Abstract
Background
Treatment of chronic severe pediatric ITP is not well studied. In a phase 1/2 12–16-week study, 15/17 romiplostim-treated patients achieved platelet counts ≥50 × 109/L, and romiplostim treatment was well tolerated. In a subsequent open-label extension (≤109 weeks), 20/22 patients received romiplostim; all achieved platelet counts >50 × 109/L. Twelve patients continued in a second extension (≤127 weeks). Longitudinal data from start of romiplostim treatment through the two extensions were evaluated to investigate the safety and efficacy of long-term romiplostim treatment in chronic severe pediatric ITP.
Procedure
Patients received weekly subcutaneous romiplostim, adjusted by 1 µg/kg/week to maintain platelet counts (50–200 × 109/L, maximum dose 10 µg/kg). Bone marrow examinations were not required.
Results
At baseline, patients were median age 10.0 years; median ITP duration 2.4 years; median platelet count 13 × 109/L; 73% were male; and 36% had prior splenectomy. Median romiplostim treatment duration was 167 weeks (Q1, Q3: 78,227 weeks), and median average weekly dose was 5.4 µg/kg (Q1, Q3: 4.3, 8.0 µg/kg). Seven patients discontinued treatment: four withdrew consent, two were noncompliant, and one received alternative therapy. None withdrew because of adverse events (AEs). After the first 12 weeks, median platelet counts remained >50 × 109/L. Eight (36.4%) patients received rescue medication, and 14 (63.6%) used concurrent ITP therapy. Seven patients (31.8%) reported serious AEs, and two (9.1%) reported life-threatening AEs (both thrombocytopenia); there were no serious AEs attributed to treatment and no fatalities.
Conclusions
Long-term romiplostim treatment in this small cohort increased and maintained platelet counts for over 4 years in children with ITP with good tolerability and without significant toxicity. Pediatr Blood Cancer 2015;62:208–213. © 2014. The Authors. Pediatr Blood & Cancer published by Wiley Periodicals, Inc.
Keywords: autoimmunity, bleeding, platelets, thrombopoietin
INTRODUCTION
Immune thrombocytopenia (ITP) during childhood is an autoimmune disorder characterized by increased platelet destruction and suboptimal platelet production resulting in thrombocytopenia. The presentation of this disease varies, ranging from severe acute bleeding to mild bruising and purpura to an asymptomatic incidental diagnosis [1–4]. In contrast to adult ITP, treatment options for severe chronic ITP in children are very poorly studied; even single-arm trials are limited. Current therapeutic approaches to managing chronic pediatric ITP rely on data from adult studies further informed by small pilot studies in children and expert opinion, as there is virtually no evidence derived from randomized controlled trials [1].
Two thrombopoietin (TPO) receptor agonists, romiplostim and eltrombopag, have been approved for the treatment of chronic ITP in adults in the United States and elsewhere. Romiplostim, an Fc-peptide fusion protein that stimulates growth and maturation of megakaryocytes thus increasing platelet production, was developed to address the unmet need in the long-term treatment of adults with chronic ITP. In randomized controlled trials, romiplostim consistently demonstrated a high response rate and a good safety and tolerability profile, and reduced the need for splenectomy and use of rescue medication [5–7]. Additionally, adults with ITP who received romiplostim experienced a significant reduction in the rate of moderate and severe bleeding events and reported improved quality of life [5,8,9]. Long-term studies have shown good safety and tolerability profiles in adults over more than 5 years of continuous treatment [10].
The use of romiplostim in pediatric ITP was initially explored in a small randomized, double-blind, placebo-controlled, phase 1/2 study of patients between the ages of 1 and 18 years who had been diagnosed with ITP at least 6 months prior to enrollment [11]. The primary endpoint was the mean percentage of patients who achieved a platelet count ≥50 × 109/L for 2 consecutive weeks, which was achieved in 15 of 17 patients who received romiplostim compared with none who received placebo (P = 0.0008) [11]. Likewise, the percentage of patients who received rescue medication during the treatment period was lower in the romiplostim-treated group than in the placebo-treated group [11]. Overall, the response of pediatric patients to romiplostim was similar to that of adults observed in two phase 3 studies of romiplostim in patients with ITP [5,11]. In addition, romiplostim was well tolerated and there were no safety issues. Another small study had similar findings, although with a slightly lower response rate [12]. However no data on long-term use of romiplostim in children exist.
