD8 T cell subset was used as a control. After 48 hours, nonadherent cells were removed and supernatants were collected for measurement of cytokines (IFNγ and TGFβ1) by ELISA (BioLegend). The adherent MSCs were washed 3 times with phosphate buffered saline (PBS) and lysed with TRIzol (Takara) for real-time polymerase chain reaction (PCR) analysis. In some experiments, a Transwell system (0.4 μm pore size; Millipore) was used to block cell–cell contact.
Treg cell differentiation in vitro
CD4+CD25− T cells were cultured with soluble anti-CD3 (2 μg/ml) and anti-CD28 (2 μg/ml) antibodies, with the addition of recombinant human TGFβ1 (10 ng/ml) and IL-2 (100 IU/ml) to induce Treg cell conversion (22,23). In some cultures, allogeneic human MSCs or human lung fibroblast (HLF) cells were initially included. Cells were cultured for 5 or 6 days and collected for measurement of Treg cells.
T cell proliferation assay
For the carboxyfluorescein succinimidyl ester (CFSE)–labeling assay, 106 cells/ml of PBMCs or purified T cells were incubated with 3 μmoles/liter of CFSE in PBS/0.5% bovine serum albumin (BSA) at 37°C for 15 minutes. Cells were washed 3 times with fresh, ice-cold complete 1640 medium and resuspended in complete 1640 medium for further culture. After the cells were cultured for several days as indicated, cells were harvested to examine the CFSE-negative cells using flow cytometry.
Flow cytometric analysis
PBMCs or purified T cells were resuspended in PBS containing 1% BSA and 0.1% sodium azide. For the staining of surface antigens, cells were incubated with FITC-conjugated, PE-conjugated, or APC-conjugated monoclonal antibodies or their negative control antibodies for 30 minutes on ice as indicated. Intracellular staining of FoxP3 and IFNγ was performed as described previously (3,24).
Real-time quantitative PCR
Complementary DNA (cDNA) was synthesized from TRIzol-isolated total RNA using a SuperScript III First Strand Synthesis SuperMix for quantitative reverse transcription–PCR (Takara). For real-time PCR experiments, reactions containing SYBR Premix EX Taq (Takara), ROX Reference Dye (50×; Takara), cDNA, and gene primers were run on a StepOnePlus real-time PCR system and analyzed using StepOne Software, version 2.1 (Applied Biosystems). Gene primers are available upon request from the corresponding author. Relative gene quantification was calculated by the 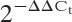 method and then normalized to the level of GAPDH (25).
method and then normalized to the level of GAPDH (25).
Western blot analysis and ELISA
We used antibodies recognizing human STAT-1, STAT-3, STAT-5, Akt, IκB, ERK and their phosphorylation forms, p52, p65, and GAPDH (1:1,000; Cell Signaling Technology) to examine the concentrations of proteins in MSCs lysates. The concentration of IDO protein in MSCs (1:400 dilution; Epitomics Technology) was also determined (methods are available upon request from the corresponding author).
We detected amounts of HGF, TGFβ1, and IFNγ in the conditioned media and/or human serum using ELISA kits (eBioscience or BioLegend) according to the manufacturer's instructions.
High-performance liquid chromatography
Kynurenine and tryptophan concentrations were analyzed by high-performance liquid chromatography as reported (26) (methods are available upon request from the corresponding author).
Statistical analysis
We used a t-test for statistical analysis of parametric data and the Mann-Whitney test for analysis of nonparametric data. One-way analysis of variance was used when there were >2 groups, followed by the Bonferroni test. Statistical analyses were performed with SPSS version 16.0 and GraphPad Prism version 4.3 software packages. Data are presented as the mean ± SEM. P values less than 0.05 were considered significant.
Results
Allogeneic UC-MSC inhibition of the proliferation of T cells from lupus patients
MSCs have been reported to inhibit T cell proliferation in healthy subjects (27,28), but whether this can occur in patients with lupus remains largely unknown. We first investigated whether allogeneic UC-MSCs regulated T cell proliferative responses in lupus patients. We found that UC-MSCs significantly inhibited the proliferation of anti-CD3 and anti-CD28–activated CD4+ T lymphocytes from both healthy controls and lupus patients (Figure 1A). The allogeneic normal human fibroblasts (HLF cells) that served as controls, however, exhibited no suppression of T cell proliferation (Figure 1A). To determine which subset of CD4+T cells was inhibited by UC-MSCs, we separated CD4+CD25− (responder) and CD4+CD25+ (predominantly regulatory) T cells from the peripheral CD4+ T cells of patients and found that UC-MSCs efficiently inhibited CD4+CD25− T cell proliferation (Figure 1B), while they promoted CD4+CD25+ Treg cell proliferation and maintained their survival in vitro (Figures 1C and D).
Figure 1.
