SUMMARY
Melanoma brain metastasis (MBM) is frequent and has a very poor prognosis with no current predictive factors or therapeutic molecular targets. Our study unravels the molecular alterations of cell-surface glycoprotein CD44 variants during melanoma progression to MBM. High expression of CD44 splicing variant 6 (CD44v6) in primary melanoma (PRM) and regional lymph node metastases from AJCC Stage IIIC patients significantly predicts MBM-development. The expression of CD44v6 also enhances the migration of MBM cells by hyaluronic acid and hepatocyte growth factor exposure. Additionally, CD44v6-positive MBM migration is reduced by blocking with CD44v6-specific monoclonal antibody or knocking down CD44v6 by siRNA. ESRP1 and ESRP2 splicing factors correlate with CD44v6 expression in PRM, and ESRP1 knockdown significantly decreases CD44v6 expression. However, an epigenetic silencing of ESRP1 is observed in metastatic melanoma, specifically in MBM. In advanced melanomas, CD44v6 expression is correlated with PTBP1 and U2AF2 splicing factors, and we found that PTBP1 knockdown also significantly decreases CD44v6 expression. Overall, these findings open up a new avenue for understanding the high affinity of melanoma to MBM, suggesting CD44v6 as a potential MBM-specific factor with theranostic utility for stratifying patients.
Keywords: CD44v6, alternative splicing, spliceosome, brain metastasis, melanoma
INTRODUCTION
Cutaneous melanoma is the most deadly type of skin cancer (Bristow et al., 2013) and its incidence has been steadily rising for the last three decades (Siegel et al., 2014). In addition, melanoma cells have a high affinity to metastasize the brain (Sundstrom et al., 2013). More than 75% of Stage IV melanoma patients will eventually develop brain metastases (MBMs) with less than one year of median survival (de la Monte et al., 1983; Sampson et al., 1998). Explanations for this high affinity could stem from the fact that melanoma and brain cells both derive from the neuroectoderm and thrive in a similar microenvironment niche (Fidler et al., 2010; Izraely et al., 2012). However, the ability of cancer cells to acquire metastatic traits remains unclear. Because of melanoma's high propensity to metastasize to the brain and the resulting poor prognosis, there is a major need to better understand the molecular mechanisms that allow melanoma cells to metastasize and survive in the brain and identify potential theranostic cell surface markers.
CD44 glycoproteins, a family of cell surface adhesion molecules, are key receptors of hyaluronic acid (HA), which is one of the most important components of the extracellular matrix with a high concentration in brain tissue (Ananthanarayanan et al., 2011; Aruffo et al., 1990). Activation of CD44 by HA leads to an enhanced migration of melanoma and other tumor cells (Ichikawa et al., 1999). The blocking of the interaction between CD44 and HA by CD44-specific antibodies was recently identified as a therapeutic target for colon cancer (Misra et al., 2011). CD44 is also necessary for the induction of metastatic migration and invasion by hepatocyte growth factor (HGF), a factor released during inflammation or tissue disruption (Orian-Rousseau et al., 2002). Different isoforms of this glycoprotein are generated by alternative splicing of CD44 gene with diverse biological properties (Misra et al., 2011; Screaton et al., 1992). Alternative splicing of the CD44 gene generates two main types of CD44 proteins, one containing only standard exons (CD44s), and the other including diverse combinations of 9 variant exons (CD44v) (Goodison et al., 1999). Clinically, an increment in the ratio CD44v/CD44s enhances the tumorigenicity and metastatic potential of colon cancer cells (Choi et al., 2000). In melanoma, in vivo and in vitro models have indicated that the expression of specific CD44v is related with tumor progression (Raso-Barnett et al., 2013). Different mediators of CD44 alternative splicing have been described, including epithelial splicing regulatory proteins (ESRPs), serine/arginine repetitive matrix 1 (SRRM1), transformer 2 beta homolog (TRA2B), and Y box binding protein 1 (YBX1) (Cheng and Sharp, 2006; Stickeler et al., 2001; Watermann et al., 2006; Yae et al., 2012). The core spliceosome is a large ribonucleoprotein (RNP) complex that comprises the U1, U2, U4/6 and U5 small nuclear RNPs (snRNPs) (Wahl et al., 2009). Recent studies indicate that the expression level of splicing factors can affect splice site choice and generate tissue-specific splicing programs (Wahl et al., 2009). To date, very little is known about the role of splicing mediators in neuroectoderm-derived cancers such as melanoma and their role in tumor progression.
In this study, we screened CD44 isoforms in melanoma progression to MBMs using clinical specimens (n=195) and cell lines (n=41). CD44v6 isoform expression was significantly higher in MBMs than in other organ metastases. In primary melanomas (PRMs) as well as lymph node metastases (LNMs) resected from AJCC Stage IIIC patients, the expression of CD44v6 significantly predicted MBM progression. The functional role of CD44v6 was uncovered through the detection of an enhanced migration potential of MBM cells in response to hyaluronic acid (HA) and hepatocyte growth factor (HGF) stimulation. Importantly, the migration was significantly decreased with a CD44v6-specific antibody and siRNA-mediated knockdown. Gene expression and DNA methylation of 190 splicing factors were evaluated in the progression to MBM. ESRP1 and ESRP2 played a significant role in CD44v6 expression in PRM; however, ESRP1 was epigenetically silenced in advanced melanoma. In distant organ metastases, CD44v6 expression was correlated with two different splicing factors, PTBP1 and U2AF2. Furthermore, PTBP1 knockdown significantly decreased CD44v6 expression on MBM cell lines. This study reveals a new avenue for understanding the high affinity between melanoma cells and the human brain, and offers CD44v6 as a potential MBM-specific factor with theranostic utility for melanoma patients.
RESULTS
CD44v/CD44s ratio is increased in melanoma brain metastasis
To identify the transcript variants generated by alternative splicing of CD44, a compilation of experimentally-validated and computationally-predicted CD44 isoforms was performed. Based on the Ref-Seq data, three alternative isoforms exclusively containing standard exons (s1 to s10) and nine alternative isoforms involving variant exons (v2 to v10) were found. Using the Gnomon predictive methods, six additional isoforms containing combinations of variant exons were also identified (Fig. 1A). To evaluate the expression level of standard and variant exons, 41 early-passage metastatic melanoma cell lines were analyzed by Human Exon 1.0 (Affymetrix) microarray (Supplemental Table 1). Overall, standard exons presented significantly higher expression levels than variant exons. Interestingly, the expression of variant exons presented a larger variance than standard exons, suggesting higher variability in the expression of this region of the CD44 gene among metastatic melanoma cells (Fig. 1B). Cell lines were divided into AJCC Stage III lymph node metastases (LNMs) and Stage IV melanoma brain metastases (MBMs). The average expression of variant exons was higher in MBM than in LNM cell lines (Fig. 1B). By applying the formula: mean expression of variant exons / (mean expression of 5’-standard exons + mean expression of 3’-standard exons), the CD44 variant/standard (CD44v/s) ratio was calculated for each specimen. MBM cell lines presented a significantly higher CD44v/s ratio than LNM cell lines (W, p=0.001; Fig. 1C). Furthermore, the ratio for each variant exon and each CD44v isoform was calculated. Most of the variant exons had significantly higher expression in MBM than in LNM lines, with exon v6, as well as the isoforms containing exon v6, presenting the largest difference in expression level (Fig. 1D). Because of this significantly higher expression, further studies focused on the role of CD44v6 in MBM were performed.
