Abstract
Patient experience is a critical dimension of cancer care quality. Understanding variation in experience among patients with different cancers and characteristics is an important first step for designing targeted improvement interventions. We analysed data from the 2011/2012 English Cancer Patient Experience Survey (n = 69 086) using logistic regression to explore inequalities in care experience across 64 survey questions. We additionally calculated a summary measure of variation in patient experience by cancer, and explored inequalities between patients with cancers treated by the same specialist teams. We found that younger and very old, ethnic minority patients and women consistently reported worse experiences across questions. Patients with small intestine/rarer lower gastrointestinal, multiple myeloma and hepatobiliary cancers were most likely to report negative experiences whereas patients with breast, melanoma and testicular cancer were least likely (top-to-bottom odds ratio = 1.91, P < 0.0001). There were also inequalities in experience among patients with cancers treated by the same specialty for five of nine services (P < 0.0001). Specifically, patients with ovarian, multiple myeloma, anal, hepatobiliary and renal cancer reported notably worse experiences than patients with other gynaecological, haematological, gastrointestinal and urological malignancies respectively. Initiatives to improve cancer patient experience across oncology services may be suitably targeted on patients at higher risk of poorer experience.
Keywords: oncology service, hospital, quality of health care, healthcare inequalities, patient satisfaction, neoplasms
Introduction
Patient experience is a central dimension of healthcare quality (IOM 2001; ASCO 2006; Ludwig et al. 2006; NHS 2013–2014). Consequently, measurement of patient experience in large nationwide samples of patients is becoming common practice in several countries. For example, in the United States, the Hospital Consumer Assessment of Healthcare Providers and Systems survey measures the experience of over a million hospital patients every year (Goldstein et al. 2013; Medicare.Gov 2014). A potential barrier in translating patient experience information into improvement actions is that data often relate to patients with a wide spectrum of conditions. Patients with cancer, in particular, have very different care pathways compared with other inpatients with non-neoplastic disease (e.g., elective surgery patients or those with exacerbations of a chronic disease). Therefore, experience surveys of patients with cancer are increasingly being considered in different countries (Iversen et al. 2012; Evensen et al. 2013; Garfinkel et al. 2013).
In England, a national programme of repeatable surveys specifically dedicated to the measurement of the experience of patients with cancer was introduced recently (Department of Health 2011–2012a). Cancer charities and consumer groups strongly support and engage with the dissemination of the results of these surveys, which cover a wide spectrum of items on different domains of experience (Macmillan Cancer Support 2012–2013). Because this survey encompasses patients with any type of cancer, it provides an opportunity to explore variation among patients with different diagnoses (including common, rarer and rare cancers; Department of Health 2011–2012a; Lyratzopoulos et al. 2012b; El Turabi et al. 2013).
Appreciating how patient experience varies among patients with different socio-demographic characteristics, and crucially, different cancers, is an important first step in helping to understand responsible mechanisms and the development of targeted interventions for improvement. Because large national surveys of the experience of patients with many different cancers are a recent development, our current understanding of variation in experience by cancer diagnosis is limited. The ease and speed by which a diagnosis can be established, the treatment burden and the prognosis of different cancers vary substantially. We would hypothesise that the experience of care will vary substantially among patients with different cancers; specifically, patients with prolonged diagnostic intervals, higher treatment burden and worse prognosis may provide more critical evaluations of their care. Although inequalities in reported experience among socio-demographic groups may be driven by differential prior expectations of quality, variation in experience among patients with different cancers should be relatively robust to such confounding, more directly reflecting disease factors.
Further, as results for the Cancer Patient Experience Survey are reported publicly for each survey item, we were a priori interested in potential inequalities in respect of each survey item; and on the degree of consistency in patterns of variation across different research questions and aspects of experience.
With these prior considerations, we analysed data from the English Cancer Patient Experience Survey 2011/2012 to explore variation in the experience of care between patients with cancer for all survey questions. Specifically, our objective was to describe and summarise variation in patient experience by age, gender, deprivation, ethnicity and cancer diagnosis across all survey questions.
Methods
Data
We used anonymous data from the Cancer Patient Experience Survey 2011/2012, a national postal survey of patients with cancer treated in English National Health Service hospitals commissioned by the UK Department of Health and undertaken by Quality Health (Chesterfield, UK), a specialist survey provider (Department of Health 2011–2012a). Patients were included in the survey sampling frame if cancer was recorded as the primary diagnosis in any hospital care record during September to November 2011. Primary data collection for the survey was approved by the Ethics and Confidentiality Committee of the National Information Governance Board (reference ECC 3-04(d)/2011; Department of Health 2011–2012a). Anonymous data from the survey are available for not-for-profit research from the UK Data Archive (http://www.data-archive.ac.uk/) – the source of data used in the present study (Department of Health 2011–2012b).
The survey questionnaire included evaluative questions that covered patient experience along the care pathway of patients with cancer, encompassing experience of presentation, diagnostic testing, treatment decisions, doctor and nurse communication, informational integration between hospital and community services after discharge, experience of chemotherapy and radiotherapy treatment, and outpatient follow-up care (Appendix 1). Cognitive testing of survey items was carried out by the survey provider in panels of volunteers identified by Macmillan Cancer Support, a national cancer charity (Department of Health 2011–2012a). The same postal methodology used for the final survey was used during cognitive testing, with interview follow-up. Testing encompassed the assessment of patients' ability to complete the questionnaire, their understanding of questions and general exploration of patient views about the survey.
Most questions had four or five ordered (Likert) response options, ranging from very positive to very negative experience, although some report-type questions had a binary (Yes/No) format. For each survey question, public reporting is based on the proportion of patients who provided responses indicating ‘good’ or ‘very good’ experience (Department of Health 2011–2012a). As this is how the survey findings are used by clinicians and managers to inform quality improvement efforts, and also by members of the public when accessing information about the comparative performance of different English hospitals, in this analysis, we used the same binary categorisation of experience (positive/negative) as used in public reporting.
Hospital record recorded cancer diagnosis classified using the International Classification of Diseases 10th edition (ICD-10) diagnostic code, patient age and gender, and hospital of treatment were available for all respondents. Self-reported ethnic group information was used in the analysis (Office of National Statistics classification) as the gold-standard approach to ethnic group assignment (Saunders et al. 2013). Therefore, and in the context of highly complete information (96.3%), the analysis was restricted to survey respondents for whom information on self-reported ethnicity was available. Age was categorised into eight groups (16–24, 25–34 in 10 year groups to 75–84 and 85+). Socio-economic status information was not available in the 2011/2012 dataset; however, patients' deprivation score (Index of Multiple Deprivation 2007 score of lower super output area of residence) was available for the 2010 survey (Indices of Deprivation 2007; Department of Health 2010). Consequently, in supplementary analysis, we examined socio-economic inequalities in cancer patient experience using the latter dataset.
