Abstract
Objective
To describe the rates of elevated inflammation, obesity and metabolic syndrome (MetS) within a large cohort of individuals with depression and to examine the inter-relationships of inflammation and metabolic syndrome in depressed individuals.
Method
Analyses were conducted on study participants from the 2009–2010 National Health and Nutrition Survey (NHANES) with Patient Health Questionnaire (PHQ)-9 depression scores ≥ 10 to: 1) examine the relationship of inflammation (C-reactive protein; CRP) with demographic and clinical characteristics and 2) examine the prevalence of MetS criteria within CRP groups.
Results
5579 participants provided PHQ-9 data; of those, 606 had PHQ-9 scores ≥ 10 and were included in further analysis. Of the 606 depressed participants, 585 participants had valid CRP data; 275 participants (47.01%) had CRP levels ≥ 3.0 mg/L, while 170 (29.06%) had CRP levels ≥ 5.0 mg/L. Elevated inflammation was significantly correlated with body weight, waist circumference, BMI, insulin, 2-hour glucose tolerance, and self-report general health (p’s < 0.05). 112 subjects (41.18%) met AHA/NHLBI criteria for metabolic syndrome (MetS). Those with elevated CRP were more likely to meet criteria for MetS (Odds Ratios of 2.81 for those with CRP levels ≥ 3.0 mg/L and 1.94 for those with CRP levels ≥ 5.0 mg/L).
Conclusion
Over 29% of depressed individuals have elevated levels of CRP and 41% met criteria for MetS. Individuals with elevated inflammation are more likely to be obese and meet criteria for MetS. These results highlight the significant inflammatory and metabolic burden of individuals with depression.
Keywords: Depression, Inflammation, Obesity, Metabolic Syndrome
Introduction
Major Depressive Disorder (MDD) is a chronic, recurring disorder that results in significant emotional and socioeconomic burden1,2. This burden is due, in part, to the lack of a clear understanding of biological sub-groups and shortcomings of available treatments to match subtypes of depression. Many patients with MDD do not receive adequate care and among those who do, a significant portion fail to achieve remission 3–5. These poor outcomes underscore the need for novel treatment options for patients with MDD. To this end, recent research has aimed to identify specific biomarkers indicative of reliable subtypes in the hopes that patients can ultimately be matched to treatments most likely to illicit a positive treatment response.
Inflammation has been implicated in the etiology of MDD. Pro-inflammatory cytokines, specifically interleukin-6 (IL-6) and factor-α (TNF-α) and IL-1B, are elevated in patients with MDD compared to healthy controls6,7. Furthermore, studies of interferon-α-induced MDD in Hepatics C and cancer patients implicate inflammation in the development and recurrence of MDD8. Research also indicates an effect of antidepressant medications on inflammatory cytokines. A meta-analysis by Hannestad et al. 9 reports reductions in IL-6 and IL-1β following antidepressant treatment; while other studies have reported correlations between reductions in IL-1β and reductions in depressive symptoms. Importantly, elevated baseline levels of inflammation are predictive of treatment non-response to a variety of antidepressant medications 10–14.
The role of inflammation in MDD treatment response suggests MDD in the presence of inflammation may be a distinct subtype of MDD requiring a targeted treatment approach. While previous epidemiological work has shown a relationship between inflammation and MDD, the rates of inflammation among those with MDD has not been well characterized. Inflammation accompanying MDD may also be related to important demographic and clinical characteristics that may impact treatment selection and response. For example, there is a bidirectional relationship between MDD and metabolic syndrome (MetS) 15–17 and it has been proposed that inflammation is the underlying link between MDD and MetS 18.
The purpose of this paper is to describe the rates of elevated inflammation among those with depression using the 2009–2010 National Health and Nutrition Examination Survey (NHANES). Furthermore, we will describe the clinical characteristics, including the presence of MetS, of those with depression and elevated inflammation.