Figure 1. Alternative transcripts generated from CD44 gene on brain and lymph node metastatic melanoma cell lines.
A. Experimentally validated (Ref-Seq data) and computationally predicted (Gnomon method) CD44 isoforms. Variant exons v2 to v10 are highlighted (not a scale representation of CD44 gene). B. Expression level of each (standard and variant) CD44 exons in metastatic melanoma cell lines (n=41; Supplemental Table 1) derived from lymph node metastases (LNMs) and melanoma brain metastases (MBMs). The mean of exon expression for LNM (orange line) and MBM (red line) and the standard error of the mean (S.E.M.) are represented in the plot. C. Box plot comparing the ratio of CD44v exon and standard exon (CD44v/s) expression between LNM (orange) and MBM (red). D. Box plot comparing the CD44v/s ratio for each individual variant exon and isoforms combining variant exons between LNM (orange) and MBM (red). * is p≤0.05 and ** is p≤0.01.
Expression of CD44v6 increases in MBMs
To evaluate CD44v6 differential expression at protein level, flow cytometry analysis using a CD44v6-specific antibody (Ab) was carried out in 10 melanoma cell lines (5 LNMs with low expression of exon v6 mRNA and 5 MBMs with high expression of exon V6 mRNA; Supplemental Table 1). In concordance with exon expression analyses, a significantly higher expression of CD44v6 protein was detected in the plasma membrane of MBM than in LNM cell lines (W, p=0.008; Fig. 2A-B). Subsequently, extending this analysis to tissue specimens, CD44v6 protein expression was analyzed by immunohistochemistry (IHC) in a large cohort (n=195) of clinically well-annotated paraffin-embedded archival tissue (PEAT) melanoma specimens. Initially, the assessment involved IHC detection of CD44v6 protein in LNMs (n=42), MBMs (n=52) and non-brain metastasis (non-MBMs; n=50) specimens. The results were scored (IHC score = tumor intensity – background intensity) for each tissue type, as described in the methods section (Fig. 2C). In accordance with the results observed in cell lines, MBM PEAT specimens presented a significantly higher expression of CD44v6 than LNM PEAT specimens (W; p=6.12×10−5; Fig. 2D). Interestingly, among AJCC Stage IV specimens, the level of CD44v6 expression was significantly higher in MBM than in non-MBM specimens (W; p=8.65×10−7; Fig. 2D). Specifically, CD44v6 expression was significantly higher in MBM than bowel and lung melanoma metastases (W; p=3.0×10−5 and p=0.02, respectively; Supplemental Fig. 1A). To identify how early CD44v6 could be detected in the melanoma progression to MBM, the IHC analysis was extended to primary melanomas (PRMs; n=51). Two long-term followed-up patient cohorts were evaluated, PRMs from patients with (n=28) and without (n=23) progression to MBM. The expression of CD44v6 was significantly higher in PRMs from patients that developed MBM than in PRMs from patients with MBM-free survival (W, p=0.02; Fig. 2E). This finding suggests that certain PRMs may be prone to develop MBM.
Figure 2. CD44v6 protein expression on melanoma progression to brain metastasis.
A. Histograms showing the quantitative FACS analysis of anti-CD44v6 antibody (Ab) reactivity for lymph node metastasis (LNM; upper panel) and melanoma brain metastasis (MBM; lower panel) cell lines. The percentage of CD44v6-specific signal is represented in each histogram. The ratio between CD44 exon variant 6 and standard exons (v6/s) is represented between brackets for each cell line. B. Box plot comparing the expression of CD44v6 protein evaluated by quantitative FACS analysis in MBM (red) and LNM (orange) melanoma cell lines. C. Representative photographs of IHC staining with anti-CD44v6 Ab for PRM, LNM, non-MBM (liver metastasis) and MBM. Bars indicate 100 μm in length. D. Box plot comparing the IHC score for LNM (orange), MBM (red) and non-MBM (blue). E. Box plot comparing the IHC score for PRM (brown) from patients with and without development of clinical MBM PEAT specimens. * is p≤0.05 and ** is p≤0.01.
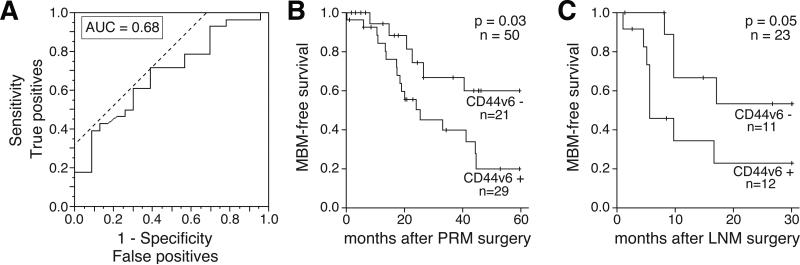
CD44v6 expression in PRMs and LNMs predicts MBM-development
Based on the significantly higher expression of CD44v6 protein detected in PRMs from patients that developed MBM, the MBM-predictive potential of CD44v6 was analyzed. Receiver operating characteristic (ROC) curve analysis was used to classify PRMs from patients at high-risk for MBM-development (n=28; CD44v6 positive) or at low-risk for MBM-development (n=23; CD44v6 negative; Fig. 3A). A modest performance of CD44v6 to predict MBM occurrence was observed (AUC=0.68). Based on the IHC score threshold value (27.0), patients with a high IHC score (CD44v6-positive) were classified in the “high-risk for MBM” group and patients with a low IHC score (CD44v6-negative) were classified in the “low-risk for MBM” group, with a sensitivity of 71.4% and a specificity of 60.9%. Patients with a high CD44v6 IHC score in PRMs had a relative 2-fold higher risk of MBM-development than patients with a low CD44v6 IHC score in PRMs (95% CI, 1.05-3.83, p=0.02). Survival analysis indicated a significantly lower MBM-free survival for melanoma patients with CD44v6-positive PRMs (Log-rank, p=0.03; Fig. 3B). Multivariate analysis showed that CD44v6 expression was independently associated with MBM-free survival (p=0.01; Table 1). To evaluate the predictive potential of CD44v6 expression in regional metastatic melanomas, the analysis was extended to LNM specimens. Based on the median IHC score for CD44v6 expression on LNM (31.07), these specimens were classified into two groups (CD44v6 positive (n=21) and CD44v6 negative (n=21)). Overall, there was no significant higher risk of MBM-development in the CD44v6-positive group. However, AJCC Stage IIIC melanoma patients with CD44v6-positive LNMs (n=12) had a 1.6-times higher risk of MBM-advancement than melanoma AJCC Stage IIIC patients with CD44v6-negative LNMs (n=11; 95% CI, 1.01-2.46, p=0.03). CD44v6 expression in regional LNMs resected from AJCC Stage IIIA or B melanoma patients did not show significant association with MBM risk. Accordingly, analyses of CD44v6 expression in LNMs of Stage IIIC patients with respect to MBM-free survival were carried out. A slightly shorter MBM-free survival was identified in AJCC Stage IIIC patients with CD44v6-positive LNMs (Log-rank, p=0.05; Fig. 3C). These results propose a potential MBM-predictive utility of CD44v6 expression in two different stages of melanoma progression, PRMs and LNMs from AJCC Stage IIIC patients.
