Abstract
Organophosphate pesticides are widely used and recent studies suggest associations of in utero exposures with adverse birth outcomes and neurodevelopment. Few studies have characterized organophosphate pesticides in human plasma or established how these levels correlate to urinary measurements. We measured organophosphate pesticide metabolites in maternal urine and chlorpyrifos and diazinon in maternal and cord plasma of subjects living in an agricultural area to compare levels in two different biological matrices. We also determined paraoxonase 1 (PON1) genotypes (PON1192 and PON1-108) and PON1 substrate-specific activities in mothers and their newborns to examine whether PON1 may affect organophosphate pesticide measurements in blood and urine.
Chlorpyrifos levels in plasma ranged from 0-1726 ng/mL and non-zero levels were measured in 70.5% and 87.5% of maternal and cord samples, respectively. Diazinon levels were lower (0-0.5 ng/mL); non-zero levels were found in 33.3% of maternal plasma and 47.3% of cord plasma. Significant associations between organophosphate pesticide levels in blood and metabolite levels in urine were limited to models adjusting for PON1 levels. Increased maternal PON1 levels were associated with decreased odds of chlorpyrifos and diazinon detection (odds ratio(OR): 0.56 and 0.75, respectively). Blood organophosphate pesticide levels of study participants were similar in mothers and newborns and slightly higher than those reported in other populations. However, compared to their mothers, newborns have much lower quantities of the detoxifying PON1 enzyme suggesting that infants may be especially vulnerable to organophosphate pesticide exposures.
Keywords: paraoxonase, organophosphate pesticides, biomarkers, cord blood, maternal blood, urinary metabolites
1. Introduction
Organophosphorous pesticides are widely used in agriculture in the United States(DPR, 2008); despite the voluntary phase out of residential uses of chlorpyrifos and diazinon between 2000 and 2004 (U.S. EPA, 2000; U.S. EPA, 2001), some organophosphate pesticides are still registered for home garden use (U.S. EPA, 2006). Acute exposure to organophosphate pesticides can lead to neurotoxic effects through inhibition of the enzyme acetylcholinesterase (Costa et al., 2008). Recent epidemiologic studies suggest associations of low dose chronic prenatal exposure to organophosphate pesticides with adverse birth and neurodevelopmental outcomes including reduced birth weight and length (Whyatt et al., 2004), shorter gestational duration (Eskenazi et al., 2004), increased number of abnormal reflexes in neonates (Engel et al., 2007; Eskenazi et al., 2008), higher risk of reported attention problems (Marks et al., 2010), and lower intelligence in 7 year olds (Bouchard et al., 2011).
Although the majority of animal data provide evidence of organophosphate toxicity through cholinergic pathways, some studies suggest potential mechanisms for the adverse effects of organophosphate pesticide exposures, even at dose levels below the threshold for acetylcholinesterase inhibition (Costa, 2006). For instance, exposures to low doses of diazinon and/or chlorpyrifos in rat and or mouse models were associated with changes in neuronal cell development (Slotkin et al., 2008), changes in emotional behaviors (Roegge et al., 2008), up regulation of serotonin neurotransmitters(Aldridge et al., 2003; Slotkin et al., 2006), and changes in thyroid hormone levels and the reproductive system(Buratti et al., 2006; De Angelis et al., 2009; Haviland et al., 2010). Recent studies also provide evidence that organophosphate pesticide exposure induces oxidative stress (Samarawickrema et al., 2008; Slotkin and Seidler, 2009), a condition associated with common diseases like cardiovascular disease and diabetes (Bhattacharyya et al., 2008; Li et al., 2003).
Estimating the internal dose of organophosphate pesticide exposure in biological specimens is particularly challenging because organophosphate pesticides have relatively short half-lives and are quickly metabolized and excreted from the body (Wessels et al., 2003). Organophosphate metabolites, including dialkyl phosphates, in urine have been used as biomarkers of organophosphate pesticide exposure in many studies (Bouchard et al., 2010; Eskenazi et al., 2004; Fenske et al., 2002; Grandjean et al., 2006; Lacasana et al., 2010; Ye et al., 2009). Collection of urine specimens from study participants is relatively noninvasive and methods for analyzing organophosphate pesticide metabolites are well established (Bradman and Whyatt, 2005). Analysis of organophosphate pesticide levels in blood allows for direct measurement of parent compounds rather than metabolites and may more accurately represent the dose that reaches the target tissue (Bradman and Whyatt, 2005). Although the rate of clearance from the blood is initially quite rapid, chlorpyrifos and diazinon are lipophilic so the portion of compound that partitions into body fat may be eliminated more slowly (Eaton et al., 2008). Therefore, levels in blood may represent a steady state concentration (Needham, 2005). However, since concentrations of organophosphate pesticides in blood are much lower (by orders of magnitude) than metabolite levels in urine, very sensitive analytical methods are required to measure them (Perez et al., 2010). Thus far, only a small number of studies have measured prenatal organophosphate pesticide exposure in maternal or umbilical cord blood (Neta et al., 2010; Whyatt et al., 2003). Only one study has compared chlorpyrifos levels in blood and urine from the same subjects (mothers and infants) and reported no association between chlorpyrifos in maternal or cord blood and levels of the chlorpyrifos metabolite 3,5,6-trichloro-2-pyridinol in urine (Whyatt et al., 2009). Additionally, there are no published analytical methods for some organophosphate pesticides in blood, such as oxydemeton methyl and thus, blood measures may not fully capture exposure especially in populations exposed to multiple organophosphate pesticides. As there are strengths and weaknesses in using either of the two biological matrices, it remains unclear which measures will be more useful in epidemiological studies of prenatal organophosphate pesticide exposures and adverse health effects.
The PON1 enzyme can detoxify the oxon derivatives of some organophosphate pesticides and also acts as an antioxidant (James, 2006; Li et al., 2003). Individuals with low PON1 activity may be more susceptible to organophosphate pesticide exposures due to both decreased metabolic capacity towards organophosphate oxons and lower antioxidant defenses in comparison to those with average or high PON1 activities. In humans, PON1 enzymatic activities vary widely among adults (Deakin and James, 2004) and children (Chen et al., 2003; Huen et al., 2010), due in part to genetics (Costa et al., 2005; Deakin and James, 2004). For instance, a single nucleotide polymorphism (SNP) at position -108 in the promoter region of the PON1 gene is associated with two-fold higher levels of PON1 quantity for the PON1-108c allele compared to the PON1-108T allele (Deakin et al., 2003). The nonsynonomous coding SNP, PON1192, strongly affects substrate-specific catalytic efficiency. In vitro and in vivo studies have demonstrated that the PON1192R alloform can hydrolyze the organophosphate oxons chlorpyrifos-oxon and paraoxon more efficiently than the PON1192Q alloform, conferring a greater degree of protection from organophosphate pesticide exposures (Costa et al., 2003).
Previously, we measured urinary dialkyl phosphate metabolites of organophosphate pesticides in pregnant mothers from the Center for Health Assessment of Mothers and Children of Salinas (CHAMACOS) study and found that prenatal and postpartum metabolite levels were higher in these women than in women of childbearing age who participated in the National Health and Nutrition Examination Survey (NHANES) study (Bradman et al., 2005). In the present study, we measured levels of chlorpyrifos and diazinon in maternal and umbilical cord blood of CHAMACOS participants. Our primary aims were to describe the distribution of these measurements and compare them with urinary dialkyl phosphates in the same subjects. Since PON1 may affect an individual's ability to metabolize and excrete oxon derivatives of organophosphates, we also determined whether PON1 genotype and enzyme activity affect the levels measured in both biological matrices.
