Abstract
Stimuli previously associated with drug taking can become triggers that can elicit craving and lead to relapse of drug-seeking behavior. Here, we examined the influence of deep brain stimulation (DBS) in the nucleus accumbens shell on cue-induced reinstatement of cocaine seeking, an animal model of relapse. Rats were allowed to self-administer cocaine (0.254 mg, i.v.) for 2 h daily for 21 d, with each infusion of cocaine being paired with a cue light. After 21 d of self-administration, cocaine-taking behavior was extinguished by replacing cocaine with saline in the absence of the cue light. Next, during the reinstatement phase, DBS was administered bilaterally into the nucleus accumbens shell through bipolar stainless steel electrodes immediately prior to re-exposure to cues previously associated with cocaine reinforcement. DBS continued throughout the 2 h reinstatement session. Parallel studies examined the influence of accumbens shell DBS on reinstatement induced by cues previously associated with sucrose reinforcement. Results indicated that DBS of the nucleus accumbens shell significantly attenuated cue-induced reinstatement of cocaine and sucrose seeking. Together, these results indicate that DBS of the accumbens shell disrupts cue-induced reinstatement associated with both a drug and a natural reinforcer.
Keywords: Deep brain stimulation, relapse, addiction, cue-induced reinstatement, psychostimulant, nucleus accumbens
1. Introduction
Deep brain stimulation (DBS), originally developed in the 1950s, first achieved recognition in the 1980s as a potential therapeutic intervention for Parkinson's disease and other movement disorders[1]. Due to its highly effective outcomes, reversibility, and minimal side effects, DBS has grown in popularity over the past 25 years[2,3], garnering FDA approval for the treatment of several movement disorders.
The success of DBS in treating movement disorders paved the way for its use as a therapeutic modality in psychiatric disorders. Indeed, DBS is being studied in a number of psychiatric conditions, including obsessive-compulsive disorder, major depression, eating disorders, Tourette's syndrome, and drug addiction[1,4]. This is primarily due to the belief that DBS is relatively safe, free of unwanted side effects, and in some cases, may even have beneficial effects on attention, learning and memory, and executive function[5,6]. Although DBS is highly invasive procedure with a surgical fatality rate estimated at 0.4%, the high costs associated with severe drug addiction have led many to conclude that DBS as a therapeutic intervention is a valuable avenue of research[7-10].
Recent preclinical and clinical studies suggest that deep brain stimulation (DBS) of the nucleus accumbens, a limbic structure that is critically involved in the reinforcing and reinstating effects of drugs of abuse, may be a possible therapy in the treatment of drug addiction[8,11]. In a pilot study of DBS of the accumbens in 5 patients with severe alcohol addiction, all subjects reported complete remission of their craving for alcohol[12]. Another case study showed complete remission of heroin abuse by a patient for 6 years. Remarkably, the patient refrained from drug abuse during active stimulation for the first 2.5 years and remained abstinent for 3.5 years even after the stimulation was removed[13]. In all cases, DBS of the nucleus accumbens produced no unwanted side effects.
In animal models of addiction, DBS of the nucleus accumbens prevented morphine-conditioned place preference[14], attenuated cocaine priming-induced reinstatement of drug seeking[15,16], and decreased alcohol consumption[17]. However, although recent work indicates that accumbens DBS attenuated cue-induced reinstatement of heroin seeking[18], the influence of DBS on cue-induced reinstatement of cocaine seeking is unknown. Therefore, we examined the effects of DBS in the nucleus accumbens on cue-induced reinstatement of cocaine-seeking as well as sucrose-seeking behavior.
2. Materials and Methods
2.1 Animals and housing
Male Sprague-Dawley rats (Rattus norvegicus) weighing 250-300g were ordered from Taconic Laboratories (Germantown, NY, USA). Animals were individually housed with food and water available ad libitum. Animals in the sucrose reinstatement study received ~25 g chow per day and had water available ad libitum. A 12h light/dark cycle (lights on at 7:00 am) was used and all experiments were performed during the light cycle. All experimental procedures were consistent with the ethical guidelines of the U.S. National Institutes of Health and were approved by the University of Pennsylvania Perelman School of Medicine Institutional Animal Care and Use Committee.