Figure 3. CD44v6 expression predicts melanoma brain metastasis development.
A. ROC curve showing whether CD44v6 expression in primary melanoma (PRM) can classify patients with melanoma brain metastasis (MBM; n=27, CD44-positive) vs. patients without history of MBM (n=23, CD44-negative). The AUC is 0.68, with an optimal cutoff for CD44v6 expression at 27, at a sensitivity of 74.1% and specificity of 60.9%. B. Kaplan-Meier curves showing the MBM-development for melanoma patients with (n=21) and without (n=29) CD44v6 expression in PRM. C. Kaplan-Meier curves showing the MBM-development for AJCC Stage IIIC melanoma patients with (n=12) and without (n=11) CD44v6 expression in lymph node metastasis (LNM).
TABLE 1.
Univariate and multivariate analysis of melanoma brain metastasis free survival by Cox proportional hazard ratio
| Variable | Factor | Patients (n=50) | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | value | Hazard Ratio | 95% CI | value | |||
| Gender | 0.92 | |||||||
| M | 29 | 1.04 | (0.46-2.51) | 0.92 | ||||
| F | 21 | 1 | (Reference) | |||||
| Age | (Mean ± SD) | 62.3 ± 16.7 | 1.01 | (0.99-1.04) | 0.29 | 1.02 | (0.99-1.06) | 0.11 |
| Breslow | 0.26 | |||||||
| <1.00mm | 5 | 1 | (Reference) | |||||
| 1.01-2.00mm | 9 | 0.38 | (0.07-2.09) | 0.25 | ||||
| 2.01-4.00mm | 16 | 0.67 | (0.19-3.15) | 0.58 | ||||
| >4.00mm | 14 | 1.52 | (0.43-7.06) | 0.53 | ||||
| Unknown | 6 | 0.57 | (0.07-3.46) | 0.53 | ||||
| Primary Site | 0.33 | |||||||
| Head/Neck | 18 | 1.15 | (0.34-5.20) | 0.83 | ||||
| Trunk | 6 | 1 | (Reference) | |||||
| Extremity | 17 | 1.63 | (0.48-7.36) | 0.45 | ||||
| Mucosal | 9 | 0.45 | (0.06-2.73) | 0.38 | ||||
| Ulceration | 0.73 | |||||||
| Present | 15 | 1.01 | (0.38- 2.43) | 0.99 | ||||
| Absent | 29 | 1 | (Reference) | |||||
| Unknown | 6 | 0.58 | (0.10-2.08) | 0.44 | ||||
| CD44v6 Expression | 0.02 | 0.01 | ||||||
| Positive (>27) | 29 | 2.89 | (1.15-8.77) | 0.02 | 3.45 | (1.32-10.77) | 0.01 | |
| Negative (≤27) | 21 | 1 | (Reference) | 1 | (Reference) | |||
CD44v6 overexpression leads to an enhancement in MBM cell migration
To evaluate the biological relevance of CD44v6 protein expression in melanoma, functional analyses were carried out using two representative CD44v6-positive MBM cell lines (BD and M16) and one representative CD44v6-negative LNM cell line (SR; Fig. 2A). Hyaluronic acid (HA) is known to activate CD44v6-positive tumor cells and is highly concentrated in brain tissue (Ananthanarayanan et al., 2011). The migration of CD44v6-positive (BD and M16) cell lines significantly increased under HA treatment (W, p<0.05; Fig. 4A-C). Importantly, blocking with anti-CD44v6 antibody (Ab) significantly decreased the HA-induced migration of CD44v6-positive cell lines (W, p<0.05; Fig. 4A-C). The migration rate of the CD44v6-negative cell line (SR) was not affected by HA treatment or anti-CD44v6 Ab (Fig. 4D). HA is considered a damage-associated molecular pattern molecule (DAMP) (Srikrishna and Freeze, 2009), which has a critical role in the initiation of immune response in noninfectious inflammatory processes, such as tumor invasion and metastasis (Sato et al., 2009). Furthermore, acute brain inflammation stimulates the release of diverse mediators including hepatocyte growth factor (HGF) (McCourt et al., 2001) and, as recently demonstrated, CD44v6 is required for the tyrosine kinase receptor c-Met activation by HGF (Orian-Rousseau et al., 2002). In consequence, the influence of HGF on CD44v6-positive and CD44v6-negative melanoma cell lines was investigated. The melanoma cell lines selected for this experiment, BD, M16, and SR were positive for c-MET receptor expression. Under HGF stimulation, CD44v6-positive MBM cell lines presented a significantly increased migration, which was significantly reduced by blocking with anti-CD44v6 Ab (W, p<0.05; Fig. 4B-C). Conversely, the CD44v6-negative LNM cell line was not affected by HGF. To further understand the role of exon variant 6 during cell migration induced by HGF, a CD44 exon variant 6 small interfering RNA (siCD44v6) was used. We observed that siCD44v6 significantly reduced CD44v6 expression at mRNA (Supplemental Fig. 1B) and protein (Supplemental Fig. 1C) levels. Additionally, siCD44v6 significantly reduced CD44v6-positive melanoma cell migration treated with HGF (W, p<0.05; Fig. 4B-C) and did not affect the migration of CD44v6-negative melanoma cells (Fig. 4D). These experiments indicate an enhanced migration of CD44v6-positive cells in environments with high HA and HGF.
Figure 4. Hyaluronic acid and hepatocyte growth factor enhance effect on migration of MBM cell lines expressing CD44v6.
A. Representative migration assay for BD melanoma cell line under different treatments. B-D. Quantification of cell migration under 8 different treatments for BD (B), M16 (C) and SR (D) melanoma lines. Treatments conditions: 100μg/mL Hyaluronic acid (HA), 50ng/mL hepatocyte growth factor (HGF),10μg/mL CD44v6 antibody (Ab), 30nM siRNA control (siCTRL) and 30nM siRNA CD44v6 (siCD44v6). The data represents the average of three independent experiments ± SD. * is p≤0.05 and ** is p≤0.01.
