Abstract
Anatomical and physiological approaches are beginning to reveal the synaptic origins of parallel ON- and OFF-pathway retinal circuits for the transmission of short (S-) wavelength sensitive cone signals in the primate retina. Anatomical data suggest that synaptic output from S-cones is largely segregated; central elements of synaptic triads arise almost exclusively from the “blue-cone” bipolar cell, a presumed ON bipolar, whereas triad-associated contacts derive primarily from the “flat” midget bipolar cell, a hyperpolarizing, OFF bipolar. Similarly, horizontal cell connectivity is also segregated, with only the H2 cell-type receiving numerous contacts from S-cones. Negative feedback from long (L-) and middle (M-) wavelength sensitive cones via the H2 horizontal cells elicits an antagonistic surround in S-cones demonstrating that S versus L + M or “blue-yellow” opponency is first established in the S-cone. However, the S-cone output utilizes distinct synaptic mechanisms to create color opponency at the ganglion cell level. The blue-cone bipolar cell is presynaptic to the small bistratified, “blue-ON” ganglion cell. S versus L + M cone opponency arises postsynaptically by converging S-ON and LM-OFF excitatory bipolar inputs to the ganglion cell’s bistratified dendritic tree. The common L + M cone surrounds of the parallel S-ON and LM-OFF cone bipolar inputs appear to cancel resulting in “blue-yellow” antagonism without center-surround spatial opponency. By contrast, in midget ganglion cells, opponency arises by the differential weighting of cone inputs to the receptive field center versus surround. In the macula, the “private-line” connection from a midget ganglion cell to a single cone predicts that S versus L + M opponency is transmitted from the S-cone to the S-OFF midget bipolar and ganglion cell. Beyond the macula, OFF-midget ganglion cell dendritic trees enlarge and collect additional input from multiple L and M cones. Thus S-OFF opponency via the midget pathway would be expected to become more complex in the near retinal periphery as L and/or M and S cone inputs sum to the receptive field center. An important goal for further investigation will be to explore the hypothesis that distinct bistratified S-ON versus midget S-OFF retinal circuits are the substrates for human psychophysical detection mechanisms attributed to S-ON versus S-OFF perceptual channels.
Keywords: Primate S-Cone, Bipolar cell, Horizontal cell, Ganglion cell, Outer plexiform layer
Primate S-cone circuits afford access to distinct color-coding synaptic mechanisms
The low spatial density and relatively selective synaptic connectivity of the short wavelength sensitive (S) cone type in the primate retina has long implicated these photoreceptors as playing a distinctive, if not exclusive, role in color processing (Garrigan et al., 2010) and the circuitry for color opponency at the retinal level (Calkins, 2001). But the sparsity of the S-cones as well as the bipolar and horizontal cells they synapse with, though amenable to anatomical study, has also made them a difficult subject for physiological characterization. The goal of this short review will be to consider how the output from the primate S-cone to second order interneurons, the horizontal and bipolar cell types, determines and limits the circuitry that establishes S versus L + M or “blue-yellow” color opponent visual pathways that emerge at the ganglion cell level. As this special issue makes clear, various components of the S-cone related circuitry and blue-yellow color-opponency are a shared attribute of the structure and function of mammalian retina (Haverkamp et al., 2005; Yin et al., 2009; Sher & DeVries, 2012). Despite this commonality, it remains striking that two key ganglion cell types linked to color opponency and the S-cones in the primate, the small bistratified, blue-ON cell and the midget ganglion cell have only been recognized in human and nonhuman primate species (Dacey, 1993; Silveira et al., 1999; Percival et al., 2009). The picture that emerges from recent work, although incomplete in many details, suggests that both center-surround receptive field structure and S versus L + M opponency arise first at the level of the S-cone itself and that these properties are transmitted primarily, but not exclusively, to single ON and OFF bipolar types, the presumed ON-blue cone bipolar cells and the presumed OFF-midget bipolar cells, which in turn synapse on the blue-ON small bistratified cell and the OFF-midget ganglion cell. The circuitry that utilizes these two pathways and further elaborates “blue-yellow” opponency appears distinct for the S-ON versus the S-OFF pathway and may contribute to distinct asymmetries in the S-ON versus S-OFF channels that have been measured with psychophysical methods in human vision.
Negative feedback from H2 horizontal cells creates S versus LM opponency in S-cones
The distinctive nature of the sparse S-cone population (Marc & Sperling, 1977; de Monasterio et al., 1981; Williams et al., 1981; Curcio et al., 1991) and the presence of a bipolar cell type that selectively connect to the S-cones (Mariani, 1984) provided an early starting point for understanding S-cone circuitry in the primate. However, it is convenient to begin with the S-cone connectivity to the horizontal cell types as this provides an anatomical basis for the receptive field physiology of the S-cone itself, considered further below.
It is well established that the two horizontal cell types in primate retina, the H1 and H2 cells (Fig. 1A–1C) differ in their cone connections: the H2 but not the H1 cells make abundant contact with the S-cones by both dendritic and local “axon-like” processes. H2 cells also make sparse contact around the margins of L and M cone pedicles whereas the H1 cells densely innervate these pedicles (Ahnelt & Kolb, 1994a,b; Dacey et al., 1996; Goodchild et al., 1996; Kolb et al., 1997; Chan & Grünert, 1998). The H2 and H1 cells are primate correlates of the A-type (cone connecting only) and B-type (rod and cone connecting) horizontal cells respectively common to the mammalian retina. In some mammals, the A-type horizontal cells exclusively (horse) or probably (ground squirrel) contact only S-cones, though the general mammalian pattern is that both horizontal cell types contact all cone types without selectivity (Peichl et al., 1998; Hack & Peichl, 1999). The functional significance of the S-cone connection to A-type horizontal cells in various nonprimate mammal species has yet to be explored.