2.2 Materials
All experiments used Med-Associates (East Fairfield, VT, USA) operant chambers equipped with response levers, house light, cue light, pumps for injecting drugs intravenously, and food hoppers for dispensing sucrose pellets. Operant chambers were enclosed within ventilated, sound attenuating chambers.
2.3 Surgery
Prior to surgery, the rats were injected intraperitoneally with 80 mg/kg ketamine and 12 mg/kg xylazine (Sigma-Aldrich; St. Louis, MO, USA). An indwelling silastic catheter was placed into the right jugular vein (side opposite the heart) and sutured in place. The catheter was then threaded subcutaneously over the shoulder blade and was routed to a mesh backmount platform (CamCaths, UK) that was sutured below the skin between the shoulder blades. Catheters were flushed daily with 0.3 ml of an antibiotic (Timentin, 0.93 mg/ml; Henry Schein, Melville, NY, USA) dissolved in heparinized saline. Catheters were sealed with plastic obturators when not in use.
After catheter implantation, the rats were mounted in a stereotaxic apparatus (Kopf Instruments; Tujunga, CA, USA) and bipolar stainless steel electrodes (Plastics One; Roanoke, VA, USA) were implanted into to the nucleus accumbens shell according to the following coordinates, relative to bregma (Paxinos and Watson, 1997): + 1.0 mm anteroposterior (A/P), +/– 3.0 mm mediolateral (M/L), – 7.3 mm dorsoventral (D/V). The stereotaxic arms were set at a 17° angle. Electrodes were cemented in place by affixing dental acrylic to three stainless steel screws fastened to the skull.
2.4 Cocaine self-administration, extinction, and cue-induced reinstatement of drug seeking
Following a 7 d recovery period, the rats were placed in operant chambers and were allowed to press a lever for intravenous cocaine infusions (0.254 mg of cocaine dissolved 59 μL of saline) on a fixed ratio 1 (FR1) schedule of reinforcement. Each active lever press resulted in an infusion of cocaine and the drug-paired cue (concurrent illumination of the cue light above the active lever) for 5 s. When stable responding was achieved with the FR1 schedule (i.e., <15% variation in response rates over 3 consecutive days), they were switched to an FR5 schedule. A 20 s timeout period during which responses have no scheduled consequences followed each cocaine infusion. Active lever presses made during the time out were counted but did not result in drug delivery and inactive lever presses were of no consequence. The rats were limited to a maximum of 30 cocaine infusions per daily 2 h self-administration session.
After 21 d of cocaine self-administration, the animals underwent an extinction phase during which cocaine was replaced with saline. Additionally, presses on the active lever no longer produced presentation of the drug-paired cue light. Daily 2 h extinction sessions were conducted until responding was <15% of the response rate maintained by cocaine self-administration. Following the extinction phase, the ability of re-exposure to the cue light to reinstate drug-seeking behavior was assessed. For the reinstatement test sessions, the FR5 schedule was used where active lever presses produced the light cue that had been presented during self-administration. However, satisfaction of the response requirements for each component resulted in a saline infusion rather than a cocaine infusion. Each reinstatement session was followed by extinction sessions until responding was again <15% of the response rate maintained by cocaine self-administration. All animals underwent 2 reinstatement sessions, counterbalanced with respect to whether stimulation was given.
2.5 Sucrose self-administration, extinction and cue-induced reinstatement of sucrose seeking
Rats were trained to self-administer 45 mg sucrose pellets (Research Diets; New Brunswick, NJ, USA) using the same procedures described above. After 21 days of daily 1 h food-reinforced operant sessions, rats underwent an extinction phase where responding no longer resulted in food delivery or cue light presentation. After lever pressing decreased to 15% or less of the responding maintained by contingent sucrose reinforcement, animals began reinstatement testing. Reinstatement of sucrose seeking was promoted by presentation of the cue light. For reinstatement, the FR5 schedule was used where active lever presses produced the light cue that had been presented during self-administration. However, satisfaction of the response requirements for each component did not dispense any sucrose pellets. Each 1 h reinstatement session was followed by extinction sessions until responding was again <15% of the response rate maintained by sucrose.