Methods
Study sample
The 2009–2010 NHANES data was used for the analysis. The NHANES, conducted by the National Center for Health Statistics, is a stratified, multistage probability sample of the civilian noninstitutionalized U.S. population. Adults, age 18 and older, were included for this analysis.
Measures
Depression was assessed using the Patient Health Questionnaire (PHQ-9) 19. Only those with significant depressive symptoms were included in the analysis. Based on previous research, a cut-off of ≥ 10 was used to identify those with significant depressive symptoms. C-reactive protein (CRP) was assessed via a high sensitivity assay with latex-enhanced nephelometry. Glucose concentrations for fasting glucose and the 2-hour glucose tolerance test were determined by a hexokinase method. Insulin concentration was measured Mercodia Insulin ELISA (Uppsala, Sweeden, a two-site enzyme immunoassay. Triglycerides were assessed enzymatically using a two-reagent, endpoint reaction that is specific for triglycerides. Reported blood pressure is the average of 3 consecutive blood pressure readings collected following 5 minutes of quiet rest.
Demographic information and medical history were assessed through self-report questionnaires and a physical exam was conducted to ascertain height, body weight and waist circumference.
MetS classification was based on the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) definition 20. The AHA/NHLBI definition for MetS requires the presence of at least three of the following five CVD risk factors: (1) impaired fasting glucose (IFG) ≥ 100 milligrams per deciliter (mg/dL) or pharmacological treatment for IFG; (2) low HDL cholesterol (<40 mg/dL in men or <50 mg/dL in women) or pharmacological treatment for an abnormal HDL cholesterol level; (3) triglycerides ≥ 150 mg/dL or pharmacological treatment for hypertriglyceridemia; (4) a waist circumference (WC) ≥ 102 cm in men or ≥ 88 cm in women; and (5) blood pressure ≥ 130/85 mm Hg or pharmacological treatment for HTN.
Analysis
Pearson’s correlations were calculated to examine the relationship of CRP with demographic and clinical characteristics. CRP levels were examined as a continuous variable and then dichotomized using cutoffs of 3 mg/L and 5 mg/L. Continuity adjusted chi-square tests examined the prevalence of metabolic syndrome criteria within CRP groups. Since the analysis was conducted on small subsample of the total sample, analyses were conducted with and without the population weighting. No substantive differences in the two approaches were observed, therefore only the unweighted results are reported. Odds ratios were calculated to quantify the association of CRP with metabolic syndrome and individual metabolic syndrome criteria.
Results
5579 participants provided PHQ-9 data; of those, 606 (10.86%) had PHQ-9 scores ≥ 10 and were included in further analysis. Demographic and clinical characteristics of the sample are reported in Table 1. No substantive differences in analyses with and without the population weighting were observed, therefore only the unweighted results are reported.
Table 1.
Sample Characteristics
| Variables | n | Mean | SD |
|---|---|---|---|
| Age (yrs) | 585 | 48.74 | 15.88 |
| Gender | 585 | ||
| Female (%) | 332 | 56.75 | |
| Race | 585 | ||
| White (%) | 247 | 42.22 | |
| African American (%) | 110 | 18.80 | |
| Mexican America (%) | 127 | 21.71 | |
| Other Hispanic (%) | 67 | 11.45 | |
| Other (%) | 34 | 5.81 | |