After completing the initial first-in-children phase 1/2 study [11], patients were offered the option of entering an extension study. At the end of this first extension study, patients who were still aged <18 years could choose to enter a second extension study. Twenty of 22 patients from the 12–16 week phase 1/2 study received romiplostim in the first open-label extension study (≤109 weeks); all achieved platelet counts >50 × 109/L. Twelve patients continued in a second extension study (≤127 weeks). This report presents the results of up to 4.7 years of romiplostim treatment of pediatric patients with ITP who received romiplostim or placebo in the randomized study [11] through the first or the first and second extension studies. The objective of these post hoc analyses was to describe the safety and efficacy of long-term use of romiplostim in children with severe, chronic ITP.
METHODS
Study Design and Treatment
Study design for the initial 12–16 week phase 1/2 study [11] and first open-label extension study [13,14] has been described elsewhere. Briefly, children aged 1–18 years who had been diagnosed with ITP per American Society of Hematology guidelines [15] 6 or more months before screening were eligible to enter the initial study if the mean of two platelet counts taken within 3 weeks before enrollment was ≤30 × 109/L, with no individual platelet count exceeding 35 × 109/L. Exclusion criteria included having undergone splenectomy within 8 weeks of screening, ongoing stable treatment for ITP besides corticosteroids, and receiving rituximab within 14 weeks before the screening visit. Patients with a history of a bone marrow stem cell disorder, venous or arterial thrombotic or thromboembolic event, systemic lupus erythematosus, or Evans syndrome or other known secondary causes of thrombocytopenia were also excluded.
Patients who completed the initial phase 1/2 study were eligible to enter the first open-label extension study; the second open-label extension study was restricted so that only patients aged <18 years were able to enter. In the open-label extension studies, all patients were to receive subcutaneous romiplostim once weekly, with a starting dose of 1 µg/kg. Doses were adjusted by 1 µg/kg per week as needed to maintain platelet counts of 50–200 × 109/L to a maximum dose of 10 µg/kg, as in adults. Patients who had received romiplostim in the initial study entered the extension study at the same romiplostim dose last received in the initial study, unless more than 24 weeks had elapsed since the patient's last dose of romiplostim, in which case treatment was initiated at 1 µg/kg per week. Patients who had received placebo in the initial study started romiplostim at a dose of 1 µg/kg per week in the first extension study. Patients could continue to receive other ITP treatments (e.g., corticosteroids, danazol, or azathioprine) that had been administered at a constant dose and schedule prior to the study start. These additional treatments could be reduced or discontinued at any time after platelet counts reached 50 × 109/L. Patients could also receive rescue treatments when platelet counts were below 10 × 109/L, when there was bleeding or wet purpura, or when deemed medically necessary by the investigator. Rescue treatment was defined as any treatment administered to increase the platelet count. Permitted rescue treatments included intravenous immunoglobulin G (IVIG), anti-D, platelet transfusions, steroids, and antifibrinolytics. The studies were conducted in compliance with all regulatory obligations and institutional review board and informed consent regulations at each investigational site. Data are summarized from three sequential studies, http://ClinicalTrials.gov Identifiers NCT00116688, NCT00515203, and NCT01071954.
Assessments, Outcomes, and Statistics
Platelet counts, concurrent treatments, and adverse events were assessed at each designated visit. During the extension studies, samples were collected for complete blood counts and blood chemistry determinations every 4 weeks, and physical examinations were performed at week 1 and every 12 weeks thereafter. Efficacy outcomes included the platelet counts at each visit and the platelet response (platelet count ≥50 × 109/L without rescue medication use in the past 4 weeks). Platelet counts of individuals who received rescue medication within the previous 4 weeks were excluded from the analyses. Missing platelet counts were imputed using last observation carried forward (LOCF) during each study. Other efficacy assessments included the proportion of patients using other ITP treatments and the proportion of patients who required rescue treatment.
