Abstract
Fluorescence-labeled peptide-MHC class I multimers serve as ideal tools for the detection of antigen-specific T cells by flow cytometry, enabling functional and phenotypical characterization of specific T cells at the single cell level. While this technique offers a number of unique advantages, MHC multimer reagents can be difficult to handle in terms of stability and quality assurance. The stability of a given fluorescence-labeled MHC multimer complex depends on both the stability of the peptide-MHC complex itself and the stability of the fluorochrome. Consequently, stability is difficult to predict and long-term storage is generally not recommended. We investigated here the possibility of cryopreserving MHC multimers, both in-house produced and commercially available, using a wide range of peptide-MHC class I multimers comprising virus and cancer-associated epitopes of different affinities presented by various HLA-class I molecules. Cryopreservation of MHC multimers was feasible for at least 6 months, when they were dissolved in buffer containing 5–16% glycerol (v/v) and 0.5% serum albumin (w/v). The addition of cryoprotectants was tolerated across three different T-cell staining protocols for all fluorescence labels tested (PE, APC, PE-Cy7 and Quantum dots). We propose cryopreservation as an easily implementable method for stable storage of MHC multimers and recommend the use of cryopreservation in long-term immunomonitoring projects, thereby eliminating the variability introduced by different batches and inconsistent stability. © 2014 International Society for Advancement of Cytometry
Keywords: MHC multimer, cryopreservation, cryoprotectant, recommendations for MHC multimer storage, quality assurance, glycerol in T cell staining
The use of fluorescence-labeled peptide-MHC (pMHC) multimer complexes has become a state-of-the art technology for the detection of antigen-specific T cells in various biological materials. It was first described by Altman et al. in 1996 that tetrameric complexes of pMHC class I molecules would, when coupled to a fluorescent label, enable detection of antigen-specific T cells by flow cytometry (1). The necessity of multimerization relates to the low affinity interaction between a single T cell receptor (TCR) and one pMHC class I complex, making it impossible to generate a stable interaction unless pMHC complexes are detected in a multimeric form by several TCR, generating a high-avidity interaction (1). This groundbreaking observation opened a new era for T cell immunology, enabling description of antigen-specific T cells at a single cell level independently of functional properties. This has significantly extended our knowledge on T cell antigen recognition, and on the complex relation between specificity, phenotype and functional characteristics. Most importantly, this technology allows tracking of specific T-cell populations in different settings (2). As such, the technique has provided insights into T-cell reactivity important to fight a large number of pathogens (3–5), and an increased understanding of how cancer-specific T-cell responses can translate into therapeutic strategies (6–8).
A concern in the use of fluorescence-labeled MHC multimers relates to their stability, especially when these reagents are used for monitoring changes in T-cell populations over longer periods of time. MHC multimer stability may vary, depending on the peptide, the MHC molecule and the fluorochrome involved, and this may require stability-control of every single parameter. Unlike most monoclonal antibodies that can be tested for recognition of the cognate protein by simple ELISA or flow cytometry measurement, availability of T-cell clones for controlling MHC multimers quality is limited. Therefore, such tests are rarely conducted.
Quality assurance in T-cell assays has been a focus over the last couple of years, in concert with changes from few-color to multi-color flow cytometry-measurements (9–11). The importance of optimizing staining protocols, data acquisition and data analysis for better assay performance has been highlighted by a series of proficiency panels for MHC multimer staining that were designed and organized by the Immunoguiding Program of the Association for Cancer Immunotherapy (CIP) and the Cancer Immunotherapy Consortium (CIC) (12–15). These proficiency panels revealed a high variability between the results of different laboratories conducting MHC multimer staining, arguing for harmonization guidelines and improved quality assurance of both flow cytometry instruments and reagents. To increase performance and reproducibility, the use of single batches of reagents stored throughout the lifetime of individual monitoring projects is advisable. In addition, recent improvements in technologies of parallel detection of multiple antigen-specific T cells by e.g., combinatorial encoding of MHC multimers (16–18), and the use of numerous different fluorochromes for labeling MHC multimers is further emphasizing the need for stable storage. Stability under storage is a known issue for MHC multimers which may be adequately addressed by cryopreservation under appropriate conditions as the half-life of the peptide-MHC complex is a function of temperature (19).
Here, we investigated the possibility to cryopreserve MHC multimers from different sources by the use of albumin and glycerol as cryoprotectors. Albumin (bovine serum albumin, human serum albumin, or recombinant albumin) is a standard excipient in biotechnology and pharmaceutical industry (20). Because of its amphiphilic nature and high glass transition temperature, it is often used as stabilizer and cryoprotecting agent. Glycerol, like ethylene glycol and propylene glycol, can form strong hydrogen bonds and is known to reduce damage due to ice crystal formation during freezing (21). Glycerol is also widely used as a cryoprotecting agent.
Protocols for cryopreservation of MHC multimers have been suggested and used previously (22,23), but not yet formally tested for long-term storage across different peptide-MHC complexes and fluorochromes. We show here that MHC multimers can be stably stored at −20 to −80°C for at least 6 months, using glycerol addition of 5–16% and 0.5% albumin, and that the addition of cryoprotectants to MHC multimers is compatible with different T-cell staining protocols used across three different laboratories. This is evident across a wide range of different pMHC complexes, HLA molecules and fluorochrome labels.
Material and Methods
This section is structured as recommended by the framework for Minimal Information About T-cell Assays (24). Information for the five modules: sample, assay, data acquisition, data processing, and laboratory environment are summarized below, additional details specific to each of the three participating laboratories (Centers 1–3) are given in Supporting Information Table S1.
Participating Laboratories
All experiments were performed in an independent but concerted manner by one or several of the three participating centers. To establish the validity of our findings across laboratories, cell samples (PBMC and/or TIL), reagents, monoclonal antibodies (mAb), protocols, data acquisition, and data analysis were as per local resources and protocols; some assay parameters were harmonized as indicated.
Samples
Peripheral blood mononuclear cells (PBMC)
Leukaphereses and buffy-coats were obtained from healthy donors by venipuncture after informed consent at the Center for Clinical Transfusion Medicine of the Tübingen University Hospital or at the Department of Clinical Immunology and Blood Bank of Herlev Hospital. Low-resolution HLA-genotype was known, and samples were prescreened for T-cell responses against virus-derived epitopes. The blood products were transported to the centers at ambient temperature and processed within 6 h. Products were diluted in sterile PBS, and PBMC subsequently isolated by standard density gradient centrifugation. After separation, PBMC were washed in PBS, counted using Trypan blue and/or Turk's solution, frozen at 5–20 × 106 cells/ml in freezing containers and transferred to the gas phase of a liquid nitrogen tank or to −150°C freezers. Viability was typically >90% before freezing and >80% after thawing.
