Abstract
Angiotensin II (Ang II) plays a major role in the pathogenesis of end-organ injury in hypertension via its diverse hemodynamic and nonhemodynamic effects. Erythroblastosis virus E26 oncogen homolog-1 (ETS-1) is an important transcription factor recently recognized as an important mediator of cell proliferation, inflammation, and fibrosis. In the present studies, we tested the hypothesis that ETS-1 is a common mediator of the renal proinflammatory and profibrotic effects of Ang II. C57BL6 mice (n=6 per group) were infused with vehicle (control), Ang II (1.4 mg/kg per day), Ang II and an ETS-1 dominant-negative peptide (10 mg/kg per day), or Ang II and an ETS-1 mutant peptide (10 mg/kg per day) via osmotic minipump for 2 or 4 weeks. The infusion of Ang II resulted in significant increases in blood pressure and left ventricular hypertrophy, which were not modified by ETS-1 blockade. The administration of ETS-1 dominant-negative peptide significantly attenuated Ang II-induced renal injury as assessed by urinary protein excretion, mesangial matrix expansion, and cell proliferation. Furthermore, ETS-1 dominant-negative peptide but not ETS-1 mutant peptide significantly reduced Ang II-mediated upregulation of transforming growth factor-β, connective tissue growth factor, and α-smooth muscle actin. In addition, ETS-1 blockade reduced several proinflammatory effects of Ang II, including macrophage infiltration, nitrotyrosine expression, and NOX4 mRNA expression. Our studies suggest that ETS-1 is a common mediator of the proinflammatory and profibrotic effects of Ang II-induced hypertensive renal damage and may result in the development of novel strategies in the treatment and prevention of end-organ injury in hypertension.
Keywords: angiotensin II, hypertension (kidney), ETS-1, extracellular matrix, physiology/pathophysiology, growth factors and cytokines, oxidative stress (kidney)
Maladaptive activation of the renin-angiotensin system plays a critical role in the pathogenesis of glomerulosclerosis and chronic kidney disease of different causes, including hypertension1 and diabetes mellitus.2 Angiotensin II (Ang II) is a potent systemic vasoconstrictor and modulator of renal microcirculation.3 In addition to these hemodynamic actions, Ang II activates downstream signaling cascades that trigger the increased production of several growth factors,4,5 cytokines,6 chemokines,7 and other mediators that stimulate mesangial cell hypertrophy and proliferation,8 extracellular matrix deposition,9 and inflammation.10,11 It is not clear, however, whether these effects of Ang II are mediated via the independent activation of multiple pathways or whether alternatively common mediators induce these diverse profibrotic and proinflammatory pathways in response to Ang II.
The ETS factors are a family of transcription factors that participate in the regulation of a wide variety of biological processes, including normal development and differentiation.12 Erythroblastosis virus E26 oncogen homolog-1 (ETS-1) is a member of the ETS transcription factor family involved in the expression of a variety of genes, including growth factors, chemokines, and adhesion molecules.13 ETS-1 is also a well-known proto-oncogene in the pathogenesis of several different types of cancer.14,15 Several studies have also supported the role of ETS-1 as a mediator of vascular inflammation,16 recruitment of inflammatory cells to the vessel wall, and proliferation and migration of vascular smooth muscle cells.17 In recent studies, we demonstrated that ETS-1 mediates the expression of proinflammatory cytokines and adhesion molecules that participate in the formation of neointima after balloon injury.18 In previous studies, we also demonstrated that ETS-1 in large part mediates the production of fibronectin in response to Ang II in cultured mesangial cells.19
Herein, we performed a series of studies to determine the role of ETS-1 in the proinflammatory and profibrotic effects of Ang II in the kidney in vivo, including cell proliferation, macrophage infiltration, mesangial expansion, oxidative stress, and fibrosis. We took advantage of the availability of an ETS-1 dominant-negative (ETS-1 DN) peptide, which competes with ETS-1 for binding to the target genes.20
Methods
Animals and Treatments
Eight-week-old male C57BL6 mice (Jackson Labs, Bar Harbor, ME) were divided into the following groups (n=6 per group): group 1, control mice infused with vehicle; group 2, mice treated with Ang II (1.4 mg/kg per day) for 28 days via osmotic minipump; group 3, mice treated with Ang II and ETS-1 DN (10 mg/kg per day) for 28 days via osmotic minipump; and group 4, mice treated with Ang II and ETS-1 mutant peptide (ETS-1 MU, 10 mg/kg per day) for 28 days via osmotic minipump. Additional groups of mice were also studied after 2 weeks of infusion of Ang II only or Ang II plus the ETS-1 DN or ETS-1 MU peptide. Details on the peptide sequences and synthesis are available in the online-only Data Supplement. A urine sample was collected before euthanasia and saved for protein and creatinine measurements. The mice were euthanized by exsanguinations and kidneys and hearts collected for subsequent analysis. The animal protocols were approved by the institutional animal care and use committee at the University of Alabama at Birmingham and were consistent with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Blood Pressure Measurements
We used a radio telemetry system (DSI, St Paul, MN) to monitor blood pressure in conscious mice. Blood pressure was measured at baseline and then weekly for 6 hours continuously until euthanasia. See the online-only Data Supplement for expanded methods.
Real-Time Quantitative Reverse Transcriptase Polymerase Chain Reaction Analysis of mRNA Expression
Total RNA was extracted from renal cortices with TRIzol (Invitrogen, Carlsbad, CA), treated with DNAase I and then purified with an RNA purification kit (Invitrogen). The protein- and DNA-free RNA was reverse-transcribed to cDNA (Invitrogen), amplified by PCR with specific primers (Table S1 in the online-only Data Supplement), and quantified using SYBR Green and a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA), as previously described.21 Levels of specific mRNAs were normalized using GAPDH as an internal control.
Immunofluroscence
Five-micrometer sections of kidney cortex were prepared from paraffin-embedded tissues. Sections were incubated with a rabbit antibody to ETS-1 (sc-350, Santa Cruz Biotechnology) or a rabbit antibody to nitrotyrosine (06–284, Upstate) at 4°C overnight. See the online-only Data Supplement for expanded methods.