Umbilical cord–derived mesenchymal stem cells (UC-MSCs) inhibit lupus T cell proliferation, but not via conversion to induced Treg cells. A, UC-MSCs inhibit proliferation of T cells from patients with systemic lupus erythematosus (SLE) and healthy controls (HCs). B, UC-MSCs inhibit proliferation of CD4+CD25− responder T (Tresp) cells from patients with lupus patients and healthy controls. In A and B, ∗∗∗ = P < 0.001 by one-way analysis of variance (ANOVA) followed by Bonferroni test. C and D, UC-MSCs promote, rather than inhibit, proliferation of CD4+CD25+ Treg cells from lupus patients (C) and increase the absolute number of CD4+CD25+ Treg cells (D). Symbols represent individual subjects; horizontal lines show the mean (n = 6 per group in C and D). ∗∗ = P < 0.01; ∗ = P < 0.05, by t-test. E, UC-MSCs markedly inhibit, rather than induce, differentiation of Treg cells from lupus patients and healthy controls. ∗∗ = P < 0.01 by one-way ANOVA followed by Bonferroni test. Bars in A, B, and E show the mean ± SEM (n = 4 per group). HLF = human lung fibroblast; CFSE = carboxyfluorescein succinimidyl ester; iTreg = induced Treg cells.
To study whether UC-MSC–mediated suppression was due to conversion of induced CD4+CD25+ Treg cells (23), we stimulated CD4+CD25− T cells with anti-CD3/CD28 (2 μg/ml), TGFβ (10 ng/ml), and IL-2 (100 IU/ml) in the presence and absence of UC-MSCs or HLF cells. As expected, fewer induced Treg cells were differentiated in lupus CD4+CD25− T cells as compared to healthy control T cells (Figure 1E). Surprisingly, UC-MSCs failed to enhance and actually inhibited the conversion to induced Treg cells both in healthy controls and in lupus patients (Figure 1E), whereas HLF cells had no effect. Thus, UC-MSCs blocked T cell receptor (TCR)–driven lupus CD4+ T cell proliferation, which was not attributable to induced Treg cell conversion.
Role of IDO in UC-MSC–mediated inhibition of lupus T cell proliferation
We next investigated the underlying molecular mechanisms by which UC-MSCs suppressed lupus T cell proliferation. We hypothesized that UC-MSCs expressed or secreted molecules/factors in lupus patients that in turn inhibited T cell proliferation. To assess this, we cultured UC-MSCs with PBMCs isolated from patients with active SLE or healthy controls, in the absence or presence of soluble anti-CD3 and anti-CD28 antibodies (both 1 μg/ml). After 48 hours, PBMCs were removed through extensive washing, and the molecules and cytokines produced by UC-MSCs were determined. We examined HGF, TGFβ1, and IDO, since they have all been reported to influence MSC-mediated T cell proliferation (19,28). Although unstimulated lupus PBMCs increased HGF and TGFβ1 mRNA levels and protein levels in UC-MSCs as compared to untreated or normal PBMC–treated UC-MSCs, TCR-stimulated lupus PBMCs failed to further up-regulate the aforementioned cytokines (Supplementary Figure 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38674/abstract). Strikingly, however, TCR-stimulated lupus PBMCs drove UC-MSCs to produce extremely high levels of mRNA for IDO (>200-fold) (Figure 2A). We also saw enhanced IDO enzyme activity (Figure 2B) and larger amounts of protein (Figure 2C) as compared to untreated or healthy PBMC–treated UC-MSCs.
Figure 2.
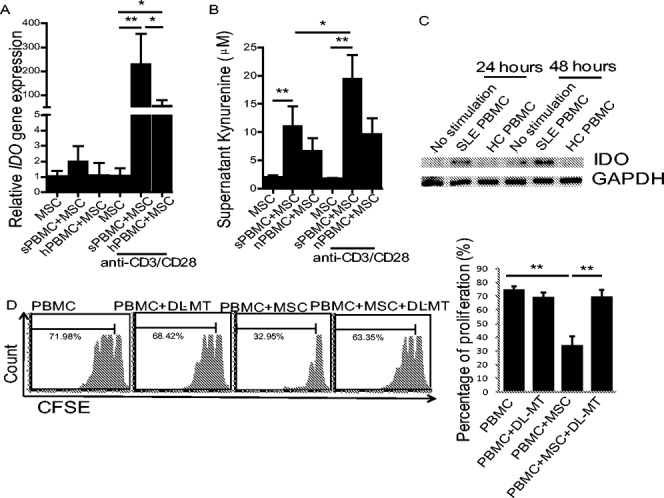
Indoleamine 2,3-dioxygenase (IDO) is key to UC-MSC–mediated inhibition of lupus T cell proliferation. A, T cell receptor–treated peripheral blood mononuclear cells from patients with active SLE (sPBMC) stimulate significant up-regulation of IDO gene expression by UC-MSCs. B, Levels of kynurenine in supernatant are increased in the presence of PBMCs from SLE patients. C, IDO protein levels produced by UC-MSCs are increased in the presence of PBMCs from SLE patients, as shown by Western blot analysis. Results are representative of 3 separate experiments. D, Treatment with 1-methyl-dl-tryptophan (DL-MT) (0.4 μM) significantly blocks UC-MSC–mediated inhibition of CD4+ T cell proliferation. Bars in A, B, and D show the mean ± SEM (n = 5 per group in A, 5 per group in B, and 4 per group in D). ∗ = P < 0.05; ∗∗ = P < 0.01, by one-way ANOVA followed by Bonferroni test. hPBMC = healthy control PBMCs (see Figure 1 for other definitions).