Tumor infiltrating T lymphocytes (TIL)
TIL were obtained from melanoma lesions after patient's informed consent. Patients were HLA-typed, either based on a TIL or a PBMC sample. After mechanical tissue disruption, TIL were cultured for 15–40 days (to reach 5 × 106 cells) in RPMI 1640 (Invitrogen) with 10% heat-inactivated human AB serum (Sigma), 1% Penicillin/Streptomycin (Invitrogen), fungizone® 1,25 μg/ml (Bristol-Myers Squibb) and IL-2 6000 IU/ml (Proleukin®, Novartis). Afterward, rapid expansion was performed for 14 days in the presence of allogenic feeders 1.3 × 106/ml (40 Gy irradiated PBMC from two healthy donors), CD3 antibody 30 ng/ml (OKT-3, Thermofisher), and IL-2 6000 IU/ml in 50% RPMI and 50% AIM-V medium (Invitrogen), supplemented with 10% heat-inactivated human AB serum (25). TIL were frozen at 15–50 × 106 cells/vial, using the same procedure as for the PBMC samples. Viability after thawing was >60% in all cases.
Assay
Peptide-MHC monomers and multimers
Biotinylated pMHC monomers were produced locally using either conventional refolding (large scale) (1) or using UV-exchange of a conditional ligand, essentially as previously described (26–28). Synthetic peptides represented known wild-type or modified CD8 T-cell epitopes derived from human viruses or tumor-associated antigens (http://www.syfpeithi.de, and Ref. 8). All pMHC-multimers used in the study, including HLA-alleles (HLA-A*0101, -A*0201, and -B*0702) and peptide sequences are listed in Supporting Information Table S2. After refolding, HLA-monomers were aliquoted and frozen at −80°C until use or multimerized immediately.
Fluorescent peptide-MHC multimers were generated at each center by coincubating monomers with streptavidin-PE, -APC, or -PE-Cy7 at a 4(streptavidin):1 (pMHC monomer) molar ratio, or with streptavidin-Quantum dots (QD) QD565, -QD585, -QD605, -QD625, -QD655, -QD705, or -QD800 at a 1(QD):30(pMHC monomer) molar ratio. They were then used either directly, after storage at 4°C or after a freezing step at −20°C or −80°C. For freezing in glycerol, a stock solution containing glycerol, albumin (human or bovine) and protease inhibitors (2 of 3 centers) was prepared and added to the fresh pMHC multimers so that the final concentration of glycerol varied between 1.25% and 16%. Aliquots were frozen at −20°C or −80°C until use. Cryopreservation was performed in either 20 mM Tris-buffer (Centers 1 and 2) or PBS (Center 3) with 0.5% HSA (Center 1) or 0.5% BSA (Centers 2 and 3). For stability testing of commercially available MHC multimers, we obtained reagents from TCMetrix (Epalinges, Switzerland), ProImmune (Oxford, the UK) and Immudex (Copenhagen, Denmark). Products were aliquoted and the following storage conditions applied for 10 days: 4°C, freezing at −80°C with or without glycerol and serum albumin (10% and 0.5% final, respectively). Frozen aliquots were either kept at −80°C or subjected to 5 thawing/freezing cycles at minimum one day interval before use.
Cell staining
PBMC or TIL prescreened for the presence of virus- or tumor-associated antigen-specific CD8 T cells by MHC-multimer staining were thawed and counted according to local protocols. Stainings were performed on 0.2–5 × 106 cells using center-specific mAb and fluorochromes, buffers, and protocols, as listed in Supporting Information Table S1. Multimers were used either directly after multimerization, after storage at 4°C, or after freezing in the absence or presence of glycerol as indicated. In all cases, incubation with MHC multimers was done before mAb staining (either at 4°C, 25°C, or 37°C). Each multimer was used at 1–5 µg/ml when labeled with one single fluorochrome and at 2–10 µg/ml final when labeled with two different fluorochromes in the combinatorial approach (16,18). Staining with commercial multimers was performed as per manufacturer's instructions. At least a CD8 mAb was systematically added. All antibodies were titrated to optimal concentrations in pilot experiments. Additionally, a dead cell dye was applied in the 1st or last step (either alone or together with mAb). After staining, cells were resuspended in staining buffer and either analyzed within 4 h or fixed and analyzed within the following 6 days. For spiking experiments, glycerol was added during the 1st staining step, together with freshly-prepared multimers.
Data Acquisition
Stained cells were acquired on Canto II or LSR II flow cytometers (BD Biosciences) equipped with the Diva software. PMT voltages were adjusted for each fluorescence channel using unstained cells, and compensations set with compensation beads (BD Biosciences or Invitrogen) labeled with antibodies, alongside with ArC Amine reactive compensation bead kit (Invitrogen) (Center 2 and 3) or with dead cells stained with the LIVE/DEAD dye (Center 1).
Data Analysis
Analysis of FCS files was performed with the softwares FACSDiva (Center 3) or FlowJo (Centers 1 and 2). Gating strategies were harmonized, but not identical: all stainings were successively gated on a time histogram, then dot-plots for singlets FSC-A/FSC-H, lymphocytes FSC-A/SSC-A, living lymphocytes FSC-A/dead cell dye, or histogram: cell viability was determined by measuring the percentage of living cells (dead cell dye-negative population) using gates.
CD8 T cells were then further selected either directly using histograms (Center 1) or as CD8+ dump channel– or as CD3+ CD8+ events using dot-plots (Centers 2 and 3). Percentage of CD8 T cells was in all cases calculated out of total living lymphocytes. CD8+, CD8+ Multimer+, and CD8+ Multimer− cells were selected by setting quadrants or gates and percentages of positive cells were recorded. Examples of analyses performed at each of the 3 labs are shown in Supporting InformationFigure S1. Staining indexes (SI) were calculated as follows: (median fluorescence positive cell subset − median fluorescence negative cell subset)/2 × fluorescence standard deviation of negative cell subset. Staining indexes are measures of fluorescence brightness over background that allow appropriate comparison of several staining conditions within one single experiment and throughout several experiments (29).
Statistical Analyses
Statistical analyses were performed using two-way ANOVA (Figs. 1A and 4C), repeated measurements ANOVA (Figs. 1C–1E) or 2-way ANOVA with Bonferroni posttests (Figs. 2, 3, and 5). Significance level is indicated by asterisk: *P < 0.05, **P < 0.01, ***P < 0.001. Number of repetitions for each experiment is indicated in figure legends by n = x.
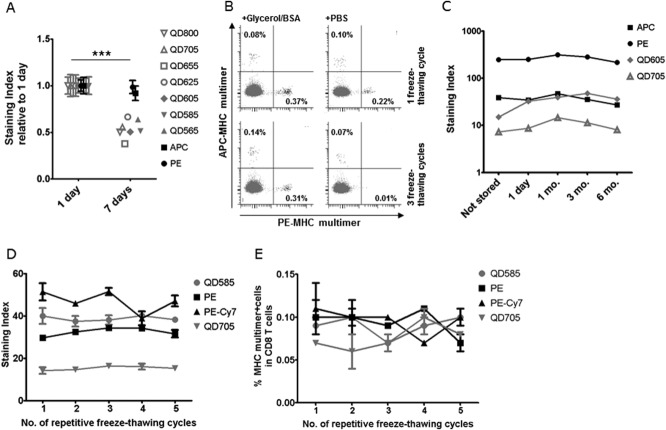
Figure 1.