Immunohistochemistry
The avidin-biotin-peroxidase immunohistochemical technique (ABC kit; Vector) was used to detect microphage infiltration, cell proliferation, and α-smooth muscle actin expression, using primary antibodies against macrophages (F4/80, Ab6640; Abcam), Ki67 (m7249; Dako), and α-smooth muscle actin (A2547; Sigma). See the online-only Data Supplement for expanded methods.
Morphometric Analysis
Light microscopy of periodic acid Schiff–stained kidney sections from the 4 experimental groups was used for morphometric analysis. See the online-only Data Supplement for expanded methods.
Western Blot
Western blot analysis was performed as described before.22 The blots were incubated with the primary antibodies against ETS-1 (sc-350; Santa Cruz Biotechnology), transforming growth factor-β (TGF-β, MAB1835; R&D), or connective tissue growth factor (CTGF, sc-14940; Santa Cruz Biotechnology) at 4°C for 24 or 48 hours. The blots were washed and incubated with secondary antibodies and the signal detected by luminol chemiluminescence. See the online-only Data Supplement for expanded methods.
Statistical Analysis
Results are expressed as mean±SEM. The data were evaluated by 1-way or 2-way ANOVA. When the overall F test result of ANOVA was significant, a multiple-comparison Dunnett test was applied. Student t test was used in 2-mean comparisons. Differences were reported as significant when P was <0.05.
Results
Blood Pressure and Left Ventricle
Blood pressure as measured by radio telemetry was increased by Ang II and not modified by the administration of either ETS-DN (Figure S1 in the online-only Data Supplement) or ETS-1 MU, an inactive peptide used as control. The administration of Ang II resulted in significant increases in left ventricular weight, which were not significantly changed by the administration of either ETS-1 DN or ETS-1 MU peptide (Table S2 in the online-only Data Supplement).
Ang II Increases Cortical ETS-1 Expression
To determine whether ETS-1 expression is increased in the renal cortex of mice infused with Ang II, we measured ETS-1 mRNA expression by real-time quantitative reverse transcriptase PCR and protein expression by Western blot. As shown in Figure 1, 12 weeks of Ang II infusion resulted in a 3-fold increase in ETS-1 mRNA expression and a 4-fold increase in ETS-1 protein expression compared with control mice. By immunofluorescence we determined that the increase in cortical ETS-1 expression was mostly glomerular (Figure 1). After 4 weeks of Ang II, the levels of ETS-1 mRNA and protein returned to baseline (Figure 1). The administration of either ETS-DN or ETS-1 MU peptide had no effect on ETS-1 expression.
Figure 1.
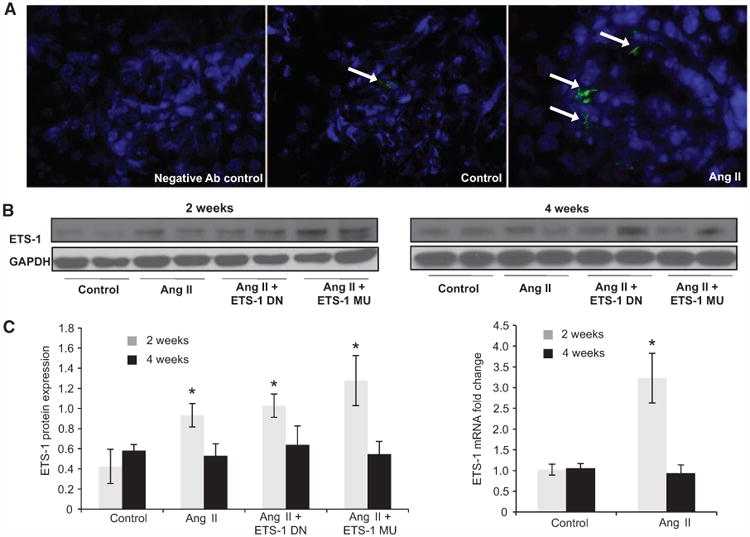
Angiotensin II (Ang I)I increases cortical ETS-1 expression. A, Representative confocal photomicrographs showing low basal expression of ETS-1 (green) in control kidney cortex, which is predominantly glomerular and increased by Ang II. B, The renal cortical ETS-1 protein expression increases after 2 weeks of Ang II as assessed by Western blot (n=6, *P<0.05 vs control) and returns to baseline after 4 weeks of Ang II. The expression of ETS-1 was not significantly modified by treatment with either ETS-1 dominant-negative (ETS-1 DN) or ETS-1 mutant (ETS-1 MU) peptide. C, Densitometric analysis for ETS-1 showing significant increases in ETS-1 protein expression after 2 but not after 4 weeks of Ang II. Neither ETS-1 DN nor ETS-1 MU modified ETS-1 protein expression. Data expressed as mean±SEM are normalized to GAPDH (*P<0.05 vs control; #P<0.05 vs Ang II group; n=6). D, The infusion of Ang II for 2 weeks resulted in increases in cortical ETS-1 mRNA expression as assessed by real-time reverse transcriptase polymerase chain reaction (n=6; *P<0.05 vs control) and returns to baseline levels after 4 weeks of Ang II.
ETS-1 Blockade Reduces Proteinuria and Matrix Expansion in Mice Infused With Ang II
As previously shown by others,23 the infusion of Ang II resulted in modest albeit significant increases in urinary protein excretion, which were reduced by ETS-1 DN but not by ETS-1 MU (Figure S2). As also previously shown by others,23 the administration of Ang II for 4 weeks resulted in significant increases in glomerular matrix expansion as assessed by morphometric analysis of periodic acid Schiff–stained kidney sections, which was significantly reduced by the administration of ETS-1 DN but not ETS-1 MU (Figure 2). By immunohistochemistry, we determined that the cortical staining of α-smooth muscle actin, a well-validated marker of renal fibrosis,24 was significantly increased by the infusion of Ang II and reduced by the administration of ETS-1 DN but not ETS-1 MU (Figure 3).
Figure 2.
