Abstract
Parking lot runoff retention ponds (PLRRP) receive significant chemical input, but the biological effects of parking lot runoff are not well understood. We used the Japanese medaka (Oryzias latipes) as a model to study the toxicity of water and sediment samples from a PLRRP in Morehead City, NC. Medaka exposed in ovo to a dilution series of PLRRP water had increased odds of death before hatching, but not teratogenesis or delayed hatching. Next, we adapted a long-amplicon quantitative PCR (LA-QPCR) assay for DNA damage for use with the Japanese medaka. We employed LA-QPCR to test the hypotheses that PLRRP water and sediments would cause nuclear and mitochondrial DNA damage with and without full-spectrum, natural solar radiation. Fluoranthene with and without natural sunlight was a positive control for phototoxic polycyclic aromatic hydrocarbon-induced DNA damage. Fluoranthene exposure did not result in detectable DNA damage by itself, but in combination with sunlight caused significant DNA damage to both genomes. PLRRP samples caused DNA damage to both genomes, and this was not increased by sunlight exposure, suggesting the DNA damage was unlikely the result of PAH phototoxicity. We report for the first time that PLRRP-associated pollutants cause both nuclear and mitochondrial DNA damage, and that fluoranthene-mediated phototoxicity results in similar levels of damage to the nuclear and mitochondrial genomes. These effects may be especially significant in sensitive marine ecosystems.
Keywords: Japanese medaka, parking lot runoff retention ponds, polycyclic aromatic hydrocarbons (PAHs), phototoxicity, DNA damage, Long amplicon QPCR assay
1. INTRODUCTION
Increasing urbanization is changing the way that water moves within watersheds and may concentrate nonpoint source pollution(Bay et al., 2003; Van Metre et al., 2000). Specifically, expanding impervious land cover leads pollutants to accumulate in major water bodies (Borden et al., 2002; Davis et al., 2001; Hwang and Foster, 2006; Karlsson et al., 2010; Weinstein et al., 2010a). Furthermore, coal tar-based sealcoat, used to protect asphalt parking lots, acts as a source of contaminants such as polycyclic aromatic hydrocarbons (PAHs). Van Metre and Mahler (2010) found that coal tar-based sealcoat was a major source of PAHs in 40 US lakes and Weinstein et al. (2010b) found that commercial runoff ponds were contaminated with levels of PAHs that exceed threshold effect concentrations, potential effect concentrations, and preliminary remediation goals by 10–90%. Watts et al. (2010) and Mahler et al. (2012) reported that use of coal tar-based sealcoat was the source of large increases of PAHs in run-off from parking lots.
Parking lot runoff retention ponds (PLRRP) are a stormwater control measure used to reduce peak flow volume to the watershed and are commonly used in strip malls (National Research Council, 2009). However, PLRRP have no filtering or treatment features and routinely contain complex mixtures of metals, salts, PAHs, heterocyclic aromatic compounds and other organic constituents (Bartlett et al., 2012; Gobel et al., 2007; McQueen et al., 2010; Wium-Andersen et al., 2011). This impaired water quality is of concern as contaminated PLRRPs routinely overflow, sending constituents into the surrounding ecosystems (Mahler et al., 2012; Wilson, 2011), and exposure to PLRRP samples causes toxicity in several aquatic species (Bommarito et al., 2010a; Bommarito et al., 2010b; McQueen et al., 2010). This source of pollution may be especially relevant in coast areas, which contain sensitive marine species. As yet, few studies have examined effects of PLRRP-derived samples on developing embryos, although PLRRP contaminants such as PAHs are well-known fish teratogens (Billiard et al., 2008) and early life stages of many organisms are particularly sensitive to contaminant exposure (Makri et al., 2004; McKim, 1977; Rand et al., 1995).
Similarly, while DNA damage is an important endpoint in ecotoxicology (Theodorakis, 2001), little is known regarding the potential for PLRRP to cause genotoxicity. The mitochondrial genome is increasingly recognized as the target of many environmental genotoxicants (Meyer et al., 2013), and there is evidence that PAHs, well-known genotoxicants (IARC, 1983), accumulate in the mitochondria and may cause more DNA damage in the mitochondrial as compared to the nuclear genome (Allen and Coombs, 1980; Backer and Weinstein, 1980). PAHs can be toxic via photo-induced toxicity, in which ultraviolet light increases the toxicity of PAHs by up to three orders of magnitude (Arfsten et al., 1996; Larson and Berenbaum, 1988). This photo-induced toxicity has been associated with nuclear DNA damage (Toyooka and Ibuki, 2007), but DNA damage has not been compared between genomes following photo-induced PAH toxicity. Given the evidence that PAHs accumulate in mitochondria, we hypothesized that damage would be greater in the mitochondrial genome. Although PAH phototoxicity is dramatic in laboratory studies, its ecological relevance is debated (McDonald and Chapman, 2002). We hypothesized that PLRRP might represent an example of ecologically relevant genotoxic phototoxicity.
Cyprus Bay Shopping Center in Morehead City, NC is a shopping center containing several major retail stores and approximately 25 local stores approximately 4 km upstream of where the Newport river flows into the Atlantic Ocean. The mall consists of 16,000 m2 of flat roof and paved parking area. Six PLRRPs, which are exposed to full sunlight and surrounded by grass edges, are located in the complex. The ponds are all directly connected to the parking lots and receive water through drains built into the pavement. The lots are treated with coal tar-based sealcoat, which was renewed in the late winter/early spring of 2009.
We report the results of two experiments designed to characterize early life stage toxicity from exposure to PLRRP samples from the Cyprus Bay Shopping Center. First, we exposed Japanese medaka (Oryzias latipes) in ovo to a dilution series of PLRRP water and assessed several outcomes including teratogenesis, hatching, and mortality. Second, we adapted the long amplicon quantitative PCR (LA-QPCR) assay for use with the medaka to study mitochondrial and nuclear DNA damage resulting from exposure to PLRRP water and sediments, and explored the role of phototoxicity in the mechanism of this toxicity.