MHC multimer staining is affected by storage conditions. (A) MHC multimers HLA-A2 CMVNLV coupled to Streptavidin QD800, 705, 655, 625, 605, 585, 565 (in gray), APC, or PE (in black) were stored at 4°C for either 1 day or 7 days without glycerol. The effect of storage time is depicted as the average staining index for the multimer specific T-cell population in two different donors, calculated relative to the average staining index at day 1. Error bars indicate range between donors. Performed at Center 2 (n = 1), with additional dataFigure S2 (n = 2). (B) Dot plots of HLA-A1 CMVVTE-PE and CMVYSE-APC multimer stainings of healthy donor PBMC after one (top row) or three (bottom row) freeze-thawing cycles with (left plots) or without (right plots) addition of 10% glycerol/0.5% BSA. Performed at Center 3 (n = 3). (C) MHC multimers: HLA-A2 EBVGLC-APC, -PE, -QD605, or -QD705 were produced and tested immediately (not stored) or frozen with 10% glycerol/0.5% BSA and tested after 1 day, 1 month, 3 months, and 6 months, respectively. Performed at Center 2 (n = 1). (D and E) The effect of repetitive freeze-thawing cycles on D) the staining index and E) the percentage of MHC multimer+ CD8 T cells when staining PBMC from one donor with: HLA-A1 FLUVSD -QD585 or -PE-Cy7 and HLA-A1 CMVYSE -PE or -QD705. MHC multimers were frozen with 10% glycerol/0.5% BSA and subjected to 1–5 freeze-thawing cycles at one-day intervals. All data points are the average of duplicates, error bars indicate range (not always visible). Performed at Center 3 (n = 1).
Figure 4.
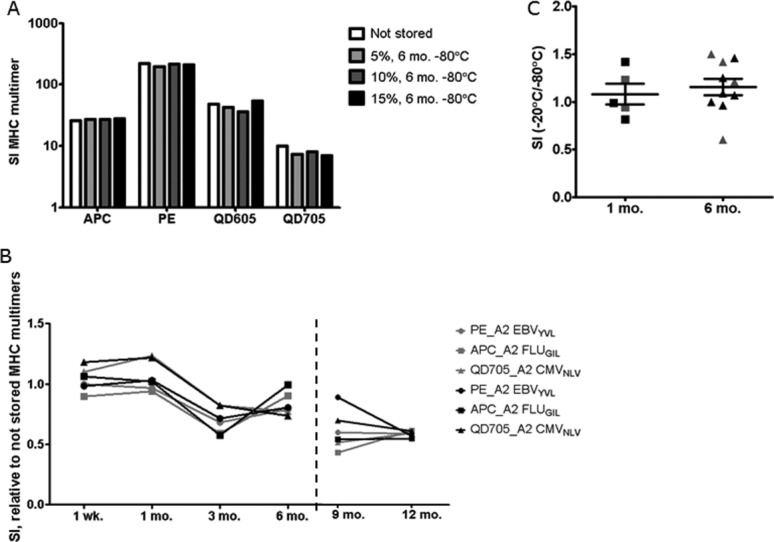
Long-term storage of cryopreserved MHC multimers. Results obtained in three independent tests performed are shown. (A) HLA-A2 EBVGLC MHC multimers with different fluorescent labels (APC, PE, QD605, QD705) were cryopreserved with 0.5% BSA and 5, 10, or 15% glycerol for 6 months. The staining index of the MHC multimer population is depicted compared to a not stored control prepared each time 1 day prior to staining (performed by Center 2, one donor, n = 1). (B) Long-term stability of three different HLA-A2 multimers (PE-EBVYVL, APC-FLUGIL, QD705 CMVNLV) was assessed after 1 week up to 13 months storage for two different donors (indicated by gray or black symbols). The staining index of the MHC multimer population is depicted relative to a not stored control of the same batch of monomers tested directly after preparation, before cryopreservation (left plot, 1 week-6 months) or compared to a freshly-prepared batch of multimers for the additional time points, 9 and 12 months (right plot), (performed by Center 1, n = 1). (C) SI after storage at −20°C vs. −80°C, for MHC multimers refolded with virus-derived epitopes: A2 CMVNLV, A2 EBVFLY, B7 CMVTPR labeled with PE or QD605, used for staining PBMC (black symbols) or MHC multimers with cancer-associated epitopes A2 MARTELA (modified) and A2 MARTEAA (wt) used for staining TILs (gray symbols) (performed by Center 3, n = 1).
Figure 2.
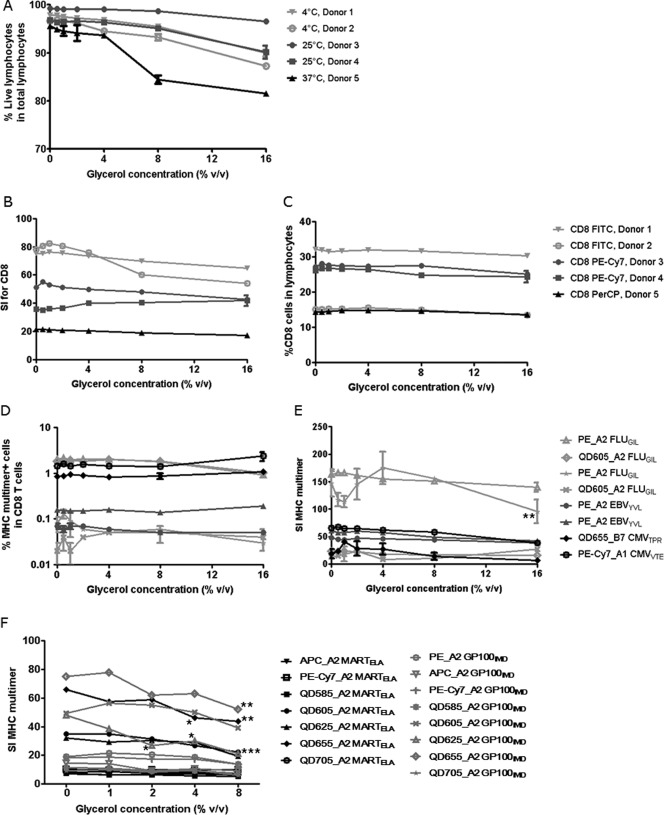
Effect of glycerol on cell viability and T-cell staining. The effect of increasing concentrations of glycerol was analyzed by addition of 0, 0.5, 1, 2, 4, 8, 16% glycerol to the first step of the MHC multimer staining reaction. MHC multimers were freshly prepared and contained no glycerol themselves. Three independent experiments including a total number of five different donors are shown: Donors 1 and 2 by Center 2 (light gray) (n = 2), Donors 3 and 4 by Center 1 (dark gray) (n = 2), Donor 5 by Center 3 (black) (n = 2). All data points are the average of duplicates, error bars indicate range (often not visible). (A) Cell viability under different staining conditions. The percentage of living lymphocytes (dead cell dye negative) in total lymphocytes (defined by FSC/SSC) is depicted in relation to increasing glycerol concentrations. Three different incubation temperatures were used based on the different staining protocols of each center. (B) Staining indexes for the CD8 staining by use of different antibodies, CD8-PE-Cy7, FITC, and PerCP and (C) Frequency of CD8 T cells in living lymphocytes for the same CD8 antibodies. (D and E) MHC multimer staining results expressed as percentages of specific T cells and staining indexes for eight different virus-specific T-cell populations. (F) MHC multimer staining of TIL specific for HLA-A2 MART-1ELA and GP100IMD with multimers labeled with PE, APC, PE-Cy7, QD585, 605, 625, 655, 705, after addition of increasing concentration of glycerol. The staining index of the MHC multimer positive population is depicted. Performed at Center 3 (n = 1).