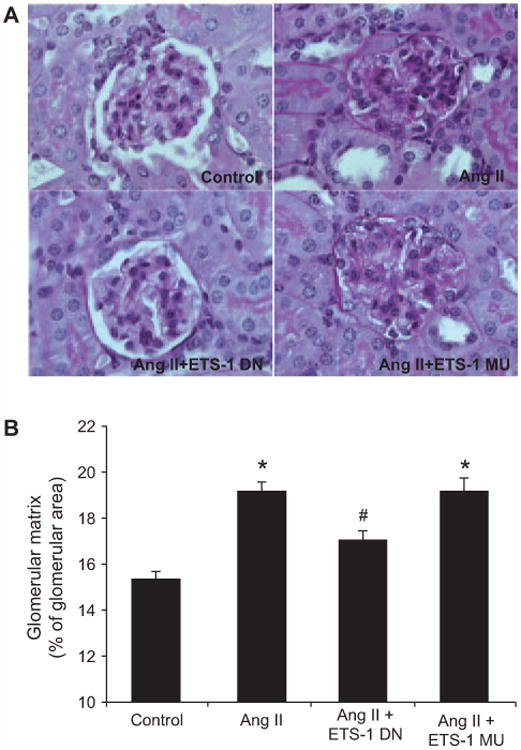
ETS-1 blockade reduces matrix expansion in angiotensin II (Ang II)–infused mice. A, Representative photomicrographs of sections stained with periodic acid Schiff to assess extracellular matrix (pink stain). B, Quantitative analysis of glomerular matrix expansion from mice infused with Ang II for 4 weeks with and without concomitant infusion of ETS-1 dominant-negative (ETS-1 DN) or ETS-1 mutant (ETS-1-MU) peptide. ETS-1 DN inhibited the Ang II–induced mesangial expansion (n=6 per group; *P<0.05 vs control; #P<0.05 vs Ang II).
Figure 3.
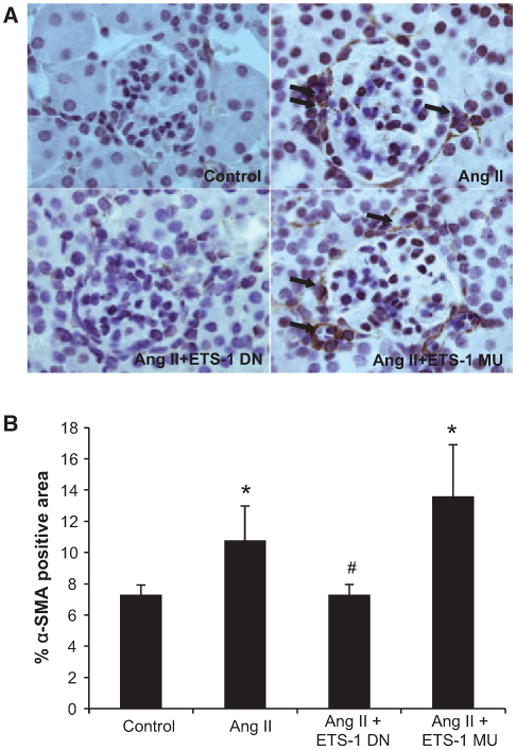
ETS-1 blockade reduces cortical α-smooth muscle actin (α-SMA) expression in angiotensin II (Ang II)–infused mice. A, Representative photomicrographs of immunohistochemistry sections for α-SMA; Brown staining is indicative of positive staining that was evident in glomerular and interstitial areas. B, Quantitative analysis of glomerular α-SMA immunohistochemistry from mice infused with Ang II for 4 weeks with and without concomitant infusion of ETS-1 dominant-negative (ETS-1 DN) or ETS-1 mutant (ETS-1-MU) peptide. ETS-1 DN inhibited the expression of α-SMA induced by Ang II (n=6 per group; *P<0.05 vs control; #P<0.05 vs Ang II).
ETS-1 Blockade Inhibited Profibrotic Gene Expression in Mice Infused With Ang II
To determine whether these changes were linked to changes in known mediators of mesangial matrix expansion, we measured the expression of TGF-β and CTGF,25 2 profibrotic cytokines with promoter sequences that indicate the existence of several ETS-1 binding sites. As shown in Figures 4 and 5, the administration of Ang II resulted in a significant increase in the mRNA expression of TGF-β and CTGF after 2 and 4 weeks of Ang II, which were significantly reduced by the administration of ETS-1 DN but not ETS-1 MU. These changes in mRNA expression were accompanied by similar directional changes in the protein expression of TGF-β and CTGF after 4 weeks of Ang II as assessed by Western blot (Figures 4 and 5).
Figure 4.
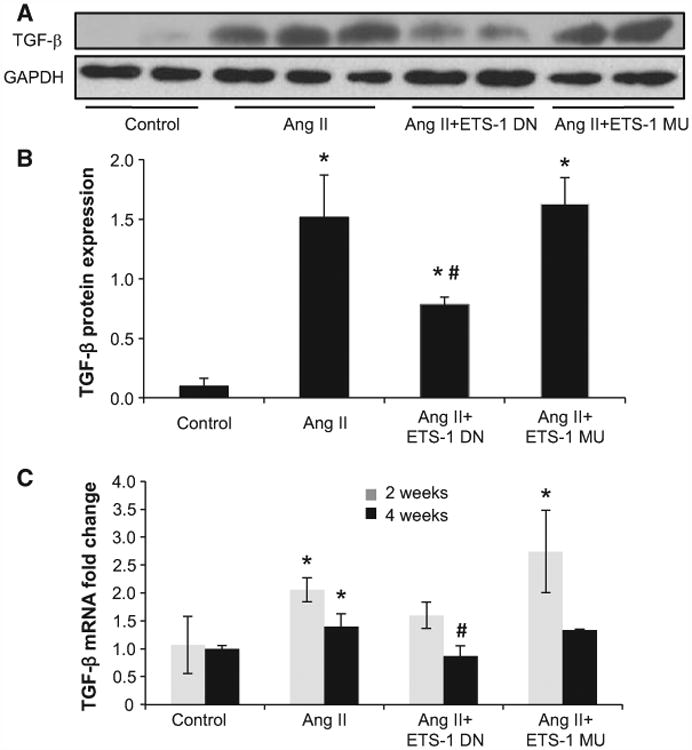
ETS-1 blockade reduced angiotensin II (Ang II)–induced transforming growth factor-β (TGF-β) upregulation. A, Representative Western blots for TGF-β. B, Densitometry data analysis for TGF-β protein expression. ETS-1 dominant-negative (ETS-1 DN) peptide inhibited the Ang II–induced TGF-β overexpression in kidney cortex. Data expressed as mean±SEM are normalized to GAPDH (*P<0.05 vs control; #P<0.05 vs Ang II group; n=6). C, TGF-β mRNA expression as assessed by real-time polymerase chain reaction after 2 and 4 weeks of Ang II infusion. ETS-1 DN inhibited Ang II–induced TGF-β mRNA expression in kidney cortex. Data are expressed as mean±SEM and normalized to GAPDH (*P<0.05 vs control; #P<0.05 vs Ang II group; n=6). ETS-1 MU indicates ETS-1 mutant.