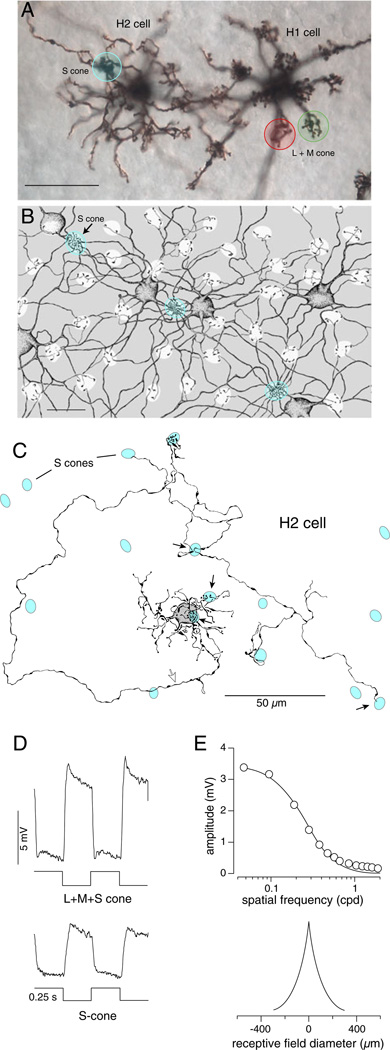
Fig. 1.
Morphology, physiology, and S-cone connectivity of the primate H2 horizontal cell. (A) Photomicrograph of horizontal cells in the flat-mounted macaque monkey retinal periphery capriciously stained with the Golgi method (Dacey, unpublished). Focus is on the dendritic contacts with cone pedicles in the outer plexiform layer (OPL). The H2 cell on the left sparsely contacts L and M cone pedicles and densely contacts S-cone pedicles (blue circle); by contrast, the H1 cell on the right densely contacts the majority L and M cone pedicles (red and green circles). (B) Camera lucida tracing of H2 cell dendritic network revealed by injection of Neurobiotin during intracellular recording (modified from Dacey et al., 1996). H2 cell bodies and dendrites are shown by stippling, and white holes in the gray background indicate position and size of cone pedicles. Consistent with the image of a single H2 cell shown in (A) the majority of cones are sparsely innervated and correspond to L and M cones. Three S-cone pedicles (blue circles; one pedicle indicated by arrow) are densely innervated. (C) Camera lucida tracing of a single H2 cell and associated “axon-like” process with surrounding S cone pedicles shown in blue (modified from Chan & Grünert, 1998). The H2 cell makes axonal contact with some of the S cones (black arrows) along its course. Axon indicated by open arrow. (D) Large hyper-polarizing voltage response to luminance modulation (upper trace; all three cone types modulated; stimulus time course shown below voltage trace). Hyperpolarizing voltage responses to S-cone isolating condition (lower trace). (E) H2 cell voltage response as a function of the spatial frequency for a luminance modulated drifting grating (upper plot). Data were fit with a single exponential function (solid curve). Exponential fit shown in the upper plot gives a receptive field extent of ~400 µm, reflecting the gap junctional coupling among highly overlapping H2 cell processes as indicated in (B).
The physiological consequence of primate horizontal cell cone connectivity is that only the H2 cells show a hyperpolarizing response to stimuli that selectively modulate the output of the S cone (Fig. 1D). H2 cells display large receptive fields that extend well beyond the dendritic field of a single H2 cell and reflect gap junctional coupling among neighboring cells (Fig. 1B and 1E). If the large coupled H2 network provides lateral negative feedback to the S-cone, it should give rise to a surround contributed by the majority of L and M cones and create S versus L + M opponency. Recordings made from S-cones in the intact macaque retina confirm this prediction (Fig. 2; Packer et al., 2010). S-cones were distinguished from L and M cones by their distinctive morphology (Fig. 2A) and a membrane current response to selective modulation of the S-cone quantal catch (Fig. 2B). The small diameter and cone purity of the S-modulated response were consistent with an origin from a single S-cone and with previous results in primates as well as other mammals showing that S-cones distinctively lack extensive coupling to other cones by telodendritic gap junctions (Ahnelt et al., 1990; Kolb et al., 1997; Hornstein et al., 2004; Li & DeVries, 2004; O’Brien et al., 2012). Chromatic opponency was shown by opposing response polarities to short- and long-wavelength flashes (Fig. 2C). In identified S-cones outward versus inward currents (hyperpolarization vs. depolarization of the voltage response) were evoked respectively by short and long wavelength light. The long-wavelength flash-evoked inward current had a large spatial extent consistent with a sign-reversed surround originating from L and M cones. These data were well fit with a receptive field model in which the sensitivities of a small center and a larger opponent surround decline exponentially with increasing distance from the receptive field center (Fig. 2D). The surround-evoked current was also abolished by an AMPA/kainate ionotropic glutamate receptor antagonist (Fig. 2C) showing that signal transmission from cones to horizontal cells was required.
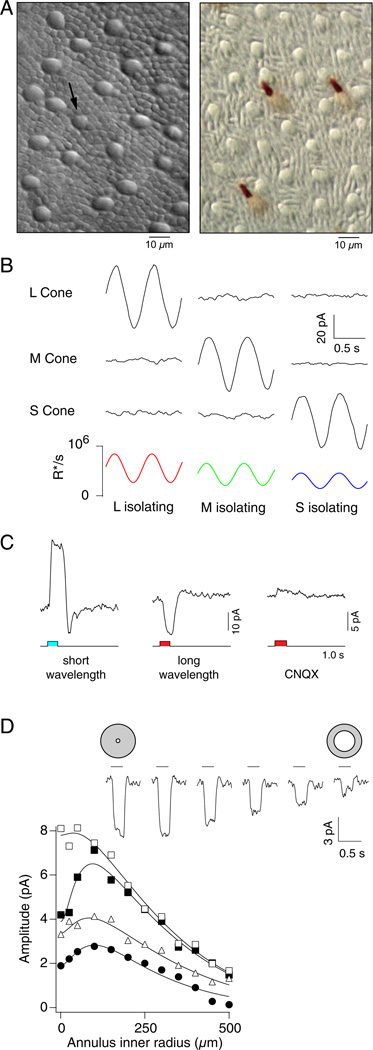
Fig. 2.