The increase in IDO levels by UC-MSCs in response to lupus PBMCs prompted us to determine whether IDO was involved in UC-MSC–mediated suppression of lupus T cell proliferation. For this, we added 1-methyl-dl-tryptophan the inhibitor of IDO enzyme activity, to the cocultures of UC-MSCs and TCR-stimulated lupus PBMCs that had been prelabeled with CFSE. While the IDO inhibitor itself had no effect on TCR-driven T cell proliferation, it completely reversed the suppression of lupus T cell proliferation mediated by UC-MSCs (Figure 2D).
We next determined whether IDO-mediated inhibition of cell proliferation by UC-MSCs was specific to T cells. We examined effects of IDO on UC-MSC–mediated B cell suppression. PBMCs from lupus patients were cocultured with allogeneic UC-MSCs, and B cells were stimulated with anti-human CD40 and anti-human IgM (F[ab′]2). We found that UC-MSCs inhibited B cell differentiation (as determined by CD138 staining), proliferation (as determined by CFSE labeling), and IgG production (as determined by ELISA) (Supplementary Figure 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38674/abstract). However, inclusion of 1-methyl-dl-tryptophan failed to reverse the inhibition of B cells by UC-MSCs (Supplementary Figure 2). Thus, we found that IDO plays a key role in MSC-mediated suppression of T cells from lupus patients, although the same is not true for B cells.
We also wanted to determine whether the enhanced proliferation of Treg cells by UC-MSCs was dependent upon IDO. UC-MSCs were cocultured in vitro with Treg cells from healthy controls or patients with active lupus. We found that healthy Treg cells failed to induce IDO in UC-MSCs, but lupus Treg cells induced some IDO gene expression in UC-MSCs (Supplementary Figure 2F). In a coculture of lupus PBMCs and UC-MSCs, the addition of the TGFβ kinase inhibitor SB431542 inhibited the proliferation of CD4+FoxP3+ Treg cells. Interestingly, the IDO inhibitor, 1-methyl-dl-tryptophan, had no effect on Treg cell proliferation (Supplementary Figure 2G); thus, the effect of MSCs on Treg cells is not IDO dependent.
Lupus CD8+ T cell–derived IFNγ promotion of IDO activity in UC-MSCs
To investigate which cell subset(s) in lupus PBMCs stimulates UC-MSC production of IDO, we first purified CD4+ T cells from the PBMCs of healthy controls and lupus patients. CD4+ T cells were cocultured with UC-MSCs for 48 hours in the presence of anti-CD3 and anti-CD28 antibodies. Non-CD4+ cells were used as controls. Unexpectedly, we found that non-CD4+ cells from lupus patients drove UC-MSCs to increase expression of mRNA for IDO and exhibited higher levels of IDO enzymatic activity when compared to CD4+ T cells from lupus patients (Supplementary Figure 3, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38674/abstract).
To identify which cell type(s) in non-CD4+ cells was responsible for the IDO production of UC-MSCs, we separated CD4+ and CD8+ T cells and the non-CD4/CD8 cell population (B cells, monocytes, and other cells) from both lupus and healthy PBMCs and repeated the experiment. We determined that CD8+ T cells from lupus patients induced the highest levels of mRNA for IDO and its enzymatic activity (Figures 3A and B). Lupus CD4+ T cells also induced increased IDO levels in UC-MSCs, although the levels were significantly lower than those induced by CD8+ T cells (Figures 3A and B). In healthy subjects, CD8+ T cells were also the most potent cells at stimulating IDO in UC-MSCs, but the levels of mRNA for IDO, as well as its activity, were significantly lower than that in lupus CD8+ T cell–treated UC-MSCs (Figures 3A and B). Interestingly, neither lupus nor healthy non-CD4/CD8 cells from PMBCs induced a significant up-regulation of IDO in UC-MSCs (Figures 3A and B). To determine whether the ability of CD8+ T cells to increase IDO mRNA levels in UC-MSCs was dependent upon cell–cell contact, we separated CD8+ T cells from UC-MSCs in a Transwell coculture system. In cell–cell contact cultures, even when separated from the UC-MSCs, lupus CD8+ T cells induced levels of IDO similar to those induced in UC-MSCs (Figure 3C). These data suggest that a soluble factor(s) secreted from lupus CD8+ T cells stimulates IDO activity in UC-MSCs.