Figure 5.
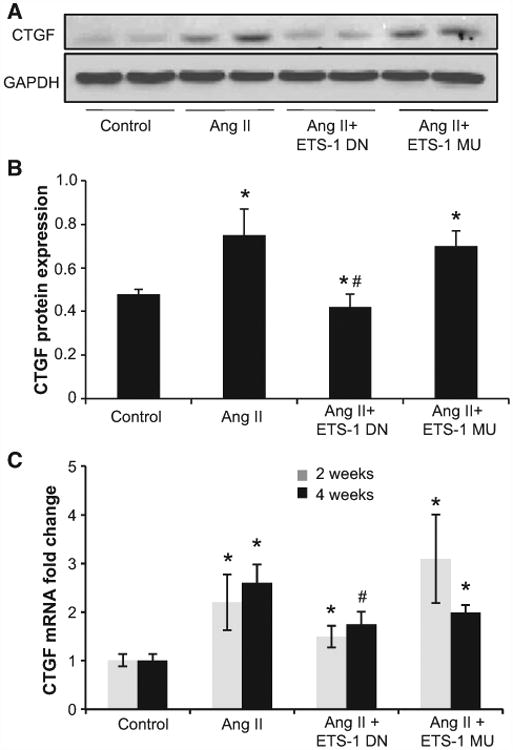
ETS-1 blockade reduced angiotensin II (Ang II)–induced connective tissue growth factor (CTGF) upregulation. A, Representative Western blots for CTGF. B, Densitometry data analysis for CTGF protein expression. ETS-1 dominant-negative (ETS-1 DN) peptide inhibited the Ang II–induced CTGF overexpression in kidney cortex. Data are expressed as mean±SEM and are normalized by GAPDH (*P<0.05 vs control; #P<0.05 vs Ang II group; n=6). C, CTGF mRNA expression as assessed by real-time polymerase chain reaction after 2 and 4 weeks of Ang II infusion. ETS-1 DN inhibited the Ang II–induced CTGF mRNA expression in mice kidney. Data are expressed as means±SEM and normalized to GAPDH (*P<0.05 vs control; #P<0.05 vs Ang II group; n=6). ETS-1 MU indicates ETS-1 mutant.
ETS-1 Mediates Proinflammatory Effects of Ang II
To evaluate the role of ETS-1 on the proinflammatory effects of Ang II, we measured macrophage infiltration as assessed by the number of F4/80-positive cells in kidney sections. In the control group, there were scattered low numbers of F4/80-positive cells, which increased significantly after 2 and 4 weeks of infusion with Ang II. The number of F4/80-positive cells was higher at 2 weeks compared with 4 weeks of Ang II infusion and was reduced by treatment with ETS-1 DN peptide but not by ETS-1 MU peptide. F4/80-positive cells were present in the tubulointerstitium, as well as in glomerular spaces (Figure 6).
Figure 6.
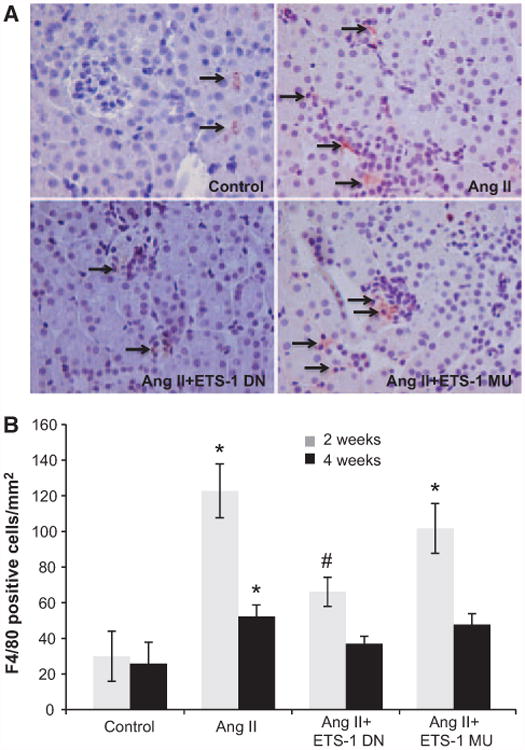
ETS-1 blockade reduces angiotensin II (Ang II)–induced macrophage infiltration. A, Representative photomicrographs of immunohistochemical staining for F4/80-positive cells (macrophages). Reddish brown color in the cytoplasm indicates positive stain. Positive staining for macrophages was found in tubulointerstitial and glomerular areas (arrows). B, Quantitative analysis of immunohistochemical staining for F4/80-positive cells. In mice infused with Ang II, the number of positive cells increased significantly (*P<0.01 vs control). The administration of ETS-1 dominant-negative (ETS-1 DN) peptide decreased macrophage infiltration in response to Ang II, whereas the ETS-1 mutant (ETS-1 MU) peptide had no effect (#P<0.05 vs Ang II; n=6 per group).
Cell Proliferation
The number of Ki-67 cells, a marker of cell proliferation, was assessed by immunohistochemistry. The infusion of Ang II for either 2 or 4 weeks resulted in significant increases in cell proliferation (Figure 7), which were ameliorated by the administration of ETS-1 DN peptide but not ETS-1 MU peptide. Ki-67–positive cells were found in the glomerular and tubulointerstitial areas.
Figure 7.