Figure 3.
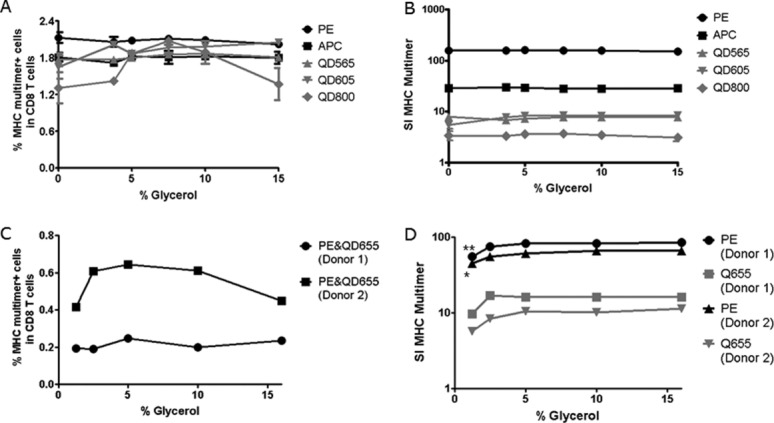
Concentration of glycerol for cryopreservation. The concentration of glycerol required for cryopreservation of MHC multimers was tested in two independent experiments (A, B) (Center 2) and (C, D) (Center 3). The x-axis of each plot shows the % glycerol used for storage, x = 0 represents the not stored sample. (A, B) HLA-A2 EBVCLG MHC multimers were generated with five different fluorescence labels, cryopreserved with 3.75, 5, 7.5, 10, or 15% glycerol for 7 days, and compared to not frozen MHC multimers in terms of (A) percentage of MHC multimer+ and (B) staining index for the MHC multimer+ CD8 T cell population in one donor. Each dot is the average of duplicates and error bars indicate range (often not visible). Residual glycerol during staining varied between 0.5 and 2.2% for this experiment. (C, D) HLA-A2 CMVNLV MHC multimers were generated with PE- and QD655-fluorescence labels (dual color coded). MHC multimers were cryopreserved with 1.25, 2.5, 5, 10, and 16% glycerol for 7 days, then (C) percentage of stained CD8 T cells and (D) staining index for the MHC multimer+ population were determined in two donors. Single values of one experiment are plotted. Residual glycerol during staining varied between 0.1 and 1.6% in this experiment.
Figure 5.
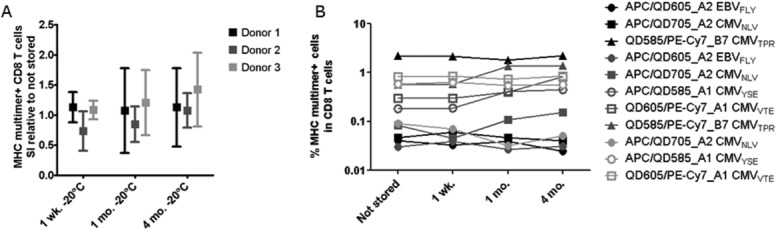
Cryopreservation of MHC multimers for large panels of combinatorial-encoded stainings. We generated a panel of MHC multimers refolded with 27 virus-derived T-cell epitopes and determined the frequency of specific T-cell populations in three different healthy donors (black, dark gray, and light gray). Only responses of >0.002% of CD8 T cells and a minimum of 10 events are included in the graphs. (A) SI of the MHC multimer positive populations when stained using MHC multimers cryopreserved at −20°C for either 1 week, 1 month, or 4 months relative to the SI obtained using not stored MHC multimers. The data represent the average relative SI of the following populations: Donor 1: APC_A2 EBVFLY, QD605_A2 EBVFLY, APC_A2 CMVNLV, QD705_A2 CMVNLV, QD585_B7 CMVTPR, PE-Cy7_B7 CMVTPR; Donor 2: APC_A2 EBVFLY, QD605_A2 EBVFLY, APC_A2 CMVNLV, QD705_A2 CMVNLV, APC_A1 CMVYSE, QD585_A1 CMVYSE, QD605_A1 CMVVTE, PE-Cy7_A1 CMVVTE, QD585_B7 CMVTPR, PE-Cy7_B7 CMVTPR; Donor 3: APC_A2 CMVNLV, QD705_A2 CMVNLV, APC_A1 CMVYSE, QD585_A1 CMVYSE, QD605_A1 CMVVTE, PE-Cy7_A1 CMVVTE. Error bars represent SD. (B) Frequency of MHC multimer+ T cells, when stained using not stored, 1 week, 1 month, or 4 month cryopreserved MHC multimers at −20°C, prepared from the same batch (performed by Center 3, n = 1).
Laboratory Environment
The 3 labs operate under exploratory research conditions with qualified assays using established protocols (n = 2) or SOP (n = 1), and experiments were performed by trained personnel. They participate regularly in proficiency panels organized by CIP or CIC.
Results
Stability of MHC Multimers is Improved by Freezing and Addition of Cryoprotectants
Storage of MHC multimers at 4°C for longer periods of time is common practice. However, we have observed before that the stability of in-house produced MHC multimers kept at 4°C can be unreliable. This may relate both to the stability of the peptide-MHC complexes themselves and/or to the fluorescent label used. As depicted in Figure 1A, we tested the effect of storage at 4°C for 9 differently labeled-MHC multimer complexes of HLA-A2 CMVNLV. The staining index (SI) determining the separation between the multimer negative and multimer positive events was significantly reduced after 1 week storage at 4°C (P < 0.0001), with a particular signal reduction observed for the QD-labeled MHC multimers. This was further confirmed in additional two experiments (Supporting InformationFig. S2). Furthermore, freezing-thawing of MHC multimers also resulted in a dramatic loss of specific staining, which could be rescued by addition of 10% glycerol and 0.5% BSA to the freezing buffer, as has been proposed by Haanen et al. (22,23) (Fig. 1B). Importantly, cryopreservation in the presence of glycerol and BSA allowed stable storage of MHC multimers over 6 months (Fig. 1C) and conduction of at least 5 freeze-thawing cycles without any changes to the staining efficacy, as determined by the staining indexes and the fraction of stained CD8 T cells (Figs. 1D and 1E).
Thus, cryoprotection of MHC multimers with glycerol and albumin seems a solution to stably store these sensitive complexes. In the following steps, we systematically tested the optimal preservation conditions and the long-term stability of MHC multimers prepared by this procedure.
Effects of Residual Glycerol on Cell Viability and Staining
An obvious consequence of using glycerol-protected MHC multimer stocks is the residual cryoprotectant remaining during cell staining. Whereas T cells are exposed to high concentrations of serum albumin under physiological conditions, toxic potential has been reported for glycerol on various cell types (30,31). The concentration of residual glycerol during multimer staining will depend on the concentration of glycerol used for cryoprotection of the MHC multimer stocks, as well as on the total concentration of MHC multimers in the staining tube.
