Abstract
Background
The plasma protease factor VII-activating protease (FSAP) can release nucleosomes from late apoptotic cells. Nucleosomes are markers of cell death, and extracellular cell-free DNA has been suggested to play an important role in inflammation and has been demonstrated to correlate with severity and outcome in sepsis patients.
Objective
To investigate FSAP activation in patients suffering from Burkholderia pseudomallei infection (melioidosis), an important cause of Gram-negative sepsis in Southeast Asia. As diabetes mellitus (DM) is the most important risk factor for both melioidosis and sepsis, we were also able to examine the role of DM in FSAP activation in this cohort of patients.
Methods
In a prospective observational study, complexes of FSAP with α2-antiplasmin (AP) were assayed in 44 patients with melioidosis, 34 of whom were classified as diabetic. Eighty-two healthy subjects served as controls (52 with DM and 30 without).
Results
FSAP–AP complex levels were markedly elevated in patients as compared with controls. The FSAP level increased by 16.82 AU mL−1 in patients with melioidosis after adjustment for the effect of DM in the regression model. As expected, FSAP activation was correlated with nucleosome release (slope = 0.74). No difference in FSAP activation on admission was seen between survivors and non-survivors, but the extent of FSAP activation correlated with stage of the disease; repeated testing during convalescence showed a return towards normal values (day 0 vs. day 28, 4.16 AU mL−1, 95% confidence interval [CI] 1.42–12.22).
Conclusion
Patients with Gram-negative sepsis caused by B. pseudomallei have abundant FSAP activation, which significantly correlates with stage of disease. The presence of DM, however, does not influence the extent of FSAP activation.
Keywords: blood coagulation; Burkholderia pseudomallei; FSAP protein, human; melioidosis; nucleosomes
Introduction
Cell death has been implicated in the pathogenesis of severe sepsis and the associated immune suppression. Circulating cell-free DNA in the form of nucleosomes is a marker of cell death [1,2], and extracellular cell-free DNA has been suggested to play an important role in inflammation and has been demonstrated to correlate with severity and outcome in sepsis patients [2,3]. Melioidosis (Burkholderia pseudomallei infection) is a major cause of severe community-acquired sepsis in Southeast Asia and northern Australia [4], and is characterized by elevated levels of granzymes [5] and interleukin-18 [6,7], both of which are known inducers of cell death.
The clinical manifestations of melioidosis range from chronic skin abscesses to acute fulminant pneumonia-derived sepsis [4,8]. Despite appropriate antibiotic treatment, melioidosis patients with bacteremia or pneumonia have a mortality rate of up to 40% [9]. We recently showed that plasma protease factor VII-activating protease (FSAP), also known as plasma hyaluronic acid binding protein 2, is activated in adults suffering from sepsis and in children with meningococcal sepsis [3]. It remains to be determined to which structure FSAP binds and how FSAP activation is achieved. RNA, histones, glycosaminoglycans (e.g. heparin) and, to a lesser extent, also DNA have ben reported to activate FSAP [10]. Activated FSAP can release nucleosomes from late apoptotic cells [11,12] and, in concert with DNase I, from necrotic cells [13]. FSAP circulates as a single-chain inactive protease in plasma, and is activated upon contact with circulating histones [14] and either late apoptotic or necrotic cells [10]. FSAP has several plasma inhibitors, including α2-antiplasmin (AP) [10] and tissue factor pathway inhibitor (TFPI) [15], which are both inhibitors of plasmin as well. Increased levels of TFPI and plasmin–AP complexes (PAPcs) are both associated with mortality in patients with melioidosis [16]. The aim of this study was to investigate FSAP activation as shown by complexes of FSAP with AP in patients suffering from Gram-negative sepsis caused by B. pseudomallei. Furthermore, as diabetes mellitus (DM) is the most important risk factor for both melioidosis and sepsis, we were also able to examine the role of DM in FSAP activation in this cohort of patients [9,17,18].