Safety
Safety assessments included review of adverse events, physical examination, vital signs, serum chemistries, complete blood count, platelet count, and antibody status. Medically significant adverse events considered to be treatment-related by the investigator were followed until they resolved or were considered stable. The investigator assigned the following attributes: description; dates of onset and resolution; severity; assessment of relatedness to treatment, other suspect drug, or device; and action taken. The severity of toxicities was assessed on the following scale with appropriate clinical definitions: 1 = mild, 2 = moderate, 3 = severe, 4 = life-threatening, and 5 = fatal. Serious adverse events included any adverse events that were fatal, were life threatening, required inpatient hospitalization, or prolonged existing hospitalization. Standard Common Terminology Criteria for Adverse Events (CTCAE) grading of adverse events was used. Thromboembolic events were assessed as part of the overall adverse event evaluation, not as a separate assessment. Bone marrow biopsies were not required at study entry or at any time, but could be performed at the investigator's discretion; a biopsy was recommended if there were abnormalities in the peripheral blood smear (e.g., nucleated red blood cells) or if a loss of response to romiplostim occurred despite increasing doses. Blood samples for assays for antibodies to TPO and romiplostim were collected at week 1, week 52, then annually thereafter, and also at the end of the study.
Data Analyses
All analyses performed for this study were post hoc. Statistical analyses were descriptive. Categorical endpoints were summarized by the number and percentage of patients in each category. Continuous endpoints were summarized by number of patients, mean, standard deviation, median, and 25th percentile (Q1) and 75th percentile (Q3), with minimum and maximum values.
RESULTS
Demographics and Disposition
Baseline characteristics when the 22 patients enrolled in the initial phase 1/2 study were median age, 10.0 years; median duration of ITP, 2.4 years; median platelet count, 13 × 109/L; 73% male; and 36% with prior splenectomy (Table I). All 22 patients completed the phase 1/2 study, 21 of whom entered the first extension study; one of these 21 patients did not receive study drug. The 20 treated patients received up to 109 weeks of romiplostim in this first extension study; four patients withdrew (two due to withdrawal of consent, one due to noncompliance, and one due to requirement for alternative therapy), leaving 17 patients (of the 21) who completed the first extension study. Of these 17 patients, three were not eligible for the second extension study (two because they were over 18 years of age). Twelve of the 14 eligible patients chose to enter the second extension study, three of whom later withdrew (two due to consent withdrawal and one due to noncompliance). In this second extension study, patients received up to 127 weeks of romiplostim.
TABLE I.
Baseline Demographics
| N = 22 | |
|---|---|
| Age group in years, n (%) | |
| 1–2 | 4 (18.2) |
| 3–11 | 10 (45.5) |
| 12–17 | 8 (36.4) |
| Age (years), median (range) | 10 (1–17) |
| Male, n (%) | 16 (72.7) |
| Race, n (%) | |
| White or Caucasian | 13 (59.1) |
| Black or African American | 5 (22.7) |
| Hispanic or Latino | 3 (13.6) |
| Other | 1 (4.5) |
| Splenectomy, n (%) | 8 (36.4) |
| Baseline concurrent ITP therapy, n (%) | 3 (13.6) |
| Duration of ITP (years), median (range) | 2.4 (1–14) |
| Platelet count x109/L, median (range) | 13 (2–29) |
Except for baseline platelet counts (which were from the start of the initial study), other demographic characteristics were as of initiating romiplostim.
Dose and Exposure
The median romiplostim treatment duration for all three studies combined was 167 weeks (Q1, Q3: 78, 227 weeks; range: 12–242 weeks or nearly 5 years) ( Fig. 1). The total duration of treatment was 63.3 patient-years for these three consecutive studies. The median average weekly dose was 5.4 µg/kg (Q1, Q3: 4.3, 8.0 µg/kg); the median average weekly dose increased over time until Weeks 85–96 and then fell ( Fig. 2). Excluding the first 12 weeks of dose finding in the first study, patients received doses within 2 µg/kg of their most frequent dose 52% of the time.
Fig. 1.
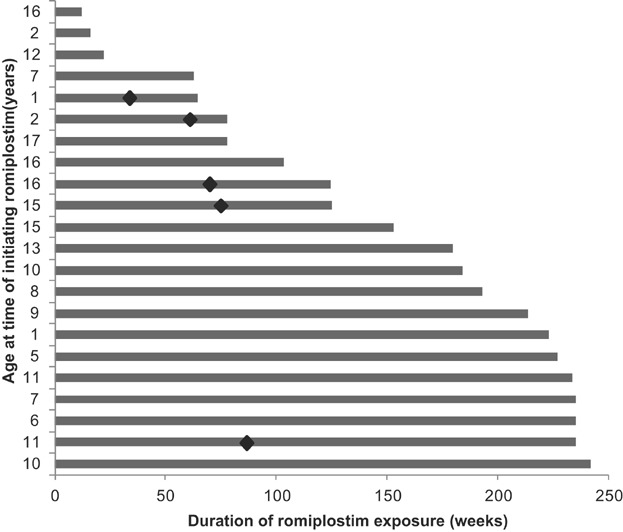
The total duration of romiplostim treatment for individual children. Diamonds indicate duration of romiplostim treatment when bone biopsies were performed.