In a series of “spike-in” experiments, we tested the effect of increasing glycerol concentration on lymphocyte viability, CD4 and CD8 T cell staining, and detection of MHC multimer+ T cells. In all cases, glycerol was added to the cells during the first step of the staining protocol, together with freshly prepared MHC multimers. Experiments were performed on altogether five different donors in three different centers. A stepwise 2-fold increase in glycerol concentration, ranging from 0.5% to 16% was used with three staining protocols, including MHC multimer stainings at different temperature conditions (4°C, 25°C, and 37°C).
As shown in Figure 2A, glycerol concentrations above 4% had a negative impact on lymphocyte viability most prominently when cells were stained at 37°C. This effect was even more evident when directly comparing the ratio between living lymphocytes and all dead cells based on the separation obtained from the FSC/SSC properties of these two cell populations (Supporting InformationFig. S3A). We next tested on the same five donors the effect of glycerol on the staining of the CD8 population. No significant effect of glycerol addition was observed, neither on the staining index nor on the frequency of CD8 T cells detected using either of the three different CD8 Ab and fluorochromes (Figs. 2B and 2C).
Finally, we assessed the frequency and staining intensity of MHC multimer-binding T-cell populations at increasing glycerol concentrations. For the same five donors, we stained a total of 15 different T-cell populations, using MHC multimers refolded with altogether seven different virus-derived epitopes, restricted to three different MHC alleles, HLA-A*0201, A*0101, and B*0702, and coupled to six different fluorochromes including Quantum dots and a tandem conjugate. Data for T-cell specificities labeled with PE, PE-Cy7, QD605, and QD655 are shown in Figures 2D and 2E, responses detected with APC and QD705-labeled multimers are shown in Supporting Information Figures S3B and S3C. No significant differences were observed with addition of glycerol up to 8%. With 16% glycerol, SI was significantly affected for two of the 15 MHC multimer/donor combinations (Fig. 2E and Supporting InformationFig. S3C).
It could be speculated that antigen-specific T-cell populations with low affinity interaction between the T-cell receptor and the MHC complex would be more affected by glycerol addition than high-affinity viral-specific T cells. Since tumor-antigen specific T cells are expected to be mostly of middle to low affinity, we tested the effect of glycerol addition during staining of a prescreened tumor infiltrating lymphocyte (TIL) population containing T cells specific for HLA-A*0201 MARTELA and GP100IMD, using multimers with eight different fluorescence labels (PE, APC, PE-Cy7, QD585, QD605, QD625, QD655, and QD705) (Fig. 2F). There was no significant difference in the percentage of specific T cells detected after addition of glycerol (data not shown). However, a lowering of the SI was observed, beginning at 2% glycerol (QD625 A2 GP100IMD), at 4% glycerol (QD655 A2 MARTELA) and at 8% (QD655A2 GP100IMD), indicating that indeed, self-antigen specific T-cell populations may be especially sensitive to residual glycerol.
On the basis of the above data on cell viability and cell staining, we concluded that up to 2% of residual glycerol can be present in the MHC staining solution without significantly affecting the quality of the staining procedure for both PBMC and TIL samples, and using MHC multimers labeled with various fluorochromes.
Optimal Glycerol Concentration for Cryoprotection of MHC Multimers
Cryopreservation of MHC multimers with 16% glycerol has previously been used to successfully stabilize the pMHC/fluorochrome complexes (22,23). We next tested which concentrations of glycerol are minimally required. We compared the staining index and the frequency of HLA-A2 EBVCLG-specific T cells in PBMC of a healthy donor stained with MHC multimers cryopreserved with 3.75%, 5.0%, 7.5%, 10%, or 15% glycerol compared to nonfrozen, freshly prepared MHC multimers. The analyses were conducted for MHC multimers conjugated to PE, APC, QD565, QD605, and QD800 (Figs. 3A and 3B). There were no significant differences observed in either the frequency of MHC multimer-binding CD8 T cells or the staining index when MHC multimers were cryopreserved in a range of 3.75% to 15% glycerol. These results were confirmed in additional two donors with stainings of HLA-A2 CMVNLV-specific T cells using a mix of QD655- and PE-labeled MHC multimers cryopreserved with 1.25, 2.5, 5, 10, and 16% glycerol. In this second experiment, we observed no difference in the frequency of MHC multimer+ T cells detected, albeit a significant decrease in PE staining intensity (SI of pMHC multimer) was noted if the glycerol concentration during cryopreservation was reduced to 1.25% (Figs. 3C and 3D).
Overall, cryopreservation with low concentrations of glycerol is feasible for PE, APC, and QD-conjugated MHC multimers; however, a minimum concentration of 3.75–5% glycerol is advisable.
Long-Term Stability of Cryopreserved MHC Multimers
Next, we analyzed the stability of cryopreserved MHC multimers over longer periods of time. In a first experiment, the stability of EBVGLC MHC multimers labeled with four different fluorochromes (APC, PE, QD605, or QD705) and cryopreserved with different concentrations of glycerol (5, 10, and 15%) at −80°C over a period of 6 months was compared to freshly-prepared MHC multimers. We observed no difference in the percentage (not shown) or in the staining index of the MHC multimer+ CD8 T-cell population using any of the glycerol concentrations or for any of the fluorescence labels (Fig. 4A). Furthermore, we tested the long-term stability of HLA-A2 multimers (PE EBVYVL, APC FLUGIL, and QD705 CMVNLV) cryopreserved at −80°C with 16% glycerol over a period of 13 months using PBMC of two different healthy donors (Fig. 4B). For each time-point, we compared the staining indices of the pMHC multimer+ populations to that obtained with the same batches of multimers prior to cryopreservation (Fig. 4B, left panel) or with freshly-made batches of pMHC multimers prepared on the day of T-cell staining from 9 months on onwards (Fig. 4B, right panel). This change in control measurement was included due to baseline changes of the flow cytometry instrument, prohibiting direct comparison to the prior day zero measurement. There were no differences in the frequency of MHC multimer-binding CD8 cells (data not shown) and staining indices appear to be stable for at least 6 months (see also Fig. 1C).
Cryopreservation was performed at different temperatures, i.e., −80°C at Centers 1 and 2, and −20°C at Center 3. Therefore, we assessed if any difference could be observed in long-term storage at these different temperatures. We compared the staining indices of T-cell populations stained with fresh, 1 month- and 6 month-cryopreserved MHC multimers stored at −20°C and at −80°C. We included MHC multimers against both virus-derived (HLA-A2 CMVNLV, HLA-A2 EBVFLY, and HLA-B7 CMVTPR) and cancer-associated antigens (HLA-A2 MARTELA ‘modified’ and -A2 MARTEAA ‘wild-type’) tested respectively on PBMC (one healthy donor) and TIL (one culture). As summarized in Figure 4C, no significant difference was observed with multimers stored either at −20°C or −80°C storage, when testing up to 6 months of storage. Exemplary dot-plots are shown in Supporting InformationFigure S4.