| Body Mass Index | 581 | 30.43 | 8.24 |
| Waist Circumference (cm) | 565 | 101.28 | 18.53 |
| CRP (mg/L) | 585 | 4.77 | 6.52 |
| >3.0 mg/L (%) | 275 | 47.01 | |
| >5.0 mg/L (%) | 170 | 29.06 | |
| Fasting Glucose (mg/dL) | 275 | 109.80 | 39.45 |
| Fasting Insulin (uU/mL) | 271 | 15.93 | 12.51 |
| 2-hour Glucose (mg/dL) | 202 | 124.42 | 54.37 |
| Systolic BP (mm Hg) | 565 | 120.26 | 18.85 |
| Diastolic BP (mm Hg) | 564 | 70.17 | 12.53 |
| HDL Cholesterl (mg/dL) | 581 | 50.79 | 15.40 |
| Triglyceride (mg/dL) | 273 | 144.97 | 178.86 |
Proportion of Depressed Sample with Elevated Inflammation
CRP was dichotomized using two established criteria. A cut-off of ≥ 3.0 mg/L is typically used to signify high-risk of cardiovascular disease 21. However, a recent study by Raison et al. suggests that a cut-off of ≥ 5.0 mg/L might predict treatment response in depression so both criteria were employed. Of the 606 depressed participants, 585 participants had valid CRP data; 275 participants (47.01%) had CRP levels ≥ 3.0 mg/L, while 170 (29.06%) had CRP levels ≥ 5.0 mg/L. Mean CRP levels did not differ in patients taking antidepressant or antipsychotic medications compared to those not taking medications (t = −0.67; p = 0.50)
Relationship of Inflammation with Demographic and Clinical Characteristics
Pearson correlation coefficients were calculated to examine the relationship of elevated inflammation with demographic and clinical characteristics (Table 2). Elevated inflammation was not significantly correlated with gender, race or age (p’s > 0.05). CRP was positively correlated with body weight, waist circumference and BMI (p’s < 0.0001). CRP also had significant correlations with insulin (p = 0.04) and 2-hour glucose tolerance (p = 0.03), as well as self-report general health (p = 0.02). As expected given these significant correlations, individuals with elevated CRP levels had significantly higher body weight, waist circumference, BMI and insulin (p’s < 0.0001).
Table 2.
Pearson Correlations of C-Reactive Protein with Clinical Characteristics
| Variables | n | CRP |
|---|---|---|
| Weight | 583 | 0.29* |
| BMI | 581 | 0.33* |
| Waist Circumference | 565 | 0.33* |
| Fasting Glucose | 275 | 0.01 |
| Insulin | 271 | 0.13 |
| 2-hour Glucose | 202 | 0.14 |
| Systolic BP | 565 | 0.11# |
| Diastolic BP | 564 | 0.05 |
| HDL Cholesterl | 581 | −0.09 |
| Triglyceride | 273 | 0.002 |
p < 0.0001
p < 0.001
p < 0.01
Inflammation and Metabolic Syndrome
Of the 272 depressed participants that provided fasting blood samples, 132 subjects (48.53%) met criteria for metabolic syndrome (MetS). Though the collection of samples was random, the subsample that provided a fasting blood sample had a significantly lower BMI (p = 0.04) and waist circumference (p = 0.02). The subsample did not differ PHQ-9 score or mean CRP level.
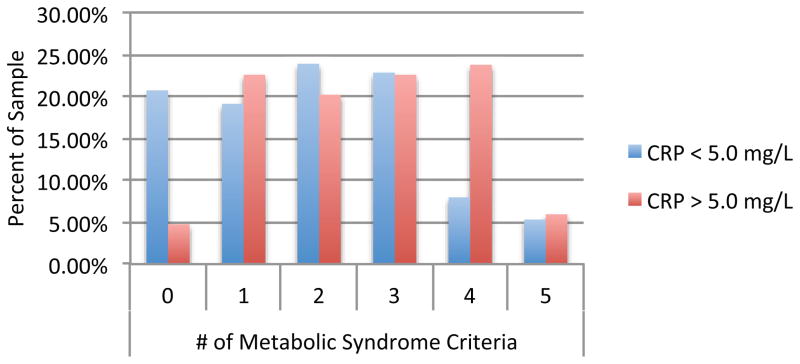
Those with elevated CRP were more likely to meet criteria for MetS. MetS was present in 53.79% of those with CRP greater than 3.0 mg/L compared to 29.29% of those with CRP less than 3.0 mg/L (OR = 2.81) and 52.38% of those with CRP greater than 5.0 mg/L compared to 36.17% among those with CRP less than 5.0 mg/L (OR= 1.94). Individuals with elevated inflammation were also more likely to meet a greater number of MetS criteria (Figure 1) and had greater prevalence of individual MetS symptoms, specifically elevated glucose, low HDL cholesterol and high waist circumference (Table 3).