(A) Left panel: flat mounted macaque monkey retina maintained in vitro, photoreceptor side-up, plane of focus on rod (small) and cone (large) inner segments, viewed with infrared optics just prior to identifying and targeting an S-cone for intracellular recording and visual stimulation. Arrow indicates the smaller inner segment of an S cone. Right panel: S-cones labeled with an antibody against human S-cone opsin. Differential interference contrast images show inner and outer segments of unlabeled cones and rods interspersed between the labeled S cones (modified from Lee et al., 2010). (B) Membrane current response of macaque monkey cones to stimuli that selectively modulate the quantal catch of L, M, or S-cones. Colored lines plot predicted photoisomerization/s (R*/s) in L(red), M(green), and S(blue) cones. (C) S-cone chromatic opponency is created by horizontal cell feedback. Short wavelength light (blue) evokes outward current (left panel); long wavelength light (red) evokes inward current (middle panel). Inward current is blocked by 20 mM CNQX, a nonspecific AMPA/kainate type glutamate receptor antagonist (right). Membrane potential voltage clamped at −40 m V. Stimulus monitor below current traces. (D) Size of opponent surround in S-cones. Top: voltage responses to flashed annuli; inner radius 25–500 µm, outer radius 1 mm; flash duration, 250 ms. Bottom plot: peak response amplitude versus inner radius from four cones. Annular flash: long wavelength flash from darkness (□, ■, Δ) evoked 3.5 × 106, 2.6 × 106, and 1.1 × 105 photoisomerizations/s in L, M, and S cones, or L + M cone modulation (●) with a background rate of ~ 1.5 × 105 photoisomerizations/s for all spectral types and a flashed increment of 5.7 × 106 (L), 5.6 × 106 (M), and 0 (S). Membrane potential −40 mV. Lines are fits from a spatial receptive field model that summed center and surround fields of opposite polarity that declined exponentially with distance from the center. Surround length constants are 172(□), 176(■), 180(Δ), and 140(●) µm. Average center length constant is 31 ± 13 µM. (B, C, and D, modified from Packer et al., 2010).
Evidence was also presented that the negative feedback that gives rise to the long-wavelength sensitive surround is mediated by a voltage-activated cone calcium current, as has been demonstrated in other vertebrate cones (Verweij et al., 1996; Thoreson & Mangel, 2012). While the surround-induced modulation of the cone calcium current is relatively small it would be expected to have a large effect on cone synaptic transmission and the receptive field structure of bipolar cells.
In sum, blue–yellow spectral opponency and center-surround organization can be demonstrated in the macaque S-cone. Since the H2 horizontal cell is the only cell that can provide negative lateral feedback from the L and M cones to the S-cone it must be the source of this surround. Presumably, both the spectral and spatial opponency of the S-cone would be transmitted to the ON and OFF bipolar cells that receive excitatory input from the S-cone.
The S-ON pathway arises largely but not exclusively from the “blue-cone” bipolar cell
Cone bipolar cell types are well characterized anatomically in the primate and show diversity parallel to that found in other mammals (Fig. 3A; Boycott & Wässle, 1991; Wassle et al., 2009; Joo et al., 2011) as well as center-surround receptive field structure (Dacey et al., 2000b). Ten cone bipolar types are distinguished by their cone connectivity and dendritic stratification: the ON- and OFF-center midget cells defined by their “private-line” connection to single cones across much of the retina, three presumed OFF-center and three presumed ON-center “diffuse” types defined by their abundant, if not exclusive, input from multiple L and M cones and a single rarely observed “giant” type that makes sparse connections to widely spaced L and/or M cones. Finally, the blue-cone bipolar cell bears dendritic processes that extend toward and selectively contact the S-cone terminal in the OPL, and axon terminals that stratify deep in the inner-ON portion of the IPL (Fig. 3A).
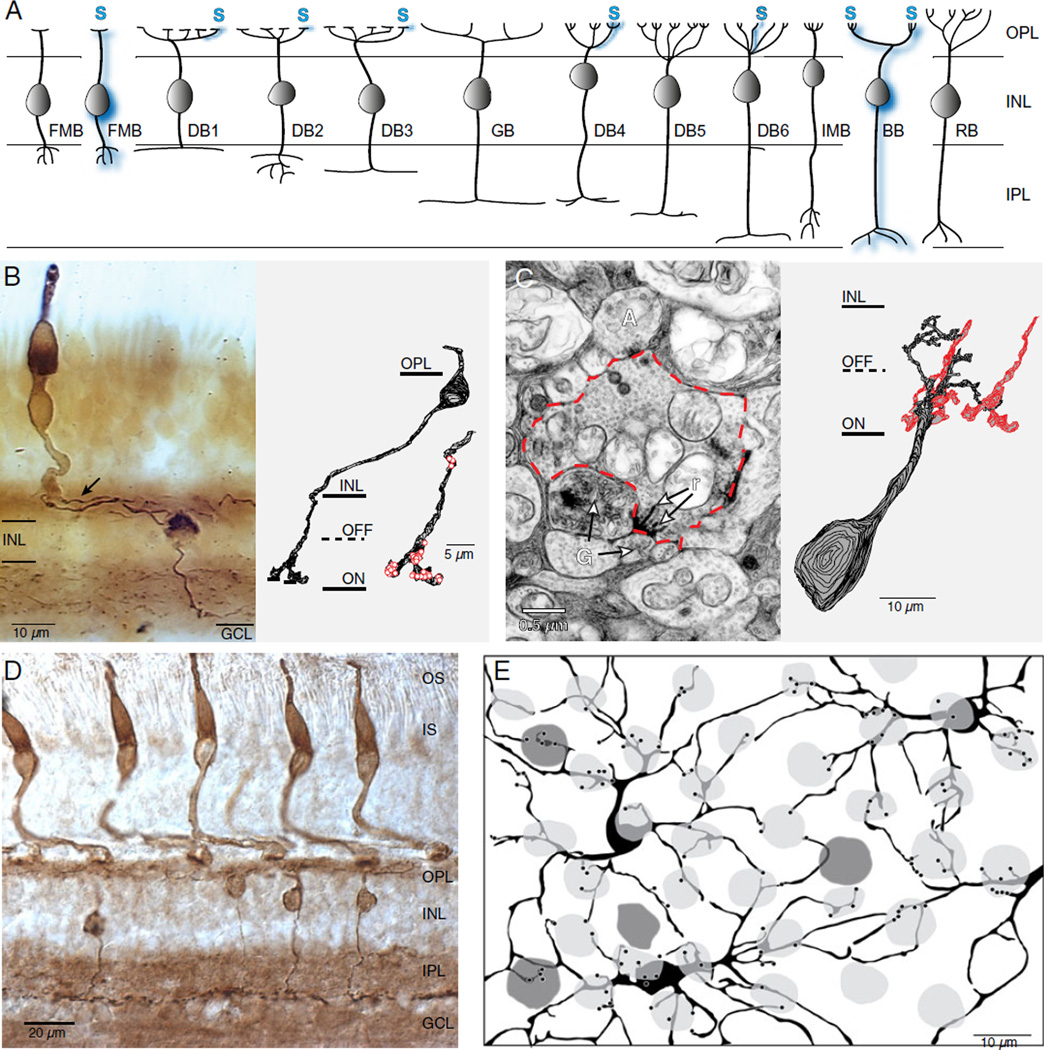
Fig. 3.