Figure 3.
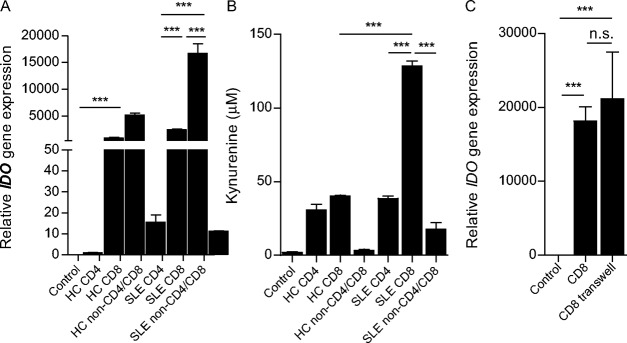
Lupus CD8+ T cells promote indoleamine 2,3-dioxygenase (IDO) activity in UC-MSCs. A, The CD8+ T cell subset from patients with active SLE is the most important factor involved in enhancing IDO mRNA expression in UC-MSCs. B, Supernatant kynurenine levels are increased in the presence of SLE CD8+ T cells, as assessed by high-performance liquid chromatography. C, Up-regulation of IDO mRNA expression in UC-MSCs is not dependent upon cell–cell contact. Bars show the mean ± SEM (n = 7 per group in A, 7 per group in B, and 4 per group in C). ∗∗∗ = P < 0.001 by one-way ANOVA followed by Bonferroni test. NS = not significant (see Figure 1 for other definitions).
Since it has been reported that IFNγ and TGFβ are important factors for the initiation of IDO activity in DCs and macrophages (18), we focused on these 2 cytokines in UC-MSCs. We found a significant increase in levels of IFNγ in the supernatants of cultures containing UC-MSCs and TCR-stimulated lupus CD8+ T cells as compared to cultures containing CD4+ T cells or non-CD4/CD8 cells (Figure 4A), while levels of TGFβ1 were unchanged (Figure 4B). This IFNγ was not produced by UC-MSCs, as the supernatants from CD8+ T cells alone contained similar amounts of IFNγ (Figure 4A). To further confirm that IFNγ was from CD8+ T cells, we examined intracellular IFNγ expression in CD4+, CD8+, and non-CD4/CD8 cells using flow cytometry. We found that lupus CD8+ T cells produced the largest amounts of intracellular IFNγ (Figure 4C). Importantly, the addition of anti-IFNγ antibody to the cocultures of UC-MSCs and lupus CD8+ T cells completely abrogated IDO gene expression (Figure 4D) as well as supernatant kynurenine levels (Figure 4E). Moreover, anti-IFNγ antibody completely restored TCR-driven lupus T cell proliferation that was inhibited by UC-MSCs; when anti-IFNγ was added to cocultures, IDO enzyme activity was no longer increased (Figures 4F–H). The effect was comparable to that found when 1-dl-MT was added to the cocultures (Figures 4F–H). In contrast, neutralization of TGFβ with anti-TGFβ antibody failed to significantly decrease IDO activity and/or restore T cell proliferation in the same UC-MSCs and lupus PBMC cocultures (Figure 4F–I). These data collectively indicate that the IFNγ that is secreted by lupus CD8+ T cells stimulates IDO activity in UC-MSCs and that enhanced levels of IDO then cause inhibition of T cell proliferation.
Figure 4.
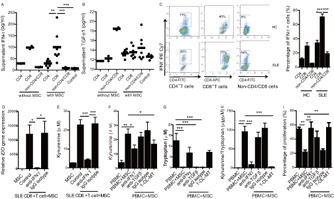
Lupus CD8+ T cell–derived interferon-γ (IFNγ) promotes indoleamine 2,3-dioxygenase (IDO) production. A and B, Levels of IFNγ in supernatant are increased in the presence of UC-MSCs and SLE CD8+ T cells (A), while no change in levels of transforming growth factor β (TGFβ) is observed (B). Symbols represent individual subjects; horizontal lines show the mean (n = 7 per group). C, CD8+ T cells derived from patients with SLE produce much higher levels of intracellular IFNγ as compared to other cell subsets. D, Recombinant anti-human IFNγ antibody significantly abrogates SLE CD+ T cell–mediated IDO mRNA expression in UC-MSCs. E, Kynurenine levels in supernatant decrease in the presence of anti-human IFNγ antibody. F–H, In cocultured lupus peripheral blood mononuclear cells (PBMCs) and UC-MSCs, anti-human IFNγ antibodies (10 μg/ml) significantly inhibit kynurenine levels in supernatant (F), while the level of tryptophan is increased (G), and the ratio of kynurenine to tryptophan is decreased (H) (effects similar to those observed when 1-methyl-dl-tryptophan [1-DL-MT] is added). I, In the coculture system described above, UC-MSC–mediated inhibition of CD4+ T cell proliferation is abrogated by the addition of anti-human IFNγ antibody. Bars in C–I show the mean ± SEM (n = 7 per group in C, 8 per group in D, 8 per group in E, 5 per group in F–H, and 5 per group in I). ∗ = P < 0.05; ∗∗ = P < 0.01; ∗∗∗ = P < 0.001, by one-way ANOVA followed by Bonferroni test. PE = phycoerythrin; FITC = fluorescein isothiocyanate; APC = allophycocyanin (see Figure 1 for other definitions). Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.38674/abstract.