2. MATERIALS AND METHODS
2.1 Parking lot runoff retention pond (PLRRP) sample collection
We collected PLRRP samples from a retention pond located on the northern edge of the Staples parking lot at the Cyprus Bay Shopping Center in Morehead City, North Carolina (34.7° N; 76.8° W). PLRRP samples were collected for the development study on January 15th, 2010; for the first DNA damage trial on September 20th, 2010; and for the second DNA damage trial on September 14th, 2011. There were no major (>0.2 cm) rain events for at least one week prior to each collecting trip, according to records from the National Oceanic and Atmospheric Administration (NOAA) station in Morehead City. Each individual PLRRP sample was taken from the pond with a pre-cleaned, 4L brown glass bottle. The water was first distributed so as to capture flocculent precipitates (“turbid water”). The container was filled (without leaving head room), and the water was covered and cooled as described by US Environmental Protection Agency (EPA) protocol (2002). Water was transported to Duke University, Durham, NC and stored in the dark at 4 °C until use.
2.2 Embryo collection
Orange-red (OR) outbred-medaka (Oryzias latipes) were maintained at the Duke University Aquatic Research Facility under standard recirculating water conditions following approved animal care and maintenance protocols (Duke University Institutional Animal Care and Use Committee). Fish were maintained at ~25°C and pH ~7.4 under a constant 16:8 light:dark cycle. Dry food (Otohime B1, Reed Mariculture, Campbell, CA) was fed several times per day via automated feeders with once daily supplementation of newly-hatched Artemia nauplii. Embryos were collected from this colony, and only embryos of stages 7–9 (i.e. <6h post fertilization (Iwamatsu, 2004)) were used (OECD, 1998).
2.3 Developmental toxicity experiment
The experiment was initiated over a two-day interval. PLRRP water was isolated from the first PLRRP sample by allowing sediments and organic material to settle out and pulling liquid from the top of the jar. Four dilutions of PLRRP water in embryo rearing medium (ERM: 17.1 mM NaCl; 272 μMCaCl2 * 2H2O, 402 μM KCl, and 661 μM MgSO4 * 7H2O (Kirchen and West, 1976)) were made using a 2-factor dilution scale (i.e 100%, 50%, 25%, and 12.5%) and are referred to as 1X, 0.5X, 0.25X, and 0.125X, respectively. Controls consisted of embryos incubated in ERM alone (i.e. 0X). For each treatment, duplicates of 24 embryos were placed, individually, in separate wells of 24-well plastic plates (BD Falcon, Franklin Lake, NJ).
PLRRP water pH, hardness, and dissolved oxygen (DO) were all measured and were suitable for morphological testing according to OECD guidelines (OECD, 1998). Similar measures were made on ERM. For mixtures of PLRRP water and ERM, the hardness and DO were calculated on a dilution scale.
As organic molecules sorb to plastic, the plates were first filled with 2 mL of their respective concentrations for three days prior to the experiment. When the experiment was initiated, the trays were emptied and each well was filled with 1 mL of respective dilution. Solutions were renewed on day seven. The plates were incubated in an environmental chamber at 25 ± 0.5° C on a 16:8 h light:dark cycle.
2.3.1 Teratogenesis, hatching, and mortality
We examined eggs for teratogenesis such as cranial-skeletal malformations, yolk-sac or pericardial edema, heart malformations, failure to inflate the swim bladder, and other gross deformities. To quantify the timing of organogenesis and hatching, seven stages of development were selected from the 40 stages described by Iwamatsu (2004), each 24 h apart in normal development: 25, 29, 32, 34, 36, 38, and 40. Each day, the stage of development and hatching of each embryo was identified with the aid of a Nikon SMZ 1500 dissecting microscope. Mortality was recorded as any egg with a brown, opaque shell or any embryo with heart but no heartbeat. Eggs that did not hatch by day 14 were considered dead. Hatching was defined as complete emergence from the chorion. After hatching, eleuthero-embryos were monitored for swimming activity and developmental abnormalities. The experiment was terminated on day 14 (OECD, 1998).
2.4 LA-QPCR development
To measure DNA damage in the nuclear and mitochondrial genomes, we adapted the LA-QPCR assay to medaka and normalizing to mitochondrial and nuclear DNA copy number as described by Rooney et al. (2014, in press). The long amplicon quantitative PCR (LA-QPCR) assay enables direct comparison of lesion frequency across the mitochondrial and nuclear genomes. LA-QPCR detects any DNA damage that inhibits the DNA polymerase, thus giving a relatively comprehensive quantification of genotoxicity. The LA-QPCR assay works by comparing amplification of a long section of sample DNA to amplification of control DNA; DNA lesions that block or slow the progression of DNA polymerase reduce amplification, and this reduced amplification can be converted mathematically to a number of lesions (sites of damage) per 10 kb (Ayala-Torres et al., 2000). A detailed summary of the benefits and limitations of this assay can be found in Meyer (2010), and details of the LA-QPCR assay development for medaka are presented in Supporting Information.
2.4.1 LA-QPCR optimization
LA-QPCR conditions were optimized as described by Meyer (2010) and are detailed in Table 1. Primers for the short and long nuclear targets were designed to amplify the DNA polymerase B gene. High transcriptional activity for this gene was confirmed using the University of Tokyo Genome Project Browser (http://utgenome.org/medaka/). Therefore, this assay will be useful in future studies of DNA repair where transcription-coupled repair is likely to be important. Primers for the short and long mitochondrial targets were designed to avoid the D-loop gene sequence and are shown schematically in Supporting Information. The primers and conditions for the short products have been described (Rooney et al., 2014, in press), and permit quantification of relative copy number of the mitochondrial and nuclear genomes, but not detection of DNA damage. DNA damage is detected by amplification of long products, with normalization to the short products (Hunter et al., 2010).
Table 1.