2. Materials and Methods
2.1 Study Population
The CHAMACOS Study is a longitudinal birth cohort study examining the effects of pesticide and other environmental exposures on children's neurodevelopment, growth, and respiratory disease (Eskenazi et al., 2003). The study is located in the Salinas Valley in Monterey County, CA, an intensively farmed region with approximately 200,000 kg of organophosphates applied annually (DPR, 2007). Women eligible to participate in the study were at least 18 years of age, spoke English or Spanish, qualified for Medicaid, were less than 20 weeks gestation, and were receiving prenatal care in one of six clinics serving the community. Participants were primarily Mexican-American, many of whom were born in Mexico. Six hundred and one pregnant women were enrolled in 1999-2000 and 526 delivered liveborn singleton newborns.
Organophosphate pesticides were measured in blood collected from mothers at the hospital shortly before delivery and in umbilical cord blood (n=234 and 256, respectively). Measurements were only made in those participants with sufficient blood volumes for the analysis. Heparinized whole blood was collected in BD vacutainers® (Becton, Dickinson and Company, Franklin Lakes, NJ), centrifuged, divided into plasma, buffy coats and red blood cells, and stored at −80°C. Serum and blood clots were collected in vacutainers containing no anticoagulant. DNA was isolated from clots as described previously (Holland et al., 2006).
Most of the mothers also provided urine specimens in the peripartum period after delivery, which were used to measure urinary dialkyl phosphate metabolites (n=221). PON1 genotypes were ascertained in 221 mothers and 244 children (PON1192 and PON1-108). In addition, 219 of these women and 236 of the newborns had adequate samples available for PON1 enzyme activity measurements in maternal and umbilical cord blood.
Study protocols were approved by the University of California, Berkeley Committee for Protection of Human Subjects. Written informed consent was obtained from all mothers.
2.2 Pesticide Exposure Measurement
2.2.1 Organophosphate Pesticide Parent Compound in blood
Frozen aliquots of heparinized plasma were shipped to the Center for Disease Control and Prevention (CDC) for analysis of parent organophosphate pesticides in maternal and umbilical cord blood on dry ice. Chlorpyrifos, diazinon, and several pyrethroid pesticides were measured in plasma specimens using solid phase extraction and gas chromatography-high resolution mass spectrometry as described previously (Perez et al., 2010). (This paper focuses solely on measurements of the organophosphorous pesticides diazinon and chlorpyrifos). Pesticide measurement results were reported for all samples and fell into three categories: 1) non-detect for which no signal was detected, 2) detectable concentrations that were below the instrument limit of quantification (Armbruster and Pry, 2008) and 3) quantifiable concentrations above the limit of quantification. Data were reported as detectable only when a peak was visibly detectable and the signal to noise ratio was greater than three. The limit of quantification for chlorpyrifos and diazinon were determined by regression techniques. The standard deviation of known concentrations was plotted against their measured values and the y-intercept of the line was extrapolated to derive the estimated standard deviation at zero concentration (s0). The limit of quantification was calculated as 3 s0. Concentrations below the limit of quantification can be detected by the instrument but have higher relative standard deviations than those above the limit of quantification (relative standard deviation=30-40% versus 10-15%, respectively) and should therefore be considered relevant measurements for use in subsequent analyses. The instrument limit of quantifications for individual samples were 21 pg/mL and 16 pg/mL for chlorpyrifos and diazinon, respectively. These quantification limits were higher than limits reported in Whyatt et al (2003), primarily due to (1) inclusion of the pyrethroid target analytes, which reduced sensitivity to chlorpyrifos and diazinon in the elution window and, (2), aging of the equipment.
2.2.2 Urinary Metabolites
For analysis of organophosphate pesticide metabolites, spot urine samples were collected from mothers soon after delivery and stored at -80°C until shipment to the CDC. Urine specimens were analyzed using gas chromatography-tandem mass spectrometry and dialkyl phosphate metabolites were then quantified using isotope dilution calibration (Bravo et al., 2002). Details of laboratory measurements and quality control are described by Bradman et al. (2005).
A total of six dialkyl phosphate metabolites were quantified: three dimethyl phosphate metabolites (dimethylphosphate, dimethyldithiophosphate, dimethylthiophosphate) and three diethyl phosphate metabolites (diethylphosphate, diethyldithiophosphate, diethylthiophosphate). Dimethyl phosphates are derived from pesticides such as malathion, oxydemeton-methyl, and dimethoate and diethyl phosphates are derived from pesticides such as diazinon, chlorpyrifos, and disulfoton. This data analysis is limited to diethyl phosphate metabolites because both of the organophosphates measured in blood, chlorpyrifos and diazinon, devolve into diethyl phosphates. Diethyl phosphate concentrations were summed on a molar basis to generate a variable for total diethyl phosphates. For eight women, levels of one urinary metabolite could not be calculated due to analytic interference. Because metabolites were correlated within the diethyl phosphate group, regression was used to impute the value of the missing metabolite based on the concentrations of the other two metabolites.
2.3 PON1 Genotypes and Enzyme Activity
PON1 polymorphisms were genotyped as previously described (Holland et al., 2006; Huen et al., 2009a). Briefly, the coding polymorphism PON1192 was analyzed using the Taqman real-time PCR method. Primers for the nucleotide sequence flanking the SNP, and probes specific for the SNPs, were custom-designed by Applied Biosystems, Inc. (Foster City, CA). The promoter SNP, PON1-108, was genotyped using a fluorogenic allele-specific assay (Amplifluor, Chemicon, Temecula, CA). It required a two-part nested PCR strategy, in which the region surrounding the SNP was pre-amplified using non-allelic flanking primers and then the amplicon was diluted and used as the template for the Amplifluor assay. Quality assurance procedures for genotyping of these PON1 SNPs included assessment of randomly distributed blank samples in each plate and duplicates of randomly selected samples with independently isolated DNA from the same subjects. Repeated analysis (4% of samples) in several runs showed a high degree (>99%) of concordance. All discrepancies were resolved with additional genotyping.
We measured PON1 enzyme activities against three different substrates (paraoxon (PO), phenyl acetate (ARY), and chlorpyrifos-oxon (CPO)) in plasma samples using spectrophotometric methods as described previously (Huen et al., 2009b). In this paper, we use these three measurements as markers of PON1 molecular phenotype. The arylesterase assay serves as an indirect measure of PON1 enzyme quantity. ELISA and Western blot based methods have been used to demonstrate a high correlation of PON1 quantity with arylesterase activity (r > 0.85) (Connelly et al., 2008; Kujiraoka et al., 2000). In contrast, the paraoxonase and chlorpyrifos-oxonase substrate-specific activities reflect both quantity and catalytic efficiency of the enzyme. All assays were performed in triplicate. Quality assurance included assessment of repeat samples (separate aliquots of the same sample run on different days) and internal controls (aliquots of the same sample run on all assay plates). We found a high degree of concordance between repeated samples (3% of samples were repeated). The average relative standard deviation for repeated samples ranged from 6-9% and the correlation coefficients between repeated runs were between 0.91-0.98 for all three assays. The same internal control samples were used on every plate for every assay and the inter-assay variability (average relative standard deviation) for these samples ranged from 7 to 9%.