Association of JAK/STAT pathways with enhanced levels of IDO activity in UC-MSCs
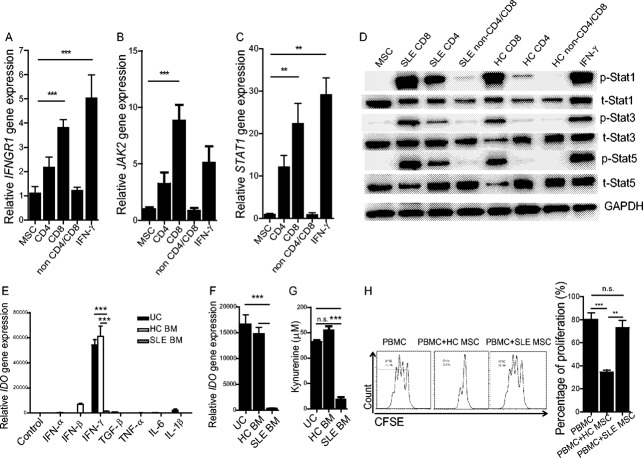
We next sought to elucidate the signaling pathways by which lupus CD8+ T cells induced IDO production in UC-MSCs. In DCs, the initiation of IDO activity is mostly dependent upon noncanonical NF-κB signaling pathways (29). On the other hand, JAK/STAT signaling activation is involved in IFNγ-induced immune responses (30). How CD8+ T cells induce IDO in UC-MSCs is unknown. In vitro stimulation by lupus CD8+ T cells resulted in a significant increase in IFNGR1 but not IFNGR2 in UC-MSCs (Figure 5A and Supplementary Figure 4A, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38674/abstract). In addition, downstream JAK-2 (although not JAK-1) gene expression in UC-MSCs was significantly up-regulated in the presence of lupus CD8+ T cells, an up-regulation which was similar to that seen when cells were stimulated with 20 ng/ml of recombinant human IFNγ (Figure 5B and Supplementary Figure 4B). Furthermore, STAT-1 gene expression was also increased in these settings (Figure 5C). Western blot analysis confirmed the activation of STAT-1, STAT-3, and STAT-5 signaling pathways following coculture of lupus CD8+ T cells with UC-MSCs (Figure 5D). We also examined NF-κB, ERK, and Akt pathways in UC-MSCs, but found no obvious activation in the presence of lupus CD8+ T cells (Supplementary Figure 4C). Thus, IFNGR1/JAK-2/STAT signaling pathways are associated with the IDO activity in UC-MSCs that is stimulated by lupus CD8+ T cells.
Figure 5.
Defective indoleamine 2,3-dioxygenase (IDO) production in lupus bone marrow–derived MSCs (BM-MSCs). A–C, Expression of IFNGR1 (A), JAK-2 (B), and STAT-1 (C) was examined by real-time quantitative polymerase chain reaction. D, STAT-1, STAT-3, STAT-5, and their phosphorylated forms were assessed by Western blot analysis after treatment with UC-MSCs alone, UC-MSCs with different cell subsets (CD4+, CD8+, and non-CD4/CD8 T cells), or recombinant human interferon-γ (IFNγ). E, IDO mRNA expression in UC-MSCs, BM-MSCs from healthy controls, and BM-MSCs from SLE patients was examined after stimulation with different cytokines for 48 hours. F and G, Peripheral blood CD8+ T cells derived from patients with lupus were purified and used to stimulate UC-MSCs and BM-MSCs from healthy controls or lupus patients. Forty-eight hours later, IDO gene expression (F) and kynurenine enzyme activity (G) were determined. H, The ability of BM-MSCs from healthy controls and lupus patients to inhibit CD4+ T cell proliferation was compared. Bars in A–C and E–H show the mean ± SEM (n = 7 per group in A–D, 6 per group in E, 3 per group in F and G, and 4 per group in H). ∗∗ = P < 0.01; ∗∗∗ = P < 0.001, by one-way ANOVA. TGFβ = transforming growth factor β; IL-6 = interleukin-6; PBMC = peripheral blood mononuclear cell; NS = not significant (see Figure 1 for other definitions).