Fig. 2.
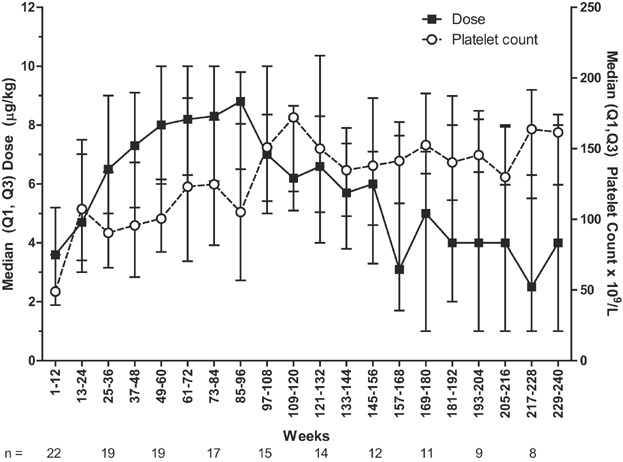
Median (Q1, Q3) average weekly romiplostim dose (left y-axis) and median (Q1, Q3) platelet counts (right y-axis) are presented over time by 12-week intervals with the number of patients with available data underneath the x-axis.
At the time of these analyses, nine children remained in the study. The median cumulative duration of treatment in these nine children was 234 weeks (range: 193–242 weeks) and the median average weekly dose was 6.0 µg/kg (range: 3.1–9.7 µg/kg). However, the median last dose was only 3.0 µg/kg (range: 0–10 µg/kg).
Efficacy
Over the course of the three studies, 21 of the 22 (95.5%) children treated had a peak platelet count ≥150 × 109/L, and 11 (50%) had a peak platelet count ≥400 × 109/L. After the first 12 weeks, median platelet counts stayed above 50 × 109/L ( Fig. 2). The median percentage of weeks that these 21 patients had a platelet response was 84.3% (Q1, Q3: 69.9%, 90.8%). Of the three patients who had baseline concurrent ITP therapy, two discontinued these therapies. Fourteen (63.6%) patients used concurrent ITP therapy during the study (Table II); the proportion of patients using concurrent ITP medications decreased gradually over time. Concurrent ITP therapy included those medications continued from baseline as well as any medications added or increased in dose to increase platelet count, with the added/increased medications considered rescue therapy. Of the 14 patients receiving concurrent ITP therapy, eight (36.4%) received rescue medication, including one platelet transfusion (Table II). Rescue medication use was low throughout romiplostim treatment—for example, rescue medication was not administered in most 12-week time periods.
TABLE II.
Concurrent and Rescue ITP Medications
| N = 22, n (%) | |
|---|---|
| Concurrent medications | |
| All | 14 (63.6) |
| Corticosteroids | 10 (45.4) |
| Prednisone | 6 (27.3) |
| Dexamethasone | 3 (13.6) |
| Hydrocortisone | 2 (9.1) |
| Methylprednisolone sodium succinate | 1 (4.5) |
| Immunoglobulin | 5 (22.7) |
| Aminocaproic acid | 1 (4.5) |
| Dapsone | 1 (4.5) |
| Immunoglobulin anti-Rh (anti-D) | 1 (4.5) |
| Vincristine | 1 (4.5) |
| Rescue medications | |
| All | 8 (36.4) |
| Immunoglobulin | 5 (22.7) |
| Dexamethasone | 2 (9.1) |
| Immunoglobulin anti-Rh (anti-D) | 1 (4.5) |
| Platelets | 1 (4.5) |
| Prednisone | 1 (4.5) |
| Vincristine | 1 (4.5) |
Safety
All patients reported adverse events. Seven patients (31.8%) reported serious adverse events, for a duration-adjusted rate of 0.43 for 100 patient-weeks. The serious adverse events were influenza (in two patients), asthma, epistaxis, hemangioma, hypotension, idiopathic thrombocytopenic purpura, lymphadenitis, pharyngitis streptococcal, pyrexia, sepsis, thrombocytopenia, and transfusion reaction (all in one patient each). There were two life-threatening adverse events (9.1%, 0.06/100 patient-weeks), which were thrombocytopenia in both cases. There were no fatalities. There were no serious adverse events attributed to treatment, and no patients withdrew from treatment due to adverse events (Table III). There were no reports of thrombotic events. Five bone marrow biopsies were performed between 34 and 87 weeks (median: 70 weeks) after the start of treatment, all in the first extension study ( Fig. 1); no bone marrow reticulin or fibrosis was observed. There were no reports of neutralizing antibodies to TPO or romiplostim. The most common adverse events were headache, upper respiratory tract infection, and pyrexia (Table IV).