Figure 1.
Metabolic Syndrome Criteria by CRP status
Table 3.
Relationship of C-Reactive Protein with Metabolic Syndrome
| Variables | Total Sample n = 272 |
CRP <3.0mg/L n = 140 |
CRP >3.0mg/L n = 132 |
X 2 | p | OR (95% CI) | CRP <5.0mg/L n = 188 |
CRP >5.0mg/L n = 84 |
X 2 | p | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolic Syndrome | 112 (41.18%) | 41 (29.29%) | 71 (53.79%) | 15.84 | < 0.0001 | 2.81 (1.71, 4.63) | 68 (36.17%) | 44 (52.38%) | 5.65 | 0.0175 | 1.94 (1.15, 3.27) |
| Glucose | 140 (51.47%) | 55 (39.29%) | 85 (64.39%) | 16.16 | < 0.0001 | 2.79 (1.71, 4.57) | 85 (45.21%) | 55 (65.48%) | 9.67 | 0.0031 | 2.30 (1.35, 3.92) |
| HDL Cholesterol | 112 (41.18%) | 42 (30.00%) | 70 (53.03%) | 13.94 | 0.0002 | 2.63 (1.60, 4.33) | 65 (34.57%) | 47 (55.98%) | 10.09 | 0.0015 | 2.40 (1.42, 4.06) |
| Triglyceride | 86 (31.62%) | 40 (28.57%) | 46 (34.85%) | 0.96 | 0.326 | 1.34 (0.80, 2.23) | 56 (29.79%) | 30 (35.71%) | 0.69 | 0.4065 | 1.31 (0.76, 2.26) |
| Waist Circumference | 163 (59.92%) | 65 (46.43%) | 98 (74.24%) | 20.74 | < 0.0001 | 3.33 (1.99, 5.55) | 103 (54.79%) | 60 (71.43%) | 6.02 | 0.0141 | 2.06 (1.19, 3.59) |
| Blood Pressure | 79 (29.04%) | 41 (29.29%) | 38 (28.79%) | 0.00 | 1.00 | 0.98 (0.58, 1.65) | 56 (29.79%) | 23 (27.38%) | 0.07 | 0.7954 | 0.89 (0.50, 1.58) |
Discussion
Our results highlight the prevalence of inflammation among those with depression. While previous research has demonstrated a relationship between depression and inflammation, the current analysis is the first to characterize inflammation within a depressed sample and to describe the clinical characteristics associated with elevated inflammation among those with depression. Approximately half of those with PHQ-9 scores indicative of significant depression had CRP levels greater than 3.0 mg/L and nearly 30% had CRP levels greater than 5.0 mg/L. Furthermore, inflammation was associated with markers of obesity, insulin resistance and MetS.
Our results also build upon an existing literature highlighting the association between depression and metabolic syndrome. Previous research suggests a bidirectional relationship between MetS and MDD, as the presence of MDD predicts future incidence of MetS16 and conversely current MetS is associated with future onset of MDD15. This would suggest that rates of MetS are higher in those with MDD. In fact, our results indicate the rate of MetS is over 41% in those with depression, while estimates of the prevalence of MetS in the general population range from 27.9% to 34.1%.22
The implications of the prevalence of inflammation and MetS in MDD are substantial. These factors help to explain the high rates of medical comorbidities and poor health outcomes in persons with depression23,24. Furthermore, inflammation and MetS are also associated with poorer treatment outcomes for MDD 10–14,25. MDD is a heterogeneous disease and it is postulated that the biological underpinnings are equally varied. As a result, there are multiple treatment targets and effective treatment of MDD likely requires several treatment options. Therefore, effective treatment of MDD will require identification of specific MDD “subtypes” that are then matched to the appropriate treatment. Given that previous research suggests that elevated inflammation and presence of MetS are associated with poorer treatment response, these factors might be indicative of one specific biological “subtype” of MDD.