Assay conditions for LA-QPCR of Oryzias latipes
| Primers | Amplicon Length | Cycle Temp. and Time | No. of Cycles | MgO(Ac)2 Conc. (mM) | Primer Conc. (ng/μL) | |
|---|---|---|---|---|---|---|
|
Long Nuclear (DNA polymerase B gene) |
F475 (cacacagtttcacacggccatcg) R13411 (aaccgcctccgactggtgatgtt) |
12,936 bp | 75° 2′ 94° 1′ 94° 15″ 67° 14′ 72° 8′ 8° ∞ |
27 | 1.2 | 10 |
| Long Mitochondrial | F3422 (tgaaaccaaccgagccccttttg) R14546 (aatgctgtggcgatgtcggatgt) |
11,124 bp | 75° 2′ 94° 1′ 94° 15″ 69° 14′ 72° 8′ 8° ∞ |
20 | 1.2 | 10 |
2.4.2 LA-QPCR validation
To confirm optimization of the assay, we exposed purified medaka DNA to 5, 10, and 20 J/m2 UVC radiation as described (Bess et al., 2012) using a UVX digital radiometer and an ultraviolet lamp with peak emission at 254 nm (UVLMS-38 EL Series 3UV Lamp, UVP, Upland, CA, USA). There is a dose-dependent increase in damage when the assay is optimized.
2.5 DNA damage experiment
Two separate trials (one using the second PLRRP sample and the latter using the third PLRRP sample) of this experiment were conducted using medaka eleuthero-embryos that had hatched within 24 hours. This life stage was chosen because of its sensitivity and tendency to swim at the surface and be exposed to sunlight. For each exposure, embryos were maintained in ERM under a constant 16:8 light:dark cycle until hatched (9 days).
A 3 × 2 factorial design (control medium, positive control fluoranthene (FLU), PLRRP (with sediments); +/− sunlight) was used. Three replicates of each treatment (FLU, PLRRP, and control; +/− sunlight) were performed resulting in 18 exposures per trial. Solutions of FLU were made by diluting 10g/L DMSO stock solution (Absolute Standards, Inc.) to 10 μg/L in ERM. The concentration was chosen based on previous studies (Barron et al., 2005; Meyer and Di Giulio, 2003). The PLRRP sample was disturbed for use in the experiment so that exposures included settled, flocculent sediments and water. Sediments were included in the DNA damage analysis because our hypothesis concerned the toxicity of PAHs, which are predominantly hydrophobic. Furthermore, we identified high levels of PAH in sediments from the PLRRP site in previous unpublished work (see Supporting Information for detail). ERM was used as the control solution.
We randomly deposited 15 eleuthero-embryos into autoclaved beakers containing 100 mL of designated solution. Fish loading was less than 0.015 g/L. The beakers were incubated in an environmental chamber at 25± 0.5° C on a 16:8 h light:dark cycle for 20h. After this time, all solutions were replaced with 100mL of ERM to remove photomodified chemicals. Three replicates of each solution were exposed to natural sunlight for three hours at an average irradiance of 726 W/m2 (based on NOAA measurements in the nearby Duke Forest on the day of exposure [http://www.ncdc.noaa.gov/crn/station.htm?stationId=1347]), amounting to a total radiant exposure of 7484 KJ/m2. The remaining replicates of each solution were maintained in the environmental chamber with laboratory light for this period.
5.1 DNA extraction and damage analysis
After being exposed to sunlight, 10 fish from each of the 18 exposures were randomly removed and flash frozen in an eppendorf tube using liquid nitrogen. DNA was extracted with the Genomic-tip 20/G kit (Qiagen Inc., Valencia, CA, USA) per Hunter et al. (2010). Mechanical homogenization was identified as the method that produced the largest amount of high molecular weight DNA (Supporting Information). Samples were mechanically homogenized by adding 0.5 mL of G2 Buffer (with RNAse A) to the eppendorf tubes containing 10 mg of frozen fish and blending until visually smooth. Then, an additional 1.5mL of G2 Buffer and 100 μL of Proteinase K solution were added. The Genomic-tip protocol was followed after this point. DNA quantification and DNA damage analysis were performed as described by Hunter et al. (2010).
6. Statistical analysis
We constructed a Cox proportional hazards model to compare hatching success and mortality between PLRRP treatments and the control group. The Cox model is a semi-parametric method of longitudinal analysis that takes into account the time to “event” in the presence of censoring (Newman, 1995). The resultant “hazard ratio” can be interpreted as an odds ratio, where the treatments of interest have a multiplicative effect on the baseline risk equal to the hazard ratio. We evaluated the global effect of treatment using the Type III maximum likelihood estimates.
The survival model assumes that the survival curve is proportionate between treatments and the control group over time. This assumption may be violated when a major change in physiologic properties occurs (i.e. hatching). Consequently, we included an interaction term to test for differences in the hazard ratio of death pre and post hatching. As interactions are poorly powered, we considered α<0.2 statistically significant evidence of a difference in hazard ratio pre and post hatch. We also tested the stratified model for linear and sigmoidal trend and present the result with the lowest Akaike’s Information Criteria (AIC). The proportional hazard assumption was tested in the final, stratified models using the ASSESS option in PHREG (SAS version 9.3, Cary, NC).
We fit a generalized linear model to quantify the effects of sunlight, genome, and chemical treatment on lesion frequency and included an interaction term between light and each chemical treatment. This model can be represented as:
where each β estimates the effect of the independent variable. We also performed global tests for treatment and the interaction between treatments and sunlight. All analyses were performed using SAS (version 9.3, Cary, NC).
3. RESULTS
3.1 DEVELOPMENTAL TOXICITY EXPERIMENT
3.1.1 PLRRP water
The pH, hardness, and dissolved oxygen (DO) of the water treatments are displayed in Table 2. Hardness and DO results of all treatments were within OECD (1998) guidelines.
Table 2.