TABLE III.
Summary of Adverse Events
| N = 22, n (%) | 3,292 patient-weeks, n (ratea) | |
|---|---|---|
| Patients reporting any adverse events (AE) | 22 (100) | 745 (22.63) |
| Patients reporting any severe AE | 9 (40.9) | 20 (0.61) |
| Patients reporting any serious AE | 7 (31.8) | 14 (0.43) |
| Patients reporting any treatment-related AE | 6 (27.3) | 37 (1.12) |
| Patients reporting any serious treatment-related AE | 0 (0) | 0 (0) |
| Fatal AE | 0 (0) | 0 (0) |
| Patients who withdrew from study or romiplostim due to AE | 0 (0) | 0 (0) |
| AEs of interest | ||
| Bone marrow fibrosis | 0 (0) | 0 (0) |
| Thrombotic events | 0 (0) | 0 (0) |
| Antibodies to romiplostim or TPO | 0 (0) | 0 (0) |
Rate is exposure-adjusted incidence for 100 patient-weeks.
TABLE IV.
Incidence of Overall Adverse Events in Descending Order of Frequency (≥25%)
| Preferred term | N = 22 (n (%)) |
|---|---|
| Headache | 14 (63.6) |
| Upper respiratory tract infection | 14 (63.6) |
| Pyrexia | 12 (54.5) |
| Oropharyngeal pain | 11 (50.0) |
| Petechiae | 11 (50.0) |
| Cough | 10 (45.5) |
| Epistaxis | 10 (45.5) |
| Contusion | 9 (40.9) |
| Fatigue | 9 (40.9) |
| Nasal congestion | 9 (40.9) |
| Rhinorrhoea | 9 (40.9) |
| Vomiting | 9 (40.9) |
| Nausea | 8 (36.4) |
| Gingival bleeding | 7 (31.8) |
| Nasopharyngitis | 7 (31.8) |
| Rash | 7 (31.8) |
| Arthralgia | 6 (27.3) |
| Mouth hemorrhage | 6 (27.3) |
| Pain | 6 (27.3) |
Bleeding adverse events occurred in 17 (77.3%) patients. The most common bleeding adverse events were petechiae (11 patients, 50.0%), epistaxis (10 patients, 45.5%), gingival bleeding (seven patients, 31.8%), and mouth hemorrhage (six patients, 27.3%). Bleeding adverse events of grade 3 or above occurred in two patients and were epistaxis in both cases.
DISCUSSION
Children with chronic ITP by definition have low, but variable, platelet counts; typically those patients with pronounced thrombocytopenia have the greatest risk for major bleeding [16]. Unfortunately, there are no randomized controlled clinical trials that clearly establish whether any of the common treatment options for ITP is preferable to another in children, or even in adults [1]. Specifically, long-term corticosteroid regimens have considerable toxicity and many patients who respond to steroids require a high dose. Splenectomy, with response rates of approximately 70% in children with chronic ITP, carries a risk of sepsis [1,17,18], which may be increased if an underlying immune deficiency disorder contributes to the persistence and/or severity of ITP. Rituximab therapy is reported to result in long-term remission in only 20–25% of patients [19]; with optimization of rituximab regimens not yet evaluated in children [20]. Danazol may impact sexual development and shorten final height in pre-pubertal children [21]. Finally, response rates to immunosuppressive agents, such as azathioprine or mycophenolate mofetil or cyclosporin, are not well defined and do not appear to be high in children with chronic ITP, and it may take months for a therapeutic effect to be seen.