S-cone connectivity to cone bipolar cell types in the primate retina. (A) Schematic that illustrates the bipolar cell types of the primate retina together with current knowledge of the distribution of S-cone connectivity among these types. DB, diffuse cone bipolar; FMB, flat OFF-midget bipolar; IMB, invaginating presumed ON-midget bipolar; GB, giant bipolar; BB, blue cone bipolar; RB, rod bipolar. A subset of flat midget bipolars and the blue cone bipolar make selective connections with the S-cone (blue shading); DB1 (see Fig. 4 also), DB4, and DB6 bipolars like other diffuse types receive input primarily from L and M cones but also make consistent but sparse S-cone connections (blue shading; schematic modified from Joo et al., 2011). (B–C) Blue-cone bipolar circuit (modified from Calkins, 2001). (B) Left panel: dendrites of a blue cone bipolar cell in macaque retina, stained using antibodies against cholecystokinin (see also Kouyama and Marshak, 1992), contact the pedicle (arrow) of an S-cone immunostained with an antibody to S-opsin. OPL, outer plexiform layer; INL, inner nuclear layer; GCL, ganglion cell layer. Right panel: reconstruction from serial electron micrographs of a blue cone bipolar cell dendrite in macaque fovea coursing through and terminating deep in the “ON” portion of the IPL and forming 30 presynaptic active zones (red circles). (C) Left panel: axon terminal of S bipolar cell outlined with red dotted line; ribbon synapses (r) are presynaptic at a “dyad” of two ganglion cell dendrites. Right panel: reconstruction from electron micrographs of synaptic contacts between a small bistratified ganglion cell and the axon terminals of two blue cone bipolar cells (red outlined processes). (D) Vertical cryostat section through central macaque monkey retina double immunostained with CD15 and an S-cone opsin antibody that also labels the cone pedicles (see Lee et al., 2004). This material was used to show connectivity between the DB6 cell (stained by CD15) and S-cones. OS, outer segments; IS, inner segments OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. (E) Triple labeling with antibodies to CD15 and S-cone opsin, and with peanut agglutinin-fluorescein (PNA), which stains all cone pedicles, reveals DB6 connectivity to S, L, and M cones. The DB6 cell bodies and dendrites are shown schematically in black, the M/L-cone pedicles are shown in light gray, and the S-cone pedicles are shown in dark gray. Dendritic endings in the same focal plane as the cone pedicles are indicated as black dots. (D, E; modified from Lee et al., 2004).
Because S-cones can be selectively labeled with S-opsin immunostaining and are morphological distinct (Ahnelt et al., 1987; Ahnelt et al., 1990; Curcio et al., 1991), a number of studies have been able to characterize the synaptic architecture of the S-cone (Kouyama & Marshak, 1992; Kolb et al., 1997; Calkins et al., 1998; Herr et al., 2003; Klug et al., 2003; Lee et al., 2004; Lee et al., 2005) and to address the question of the degree to which the S-cone connects to the diverse bipolar types beyond the blue-cone bipolar. For the presumed ON-pathway types, general consensus is that the great majority, and perhaps in some instances, all of the invaginating presumed ON-pathway contacts arise from the selective connections made by the “blue-cone bipolar” cells (Fig. 3A) and that there are no connections with the ON-midget pathway (but see Field et al., 2010, which provides evidence that ~ 10% of the ON midget ganglion cells in the far retinal periphery receive combined L, M, and S-cone inputs). The expected depolarizing S-cone mediated ON response, and an L + M cone mediated surround transmitted from the S-cone has not been confirmed but a clear sign-inverted outward current in a blue-cone bipolar in response to current injected into a synaptically connected S-cone has been shown in the ground squirrel (Li & DeVries, 2006), and more recently, an S-ON response has been found for the equivalent cell type (type 9 BC) in the mouse (Haverkamp et al., 2005; Breuninger et al., 2011).