CD44v6 isoform expression correlates with ESRP1 and ESRP2 expression in primary melanoma
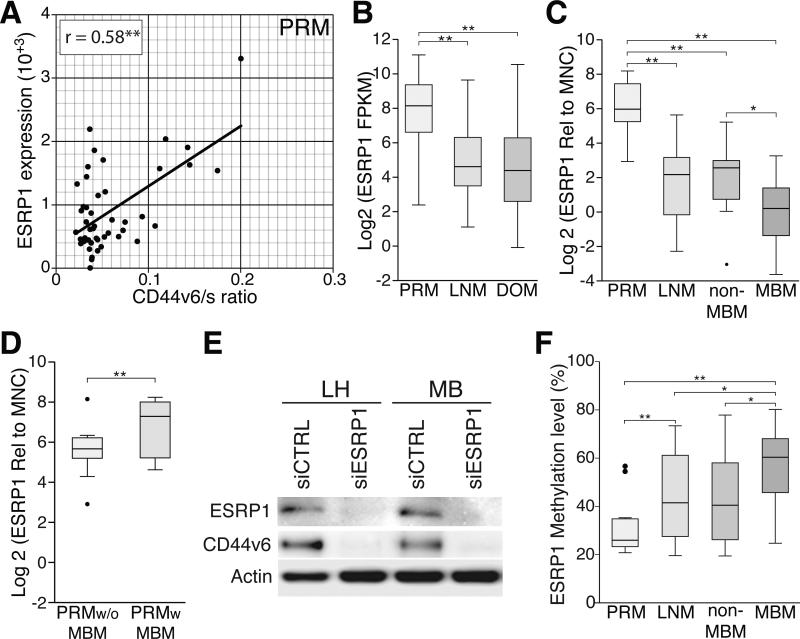
To better understand the splicing mechanisms involved in the generation of CD44v6 during melanoma progression to MBM, RNA-sequencing (RNA-Seq) data generated by The Cancer Genome Atlas (TCGA) network was analyzed (TCGA, 2008). Correlation analyses were performed between CD44v6/s ratio and the expression of five previously reported CD44 splicing factors: ESRP1 (Yae et al., 2012), ESRP2 (Yae et al., 2012), SRRM1 (Cheng and Sharp, 2006), TRA2B (Watermann et al., 2006), and YBX1 (Stickeler et al., 2001) using 239 melanoma specimens including 46 PRMs, 160 LNMs, and 33 distant organ metastases (DOMs). Among these splicing factors, only ESRP1 and ESRP2 presented significant correlation with CD44v6/s ratio in PRMs (r=0.53 and r=0.84, respectively; Fig. 5A and Supplemental Fig. 2). However, in metastatic melanomas, ESRP1 or ESRP2 were not significantly correlated with the CD44v6/s ratio (Supplemental Fig. 2). Additionally, while ESRP2 expression did not present variation, a significantly higher expression of ESRP1 in PRMs than in LNMs (W, p=1.5×10−6) and DOMs (W, p=9.7×10−8; Fig. 5B) was identified. To validate these results, a quantitative PCR (qPCR) assay for the assessment of ESRP1 mRNA was performed on an independent cohort of melanoma specimens from JWCI (n=62), which included PRMs (n=23), LNMs (n=14) and DOMs (n=25). In concordance with the data generated by TCGA network, the qPCR analysis confirmed a significantly higher expression of ESRP1 in PRMs than in LNMs (W, p=4.5×10−6) and DOMs (W, p=2×10−6; Fig. 5C). Among the DOMs, a significantly lower expression of ESRP1 was detected in MBMs than in non-MBMs (W, p=0.02; Fig. 5C). Interestingly, PRMs from patients that later developed MBM presented a significantly higher ESRP1 expression than PRMs from patients with MBM-free survival (W, p=0.01). qPCR data was further supported by ESRP1 IHC staining of melanoma tumors (Supplemental Fig. 3A-B). These experiments suggested an important role of ESRP1 in PRMs, generating melanoma cell clones that ultimately may be pre-adapted to target specific metastatic microenvironments, such as the brain.
Figure 5. ESRP1 expression influences CD44v6 expression and is epigenetically downregulated in advanced metastatic melanoma.
A. Scatter plots showing the correlation analysis of ESRP1 expression and CD44v6/s ratio using TCGA data for PRM. B. Box plot comparing the ESRP1 expression using TCGA RNA-Seq data for PRM, LNM, and DOM specimens. C. Box plots comparing the expression of ESRP1 assessed by qPCR for PRM, LNM, non-MBM, and MBM. D. Box plot comparing ESRP1 expression for PRM with and without MBM-development. E. Western blot showing ESRP1 and CD44v6 expression on two LNM cell lines (MB and LH) treated with siRNA control (siCTRL) and siRNA ESRP1 (siESRP1). F. Box plot comparing the DNA methylation level of ESRP1 gene in PRM, LNM, non-MBM, and MBM specimens. * is p ≤ 0.05 and ** is p ≤ 0.01.
To better understand the influence of ESRP1 on CD44 alternative splicing, ESRP1 and CD44v6 mRNA expression was evaluated in melanoma cell lines (n=12; Supplemental Fig 3C). Two LNM cell lines with a relative high expression of both ESRP1 and CD44v6 (MB and LH) were selected and ESRP1 expression was knocked down by ESRP1-specific siRNA (siESRP1). As previously demonstrated in epithelial-derived tumors (Brown et al., 2011; Reinke et al., 2012; Warzecha et al., 2009; Yae et al., 2012), ESRP1 downregulation significantly decreased the expression of CD44v6 in melanoma cell lines (Figure 5E). Furthermore, migration of melanoma cells under HGF treatment was significantly reduced by siESRP1 transfection (Supplemental Fig. 3D). However, since CD44 is not the only potentially affected gene by ESRP1 downregulation, it cannot be concluded that the decrease in cell migration is solely reflecting CD44v6 downregulation.
ESRP1 is epigenetically silenced in melanoma brain metastasis
To understand the mechanisms involved in ESRP1 downregulation during melanoma progression to MBM, genome-wide DNA methylation analysis of melanoma specimens (n=82) was performed. This included PRMs (n=14), LNMs (n=12), non-MBMs (n=38) and MBMs (n=18). The DNA methylation level of ESRP1 gene significantly increased during melanoma progression (KW, p=0.02; Supplemental Fig. 3E). Specifically, MBMs presented a significantly higher methylation level compared to non-MBMs (W, p=0.03; Fig. 5G).
CD44v6 isoform expression is influenced by PTBP1 in distant organ metastasis
Our results indicated that ESRP1 influenced the expression of CD44v6 in PRMs and a subset of LNM cell lines with relative high expression of CD44v6 and ESRP1. However, we detected a significant downregulation of ESRP1 in MBMs. Based on recent studies which suggest that the expression level of splicing factors can affect splicing site decisions and generate tissue-specific splicing programs (Saltzman et al., 2011), our analysis was extended to other potential splicing factors. The correlation between CD44v6/s ratio and the expression of a panel of potential candidate splicing factors (n=190) was analyzed using TCGA RNA-Seq data (Supplemental Fig. 4). Significant positive correlations in metastatic melanoma were identified between the CD44v6/s ratio and two splicing factors, PTBP1 and U2AF2 (Fig. 6A-B). Unlike ESRP1, the expression and DNA methylation levels of both PTBP1 and U2AF2 did not present significant variation during melanoma progression (Supplemental Fig. 5A-D) or between PRM with and without MBM-development (Supplemental Fig. 5E-F). Additionally, while ESRP1 recognizes GGU/UGG–rich sequences (Dittmar et al., 2012), PTBP1 recognizes CUCUCU-rich sequences (Oberstrass et al., 2005). Our mapping of the ESRP1/2 and PTBP1 recognition sites in the CD44 gene variant region suggests that both splicing factors can generate CD44 isoforms containing exon variant 6 (Supplemental Fig. 6A). PTBP1 was knocked down by siPTBP1 in BD and M16 MBM lines (Supplemental Fig. 6B) to evaluate its impact on CD44v6 expression. PTBP1 downregulation induced a significant decrease of CD44v6 expression at mRNA (Figure 6C) and protein (Figure 6D) levels. Additionally, PTBP1 knockdown significantly affected the migration of BD and M16 MBM lines treated with HGF (Supplemental Fig. 6C-D). While M16 showed a significant decrease, BD on the other hand showed a significant increase in cell migration. As in the case of ESRP1, PTBP1 downregulation may be affecting the splicing of a large number of proteins, including CD44, with positive and negative effects on cell migration. The inverse effect displayed by M16 and BD cell lines could be reflecting a different splicing and transcriptomic context between these cells.