2.6 Deep brain stimulation
For DBS experiments, we used alternating current with biphasic symmetrical pulses (60 μs pulse width and a 160 Hz frequency) and 150 μA of current, parameters that are consistent with previous work in this field[15,19,20]. Immediately before the start of a reinstatement session, 0 or 150 μA current was delivered continuously to the bipolar electrodes. The stimulation continued for the duration of the 2 h reinstatement session. In the 0 μA condition, the electrodes were attached in the exact same manner as the 150 μA condition but DBS was not administered. The 0 and 150 μA currents were administered in a counterbalanced fashion across the multiple reinstatement test days.
2.7 Verification of electrode placements
After the completion of all experiments, the animals were given an overdose of pentobarbital (100 mg/kg) and perfused intracardially with 0.9% saline followed by 10% formalin. The brains were removed and coronal sections (100 μm) were taken at the level of the nucleus accumbens with a vibratome (Technical Products International; St. Louis, MO, USA). Animals with electrode placements outside of the areas of interest, or with excessive mechanical damage, were excluded from subsequent data analysis.
2.8 Drugs
Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (Rockville, MD, USA) and dissolved in bacteriostatic 0.9% saline.
2.9 Statistics
All reinstatement experiments were analyzed with two-way ANOVAs with repeated measures over reinstatement days. Pairwise analyses were made with Bonferroni post-tests (p < 0.05).
3. Results
3.1 DBS of the nucleus accumbens shell attenuates cue-induced reinstatement of cocaine seeking
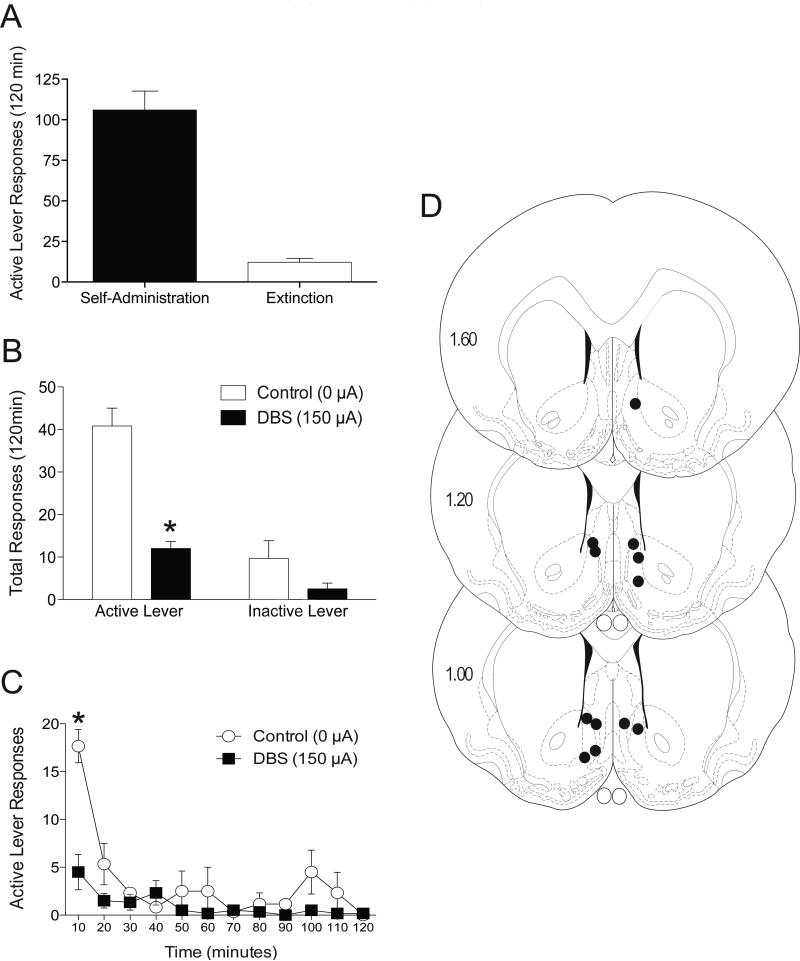
Following cocaine self-administration and extinction, deep brain stimulation of the nucleus accumbens shell (0 or 150 μA) was administered throughout the 2 h cue-induced reinstatement test session. Active lever responses (mean±SEM) during the final days of cocaine self-administration and extinction are shown in Figure 1A. Total active and inactive lever responses (mean±SEM) from the reinstatement session are presented in Figure 1B. These data were analyzed using a two-way ANOVA (both treatment and lever were within-subject factors), which revealed significant main effects of DBS treatment (F(1,5)=45.53, p<0.0011) and lever (F(1,5)=29.13, p<0.0029), as well as a significant interaction between these variables (F(1,5)=17.32, p<0.0088). Subsequent pairwise analyses indicated that the total active lever responses were significantly different between the 0 and 150 μA treatments during cue-induced reinstatement test sessions (Bonferroni, p<0.05). The time course data for active lever responding (Figure 1C) were analyzed with a two-way ANOVA (treatment and time were both within-subject), which revealed significant main effects of DBS treatment (F(1,5)=45.54, p<0.0011) and time (F(11,55)=9.474, p<0.0001) as well as a significant interaction between these variables (F(11,55)=5.115, p<0.0001). Subsequent pairwise analyses indicated that the active lever responses between 0 and 150 μA treatments were significantly different over the first 10 minutes of the reinstatement session (Bonferroni, p<0.001). The electrode placements are shown in Figure 1D (n=6). Although inactive lever responding was somewhat lower in the DBS treatment relative to control (Figure 1B), the low number of inactive responses limits the ability of this measure to accurately assess nonspecific rate suppression effects. Therefore, we also assessed the effects of DBS in the nucleus accumbens shell (0 or 150 μA) on the reinstatement of sucrose seeking.
Figure 1.
Deep brain stimulation of the nucleus accumbens shell attenuates cue-induced reinstatement of cocaine seeking. (A) Mean (±SEM) active lever responses during the final days of cocaine self-administration and extinction. (B) Mean (±SEM) active and inactive lever responses from reinstatement sessions with 0 or 150 μA stimulation aimed at the accumbens shell. (C) Time course of active lever responding from 0 or 150 μA stimulation of the accumbens shell. (D) Electrode placements from the shell (dark circles). The values are in millimeters, relative to bregma. *p < 0.001 0 μA compared to 150 μA. There were 6 animals per group.
3.2 DBS of the nucleus accumbens shell attenuates cue-induced reinstatement of sucrose seeking
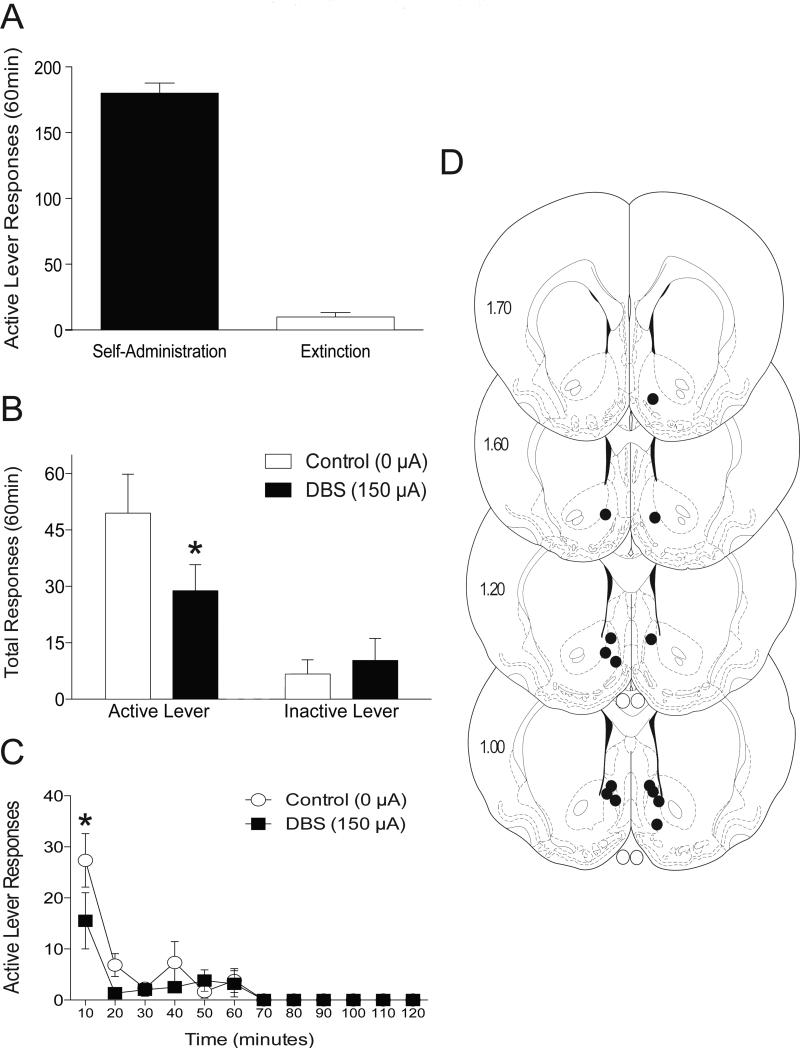
In order to determine if the effects of DBS in the accumbens shell were reinforcer specific, we tested the effect of DBS in the accumbens shell on sucrose cue-associated reinstatement. Active lever responses (mean±SEM) during the final days of sucrose self-administration and extinction are shown in Figure 2A. Total active and inactive lever responses (mean±SEM) from the reinstatement session during which DBS was administered to the accumbens shell during