We included patients with any cancer, using 36 diagnosis groups based on the ICD-10 classification (Table 1; Appendix 2). Of these, 31 were further nested within nine specialty groups (also called ‘multidisciplinary team’ or MDT groups in the United Kingdom and in other European countries, e.g., urological, gynaecological or haematological specialty groups) (European Partnership Action Against Cancer Consensus Group et al. 2014). Specialty groups encompass patients with cancers typically treated by the same oncology service and within shared premises (e.g., wards or outpatient clinics); these patients are also often treated by the same multidisciplinary group of oncologists, surgeons, nurses and other healthcare professionals, specialising in the treatment of cancers of the same body system (Table 2; Appendix 2).
Table 1.
Socio-demographic and clinical characteristics of survey respondents
| Age | All | % |
|---|---|---|
| 16–24 | 355 | 0.5 |
| 25–34 | 954 | 1.4 |
| 35–44 | 2,999 | 4.3 |
| 45–54 | 8,911 | 12.9 |
| 55–64 | 16,970 | 24.6 |
| 65–74 | 22,749 | 32.9 |
| 75–84 | 13,564 | 19.6 |
| 85+ | 2,584 | 3.7 |
| Gender | ||
| Men | 32,463 | 47.0 |
| Women | 36,623 | 53.0 |
| Ethnic group | ||
| White | 66,421 | 96.1 |
| Mixed | 278 | 0.4 |
| Asian | 1,146 | 1.7 |
| Black | 949 | 1.4 |
| Chinese | 150 | 0.2 |
| Other | 142 | 0.2 |
| Deprivation | ||
| Most affluent | 14,589 | 21.1 |
| 2nd | 14,624 | 21.2 |
| 3rd | 13,582 | 19.7 |
| 4th | 11,566 | 16.7 |
| Most deprived | 9,409 | 13.6 |
| Cancer diagnosis | ||
| Anal | 242 | 0.4 |
| Bladder | 6,503 | 9.4 |
| Bone sarcoma | 174 | 0.3 |
| Brain | 483 | 0.7 |
| Breast | 13,396 | 19.4 |
| Cervical | 405 | 0.6 |
| Colon | 5,054 | 7.3 |
| Ductal carcinoma in situ | 916 | 1.3 |
| Endometrial | 1,478 | 2.1 |
| Gynaecological NOS | 88 | 0.1 |
| Hepato-biliary | 568 | 0.8 |
| Hodgkin lymphoma | 487 | 0.7 |
| Laryngeal | 361 | 0.5 |
| Leukaemia | 2,479 | 3.6 |
| Lung | 3,698 | 5.4 |
| Melanoma | 1,546 | 2.2 |
| Mesothelioma | 392 | 0.6 |
| Multiple myeloma | 3,236 | 4.7 |
| Non-Hodgkin lymphoma | 4,290 | 6.2 |
| Oesophageal | 1,362 | 2 |
| Ophthalmic and rarer CNS | 59 | 0.1 |
| Oropharyngeal | 1,280 | 1.9 |
| Ovarian | 1,823 | 2.6 |
| Pancreatic | 673 | 1 |
| Prostate | 5,568 | 8.1 |
| Rectal | 3,541 | 5.1 |
| Renal | 950 | 1.4 |
| Secondary | 4,308 | 6.2 |
| Small-intestine | 215 | 0.3 |
| Soft tissue sarcoma | 575 | 0.8 |
| Stomach | 1,019 | 1.5 |
| Testicular | 256 | 0.4 |
| Thyroid | 493 | 0.7 |
| Ureter and rarer urological | 349 | 0.5 |
| Vulval / vaginal | 236 | 0.3 |
| Any other cancer diagnosis | 583 | 0.8 |
Ethnic group was defined using a six-group classification (White, Mixed, Asian or Asian British, Black or Black British, Chinese and Other).
Deprivation quintile groups (for data from the 2010 survey only) were defined by applying national (England) quintile-defining points (8.257, 13.525, 20.741, and 33.511) to Index of multiple deprivation (IMD) scores.
ICD-10 diagnostic codes. Anal C21; Bladder C67; Bone sarcoma C40, C41; Brain C71; Breast C50; Cervical C53; Colon C18; Ductal carcinoma in situ (DCIS) D05; Endometrial C54, C55; Gynaecological not otherwise specified (Gynaecological NOS) C57; Hepato-biliary C22, C23, C24; Hodgkin lymphoma C81; Laryngeal C32; Leukaemia C91, C92, C93, C94, C95; Lung C34, C33; Melanoma C43; Mesothelioma C45; Multiple myeloma C90; Non-Hodgkin lymphoma C82, C83, C85, C84; Oesophageal C15; Ophthalmic and rarer central nervous system (CNS) C47, C69, C70, C72; Oropharyngeal C00 – C14, C30, C31; Ovarian C56; Pancreatic C25; Prostate C61; Rectal C19, C20; Renal C64; Secondary C77, C78, C79; Small-intestine C17, C26; Soft tissue sarcoma C48, C49, C46; Stomach C16; Testicular C62; Thyroid C73; Ureter and rarer urological C60, C63, C65, C66, C68; Vulval / vaginal C51, C52; Any other cancer diagnosis C37, C38, C39, C74, C75, C76, C80, C97, C58, C88, C96.
Table 2.