The TPO-agent romiplostim has been associated with a relatively low incidence of side effects combined with a high, stable response rate in adults with ITP [13,14]. In the long-term TPO-agent study described here, romiplostim treatment consistently increased platelet counts for over 4 years in a small cohort of children with severe chronic ITP. All but one of the initial 22 patients had peak platelet counts ≥150 × 109/L in response to treatment and, after the first 12 weeks of treatment, median platelet counts remained consistently above 50 × 109/L. Romiplostim was well tolerated and without significant toxicity in this small cohort. Toward the end of the greater than 4 years of treatment, the median romiplostim dose decreased from 6 to 4 µg/kg while maintaining the platelet response. This may represent slow improvement of the disease with time; no data have clearly demonstrated improvement as a result of TPO-agent treatment.
The primary limitation of this series of consecutive studies is the small sample size, which was further reduced by patient withdrawals, lack of eligibility in three patients, and certain patients' discontinuing treatment over time due to improvement of ITP. In addition, the study protocol did not require specific evaluation of toxicities such as thromboembolic events and bone marrow reticulin fibrosis, although neither was seen (Table III). The lack of bone marrow findings is encouraging, as grade 1 reticulin may be seen in normal bone marrow (without any treatment). Romiplostim dosing in this study was designed to lessen the development of thrombocytosis, but, because of the nature of pediatric ITP and concurrent viral infections, it was not possible to avoid thrombocytosis and half of the 22 children developed platelet counts over 400 × 109/L at least once. However, even in adults, thrombocytosis is not associated with thrombosis [13,14,22], and the transient nature of the thrombocytosis renders it a minor issue. Of note, as most patients were receiving romiplostim through home administration, and thus had monthly rather than weekly assessments, the true extent of platelet fluctuations is unknown. The possibility of infection-related platelet count fluctuations seems to be greater in children, meaning that the dosing schedule may require closer monitoring and that in certain settings of clinical use, dose adjustments may need to be minimized as a result. Finally, one concern about the use of romiplostim in both children and adults with ITP is that it will need to be used for many months or years, if not indefinitely. This concern is compounded by the lack of long-term data in children. In this study of long-term romiplostim use for up to 4.7 years, several children were able to discontinue romiplostim treatment. As to how and when treatment can be discontinued, studies to address these issues are ongoing in adults.
Use of romiplostim in children with chronic ITP offers several possible advantages to other options, notwithstanding the limitations discussed above. Quality-of-life data from the original phase 1/2 study suggest that parental burden is decreased with romiplostim treatment [23]; this has not been addressed with other treatments. While romiplostim requires a weekly subcutaneous injection, it can potentially be given at home, a strategy approved in Europe although not in the United States at the time of writing. Based on data in adults and very limited data in children, the concern of bone marrow fibrosis due to treatment with romiplostim appears to be low [24]. There is no concern about hepatotoxicity with romiplostim and no restrictive dietary requirements, as opposed to those with eltrombopag [25], although platelet counts may fluctuate more [26], an issue that was seen in this study. No episodes of thromboembolic phenomena have been seen in children. There may be less thrombosis in children than that seen in adults; however, the data here are from only 63 patient-years of exposure to romiplostim, whereas for adults, data from up to 922 patient-years have been published [10]. Compared with adults, there is very little concern about myelodysplastic syndrome masquerading as ITP, resulting in treatment with romiplostim, possibly inducing leukemia. Finally, romiplostim increased platelet counts in children with chronic ITP in this study and in adults with ITP, bleeding is substantially reduced when the platelet count increases.
In conclusion, the data described here suggest that long-term romiplostim treatment in children with chronic ITP provides adverse event and platelet response outcomes similar to those seen in adults. Evaluation of patients enrolled in the ongoing phase 3 study of romiplostim in children with ITP (http://ClinicalTrials.gov NCT01444417, estimated enrolment of 60) will provide further data as to its safety and efficacy.
Acknowledgments
Medical writing assistance was provided by Susanna Mac and James O'Kelly, employees of Amgen.