cue-induced sucrose reinstatement test sessions are shown in Figure 2B. These data were analyzed with a two-way ANOVA (treatment and lever were within-subject factors), which revealed no effect of DBS treatment (F(1,5)=1.836, p<0.2334), a significant effect of lever (F(1,5)=31.62, p<0.0025) and a significant interaction between these variables (F(1,5)=9.352, p<0.0282). Subsequent pairwise analyses indicated that the total active lever responses between the 0 and 150 μA treatments were significantly different (Bonferroni, p<0.05). The time course data for active lever responding (Figure 2C) were analyzed with a two-way ANOVA (treatment and time were within-subject factors), which revealed no significant effect of DBS treatment (F(1,5)=5.875, p<0.0598), but a significant main effect of time (F(11,55)=15.73, p<0.0001) as well as a significant interaction between these variables (F(11,55)=2.094, p<0.0362). Subsequent pairwise analyses indicated that the active lever responses between 0 and 150 μA treatments were significantly different over the first 10 minutes of the reinstatement session (Bonferroni, p<0.001). All data from one animal were removed because of highly unusual time course responses in the 0 μA control condition (162 active lever presses between 40 and 60 minutes with no other presses). This is well beyond 2 SDs above the mean for this group. electrode placements for these studies are shown in Figure 2D (n=6).
Figure 2.
Deep brain stimulation of the nucleus accumbens shell attenuates cue-induced reinstatement of sucrose seeking. (A) Mean (±SEM) active lever responses during the final days of sucrose self-administration and extinction. (B) Mean (±SEM) active and inactive lever responses from reinstatement sessions with 0 or 150 μA stimulation aimed at the accumbens shell. (C) Time course of active lever responding from 0 or 150 μA stimulation of the accumbens shell. (D) Electrode placements from the shell (dark circles). The values are in millimeters, relative to bregma. *p < 0.05 0 μA compared to 150 μA. There were 6 animals per group.
4. Discussion
We have previously shown that DBS of the accumbens shell attenuated cocaine priming-induced reinstatement of drug seeking but had no influence on the reinstatement of sucrose seeking[15]. The current data expand upon these findings and indicate that DBS of the nucleus accumbens shell also attenuates cue-induced reinstatement of cocaine and sucrose seeking.