Figure 6. PTBP1 and U2AF2 expression correlates with CD44v6 in advanced metastatic melanoma.
A. Scatter plots showing the correlation analysis between PTBP1 expression and CD44v6/s ratio using TCGA data for distant organ metastasis (DOM). B. Scatter plots showing the correlation analysis between U2AF2 expression and CD44v6/s ratio using TCGA data for DOM. C. qPCR analysis of CD44v6 expression of BD and M16 cell lines treated with siRNA control (siCTRL) and siRNA PTBP1 (siPTBP1). * is p ≤ 0.05 and ** is p ≤ 0.01. D. Western blot showing PTBP1 and CD44v6 expression on BD and M16 cell lines treated with siCTRL and siPTBP1.
DISCUSSION
Brain metastasis is the most aggressive complication of cutaneous melanoma, and with frequently dismal survival rates, it is commonly associated with severe morbidity. Therefore, we are focused on unmasking molecular alterations with clinical utility as theranostic markers for melanoma progression to MBM (Marzese et al., 2014a; Marzese et al., 2014b; Marzese et al., 2014c).
In this study, we identified significantly higher expression of CD44 isoforms involving variant exons in melanoma brain metastasis (MBM) than in lymph node metastasis (LNM) cell lines. The largest difference was observed in the expression of isoforms involving exon variant 6. Different studies have reported a functional relation of CD44v6 expression and the increased metastatic potential of melanoma and other tumor types (Misra et al., 2011; Orian-Rousseau, 2010; Raso-Barnett et al., 2013). After analyzing a large cohort of clinical melanoma specimens, the expression of CD44v6 in primary melanomas (PRMs), as well as AJCC Stage IIIC LNMs significantly predicted MBM-free survival. This is an important finding since there are currently no MBM-predictive markers for PRMs or LNMs. Conversely, in LNMs from AJCC Stage IIIA/B, CD44v6 expression was not associated with MBM development. It is well known that AJCC Stage IIIC melanoma patients have a dismal disease outcome, and often succumb to distant organ metastasis (DOM) including MBM, while AJCC Stage IIIA and B involve LNMs in early stages of melanoma regional metastasis (Balch et al., 2009).
Hyaluronic acid (HA)-rich and hepatocyte growth factor (HGF)-rich microenvironments increased the migration of CD44v6-positive melanoma cells. The presence of metastatic cells in the brain can stimulate the release of damage-associated molecular pattern (DAMP) molecules, such as HA, causing inflammation. Acute inflammation has been demonstrated to increase HGF release in the brain (McCourt et al., 2001). Other physiological events may also cause inflammation of the brain tissue, such as stroke and Alzheimer's disease (Akiyama et al., 2000; Brambilla et al., 2013). Each event could contribute to a positive feedback loop promoting MBM establishment and progression. The inflamed brain tissue may provide the “soil” environment for the metastatic “seed” tumor cells to survive and spread. However, whether the seed of metastasis or the pre-niche inflammation environment comes first is still not well understood. In-vivo studies have to be designed in order to investigate whether CD44v6 increases the tropisms of MBMs in brain tissue. The successful CD44v6 blocking that reduced MBM migration under HA and/or HGF treatment may also bring the opportunity of novel therapeutic strategies for patients with MBM, similar to what was shown by Phase I trials using bivatuzumab, an anti-CD44v6 monoclonal antibody (Ab) in different cancer types (Gurtner et al., 2012; Tijink et al., 2006) even though this trial was discontinued because of serious skin toxicity (Riechelmann et al., 2008). An analogous strategy using soluble CD44 to block CD44 binding to HA has been successfully applied in metastatic melanoma tumors (Ahrens et al., 2001).
ESRP1 and ESRP2 were the only splicing factors that demonstrated a significant correlation with CD44v6 expression in PRMs, but not in distant organ metastases (DOMs). The expression of ESRP1 was higher in PRMs from patients that ultimately developed MBMs, suggesting an important role of ESRP1 in PRMs facilitating CD44v6-positive melanoma cells to target specific organ microenvironments, such as the brain. The role of ESRP1 on CD44 alternative splicing has been recently demonstrated in epithelial tumors (Brown et al., 2011; Reinke et al., 2012; Warzecha et al., 2009; Yae et al., 2012). Nevertheless, in neuroectoderm-derived tumors, such as melanoma, its functional impact has not been described. Our study showed that in a subset of LNM cell lines with relative high expression of ESRP1 and CD44v6 the inhibition of ESRP1 expression induced a significant downregulation of CD44v6 expression. Additionally, we identified a progressive decrease in the expression of ESRP1 that was accompanied by a significant increase in ESRP1 gene hypermethylation, similar to events we have previously identified for several other melanoma progression-related genes such as ESR1, RARB and RASSF1 (Marzese et al., 2014c; Mori et al., 2006; Tanemura et al., 2009). Our study suggests that the hypermethylation of the ESRP1 gene, a novel epigenetic alteration in melanoma, is an alternative mechanism to induce changes in the splicing machinery in response to changes in the tissue microenvironment. Indeed, in metastatic melanomas, CD44v6 expression was correlated with the splicing factor PTBP1 and the splicing auxiliary factor U2AF2. Importantly, PTBP1-specific knockdown induced a downregulation of the CD44v6 isoform. These experiments revealed a functional link between PTBP1 and CD44 splicing regulation. Our study indicates that ESRP1 may be important for CD44v6 expression in PRM cells, but further MBM progression may depend on its decrease, which could subsequently reorganize the core spliceosome through PTBP1 and U2AF2. This observation agrees with the previously reported exceptional compositional and structural dynamic of the spliceosome, which creates tissue-specific splicing programming according to the cellular context (Wahl et al., 2009). The changes in the spliceosome described in this study offer a novel approach to understanding melanoma progression to MBM and a new potential target to override the effects of its aggressive behavior.
In summary, CD44v6 expression could be utilized as a potential theranostic cell surface marker for patients with MBM and for those that are at high-risk for MBM development. These are critical findings as there are currently no predictive factors for discerning patients at high-risk for MBM progression to date or specific molecular targets for MBMs.
MATERIALS AND METHODS
Clinical tissue specimens
Melanoma patients in this study were treated at Saint John's Health Center (SJHC) and included based on clinical pathology follow-up. Melanoma PEAT from the patients was obtained under an institutional review board (IRB) protocol that was approved by the SJHC/John Wayne Cancer Institute (JWCI) joint IRB and Western IRB.