Materials and methods
The study included a Thai patient population (Table1) detailed previously [19]: 44 patients with culture-proven melioidosis and sepsis (18–75 years, 30 males), from whom citrate plasma samples were obtained on the day of recruitment, 7 days thereafter, and at the first clinical follow-up ≥ 28 days after discharge, and stored at − 70 °C pending analysis. Eligible patients had received active antimicrobial chemotherapy for < 48 h (ceftazidime, amoxicillin–clavulanate, meropenem, or imipenem) and had two of four criteria for systemic inflammatory response syndrome. Thirty-four patients were classed as diabetic if they had an HbA1c of ≥ 7.8% at enrollment, or a diagnosis of DM made prior to admission. Eighty-two healthy subjects served as controls (52 with DM and 30 without).
Table 1.
Patient baseline characteristics
| Controls |
Melioidosis |
|||
|---|---|---|---|---|
| Blood donors, n = 30 | Diabetes patients, n = 52 | No diabetes, n = 10 | Diabetes, n = 34* | |
| Age (years), mean (95% CI) | 41.5 (37.5–45.4) | 57.5 (54.1–60.9) | 51.6 (40.9–62.3) | 52.9 (49.8–56.0) |
| Male sex, % (no.) | 80.0 (24/30) | 34.6 (18/52) | 90.0 (9/10) | 61.8 (21/34) |
| Glucose (mg L−1), mean (95% CI) | 101 (87–117) | 126 (117–136) | 124 (97–159) | 214 (188–244) |
| HbA1c (%), mean (95% CI) | 5.8 (5.4–6.3) | 8.2 (7.8–8.5) | 6.0 (5.5–6.5) | 10.6 (9.6–11.7) |
| Mortality, % (no.) | – | – | 0.0 (0/0) | 35.2 (12/34) |
FSAP–AP complexes were determined in citrate plasma by ELISA, as described previously [10,20]. Nucleosome levels were determined in EDTA plasma by ELISA, as described previously [11,21]. We log-transformed FSAP–AP complex and nucleosome levels to correct for heteroscedasticity. We performed multivariable linear regression to determine the effect of melioidosis on FSAP–AP complexes, adjusting for the effect of DM as a covariate. For linear regression analysis, the slope, 95% confidence interval (CI) and P-value are reported. An unpaired Student's t-test (for two groups) and one-way anova (for three groups or more) were used for comparison between groups. Analyses were performed in graphpad prism version 6.0 for Mac OS X (GraphPad Software, La Jolla, CA, USA).
Results and discussion
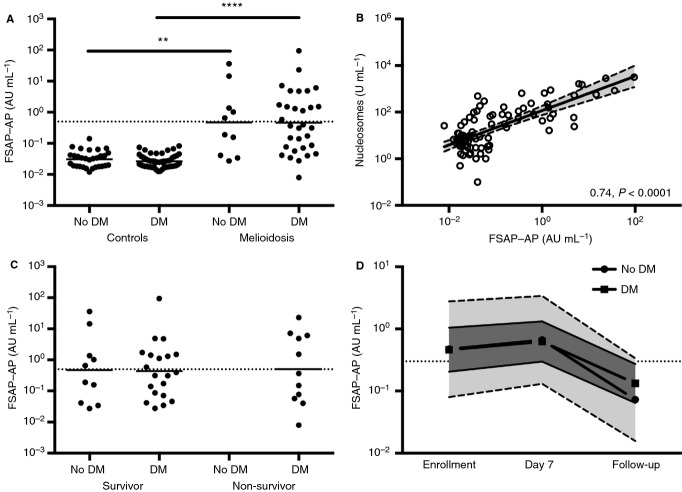
FSAP has recently been identified as the plasma serine protease responsible for nucleosome release from late apoptotic cells. As activation of this protein during sepsis might be involved in nucleosome release and thereby contribute to morbidity [10], we first determined FSAP–AP complex levels in our cohort of patients with melioidosis. FSAP–AP complex levels were significantly elevated (P < 0.007; Fig.1A) in patients (mean 0.47 AU mL−1; 95% CI 0.08–2.79) with melioidosis (without DM) as compared with healthy controls (mean 0.03 AU mL−1, 95% CI 0.03–0.04; without DM). The FSAP level increased by 16.82 AU mL−1 (95% CI 9.93–28.49) in melioidosis after adjustment for the effect of DM in the regression model. FSAP activation strongly correlated with nucleosome release (slope 0.74, 95% CI 0.59–0.89; P < 0.0001; Fig.1B), which is as expected, given the function of FSAP. The severity of illness and the high mortality rate of 27% in this cohort of patients enabled us to correlate the presence of FSAP–AP complexes with disease severity. No difference in FSAP activation on admission was seen between survivors and non-survivors (Fig.1C). Evidence for an association