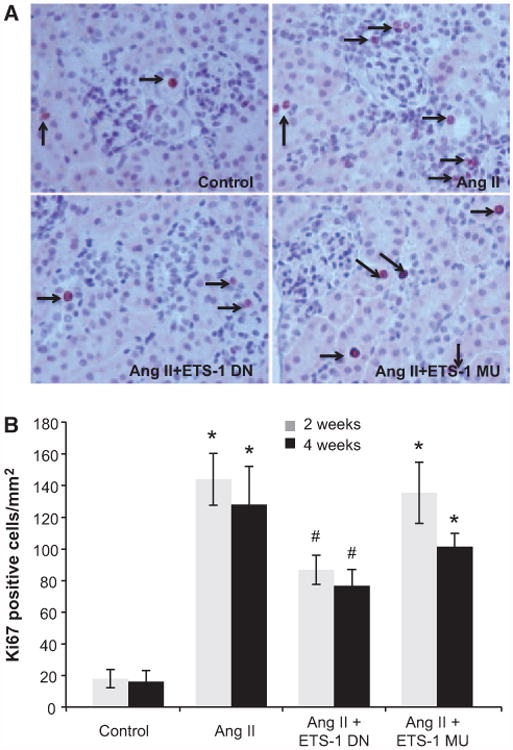
ETS-1 blockade reduces angiotensin II (Ang II)–induced cell proliferation. A, Representative photomicrographs of immunohistochemical staining for Ki-67–positive cells as a marker of cell proliferation. Nuclear reddish brown color is considered positive. Positive cells were found in tubulointerstitial area and glomerular areas (arrows). B, Quantitative analysis of immunohistochemical staining for Ki-67 cells. In mice infused with Ang II, the number of Ki-67–positive cells increased significantly (*P<0.01, control vs Ang II). The administration of ETS-1 dominant-negative (ETS-1 DN) peptide decreased cell proliferation in response to Ang II, whereas the ETS-1 mutant (ETS-1 MU) peptide had no effect (#P<0.05 vs Ang II; n=6 per group).
Nitrotyrosine Formation
Nitrotyrosine, a marker of oxidative stress, was detected by immunofluorescence. Kidneys from mice infused with Ang II for 4 weeks had increased nitrotyrosine immunofluorescence, both interstitial and glomerular (Figure 8). Treatment with ETS-1 DN but not with ETS-1 MU resulted in significant reductions in the intensity of immunofluorescence. These changes in nitrotyrosine formation were accompanied by concomitant increases in NOX4 mRNA expression, which were reduced by treatment with ETS-1 DN but not ETS-1 MU peptide after 2 and 4 weeks of Ang II (Figure 8). The infusion of Ang II for 2 weeks resulted in significant increases in NOX2 mRNA that were not modified by ETS-1 DN: vehicle, 1.05 ± 0.21; Ang II, 1.86 ± 0.23; Ang II+ETS-DN, 2.24 ± 0.47 (mRNA relative expression, n=6; P<0.05 versus vehicle). After 4 weeks of Ang II, the mRNA levels of NOX2 were no different from the control: vehicle 1.1 ± 0.14 versus Ang II 1.17 ± 0.2 (mRNA relative expression, n=6; P value was not significant). The mRNA expression for NOX1 was detected only after 35 cycles of PCR amplification in both vehicle and Ang II–infused mice, indicating low expression of this NOX isoform.
Figure 8.
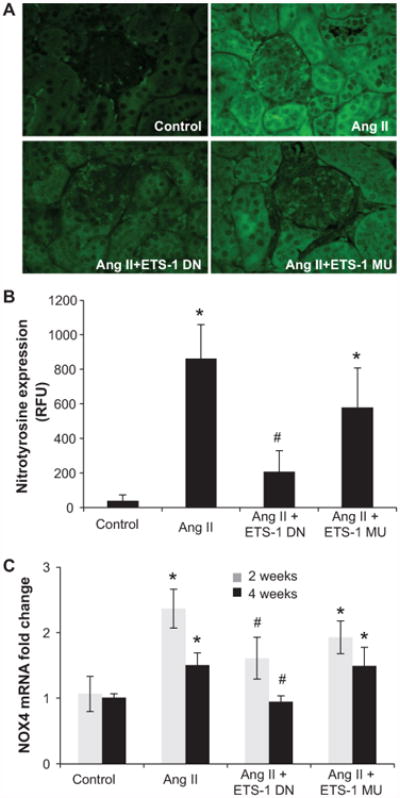
ETS-1 blockade reduces angiotensin II (Ang II)–induced nitrotyrosine formation and NOX4 expression. A, Representative photomicrographs of immunofluorescence for nitrotyrosine. B, Quantitative analysis of the intensity of cortical nitrotyrosine immunofluoroscence from mice infused with Ang II for 4 weeks with and without concomitant infusion of ETS-1 dominant-negative (ETS-1 DN) or ETS-1 mutant (ETS-1-MU) peptide showing significant increase in nitrotyrosine in response to Ang II, which is significantly reduced by ETS-1 DN but not by ETS-1 MU (n=6 per group; *P<0.05 vs control; #P<0.05 vs Ang II). C, NOX4 mRNA expression as assessed by real-time polymerase chain reaction after 2 and 4 weeks of Ang II, which is increased by Ang II and reduced by ETS-1 DN but not by ETS-MU (*P<0.01 vs control; #P<0.01 vs Ang II and Ang II+ETS-1 MU).
Discussion
ETS-1 is a member of the ETS family of transcription factors that share a highly conserved DNA-binding domain (ETS domain).26 The ETS originates from the sequence described in the E26 avian erythroblastosis virus (E26 transformation-specific sequence). ETS-1 is involved in the regulation of normal development and differentiation12,27 and as a proto-oncogene28 is implicated in the pathogenesis of different types of cancer.14 Several studies have shown that ETS-1 is required for the normal development of the mammalian kidney and for the maintenance of glomerular integrity.29,30 Indeed, ETS-1 knockout animals have fewer glomeruli, and among the existing glomeruli, a higher percentage are immature.31 The renal expression of ETS-1 is increased in several models of acute kidney injury, including ischemia-reperfusion and cisplatin toxicity,32 and in models of glomerular injury, including the anti-Thy model of glomerulonephritis33 and antiglomerular basement-induced glomerulonephritis.34 In previous studies, we also demonstrated that Ang II increases the cortical expression of ETS-1 in rats and that knockdown of ETS-1 reduces Ang II–stimulated fibronectin production in rat mesangial cells.19 In the current studies, we observed that the expressions of ETS-1 mRNA and protein were increased after 2 weeks but returned to baseline at week 4. Similarly studies by others have demonstrated that some of the proinflammatory effects of Ang II are more pronounced during the first 2 weeks of infusion with Ang II and then reduced at later time points.23 We hypothesize that ETS-1 functions as an initiator of proinflammatory and profibrotic pathways, which at the same time trigger feedback mechanisms that modulate these responses.