By contrast, with the ON-midget bipolar, it is now well established that at least two of the three ON-diffuse bipolar types (DB4 and DB6) that are dominated by the L + M cone contacts also make consistent, albeit sparse, connections with the S-cones (Fig. 3D–3E; Lee et al., 2004; Lee & Grunert, 2007). Such mixing of S-cone and M-cone inputs have also been observed for at least one ON bipolar type in both mouse (Breuninger et al., 2011) and ground squirrel (Li & DeVries, 2006). The significance of these other S-cone connections to presumed ON pathway bipolars is not clear, and it is worth noting that a minor input from the S-cones to the L + M cone-dominated H1 horizontal cells (Goodchild et al., 1996; Chan et al., 1997) was not detected physiologically (Dacey et al., 1996; Verweij et al., 1999a; Dacey et al., 2000a); similarly, the DB4 bipolar cell likely provides output to the ON-parasol ganglion cell (Marshak et al., 2002), and there is little evidence of a measurable S-cone input to this nonopponent pathway (Lee et al., 1988, 1989; Dacey & Lee, 1994; Sun et al., 2006a,b; Field et al., 2010) or its likely contribution to the luminance channel of human vision (Lennie et al., 1993; Lennie & Movshon, 2005). On the other hand, the S-cone connectivity does appear to be a very clear property of these diffuse bipolar cells. In this regard, it has been shown that the DB6 cell connects at both the inner and outer borders of the IPL with the intrinsically photosensitive melanopsin-expressing ganglion cells (M1 and M2 types) (Dacey et al., 2006; Grunert et al., 2011). In addition to the melanopsin-mediated photoresponse, these ganglion cells show S-OFF versus L + M ON color opponency (Dacey et al., 2005) suggesting the interesting speculation that the sparse contacts with S-cones may utilize ionotropic glutamate receptors and generate a hyperpolarizing-OFF response and the more numerous contacts with L + M cones generate a depolarizing-ON response. There is a precedent for such an ON–OFF color opponent bipolar cell in the nonmammalian retina (Haverkamp et al., 1999; Wong & Dowling, 2005), but as yet this pattern has not been observed in a mammalian retina.
In sum, a single S-cone selective bipolar provides the major excitatory pathways for an S-ON signal to the inner retina in both primates and nonprimate mammals. By contrast, the primate invaginating ON-midget bipolar avoids S-cone input entirely, or almost entirely. The presumed ON-diffuse types (DB4, 5, and 6) are dominated by L + M cone input but at least two types, DB4 and DB6 make consistent, though sparse, connections with the S-cones. Similar mixing of sparse S-cone and more numerous non-S-cone input to ON bipolar types in other mammals have been observed; the significance of these “minor” S-cone outputs will remain obscure until the light-evoked responses of the bipolar types and their ganglion cell targets are characterized in detail.
The S-OFF pathway arises largely, but not exclusively, from the “OFF-midget” bipolar cell
Initially, the lack of a symmetrical, OFF-pathway partner of the blue-cone bipolar cell, together with an apparent attenuation of an S-OFF component in ERG recordings led to the early suggestion that, similar to the rod pathway, an S-OFF-bipolar pathway may be absent or minor (de Monasterio, 1979; Evers & Gouras, 1986). However, basal or flat contacts, normally arising from hyperpo-larizing OFF-center bipolar cells, have been consistently observed at the S-cone synaptic complex though possibly at a reduced density relative to that found at L and M cone pedicles (Kouyama & Marshak, 1992; Kolb et al., 1997; Calkins et al., 1998; Calkins, 2000; Lee & Grunert, 2007). In addition, S-OFF receptive fields, though less frequently sampled, have been consistently identified at the retinal and LGN level in many studies (e.g., Valberg et al., 1986; for a list see, Calkins, 2001). Recently, clear differences in the spatial and chromatic properties of S-ON versus S-OFF cells have been characterized at the LGN level (Szmajda et al., 2006; Tailby et al., 2008a,b), and it is possible that these differences contribute to or underlie asymmetries identified in the human S-ON and S-OFF visual channels characterized with psychophysical methods (Shinomori et al., 1999; McLellan & Eskew, 2000; Shinomori & Werner, 2008). Thus clear identification and characterization of an S-OFF pathway could shed light on how synaptic mechanisms and circuits at the retinal level link to human color vision.
There is strong anatomical evidence that in the macaque monkey foveal retina each S-cone makes a cluster of synapses with the dendritic terminal of a single OFF-midget bipolar cell and that this presumed S-OFF bipolar forms a private-line connection from an S-cone to an OFF-midget ganglion cell (Fig. 4A; Klug et al., 2003). However, this apparently clear picture took a somewhat upsetting turn when it was shown that in another monkey species, a New World marmoset (Callithrix jacchus), the S-cones, identified by opsin immunolabeling, clearly avoided any contact with the flat midget bipolar, also identified by immunolabeling (Lee et al., 2005). This was doubly surprising as many other aspects of the S-cone circuit appear similar, if not identical, in macaque and marmoset (Chan & Grünert, 1998; Ghosh & Grunert, 1999; Percival et al., 2009). However, a true species difference does appear likely as AMPA-type glutamate receptor localization (a marker for midget bipolar flat contacts) is reduced at S-cone terminals in marmoset but not macaque (Haverkamp et al., 2001; Puller et al., 2007). Moreover, the S-cone to OFF-midget pathway connection has recently been detected in high-density multielectrode array recordings from large patches of midget ganglion cells in the periphery of the macaque retina (Fig. 4B; Field et al., 2010). In the retinal periphery, midget ganglion cells have large dendritic trees that gather convergent input from several midget bipolar cells, each of which connects to a single cone. Strikingly, ~ 60% of the OFF-midget ganglion cells received combined input from L, M, and S cones, whereas ~ 10% of the ON-midget ganglion cells are connected to an S-cone. This picture from the retinal periphery appears to echo the similar restriction of S-cone connections to presumed OFF-midget bipolar cells described above for macaque fovea (Klug et al., 2003).