Cell culture, HA and HGF stimulation and siRNA transfection
Established human metastatic melanoma cell lines were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (Gemini Bio-Products, Sacramento, CA), HEPES buffer (Cellgro, Manassas, VA), penicillin-streptomycin and fungizone (Gemini Bio-Products, Sacramento, CA). Early passaged cultures were maintained at 37°C with 5% CO2. For the migration experiments, the cells were harvested with 5mM EDTA instead of trypsin-EDTA to minimize the alteration on the structure of CD44 and other membrane proteins. Recombinant hepatocyte growth factor (HGF, R&D Systems, Inc., Minneapolis, MN) was reconstituted to 50μg/μL in sterile physiological phosphate buffered saline (PBS; Life Technologies, Carlsbad, CA) containing 0.1% bovine serum albumin (BSA; Applied Biosystems, Foster City, CA). Hyaluronic acid (HA, Sigma-Aldrich, St. Louis, MO) was reconstituted to 100μg/mL in sterile PBS. Melanoma cells were grown in culture medium for 24h and starved for an additional 24h using RPMI 1640 without serum. After starvation, the cells were treated with or without HA or recombinant HGF protein and incubated for 24h in specific experiments as outlined below. siRNA transfection was performed using Jetprime reagent (Polyplus-transfection Inc., New York, NY) following the manufacturer's protocol. Sequences for siRNA against CD44 exon variant 6, PTBP1, ESRP1, and control are described in Supplemental Table S2.
Flow cytometry
Melanoma cells were harvested and suspended in sterile PBS with 0.5% BSA and then diluted to a concentration of 4×106 cells/mL. The cells were washed and then incubated with H-CAM antibody (Ab) (VFF-7, Santa Cruz Biotechnology, Santa Cruz, CA) at the recommended concentration of 1μg per 106 cells for 1h on ice. This was followed by incubation with phycoerythrin conjugated goat to mouse IgG secondary Ab (Abcam, Cambridge, MA) for 45min at 4°C. After washing, 104 cells were analyzed by flow cytometry using a BD FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ). All data was analyzed using the BD Cell Quest Software (BD Biosciences, Franklin Lakes, NJ). Normal mouse IgG1 (Santa Cruz Biotechnology, Santa Cruz, CA) was used as a negative isotype Ab control.
Migration assay
Melanoma cell migration ability was determined using a transwell chamber with an 8.0μm polyethylene terephythalate membrane following the manufacturer's instructions (BD Biosciences, San Diego, CA). Melanoma lines BD, M16, and SR were seeded onto the upper chamber in triplicate at 105 cells per well with serum-free RPMI 1640 medium. The lower chamber contained RPMI 1640 with 10% fetal bovine serum with either a control medium, HA at 100μg/mL, or recombinant HGF protein at 50ng/mL. The chambers were incubated for 24h at 37°C and the cells were fixed and stained with methanol and crystal violet solution (2% crystal violet, 20% ethanol in PBS). For the cell migration blocking assay, the cells were pre-treated with 10μg/mL human anti-CD44v6 Ab (BBA13, R&D Systems, Inc., Minneapolis, MN) and placed on a rotator for 1 h in the dark at 4°C before treatment with HA and HGF. For the siRNA knockdown assays, the cells were treated with corresponding siRNAs for 16 h and the cell lines were refreshed with new medium for 72 h before the migration assay. All migration assays were performed in triplicate for verification and after fixing and staining, the photographs were taken under a Nikon Eclipse Ti microscope (Nikon, Melville, NY) at 10X, with four fields taken of each triplicate sample of each condition. The number of migrated cells was tallied by two independent surveyors. Averages were taken for each set of four photographic fields between both readers for analysis.
Protein extraction and western blot analysis
Protein extraction and western blots were performed as previously described (Marzese et al., 2014b; Marzese et al., 2014c). The membranes were immunoblotted with primary antibodies against CD44v6 (H-CAM; 1:500; Santa Cruz Biotechnology, Santa Cruz, CA), ESRP1 (1:1,000; Pierce Biotechnology, Rockford, IL. Cat#PA5-25833), PTBP1 (1:1,000; Invitrogen, Camarillo, CA. Cat#32-4800) and β-actin (1:5,000, Sigma-Aldrich, St. Louis, MO. Cat#A1978-200UL) overnight at 4°C followed by peroxidase-conjugated secondary antibody against rabbit IgG for ESRP1 (1:4,000; GE healthcare, Pittsburgh, PA) and against mouse IgG for CD44v6, PTBP1 and β-actin (1:6,000 GE healthcare). Detection was carried out using the Pierce SuperSignal West Femto Maximum Sensitivity chemiluminescent substrate (Pierce Biotechnology) followed by scanning using MyECL Imager (Thermo Scientific, Rockford, IL).
Immunohistochemistry
IHC was performed on 5μm PEAT sections as previously described (Kagara et al., 2012). CD44v6 protein expression was assessed using 1μg/mL of the monoclonal CD44v6 Ab (H-CAM; Santa Cruz Biotechnology, Santa Cruz, CA) or the polyclonal ESRP1 Ab (PA5-25833; Thermo Scientific). The IHC protocol was optimized using the DAKO CSAII kit (Dako, Carpinteria, CA). Slides were deparaffinized, rehydrated, and then washed in 1X PBS. Antigen retrieval was performed using 1X citrate, pH=6.0, in a 100°C water bath for 20 min as previously described (Nguyen et al., 2011). Tissue sections were stained with Vector VIP and counterstained with hematoxylin. As a negative control, PEAT specimens were treated with Dako Universal Negative Control (Dako, Carpinteria, CA) instead of the primary Ab. Photographs were taken using a Nikon Eclipse Ti microscope (Nikon, Melville, NY). Tumor areas were identified and the intensity was quantified using ImageJ software (National Institute of Health, Bethesda, MD), as previously described (Nguyen et al., 2011). Ten tumor fields of each slide were measured and averaged, and then the IHC score was calculated as: tumor intensity - background intensity. Background intensity was estimated by evaluating connective tissue with no tumor cells. Each tissue section was individually scored by two investigators (D.C. and R.T.) and then averaged. The Cohen's kappa agreement coefficient between the readers (for the categorization of the data into CD44v6-positive and CD44v6-negative groups) was 0.91.
Quantitative real-time PCR
Total RNA was extracted from cell lines using TriReagent (Molecular Research Center, Inc., Cincinnati, OH) and from tissues using RNA-Solv Reagent (Omega Bio-Tek, Norcross, GA) according to the manufacturers’ instructions. RNA concentration was qualified and quantified by UV absorption spectrophotometry and the Quant-iT RiboGreen RNA Assay Kit (Life Technologies, Carlsbad, CA). Reverse-transcription of total RNA was performed using Moloney murine leukemia virus reverse-transcriptase with oligo-dT and random hexamers as previously described (Hoshimoto et al., 2012; Koyanagi et al., 2010). The qPCR of CD44 standard, CD44v6, ESRP1, ESRP2, PTBP1, and U2AF2 mRNAs was performed on the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA) by using the primers described in the Supplemental Table S2. The amount of cDNA was normalized using SDHA mRNA (Supplemental Table S2) expression as an internal control. A melanocyte cell line (HEMn-LP, Life Technologies, Carlsbad, CA) was used as an external reference for gene expression. RNA extracted from the established melanoma cell line LF-0023 was used as an internal control to normalize the variation in qPCR assays. qPCR data was analyzed using the ddCq method as previously described (Heid et al., 1996).
Exon expression by microarray analysis
The Human Exon 1.0 ST array (Affymetrix, Santa Clara, CA) was performed at the Genome Core of the USC Children's Hospital using RNA extracted from 41 early passage established metastatic melanoma lines (34 LNMs and 7 MBMs). Data was analyzed in the Expression Console 1.1 software (Affymetrix, Santa Clara, CA) using the core transcript set and employing the robust multi-array average algorithm (RMA) for background correction and normalization of data as previously shown (Marzese et al., 2014a; Marzese et al., 2014b).