REFERENCES
- 1.Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB, Godeau B, Grainger J, Greer J, Greer I, Hunt BJ, Imbach PA, Lyons G, McMillan R, Rodeghiero F, Sanz MA, Tarantino M, Watson S, Young J, Kuter DJ. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 2.Aledort LM, Hayward CP, Chen MG, Nichol JL, Bussel J; ITP Study Group. Prospective screening of 205 patients with ITP, including diagnosis, serological markers, and the relationship between platelet counts, endogenous thrombopoietin, and circulating antithrombopoietin antibodies. Am J Hematol. 2004;76:205–213. doi: 10.1002/ajh.20104. [DOI] [PubMed] [Google Scholar]
- 3.Psaila B, Petrovic A, Page LK, Menell J, Schonholz M, Bussel JB. Intracranial hemorrhage (ICH) in children with immune thrombocytopenia (ITP): Study of 40 cases. Blood. 2009;114:4777–4783. doi: 10.1182/blood-2009-04-215525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imbach P. Refractory idiopathic immune thrombocytopenic purpura in children: Current and future treatment options. Paediatric Drugs. 2003;5:795–801. doi: 10.2165/00148581-200305120-00003. [DOI] [PubMed] [Google Scholar]
- 5.Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, Aledort LM, George JN, Kessler CM, Sanz MA, Liebman HA, Slovick FT, de Wolf JT, Bourgeois E, Guthrie TH, Jr, Newland A, Wasser JS, Hamburg SI, Grande C, Lefrère F, Lichtin AE, Tarantino MD, Terebelo Hr, Viallard JF, Cuevas FJ, Go RS, Henry DH, Redner RL, Rice L, Schipperus Mr, Guo DM, Nichol JL. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: A double-blind randomised controlled trial. Lancet. 2008;371:395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 6.Kuter DJ, Rummel M, Boccia R, Macik BJ, Pabinger I, Selleslag D, Rodeghiero F, Chong BH, Wang X, Berger DP. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363:1889–1899. doi: 10.1056/NEJMoa1002625. [DOI] [PubMed] [Google Scholar]
- 7.Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, Lichtin AE, Lyons RM, Nieva J, Wasser JS, Wiznitzer I, Kelly R, Chen CF, Nichol JL. AMG531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355:1672–1681. doi: 10.1056/NEJMoa054626. [DOI] [PubMed] [Google Scholar]
- 8.George JN, Mathias SD, Go RS, Guo M, Henry DH, Lyons R, Redner RL, Rice L, Schipperus MR. Improved quality of life for romiplostim-treated patients with chronic immune thrombocytopenic purpura: Results from two randomized, placebo-controlled trials. Br J Haematol. 2009;144:409–415. doi: 10.1111/j.1365-2141.2008.07464.x. [DOI] [PubMed] [Google Scholar]
- 9.Gernsheimer TB, George JN, Aledort LM, Tarantino MD, Sunkara U, Matthew Guo D, Nichol JL. Evaluation of bleeding and thrombotic events during long-term use of romiplostim in patients with chronic immune thrombocytopenia (ITP) J Thromb Haemost. 2010;8:1372–1382. doi: 10.1111/j.1538-7836.2010.03830.x. [DOI] [PubMed] [Google Scholar]
- 10.Rodeghiero F, Stasi R, Giagounidis A, Viallard JF, Godeau B, Pabinger I, Cines D, Liebman H, Wang X, Woodard P. Long-term safety and tolerability of romiplostim in patients with primary immune thrombocytopenia (ITP): A pooled analysis of 13 clinical trials. Eur J Haematol. 2013:423–436. doi: 10.1111/ejh.12181. [DOI] [PubMed] [Google Scholar]
- 11.Bussel JB, Buchanan GR, Nugent DJ, Gnarra DJ, Bomgaars LR, Blanchette VS, Wang YM, Nie K, Jun S. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 2011;118:28–36. doi: 10.1182/blood-2010-10-313908. [DOI] [PubMed] [Google Scholar]
- 12.Mokhtar GM, Tantawy AA, El Sherif NH. Romiplostim therapy in children with unresponsive chronic immune thrombocytopenia. Platelets. 2012;23:264–273. doi: 10.3109/09537104.2011.619601. [DOI] [PubMed] [Google Scholar]