2.4 Statistical Analysis
To determine whether there were systematic differences between participants and non-participants in this study, we performed logistic regression to assess whether missingness was associated with numerous variables related to sociodemographics, exposures, and genotype frequency including: mother's country of birth, length of time in the United States, sex of the child, poverty levels, alcohol and tobacco use during pregnancy, and maternal work in agriculture.
To examine the relationship between organophosphate pesticides in maternal and umbilical cord blood (chlorpyrifos or diazinon), we first computed Pearson's correlation coefficients (r). Fischer's transformation was used to compute a 95% confidence interval for the correlation. Chlorpyrifos and diazinon levels were expressed continuously. To deal with the high proportion of non-detectable concentrations, we used a multiple imputation procedure to estimate our coefficients of interest. We employed a variation of the method described by Lubin et al. (2004) which randomly imputes values less than the limit of quantification for all non-detects based on a log-normal probability distribution whose parameters were determined by maximum likelihood estimation. We performed 20 imputation cycles, generating 20 individual imputed data sets for which parameter estimates and standard errors were calculated. We combined the 20 individual estimates to obtain the multiple imputation estimate using Rubin's rules as described in Schafer et al (2002). This method has been shown to yield reasonable estimates when detection frequencies are > 70%; when detection frequencies are <70% — which is the case for diazinon — this method produces unbiased parameter estimates but may yield biased variance estimates.
In subsequent analyses, blood organophosphate levels were expressed both continuously using the multiple imputation procedures described above and categorically. They were classified categorically in three ways: (1) as (a)nondetectable and (b)detectable (includes both detectable levels <limit of quantification and levels>limit of quantification) values; (2) as (a)nondetectable, (b) signal detected but <limit of quantification, (c)and >limit of quantification; and (3) as values (a)above and (b)below the limit of quantification (only for chlorpyrifos). Results were similar using all classifications, therefore only the data for nondetectable versus detectable (categorical classification # 1) values are shown.
Logistic regression models were constructed to examine the association between PON1 genotypes (PON1192 and PON1-108), enzyme activities (arylesterase, chlorpyrifos-oxonase, and paraoxonase), or PON1 status (arylesterase and PON1192) and the dependent variable, chlorpyrifos or diazinon detection (nondetectable vs detectable levels). PON1 status, which accounts for both PON1 enzyme quantity and PON1192 genotype, can be more informative than looking at PON1 genotype alone in epidemiologic studies (Richter and Furlong, 1999). Odds ratios (ORs) for arylesterase, chlorpyrifos-oxonase, and paraoxonase were expressed as a change in odds of chlorpyrifos or diazinon detection per increase by one standard deviation of enzyme activity (arylesterase, chlorpyrifos-oxonase, or paraoxonase). In models testing for associations with PON1 genotype, odds ratios were expressed as the odds of chlorpyrifos or diazinon detection compared to the reference group (PON1192QQ or PON1-108cc). For the models including PON1 status, PON1192 genotype was coded 0, 1, or 2, for the number of R alleles. We included an interaction term for arylesterase x PON1192, however the likelihood ratio test indicated that this interaction was not significant (p>0.20) and results reported are for models without the interaction terms.
We constructed separate regression models to examine the relationship between PON1 genotypes, enzyme activities or status (e.g. arylesterase and PON1192 in the same model) with levels of maternal urinary diethyl phosphates (dependent variable expressed continuously). Diethyl phosphate measurements (nmol/L) were log10 transformed to normalize their distribution. We also used linear regression models to determine the relationship between organophosphate pesticides in blood (chlorpyrifos and diazinon, expressed categorically as nondetectable versus detectable levels) and urinary diethyl phosphates (expressed continuously) in an unadjusted model. Since urinary metabolites were not measured in newborns, these models were only constructed for maternal measurements.
To determine whether PON1 modifies the association between organophosphate pesticides in blood on urinary dialkyl phosphates, additional regression models were constructed where urinary dialkyl phosphates (expressed continuously) was the dependent variable and the independent variables included organophosphate pesticides in blood (chlorpyrifos or diazinon, expressed categorically as nondetectable versus detectable levels) and either PON1 genotypes (coded as 0, 1, or 2 for the number of PON1192R or PON1-108T alleles), activity (arylesterase, chlorpyrifos-oxonase, or paraoxonase expressed continuously), or status (arylesterase and PON1192). In additional models, we incorporated an interaction term between the genotype or enzyme activity and chlorpyrifos or diazinon detection (nondetectable vs. detectable levels). Models investigating PON1 status included three 2-way interaction terms (arylesterase x organophosphates in blood, arylesterase x PON1192 genotype, and PON1192 genotype x organophosphates in blood) and one three-way interaction term (arylesterase x PON1192 genotype x organophosphates in blood) all in the same model. Interaction terms remained in the model if the f-test comparing the full model with interaction terms to the nested model with no interaction terms was statistically significant (p<0.20). We also used regression models to examine the association between organophosphates in blood and urinary dialkyl phosphates stratifying by tertile of PON1 enzyme activity (interaction between organophosphates in blood and tertiles of PON1 enzyme activity).
All statistical analyses were performed using STATA 11.1 (College Station, TX).
3. Results
There were no differences in those women and children who had sufficient plasma available for organophosphate measurements compared to those who did not except that mothers who gave birth to girls were more likely to have sufficient specimen volumes (OR(95% confidence interval (CI)):1.58(1.12,2.22)). Demographic characteristics of CHAMACOS mothers and their children with adequate specimen for analysis are described in Table 1. Mothers were primarily young (mean ± standard deviation:25.6 ±5.3 years), married, low-income, Mexican-born, and Spanish-speaking. Many were farm workers themselves (40%) and/or lived with farm workers at the time of enrollment (83%).
Table 1. Demographic and Exposure Characteristics of CHAMACOS Participants.
| Na | % | |
|---|---|---|
| Country of Birth | ||
| Mexico | 315 | 84.7 |
| US | 49 | 13.2 |
| Other | 8 | 2.2 |
| Household Income | ||
| At or below Poverty Level | 208 | 59.4 |
| Within 200% Poverty Level | 131 | 37.4 |
| Above 200% Poverty Level | 11 | 3.1 |
| Infant Sex | ||
| Boy | 174 | 46.8 |
| Girl | 198 | 53.2 |
| Lived with agriculture worker during pregnancy | ||
| Yes | 311 | 83.8 |
| No | 60 | 16.2 |
| Work Status During Pregnancy | ||
| Did not work | 129 | 35.3 |
| Some field work | 98 | 26.9 |
| Some agriculture work | 49 | 13.4 |
| Other work only | 89 | 24.4 |
| Lived within 200ft of a field | ||
| Yes | 45 | 12.1 |
| No | 327 | 87.9 |
Total number of observations vary due to missing data (table entries do not include imputed values).