Genome-wide DNA methylation analysis
The genome-wide DNA methylation status of 82 melanoma PEAT specimens [14 PRMs, 12 LNMs, 18 MBMs and 38 non-brain metastases (non-MBMs)] were assessed by using the Infinium HumanMethylation450 BeadChip (HM450K) following the manufacturer's protocol (Illumina, Inc.) as previously shown (Marzese et al., 2014b). The chips were scanned with Illumina iScan, and the data was extracted using the R package methylumi on Bioconductor. The methylation status was reported as a β-value [β=intensity of the Methylated allele/(intensity of the Unmethylated allele + intensity of the Methylated allele)] as previously described (Marzese et al., 2014b) (Marzese et al., 2014a).
Biostatistical analysis
To determine the normal distribution of the data, Kolmogorov–Smirnov test was applied. The parametric student's t test was used for normally distributed data and the non-parametric Wilcoxon rank-sum (W) and Kruskal-Wallis (KW) tests were used for non-normally distributed data. Determination of a CD44v6 expression cutoff to predict MBM recurrence was conducted using a ROC curve and calculating the area under the curve (AUC). Multivariate analysis was performed by the Cox proportional hazard model including CD44v6 expression, Breslow thickness, ulceration, primary tumor location, age, and gender. MBM progression-free survival analyses were conducted using the Kaplan–Meier method and comparisons among groups were performed using log-rank tests. Correlation analysis was performed using Pearson's r correlation coefficient (r). Experimentally validated CD44 isoforms were identified from Ref-Seq database (http://www.ncbi.nlm.nih.gov/refseq/) and computationally predicted isoforms were identified using the Gnomon method (http://www.ncbi.nlm.nih.gov/genome/guide/gnomon.shtml).
Supplementary Material
SIGNIFICANCE.
CD44v6-positive melanoma cells have a tendency to metastasize to the brain. Detection of high CD44v6 expression in early stages significantly predicts melanoma brain metastasis (MBM) development. Functionally, this alteration increases melanoma cell migration that can be blocked by targeting CD44v6. Epigenetic alterations of spliceosome factors were also identified during melanoma progression from primary to MBM. Hypermethylation of ESRP1 gene is a novel alteration in melanoma that provides alternative mechanisms of melanoma progression to MBM through the epigenetic modulation of spliceosome factors. Interestingly, in MBM, CD44v6 expression is induced by the splicing factor PTBP1.
Acknowledgments
Financial support: This work was supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (AMFR; D.H.), Ruth and Martin H. Weil Foundation (D.H.), and National Institute of Health, National Cancer Institute, USA the Award Number [1R01CA167967-01A1(D.H.), P01CA029605 Project II/Core C (D.H.)].
Abbreviations
- Ab
antibody
- AJCC
American Joint Committee on Cancer
- CD44s
CD44 standard exons
- CD44v
CD44 variant exons
- CD44v6
CD44 variant 6
- DAMP
damage-associated molecular pattern
- DOM
distant organ metastasis
- HA
hyaluronic acid
- HGF
hepatocyte growth factor
- hnRNP
heterogeneous nuclear ribonucleo protein
- KW
Kruskal-Wallis
- LNM
lymph node metastasis
- MBM
melanoma brain metastasis
- PBS
phosphate buffered saline
- PEAT
paraffin-embedded archival tissue
- PRM
primary melanoma
- r
Pearson's R correlation coefficient
- RNP
ribonucleo protein
- siCTRL
small interfering RNA control
- siESRP1
small interfering RNA ESRP1
- siPTBP1
small interfering RNA PTBP1
- snRNP
small nuclear ribonucleo protein
- TCGA
The Cancer Genome Atlas
- W
Wilcoxon rank-sum
Footnotes
Conflicts of interest: The authors have declared that no competing interest exists.
REFERENCES
- Ahrens T, Sleeman JP, Schempp CM, Howells N, Hofmann M, Ponta H, Herrlich P, Simon JC. Soluble CD44 inhibits melanoma tumor growth by blocking cell surface CD44 binding to hyaluronic acid. Oncogene. 2001;20:3399–408. doi: 10.1038/sj.onc.1204435. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer's disease. Neurobiology of aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthanarayanan B, Kim Y, Kumar S. Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials. 2011;32:7913–23. doi: 10.1016/j.biomaterials.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, et al. Final version of 2009 AJCC melanoma staging and classification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Couch Y, Lambertsen KL. The effect of stroke on immune function. Molecular and cellular neurosciences. 2013;53:26–33. doi: 10.1016/j.mcn.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Bristow BN, Casil J, Sorvillo F, Basurto-Davila R, Kuo T. Melanoma-related mortality and productivity losses in the USA, 1990-2008. Melanoma research. 2013;23:331–5. doi: 10.1097/CMR.0b013e328361926c. [DOI] [PubMed] [Google Scholar]
- Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, Cheng C. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121:1064–74. doi: 10.1172/JCI44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Sharp PA. Regulation of CD44 alternative splicing by SRm160 and its potential role in tumor cell invasion. Molecular and cellular biology. 2006;26:362–70. doi: 10.1128/MCB.26.1.362-370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Takahashi K, Eto H, Yoon SS, Tanabe KK. CD44s expression in human colon carcinomas influences growth of liver metastases. International journal of cancer. Journal international du cancer. 2000;85:523–6. doi: 10.1002/(sici)1097-0215(20000215)85:4<523::aid-ijc13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- De La Monte SM, Moore GW, Hutchins GM. Patterned distribution of metastases from malignant melanoma in humans. Cancer research. 1983;43:3427–33. [PubMed] [Google Scholar]
- Dittmar KA, Jiang P, Park JW, Amirikian K, Wan J, Shen S, Xing Y, Carstens RP. Genome-wide determination of a broad ESRP-regulated posttranscriptional network by high-throughput sequencing. Molecular and cellular biology. 2012;32:1468–82. doi: 10.1128/MCB.06536-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ, Balasubramanian K, Lin Q, Kim SW, Kim SJ. The brain microenvironment and cancer metastasis. Molecules and cells. 2010;30:93–8. doi: 10.1007/s10059-010-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Molecular pathology : MP. 1999;52:189–96. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner K, Hessel F, Eicheler W, Dorfler A, Zips D, Heider KH, Krause M, Baumann M. Combined treatment of the immunoconjugate bivatuzumab mertansine and fractionated irradiation improves local tumour control in vivo. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012;102:444–9. doi: 10.1016/j.radonc.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome research. 1996;6:986–94. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Hoshimoto S, Faries MB, Morton DL, Shingai T, Kuo C, Wang HJ, Elashoff R, Mozzillo N, Kelley MC, Thompson JF, et al. Assessment of prognostic circulating tumor cells in a phase III trial of adjuvant immunotherapy after complete resection of stage IV melanoma. Annals of surgery. 2012;255:357–62. doi: 10.1097/SLA.0b013e3182380f56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Itano N, Sawai T, Kimata K, Koganehira Y, Saida T, Taniguchi S. Increased synthesis of hyaluronate enhances motility of human melanoma cells. The Journal of investigative dermatology. 1999;113:935–9. doi: 10.1046/j.1523-1747.1999.00804.x. [DOI] [PubMed] [Google Scholar]