Physical Properties of the Water Samples. The EPA describes 120–124 ppm CaCO3 as between Medium Hard and Hard (EPA, 1986).
| pH | Hardness (ppm CaCO3) | Dissolved Oxygen (ppm) | |
|---|---|---|---|
| Control | 6.00 | 124 | 7.4 |
| .125x | 7.35 | 123.5* | 7.9* |
| .25x | 7.54 | 123* | 8.4* |
| .5x | 7.79 | 122* | 9.3* |
| 1x | 7.96 | 120 | 11.2 |
values marked with an asterisk were calculated based on dilution.
3.1.2 Teratogenesis, hatching, and mortality
We examined teratogenesis, hatching, and mortality differences in embryos raised in a dilutions series of PLRRP water compared to control embryos (Table 3). Treatment with PLRRP water did not result in detectable differences in teratogenesis (p=0.56). The average times to hatch ranged from 8.2–8.9 days. The proportional hazard model did not find a significant effect of dilution level of PLRRP water on the timing of embryo development (p=0.42; global effect of treatment).
Table 3.
Summary statistics detailing number and percent of embryos experiencing teratogenesis, hatching, and mortality and the mean time (SD) to oogenesis and mortality.
| Teratogenesis | Hatching | Mortality | ||||
|---|---|---|---|---|---|---|
| N | no. (%) | no. (%) | Mean day (SD) | no. (%) | Mean day (SD) | |
| 0X | 48 | 3 (6) | 35 (73) | 8.7 (1.2) | 12 (25) | 8.8 (5.9) |
| 0.125X | 48 | 2 (4) | 28 (58) | 8.4 (0.6) | 20 (42) | 6.6 (3.6) |
| 0.25X | 48 | 1 (2) | 34 (71) | 8.2 (0.5) | 14 (29) | 7.0 (5.0) |
| 0.5X | 48 | 5 (10) | 24 (50) | 8.4 (1.0) | 26 (54) | 6.3 (4.4) |
| 1X | 48 | 3 (6) | 28 (58) | 8.9 (1.3) | 20 (42) | 5.1 (3.8) |
PLRRP water treatments had lower final survivorship than the control, which had a final survivorship of 75%. The 0.125X, 0.25X, and 1X treatments had 58%, 71%, and 58% survivorship (respectively). The 0.5X treatment had the lowest survivorship of 46%. The hazard ratios of each treatment in the unstratified and stratified by pre and post hatching models are presented in Table 4. In the unstratified model, only the 0.5X treatment resulted in statistically significant higher mortality. The hazard ratios were all higher in the pre-hatch phase as compared to after hatching.
Table 4.
Hazard ratio (HR) and 95% confidence intervals (95% CI) for each treatment in the unstratified and stratified by pre and post hatching models. P-value for the difference between pre and post hatch hazard ratios.
| Unstratified Model | Pre-Hatch | Post-Hatch | Difference | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | p-value | |
| 0.125X | 1.54 (0.77, 3.12) | 2.84 (1.02, 7.89)* | 0.74 (0.26, 2.12) | 0.072 |
| 0.25X | 1.07 (0.51, 2.29) | 1.81 (0.61, 5.39) | 0.62 (0.20, 1.89) | 0.178 |
| 0.5X | 2.03 (1.04, 3.95)* | 3.48 (1.28, 9.44)* | 1.12 (0.43, 2.91) | 0.107 |
| 1X | 1.54 (0.77, 3.11) | 3.26 (1.19, 8.90)* | 0.48 (0.15, 1.62) | 0.017 |
p-value<0.05
After stratification, the 0.125X, 0.5X and 1X treatments exhibited elevated hazard ratios. The dose response structure of these results are presented in Figure 1. The pre-hatching model had a significant sigmoidal trend for treatment (p=0.04), but the post hatching model had no significant trend for treatment (p=0.45). Mortality was not statistically significantly different from the control in any of the treatments after hatching. The global effect of treatment was marginally significant pre-hatch (p=0.08) and not significant post-hatch (p=0.19).
Figure 1.
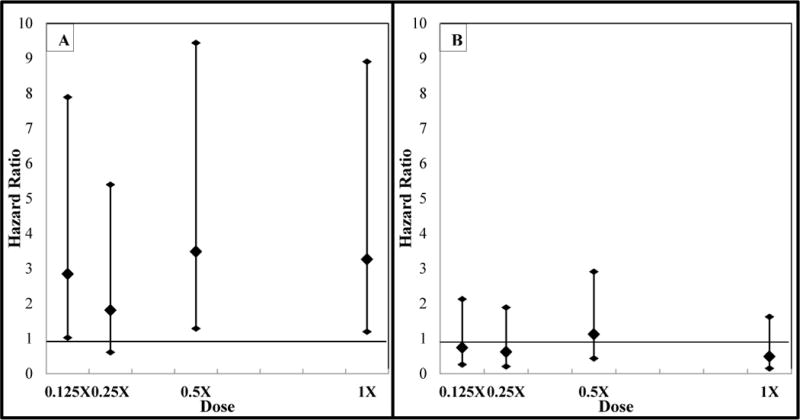
Dose response curve of the hazard ratios from 0.125X, 0.25X, 0.5X, and 1X dilution series of PLRRP water (A) before hatching and (B) after hatching. Error bars represent 95% confidence intervals.
3.2 DNA Damage from exposure to PLRRP, fluoranthene and sunlight
3.2.2 LA-QPCR validation
To confirm the ability of the assay to detect DNA damage, LA-QPCR was performed using DNA damaged by exposure to 5, 10, and 20 J/m2 UVC radiation. Lesions/10 kb increased linearly with UVC dose in both nuclear and mitochondrial DNA (Figure 2), and the number of lesions detected was in agreement with previous experiments using extracted DNA (Jung et al., 2009).
Figure 2.