Survey question 70: Overall rating of care. Clinical and socio-demographic variation
| Comparison of experience by socio-demographic characteristic |
Comparison of experience by cancer |
Comparison of experience between patients treated by the same specialty |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | Joint P-value | Cancer diagnosis | Odds ratio (95% CI) | Joint P-value | Specialty team | Cancer diagnosis (comparisons within MDT) | Within MDT odds ratio (95% CI) | Within group P-value | |
| Age (years) | Ductal carcinoma in situ | 0.40 (0.31–0.53) | <0.0001 | Breast | Breast | Baseline | 0.021 | ||
| 16–24 | 1.77 (1.29–2.44) | <0.0001 | Breast | 0.55 (0.48–0.62) | Ductal carcinoma in situ | 0.74 (0.57–0.96) | |||
| 25–34 | 1.66 (1.37–2.01) | Non-Hodgkin lymphoma | 0.58 (0.50–0.68) | Gynaecology | Ovarian | Baseline | 0.396 | ||
| 35–44 | 1.73 (1.54–1.94) | Leukaemia | 0.59 (0.5–0.71) | Endometrial | 0.90 (0.73–1.11) | ||||
| 45–54 | 1.42 (1.32–1.54) | Hodgkin lymphoma | 0.6 (0.44–0.82) | Cervical | 0.90 (0.65–1.24) | ||||
| 55–64 | 1.31 (1.23–1.40) | Testicular | 0.70 (0.46–1.06) | Vulval/vaginal | 1.19 (0.80–1.76) | ||||
| 65–74 | Reference | Melanoma | 0.71 (0.58–0.86) | Gynaecological NOS | 1.40 (0.78–2.50) | ||||
| 75–84 | 1.10 (1.03–1.18) | Bone sarcoma | 0.74 (0.45–1.21) | Head and neck | Thyroid | Baseline | 0.0002 | ||
| 85+ | 1.47 (1.31–1.66) | Laryngeal | 0.74 (0.51–1.08) | Laryngeal | 0.51 (0.34–0.79) | ||||
| Gender | Cervical | 0.76 (0.56–1.04) | Oropharyngeal | 0.57 (0.43–0.76) | |||||
| Men | Reference | <0.0001 | Endometrial | 0.76 (0.63–0.93) | Haematology | Non-Hodgkin lymphoma | Baseline | <0.0001 | |
| Women | 1.34 (1.26–1.42) | Mesothelioma | 0.79 (0.55–1.14) | Multiple myeloma | 1.56 (1.34–1.82) | ||||
| Ethnic group | Oropharyngeal | 0.82 (0.67–1.01) | Leukaemia | 1.02 (0.85–1.22) | |||||
| White | Reference | <0.0001 | Any other cancer diagnosis | 0.83 (0.63–1.10) | Hodgkin lymphoma | 1.03 (0.75–1.42) | |||
| Mixed | 1.50 (1.08–2.09) | Ovarian | 0.85 (0.71–1.01) | Lower gastrointestinal | Rectal | Baseline | 0.753 | ||
| Asian | 2.67 (2.32–3.07) | Multiple myeloma | 0.91 (0.78–1.05) | Colon | 0.99 (0.87–1.13) | ||||
| Black | 2.03 (1.96–2.70) | Lung | 0.95 (0.82–1.10) | Anal | 1.21 (0.84–1.74) | ||||
| Chinese | 3.49 (2.45–4.97) | Colon | 0.99 (0.87–1.13) | Small-intestine | 1.06 (0.71–1.59) | ||||
| Other | 2.30 (1.55–3.42) | Rectal | Baseline | Lung | Lung | Baseline | 0.321 | ||
| Vulval/vaginal | 1.01 (0.69–1.48) | Mesothelioma | 0.83 (0.57–1.20) | ||||||
| Soft-tissue sarcoma | 1.02 (0.78–1.33) | Neurology | Brain | Baseline | 0.914 | ||||
| Oesophageal | 1.03 (0.85–1.25) | Ophthalmic and rarer CNS | 1.04 (0.50–2.17) | ||||||
| Small-intestine | 1.06 (0.71–1.59) | Upper gastrointestinal | Oesophageal | Baseline | 0.064 | ||||
| Bladder | 1.18 (1.05–1.34) | Stomach | 1.23 (0.97–1.57) | ||||||
| Gynaecological NOS | 1.19 (0.67–2.11) | Pancreatic | 1.28 (0.98–1.67) | ||||||
| Secondary | 1.19 (1.04–1.36) | Hepatobiliary | 1.42 (1.07–1.88) | ||||||
| Anal | 1.21 (0.84–1.74) | Urology | Bladder | Baseline | 0.038 | ||||
| Prostate | 1.21 (1.06–1.37) | Prostate | 1.02 (0.91–1.14) | ||||||
| Brain | 1.25 (0.96–1.62) | Renal | 1.18 (0.97–1.42) | ||||||
| Stomach | 1.27 (1.03–1.55) | Testicular | 0.59 (0.39–0.89) | ||||||
| Ophthalmic and rarer CNS | 1.30 (0.64–2.62) | Ureter and rarer urological | 1.11 (0.82–1.51) | ||||||
| Ureter and rarer urological | 1.31 (0.96–1.80) | ||||||||
| Pancreatic | 1.32 (1.04–1.66) | ||||||||
| Renal | 1.39 (1.14–1.70) | ||||||||
| Thyroid | 1.44 (1.12–1.84) | ||||||||
| Hepatobiliary | 1.46 (1.14–1.87) | ||||||||
CI, confidence interval; Ophthalmic and rarer CNS, ophthalmic and rarer central nervous system; Gynaecological NOS, gynaecological not otherwise specified; MDT, multidisciplinary team.
Analysis
Initially, we calculated the unadjusted proportion of positive responses for each question and by each variable group. Then, for each (of 65) evaluative questions separately, we fitted a mixed-effect logistic regression model with positive/negative experience categories as the outcome, and adjusting for age group, sex, ethnicity and cancer diagnosis as categorical variables. A random effect for hospital of treatment was also included in these models, to account for potential confounding by differential concentration of patients with different characteristics (e.g., ethnic minority patients) in differentially performing hospitals. For one question (question 28, whether a patient was happy to be asked to take part in research) the model did not converge, and this question was excluded from further analyses; patient experience for this question was highly positive (95.5%).
We visually summarised patterns of inequality across all questions – similar approaches for visually summarising multiple observations across many cancer sites have been used previously (Moller et al. 2009). By survey question, for each variable we assigned ranks to each one of its categorical groups (i.e., among the 36 cancers, we assigned ranks of 1 and 36 to those cancers associated with the best and worst reported experience, respectively, and similarly for all other variable categories by question). We subsequently used a red-amber-green colour coding convention (red = most negative experience rank/group, green = most positive experience rank/group) to further visually summarise the findings across questions, reporting odds ratios (ORs) and a rank-based colour code. We used a joint (Wald) test to examine whether each variable improved the fit of model to the data, and where it did not we did not apply the colour coding.
Subsequently, as we observed overall consistent patterns of inequalities in patient experience by cancer, we estimated the average association between cancer diagnosis and patient experience across the entire survey. For this analysis we included all responses to all questions and adjusted for question to account for the fact that the proportion of patients reporting positive experience varies by question; and that some questions do not apply to all patients (see Appendix 1). We also adjusted for age, gender and ethnicity, and accounted for within-respondent correlation of responses using a linear regression within a generalised estimating equation approach. This approach increases power to explore within specialty group (i.e., within MDT) variation, enabling us to better distinguish inequalities even between cancers with relatively small sample sizes. To explore whether there are inequalities in cancer patient experience among patients with cancers treated by the same specialty groups we tested the equality of ORs for diagnoses within each specialty group using a Wald test.