Recent research has begun to identify potential novel treatment options for depression in patients with elevated inflammation. A recent study by Raison et al. suggests the TNF-a antagonist infliximab, a TNF antagonist, may be efficacious in those with elevated inflammation. In a randomized controlled trial in treatment-resistant depression, infliximab resulted in a better treatment response in patients whose baseline CRP levels were greater than 5.0 mg/L 26. Furthermore, genetic transcription factors related to glucose and lipid metabolism were predictive of treatment response to infliximab 27. Similarly, a trial examining exercise augmentation in non-responders to SSRIs observed favorable findings in treating patients with elevated inflammation. Specifically, elevated pre-treatment levels of TNF-a were predictive of improved remission rates28. Higher BMI was also associated with better treatment outcomes following the exercise intervention.29
Continued research is necessary to better characterize this potential biological subtype of MDD. Results of a recent latent class analysis of the Netherlands Study of Depression and Anxiety cohort identified two classes of severe depression based on depressive symptom profiles: melancholic and atypical 30. Atypical depression, characterized by increased weight, increased appetite and leaden paralysis. Atypical depression was also related to elevations in multiple inflammatory markers (CRP, IL-6 and TNF-a), higher BMI, and prevalence of MetS 31. The association between obesity and atypical depressive symptoms has also been reported elsewhere 32. Therefore, it is possible that the potential biological “subtype” of MDD, characterized by inflammation and MetS, may overlap with the clinical “subtype” of MDD, characterized by atypical depressive symptoms. A limitation of the current analysis is we are unable to assess the relationship of atypical depressive symptoms with inflammation and MetS in this sample because the PHQ-9 does not assess for atypical depressive symptoms.
While the NHANES data provide a large, nationally representative sample, there are limitations of the data collection methods that could influence our results. First is the identification of depression using the PHQ-9. The PHQ-9 has been validated as a screening tool with acceptable sensitivity and specificity 19; however, it is not a diagnostic tool. Though our findings may not reflect the exact portion of individuals with MDD that also have high inflammation and MetS, our results do highlight the significant portion of individuals with elevated depressive symptoms also have elevated inflammation and MetS. Second, only a subset of the sample provided fasting blood samples for allowing classification of MetS and those who provided samples had significantly lower BMI and waist circumference. While these differences potentially bias our findings, the fact that those who provided fasting samples had lower BMI and waist circumference would suggest that the high rate of MetS observed in our sample might in fact underestimate the true prevalence of MetS in those with MDD.
Another important topic for future research is to identify factors that may affect the relationship of inflammation and MetS with depression. Previous research indicates psychiatric medications may be in part responsible for higher rates of MetS in persons with MDD 33,34. Our analyses support this, as the use of psychiatric medications was associated with higher BMI and waist circumference. However, levels of CRP were not higher in those taking psychiatric medication. Similarly, diet and exercise are clearly linked to MetS and inflammation 35,36 and engaging in physical activity may affect the relationship between inflammation and depressive symptoms 37. Though examination of these factors are beyond the scope of the current analysis, identification of these and other factors that may influence the relationship of inflammation and MetS with depression is an important area for future research.
The current analysis highlights prevalence of elevated inflammation in a depressed sample along with the links among inflammation, obesity, insulin resistance, and metabolic symptoms. Previous research suggests that these biological markers may be associated with atypical depressive symptoms. Furthermore, these biological and psychological symptoms may be indicative of treatment resistance of depression. Future work should continue to characterize the clinical and biological characteristics that may distinguish specific MDD subtypes and to identify efficacious treatment options for those subtypes.
Clinical points.
This study highlights the significant prevalence of inflammation, obesity, and MetS in persons with MDD.