The role of the accumbens core in cue-induced reinstatement of drug seeking is well established in the literature. Administration of an AMPA/kainate receptor antagonist in the nucleus accumbens core, but not the shell, reduced cue-induced cocaine-seeking behavior[21]. Additionally, excitotoxic lesions of the core, but not the shell, markedly attenuated cue-induced reinstatement of cocaine seeking[22]. Indeed, the accumbens core plays a critical role in cue-induced reinstatement of other drugs of abuse as well, including heroin, amphetamine, and even natural rewards such as sucrose[23]. While much is known about the accumbens core and its role in cue-induced reinstatement of drug seeking, the role of the accumbens shell in cue-induced reinstatement of drug seeking is much more complex.
Most studies have reported no effect or an attenuated effect in cue-induced reinstatement of drug or food seeking following pharmacological inactivation of the accumbens shell[9,24,25]. Others have even demonstrated potentiated cue-induced reinstatement of food seeking following inactivation of the accumbens shell[26]. Our results may differ from these studies as DBS has multiple potential mechanisms of action, including, but not limited to, inactivation of target brain regions. Additionally, our findings suggest that while accumbal shell DBS is effective at attenuating priming-induced reinstatement[15], it seems to be a questionable treatment for cue-induced reinstatement, as it also attenuated cue-induced reinstatement of sucrose seeking (Figure 2B). Although we show nonspecific behavioral effects of accumbal shell DBS, we do not believe that these effects are due to generalized motor inhibition, as we do not see any attenuation in inactive lever responding in either group. Additionally, we have previously shown that although DBS of the nucleus accumbens shell attenuates the priming-induced reinstatement of cocaine seeking, it has no effect on the priming-induced reinstatement of sucrose seeking[15,16]. These findings lead us to believe that the nonspecific behavioral effects elicited by accumbal shell DBS are not due to generalized motor inhibition, but rather to the differences between priming-induced and cue-induced reinstatement tasks.
Our present findings show that DBS of the accumbens shell attenuates cue-induced reinstatement of cocaine and sucrose seeking. As previously stated, preclinical and clinical literature suggest that DBS of the accumbens is a promising therapeutic modality in the treatment of addiction, especially due to the lack of unwanted side effects. These findings suggest that while DBS of the nucleus accumbens shell is a promising therapeutic modality for the treatment of severe cocaine addiction, clinical trials should proceed with caution, as there may be nonselective effects of accumbal DBS.
Highlights.
DBS of the accumbens shell attenuates cue-induced reinstatement of cocaine seeking
DBS of the accumbens shell attenuates cue-induced reinstatement of sucrose seeking
DBS of the accumbens shell non-selectively reduces cue-induced reward seeking behavior
Acknowledgements
We would like to thank Adrian Arreola and Julia Sigman for their technical assistance while performing experiments. Additionally, we would like to thank Dr. Mathieu Wimmer for his insightful comments on this manuscript. This work was generously supported by grants from the National Institutes of Health (NIH) R01 DA15214, K02 DA18678, and R01 DA22339 (RCP). H.D.S. was supported by an individual K01 award (DA030445). L.A.G. was supported in part by the Translational Addiction Research Fellowship Program Grant (T32 DA028874).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77:406–424. doi: 10.1016/j.neuron.2013.01.020. doi:10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Carroll KM. One-Year Follow-up of Psychotherapy and Pharmacotherapy for Cocaine Dependence. Arch Gen Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. doi:10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- 3.Hariz M. Twenty-five years of deep brain stimulation: Celebrations and apprehensions. Mov. Disord. 2012;27:930–933. doi: 10.1002/mds.25007. doi:10.1002/mds.25007. [DOI] [PubMed] [Google Scholar]
- 4.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. doi:10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grubert C, Hurlemann R, Bewernick BH, Kayser S, Hadrysiewicz B, Axmacher N, et al. Neuropsychological safety of nucleus accumbens deep brain stimulation for major depression: Effects of 12-month stimulation. World J Biol Psychiatry. 2011;12:516–527. doi: 10.3109/15622975.2011.583940. doi:10.3109/15622975.2011.583940. [DOI] [PubMed] [Google Scholar]