between FSAP activation and stage of disease, however, was obtained in patients who survived and from whom a second blood sample was drawn during treatment (at day 7) and after successful completion of therapy (≥ 28 days after discharge): in all surviving patients (without DM/with DM), a strong decrease towards complete normalization of FSAP activation was detected (one-way anova, Dunnet's post hoc test: day 0 vs. day 7, 0.73 AU mL−1 [95% CI 0.26–2.10]; day 0 vs. day 28, 4.16 AU mL−1 [95% CI 1.42–12.22]; Fig.1D).
Figure 1.
There is a high level of factor VII-activating protease (FSAP) activation in plasma from patients with melioidosis with or without diabetes mellitus (DM). (A) Increased levels of the apoptosis marker FSAP–α2-antiplasmin (AP) complex are present in plasma of patients with sepsis caused by Burkholderia pseudomallei (melioidosis) on the day of initial presentation as compared with healthy control subjects. No difference was found between melioidosis patients with or without DM. Straight lines represent the mean; FSAP–AP complex levels of > 0.5 AU mL−1 are considered to represent FSAP activation (dotted line). **P < 0.01 and ****P < 0.0001 (unpaired t-test). (B) Correlation curve for FSAP–AP complexes vs. nucleosomes. Slopes and P-values are reported (linear regression analysis). (C) No difference was seen between melioidosis survivors and non-survivors. (D) FSAP–AP complex levels measured in all surviving patients on the day of enrollment, day 7, and day 28 (follow up): in all surviving patients (no DM/DM), a strong decrease towards complete normalization (dotted line) of FSAP–AP complex levels was detected. Means (circle, no DM; square, DM) and 95% confidence intervals (light gray, no DM; dark gray, DM) are shown.
As DM is the main risk factor for melioidosis, and DM is known to negatively influence neutrophil function [9,18], we next sought to determine whether the presence of DM influenced FSAP–AP complexes in our cohort of patients, 72% of whom were diabetic (Table1). We found no difference in the release of FSAP–AP complexes in melioidosis patients when we compared patients with or without DM on the day of recruitment, but a significant difference in the release of FSAP–AP complexes between healthy volunteers (0.03 AU mL−1 [95% CI 0.02–0.03]) and melioidosis patients (0.46 AU mL−1 [95% CI 0.21–1.05]), both with DM, remained (P < 0.0001; Fig.1A). In addition, FSAP–AP complex levels were not different between healthy controls with or without DM.
In this cohort of patients with melioidosis, we see that there is significant FSAP activation, which might be involved in nucleosome release during sepsis and thereby might contribute to lethality. Even though FSAP activation and nucleosome release were correlated, and even though FSAP–AP complex levels in patients with melioidosis correlated with stage of disease, no differences were seen between survivors and non-survivors. Interestingly, our previous findings showed that inhibition of endogenous activated protein C, which has a cytoprotective effect against nucleosomes by cleavage of histones [22], leads to excessive nucleosome release in bronchoalveolar fluid during murine experimental melioidosis and increased mortality [23]. Furthermore, increased levels of plasma inhibitors of FSAP, i.e. AP (in complex with plasmin, PAPc) [10] and TFPI [15], are associated with mortality in patients with melioidosis [16].