Altogether, these results indicate that it is feasible to cryopreserve MHC multimers with glycerol for at least 6 months over a broad range of different fluorescence labels.
Cryopreservation for Large Panels of Combinatorial Color-Encoded MHC Multimers
We next proceeded to cryopreserve large panels of combinatorial encoded MHC multimers, i.e., mix of two differently labeled pMHC multimers holding the same peptide sequence, to be used for parallel detection of numerous T-cell populations (16). In this setting, up to 27 different T-cell specificities can be identified in a single cell sample, using a unique two-color code for each pMHC specificity as reported previously (18). Because of a high MHC multimer concentration in such multiplex T-cell stainings, the impact of residual glycerol and the determination of minimal glycerol required for stable cryopreservation become increasingly important.
We generated MHC multimers containing 27 different virus-derived T-cell epitopes. For each of the specificities, MHC multimers were labeled with two-different fluorochromes, forming a unique two-color code. On the basis of the above described data, we cryopreserved these MHC multimers at −20°C, with 5% glycerol and 0.5% BSA, keeping each pMHC specificity separated until cell staining. This resulted in a residual glycerol concentration of 2.5% when performing the T-cell staining. A total of five different T-cell responses directed against CMV (HLA-A1, -A2, and -B7 restricted) and EBV (HLA-A2 restricted) were tested in three different donors. We compared the staining indices and the frequencies of MHC multimer+ T-cell populations when stained with a library of MHC multimers either stored overnight at 4°C or cryopreserved for 1 week, 1 month, or 4 months at −20°C (Figs. 5A and 5B). In all cases, the 27 multimers were mixed just prior to T-cell staining. Interexperiment instrument stability was assessed by CS&T beads (BD) (data not shown). Although some variations were detected for individual populations, we did not observed any trend towards increased background level or decreased signal intensity, and both the staining indices (Fig. 5A) and the percentage of stained CD8 T cells (Fig. 5B) were stable for a wide range of fluorochromes and over time. Exemplary dot-plots for one of the donors are shown in Supporting InformationFigure S5. We concluded that cryopreservation is feasible for MHC multimers for later use in combinatorial-encoded stainings.
Cryopreservation of Commercially Available MHC Multimers
Since all previous experiments were conducted with MHC multimers generated in-house at the participating laboratories, we finally tested the feasibility of cryopreservation for a range of commercially available MHC multimers (MHC dextramers from Immudex, MHC tetramers from TCMetrix and MHC pentamers from ProImmune). Since commercial MHC multimers labeled with Qdot labels are not available, testing for cryo-feasibility was only analyzed using PE and APC fluorescence labels. We assessed the SI and the frequency of MHC multimer+ T cells after receiving the reagents, and after 10 days storage at either 4°C or frozen (−80°C) with or without glycerol, including additional 5 freeze-thawing cycles (Fig. 6). Results from these analyses showed some loss of staining efficacy during 10 days storage at 4°C that for the affected reagents could be rescued by cryopreservation (Figs. 6A and 6B, and Supporting InformationFig. S6). Cryopreservation of commercially available MHC multimers seems therefore feasible, with no apparent reduction in SI compared to 4°C storage (Figs. 6A and 6B) or in the frequency of MHC multimer+ T cells detected (Fig. S6). Glycerol as cryopreservation agent seems to be required only for the in-house produced MHC multimers and the MHC pentamers, and most evidently when repeated freeze-thawing cycles were introduced. However, in all cases cryopreservation with glycerol including repeated freeze-thawing cycles were feasible for all tested reagents.
Figure 6.
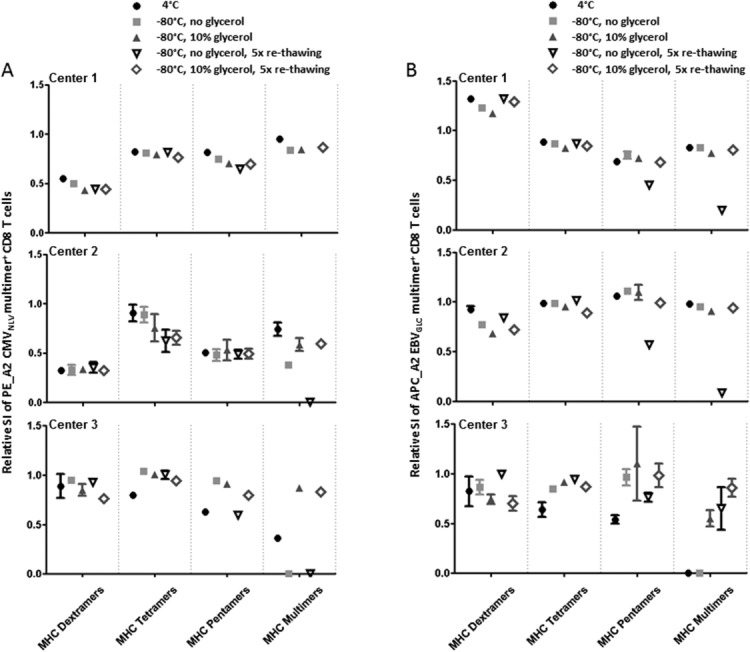
Testing of different storage conditions for commercially available and in-house generated MHC-multimers. We measured the SI of the MHC multimer+ T cell populations after staining using three different commercial MHC multimers: MHC dextramers (Immudex), MHC tetramers (TCMetrix), and MHC pentamers (ProImmune), in parallel with in-house produced MHC multimers from the three different centers. MHC multimers PE_A2 CMVNLV and APC A2_EBVGLC were included and tested shortly after purchase and following 10 days storage under the indicated conditions. (A) The SI of PE_A2 CMVNLV positive T cells at day 10 calculated relative to the SI at day 1, respectively shown for the different storage conditions, separately for each of the three centers. (B) The SI of APC A2_EBVGLC positive T cells at day 10 calculated relative to the SI at day 1, respectively shown for the different storage conditions, separately for each of the three centers. Performed as three independent, but parallel experiments at Centers 1–3. Relative SI = 0 means that no visible MHC multimer+ cells were detected. Each dot is the average of duplicates except staining with the MHC dextramers for Center 1, and error bars indicate range (often not visible).
Although these data indicate that commercially available MHC multimers may possess increased short-term stability as compared to the in-house generated MHC reagents, we show that that they can likewise can likewise be cryopreserved with glycerol, without significant loss of staining efficiency.
Discussion
Quality assurance in immunomonitoring is an area of increasing focus, as this is an absolute requirement for conducting long-term studies. Especially in the fast growing field of cancer immunotherapy where T-cell responses are often of low avidity and low frequency, it is extremely important to assure the optimal setting of the flow cytometer, assays and reagents used for T-cell analyses. A challenge in the use of MHC multimers for immunological monitoring is the undefined and variable stability of each peptide/MHC/fluorochrome complex.
The need for robust reagents becomes a mandatory requirement when moving from a pure research setting to analyzing sample specimens from clinical development programs. Here, the tight control of the whole experimental setup is needed due to a possible impact either on a patient's treatment or on the strategy for clinical development of immune-modulatory compounds. Therefore, only the access to stable MHC multimers will allow the availability of large batches of well-controlled reagents that are required for use in long-term and multicentric monitoring studies.