We chose the largest cancer diagnosis group with approximately balanced gender distribution (rectal) as the baseline, while for further analysis comparing cancer experience within specialty group, we chose the largest bi-gender cancer diagnosis in each group. All analyses were carried out using Stata 11.2 (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP). Colour coding was applied using MS Excel 2010 (Microsoft Excel (2010) [computer software]).
Results
Of an initial total of 113 808 patients, 71 793 completed the survey (a response rate of 68%, after excluding patients who died soon after their inclusion in the sampling frame); 69 086 responses with valid self-reported ethnicity were included in the final analysis sample.
The sample characteristics and number of responses by survey question are shown in Table 1 and Appendix 1. The unadjusted proportion of patients reporting positive experience ranged from 24% (q68 – was the patient offered a written care plan) to 95% (q63 – correct patient records available at outpatient appointment).
Hereafter and unless otherwise noted, the adjusted ORs for reporting a more negative experience of care compared with the baseline group are presented for question 70 (overall rating of care, Table 2). The results for this question are a typical reflection of inequalities observed in respect of other survey questions (Tables 3 and 4 and Appendix 3).
Table 3.
Association between patient socio-demographic characteristics and cancer patient experience
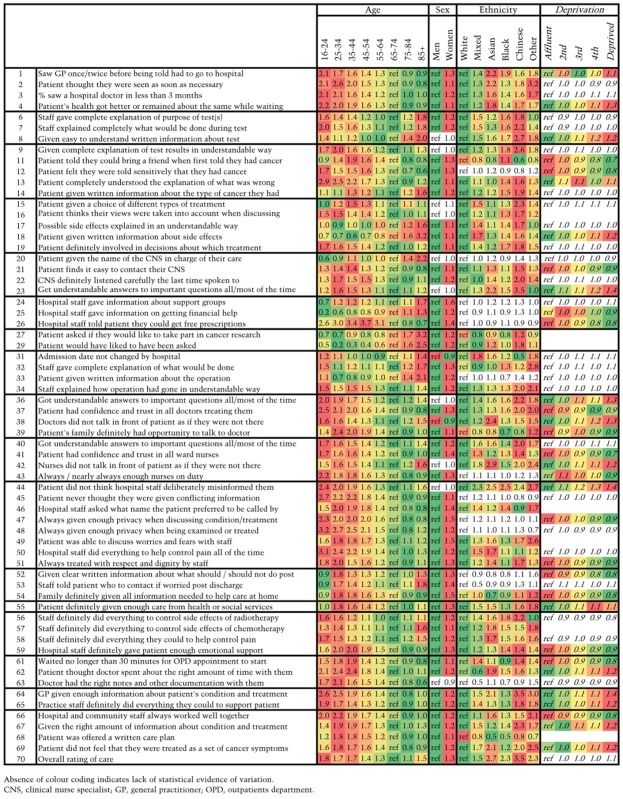 |
Table 4.
Association between cancer diagnosis and patient experience
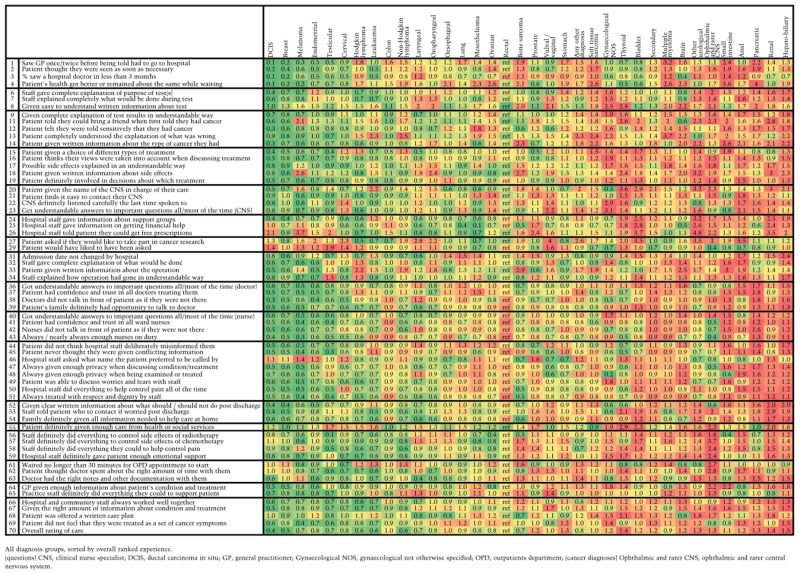 |
Patients in the 65–74-year group reported a positive experience more often than any other age group, while younger patients, and also the very old, reported comparatively poorer experience. Patients from ethnic minorities and women were also more likely to report poor experience (P < 0.0001 for all).
There was also evidence (P < 0.0001) of considerable inequalities in experience across cancer diagnoses, with patients with ductal carcinoma in situ and breast cancer being the most likely to report positive experiences and patients with thyroid and hepatobiliary cancer the least likely to do so [OR for negative experience compared with rectal cancer; ductal carcinoma in situ OR = 0.40 (95% confidence interval 0.31–0.53), breast OR = 0.55 (0.48–0.62), thyroid OR = 1.44 (1.12–1.84), hepatobiliary cancer OR = 1.46 (1.14–1.87) ]. There was evidence (P < 0.05) of inequalities within cancer specialty for four of the nine specialty groups. For example, among patients with haematological cancers, patients with multiple myeloma were most likely to report poor experience (OR compared with non-Hodgkin lymphoma patients 1.56, 95% confidence interval 1.34–1.82) and within the urology group, compared with patients with bladder cancer, patients with testicular cancer had substantially lower odds of reporting a negative overall experience (OR = 0.59, 95% confidence interval 0.39–0.89) – noting that this difference was adjusted for age.
To aid interpretation, the full crude and case-mix adjusted proportions of patients reporting a negative experience by variable category (age group, gender, ethnic group and cancer diagnosis) for question 70 are shown in Appendix 4. For example, the case-mix adjusted proportion of participants reporting a negative experience was 6% and 8% for patients with ductal carcinoma in situ or breast cancer, whereas it was 18% and 19% for patients with thyroid and hepatobiliary cancer respectively.
Visual summaries of inequalities in patient experience by age, gender, deprivation, ethnic group and cancer diagnosis across survey questions is provided by Tables 3 and 4 and Appendix 3. A brief summary is presented in Box 1. Questions are grouped by domain of care (full details in Appendix 1).