The innate immune response can trigger different forms of cell death in order to release cytotoxic or inflammatory contents and enhance exposure of intracellular bacteria such as B. pseudomallei to phagocytic cells [24]. Apoptosis (or programmed cell death) has been shown to play an important role in the pathogenesis of severe sepsis, and is reflected by circulating nucleosomes [1,2]. ELISAs detecting nucleosomes have proven to constitute a good indicator of extracellular DNA released by immune cells in sepsis patients [25], but are not specific for nucleosomes released by neutrophils. However, the positive correlation with neutrophil-elastase together with the marked upregulation of genes encoding the central neutrophil-associated proteins does suggest that neutrophils constitute the main source of the detected nucleosomes during melioidosis [26]. FSAP has been shown to be activated upon contact with either late apoptotic or necrotic cells [10] and circulating histones [14], thereby releasing nucleosomes (Fig.2). In our cohort of patients, we found increased FSAP–AP levels and increased nucleosome levels [26], which were both strongly correlated, but found, unexpectedly, no association with patient mortality. We may speculate on the reasons why this may be the case. After incubation, plasma-derived FSAP binds to dead cells and, when activated, results in the release of nucleosomes [10]. Activated FSAP, which detaches from dead cells, will immediately be inactivated by plasma inhibitors, such as AP. Therefore, FSAP–AP complexes provide an indirect measure of FSAP activation, as activated FSAP bound to dead cells (and not released into plasma) is not measured with this approach (Fig.2). Increased severity of melioidosis may result in more tissue damage and cell death, a condition that favors FSAP binding to damaged tissue and nucleosome release. This is supported by the fact that nucleosome levels do indeed increase with severity [2,26]. However, the value of nucleosomes as a marker for severity and outcome is multifaceted. Recent literature shows that nucleosomes correlate with disease severity in patients with severe sepsis and septic shock, and in children suffering from meningococcal sepsis [3]. However, only in the latter group has a significant correlation between nucleosomes and outcome been observed. Most probably, there is a hierarchy in the dynamics and source of nucleosome release. Preliminary results demonstrate that, upon inflammation, nucleosome release occurs in a biphasic pattern, immune cells (e.g. neutrophils) being responsible for the initial release of nucleosomes, and parenchymal cells being mostly responsible in the later stages. In this light, meningococcal disease significantly differs from ‘classic sepsis’ with regard to the dynamics; young patients with meningococcal infection who die most often succumb during the state of ‘hyperinflammation’, whereas older patients with non-meningococcal sepsis who do not survive their critical illness almost universally show signs of a deactivated immune system or ‘immunosuppression’. Therefore, in fulminant meningococcal sepsis, the circulating nucleosomes most likely reflect both the activation of immune cells (most notably neutrophils) and extensive nucleosome release following tissue damage resulting from microvascular thrombosis. In sepsis, nucleosomes can originate from either polymorphonuclear neutrophils (PMNs) or parenchymal cells. The source of nucleosomes most likely also determines the degree of toxicity of nucleosomes. This is illustrated by the fact that nucleosomes exposed (and released) by PMNs have been reported to be highly cytotoxic and procoagulant [27]. In contrast, nucleosomes isolated from chicken erythrocytes seem to cause no harm when administered to mice [28]. In that light, the source of nucleosomes and the association of nucleosomes with cells may influence the cytotoxicity of nucleosomes.
Figure 2.