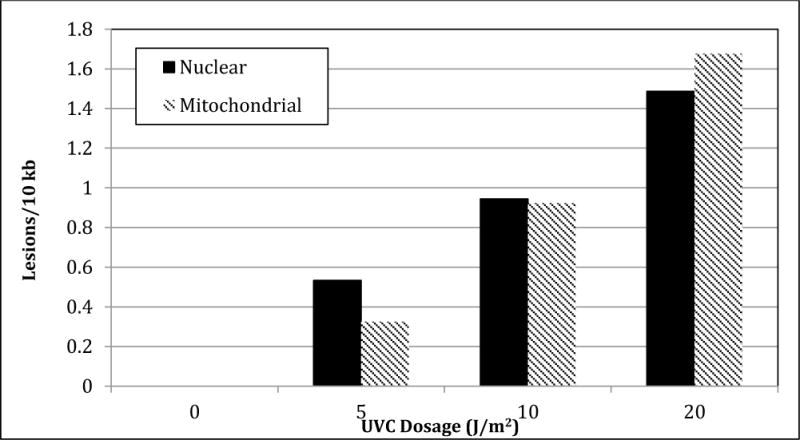
Lesions/10kb in medaka DNA exposed to 5 J/m2, 10 J/m2, and 20 J/m2 of direct UVC radiation.
3.2.3 LA-QPCR assay
We used the LA-QPCR assay to test the ability of a pure PAH, FLU and/or PLRRP sample (water with sediments), to cause DNA damage in the mitochondrial and nuclear genomes, with and without the addition of sunlight. The results are detailed in Figure 3.
Figure 3.
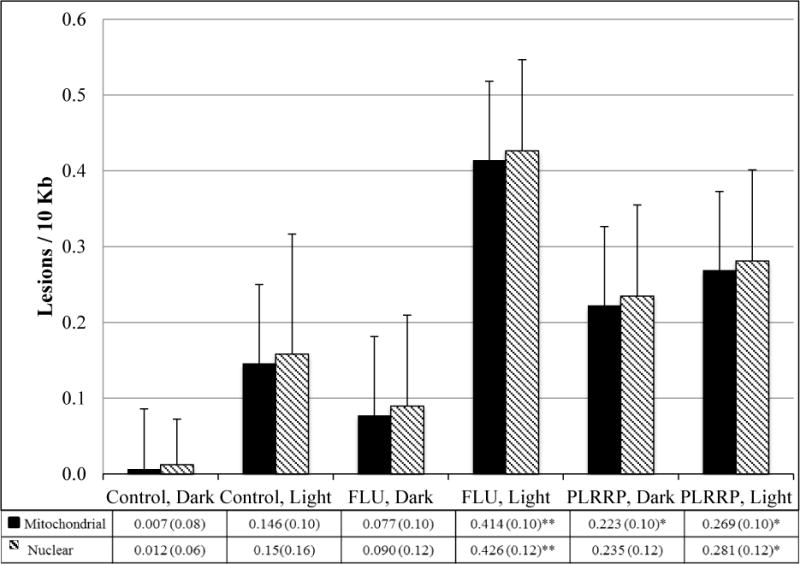
Estimated lesions/10kb (SD) in medaka DNA exposed to control (ERM), fluoranthene (FLU), and parking lot runoff retention pond (PLRRP) solutions, ± natural solar radiation (light/dark). Error bars represent the standard error of the mean.
*p ≤ 0.05
**p ≤ 0.001
Treatment (control, PLRRP, or FLU; p=0.031) and sunlight (p=0.005) but not genome (p=0.840) had significant main effects. There was also a statistically significant interaction between light and chemical treatment (p=0.045).
The largest amount of lesions resulted from exposure to FLU with full-spectrum natural solar radiation (Fig. 3). Notably, FLU by itself caused no detectable DNA damage. Both PLRRP treatments resulted in statistically significant damage, regardless of the presence of light (p=0.012 with sunlight, p=0.036 in the dark). Neither control treatment was statistically different from 0 lesions/10kb (p=0.164 with sunlight, p=0.934 in the dark).
4. DISCUSSION
We describe embryonic mortality and mitochondrial and nuclear DNA damage in Japanese medaka after exposure to PLRRP samples. To our knowledge, this is the first time that these endpoints have been studied in the context of PLRRP. We also report the adaptation of the LA-QPCR assay to Japanese medaka. Finally, we report for the first time that PAH-mediated phototoxicity resulted in similar levels of DNA damage in the mitochondrial and nuclear genomes.
4.1 Developmental toxicity experiment
PLRRP resulted in increased pre-hatching mortality but did not cause significant teratogenesis or delayed development. This result extends previous reports of toxicity in similar samples (Bommarito et al., 2010a; Bommarito et al., 2010b; McQueen et al., 2010) to another organism, and to a life stage that is frequently particularly vulnerable to toxicant exposure (Lanphear et al., 2005; McKim, 1977; Rand et al., 1995).
Differences in DO and pH in the test solutions compared to the control have the potential to affect the results of this assay, however all treatments were within OECD (1998) guidelines for DO and medaka are shown to develop normally between pH of 5.6 and 8.4 (Benoit et al., 1991; Murano et al., 2007). Additionally, we have found that medaka embryos develop normally in a broad range of solutions, including distilled water or full strength seawater (), and we do not believe the observed effects to be a result of these differences in solutions.
It was surprising to us that we observed mortality in the absence of significant teratogenesis. Previous work has shown similar normal development timing with increased mortality in medaka embryos exposed to polycyclic aromatic hydrocarbons (PAHs); however, these studies also found an increase in morphological abnormalities (Farwell et al., 2006; Gonzalez-Doncel et al., 2008). PLRRPs contain many constituents (Davis et al., 2001; Gobel et al., 2007; Weinstein et al., 2010a), including both herbicides and metals, which may act antagonistically to conceal any effects on developmental timing. For example, the herbicide Thiobencarb delays hatching in medaka (Villalobos et al., 2000), while copper accelerates hatching in the Fathead minnow, Pimephales promelas,(Scudder et al., 1988). Death is a competing risk in time to hatch analysis, and consequently, our interpretation of the time to hatch is limited to medaka that survived to hatch. We hypothesize that delays in hatching lead to a greater incidence of mortality and the full effect of contaminants on hatching timing is not elucidated in this analysis.