The high rates of inflammation, obesity and MetS likely contribute to the elevated risk of developing medical comorbidities in persons with MDD
Acknowledgments
Source of Funding:
Chad D. Rethorst is supported by the National Institute Of Mental Health of the National Institutes of Health under Award Number K01MH097847. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Ira Bernstein serves on the Joint Research Committee of the National Council of State Boards of Nursing.
Madhukar H. Trivedi is or has been an advisor/consultant to: Abbott Laboratories, Inc., Abdi Ibrahim, Akzo (Organon Pharmaceuticals Inc.), Alkermes, AstraZeneca, Axon Advisors, Bristol-Myers Squibb Company, Cephalon, Inc., Cerecor, Concert Pharmaceuticals, Inc., Eli Lilly & Company, Evotec, Fabre Kramer Pharmaceuticals, Inc., Forest Pharmaceuticals, GlaxoSmithKline, Janssen Global Services, LLC, Janssen Pharmaceutica Products, LP, Johnson & Johnson PRD, Libby, Lundbeck, Meade Johnson, MedAvante, Medscape, Medtronic, Merck, Mitsubishi Tanabe Pharma Development America, Inc., Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Parke-Davis Pharmaceuticals, Inc., Pfizer Inc., PgxHealth, Phoenix Marketing Solutions, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Products Ltd., Sepracor, SHIRE Development, Sierra, SK Life and Science, Sunovion, Takeda, Tal Medical/Puretech Venture, Targacept, Transcept, VantagePoint, Vivus, and Wyeth-Ayerst Laboratories. In addition, he has received research support from: Agency for Healthcare Research and Quality (AHRQ), Corcept Therapeutics, Inc., Cyberonics, Inc., National Alliance for Research in Schizophrenia and Depression, National Institute of Mental Health, National Institute on Drug Abuse, Novartis, Pharmacia & Upjohn, Predix Pharmaceuticals (Epix), and Solvay Pharmaceuticals, Inc.
Role of the Sponsor: NIH had no role in the design of the study, data collection, data analysis, or manuscript preparation.
References
- 1.Greenberg PE, Kessler RC, Birnbaum HG, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006 May 27;367(9524):1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 3.Rush A, Trivedi M, Wisniewski S, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. A J Psychiatry. 2006;163(11):1905. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi M, Rush A, Wisniewski S, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. A J Psychiatry. 2006;163(1):28. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Andrews G, Sanderson K, Corry J, Lapsley H. Using epidemiological data to model efficiency in reducing the burden of depression. The Journal of Mental Health Policy and Economics. 2000;3(4):175. doi: 10.1002/mhp.96. [DOI] [PubMed] [Google Scholar]
- 6.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010 Mar 1;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009 Feb;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 8.Prather AA, Carroll JE, Fury JM, McDade KK, Ross D, Marsland AL. Gender differences in stimulated cytokine production following acute psychological stress. Brain Behav Immun. 2009;23(5):622–628. doi: 10.1016/j.bbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011 Nov;36(12):2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 11.Benedetti F, Lucca A, Brambilla F, Colombo C, Smeraldi E. Interleukine-6 serum levels correlate with response to antidepressant sleep deprivation and sleep phase advance. Prog Neuropsychopharmacol Biol Psychiatry. 2002 Oct;26(6):1167–1170. doi: 10.1016/s0278-5846(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 12.Mikova O, Yakimova R, Bosmans E, Kenis G, Maes M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur Neuropsychopharmacol. 2001 Jun;11(3):203–208. doi: 10.1016/s0924-977x(01)00081-5. [DOI] [PubMed] [Google Scholar]
- 13.Eller T, Vasar V, Shlik J, Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008 Feb 15;32(2):445–450. doi: 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Sluzewska A, Sobieska M, Rybakowski JK. Changes in acute-phase proteins during lithium potentiation of antidepressants in refractory depression. Neuropsychobiology. 1997;35(3):123–127. doi: 10.1159/000119332. [DOI] [PubMed] [Google Scholar]