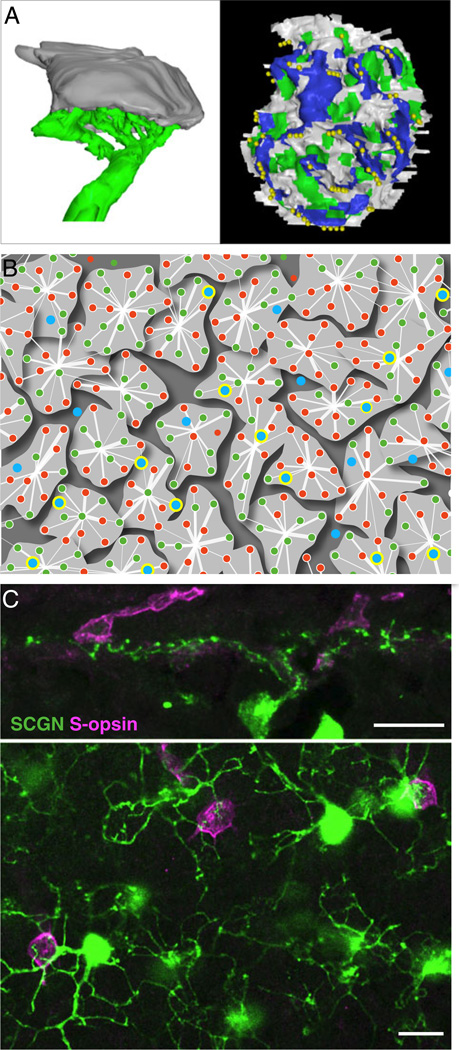
Fig. 4.
S-cone connectivity to the OFF visual pathway in primates. (A) An S-cone OFF midget bipolar cell receives all input from one S cone. Left: the synaptic terminal of an S-cone pedicle (gray) and the dendritic arbor of its OFF midget bipolar cell (green) were reconstructed at the ultrastructural level from vertical sections. Right: the presynaptic surface of the cone terminal, viewed en face. Synaptic contacts between the cone terminal and blue cone bipolar cells (dark blue regions) form the central elements of a synaptic triad. Synaptic contacts with the dendrites of the OFF midget bipolar cell (green) are adjacent to these central elements, in the triad-associated position. Gray regions identify contacts with other presumed diffuse bipolar cells. Yellow spheres mark sites at which synaptic ribbons are anchored to the presynaptic membrane (modified from Klug et al., 2003). (B) Functional sampling of L, M, and S cones (red, green, and blue dots, respectively) by a patch of neighboring OFF midget ganglion cell receptive field centers in the far retinal periphery using a high-density multielectrode array (modified from Field et al., 2010). Midget ganglion cell receptive field centers “tile” the retina (irregular light gray contours); cones with input to at least one midget ganglion cell are highlighted with an annulus. The thickness of the straight lines linking cones to the center of each field denotes relative input strength (see Field et al., 2010 for detailed methods). Note that in OFF-midget cells, ~ 56% of the S-cones are functionally linked to a midget receptive field, though the S-cone connections are generally weaker (thinner lines) than the L- and M-cone connections. Such S-cones in this OFF-midget patch are indicated with a thick yellow outline. (C) DB1, presumed OFF-center bipolar cells with axonal stratification at the outermost border of the IPL (see Fig. 3A) frequently contact S-cones, in addition to L and M cones. Upper panel: confocal projection (3 × 1.0 µm) of a peripheral retinal section stained for secretagogin (SCGN) and S-opsin showing that SCGN-labeled bipolar cell dendrites contact two S-cone pedicles. Lower panel: a single confocal section of a retinal flat-mount showing double labeling of SCGN and S-opsin. SCGN-labeled DB1 bipolar cells nonselectively contact M/L cones and S-cone pedicles within their dendritic arbors (scale bars = 10 µm) (modified from Puthussery et al., 2011).
Like the presumed ON-diffuse bipolar types, minor contacts from S-cones to presumed OFF-diffuse bipolar types DB1, DB2, and DB3 have been observed or inferred as likely (Calkins, 2000; Chan et al., 2001; Lee & Grunert, 2007). Most recently fortuitous and selective immunostaining of the DB1 cell population in macaque retina combined with S-opsin staining showed conclusively that this bipolar type consistently and frequently contacts S-cones in addition to L and M cones (Fig. 4C; Puthussery et al., 2011). Mixed S- and M-cone input to at least one OFF bipolar type in ground squirrel (Li & DeVries, 2006) and multiple types in mouse have also been identified (Wassle et al., 2009; Breuninger et al., 2011).
As for the ON-diffuse bipolar types, the significance of the S-cone contribution to these bipolar types remains unclear. The OFF-parasol cells receive input from the DB3 (Jacoby et al., 2000) cells, but there is strong evidence against a physiologically significant S-cone input to the OFF-parasol cells (Sun et al., 2006a; Field et al., 2010). The output of the DB1 cell is at the outer border of the IPL, however the only ganglion cell type identified thus far in the primate retina that stratifies at the outer IPL border is the melanopsin-expressing cell (Dacey et al., 2005), which is an unlikely recipient of synaptic input from the DB1 cell.
Transfer of S-ON signals from outer to inner retina
How does the creation of S versus L + M opponency at the level of the S-cone contribute to color opponency found at the ganglion cell level? Two S-ON ganglion cell types, the large and small bistra-tified cells, have been clearly identified (Dacey & Packer, 2003; Dacey et al., 2003), but only the small bistratified cell has now been characterized at the level of microcircuitry and synaptic physiology. The morphology, physiology, and synaptic connections of the Blue-ON, small bistratified ganglion cell provided a seductively simple “ON–OFF pathway hypothesis” (Dacey, 1996; Calkins et al., 1998) whereby the small bistratified cell receives parallel, converging excitatory ON-pathway input from the blue-cone bipolar cell and an L + M cone OFF-pathway input from the DB2 diffuse bipolar type (Calkins et al., 1998; Ghosh & Grunert, 1999; Percival et al., 2009). This picture is consistent with the original (Wiesel & Hubel, 1966; de Monasterio & Gouras, 1975; Derrington et al., 1984) and more recent physiological observations (Solomon et al., 2005; Crook et al., 2009) that blue-ON cells show “spatially coextensive” blue-ON and yellow-OFF receptive fields, which can thus be interpreted as opposing ON and OFF center mechanisms of roughly similar spatial extent (Fig. 5).