In our studies, ETS-1 blockade in mice infused with Ang II did not modify blood pressure or left ventricular hypertrophy, but the severity of kidney damage was significantly reduced compared with Ang II alone, suggesting that these effects were independent of the hemodynamic effects of Ang II. As we and others have demonstrated, Ang II has important proinflammatory effects by promoting the expression of several proinflammatory mediators, including monocyte chemotactic protein 1, tumor necrosis factor-α, interleukin 1, reactive oxygen species (ROS), and cyclooxygenase 2 among others.22,35,36 In addition, Ang II has profibrotic effects that are mediated via increases in the expression of growth factors such as TGF-β37 and CTGF.38 In agreement with previous reports by others, the infusion of pressor doses of Ang II for 4 weeks resulted in significant increases in proteinuria, mesangial expansion,23 and α-smooth muscle actin expression,24 an important marker of renal fibrosis. These renal effects of Ang II were significantly reduced by the administration of an ETS-1 DN peptide but not by an ETS-1 MU peptide, suggesting that ETS-1 regulates and initiates the activation of pathways involved in the development of fibrosis in response to Ang II. TGF-β and CTGF are 2 important growth factors that mediate the profibrotic effects of Ang II.25,38,39 Analysis of the TGF-β and CTGF promoter sequences reveals the existence of several ETS-1 binding sites. Based on our results, we hypothesize that ETS-1 may be directly regulating the expression of these growth factors.
The infusion of Ang II resulted in significant increases in the number of macrophages in the renal cortex, which were also reduced by ETS-1 blockade. Most of the macrophage infiltration was found in the tubulointerstitial space and to a lesser degree in the glomeruli. In the kidney, Ang II induces inflammation by promoting the production of ROS and proinflammatory cytokines and chemokines, leading to increased chemotaxis and macrophage infiltration.35,36 Macrophages subsequently secrete a variety of mediators that participate in the pathogenesis of renal damage in response to Ang II.23 Our results indicate that ETS-1 is an important mediator of the proinflammatory effects of Ang II in the kidney cortex, likely by regulating the expression of chemokines and cytokines involved in macrophage infiltration.13,18
The administration of Ang II also resulted in significant increases in renal cell proliferation, which was predominantly tubulointerstitial and significantly reduced by ETS-1 blockade. As we and others have shown, ROS are important mediators of cell proliferation in response to Ang II.40,41 In our studies, we observed significant increases in nitrotyrosine expression, a well-validated marker of oxidative stress.42 The expression of nitrotyrosine was accompanied by concomitant increases in the mRNA expression of NOX4, the most abundant NOX isoform and source of ROS in response to Ang II in the kidney.43 Blockade of ETS-1 resulted in significant reductions in nitrotyrosine and NOX4 mRNA expression, suggesting that ETS-1 may be directly regulating NOX4 expression or alternatively regulating the expression of proinflammatory mediators that stimulate the production of ROS in response to Ang II. In contrast, although the administration of Ang II increased NOX2 mRNA expression, treatment with ETS-DN did not modify its expression, suggesting that ETS-1 does not regulate this NOX isoform.
Perspectives
In these studies, we have unveiled the role of the transcription factor ETS-1 as a common mediator in the simultaneous activation of profibrotic and proinflammatory pathways in the presence of increased activation of the renin-angiotensin system. Given its a role as a common mediator of multiple pathways, we postulate that ETS-1 may be a target for novel strategies in the prevention and treatment of end-organ injury in hypertension, as well as other conditions characterized by increased activation of profibrotic and proinflammatory pathways, including chronic kidney disease of different causes, glomerulonephritis, acute kidney injury, and diabetic nephropathy, among others.
Supplementary Material
Figure S1. Effects of ETS-1 blockade on blood pressure.
The infusion of Ang II resulted in significant elevations in blood pressure that were not modified by the administration of the active ETS-1 DN peptide and the inactive ETS-1 MU peptide. (N=6 per group, * P < 0.05 vs. Ang II, Ang II + DN and Ang II + MU)
Figure S2. Effects of ETS-1 Blockade on Proteinuria
A. The infusion of Ang II resulted in significant increases in urinary protein excretion that were normalized by ETS-1 blockade with ETS-1 DN but not by the administration of an inactive mutant peptide (ETS-1 MU) (* P < 0.05 vs.Control, # P < 0.05 vs Ang II, N=6).
B. These changes in urinary excretion of protein were not secondary to changes in the urinary concentration of creatinine as they were not significantly different among all groups (P=NS, N=6).
Table S1. Primers Used for Real-Time RT-PCR Analysis
Table S2. ETS-1 blockade has no effect on Ang II induced LVH.
Novelty and Significance.
What Is New?
These studies establish the role of the transcription factor ETS-1 as a mediator of the proinflammatory and profibrotic effects of angiotensin II.
What Is Relevant?
In these studies, we have identified ETS-1 as a potential new target for the treatment and prevention of renal injury in hypertension.
Summary
In conclusion, we have characterized the transcription factor ETS-1 as a mediator of several effects of angiotensin II involved in the pathogenesis of renal injury in hypertension. These studies may result in the development of novel strategies in the treatment and prevention of end-organ injury in hypertension.
Acknowledgments
We are grateful for the support of core facilities at the O'Brien Kidney Center (P30 DK079337) at the University of Alabama at Birmingham.
Sources of Funding: These studies were funded by a Merit Review Award of the Veterans Affairs Administration (E.A. Jaimes).
Footnotes
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.112.197871/-/DC1.