- 15.Koponen H, Jokelainen J, Keinanen-Kiukaanniemi S, Kumpusalo E, Vanhala M. Metabolic syndrome predisposes to depressive symptoms: a population-based 7-year follow-up study. J Clin Psychiatry. 2008 Feb;69(2):178–182. doi: 10.4088/jcp.v69n0202. [DOI] [PubMed] [Google Scholar]
- 16.Goldbacher EM, Bromberger J, Matthews KA. Lifetime history of major depression predicts the development of the metabolic syndrome in middle-aged women. Psychosom Med. 2009 Apr;71(3):266–272. doi: 10.1097/PSY.0b013e318197a4d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinder LS. Depression and the Metabolic Syndrome in Young Adults: Findings From the Third National Health and Nutrition Examination Survey. Psychosom Med. 2004;66(3):316–322. doi: 10.1097/01.psy.0000124755.91880.f4. [DOI] [PubMed] [Google Scholar]
- 18.Capuron L, Su S, Miller AH, et al. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry. 2008 Nov 15;64(10):896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 21.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 22.Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among US adults: NHANES III to NHANES 1999 2006. Diabetes Care. 2011;34(1):216–219. doi: 10.2337/dc10-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. The Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 24.Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord. 2002;72(3):227. doi: 10.1016/s0165-0327(01)00413-x. [DOI] [PubMed] [Google Scholar]
- 25.Toups MS, Trivedi MH. The role of metabolic dysfunction in treatment resistance of major depressive disorder. Neuropsychiatry. 2011;1(5):441–455. [Google Scholar]
- 26.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013 Jan;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta D, Raison CL, Woolwine BJ, et al. Transcriptional signatures related to glucose and lipid metabolism predict treatment response to the tumor necrosis factor antagonist infliximab in patients with treatment-resistant depression. Brain Behav Immun. 2013 Jul;31:205–215. doi: 10.1016/j.bbi.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rethorst CD, Toups MS, Greer TL, et al. Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol Psychiatry. 2013 Oct;18(10):1119–1124. doi: 10.1038/mp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toups MS, Greer TL, Kurian BT, et al. Effects of serum Brain Derived Neurotrophic Factor on exercise augmentation treatment of depression. J Psychiatr Res. 2011 Jun 2; doi: 10.1016/j.jpsychires.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamers F, de Jonge P, Nolen WA, et al. Identifying depressive subtypes in a large cohort study: results from the Netherlands Study of Depression and Anxiety (NESDA) The Journal of clinical psychiatry. 2010 Dec;71(12):1582–1589. doi: 10.4088/JCP.09m05398blu. [DOI] [PubMed] [Google Scholar]
- 31.Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2013 Jun;18(6):692–699. doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- 32.Chou KL, Yu KM. Atypical depressive symptoms and obesity in a national sample of older adults with major depressive disorder. Depress Anxiety. 2013 Jun;30(6):574–579. doi: 10.1002/da.22098. [DOI] [PubMed] [Google Scholar]
- 33.Fava M. Weight gain and antidepressants. The Journal of clinical psychiatry. 2000;61:37. [PubMed] [Google Scholar]
- 34.Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. The Journal of clinical psychiatry. 2006;68:8–13. [PubMed] [Google Scholar]
- 35.Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002 Sep;13(5):561–568. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Ford ES, Kohl HW, Mokdad AH, Ajani UA. Sedentary behavior, physical activity, and the metabolic syndrome among US adults. Obes Res. 2005;13(3):608–614. doi: 10.1038/oby.2005.65. [DOI] [PubMed] [Google Scholar]
- 37.Rethorst CD, Moynihan J, Lyness JM, Heffner KL, Chapman BP. Moderating effects of moderate-intensity physical activity in the relationship between depressive symptoms and interleukin-6 in primary care patients. Psychosom Med. 2011 Apr;73(3):265–269. doi: 10.1097/PSY.0b013e3182108412. [DOI] [PMC free article] [PubMed] [Google Scholar]