3.1 Organophosphate parent compound concentration data
Table 2 presents the means, ranges and percentiles for parent chlorpyrifos and diazinon measured in maternal and umbilical cord blood plasma. The percent of detectable chlorpyrifos and diazinon concentrations was slightly higher in cord blood (87.5% and 47.3%) than in maternal blood (70.5% and 33.3%), whereas the percent >limit of quantification was higher in maternal blood for chlorpyrifos (17.9% and 11.3%, respectively) and identical for maternal and cord blood for diazinon (1.7%). There was a relatively narrow range of diazinon levels in both newborns and mothers from non-detect - 0.5 and nondetect - 0.03 ng/mL in mothers and newborns, respectively. Chlorpyrifos levels in blood ranged from nondetect -1385 ng/mL in mothers and from nondetect -1726 ng/mL in newborns. Among mothers, there was one extreme outlier (1385 ng/mL). There was also one extreme outlier among newborns (1726 ng/mL). These levels are more than 100-fold greater than levels measured at the 95th percentile. The mother and newborn were unrelated. The mother with the high level did not work in agriculture but pesticides were found in her home during pregnancy; interestingly her newborn did not have unusually high organophosphate levels in blood. For the newborn with the high level, maternal levels were not measured; pesticides were found in the home and agriculture workers lived in the home. All subsequent analyses do not include these two extreme outliers.
Table 2. Concentrations of Chlorpyrifos and Diazinon (ng/mL) in Plasma and Diethyl Phosphates (nmol/L) in Urine from Mothers and Newborns.
| N | Percent Detectablea |
Percent> Limit of quantificationb |
Range | Percentilec | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | 90 | 95 | |||||
| Mothers at Delivery | |||||||||
| Chlorpyrifos | 234 | 70.5 | 17.9 | nondetect-1385.1 | nondetect | <limit of quantification | <limit of quantification | 0.07 | 0.40 |
| Diazinon | 234 | 33.3 | 1.7 | nondetect-0.5 | nondetect | nondetect | <limit of quantification | <limit of quantification | <limit of quantification |
| Diethyl phosphates | 347 | 99.8 | 99.8 | 1.7-596.5 | 11.8 | 28.5 | 61.5 | 128.8 | 162.3 |
| Newborn children | |||||||||
| Chlorpyrifos | 256 | 87.5 | 11.3 | nondetect-1726.1 | <limit of quantification | <limit of quantification | <limit of quantification | 0.03 | 1.33 |
| Diazinon | 256 | 47.3 | 1.7 | nondetect-0.03 | nondetect | nondetect | <limit of quantification | <limit of quantification | <limit of quantification |
Detectable values include all non-zero values both above and below the limit of quantification.
Limit of quantification for the instrument method used to measure chlorpyrifos and diazinon in plasma was 0.021 and 0.016 ng/mL, respectively.
For descriptive purposes, only values greater than the instrument limit of quantification are reported.
Maternal blood levels of chlorpyrifos were not correlated with levels of diazinon (r(95%CI)=0.07(-0.11, 0.24)) nor were they highly correlated in cord blood (r(95%CI)=0.14(-0.04,0.33)). Additionally, organophosphate pesticide levels were not correlated among mother-newborn pairs (n=125;r(95%CI) = 0.05(-0.16,0.26) and 0.06(-0.18, 0.29) for chlorpyrifos and diazinon, respectively).
3.2 Demographic and exposure characteristics
Blood organophosphate levels (nondetectable vs detectable) did not differ significantly by most demographic characteristics including sex of the child and poverty level. Determinants that could potentially affect exposure to pesticides such as whether the mother worked in agriculture, lived with an agriculture worker, or lived near a field during pregnancy were not associated with blood organophosphates in mothers or newborns.
3.3 Organophosphate metabolites (dialkyl phosphates) in urine and comparisons to organophosphate parent compound in blood
Means and ranges for diethyl phosphate metabolites in urine are presented in Table 2. Diethyl phosphate levels ranged from 1.7-596.5 nmol/L with a median of 28.5 nmol/L. While over 99.8% of mothers have diethyl phosphates measurements above the limit of quantification, much fewer mothers and newborns had quantifiable levels of chlorpyrifos and diazinon in their blood. We observed no significant association between detection of chlorpyrifos and diazinon in maternal blood and postpartum diethyl phosphate metabolites in maternal urine (Table 3).
Table 3. Associations between Organophosphates in Plasma (Maternal and Cord Blood) and Postpartum Urinary Diethyl Phosphates (nmol/L).
| Maternal Diethyl Phosphate Metabolites (nmol/L) | ||
|---|---|---|
| N | Change in Urinary Diethyl Phosphates for Detectable versus Nondetectable Plasma Organophosphate Levels (95% Confidence Interval) | |
| Maternal Diazinon (Nondetect vs Detectable) | 221 | -0.02(-0.17,0.13) |
| Cord Diazinon (Nondetect vs Detectable) | 246 | -0.04(-0.18,0.10) |
| Maternal Chlorpyrifos (Nondetect vs Detectable) | 220 | 0.01(-0.15,0.16) |
| Cord Chlorpyrifos (Nondetect vs Detectable) | 245 | -0.03(-0.24,0.18) |
3.4 PON1 genotypes and activities
PON1 genotypes and substrate-specific activities in CHAMACOS mothers and their newborns have been described previously (Holland et al., 2006; Huen et al., 2010). For the subset of subjects with measured organophosphate pesticides in blood presented in this paper, all PON1 values were similar to those assessed for the larger subset of the CHAMACOS cohort (n=336 cords and n=382 mothers). Allele frequencies in both mothers and their children were close to 0.5 for both SNPs (PON1-108 and PON1192). Arylesterase ranged from 27-346 U/mL in mothers and 4-98 U/mL in newborns. Chlorpyrifos-oxonase ranged from 1,766-14,676 U/L and 154-5,255 U/L in mothers and newborns, respectively. Maternal paraoxonase measures ranged from 75 to 3,538 U/L and levels in newborns ranged from 7 to 1,018 U/L. For all three substrate-specific activities, maternal measures were on average four-fold higher than those in newborns.
3.5 Organophosphate pesticides in blood and PON1
Maternal PON1 activity was inversely associated with the odds of organophosphate pesticide detection in cord blood (Table 4). An increase of maternal arylesterase and chlorpyrifos-oxonase by one standard deviation (44.5 U/mL and 2235.4 U/L, respectively) was associated with a decreased odds of detectable chlorpyrifos levels in cord blood (OR(95% CI)=0.56 (0.39-0.82) and 0.50 (0.33-0.77), respectively), suggesting that increased maternal PON1 activity may result in a lower internal dose to the fetus as measured in cord blood. A similar trend was seen for diazinon, i.e., a decreased odds of detection in cord blood with an standard deviation increase of arylesterase, chlorpyrifos-oxonase, and paraoxonase activities; however only the association with paraoxonase reached statistical significance. We did not observe any association between maternal PON1 enzyme activity and presence of organophosphate pesticides in maternal blood or between newborn PON1 activity and detectable organophosphate pesticides in cord blood.