Disclosures: None.
Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Hypertension can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document.
Reprints: Information about reprints can be found online at: http://www.lww.com/reprints
References
- 1.Zhou MS, Hernandez Schulman I, Pagano PJ, Jaimes EA, Raij L. Reduced NAD(P)H oxidase in low renin hypertension: link among angiotensin II, atherogenesis, and blood pressure. Hypertension. 2006;47:81–86. doi: 10.1161/01.HYP.0000197182.65554.c7. [DOI] [PubMed] [Google Scholar]
- 2.Anderson S, Jung FF, Ingelfinger JR. Renal renin-angiotensin system in diabetes: functional, immunohistochemical, and molecular biological correlations. Am J Physiol. 1993;265(4 pt 2):F477–F486. doi: 10.1152/ajprenal.1993.265.4.F477. [DOI] [PubMed] [Google Scholar]
- 3.Ito S, Johnson CS, Carretero OA. Modulation of angiotensin II-induced vasoconstriction by endothelium-derived relaxing factor in the isolated microperfused rabbit afferent arteriole. J Clin Invest. 1991;87:1656–1663. doi: 10.1172/JCI115181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floege J, Burns MW, Alpers CE, Yoshimura A, Pritzl P, Gordon K, Seifert RA, Bowen-Pope DF, Couser WG, Johnson RJ. Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int. 1992;41:297–309. doi: 10.1038/ki.1992.42. [DOI] [PubMed] [Google Scholar]
- 5.Floege J, Johnson RJ, Gordon K, Yoshimura A, Campbell C, Iruela-Arispe L, Alpers CE, Couser WG. Altered glomerular extracellular matrix synthesis in experimental membranous nephropathy. Kidney Int. 1992;42:573–585. doi: 10.1038/ki.1992.321. [DOI] [PubMed] [Google Scholar]
- 6.Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mervaala EM, Müller DN, Park JK, Schmidt F, Löhn M, Breu V, Dragun D, Ganten D, Haller H, Luft FC. Monocyte infiltration and adhesion molecules in a rat model of high human renin hypertension. Hypertension. 1999;33(1 pt 2):389–395. doi: 10.1161/01.hyp.33.1.389. [DOI] [PubMed] [Google Scholar]
- 8.Jaimes EA, Galceran JM, Raij L. Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int. 1998;54:775–784. doi: 10.1046/j.1523-1755.1998.00068.x. [DOI] [PubMed] [Google Scholar]
- 9.Tharaux PL, Chatziantoniou C, Fakhouri F, Dussaule JC. Angiotensin II activates collagen I gene through a mechanism involving the MAP/ER kinase pathway. Hypertension. 2000;36:330–336. doi: 10.1161/01.hyp.36.3.330. [DOI] [PubMed] [Google Scholar]
- 10.Kranzhöfer R, Browatzki M, Schmidt J, Kübler W. Angiotensin II activates the proinflammatory transcription factor nuclear factor-kappaB in human monocytes. Biochem Biophys Res Commun. 1999;257:826–828. doi: 10.1006/bbrc.1999.0543. [DOI] [PubMed] [Google Scholar]
- 11.Kranzhöfer R, Schmidt J, Pfeiffer CA, Hagl S, Libby P, Kübler W. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1623–1629. doi: 10.1161/01.atv.19.7.1623. [DOI] [PubMed] [Google Scholar]
- 12.Wasylyk B, Hahn SL, Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993;211:7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- 13.Oettgen P. Regulation of vascular inflammation and remodeling by ETS factors. Circ Res. 2006;99:1159–1166. doi: 10.1161/01.RES.0000251056.85990.db. [DOI] [PubMed] [Google Scholar]
- 14.Seth A, Watson DK. ETS transcription factors and their emerging roles in human cancer. Eur J Cancer. 2005;41:2462–2478. doi: 10.1016/j.ejca.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34. doi: 10.1016/s0378-1119(02)01156-3. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Brown C, Ni W, Maynard E, Rigby AC, Oettgen P. Critical role for the Ets transcription factor ELF-1 in the development of tumor angiogenesis. Blood. 2006;107:3153–3160. doi: 10.1182/blood-2005-08-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hultgårdh-Nilsson A, Cercek B, Wang JW, Naito S, Lövdahl C, Sharifi B, Forrester JS, Fagin JA. Regulated expression of the ets-1 transcription factor in vascular smooth muscle cells in vivo and in vitro. Circ Res. 1996;78:589–595. doi: 10.1161/01.res.78.4.589. [DOI] [PubMed] [Google Scholar]
- 18.Feng W, Xing D, Hua P, Zhang Y, Chen YF, Oparil S, Jaimes EA. The transcription factor ETS-1 mediates proinflammatory responses and neointima formation in carotid artery endoluminal vascular injury. Hypertension. 2010;55:1381–1388. doi: 10.1161/HYPERTENSIONAHA.110.150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearse DD, Tian RX, Nigro J, Iorgulescu JB, Puzis L, Jaimes EA. Angiotensin II increases the expression of the transcription factor ETS-1 in mesangial cells. Am J Physiol Renal Physiol. 2008;294:F1094–F1100. doi: 10.1152/ajprenal.00458.2007. [DOI] [PubMed] [Google Scholar]
- 20.Ni W, Zhan Y, He H, Maynard E, Balschi JA, Oettgen P. Ets-1 is a critical transcriptional regulator of reactive oxygen species and p47(phox) gene expression in response to angiotensin II. Circ Res. 2007;101:985–994. doi: 10.1161/CIRCRESAHA.107.152439. [DOI] [PubMed] [Google Scholar]
- 21.Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, Oparil S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation. 2004;110:1664–1669. doi: 10.1161/01.CIR.0000142050.19488.C7. [DOI] [PubMed] [Google Scholar]
- 22.Jaimes EA, Zhou MS, Pearse DD, Puzis L, Raij L. Upregulation of cortical COX-2 in salt-sensitive hypertension: role of angiotensin II and reactive oxygen species. Am J Physiol Renal Physiol. 2008;294:F385–F392. doi: 10.1152/ajprenal.00302.2007. [DOI] [PubMed] [Google Scholar]