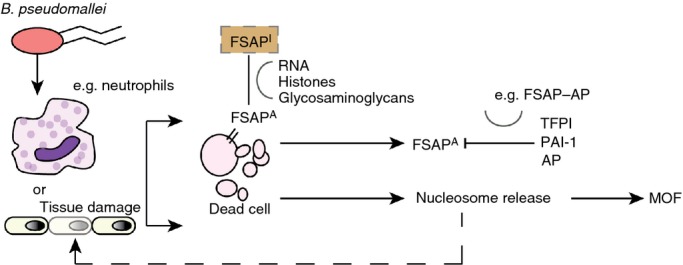
Simplified model of factor VII-activating protease (FSAP) activation during melioidosis. During infection, Burkholderia pseudomallei is recognized by innate immune effector cells such as neutrophils, which in turn release proinflammatory cytokines. When initiated, the immune cascade leads to programmed cell death, activation of the complement system, and activation of coagulation. The last of these can cause microvascular thrombosis, which can lead to extensive tissue damage and cell death. FSAP circulates as a single-chain inactive protease in plasma, and is activated upon contact with circulating histones and either late apoptotic or necrotic cells. Plasma-derived FSAP binds to dead cells and, when activated, results in the release of nucleosomes. FSAP-induced nucleosome release might be harmful to the host by propagating the proinflammatory response, and nucleosomes, in turn, can cause further tissue damage, contributing to multiorgan failure (MOF). Activated FSAP (FSAPA), which detaches from dead cells, will immediately be inactivated by plasma inhibitors, such as α2-antiplasmin (AP), tissue factor pathway inhibitor (TFPI), and plasminogen activator inhibitor-1 (PAI-1). FSAPI, inactivated FSAP.
DM is present in 23–60% of patients with melioidosis [8,9]. An explanation for this increased susceptibility was sought in the function of neutrophils from diabetic patients, which showed impaired phagocytosis of B. pseudomallei, reduced migration, and an inability to produce an oxidative burst to kill intracellular bacteria [29]. Furthermore, it was shown that isolated neutrophils from patients with DM released less extracellular DNA upon stimulation with B. pseudomallei and were associated with reduced bacterial killing relative to that by neutrophils from healthy controls [30]. In our study, we have now shown that FSAP activation is not dependent on DM, and therefore does not provide an explanation for the markedly increased susceptibility to B. pseudomallei infection in patients with DM.
In conclusion, patients with Gram-negative sepsis caused by B. pseudomallei have abundant FSAP activation, which significantly correlates with stage of disease. However, no differences were seen between survivors and non-survivors or between patients with or without DM.
Addendum
H. K. de Jong, G. C. K. W. Koh, W. J. Wiersinga, and S. S. Zeerleder conceived and designed the experiments. H. K. de Jong and G. C. K. W. Koh performed the experiments. H. K. de Jong, G. C. K. W. Koh, I. Bulder, F. Stephan, W. J. Wiersinga, and S. S. Zeerleder analyzed the data. G. C. K. W. Koh and S. S. Zeerleder contributed reagents/materials/analysis tools. H. K. de Jong and W. J. Wiersinga wrote the first draft. H. K. de Jong, G. C. K. W. Koh, I. Bulder, F. Stephan, W. J. Wiersinga, and S. S. Zeerleder contributed to the writing of the manuscript and agreed with the final results and conclusions. G. C. K. W. Koh enrolled patients.
Acknowledgments
We are grateful for the valuable contributions of past and present group members for their work in contributing to the understanding of nucleosomes and bacteria, in particular T. van der Poll. We would also like to thank G. van Mierlo and S. Solati for their expert technical assistance. We also wish to thank the nurses and doctors at Sappasithiprasong Hospital, Ubon Ratchathani, who were responsible for providing all clinical care for the patients in the study.
Source of Funding
H. K. de Jong: unrestricted funding for this project from the Academic Medical Center (AMC PhD Scholarship). G. C. K. W. Koh: Wellcome Trust of Great Britain (086532/Z/08/Z). W. J. Wiersinga: Clinical Fellowship from the Netherlands Organization for Health Research and Development (ZonMw; Clinical Fellowship grant number 90700424) and the Netherlands Organization for Scientific Research (NWO; VENI grant number 91610008).