- Izraely S, Sagi-Assif O, Klein A, Meshel T, Tsarfaty G, Pasmanik-Chor M, Nahmias C, Couraud PO, Ateh E, Bryant JL, et al. The metastatic microenvironment: brain-residing melanoma metastasis and dormant micrometastasis. International journal of cancer. Journal international du cancer. 2012;131:1071–82. doi: 10.1002/ijc.27324. [DOI] [PubMed] [Google Scholar]
- Kagara N, Huynh KT, Kuo C, Okano H, Sim MS, Elashoff D, Chong K, Giuliano AE, Hoon DS. Epigenetic regulation of cancer stem cell genes in triple-negative breast cancer. The American journal of pathology. 2012;181:257–67. doi: 10.1016/j.ajpath.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Koyanagi K, O'day SJ, Boasberg P, Atkins MB, Wang HJ, Gonzalez R, Lewis K, Thompson JA, Anderson CM, Lutzky J, et al. Serial monitoring of circulating tumor cells predicts outcome of induction biochemotherapy plus maintenance biotherapy for metastatic melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:2402–8. doi: 10.1158/1078-0432.CCR-10-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzese DM, Huynh JL, Kawas NP, Hoon DSB. Multi-platform genome-wide analysis of melanoma progression to brain metastasis. Genomics Data. 2014a;2:150–152. doi: 10.1016/j.gdata.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzese DM, Scolyer RA, Huynh JL, Huang SK, Hirose H, Chong KK, Kiyohara E, Wang J, Kawas NP, Donovan NC, et al. Epigenome-wide DNA methylation landscape of melanoma progression to brain metastasis reveals aberrations on homeobox D cluster associated with prognosis. Human molecular genetics. 2014b;23:226–38. doi: 10.1093/hmg/ddt420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzese DM, Scolyer RA, Roque M, Vargas-Roig LM, Huynh JL, Wilmott JS, Murali R, Buckland ME, Barkhoudarian G, Thompson JF, et al. DNA methylation and gene deletion analysis of brain metastases in melanoma patients identifies mutually exclusive molecular alterations. Neuro-oncology. 2014c doi: 10.1093/neuonc/nou107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccourt M, Wang JH, Sookhai S, Redmond HP. Activated human neutrophils release hepatocyte growth factor/scatter factor. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2001;27:396–403. doi: 10.1053/ejso.2001.1133. [DOI] [PubMed] [Google Scholar]
- Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR, Ghatak S. Hyaluronan-CD44 interactions as potential targets for cancer therapy. The FEBS journal. 2011;278:1429–43. doi: 10.1111/j.1742-4658.2011.08071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Martinez SR, O'day SJ, Morton DL, Umetani N, Kitago M, Tanemura A, Nguyen SL, Tran AN, Wang HJ, et al. Estrogen receptor-alpha methylation predicts melanoma progression. Cancer research. 2006;66:6692–8. doi: 10.1158/0008-5472.CAN-06-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Kuo C, Nicholl MB, Sim MS, Turner RR, Morton DL, Hoon DS. Downregulation of microRNA-29c is associated with hypermethylation of tumor-related genes and disease outcome in cutaneous melanoma. Epigenetics : official journal of the DNA Methylation Society. 2011;6:388–94. doi: 10.4161/epi.6.3.14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstrass FC, Auweter SD, Erat M, Hargous Y, Henning A, Wenter P, Reymond L, Amir-Ahmady B, Pitsch S, Black DL, et al. Structure of PTB bound to RNA: specific binding and implications for splicing regulation. Science. 2005;309:2054–7. doi: 10.1126/science.1114066. [DOI] [PubMed] [Google Scholar]
- Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. European journal of cancer. 2010;46:1271–7. doi: 10.1016/j.ejca.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes & development. 2002;16:3074–86. doi: 10.1101/gad.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raso-Barnett L, Banky B, Barbai T, Becsagh P, Timar J, Raso E. Demonstration of a melanoma-specific CD44 alternative splicing pattern that remains qualitatively stable, but shows quantitative changes during tumour progression. PloS one. 2013;8:e53883. doi: 10.1371/journal.pone.0053883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke LM, Xu Y, Cheng C. Snail represses the splicing regulator epithelial splicing regulatory protein 1 to promote epithelial-mesenchymal transition. J Biol Chem. 2012;287:36435–42. doi: 10.1074/jbc.M112.397125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechelmann H, Sauter A, Golze W, Hanft G, Schroen C, Hoermann K, Erhardt T, Gronau S. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral oncology. 2008;44:823–9. doi: 10.1016/j.oraloncology.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Saltzman AL, Pan Q, Blencowe BJ. Regulation of alternative splicing by the core spliceosomal machinery. Genes & development. 2011;25:373–84. doi: 10.1101/gad.2004811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JH, Carter JH, Jr., Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. Journal of neurosurgery. 1998;88:11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- Sato Y, Goto Y, Narita N, Hoon DS. Cancer Cells Expressing Toll-like Receptors and the Tumor Microenvironment. Cancer microenvironment : official journal of the International Cancer Microenvironment Society. 2009;2(Suppl 1):205–14. doi: 10.1007/s12307-009-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:12160–4. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11:615–28. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickeler E, Fraser SD, Honig A, Chen AL, Berget SM, Cooper TA. The RNA binding protein YB-1 binds A/C-rich exon enhancers and stimulates splicing of the CD44 alternative exon v4. The EMBO journal. 2001;20:3821–30. doi: 10.1093/emboj/20.14.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom T, Daphu I, Wendelbo I, Hodneland E, Lundervold A, Immervoll H, Skaftnesmo KO, Babic M, Jendelova P, Sykova E, et al. Automated tracking of nanoparticle-labeled melanoma cells improves the predictive power of a brain metastasis model. Cancer research. 2013;73:2445–56. doi: 10.1158/0008-5472.CAN-12-3514. [DOI] [PubMed] [Google Scholar]
- Tanemura A, Terando AM, Sim MS, Van Hoesel AQ, De Maat MF, Morton DL, Hoon DS. CpG island methylator phenotype predicts progression of malignant melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:1801–7. doi: 10.1158/1078-0432.CCR-08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCGA Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijink BM, Buter J, De Bree R, Giaccone G, Lang MS, Staab A, Leemans CR, Van Dongen GA. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:6064–72. doi: 10.1158/1078-0432.CCR-06-0910. [DOI] [PubMed] [Google Scholar]
- Wahl MC, Will CL, Luhrmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–18. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Warzecha CC, Shen S, Xing Y, Carstens RP. The epithelial splicing factors ESRP1 and ESRP2 positively and negatively regulate diverse types of alternative splicing events. RNA Biol. 2009;6:546–62. doi: 10.4161/rna.6.5.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watermann DO, Tang Y, Zur Hausen A, Jager M, Stamm S, Stickeler E. Splicing factor Tra2-beta1 is specifically induced in breast cancer and regulates alternative splicing of the CD44 gene. Cancer research. 2006;66:4774–80. doi: 10.1158/0008-5472.CAN-04-3294. [DOI] [PubMed] [Google Scholar]
- Yae T, Tsuchihashi K, Ishimoto T, Motohara T, Yoshikawa M, Yoshida GJ, Wada T, Masuko T, Mogushi K, Tanaka H, et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nature communications. 2012;3:883. doi: 10.1038/ncomms1892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.