Table 4. Odds ratios for presence of organophosphates in plasma associated with PON1 activities in mothers (n=218) and newborns (n=223).
| Detectable Diazinon | Detectable Chlorpyrifos | |||
|---|---|---|---|---|
| Maternal Blood Odds Ratio(95% Confidence Interval) | Cord Blood Odds Ratio(95% Confidence Interval) | Maternal Blood Odds Ratio(95% Confidence Interval) | Cord Blood Odds Ratio(95% Confidence Interval) | |
| Maternal Arylesterase Activity | 0.93(0.70,1.23) | 0.76(0.57,1.01) | 1.16(0.86,1.56) | 0.56(0.39,0.82) |
| Cord Arylesterase Activity | — | 0.97(0.76,1.24) | — | 0.92(0.62,1.37) |
| Maternal Chlorpyrifos-oxonase Activity | 0.90(0.67,1.20) | 0.79(0.6,1.04) | 1.21(0.89,1.64) | 0.50(0.33,0.77) |
| Cord Chlorpyrifos-oxonase Activity | — | 1.01(0.79,1.29) | — | 0.97(0.65,1.46) |
| Maternal Paraoxonase Activity | 1.07(0.8,1.43) | 0.75(0.57,1.00) | 1.18(0.86,1.61) | 0.87(0.58,1.29) |
| Cord Paraoxonase Activity | — | 1.03(0.80,1.32) | — | 1.18(0.76,1.82) |
Odds ratios are for a standard deviation change in PON1 Activity. The standard deviation for maternal arylesterase. chlorpyrifos-oxonase, and paraoxonase activities was 44.5 U/mL, 2235.4 U/L, and 621.6 U/L, respectively. The standard deviation for cord arylesterase, chlorpyrifos-oxonase, and paraoxonase activities was 14.5 U/mL, 860.7 U/L,and 162.4 U/L, respectively.
For PON1 genotypes, we found a non-significant lower odds of detection of diazinon in cord blood among mothers with the PON1192R allele compared to mothers with PON1192Q ((OR(95% CI) for PON1192RR versus PON1192QQ: 0.56(0.28-1.14)). We found no difference in odds of detection of chlorpyrifos in cord blood by maternal PON1192 genotype. No significant associations were found between blood chlorpyrifos or diazinon and PON1-108 genotype. We found no associations between maternal genotypes and the presence of organophosphates in maternal blood or between child genotypes and organophosphates in cord blood.
To consider the effect of PON1 status (Costa et al., 2005; Richter and Furlong, 1999) on chlorpyrifos and diazinon levels in blood, we included both arylesterase and PON1192 genotype in the same model. We again observed a significant association between increasing maternal arylesterase and decreased odds of both cord chlorpyrifos and diazinon detection (OR(95%CI):0.56(0.39,0.82) and 0.76(0.57,1.01), respectively). After adjusting for maternal arylesterase activity, we also observed a decreased odds of diazinon levels in cord blood if mothers had the PON1192R allele (OR(95% CI)= 0.65(0.44-0.95)), confirming the suggestive trend yielded in the models examining just PON1192 genotype (without arylesterase in the model); the same association was not seen for chlorpyrifos levels in cord blood. The interaction between PON1192 and arylesterase was not significant in any of the models. Similar to the models of PON1 activity or genotype alone, we found no associations of maternal PON1 status with organophosphate pesticides in maternal blood and no associations of newborn PON1 status with organophosphate pesticides in cord blood. Since both arylesterase and PON1192 were significant in the same model, these results suggest that maternal PON1 status, which considers both PON1 quantity and quality (catalytic efficiency due primarily to PON1192), may be protective and is associated with non-detection of diazinon but not chlorpyrifos in cord blood.
3.6 Maternal urinary organophosphate metabolites, organophosphate pesticides in blood, and PON1
We found no association of maternal PON1 genotypes, activities, or status (arylesterase and PON1192 genotype) with maternal diethyl phosphates measured around the time of delivery. As mentioned earlier, we observed no association between maternal diethyl phosphates and chlorpyrifos or diazinon detection in blood using crude models; however after adjusting for maternal arylesterase activity, we found a positive association (change in diethyl phosphates(95%CI) =0.61(0.13, 1.10)) between diazinon presence in maternal plasma and postpartum diethyl phosphates (Table 5) and a significant interaction between diazinon and arylesterase activity (change in diethyl phosphates (95%CI)=-0.20(-0.35,-0.05)); a similar trend was found for chlorpyrifos detection however its association with diethyl phosphates was not significant. When we categorized chlorpyrifos levels as above and below the limit of quantification (data not shown), we found similar results (change in diethyl phosphates (95%CI) = 0.67(-0.06,1.41)).
Table 5. Association of Chlorpyrifos and Diazinon Detection in Plasma and PON1 Activities on Maternal Urinary Diethyl Phosphate Metabolites (n=206).
| Diethyl Phosphate Metabolites (nmol/L) | ||
|---|---|---|
| Change in Urinary Diethyl Phosphates (95% Confidence Interval) | ||
| Diazinon | ||
| Arylesterase | ||
| Diazinon(Nondetect vs Detectable) | 0.61(0.13,1.09) | |
| Maternal Arylesterase Activity | 0.05(-0.05,0.14) | |
| Diazinon x Arylesterase Activity | -0.20(-0.35,-0.05) | |
| Chlorpyrifos-oxonase | ||
| Diazinon(Nondetect vs Detectable) | 0.002(-0.15,0.16) | |
| Maternal Chlorpyrifos-oxonase Activity | 0.003(-0.07,0.08) | |
| Paraoxonase | ||
| Diazinon(Nondetect vs Detectable) | -0.002(-0.16,0.15) | |
| Maternal Paraoxonase Activity | 0.07(-0.010,0.14) | |
| Chlorpyrifos | ||
| Arylesterase | ||
| Chlorpyrifos(Nondetect vs Detectable) | 0.46(-0.14,1.06) | |
| Maternal Arylesterase Activity | 0.09(-0.08,0.27) | |
| Chlorpyrifos x Arylesterase Activity | -0.15(-0.35,0.04) | |
| Chlorpyrifos-oxonase | ||
| Chlorpyrifos(Nondetect vs Detectable) | 0.01(-0.16,0.17) | |
| Maternal Chlorpyrifos-oxonase Activity | 0.004(-0.07,0.08) | |
| Paraoxonase | ||
| Chlorpyrifos (Nondetect vs Detectable) | -0.003(-0.17,0.16) | |
| Maternal Paraoxonase Activity | 0.07(-0.01,0.14) | |
Change in urinary diethyl phosphates for PON1 Activity and for interaction terms are expressed per standard deviation change in PON1 Activity. The standard deviations for maternal arylesterase. chlorpyrifos-oxonase, and paraoxonase activities were 44.5 U/mL, 2235.4 U/L, and 621.6 U/L, respectively.
We also modeled the association of organophosphate detection in maternal blood with diethyl phosphates stratified by tertile of arylesterase activity to better understand the significant interaction between arylesterase and presence of organophosphates in blood (Figure 1). The association between diazinon and diethyl phosphates was positive but non-significant for the lowest two tertiles. However, a statistically significant negative association was present for the highest tertile of arylesterase activity (change in diethyl phosphates (95%CI):-0.36(-0.64,-0.07). These data indicate that for mothers with higher levels of arylesterase, we see lower diethyl phosphate metabolites in urine as diazinon in plasma increases. Similar albeit weaker trends were also seen with detectable chlorpyrifos levels in plasma (change in diethyl phosphates(95%CI):-0.32(-0.65, 0.01)) for the highest tertile; no significant associations for the lowest and medium tertiles).
Figure 1.