A number of optimization procedures for multicolor flow cytometry have been published recently, as valuable tools for optimizing performance and quality assurance of protocols and instrumentation (9,32). However, so far best strategies to maintain stability of MHC multimers have not been studied. Bovine serum albumin and glycerol are well-known protein preservators and have been previously used for cryopreservation of MHC-multimers (22,23), however the exact concentration of glycerol and the duration of stable storage have not been investigated.
In this study, we show that MHC multimers may be unstable at 4°C, and that serum albumin (of human or bovine origin) together with glycerol are indeed appropriate for cryopreservation, especially when multiple freeze-thawing cycles are applied (Fig. 1). Furthermore cryopreservation of MHC multimers is feasible across a wide range of different fluorochromes (PE, APC, Quantum dots and a tandem dye), HLA molecules (HLA-A*0201, A*0101, and B*0702) and TCR-pMHC affinities and specificities (both cancer-associated and virus-restricted), for both in-house generated as well as commercially available MHC multimers. However, as glycerol may be detrimental for cell viability and possibly affect the binding of antibodies and MHC multimers, we analyzed the effect of glycerol addition to the T-cell staining buffer, mimicking the presence of residual glycerol derived from cryopreserved multimers (Fig. 2). We conclude that with a maximum of 2% glycerol, no adverse effect can be observed neither in terms of cell viability nor on the antibody and MHC multimer binding (frequency of positive cells and fluorescence staining index). The viability seems to depend greatly on the staining protocol used, and incubation of cells in presence of glycerol at 37°C may enhance cell death (Fig. 2 and Supporting InformationFig. S3). A similar effect has been observed with prolonged incubation periods at lower temperatures (data not shown). Moreover, glycerol concentrations far lower than the previously described 16% can be used (Figs. 5), which is of great interest for new technologies enabling the screening of large panels of T-cell responses with a multitude of combinatorial-encoded MHC reagents (16). Finally, we found that cryopreserved MHC multimers can be stably stored at both −20°C and −80°C over a period of at least 6 months, leading to satisfactory staining-efficacy for a number of complexes for up to one year (Fig. 4). The fact that these experiments have been conducted in three different laboratories using different protocols also accounts for the robustness and broad applicability of the cryopreservation procedure.
Most tests conducted in this study made use of MHC monomers refolded by the original method of Altman et al. (1) or generated by UV-induced exchange of a conditional ligand (33) that were produced and multimerized in-house. In addition, a set of experiments was conducted to assess the feasibility of cryopreservation using three different formats of commercially available multimers (MHC tetramers, pentamers, and dextramers) (Fig. 6). All these multimers, could be successfully cryopreserved and refrozen using glycerol and albumin as cryoprotectors, albeit we noted that the addition of cryoprotectors was not a necessity for stability in all cases. Thus, although these data do not prove that the freezing procedure is indeed a requirement for stable storage over longer periods of time for all commercial reagents, they support the feasibility of this approach for reagents of all sources tested. In this regard, commercial providers make use of different strategies for multimerization that may affect stabilization e.g., the polysaccharide dextran backbone of MHC dextramers by Immudex, the coiled-coil multimerization domain in the MHC pentamers by ProImmune, and beta-2-microglobulin stabilization by TCMetrix. These commercially available MHC multimers are different in both linkage strategy and stoichiometry, and the backbone may by itself serve to support stability of the MHC complexes (34).
On the basis of the investigations presented here, a set of technical recommendations for MHC multimer cryopreservation is proposed in Table 1. We show here that cryopreservation may provide an efficient strategy for stable storage of MHC multimers, allowing to reach better stability, to minimize batch-to-batch variations in long-term monitoring projects and to increase quality assurance of the MHC multimer assay. These properties are well in line with good laboratories practices and assay standardization efforts and should help to pave the way for a more robust use of MHC multimers especially in the context of T-cell based immunotherapy trials.
Table 1.
Recommendations for pMHC multimer cryopreservation
| Task | Recommended values | Comments |
|---|---|---|
| Glycerol concentration for cryopreservation | 5–10% | Higher concentrations of glycerol may preserve the MHC multimer equally good, but to limit the residual glycerol during T-cell staining we recommend a use of 5–10% |
| Serum albumin (BSA or HSA) concentration for cryopreservation | 0.5% | The effect of this cryoprotector was not individually tested. No effect of residual albumin during T-cell staining is expected as albumin is present under physiological conditions and a common staining buffer constituent |
| Glycerol concentration during MHC multimer staining | Maximum 2% | Depending on the temperature for MHC multimer staining. A higher residual concentration can be allowed at 4°C. |
| Period of storage | Stable for at least 6 months | For some complexes and fluorescence labels prolonged storage is feasible |
| Temperature of cryopreservation | −80°C | No difference was observed between −20°C and −80°C storage |
| Repeated freeze-thawing cycles | At least 5 cycles | No more than 5 freeze-thawing cycles were tested |
Acknowledgments
The authors thank S. Stevanović for providing synthetic peptides, D. Wernet, for blood products, IM. Svane for tumor infiltrating lymphocyte cultures, S. Heidu and Tina Seremet for excellent technical assistance, and T. Schumacher for discussion and scientific advice. They also thank Immudex and TCMetrix for providing multimers at no or reduced fees.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Information
Literature Cited
- 1.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Davis MM, Altman JD, Newell EW. Interrogating the repertoire: broadening the scope of peptide-MHC multimer analysis. Nat Rev Immunol. 2011;11:551–558. doi: 10.1038/nri3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 4.Tan LC, Gudgeon N, Annels NE, Hansasuta P, O'Callaghan CA, Rowland-Jones S, McMichael AJ, Rickinson AB, Callan MF. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 5.Khan N, Cobbold M, Cummerson J, Moss PAH. Persistent viral infection in humans can drive high frequency low-affinity T-cell expansions. Immunology. 2010;131:537–548. doi: 10.1111/j.1365-2567.2010.03326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich P-Y, Mendrzyk R, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 7.Van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJA, Behjati S, Hilkmann H, El Atmioui D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31:e439–e442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen RS, Thrue CA, Junker N, Lyngaa R, Donia M, Ellebæk E, Svane IM, Schumacher TN, Thor Straten P, Hadrup SR. Dissection of T-cell antigen specificity in human melanoma. Cancer Res. 2012;72:1642–1650. doi: 10.1158/0008-5472.CAN-11-2614. [DOI] [PubMed] [Google Scholar]