Box 1. Summary of disparities in the experience of patients with cancer by cancer diagnosis and socio-demographic characteristics (full visual summary of results in Tables 3 and 4 and Appendix 3)
Age: Poor experience across the cancer patient journey is consistently more common among younger ages than older groups, and decreases in older age groups. This is particularly consistent for questions relating to experience of inpatient hospital care. However, there are some notable exceptions.
Very old patients (i.e., aged 85+) tend to report slightly poorer experience than 75–84-year-olds.
Younger patients more often report a positive experience for questions relating to provision of information or explanation than older patients, for example regarding treatment side effects (questions 17–18). They also report more positive experience of being offered financial support (question 25) and experience of cancer research (questions 27–29).
Gender: Women consistently report poor cancer patient experience more often than men. After adjustment for age, ethnicity and cancer, this gender inequality becomes more apparent than it could be appreciated by simply observing crude patterns of variation. This is partly explained by the overall positive experience of women with breast cancer, masking the poorer experience of female patients with cancer overall.
Ethnic group: Patients from White ethnic groups report more positive experiences than patients from ethnic minority groups. The only question where patients from all ethnic minority groups had a more positive experience was for the provision of a written care plan (question 68). Chinese ethnic group patients report the worst experience across most questions.
Deprivation: There was evidence of (relatively small) disparities in patient experience by socio-economic group for about half the survey items; however, there is no consistent pattern in the direction of this effect.
Cancer diagnosis: Positive experiences are most often reported among patients with ductal carcinoma in situ, breast, melanoma, endometrial, cervical and testicular cancers. It should be noted that in crude analysis, two of these cancers (cervical and testicular) are associated with relatively poor reported experience, before adjustment is made for age and other variables (Appendix 5). On the other hand, positive experience was least often reported by patients with anal, pancreatic, renal and hepatobiliary/gall bladder cancers and multiple myeloma.
There are disparities among specialty groups, for example we note particularly that overall inpatient experience (questions 31–54) among patients with gynaecological cancers is more often positive than for patients with lower gastrointestinal cancers.
There are also substantial disparities within cancer specialty groups, consistent across questions.
Finally, there are a few notable associations for individual questions, for example, patients with mesothelioma are very likely to report positive experiences of information about financial support (question 25), reflecting established statutory compensation schemes for this occupational cancer.
There are only a few questions where the overall patterns of inequality in experience by cancer differ from the general trend. Experience of cancer research (question 27) is one of these; this is perhaps explained by patients with common cancers (such as breast cancer) being less likely to be invited to take part in research than patients with rarer cancers, where the pool of potential research participants is smaller. Experience of outpatient waiting times (question 61) is a further question where inequalities do not follow the general pattern of variation by cancer, but overall variation in experience of outpatient waiting times is not strongly associated with any particular diagnosis.
Lastly, given overall consistent inequalities across questions, we examined the average experience of patients across the whole survey. We found significant variation in patient experience by socio-demographic characteristics (P < 0.0001 for all), cancer diagnosis (Fig. 1, P < 0.0001) and within cancer specialty group (Fig. 2, P < 0.0001 for five of nine specialty groups, Appendix 5–7). On average, patients with ductal carcinoma in situ, breast cancer and melanoma report the most positive patient experience and those with anal, hepatobiliary, multiple myeloma and small-intestine cancers the least positive, overall consistent with the patterns observed across individual questions. Regarding inequalities within cancer specialty groups, patients with endometrial cancer tended to report more positive experience than patients with ovarian cancer [OR for reporting a poor experience = 0.81 (0.75–0.86), P < 0.0001]. There was also heterogeneity in experience among patients with haematological malignancies (P < 0.0001), with patients with multiple myeloma reporting the poorest experience [OR for reporting a poorer experience = 1.25 (1.19–1.31) compared with patients with non-Hodgkin lymphoma]. Patients with anal, hepatobiliary and renal cancer also tended to report notably worse experiences compared with patients with other gastrointestinal and urological cancers respectively (P < 0.001).
Figure 1.
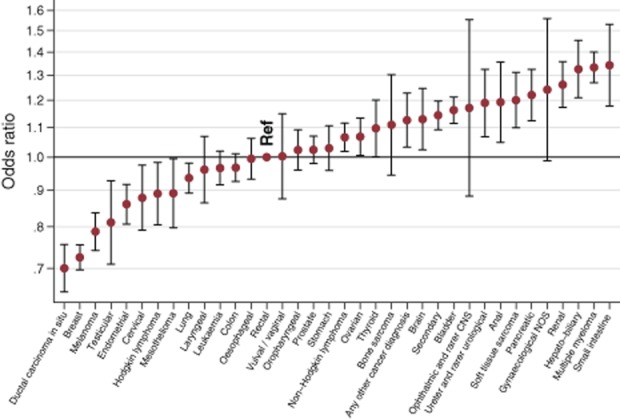
Association between cancer diagnosis and overall patient experience (average) across the survey/patient journey. Results are compared with the experience of patients with rectal cancer as a baseline, with a lower odds ratio indicating more positive experiences. Ophthalmic and rarer CNS, ophthalmic and rarer central nervous system; Gynaecological NOS, gynaecological not otherwise specified.
Figure 2.
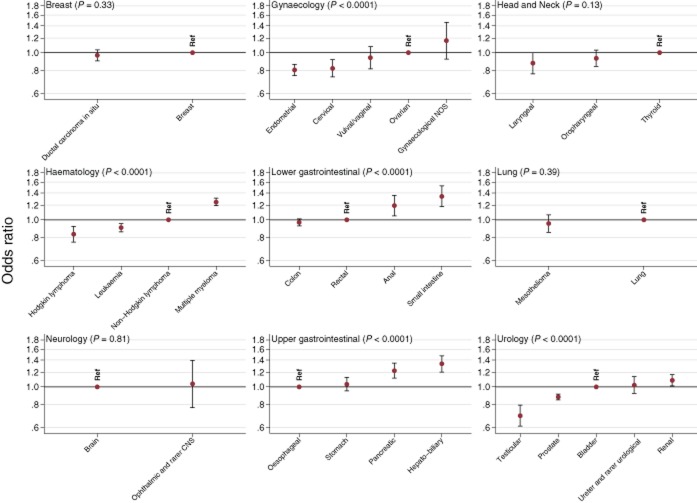
Association between cancer diagnosis and overall patient experience across the patient journey. Results are presented making comparisons in experience among cancer diagnoses within cancer specialty group. A lower odds ratio indicates more positive experiences. P-values are presented, and are a Wald test of whether odds ratios vary within cancer specialty group. Ophthalmic and rarer CNS, ophthalmic and rarer central nervous system; Gynaecological NOS, gynaecological not otherwise specified.