Our dilution series experiment did not show a typical dose response curve. The reason for this is not clear, but we note that a lack of dose-response relationship between dilutions and mortality has been observed in the past in the context of low-dose, complex mixtures. For example, exposure to intermediate concentrations (10mg/L) of thiobencarb caused increased survivorship as compared to lower concentrations (2.5 and 5mg/L) (Villalobos et al., 2000), and Scudder et al. (1988) did not find a dose dependent relationship between survivorship of the fathead minnow at total copper concentrations between 61μg/L and 338μ/L.
There are several limitations to this study that result from working with a complex and incompletely characterized mixture. For example, we cannot be sure of the final concentrations of pollutants in the solutions and we cannot characterize how pollutant mixtures, pH, or DO interfere with contaminant release or bioavailability. We believe that this logistical disadvantage is offset by the environmental realism of working with real-world samples, and these results provide impetus for future work including quantitative analysis of the complex chemical mixtures present in these samples.
4.2 DNA damage experiment
Consistent with a previous study (Stocker et al., 1996), we did not detect DNA damage from FLU in the dark. Also consistent with previous work (Toyooka and Ibuki, 2007), combined exposure to FLU and natural solar radiation resulted in significant DNA damage. PLRRP caused significant DNA damage with sunlight and elevated (though only marginally significant p=0.058) without sunlight. Previous work has identified genotoxic effects of stormwater runoff (Marsalek et al., 1999). Stormwater runoff is likely to contain many DNA-damaging chemicals other than strictly photo-activated genotoxins, including metals and pesticides. Our results suggest that photo-activatable PAHs were not the major contributors to PLRRP genotoxicity, at least in these samples.
Delivery of PAHs to PLRRPs is episodic, directly associated with precipitation events. The water used in these experiments was collected over one week after the last significant rainfall events. Because parking lot runoff ponds receive constant, unobstructed sunlight, PAHs in PLRRP were likely photomodified before use in these experiments, which may explain why no interaction with sunlight was seen.
That no difference was found between genomes for either PLRRP or FLU was surprising, since some PAHs accumulate preferentially in mitochondria, and can cause more mitochondrial than nuclear DNA damage (Allen and Coombs, 1980; Backer and Weinstein, 1980). It has been suggested that this results from a combination of accumulation of lipophilic PAHs in membrane-rich mitochondria, the capacity of mitochondrial P450s to activate parent PAHs, and the absence of the nucleotide excision repair, which handles bulky PAH adducts, in mitochondria (Meyer et al., 2013). However, our results, combined with other reports of relatively small differences for genotoxic PAHs (eg, Jung et al., 2009; Ayala-Torres et al., 2000; reviewed in Meyer at al., 2013), suggest that PAHs’ greater toxicity to the mitochondrial genome is dependent on context. Of note, photoactivated FLU-mediated DNA damage would be largely oxidative in nature, and most oxidative DNA damage can be repaired in mtDNA due to the presence of the base excision repair pathway in mitochondria (Meyer et al., 2013); thus, in that case, perhaps it is not surprising that there was not a genome difference. With respect to the PLRRP results, of course, it is also possible that the damage we measured in both genomes derived from chemical constituents other than PAHs.
5. Conclusions
Exposure to PLRRP caused an increase in the odds of early embryo mortality and DNA damage in Japanese medaka. PLRRP water is chemically complex and varies with time, and is therefore inherently difficult to study. However, the biological effects that we and others have reported, coupled with the knowledge that contaminants from these ponds enter larger ecosystems, indicate that further study and characterization of the chemistry and toxicity of PLRRPs is warranted. The toxicity of PLRRP may be especially significant in sensitive marine ecosystems such as Morehead City, NC. As PLRRP are increasingly extensive habitats with resident and visiting constituents, these kinds of studies would be valuable in informing policy.
Supplementary Material
Acknowledgments
We thank Heather Stapleton for advice on chemical analysis. This work was supported by P42 ES010356-10A2, and the National Science Foundation (NSF) and the Environmental Protection Agency under NSF Cooperative Agreement EF-0830093, Center for the Environmental Implications of NanoTechnology (CEINT). Any opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NSF or the EPA. This work has not been subjected to EPA review and no official endorsement should be inferred. The funding sources had no role in experimental design, data collection, or interpretation.
Footnotes
Author Contributions: MDC designed this experiment with guidance from JNM, DR, and DEH. MDC, KHWK, ITR, JAB, IHW, and EMC gathered samples and performed experiments. Statistical analysis was performed by MDC and JNM. The paper was written by MDC and JNM.