- 9.Perfetto SP, Ambrozak D, Nguyen R, Chattopadhyay PK, Roederer M. Quality assurance for polychromatic flow cytometry using a suite of calibration beads. Nat Protoc. 2012;7:2067–2079. doi: 10.1038/nprot.2012.126. [DOI] [PubMed] [Google Scholar]
- 10.Chattopadhyay PK, Melenhorst JJ, Ladell K, Gostick E, Scheinberg P, Barrett AJ, Wooldridge L, Roederer M, Sewell AK, Price DA. Techniques to improve the direct ex vivo detection of low frequency antigen-specific CD8+ T cells with peptide-major histocompatibility complex class I tetramers. Cytometry A. 2008;73A:1001–1009. doi: 10.1002/cyto.a.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britten CM, Gouttefangeas C, Welters MJP, Pawelec G, Koch S, Ottensmeier C, Mander A, Walter S, Paschen A, Müller-Berghaus J, et al. The CIMT-monitoring panel: a two-step approach to harmonize the enumeration of antigen-specific CD8+ T lymphocytes by structural and functional assays. Cancer Immunol Immunother. 2008;57:289–302. doi: 10.1007/s00262-007-0378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Britten CM, Janetzki S, Ben-Porat L, Clay TM, Kalos M, Maecker H, Odunsi K, Pride M, Old L, Hoos A, et al. Harmonization guidelines for HLA-peptide multimer assays derived from results of a large scale international proficiency panel of the Cancer Vaccine Consortium. Cancer Immunol Immunother. 2009;58:1701–1713. doi: 10.1007/s00262-009-0681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Burg SH, Kalos M, Gouttefangeas C, Janetzki S, Ottensmeier C, Welters MJP, Romero P, Britten CM, Hoos A. Harmonization of immune biomarker assays for clinical studies. Sci Transl Med. 2011;3:108–144. doi: 10.1126/scitranslmed.3002785. [DOI] [PubMed] [Google Scholar]
- 15.Attig S, Price L, Janetzki S, Kalos M, Pride M, McNeil L, Clay T, Yuan J, Odunsi K, Hoos A, et al. A critical assessment for the value of markers to gate-out undesired events in HLA-peptide multimer staining protocols. J Transl Med. 2011;9(108) doi: 10.1186/1479-5876-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadrup SR, Bakker AH, Shu CJ, Andersen RS, van VJ, Hombrink P, Castermans E, Thor SP, Blank C, Haanen JB, et al. Parallel detection of antigen-specific T-cell responses by multidimensional encoding of MHC multimers. Nat Methods. 2009;6:520–526. doi: 10.1038/nmeth.1345. [DOI] [PubMed] [Google Scholar]
- 17.Newell EW, Klein LO, Yu W, Davis MM. Simultaneous detection of many T-cell specificities using combinatorial tetramer staining. Nat Methods. 2009;6:497–499. doi: 10.1038/nmeth.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen RS, Kvistborg P, Moerch TF, Pedersen NW, Lyngaa R, Bakker AH, Shu CJ, thor Straten P, Schumacher TN, Hadrup SR. Parallel detection of antigen-specific T-cell responses by combinatorial encoding of MHC multimers. Nat Protoc. 2012;7:891–902. doi: 10.1038/nprot.2012.037. [DOI] [PubMed] [Google Scholar]
- 19.Schumacher TN, Heemels MT, Neefjes JJ, Kast WM, Melief CJ, Ploegh HL. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell. 1990;62:563–567. doi: 10.1016/0092-8674(90)90020-f. [DOI] [PubMed] [Google Scholar]
- 20.Emerson TE. Unique features of albumin: A brief review. Crit Care Med. 1989;17:690–694. doi: 10.1097/00003246-198907000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Fuller BJ. Cryoprotectants: The essential antifreezes to protect life in the frozen state. Cryo Letters. 2004;25:375–88. [PubMed] [Google Scholar]
- 22.Haanen JB, Wolkers MC, Kruisbeek AM, Schumacher TN. Selective expansion of cross-reactive CD8(+) memory T cells by viral variants. J Exp Med. 1999;190:1319–1328. doi: 10.1084/jem.190.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haanen JB, van Oijen MG, Tirion F, Oomen LC, Kruisbeek AM, Vyth-Dreese FA, Schumacher TN. In situ detection of virus- and tumor-specific T-cell immunity. Nat Med. 2000;6:1056–1060. doi: 10.1038/79573. [DOI] [PubMed] [Google Scholar]
- 24.Britten CM, Janetzki S, Butterfield LH, Ferrari G, Gouttefangeas C, Huber C, Kalos M, Levitsky HI, Maecker HT, Melief CJM, et al. T cell assays and MIATA: The essential minimum for maximum impact. Immunity. 2012;37:1–2. doi: 10.1016/j.immuni.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Ellebaek E, Iversen TZ, Junker N, Donia M, Engell-Noerregaard L, Met O, Hölmich LR, Andersen RS, Hadrup SR, Andersen MH, et al. Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients. J Transl Med. 2012;10::169. doi: 10.1186/1479-5876-10-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinschenk T, Gouttefangeas C, Schirle M, Obermayr F, Walter S, Schoor O, Kurek R, Loeser W, Bichler K, Wernet D, et al. Integrated functional genomics approach for the design of patient-individual antitumor vaccines integrated functional genomics approach for the design of patient-individual. 2002;62:5818–5827. [PubMed] [Google Scholar]
- 27.Walter S, Bioley G, Bühring H-J, Koch S, Wernet D, Zippelius A, Pawelec G, Romero P, Stevanović S, Rammensee H-G, et al. High frequencies of functionally impaired cytokeratin 18-specific CD8+ T cells in healthy HLA-A2+ donors. Eur J Immunol. 2005;35:2876–2885. doi: 10.1002/eji.200526207. [DOI] [PubMed] [Google Scholar]
- 28.Hadrup SR, Toebes M, Rodenko B, Bakker AH, Egan DA, Ovaa H, Schumacher TNM. High-throughput T-cell epitope discovery through MHC peptide exchange. Methods Mol Biol. 2009;524:383–405. doi: 10.1007/978-1-59745-450-6_28. [DOI] [PubMed] [Google Scholar]
- 29.Maecker HT, Frey T, Nomura LE, Trotter J. Selecting fluorochrome conjugates for maximum sensitivity. Cytometry A. 2004;62A:169–173. doi: 10.1002/cyto.a.20092. [DOI] [PubMed] [Google Scholar]
- 30.Fishman AI, Alexander B, Eshghi M, Choudhury M, Konno S. Nephrotoxin-induced renal cell injury involving biochemical alterations and its prevention with antioxidant. J Clin Med Res. 2012;4:95–101. doi: 10.4021/jocmr833w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fahning ML, Garcia MA. Status of cryopreservation of embryos from domestic animals. Cryobiology. 1992;29:1–18. doi: 10.1016/0011-2240(92)90002-j. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen R, Perfetto S, Mahnke YD, Chattopadhyay P, Roederer M. Quantifying spillover spreading for comparing instrument performance and aiding in multicolor panel design. Cytometry A. 2013;83A:306–315. doi: 10.1002/cyto.a.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toebes M, Coccoris M, Bins AD, Rodenko B, Gomez R, Nieuwkoop NJ, van de Kasteele W, Rimmelzwaan G, Haanen JB, Schumacher TN. Design and use of conditional MHC class I ligands. Nat.Med. 2006;12:246–251. doi: 10.1038/nm1360. [DOI] [PubMed] [Google Scholar]
- 34.Bakke M, Kajiyama N. Improvement in thermal stability and substrate binding of pig kidney D-amino acid oxidase by chemical modification. Appl. Biochem. Biotechnol. 2004;112:123–31. doi: 10.1385/abab:112:3:123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information