Discussion
Using data from a large nationwide survey with a relatively high response rate, we describe overall consistent inequalities in the experience of patients with cancer across different survey items. Younger and very old patients and those belonging to ethnic minorities tended to evaluate their care experience more critically across the great majority of questions; to a lesser degree, this is also true for women. There were overall consistent differences in experience among patients with different cancers. For example, among patients with 36 distinct diagnostic groups, patients with melanoma and breast cancer tended to most often report positive experiences and those with hepatobiliary cancer and multiple myeloma were more likely to report negative experiences. Further, there were also substantial differences among patients with different cancers treated by the same clinical specialties, and therefore, within the same MDT service environments. Specifically, patients with ovarian, multiple myeloma, anal, hepatobiliary and renal cancer tended to report worse experiences compared with patients with other gynaecological, haematological, upper gastrointestinal and urological cancers respectively.
The observed socio-demographic differences in experience are concordant with other patient survey evidence (note: not necessarily from patients with cancer) generally suggesting that younger and ethnic minority patients and women provide more critical evaluations of their care (Campbell et al. 2001; Weech-Maldonado et al. 2003; Mead & Roland 2009; Goldstein et al. 2010; Elliott et al. 2012; Lyratzopoulos et al. 2012a). There are only few previous reports of experience surveys specific to patients with cancer. A US study of the experience of patients with lung and colorectal cancer also indicates worse experiences by ethnic minority patients (Ayanian et al. 2010). Using data from the English Cancer Patient Experience surveys, inequalities in the experience of promptness of diagnostic suspicion in primary care, shared decision making and ‘overall’ evaluation of care have been previously reported (Lyratzopoulos et al. 2012b; El Turabi et al. 2013; Bone et al. 2014). The present study substantially amplifies these previous analyses as it explores variation in experience across every survey question and all experience domains (including the experience of diagnosis, treatment and community care, across 64 items). In spite of the observed inequalities among different patient groups, hospital performance in respect of cancer patient experience scores is only marginally affected by patient case-mix; this finding principally reflects the homogeneity of patient case-mix among most English hospitals (Abel et al. 2014).
Strengths of our study include its high (for a postal patient survey) response rate; its nationwide sample; its large population; the inclusion of patients with any type of cancer; and the relatively high completeness of information on exposure variables. Certain limitations ought to also be borne in mind in interpretation. We had no information on a range of variables that may influence experience, for example, on health performance status, or the influence of the actual treatments and care experienced by the patients (Ayanian et al. 2010). It should also be noted that the proportion of patients in active treatment or in remission (under surveillance) will vary across cancers. Even in the context of a survey with a relatively high response rate, there is potential for non-response bias. Specifically regarding the overall summary analysis of inequalities by caner type, it should be noted that it is based on a linear regression model, which essentially represents the adjusted mean variation by cancer across all survey questions. These mean-reported values are therefore susceptible to the influence of outliers and for some cancers, the mean variation in experience may not be the same as the typical variation (e.g., as observed for patients with mesothelioma). In order to characterise variation across all survey questions, it was necessary to consider a large number of models (i.e., for each outcome). We have made no attempt to correct for multiple testing as this concern is inapplicable. Standard corrections for multiple testing are designed to avoid undue attention being given to a small number of significant results that are observed when a large number of tests are considered (often also assuming that tests are independent). This consideration does not apply here as almost all associations presented are statistically significant, which strongly argues against chance findings. Length of time since diagnosis may also differentially influence recall of care experiences; however, previous work found minimal differences in overall patterns of variation by cancer when restricting the analysis to patients diagnosed within the last year (Lyratzopoulos et al. 2012b).
Being diagnosed with cancer in a young age is associated with particular practical and psychological difficulties (NICE 2005; Harrison et al. 2009; IOM 2013). It is therefore plausible that younger patients have greater needs for information about their tests, condition and treatment, and communication with their providers. Our findings would indicate that these needs are on the whole not currently being fully met. It is, however, encouraging that, against the overall pattern of differences in experience by age, younger patients reported better experiences in respect of information about their treatment, access to a specialist nurse, information about access to peer and financial support, and participation in cancer research (questions 15, 20, 24, 25 and 27 respectively), concordant with national guidance supporting access to key workers and participation to clinical trials for young patients with cancer (NICE 2005).
Inequalities in experience among different socio-demographic groups, and particularly among patients of different ethnicity, may reflect either variation in actual provision of care or differences in expectations of quality (the so-called ‘same care worse experience’ hypothesis) (Mead & Roland 2009; Lyratzopoulos et al. 2012a). Understanding the proportion of inequalities that is due to either of these two potential sources of variation is evidently critical for helping to inform appropriate improvement strategies. Distinguishing among differences in expectations of quality and actual differences in delivered care is, however, challenging, and it is likely that ethnic differences in patient experience may be the result of both differences in expectations of care quality and the care that is actually delivered (Mead & Roland 2009; Weinick et al. 2011; Lyratzopoulos et al. 2012a; Saunders et al. 2014). Communication difficulties, either because of differences in socio-cultural norms of medical consultation or language barriers, can commonly result in poorer experience. Use of translators for patients with limited English language skills can help to improve communication and satisfaction with care (Karliner et al. 2007). Hospitals with higher levels of ‘cultural competency’ scores have lower levels of ethnic disparity in patient experience (Weech-Maldonado et al. 2012).
We observed substantial inequalities in experience among patients with different cancer diagnoses. It would be unreasonable to assume that, after adjusting for age, gender and ethnicity, patients with different cancers have different prior expectations of care quality. It is theoretically possible that some of the differences may relate to inequalities in the quality of care provided by different specialist services (e.g., haematology compared with urology). However, notable inequalities in experience are observed even between patients with different cancers treated by the same clinical specialty and MDT service environments. Therefore, the observed differences in experience among patients with different cancers are likely to chiefly reflect disease-specific factors; differences in the promptness of diagnosis, treatment burden and prognosis are likely to be important contributors. For example, among patients with haematological cancers, those with multiple myeloma report the most critical experience, and they are also the patients who are likely to have experienced a greater number of pre-referral consultations with a general practitioner with relevant symptoms (Lyratzopoulos et al. 2012b, 2013). Similarly, among patients with lower gastrointestinal tract cancers, those with anal cancer tend to report the most critical experiences, possibly a reflection of associated treatment burden, as (compared with patients with colon cancer). Appreciation of worse prognosis may also be a factor, as advanced stage is associated with poorer experience of cancer care (Ayanian et al. 2010). Independent of their exact causes (in respect of the precise influence of promptness of diagnosis, treatment burden and prognosis), large within specialty group variations in experience should be considered as indicators of different healthcare needs of patients with different diagnoses. Specialty teams may therefore be able to prioritise interventions to improve the experience of their patients specifically targeting those patients within their specialty with greater needs for patient-centred care. Such interventions can, for example, include the provision of additional information and emotional or peer-support, or additional access and time for communication with nurses or doctors, for patients with diagnoses that confer a higher risk of poorer experience. Although the findings are broadly consistent across the survey, appreciation of patterns of variation in respect of each question (Tables 3 and 4) may help to identify priorities for improving specific aspects of experience for different patient groups. Given the findings, oncology teams specialising in gynaecological, haematological, gastrointestinal and urological cancers could prioritise the development and evaluation of interventions to improve the experience of patients with ovarian, multiple myeloma, anal, hepatobiliary and renal cancer respectively.