References
- Allen J, Coombs M. Covalent binding of polycyclic aromatic compounds to mitochondrial and nuclear DNA. 1980. [DOI] [PubMed] [Google Scholar]
- Arfsten DP, Schaeffer DJ, Mulveny DC. The Effects of Near Ultraviolet Radiation on the Toxic Effects of Polycyclic Aromatic Hydrocarbons in Animals and Plants: A Review. Ecotoxicology and Environmental Safety. 1996;33:1–24. doi: 10.1006/eesa.1996.0001. [DOI] [PubMed] [Google Scholar]
- Ayala-Torres S, Chen Y, Svoboda T, Rosenblatt J, Van Houten B. Analysis of gene-specific DNA damage and repair using quantitative polymerase chain reaction. Methods. 2000;22:135–147. doi: 10.1006/meth.2000.1054. [DOI] [PubMed] [Google Scholar]
- Backer J, Weinstein I. Mitochondrial DNA is a major cellular target for a dihydrodiol-epoxide derivative of benzo[a]pyrene. Science. 1980;209:297–299. doi: 10.1126/science.6770466. [DOI] [PubMed] [Google Scholar]
- Barron MG, Carls MG, Short JW, Rice SD, Heintz RA, Rau M, Di Giulio RT. Assessment of the phototoxicity of weathered Alaska North Slope crude oil to juvenile pink salmon. Chemosphere. 2005;60:105–110. doi: 10.1016/j.chemosphere.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Bartlett AJ, Rochfort Q, Brown LR, Marsalek J. Causes of toxicity to Hyalella azteca in a stormwater management facility receiving highway runoff and snowmelt. Part I: polycyclic aromatic hydrocarbons and metals. The Science of the total environment. 2012;414:227–237. doi: 10.1016/j.scitotenv.2011.11.041. [DOI] [PubMed] [Google Scholar]
- Bay S, Jones BH, Schiff K, Washburn L. Water quality impacts of stormwater discharges to Santa Monica Bay. Marine Environmental Research. 2003;56:205–223. doi: 10.1016/S0141-1136(02)00331-8. [DOI] [PubMed] [Google Scholar]
- Benoit D, Holcombe G, Spehar R. In: Guidelines for Conducting Early Life Stage Toxicity Tests with Japanese Medaka (Oryzias Latipes) Agency, U.E.P, editor. US Environmental Protection Agency; Duluth, MN: 1991. [Google Scholar]
- Bess AS, Crocker TL, Ryde IT, Meyer JN. Mitochondrial dynamics and autophagy aid in removal of persistent mitochondrial DNA damage in Caenorhabditis elegans. Nucleic acids research. 2012;40:7916–7931. doi: 10.1093/nar/gks532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiard SM, Meyer JN, Wassenberg DM, Hodson PV, Di Giulio RT. Nonadditive effects of PAHs on Early Vertebrate Development: mechanisms and implications for risk assessment. Toxicological sciences : an official journal of the Society of Toxicology. 2008;105:5–23. doi: 10.1093/toxsci/kfm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommarito T, Sparling D, Halbrook R. Toxicity of coal-tar pavement sealants and ultraviolet radiation to Ambystoma Maculatum. Ecotoxicology. 2010a;19:1147–1156. doi: 10.1007/s10646-010-0498-8. [DOI] [PubMed] [Google Scholar]
- Bommarito T, Sparling DW, Halbrook RS. Toxicity of coal-tar and asphalt sealants to eastern newts, Notophthalmus viridescens. Chemosphere. 2010b;81:187–193. doi: 10.1016/j.chemosphere.2010.06.058. [DOI] [PubMed] [Google Scholar]
- Borden RC, Black DC, Mcblief KV. MBTE and aromatic hydrocarbons in North Carolina stormwater runoff. Environmental Pollution. 2002;118:141–152. doi: 10.1016/s0269-7491(01)00204-4. [DOI] [PubMed] [Google Scholar]
- Davis AP, Shokouhian M, Ni S. Loading estimates of lead, copper, cadmium, and zinc in urban runoff from specific sources. Chemosphere. 2001;44:997–1009. doi: 10.1016/s0045-6535(00)00561-0. [DOI] [PubMed] [Google Scholar]
- EPA, U, editor. EPA, U. Quality criteria for water. Washington, DC 20460: 1986. [Google Scholar]
- EPA, U. Methods for Measuring the Acute Toxicity of Effluents and Receiving Water to Freshwater and Marine Organisms. Washington, DC: 2002. [Google Scholar]
- Farwell A, Nero V, Croft M, Bal P, Dixon D. Modified Japanese Medaka Embryo-Larval Bioassay for Rapid Determination of Developmental Abnormalities. Archives of Environmental Contamination and Toxicology. 2006;51:600–607. doi: 10.1007/s00244-005-0319-x. [DOI] [PubMed] [Google Scholar]
- Gobel P, Dierkes C, Coldewey W. Storm water runoff concentration matrix for urban areas. Journal of Contaminant Hydrogeology. 2007;91:26–42. doi: 10.1016/j.jconhyd.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Doncel M, Gonzales L, Fernandez-Torija C, Navas JM, Tarazona JV. Toxic effects of an oil spill on fish early life stages may not be exclusively associated to PAHs: studies with Prestig oil and medaka (Oryzias latipes) Aquatic Toxicology. 2008;87:280–288. doi: 10.1016/j.aquatox.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Hunter SE, Jung D, Di Giulio RT, Meyer JN. The QPCR assay for analysis of mitochondrial DNA damage, repair, and relative copy number. Methods. 2010;51:444–451. doi: 10.1016/j.ymeth.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HM, Foster GD. Characterization of polycyclic aromatic hydrocarbons in urban stormwater runoff flowing into the tidal Anacostia River, Washington, DC, USA. Environmental Pollution. 2006;140:416–426. doi: 10.1016/j.envpol.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Organization, W.H, editor. IARC, I.A.f.R.o.C. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Lyon, France: 1983. [Google Scholar]
- Iwamatsu T. Stages of normal development in the medaka Oryzias latipes. Mechanisms of Development. 2004;121 doi: 10.1016/j.mod.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Jung D, Cho Y, Meyer JN, Di Giulio RT. The long amplicon quantitative PCR for DNA damage assay as a sensitive method of assessing DNA damage in the environmental model, Atlantic killifish (Fundulus heteroclitus) Comparative Biochemistry and Physiology, Part C. 2009;149:182–186. doi: 10.1016/j.cbpc.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K, Viklander M, Scholes L, Revitt M. Heavy metal concentrations and toxicity in water and sediment from stormwater ponds and sedimentation tanks. Journal of Hazardous Materials. 2010;178:612–618. doi: 10.1016/j.jhazmat.2010.01.129. [DOI] [PubMed] [Google Scholar]
- Kirchen R, West W. The Japanese medaka. In: Supply, C.B, editor. Its care and development. Burlington, NC: 1976. [Google Scholar]