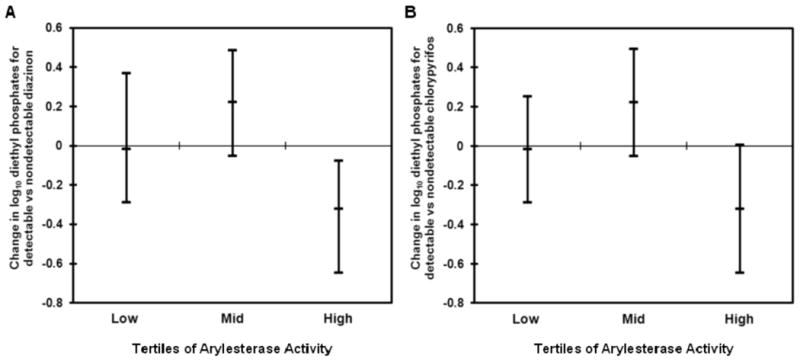
Association of detectable (A) diazinon and (B) chlorpyrifos (non-detect/detect; independent variable) in maternal plasma and log10 diethyl phosphates (dependent variable) and stratified by low, mid, and high arylesterase activity. The change in log10 diethyl phosphates for detectable versus nondetectable plasma organophosphate levels and their 95% confidence intervals are plotted for each tertile of arylesterase activity. We observed positive but nonsignificant associations between diethyl phosphates and diazinon in the first two tertiles of arylesterase. However, in the highest tertile of arylesterase activity, there was a negative association between diethyl phosphates and maternal diazinon in plasma (change in diethyl phosphates (95%CI):-0.36(-0.64,-0.07). Similarly, we found arylesterase significantly modifies the association between diethyl phosphates and diazinon in maternal plasma (change in diethyl phosphates(95%CI):-0.20(-0.35,-0.05)) in the full regression model including the following covariates: maternal diazinon detection frequency, maternal arylesterase (U/mL) activity, and the interaction term. Similarly, for chlorpyrifos, we saw an inverse relationship with diethyl phosphates in the highest tertile of arylesterase (change in diethyl phosphates(95%CI):-0.32(-0.65, 0.01)).
4. Discussion
In this study, we measured levels of chlorpyrifos and diazinon in maternal and umbilical cord blood collected at the time of delivery from a primarily Mexican-American cohort from the agricultural region of the Salinas Valley, California. To our knowledge, no other studies have examined the association of maternal or newborn PON1 genotype or enzyme activities on levels of organophosphate pesticides in blood and metabolites in urine. We found that elevated maternal PON1 enzyme activities may protect the fetus resulting in lower levels of chlorpyrifos and diazinon measured in umbilical cord blood.
Few studies have measured organophosphate pesticides in maternal or cord blood. Neta et al (2010) used the same analytical method that we employed in our study to measure chlorpyrifos and diazinon in cord serum in an urban population from Baltimore, Maryland. No newborns had diazinon above the limit of quantification and only 3% had chlorpyrifos levels above the limit of quantification (range: <limit of quantification - 0.014 ng/mL), indicating lower exposures in this population compared to CHAMACOS newborns. Whyatt et al (2003), utilizing laboratory methods with lower limits of detection (0.5-1.1 pg/mL and 0.5-1.6 pg/mL for chlorpyrifos and diazinon, respectively) in 211 newborns from a minority cohort in New York City, reported ranges of <limit of quantification - 67 pg/mL and 14 pg/mL for chlorpyrifos and diazinon, respectively. While the limit of quantification was higher in our study, levels above the 93rd percentile of our distribution exceeded the maximum levels measured in their study, suggesting higher exposures in some CHAMACOS participants; medians levels were also higher in CHAMACOS mothers and newborns than in the NYC study, however the CHAMACOS medians (0.006 and 0.004 pg/mL) were below the instrument limit of quantification. Although Whyatt et al.(2004) reported a strong correlation between maternal and cord blood chlorpyrifos levels, we did not. Neither study – ours or that reported by Whyatt et al. (2009) – observed a significant crude correlation between maternal urinary metabolites with organophosphates in maternal or cord blood. However, after adjusting for maternal arylesterase activity, we did observe a positive association between diazinon presence in maternal plasma and postpartum diethyl phosphates.
Both the Whyatt et al (2003; 2009) and the Neta et al studies (2010) were conducted in urban cohorts where exposure was predominantly due to indoor organophosphate pesticide use; this use declined after a voluntary residential restriction in 2000 (Whyatt et al., 2003). However, both pesticides were widely used in agriculture at the time of cord blood collection for this study (1999-2000). In fact, while overall use of organophosphate pesticides has decreased in the state of California, in the Salinas Valley where our study participants work and live, organophosphate pesticide use has been stable between 1999 and 2006 (DPR, 2008).
Only a few studies of pharmacokinetics of chlorpyrifos and/or diazinon are available in humans (Eaton et al., 2008). Two small studies of human volunteers (n=6 and n=12) have measured chlorpyrifos in blood and urine following controlled oral exposures and reported a very rapid initial metabolism of chlorpyrifos (half-life close to 1 hr) but much slower elimination of metabolites (measured by appearance in the urine; half-life of 24-27 hr) (Nolan et al., 1984; Timchalk et al., 2002). For diazinon, urinary dialkyl phosphate levels were highest between 2 and 12 hours after exposure(Garfitt et al., 2002). However since chlorpyrifos is highly lipophilic, there is likely a second compartment whereby a portion of the chlorpyrifos dose which partitions into body fat and/or binds to plasma proteins may be eliminated more slowly and can reach a steady-state level. This may be especially relevant to levels of chlorpyrifos and diazinon in the plasma of CHAMACOS mothers and children who may be chronically exposed to low levels of organophosphate pesticides (Eaton et al., 2008).
Although tissue distribution of chlorpyrifos in humans is not well characterized (Eaton et al., 2008), the tissue distribution of chlorpyrifos in pregnant rats was described in maternal blood, liver, brain, placenta, and the fetus (Abdel-Rahman et al., 2002) providing evidence that indeed maternal exposure and PON1 activities or status could affect the presence of organophosphates in the fetus. This is consistent with our data showing an inverse association between maternal PON1 activity and detectable levels of chlorpyrifos and diazinon in umbilical cord blood, which suggest that maternal PON1 may protect the fetus and result in a lower internal dose. PON1 arylesterase activity may also play an important role in the relationship between organophosphate pesticide levels in blood and urinary dialkyl phosphates. We only observed associations between diethyl phosphates in urine and organophosphate pesticides in blood after adjusting for arylesterase activity and including an interaction term (organophosphate detection x arylesterase). In contrast to our expectation that given the same level of organophosphate detection, we would observe increased diethyl phosphate levels in individuals with higher PON1 arylesterase, our data suggest that at similar levels of blood organophosphate pesticides, those with higher arylesterase may excrete lower levels of dialkyl phosphates. However, it should be noted that these models accounted only for a small portion of the total variability in dialkyl phosphates (r2 = 0.04). Other factors that may contribute to urinary diethyl phosphate concentrations include activities of cytochrome P450's, which are also involved in the organophosphate detoxification pathway and other parts of the oxidative stress network.