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–51. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Zeerleder S, Zwart B, Wuillemin WA, Aarden LA, Groeneveld AB, Caliezi C, van Nieuwenhuijze AE, van Mierlo GJ, Eerenberg AJ, Lammle B, Hack CE. Elevated nucleosome levels in systemic inflammation and sepsis. Crit Care Med. 2003;31:1947–51. doi: 10.1097/01.CCM.0000074719.40109.95. [DOI] [PubMed] [Google Scholar]
- 3.Zeerleder S, Stephan F, Emonts M, de Kleijn ED, Esmon CT, Varadi K, Hack CE, Hazelzet JA. Circulating nucleosomes and severity of illness in children suffering from meningococcal sepsis treated with protein C. Crit Care Med. 2012;40:3224–9. doi: 10.1097/CCM.0b013e318265695f. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauw FN, Simpson AJ, Hack CE, Prins JM, Wolbink AM, van Deventer SJ, Chaowagul W, White NJ, van der Poll T. Soluble granzymes are released during human endotoxemia and in patients with severe infection due to gram-negative bacteria. J Infect Dis. 2000;182:206–13. doi: 10.1086/315642. [DOI] [PubMed] [Google Scholar]
- 6.Wiersinga WJ, Wieland CW, van der Windt GJ, de Boer A, Florquin S, Dondorp A, Day NP, Peacock SJ, van der Poll T. Endogenous interleukin-18 improves the early antimicrobial host response in severe melioidosis. Infect Immun. 2007;75:3739–46. doi: 10.1128/IAI.00080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, Re F. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1beta is deleterious. PLoS Pathog. 2011;7:e1002452. doi: 10.1371/journal.ppat.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035–44. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 9.Koh GC, Maude RR, Schreiber MF, Limmathurotsakul D, Wiersinga WJ, Wuthiekanun V, Lee SJ, Mahavanakul W, Chaowagul W, Chierakul W, White NJ, van der Poll T, Day NP, Dougan G, Peacock SJ. Glyburide is anti-inflammatory and associated with reduced mortality in melioidosis. Clin Infect Dis. 2011;52:717–25. doi: 10.1093/cid/ciq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephan F, Hazelzet JA, Bulder I, Boermeester MA, van Till JO, van der Poll T, Wuillemin WA, Aarden LA, Zeerleder S. Activation of factor VII-activating protease in human inflammation: a sensor for cell death. Crit Care. 2011;15:R110. doi: 10.1186/cc10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeerleder S, Zwart B, te Velthuis H, Manoe R, Bulder I, Rensink I, Aarden LA. A plasma nucleosome releasing factor (NRF) with serine protease activity is instrumental in removal of nucleosomes from secondary necrotic cells. FEBS Lett. 2007;581:5382–8. doi: 10.1016/j.febslet.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 12.Zeerleder S, Zwart B, te Velthuis H, Stephan F, Manoe R, Rensink I, Aarden LA. Nucleosome-releasing factor: a new role for factor VII-activating protease (FSAP) FASEB J. 2008;22:4077–84. doi: 10.1096/fj.08-110429. [DOI] [PubMed] [Google Scholar]
- 13.Stephan F, Marsman G, Bakker LM, Bulder I, Stavenuiter F, Aarden LA, Zeerleder S. Cooperation of factor VII-activating protease and serum DNase I in the release of nucleosomes from necrotic cells. Arthritis Rheumatol. 2014;66:686–93. doi: 10.1002/art.38265. [DOI] [PubMed] [Google Scholar]
- 14.Yamamichi S, Fujiwara Y, Kikuchi T, Nishitani M, Matsushita Y, Hasumi K. Extracellular histone induces plasma hyaluronan-binding protein (factor VII activating protease) activation in vivo. Biochem Biophys Res Commun. 2011;409:483–8. doi: 10.1016/j.bbrc.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Stephan F, Dienava-Verdoold I, Bulder I, Wouters D, Mast AE, Te Velthuis H, Aarden LA, Zeerleder S. Tissue factor pathway inhibitor is an inhibitor of factor VII-activating protease. J Thromb Haemost. 2012;10:1165–71. doi: 10.1111/j.1538-7836.2012.04712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiersinga WJ, Meijers JC, Levi M, van ‘t Veer C, Day NP, Peacock SJ, van der Poll T. Activation of coagulation with concurrent impairment of anticoagulant mechanisms correlates with a poor outcome in severe melioidosis. J Thromb Haemost. 2008;6:32–9. doi: 10.1111/j.1538-7836.2007.02796.x. [DOI] [PubMed] [Google Scholar]