- 7.Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. doi:10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 8.Müller UJ, Voges J, Steiner J, Galazky I, Heinze H-J, Möller M, et al. Deep brain stimulation of the nucleus accumbens for the treatment of addiction. Annals of the New York Academy of Sciences. 2013;1282:119–128. doi: 10.1111/j.1749-6632.2012.06834.x. doi:10.1111/j.1749-6632.2012.06834.x. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. doi:10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- 10.McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. doi:10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce RC, Vassoler FM. Deep brain stimulation for the treatment of addiction: basic and clinical studies and potential mechanisms of action. Psychopharmacology. 2013;229:487–491. doi: 10.1007/s00213-013-3214-6. doi:10.1007/s00213-013-3214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller UJ, Sturm V, Voges J, Heinze H-J, Galazky I, Heldmann M, et al. Successful treatment of chronic resistant alcoholism by deep brain stimulation of nucleus accumbens: first experience with three cases. Pharmacopsychiatry. 2009;42:288–291. doi: 10.1055/s-0029-1233489. doi:10.1055/s-0029-1233489. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Xu J, Jiang J. Deep brain stimulation of nucleus accumbens on heroin- seeking behaviors: a case report. Biol. Psychiatry. 2011;69:e41–2. doi: 10.1016/j.biopsych.2011.02.012. doi:10.1016/j.biopsych.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Liu H-Y, Jin J, Tang J-S, Sun W-X, Jia H, Yang X-P, et al. Chronic deep brain stimulation in the rat nucleus accumbens and its effect on morphine reinforcement. Addict Biol. 2008;13:40–46. doi: 10.1111/j.1369-1600.2007.00088.x. doi:10.1111/j.1369-1600.2007.00088.x. [DOI] [PubMed] [Google Scholar]
- 15.Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J. Neurosci. 2008;28:8735–8739. doi: 10.1523/JNEUROSCI.5277-07.2008. doi:10.1523/JNEUROSCI.5277-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassoler FM, White SL, Hopkins TJ, Guercio LA, Espallergues J, Berton O, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine reinstatement through local and antidromic activation. J. Neurosci. 2013;33:14446–14454. doi: 10.1523/JNEUROSCI.4804-12.2013. doi:10.1523/JNEUROSCI.4804-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knapp CM, Tozier L, Pak A, Ciraulo DA, Kornetsky C. Deep brain stimulation of the nucleus accumbens reduces ethanol consumption in rats. Pharmacol. Biochem. Behav. 2009;92:474–479. doi: 10.1016/j.pbb.2009.01.017. doi:10.1016/j.pbb.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo L, Zhou H, Wang R, Xu J, Zhou W, Zhang F, et al. DBS of nucleus accumbens on heroin seeking behaviors in self-administering rats. Drug Alcohol Depend. 2013;129:70–81. doi: 10.1016/j.drugalcdep.2012.09.012. doi:10.1016/j.drugalcdep.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Chang J-Y, Shi L-H, Luo F, Woodward DJ. High frequency stimulation of the subthalamic nucleus improves treadmill locomotion in unilateral 6-hydroxydopamine lesioned rats. Brain Research. 2003;983:174–184. doi: 10.1016/s0006-8993(03)03053-1. [DOI] [PubMed] [Google Scholar]
- 20.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. doi:10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Di Ciano P. Dissociable Effects of Antagonism of NMDA and AMPA/KA Receptors in the Nucleus Accumbens Core and Shell on Cocaine-seeking Behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. doi:10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 22.Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. doi:10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- 23.Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self- administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004 doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential Effects of Blockade of Dopamine D1-Family Receptors in Nucleus Accumbens Core or Shell on Reinstatement of Heroin Seeking Induced by Contextual and Discrete Cues. 2007 doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin P, Pratt WE. PLOS ONE: Inactivation of the Nucleus Accumbens Core or Medial Shell Attenuates Reinstatement of Sugar-Seeking Behavior following Sugar Priming or Exposure to Food-Associated Cues. PloS One. 2014 doi: 10.1371/journal.pone.0099301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience. 2008;154:877–884. doi: 10.1016/j.neuroscience.2008.04.004. doi:10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]