Defective IDO activity in lupus BM-MSCs
The profound IDO production by allogeneic UC-MSCs in response to lupus CD8+ T cells prompted us to explore whether MSCs in patients with active lupus had defective IDO activity. We therefore isolated MSCs from the BM of patients with active lupus and healthy subjects and cultured the cells in vitro. The expanded BM-MSCs were stimulated with IFNγ, and UC-MSCs were used as controls. We found that lupus BM-MSCs had significantly lower IDO mRNA levels compared to healthy BM-MSCs or UC-MSCs, in response to IFNγ stimulation (Figure 5E). When cocultured with allogeneic TCR-activated lupus CD8+ T cells, lupus BM-MSCs also exhibited a profound defect in IDO secretion and activity compared to normal BM-MSCs (Figures 5F and G). Importantly, when cocultured with allogeneic lupus PBMCs, lupus BM-MSCs had a reduced ability to suppress T cell proliferation as compared to normal BM-MSCs (Figure 5H). These data demonstrate a decrease in IDO levels in MSCs derived from lupus patients in response to IFNγ and CD8+ T cells.
Increased circulating IDO activity after UC-MSC transplantation in lupus patients
Since IFNγ and CD8+ T cells trigger allogeneic MSCs to produce IDO and inhibit lupus T cell proliferation, we assessed whether IFNγ and CD8+ T cells were increased in lupus patients. We therefore analyzed a clinical index in lupus patients in vivo. First, we compared circulating levels of IFNγ, CD4+, and CD8+ T cell subsets in the peripheral blood of lupus patients and healthy controls. We found that lupus patients had a significantly higher frequency and total number of peripheral CD8+ T cells and increased levels of circulating IFNγ (Figures 6A–C). Moreover, there was an increased frequency and absolute number of IFNγ+CD8+ T cells from lupus patients compared to those from healthy controls (Figures 6D and E). These data suggest that the lupus microenvironment can initiate the function of allogeneic MSCs in vivo. Importantly, we observed that serum IDO activity (as evidenced by kynurenine concentrations) was significantly increased in 6 lupus patients 1 month after intravenous UC-MSC transplantation (Figure 6F). Serum tryptophan levels, however, did not change (Figure 6G), while the ratio of kynurenine to tryptophan was markedly increased (Figure 6H). Furthermore, in another clinical study, we found that percentages of peripheral blood CD3+CD4+ T cells decreased after UC-MSC transplantation in patients (Supplementary Figure 5, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38674/abstract). These data are consistent with our in vitro data and suggest that IDO plays a pivotal role in allogeneic MSC treatment in lupus patients.
Figure 6.
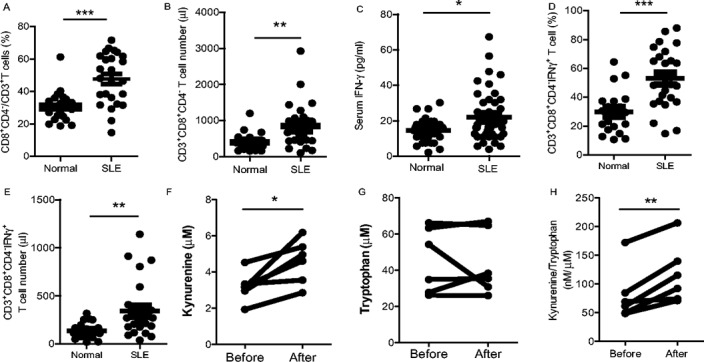
Serum indoleamine 2,3-dioxygenase (IDO) activity increases after UC-MSC transplantation in lupus patients. Peripheral blood mononuclear cells were isolated from lupus patients and healthy controls, and levels of CD8+ T cell subsets and interferon-γ (IFNγ) were compared. A–C, The percentage (A) and total number (B) of CD8+CD4−/CD3+ T cells, as well as serum levels of IFNγ (C) are significantly increased in SLE patients. D and E, The percentage (D) and total number (E) of intracellular IFNγ+CD8+ T cells are also increased in SLE patients. F–H, Serum kynurenine levels are increased (F), tryptophan levels remain unchanged (G), and the ratio of kynurenine to tryptophan is increased (H) 1 month after UC-MSC transplantation in lupus patients. ∗ = P < 0.05; ∗∗ = P < 0.01; ∗∗∗ = P < 0.001. Symbols in A–E represent individual subjects; horizontal lines show the mean. See Figure 1 for other definitions.
Discussion
The molecules that mediate MSC inhibition of lupus inflammatory cells remain incompletely understood. Herein we show that UC-MSC–produced IDO was critical for the inhibition of T cells and that IFNγ produced by lupus CD8+ T cells was the main factor driving IDO induction by MSCs. We have highlighted a novel mechanism by which allogeneic MSCs regulate lupus T cells in the disease microenvironment.