In conclusion, we report inequalities in experience among patients with cancer with different characteristics and diagnosis. The findings could guide improvement efforts targeting patients with cancer who are at greater risk of poorer experience of care. Specialty teams may be able to provide additional (targeted and tailored) support to improve the experience of patients with those cancers associated with greater care needs. Data from the English Cancer Patient Experience Survey provide an example of how nationwide surveys can provide intelligence to help inform and motivate both national and local initiatives to improve the care experience of patients with cancer. Identifying patterns of variation that we describe in other country populations and healthcare settings will be particularly useful.
Acknowledgments
The authors wish to thank the UK Data Archive for access to the anonymous survey data (UKDA study numbers: 7134 and 6742 for the Cancer Patient Experience Surveys 2011/2012 and 2010 respectively), the Department of Health as the depositor and principal investigator of the Cancer Patient Experience Survey, Quality Health as the data collector, and all the National Health Service Acute Trusts in England for provision of data samples. The authors are grateful to all patients who participated in any of the surveys.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Appendix 1. Questions, item response and score for all questions.
Appendix 2. Cancer ICD-10 codes, diagnosis groups and MDT classifications.
Appendix 3. Association between cancer diagnosis and patient experience. Cancers arranged in specialty groups, and sorted within specialty by ranked experience.
Appendix 4. Adjusted percentage of patients reporting a negative experience of care (question 70) results come from the model for which odds ratios are presented in Table 2.
Appendix 5. Unadjusted and adjusted association between patient socio-demographic characteristics and experience (average measure across all survey questions/the whole patient journey).
Appendix 6. Unadjusted and adjusted association between patient cancer diagnosis and experience (average measure across all survey questions/the whole patient journey).
Appendix 7. Adjusted ORs for ‘overall’ experience by cancer diagnosis (average measure across all survey questions/the whole patient journey).
References
- Abel GA, Saunders CL. Lyratzopoulos G. Cancer patient experience, hospital performance and case mix: evidence from England. Future Oncology. 2014;10:1589–1598. doi: 10.2217/fon.13.266. [DOI] [PubMed] [Google Scholar]
- American Society of Clinical Oncology (ASCO) ASCO–ESMO consensus statement on quality cancer care. Journal of Clinical Oncology. 2006;24:3498–3499. doi: 10.1200/JCO.2006.07.4021. [DOI] [PubMed] [Google Scholar]
- Ayanian JZ, Zaslavsky AM, Arora NK, Kahn KL, Malin JL, Ganz PA, Van Ryn M, Hornbrook MC, Kiefe CI, He Y, Urmie JM, Weeks JC. Harrington DP. Patients' experiences with care for lung cancer and colorectal cancer: findings from the Cancer Care Outcomes Research and Surveillance Consortium. Journal of Clinical Oncology. 2010;28:4154–4161. doi: 10.1200/JCO.2009.27.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone A, Mc Grath-Lone L, Day S. Ward H. Inequalities in the care experiences of patients with cancer: analysis of data from the National Cancer Patient Experience Survey 2011–2012. BMJ Open. 2014;4:e004567. doi: 10.1136/bmjopen-2013-004567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JL, Ramsay J. Green J. Age, gender, socioeconomic, and ethnic differences in patients' assessments of primary health care. Quality in Health Care. 2001;10:90–95. doi: 10.1136/qhc.10.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health. 2010. National Cancer Patient Experience Survey. [computer file] Colchester, Essex: UK Data Archive [distributor], April 2011. SN: 6742. Available at: http://dx.doi.org/10.5255/UKDA-SN-6742-1.
- Department of Health. 2011. –2012a National Cancer Patient Experience Survey – National Report http://dx.doi.org/10.5255/UKDA-SN-7134-1.
- Department of Health. 2011. –2012b National Cancer Patient Experience Survey. [computer file] Colchester, Essex: UK Data Archive [distributor], October 2012. SN: 7134. Available at: http://dx.doi.org/10.5255/UKDA-SN-7134-1.
- El Turabi A, Abel GA, Roland M. Lyratzopoulos G. Variation in reported experience of involvement in cancer treatment decision making: evidence from the National Cancer Patient Experience Survey. British Journal of Cancer. 2013;109:780–787. doi: 10.1038/bjc.2013.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MN, Lehrman WG, Beckett MK, Goldstein E, Hambarsoomian K. Giordano LA. Gender differences in patients' perceptions of inpatient care. Health Services Research. 2012;47:1482–1501. doi: 10.1111/j.1475-6773.2012.01389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Partnership Action Against Cancer Consensus Group. Borras JM, Albreht T, Audisio R, Briers E, Casali P, Esperou H, Grube B, Hamoir M, Henning G, Kelly J, Knox S, Nabal M, Pierotti M, Lombardo C, Van Harten W, Poston G, Prades J, Sant M, Travado L, Valentini V, Van De Velde C, Van Den Bogaert S, Van Den Bulcke M, Van Hoof E, Van Den Neucker I. Wilson R. Policy statement on multidisciplinary cancer care. European Journal of Cancer. 2014;50:475–480. doi: 10.1016/j.ejca.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Evensen C, Keller S, Arora N, Garfinkel S, Yost K. Frentzel E. Measuring cancer care quality: psychometric properties of an instrument for assessing patient experience with cancer care. 2013. Presentation at the International Society for Quality of Life Research (ISOQOL) Annual Meeting, Miami, FL.