- 23.Liao TD, Yang XP, Liu YH, Shesely EG, Cavasin MA, Kuziel WA, Pagano PJ, Carretero OA. Role of inflammation in the development of renal damage and dysfunction in angiotensin II-induced hypertension. Hypertension. 2008;52:256–263. doi: 10.1161/HYPERTENSIONAHA.108.112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong J, Guo D, Chen CB, Wang W, Schuster M, Loibner H, Penninger JM, Scholey JW, Kassiri Z, Oudit GY. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension. 2011;57:314–322. doi: 10.1161/HYPERTENSIONAHA.110.164244. [DOI] [PubMed] [Google Scholar]
- 25.Qi W, Chen X, Poronnik P, Pollock CA. Transforming growth factor-beta/connective tissue growth factor axis in the kidney. Int J Biochem Cell Biol. 2008;40:9–13. doi: 10.1016/j.biocel.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Nunn MF, Seeburg PH, Moscovici C, Duesberg PH. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983;306:391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- 27.Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci. 1998;23:213–216. doi: 10.1016/s0968-0004(98)01211-0. [DOI] [PubMed] [Google Scholar]
- 28.Dittmer J. The biology of the Ets1 proto-oncogene. Mol Cancer. 2003;2:29. doi: 10.1186/1476-4598-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chernavsky DFL, Barton K, Muthusamy N, Leiden J, Gomez R. Expression and function of ets-1 in the developing kidney. J Am Soc Nephrol. 1998;9:360. [Google Scholar]
- 30.Cederberg A, Hulander M, Carlsson P, Enerbäck S. The kidney-expressed winged helix transcription factor FREAC-4 is regulated by Ets-1. A possible role in kidney development. J Biol Chem. 1999;274:165–169. doi: 10.1074/jbc.274.1.165. [DOI] [PubMed] [Google Scholar]
- 31.Gomez RA, Norwood VF. Recent advances in renal development. Curr Opin Pediatr. 1999;11:135–140. doi: 10.1097/00008480-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka H, Terada Y, Kobayashi T, Okado T, Inoshita S, Kuwahara M, Seth A, Sato Y, Sasaki S. Expression and function of Ets-1 during experimental acute renal failure in rats. J Am Soc Nephrol. 2004;15:3083–3092. doi: 10.1097/01.ASN.0000145459.54236.D3. [DOI] [PubMed] [Google Scholar]
- 33.Raffetseder U, Wernert N, Ostendorf T, van Roeyen C, Rauen T, Behrens P, Floege J, Mertens PR. Mesangial cell expression of proto-oncogene Ets-1 during progression of mesangioproliferative glomerulonephritis. Kidney Int. 2004;66:622–632. doi: 10.1111/j.1523-1755.2004.00782.x. [DOI] [PubMed] [Google Scholar]
- 34.Naito T, Razzaque MS, Nazneen A, Liu D, Nihei H, Koji T, Taguchi T. Renal expression of the Ets-1 proto-oncogene during progression of rat crescentic glomerulonephritis. J Am Soc Nephrol. 2000;11:2243–2255. doi: 10.1681/ASN.V11122243. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl. 2002;82:S12–S22. doi: 10.1046/j.1523-1755.62.s82.4.x. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Ortega M, Lorenzo O, Rupérez M, Esteban V, Mezzano S, Egido J. Renin-angiotensin system and renal damage: emerging data on angiotensin II as a proinflammatory mediator. Contrib Nephrol. 2001;135:123–137. doi: 10.1159/000060153. [DOI] [PubMed] [Google Scholar]
- 37.Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension. 2001;38(3 pt 2):635–638. doi: 10.1161/hy09t1.094234. [DOI] [PubMed] [Google Scholar]
- 38.Chen XM, Qi W, Pollock CA. CTGF and chronic kidney fibrosis. Front Biosci (Schol Ed) 2009;1:132–141. doi: 10.2741/S13. [DOI] [PubMed] [Google Scholar]
- 39.Yang F, Chung AC, Huang XR, Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: the role of Smad3. Hypertension. 2009;54:877–884. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]
- 40.Zhou MS, Schuman IH, Jaimes EA, Raij L. Renoprotection by statins is linked to a decrease in renal oxidative stress, TGF-beta, and fibronectin with concomitant increase in nitric oxide bioavailability. Am J Physiol Renal Physiol. 2008;295:F53–F59. doi: 10.1152/ajprenal.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf G. Role of reactive oxygen species in angiotensin II-mediated renal growth, differentiation, and apoptosis. Antioxid Redox Signal. 2005;7:1337–1345. doi: 10.1089/ars.2005.7.1337. [DOI] [PubMed] [Google Scholar]
- 42.Eleuteri E, Magno F, Gnemmi I, Carbone M, Colombo M, La Rocca G, Anzalone R, Tarro Genta F, Zummo G, Di Stefano A, Giannuzzi P. Role of oxidative and nitrosative stress biomarkers in chronic heart failure. Front Biosci. 2009;14:2230–2237. doi: 10.2741/3375. [DOI] [PubMed] [Google Scholar]
- 43.Sedeek M, Hébert RL, Kennedy CR, Burns KD, Touyz RM. Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr Opin Nephrol Hypertens. 2009;18:122–127. doi: 10.1097/MNH.0b013e32832923c3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effects of ETS-1 blockade on blood pressure.
The infusion of Ang II resulted in significant elevations in blood pressure that were not modified by the administration of the active ETS-1 DN peptide and the inactive ETS-1 MU peptide. (N=6 per group, * P < 0.05 vs. Ang II, Ang II + DN and Ang II + MU)
Figure S2. Effects of ETS-1 Blockade on Proteinuria
A. The infusion of Ang II resulted in significant increases in urinary protein excretion that were normalized by ETS-1 blockade with ETS-1 DN but not by the administration of an inactive mutant peptide (ETS-1 MU) (* P < 0.05 vs.Control, # P < 0.05 vs Ang II, N=6).
B. These changes in urinary excretion of protein were not secondary to changes in the urinary concentration of creatinine as they were not significantly different among all groups (P=NS, N=6).
Table S1. Primers Used for Real-Time RT-PCR Analysis
Table S2. ETS-1 blockade has no effect on Ang II induced LVH.