SLE is a typical autoimmune disease, characterized by abnormal T and B cell functions. Recently, a defect in Treg cell number and function was reported in patients with active lupus, which correlated with disease onset and progression (31). Current immunosuppressive drugs used to treat lupus inhibit T and B lymphocytes in vivo indiscriminately, which may increase drug-related adverse events, such as infection (32,33). New biologic drugs that target B cells, such as anti-CD20 monoclonal antibody (rituximab) and anti-BAFF monoclonal antibody (belimumab), have shown satisfactory clinical efficacy in patients with refractory disease, but treatment-related adverse events occurred after long-term application and followup (34,35). MSCs, however, may selectively inhibit activated lymphocytes and have therefore been proposed as an alternative treatment option for patients with lupus and other autoimmune diseases. The activity of MSCs is affected by the microenvironment into which they are transferred. As such, establishing how MSCs act within a diseased environment and, more specifically, how they mediate immune tolerance in lupus patients, is pivotal to improving our understanding of MSC transplantation and identifying the patients in whom an MSC transplant would provide the most clinical benefit. Our findings reveal a CD8+ T cell/IFNγ/IDO axis, by which allogeneic MSCs inhibit T cell proliferation.
IFN signaling pathways are activated in lupus patients and are tightly correlated with disease activity (36). It has been reported that circulating IFNγ is increased in lupus patients and can facilitate B cell activation and antibody production (37,38). Previously, it was reported that the high levels of IFNγ in lupus patients were mainly produced by DCs (39) or natural killer cells (40). Not only have we identified CD8+ T cells as dominant cellular sources of IFNγ in lupus patients, but we have also importantly discovered that CD8+ T cells are the major stimulus for induction of IDO in UC-MSCs. Furthermore, we observed significantly elevated levels of circulating IFNγ produced by CD8+ T cells in lupus patients. More research on lupus is needed to determine whether circulating IFNγ levels are positively correlated with allogeneic MSC treatment efficacy. In the present study, we also found that lupus Treg cells enhanced UC-MSCs–mediated IDO productions, but IDO was not involved in Treg proliferation. This increase in IDO expression by UC-MSCs cocultured with lupus Treg cells might be due to a slight increase in IFNγ production by these lupus Treg cells (Wang D, et al: unpublished observations), although this needs to be further confirmed in future studies.
Our previous studies showed that BM-MSCs from lupus patients functioned abnormally (7,41) and that autologous BM-MSC infusion had no significant effect on animal models of lupus (42). In this study, we found that BM-MSCs from patients with active SLE were much less responsive to recombinant IFNγ or allogeneic CD8+ T cell stimulation and, importantly, failed to inhibit allogeneic T cell proliferation. This defect in suppressing T cell proliferation is at least partly attributed to their reduced ability to produce IDO in response to IFNγ and/or lupus CD8+ T cells. These findings explain why activated T cells were elevated in lupus patients and why autologous lupus BM-MSC transplantation was less effective at treating lupus patients. Many patients have received allogeneic MSC transplantation in our facility, and we have shown a good clinical safety profile as well as treatment efficacy (43). However, because the outcome of treatment with the infused allogeneic cells is unknown, long-term clinical safety needs further investigation.
We recently examined the characteristics of BM-MSCs from SLE patients and healthy controls. Our data showed that there were no significant differences in the surface phenotype of CD29, CD44, CD105, CD14, CD34, CD45, and HLA–DR cells (41). We suggest that the decreased IDO from SLE-derived MSCs may be a result of some intrinsic factors that occur in lupus disease progression, but which do not necessarily cause changes in the surface phenotype of MSCs. Moreover, IFNγ treatment in vitro had no effect on MSC surface HLA–DR, CD80, and CD86 expression, although it enhanced IDO activity in a dose-dependent manner (Wang D, et al: unpublished observations). Furthermore, a study has demonstrated that BM-MSCs from young (NZB × NZW)F1 lupus mice (5–6 weeks old) efficiently reduced severity of lupus in an MRL/lpr mouse model (42). However, BM-MSCs from old (NZB × NZW)F1 mice (26–27 weeks old) failed to ameliorate disease, indicating that BM-MSCs lose their suppressive capabilities as the disease progresses. Loss of regulatory activity by BM-MSCs is likely caused by the inflammatory milieu present in the host, which could well mediate epigenetic changes and in consequence yield changes in protein expression in BM-MSCs from lupus patients.
Taken together, our findings reveal novel mechanistic insight into how UC-MSC–mediated immunosuppression occurs in lupus patients. Additionally, our data suggest that allogeneic MSCs are more appropriate for clinical transplantation in lupus patients, although autologous MSCs are not.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Sun had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design.Wang, Feng, Gao, Lu, Shi, W. Chen, Sun.
Acquisition of data.Wang, Feng, Lu, Konkel, Zhang, Z. Chen, Li.
Analysis and interpretation of data.Wang, W. Chen, Sun.
Acknowledgments
We thank Drs. DaWei Xiao and MingWei Yang (Department of Pharmacy and Phase I Clinical Trial Base, The Affiliated Drum Tower Hospital of Nanjing University Medical School) for technical assistance with high-performance liquid chromatography. We also thank the patients and healthy volunteers for their cooperation and for consenting to participate in this study.