- 17.Suputtamongkol Y, Chaowagul W, Chetchotisakd P, Lertpatanasuwun N, Intaranongpai S, Ruchutrakool T, Budhsarawong D, Mootsikapun P, Wuthiekanun V, Teerawatasook N, Lulitanond A. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis. 1999;29:408–13. doi: 10.1086/520223. [DOI] [PubMed] [Google Scholar]
- 18.Koh GC, Peacock SJ, van der Poll T, Wiersinga WJ. The impact of diabetes on the pathogenesis of sepsis. Eur J Clin Microbiol Infect Dis. 2012;31:379–88. doi: 10.1007/s10096-011-1337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh GC, Meijers JC, Maude RR, Limmathurotsakul D, Day NP, Peacock SJ, van der Poll T, Wiersinga WJ. Diabetes does not influence activation of coagulation, fibrinolysis or anticoagulant pathways in Gram-negative sepsis (melioidosis) Thromb Haemost. 2011;106:1139–48. doi: 10.1160/TH11-07-0504. [DOI] [PubMed] [Google Scholar]
- 20.van Montfoort ML, Stephan F, Lauw MN, Hutten BA, van Mierlo GJ, Solati S, Middeldorp S, Meijers JC, Zeerleder S. Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2013;33:147–51. doi: 10.1161/ATVBAHA.112.300498. [DOI] [PubMed] [Google Scholar]
- 21.van Nieuwenhuijze AE, van Lopik T, Smeenk RJ, Aarden LA. Time between onset of apoptosis and release of nucleosomes from apoptotic cells: putative implications for systemic lupus erythematosus. Ann Rheum Dis. 2003;62:10–14. doi: 10.1136/ard.62.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kager LM, Wiersinga WJ, Roelofs JJ, Meijers JC, Zeerleder SS, Esmon CT, van't Veer C, van der Poll T. Endogenous protein C has a protective role during Gram-negative pneumosepsis (melioidosis) J Thromb Haemost. 2013;11:282–92. doi: 10.1111/jth.12094. [DOI] [PubMed] [Google Scholar]
- 24.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–16. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, Schatzberg D, Cifuni SM, Fuchs TA, von Andrian UH, Hartwig JH, Aster RH, Wagner DD. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335–43. doi: 10.1182/blood-2012-01-405183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Jong HK, Koh GCKW, Achouiti A, van der Meer AJ, Bulder I, Stephan F, Roelofs JJTH, Day NPJ, Peacock SJ, Zeerleder S, Wiersinga WJ. Neutrophil extracellular traps in the host defense against sepsis induced by Burkholderia pseudomallei (melioidosis) Intensive Care Med Exp. 2014;2 doi: 10.1186/s40635-014-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–96. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 28.Gauthier VJ, Tyler LN, Mannik M. Blood clearance kinetics and liver uptake of mononucleosomes in mice. J Immunol. 1996;156:1151–6. [PubMed] [Google Scholar]
- 29.Chanchamroen S, Kewcharoenwong C, Susaengrat W, Ato M, Lertmemongkolchai G. Human polymorphonuclear neutrophil responses to Burkholderia pseudomallei in healthy and diabetic subjects. Infect Immun. 2009;77:456–63. doi: 10.1128/IAI.00503-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riyapa D, Buddhisa S, Korbsrisate S, Cuccui J, Wren BW, Stevens MP, Ato M, Lertmemongkolchai G. Neutrophil extracellular traps exhibit antibacterial activity against Burkholderia pseudomallei and are influenced by bacterial and host factors. Infect Immun. 2012;80:3921–9. doi: 10.1128/IAI.00806-12. [DOI] [PMC free article] [PubMed] [Google Scholar]