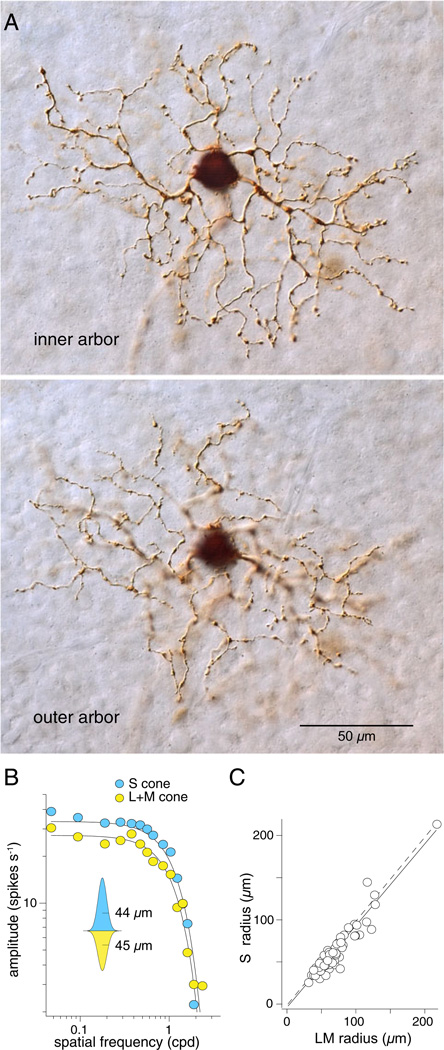
Fig. 5.
Morphology and receptive field structure of the small bistratified blue-ON ganglion cell in macaque monkey retina. (A) The bistratified dendritic tree of the blue-ON cell (wholemount; Neurobiotin intracellular fill; 6.7 mm retinal eccentricity) with separate arbors in inner-ON (upper panel; plane of focus near ganglion cell layer) and outer-OFF (lower panel; plane of focus near amacrine cell layer) portion of the IPL led to the hypothesis that parallel ON and OFF cone bipolar input to the dendritic tree could underlie spatially coextensive S ON versus LM OFF cone opponency. (B) Spatial frequency tuning of a small bistratified cell in response to S versus L + M cone selective drifting sine wave gratings. Data are fit with Gaussian functions of comparable radii (inset); S ON and L + M OFF fields are spatially coextensive. (C) Estimated L + M versus S receptive field Gaussian radii (µm) for 55 blue-ON cells (open circles; mean ± s.d.. = 1.12 ± 0.19). The dashed line shows the unity slope and the solid line, the actual fit to the data (slope = 0.97; R2 = 0.90; modified from Crook et al., 2009).
The ON–OFF pathway scheme however leaves out two fundamental features of retinal organization. First, it would be expected that the ON- and OFF-center bipolar cells presynaptic to the blue-ON ganglion cell have surrounds and that this major property would contribute to, or modify, color opponency (Dacey et al., 2000b; Fahey & Burkhardt, 2003; Thoreson & Mangel, 2012). More specifically, how can center-surround organization, established at the photoreceptor and bipolar cell level be apparently absent postsynaptically in the ON–OFF receptive field of the bistratified ganglion cells? Second, the small bistratified dendritic tree, like the majority of other ganglion cell types, is dominated by input from amacrine cells that presumably give rise to diverse types of GABAergic and glycinergic synaptic inhibition (Ghosh & Grunert, 1999). What role then does synaptic inhibition play in establishing or modulating blue-yellow opponency in this circuitry.
To begin to address these questions, a straightforward test of the ON–OFF pathway hypothesis was performed in two separate studies by selectively attenuating ON pathway transmission with the MGluR6 receptor agonist L-AP4 (Field et al., 2007; Crook et al., 2009). Field et al. found that ON pathway block completely abolished both the S-ON and L + M OFF evoked spike discharge of the blue-ON ganglion cell. The authors concluded that both response polarities must therefore originate in the S-ON pathway and that the L + M OFF response would likely originate in the outer retina as the surround of the blue-cone bipolar cell. This suggestion is certainly in concert with the presence of an L + M opponent surround in the S-cone itself. However in the other study, Crook et al. also applied L-AP4 while recording extracellularly from blue-ON ganglion cells. The S-ON response was abolished as expected, but the L + M-OFF response was fully maintained as predicted by the ON–OFF pathway hypothesis (Fig. 6). In addition, this study showed that the L + M OFF response was also preserved after block of synaptic inhibition and also after retinal buffering with HEPES [a condition that attenuated surrounds in parasol ganglion cells (Davenport et al., 2008) and midget ganglion cells (Crook et al., 2011)]. Why the Field et al. study failed to record an L + M OFF response during ON pathway block is unclear, but since L-AP4 can significantly hyperpolarize the resting membrane potential, it is possible that under the adaptation conditions used in that study, the excitatory OFF depolarization, while present, failed to reach spike threshold.
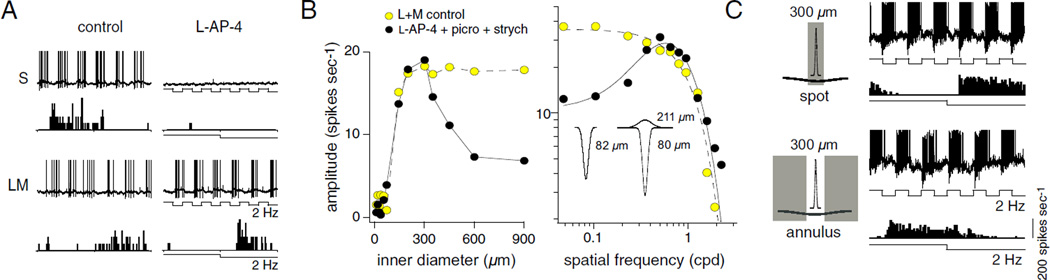
Fig. 6.