This study has several limitations. Mothers who gave birth to girls were more likely to have adequate blood specimens available for use in this study, suggesting a potential for participation bias. However, we did not identify any factors associated with participation rates in children. Furthermore, the association between mother's participation and child sex may be spurious as biologically, it is unlikely that the sex of the child would affect whether or not blood was collected for a particular mother. Recent research suggests that urinary metabolites may reflect not only an individual's contact with pesticide parent compounds, but possibly exposure to preformed metabolites present in food or the environment (Lu et al., 2005; Quirós-Alcalá et al., 2012; Weerasekera et al., 2009; Zhang et al., 2008) thus overestimating organophosphate pesticide exposure. This study is also limited by the relatively high limit of quantification for the method we used to measure chlorpyrifos and diazinon in CHAMACOS blood specimens. The available CDC method, which included pyrethoid target analytes, resulted in higher detection limits compared to Whyatt et al. (2003), who utilized a more sensitive analytical method with quantification limits close to 1 pg/mL. Finally, maternal urinary metabolites were measured during the postpartum period; we previously showed urinary dialkyl phosphates to be particularly high during this time (Bradman et al., 2005). Therefore, our findings on the associations of urinary diethyl phosphates and presence of organophosphates in blood in relation to PON1 may not be generalizable to other time periods (e.g. not postpartum).
Measurements of organophosphate pesticides in blood are sometimes considered a better biomarker for organophosphate exposure than urine because they may reflect internal dose more directly (Wessels et al., 2003). However, for many organophosphates that devolve to dialkyl phosphates, such as oxydemeton-methyl, there are no published methods to measure the parent compounds in blood. Due to the constraints of the currently available analytical methods, we had limited power to detect chlorpyrifos and diazinon in blood. We chose to include detectable levels of chlorpyrifos and diazinon below the limit of quantification in our statistical analyses even though these concentrations have higher relative standard deviations. Overall, we observed similar results when analyses were repeated with values above the limit of quantification.
4.1 Conclusions
In summary, our study suggests that PON1 enzyme quantity modifies the fetal dose of chlorpyrifos and diazinon in women exposed to these pesticides. Given that exposures to these organophosphate pesticides has been associated with adverse health effects (Eskenazi et al., 2007; Marks et al., 2010; Whyatt et al., 2004), more research is needed to confirm our findings.
Highlights.
We measured organophosphate pesticides in blood and urine of mothers and newborns.
We determined paraxonase 1 genotypes and enzyme activities.
Chlorpyrifos was detectable in 70.5 and 87.5% of maternal and cord blood samples.
Increased maternal paraoxonase 1 levels resulted in lower organophosphate detection in cord blood.
Acknowledgments
We gratefully acknowledge the contributions of the CHAMACOS staff, students and staff from the Children's Environmental Health Laboratory and the Center for Environmental Research and Children's Health, community partners, and especially the CHAMACOS participants. We would also like to thank Dr. Raul Aguilar Schall and Dr Jonathan Chevrier for their help with statistical analyses.
Funding Sources: This publication was made possible by grant numbers R826886 and R82670901 from the U.S. Environmental Protection Agency (EPA) and R01ESO12503-03 and PO1 ES009605 from the National Institute of Environmental Health Science (NIEHS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS and the EPA.
Abbreviations
- CHAMACOS
Center for Health Assessment of Mothers and Children of the Salinas Valley
- CI
confidence interval
- EPA
Environmental Protection Agency
- NHANES
National Health and Nutrition Examination Survey
- OR
odds ratio
- PON1
paraoxonase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Karen Huen, Email: khuen@berkeley.edu.
Asa Bradman, Email: abradman@berkeley.edu.
Kim Harley, Email: kharley@berkeley.edu.
Paul Yousefi, Email: yousefi@berkeley.edu.
Dana Boyd Barr, Email: dbbarr@emory.edu.
Brenda Eskenazi, Email: eskenazi@berkeley.edu.
Nina Holland, Email: ninah@berkeley.edu.
References
- Abdel-Rahman AA, et al. Pharmacokinetic profile and placental transfer of a single intravenous injection of [(14)C]chlorpyrifos in pregnant rats. Arch Toxicol. 2002;76:452–9. doi: 10.1007/s00204-002-0366-2. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, et al. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ Health Perspect. 2003;111:1736–43. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29(Suppl 1):S49–52. [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya T, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–76. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, et al. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125:e1270–7. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect. 2011;119:1189–95. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, et al. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect. 2005;113:1802–7. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Whyatt RM. Characterizing exposures to nonpersistent pesticides during pregnancy and early childhood in the National Children's Study: a review of monitoring and measurement methodologies. Environ Health Perspect. 2005;113:1092–9. doi: 10.1289/ehp.7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, et al. Quantitation of dialkyl phosphate metabolites of organophosphate pesticides in human urine using GC-MS-MS with isotopic internal standards. J Anal Toxicol. 2002;26:245–52. doi: 10.1093/jat/26.5.245. [DOI] [PubMed] [Google Scholar]
- Buratti FM, et al. Foetal and adult human CYP3A isoforms in the bioactivation of organophosphorothionate insecticides. Toxicol Lett. 2006;167:245–55. doi: 10.1016/j.toxlet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Increased influence of genetic variation on PON1 activity in neonates. Environ Health Perspect. 2003;111:1403–9. doi: 10.1289/ehp.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly PW, et al. Development of an immunoblot assay with infrared fluorescence to quantify paraoxonase 1 in serum and plasma. J Lipid Res. 2008;49:245–50. doi: 10.1194/jlr.D700022-JLR200. [DOI] [PubMed] [Google Scholar]
- Costa L, et al. Paraoxonase (PON1) and Organophosphate Toxicity. In: Mackness B, editor. The Paraoxonases: Their Role in Disease Development and Xenobiotic Metabolism. Springer; 2008. pp. 209–220. [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Costa LG, et al. Measurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicity. Clin Chim Acta. 2005;352:37–47. doi: 10.1016/j.cccn.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Costa LG, et al. Paraoxonase (PON 1) as a biomarker of susceptibility for organophosphate toxicity. Biomarkers. 2003;8:1–12. doi: 10.1080/13547500210148315. [DOI] [PubMed] [Google Scholar]
- De Angelis S, et al. Developmental exposure to chlorpyrifos induces alterations in thyroid and thyroid hormone levels without other toxicity signs in CD-1 mice. Toxicol Sci. 2009;108:311–9. doi: 10.1093/toxsci/kfp017. [DOI] [PubMed] [Google Scholar]
- Deakin S, et al. Paraoxonase-1 promoter haplotypes and serum paraoxonase: a predominant role for polymorphic position - 107, implicating the Sp1 transcription factor. Biochem J. 2003;372:643–9. doi: 10.1042/BJ20021670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin SP, James RW. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin Sci (Lond) 2004;107:435–47. doi: 10.1042/CS20040187. [DOI] [PubMed] [Google Scholar]
- DPR. Pesticide Use Report, Annual 2007. Department of Pesticide Regulation, California Environmental Protection Agency; Sacramento, CA: 2007. [Google Scholar]
- DPR. Pesticide Use Report, Annual 2008. Department of Pesticide Regulation, California Environmental Protection Agency; Sacramento, CA: 2008. [Google Scholar]