- 13.Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113:2161–2171. doi: 10.1182/blood-2008-04-150078. [DOI] [PubMed] [Google Scholar]
- 14.Kuter DJ, Bussel JB, Newland A, Baker RI, Lyons RM, Wasser J, Viallard JF, Macik G, Rummel M, Nie K, Jun S. Long-term efficacy and safety of romiplostim treatment of adult patients with chronic immune thrombocytopenia (ITP): Final report from an open-label extension study. Br J Haemat. 2013;161:411–423. doi: 10.1111/bjh.12260. [DOI] [PubMed] [Google Scholar]
- 15.George JN, Woolf SH, Raskob GE, Wasser JS, Aledort LM, Ballem PJ, Blanchette VS, Bussel JB, Cines DB, Kelton JG, Lichten AE, McMillan R, Okerbloom JA, Regan DH, Warrier I. Idiopathic thrombocytopenic purpura: A practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88:3–40. [PubMed] [Google Scholar]
- 16.Flores A, Buchanan GR. Bleeding severity as an important outcome in childhood immune thrombocytopenia. Pediatr Blood Cancer. 2013;60:S8–11. doi: 10.1002/pbc.24344. [DOI] [PubMed] [Google Scholar]
- 17.Ghanima W, Godeau B, Cines DB, Bussel JB. How I treat immune thrombocytopenia: The choice between splenectomy or a medical therapy as a second-line treatment. Blood. 2012;120:960–969. doi: 10.1182/blood-2011-12-309153. [DOI] [PubMed] [Google Scholar]
- 18.Cooper N. A review of the management of childhood immune thrombocytopenia: How can we provide an evidence-based approach. Br J Haematol. 2014 doi: 10.1111/bjh.12889. [DOI] [PubMed] [Google Scholar]
- 19.Patel VL, Mahevas M, Lee SY, Stasi R, Cunningham-Rundles S, Godeau B, Kanter J, Neufeld E, Taube T, Ramenghi U, Shenoy S, Ward MJ, Mihatov N, Patel VL, Bierling P, Lesser M, Cooper N, Bussel JB. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119:5989–5995. doi: 10.1182/blood-2011-11-393975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper N, Bussel JB. The long-term impact of rituximab for childhood immune thrombocytopenia. Curr Rheumatol Rep. 2010;12:94–100. doi: 10.1007/s11926-010-0090-5. [DOI] [PubMed] [Google Scholar]
- 21. Martindale: The Complete Drug Reference: Danazol Drug Monograph 2014 [cited 2014 April 1, 2014]. Available from http://www.medicinescomplete.com/mc/martindale/current/9035-v.htm?q=danazol&t=search&ss=text&p=1#m9035-a1-d.
- 22.Saleh MN, Bussel JB, Cheng G, Meyer O, Bailey CK, Arning M, Brainsky A; EXTEND Study Group. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: Results of the long-term, open-label EXTEND study. Blood. 2013;121:537–545. doi: 10.1182/blood-2012-04-425512. [DOI] [PubMed] [Google Scholar]
- 23.Klaassen RJ, Mathias SD, Buchanan G, Bussel J, Deuson R, Young NL, Collier A, Bomgaars L, Blanchette V. Pilot study of the effect of romiplostim on child health-related quality of life (HRQoL) and parental burden in immune thrombocytopenia (ITP) Pediatr Blood Cancer. 2012;58:395–398. doi: 10.1002/pbc.23312. [DOI] [PubMed] [Google Scholar]
- 24.Kuter DJ, Mufti GJ, Bain BJ, Hasserjian RP, Davis W, Rutstein M. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood. 2009;114:3748–3756. doi: 10.1182/blood-2009-05-224766. [DOI] [PubMed] [Google Scholar]
- 25. GlaxoSmithKline. PROMACTA® (eltrombopag) prescribing information 2012 [updated November 2012; cited 2013 July 26]. Available from http://us.gsk.com/products/assets/us_promacta.pdf.
- 26.Khellaf M, Viallard JF, Hamidou M, Cheze S, Roudot-Thoraval F, Lefrere F, Fain O, Audia S, Abgrall JF, Michot JM, Dauriac C, Lefort S, Gyan E, Niault M, Durand JM, Languille L, Boutboul D, Bierling P, Michel M, Godeau B. A retrospective pilot evaluation of switching thrombopoietic receptor-agonists in immune thrombocytopenia. Haematologica. 2013;98:881–887. doi: 10.3324/haematol.2012.074633. [DOI] [PMC free article] [PubMed] [Google Scholar]