Supporting Information
References
- 1.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–96. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 2.Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69:1423–9. doi: 10.1136/ard.2009.123463. [Ann Rheum Dis 2011;70:237]. [DOI] [PubMed] [Google Scholar]
- 3.Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–75. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–55. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood. 2012;120:3142–51. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Zhang H, Cao M, Tang Y, Liang J, Feng X. Efficacy of allogeneic mesenchymal stem cell transplantation in patients with drug-resistant polymyositis and dermatomyositis. Ann Rheum Dis. 2011;70:1285–8. doi: 10.1136/ard.2010.141804. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Liu L, Meng D, Wang D, Zhang J, Shi D. Enhanced apoptosis and senescence of bone-marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Stem Cells Dev. 2012;21:2387–94. doi: 10.1089/scd.2011.0447. [DOI] [PubMed] [Google Scholar]
- 8.Carrion F, Nova E, Ruiz C, Diaz F, Inostroza C, Rojo D. Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus. 2010;19:317–22. doi: 10.1177/0961203309348983. [DOI] [PubMed] [Google Scholar]
- 9.Nie Y, Lau C, Lie A, Chan G, Mok M. Defective phenotype of mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus. 2010;19:850–9. doi: 10.1177/0961203309361482. [DOI] [PubMed] [Google Scholar]
- 10.English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7:431–42. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–16. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 13.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;9:667–79. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–34. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 15.Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–4. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 16.Selmani Z, Naji A, Gaiffe E, Obert L, Tiberghien P, Rouas-Freiss N. HLA-G is a crucial immunosuppressive molecule secreted by adult human mesenchymal stem cells. Transplantation. 2009;87:S62–6. doi: 10.1097/TP.0b013e3181a2a4b3. [DOI] [PubMed] [Google Scholar]
- 17.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 18.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–8. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 19.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 20.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–22. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 21.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–50. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Qian X, Wang K, Wang X, Zheng SG, Lu L. Generation of human regulatory T cells de novo with suppressive function prevent xenogeneic graft versus host disease. Int Immunopharmacol. 2011;11:630–7. doi: 10.1016/j.intimp.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–32. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Mellor AL, Munn DH, Blazar BR. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood. 2009;114:5062–70. doi: 10.1182/blood-2009-06-227587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424–6. [PubMed] [Google Scholar]
- 27.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–7. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 29.Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-κB activation. Nat Rev Immunol. 2007;7:817–23. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- 30.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-γ: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–50. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheinecker C, Bonelli M, Smolen JS. Pathogenetic aspects of systemic lupus erythematosus with an emphasis on regulatory T cells. J Autoimmun. 2010;35:269–75. doi: 10.1016/j.jaut.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Crispin JC, Kyttaris VC, Terhorst C, Tsokos GC. T cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:317–25. doi: 10.1038/nrrheum.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:326–37. doi: 10.1038/nrrheum.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE BLISS-52 Study Group. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–31. doi: 10.1016/S0140-6736(10)61354-2. et al, for the. [DOI] [PubMed] [Google Scholar]
- 35.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III Systemic Lupus Erythematosus Evaluation of Rituximab trial. Arthritis Rheum. 2010;62:222–33. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi T. Therapeutic strategies for SLE involving cytokines: mechanism-oriented therapies especially IFN-γ targeting gene therapy. J Biomed Biotechnol. 2010;2010:461641. doi: 10.1155/2010/461641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J Immunol. 2008;181:2211–9. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- 38.Karonitsch T, Feierl E, Steiner CW, Dalwigk K, Korb A, Binder N. Activation of the interferon-γ signaling pathway in systemic lupus erythematosus peripheral blood mononuclear cells. Arthritis Rheum. 2009;60:1463–71. doi: 10.1002/art.24449. [DOI] [PubMed] [Google Scholar]
- 39.Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33:967–78. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hervier B, Beziat V, Haroche J, Mathian A, Lebon P, Ghillani-Dalbin P. Phenotype and function of natural killer cells in systemic lupus erythematosus: excess interferon-γ production in patients with active disease. Arthritis Rheum. 2011;63:1698–706. doi: 10.1002/art.30313. [DOI] [PubMed] [Google Scholar]
- 41.Sun LY, Zhang HY, Feng XB, Hou YY, Lu LW, Fan LM. Abnormality of bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus. 2007;16:121–8. doi: 10.1177/0961203306075793. [DOI] [PubMed] [Google Scholar]
- 42.Gu F, Molano I, Ruiz P, Sun L, Gilkeson GS. Differential effect of allogeneic versus syngeneic mesenchymal stem cell transplantation in MRL/lpr and (NZB/NZW)F1 mice. Clin Immunol. 2012;145:142–52. doi: 10.1016/j.clim.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Wang D, Zhang H, Liang J, Li X, Feng X, Wang H. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years experience. Cell Transplant. 2013;22:2267–77. doi: 10.3727/096368911X582769c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.