- Lanphear BP, Vorhees CV, Bellinger DC. Protecting children from environmental toxins. PLoS medicine. 2005;2:e61. doi: 10.1371/journal.pmed.0020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson RA, Berenbaum MR. Environmental phototoxicity. Environmental Science & Technology. 1988;22:354–360. [Google Scholar]
- Mahler BJ, Metre PC, Crane JL, Watts AW, Scoggins M, Williams ES. Coal-tar-based pavement sealcoat and PAHs: implications for the environment, human health, and stormwater management. Environ Sci Technol. 2012;46:3039–3045. doi: 10.1021/es203699x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makri A, Goveia M, Balbus J, Parkin R. Children’s susceptibility to chemicals: a review by developmental stage. Journal of toxicology and environmental health. Part B, Critical reviews. 2004;7:417–435. doi: 10.1080/10937400490512465. [DOI] [PubMed] [Google Scholar]
- Marsalek J, Rochfort Q, Brownlee B, Mayer T, Servos M. An exploratory study of urban runoff toxicity. Water Science and Technology. 1999;39:33–39. [Google Scholar]
- McDonald BG, Chapman PM. PAH phototoxicity– an ecologically irrelevant phenomenon? Marine Pollution Bulletin. 2002;44:1321–1326. doi: 10.1016/s0025-326x(02)00358-2. [DOI] [PubMed] [Google Scholar]
- McKim JM. Evaluation of tests with early life stages of fish for predicting long-term toxicity. Journal of the Fisheries Research Board of Canada. 1977;34:1148–1154. [Google Scholar]
- McQueen AD, Johnson BM, Rodgers JH, Jr, English WR. Campus parking lot stormwater runoff: Physicochemical analyses and toxicity tests using Ceriodaphnia dubia and Pimephales promelas. Chemosphere. 2010;79:561–569. doi: 10.1016/j.chemosphere.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Meyer JN. QPCR: a tool for analysis of mitochondrial and nuclear DNA damage in ecotoxicology. Ecotoxicology. 2010;19:804–811. doi: 10.1007/s10646-009-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Di Giulio RT. Heritable adaptation and fitness costs in Killifish (Fundulus Heteroclitus) inhabiting a polluted estuary. Ecological Applications. 2003;13:490–503. [Google Scholar]
- Meyer JN, Leung MC, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS. Mitochondria as a target of environmental toxicants. Toxicological sciences : an official journal of the Society of Toxicology. 2013;134:1–17. doi: 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murano H, Matsuzaki K, Shiraishi H, Wakabayashi M. Effects of heavy metals in river waters in Japan on immobility and mortality of Daphnia magna and Oryzias latipes larvae. Fisheries Science (Tokyo) 2007;73:1078–1086. [Google Scholar]
- National Research Council, C.o.R.S.D.C.t.W.P. Urban stormwater management in the United States. National Acadamies Press; Washington DC; 2009. [Google Scholar]
- Newman MC. Quantitative methods in aquatic ecotoxicology. CRC press; 1995. [Google Scholar]
- Directorate, E, editor. OECD, O.f.E.C.-o.a.D. OECD Guideline for testing of chemicals. 1998. [Google Scholar]
- Rand G, Wells P, McCarty L. Fundamentals of aquatic toxicology effects, environmental fate, and risk assessment. Taylor and Francis Publishers; North Palm Beach, Florida, USA: 1995. [Google Scholar]
- Rooney J, Ryde I, Saunders L, Colton M, Germ K, Mater G, Greenamyr J, Meyer J. PCR-based determination of mitochondrial DNA copy number in multiple species, Methods in Molecular Biology: Mitochondrial Regulation: Methods and Protocols. 2014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder BC, Carter JL, Leland HV. Effects of copper on development of the fathead minnow, Pimephales promelas Rafinesque. Aquatic Toxicology. 1988;12:107–124. [Google Scholar]
- Stocker KJ, Howard WR, Statham J, Proudlock RJ. Assessment of the potential in vivo genotoxicity of fluoranthene. Mutagenesis. 1996;11:493–496. doi: 10.1093/mutage/11.5.493. [DOI] [PubMed] [Google Scholar]
- Theodorakis CW. Integration of genotoxic and population genetic endpoints in biomonitoring and risk assessment. Ecotoxicology. 2001;10:245–256. doi: 10.1023/a:1016677629442. [DOI] [PubMed] [Google Scholar]
- Toyooka T, Ibuki Y. DNA damage induced by coexposure to PAHs and light. Environmental Toxicology and Pharmacology. 2007;23:256–263. doi: 10.1016/j.etap.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ. Contribution of PAHs from coal-tar pavement sealcoat and other sources in 40 US lakes. Science of the Total Environment. 2010;409:334–344. doi: 10.1016/j.scitotenv.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ, Furlong ET. Urban Sprawl Leaves Its PAH Signature. Environmental Science & Technology. 2000;34:4064–4070. [Google Scholar]
- Villalobos A, Hamm JT, Teh SJ, Hinton DE. Thiobencarb-induced embryotoxicity in medaka (Oryzias latipes): stage-specific toxicity aand the protective role of chorion. Aquatic Toxicology. 2000;48:309–326. doi: 10.1016/s0166-445x(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Watts AW, Ballestero TP, Roseen RM, Houle JP. Polycyclic aromatic hydrocarbons in stormwater runoff from sealcoated pavements. Environ Sci Technol. 2010;44:8849–8854. doi: 10.1021/es102059r. [DOI] [PubMed] [Google Scholar]
- Weinstein J, Crawford K, Garner T. Polycyclic aromatic hydrocarbon contamination in stormwater detention pond sediments in coastal South Carolina. Environmental Monitoring and Assessment. 2010a;162:21–35. doi: 10.1007/s10661-009-0773-4. [DOI] [PubMed] [Google Scholar]
- Weinstein JE, Crawford KD, Garner TR, Flemming AJ. Screening-level ecological and human health risk assessment of polycyclic aromatic hydrocarbons in stormwater detention pond sediments of Coastal South Carolina, USA. Journal of Hazardous Materials. 2010b;178:906–916. doi: 10.1016/j.jhazmat.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Wilson W. Constructed Climates: A primer on urban environment. The University of Chicago Press; Chicago: 2011. [Google Scholar]
- Wium-Andersen T, Nielsen AH, Hvitved-Jakobsen T, Vollertsen J. Heavy metals, PAHs and toxicity in stormwater wet detention ponds. Water science and technology : a journal of the International Association on Water Pollution Research. 2011;64:503–511. doi: 10.2166/wst.2011.075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