- Garfinkel S, Frentzel E, Evensen C, Keller S, Yost KJ, Sangl J. Arora NK. Developing CAHPS for cancer care prototype: progress and next steps. Journal of Clinical Oncology. 2013;31 (suppl 31; abstr 134) [Google Scholar]
- Goldstein E, Farquhar M, Crofton C, Darby C. Garfinkel S. Measuring hospital care from the patients' perspective: an overview of the CAHPS Hospital Survey development process. Health Services Research. 2005;40:1977–1995. doi: 10.1111/j.1475-6773.2005.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E, Elliott MN, Lehrman WG, Hambarsoomian K. Giordano LA. Racial/ethnic differences in patients' perceptions of inpatient care using the HCAHPS survey. Medical Care Research and Review. 2010;67:74–92. doi: 10.1177/1077558709341066. [DOI] [PubMed] [Google Scholar]
- Harrison JD, Young JM, Price MA, Butow PN. Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Supportive Care in Cancer. 2009;17:1117–1128. doi: 10.1007/s00520-009-0615-5. [DOI] [PubMed] [Google Scholar]
- Indices of Deprivation. 2007. Indices of deprivation. Available at: http://webarchive.nationalarchives.gov.uk/20100410180038/http://communities.gov.uk/communities/neighbourhoodrenewal/deprivation/deprivation07/
- Institute of Medicine (IOM) 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. Available at: http://www.iom.edu/Reports/2001/Crossing-the-Quality-Chasm-A-New-Health-System-for-the-21st-Century.aspx.
- Institute of Medicine (IOM) Identifying and Addressing the Needs of Adolescents and Young Adults with Cancer – Workshop Summary. The National Academies Press; 2013. Available at: http://www.iom.edu/Reports/2013/Identifying-and-Addressing-the-Needs-of-Adolescents-and-Young-Adults-with-Cancer.aspx. [PubMed] [Google Scholar]
- Iversen HH, Holmboe O. Bjertnaes OA. The Cancer Patient Experiences Questionnaire (CPEQ): reliability and construct validity following a national survey to assess hospital cancer care from the patient perspective. BMJ Open. 2012;2:e001437. doi: 10.1136/bmjopen-2012-001437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karliner LS, Jacobs EA, Chen AH. Mutha S. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Services Research. 2007;42:727–754. doi: 10.1111/j.1475-6773.2006.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H, Mok T, Stovall E, Ganz P, Armitage J, Catane R, Adamou A. Redmond K. ASCO–ESMO consensus statement on quality cancer care. Annals of Oncology. 2006;17:1063–1064. doi: 10.1093/annonc/mdl152. [DOI] [PubMed] [Google Scholar]
- Lyratzopoulos G, Elliott M, Barbiere JM, Henderson A, Staetsky L, Paddison C, Campbell J. Roland M. Understanding ethnic and other socio-demographic differences in patient experience of primary care: evidence from the English General Practice Patient Survey. BMJ Quality and Safety. 2012a;21:21–29. doi: 10.1136/bmjqs-2011-000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyratzopoulos G, Neal RD, Barbiere JM, Rubin GP. Abel GA. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. The Lancet Oncology. 2012b;13:353–365. doi: 10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
- Lyratzopoulos G, Abel GA, McPhail S, Neal RD. Rubin GP. Measures of promptness of cancer diagnosis in primary care: secondary analysis of national audit data on patients with 18 common and rarer cancers. British Journal of Cancer. 2013;108:686–690. doi: 10.1038/bjc.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan Cancer Support. 2012. –2013 Cancer Patient Experience Survey: Insight Report and League Table. Available at: http://www.macmillan.org.uk/Documents/AboutUs/Research/Keystats/2013CPESInsightBriefingFINAL.pdf.
- Mead N. Roland M. Understanding why some ethnic minority patients evaluate medical care more negatively than white patients: a cross sectional analysis of a routine patient survey in English general practices. BMJ (Clinical Research Ed.) 2009;339:b3450. doi: 10.1136/bmj.b3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicare.Gov. 2014. Hospital Compare. The Official U.S. Government Site for Medicare. Available at: http://www.medicare.gov/hospitalcompare/Data/Overview.html.
- Moller H, Linklater KM. Robinson D. A visual summary of the EUROCARE-4 results: a UK perspective. British Journal of Cancer. 2009;101(Suppl. 2):S110–S114. doi: 10.1038/sj.bjc.6605400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (NICE) 2005. Guidance on Cancer Services Improving Outcomes in Children and Young People with Cancer. Available at: http://www.nice.org.uk/nicemedia/live/10899/28876/28876.pdf.
- Saunders CL, Abel GA, El Turabi A, Ahmed F. Lyratzopoulos G. Accuracy of routinely recorded ethnic group information compared with self-reported ethnicity: evidence from the English Cancer Patient Experience survey. BMJ Open. 2013;3:e002882. doi: 10.1136/bmjopen-2013-002882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders CL, Abel GA. Lyratzopoulos G. What explains worse patient experience in London? Evidence from secondary analysis of the Cancer Patient Experience Survey. BMJ Open. 2014;4:e004039. doi: 10.1136/bmjopen-2013-004039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Service (NHS) 2013. –2014 The NHS Outcomes Framework. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/213055/121109-NHS-Outcomes-Framework-2013-14.pdf.
- Weech-Maldonado R, Morales LS, Elliott M, Spritzer K, Marshall G. Hays RD. Race/ethnicity, language, and patients' assessments of care in Medicaid managed care. Health Services Research. 2003;38:789–808. doi: 10.1111/1475-6773.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weech-Maldonado R, Elliott M, Pradhan R, Schiller C, Hall A. Hays RD. Can hospital cultural competency reduce disparities in patient experiences with care? Medical Care. 2012;50(Suppl):S48–S55. doi: 10.1097/MLR.0b013e3182610ad1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinick RM, Elliott MN, Volandes AE, Lopez L, Burkhart Q. Schlesinger M. Using standardized encounters to understand reported racial/ethnic disparities in patient experiences with care. Health Services Research. 2011;46:491–509. doi: 10.1111/j.1475-6773.2010.01214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Questions, item response and score for all questions.
Appendix 2. Cancer ICD-10 codes, diagnosis groups and MDT classifications.
Appendix 3. Association between cancer diagnosis and patient experience. Cancers arranged in specialty groups, and sorted within specialty by ranked experience.
Appendix 4. Adjusted percentage of patients reporting a negative experience of care (question 70) results come from the model for which odds ratios are presented in Table 2.
Appendix 5. Unadjusted and adjusted association between patient socio-demographic characteristics and experience (average measure across all survey questions/the whole patient journey).
Appendix 6. Unadjusted and adjusted association between patient cancer diagnosis and experience (average measure across all survey questions/the whole patient journey).
Appendix 7. Adjusted ORs for ‘overall’ experience by cancer diagnosis (average measure across all survey questions/the whole patient journey).