- Eaton DL, et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol. 2008;38(Suppl 2):1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Engel SM, et al. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am J Epidemiol. 2007;165:1397–404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, et al. CHAMACOS, a longitudinal birth cohort study: lessons from the fields. J Childrens Healt. 2003;1:3–27. [Google Scholar]
- Eskenazi B, et al. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2004;112:1116–24. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–8. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, et al. Pesticide toxicity and the developing brain. Basic Clin Pharmacol Toxicol. 2008;102:228–36. doi: 10.1111/j.1742-7843.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Fenske RA, et al. Children's exposure to chlorpyrifos and parathion in an agricultural community in central Washington State. Environ Health Perspect. 2002;110:549–53. doi: 10.1289/ehp.02110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfitt SJ, et al. Exposure to the organophosphate diazinon: data from a human volunteer study with oral and dermal doses. Toxicol Lett. 2002;134:105–13. doi: 10.1016/s0378-4274(02)00178-9. [DOI] [PubMed] [Google Scholar]
- Grandjean P, et al. Pesticide exposure and stunting as independent predictors of neurobehavioral deficits in Ecuadorian school children. Pediatrics. 2006;117:e546–56. doi: 10.1542/peds.2005-1781. [DOI] [PubMed] [Google Scholar]
- Haviland JA, et al. Long-term sex selective hormonal and behavior alterations in mice exposed to low doses of chlorpyrifos in utero. Reprod Toxicol. 2010;29:74–9. doi: 10.1016/j.reprotox.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Holland N, et al. Paraoxonase polymorphisms, haplotypes, and enzyme activity in Latino mothers and newborns. Environ Health Perspect. 2006;114:985–91. doi: 10.1289/ehp.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, et al. Longitudinal changes in PON1 enzymatic activities in Mexican-American mothers and children with different genotypes and haplotypes. Toxicol Appl Pharmacol. 2010;244:181–9. doi: 10.1016/j.taap.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, et al. Developmental Changes in PON1 Enzyme Activity in Young Children and Effects of PON1 Polymorphisms. Environmental Health Perspectives. 2009a;117:1632–1638. doi: 10.1289/ehp.0900870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, et al. Validation of PON1 enzyme activity assays for longitudinal studies. Clin Chim Acta. 2009b;402:67–74. doi: 10.1016/j.cca.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RW. A long and winding road: defining the biological role and clinical importance of paraoxonases. Clin Chem Lab Med. 2006;44:1052–9. doi: 10.1515/CCLM.2006.207. [DOI] [PubMed] [Google Scholar]
- Kujiraoka T, et al. A sandwich enzyme-linked immunosorbent assay for human serum paraoxonase concentration. J Lipid Res. 2000;41:1358–63. [PubMed] [Google Scholar]
- Lacasana M, et al. Association between organophosphate pesticides exposure and thyroid hormones in floriculture workers. Toxicol Appl Pharmacol. 2010;243:19–26. doi: 10.1016/j.taap.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Li HL, et al. Paraoxonase gene polymorphisms, oxidative stress, and diseases. J Mol Med. 2003;81:766–79. doi: 10.1007/s00109-003-0481-4. [DOI] [PubMed] [Google Scholar]
- Lu C, et al. The presence of dialkylphosphates in fresh fruit juices: implication for organophosphorus pesticide exposure and risk assessments. J Toxicol Environ Health A. 2005;68:209–27. doi: 10.1080/15287390590890554. [DOI] [PubMed] [Google Scholar]
- Lubin JH, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691–6. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, et al. Organophosphate Pesticide Exposure and Attention in Young Mexican-American Children. Environmental Health Perspectives. 2010 doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham LL. Assessing exposure to organophosphorus pesticides by biomonitoring in epidemiologic studies of birth outcomes. Environ Health Perspect. 2005;113:494–8. doi: 10.1289/ehp.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta G, et al. Distribution and determinants of pesticide mixtures in cord serum using principal component analysis. Environ Sci Technol. 2010;44:5641–8. doi: 10.1021/es1009778. [DOI] [PubMed] [Google Scholar]
- Nolan RJ, et al. Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol Appl Pharmacol. 1984;73:8–15. doi: 10.1016/0041-008x(84)90046-2. [DOI] [PubMed] [Google Scholar]
- Perez JJ, et al. Measurement of pyrethroid, organophosphorus, and carbamate insecticides in human plasma using isotope dilution gas chromatography-high resolution mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010 doi: 10.1016/j.jchromb.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirós-Alcalá L, et al. Organophosphorous pesticide breakdown products in house dust and children's urine. Journal of Exposure Science and Environmental Epidemiology. 2012 doi: 10.1038/jes.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter RJ, Furlong CE. Determination of paraoxonase (PON1) status requires more than genotyping. Pharmacogenetics. 1999;9:745–53. [PubMed] [Google Scholar]
- Roegge CS, et al. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res Bull. 2008;75:166–72. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarawickrema N, et al. Fetal effects of environmental exposure of pregnant women to organophosphorus compounds in a rural farming community in Sri Lanka. Clin Toxicol (Phila) 2008;46:489–95. doi: 10.1080/15563650701837030. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–77. [PubMed] [Google Scholar]
- Slotkin TA, et al. Neonatal exposure to low doses of diazinon: long-term effects on neural cell development and acetylcholine systems. Environ Health Perspect. 2008;116:340–8. doi: 10.1289/ehp.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Oxidative stress from diverse developmental neurotoxicants: Antioxidants protect against lipid peroxidation without preventing cell loss. Neurotoxicol Teratol. 2009 doi: 10.1016/j.ntt.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, et al. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ Health Perspect. 2006;114:1542–6. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchalk C, et al. A Physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model for the organophosphate insecticide chlorpyrifos in rats and humans. Toxicol Sci. 2002;66:34–53. doi: 10.1093/toxsci/66.1.34. [DOI] [PubMed] [Google Scholar]
- Office of Prevention, P; Toxic Substances, editor. U.S. EPA, Chlorpyrifos. Revised Risk Assessment and Agreement with Registrants. Washington, DC: 2000. pp. 1–4. [Google Scholar]
- U.S. EPA. Office of Prevention, Pesticides, and Toxic Substances. Washington, DC: 2001. Diazinon Revised Risk Assessment and Agreement with Registrants; pp. 1–4. [Google Scholar]
- U.S. EPA. Office of Prevention, Pesticides, and Toxic Substances. Washington, DC: 2006. Reregistration eligibility decision (RED) for malathion. [Google Scholar]
- Weerasekera G, et al. A mass spectrometry-based method to measure dialkylphosphate degradation products of organophosphorous insecticides in dust and orange juice. J Environ Monit. 2009;11:1345–51. doi: 10.1039/b821841b. [DOI] [PubMed] [Google Scholar]
- Wessels D, et al. Use of biomarkers to indicate exposure of children to organophosphate pesticides: implications for a longitudinal study of children's environmental health. Environ Health Perspect. 2003;111:1939–46. doi: 10.1289/ehp.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111:749–56. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, et al. A biomarker validation study of prenatal chlorpyrifos exposure within an inner-city cohort during pregnancy. Environ Health Perspect. 2009;117:559–67. doi: 10.1289/ehp.0800041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, et al. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112:1125–32. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, et al. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa) Int J Hyg Environ Health. 2009;212:481–91. doi: 10.1016/j.ijheh.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, et al. Dialkylphosphates (DAPs) in fruits and vegetables may confound biomonitoring in organophosphorus insecticide exposure and risk assessment. J Agric Food Chem. 2008;56:10638–45. doi: 10.1021/jf8018084. [DOI] [PubMed] [Google Scholar]