Probing the blue-yellow receptive field: isolation and spatial structure of the L + M excitatory OFF input to the small bistratified ganglion cell. (A) Spike discharge to full field cone isolating pulses (2 Hz, 16% cone contrast), before (left) and after (right) the addition of L-AP-4, the MGluR6 glutamate receptor agonist. L + M-OFF response is preserved after S-ON pathway attenuation by L-AP4. (B) Plots showing individual L + M-OFF receptive fields mapped with spots of increasing diameter (left) or drifting gratings before (yellow circles) and after (solid circles) the addition of L-AP-4 together with picrotoxin and strychnine to attenuate GABAergic and glycinergic synaptic inhibition. Prior to silencing S-ON pathway transmission, the LM receptive field was low pass and fit with a single Gaussian (dotted line), whereas in the presence of L-AP-4 and block of inhibition, the isolated L + M receptive field was band pass and fit with a Difference-of-Gaussians center-surround receptive field model (solid line). Insets show 2D receptive field profiles for both conditions with the estimated L + M center and surround Gaussian radii (µm) indicated. (C) In the presence of L-AP-4, the isolated LM input responds to the offset of a 300 µm spot and to the onset of an annulus with a 300 µm inner diameter as indicated by the phase of the response traces and spike histograms, confirming the presence of a surround as demonstrated in B (A, B, and C modified from Crook et al., 2009).
The question of whether these parallel bipolar inputs actually show center-surround organization was also partially answered in the Crook et al. study. After the S-ON input was attenuated with L-AP-4 and synaptic inhibition was blocked, an ON-surround was indeed evident in spatial maps of the L + M OFF response (Fig. 6B and 6C), and this surround was subsequently attenuated by enriching the retinal buffering capacity with HEPES, suggesting an origin in horizontal cell feedback to cones (Hirasawa & Kaneko, 2003; Davenport et al., 2008). These results suggest that an L + M OFF surround in the S ON excitatory pathway normally cancels an L + M ON surround in the L + M OFF excitatory pathway. This is consistent with the L + M surrounds described above for the S cones and also previously for the L and M cones (Verweij et al., 2003; Packer et al., 2010).
Finally, to more directly test the ON–OFF pathway hypothesis, a recent study used the voltage clamp to measure the excitatory and inhibitory synaptic currents evoked in the small bistratified cell by S or L + M cone selective stimuli (Crook et al., 2010; Dacey et al., 2011). Surprisingly, selective modulation of the S cone evoked a remarkably pure increase in excitation at light increment and a decrease in excitation at light decrement (Fig. 7). Indeed, synaptic inhibition did not appear to contribute to the S-cone evoked synaptic conductance (Fig. 7B). The L + M OFF light evoked current was dominated by synaptic excitation as predicted from the ON–OFF pathway hypothesis and the known anatomical wiring, but in contrast to the S cone-driven input, the L + M OFF input was also associated with clear feedforward inhibition and an additional inhibitory input at the onset of the L + M stimulus commonly referred to as “crossover” inhibition (Fig. 7C). As found for the extracellular spike recordings, S cone-evoked excitatory synaptic conductance is attenuated by L-AP4 application, while the excitatory L + M OFF response was preserved and further enhanced by antagonism of GABAergic and glycinergic receptors (Fig. 7D–7F).
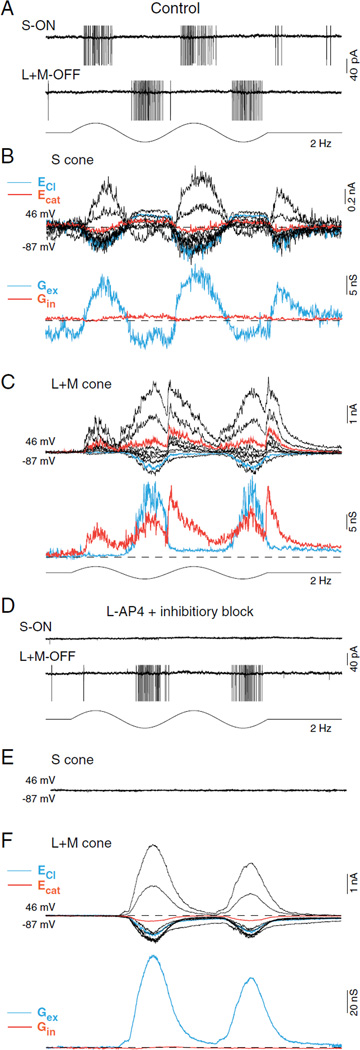
Fig. 7.
S versus L + M cone-opponent receptive field structure arises postsynaptically by convergence of ON- and OFF-bipolar input to the small bistratified dendritic tree. (A) S-ON versus L + M-OFF spike discharge of a small bistratified cell in response to cone selective S and L + M stimuli (2 Hz sinusoidal modulation; ~ 50% cone contrast; see Crook et al., 2009 for stimulus characterization). (B) Top: family of synaptic currents evoked by S-cone selective modulation, same stimulus as in (A) at holding potentials ranging from ~ −87 mV to +46 mV; currents at or closest to the chloride and cation equilibrium potentials are indicated in blue and red, respectively (see Crook et al., 2011 for detailed methods). Bottom: excitatory (blue) and inhibitory (red) synaptic conductances calculated from S cone-evoked synaptic currents shown above. S-cone stimulus evokes a pure modulation of an excitatory conductance with no evidence for either feedforward or “crossover inhibition.” (C) Top: synaptic currents evoked by L + M cone modulation; holding potentials as in (B). Bottom: excitatory (blue) and inhibitory (red) synaptic conductances derived from synaptic current family; synaptic excitation and feedforward inhibition are present during stimulus OFF-phase, and “crossover” inhibition is present during stimulus ON-phase. (D) Same experiment as in (A–C) but in the presence of bath applied L-AP4 (40 µM; MGluR6 receptor agonist, attenuates ON-pathway transmission) and block of synaptic inhibition: GABAzine (5 µM; GABAA receptor antagonist), TPMPA [50 µM; (1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid; GABA C receptor antagonist] and strychnine (1 µM; glycine receptor antagonist). S-ON spike discharge is abolished; L + M-OFF spike discharge is maintained. (E) S-cone evoked synaptic currents are completely suppressed. (F) Top: L + M OFF evoked synaptic currents persist and increase in amplitude relative to control (C). Bottom: L-AP4 and block of synaptic inhibition (red) isolates a pure excitatory conductance (blue) that underlies the L + M OFF spike discharge shown in (D).
The voltage clamp results show conclusively therefore that the fundamental S ON versus L + M OFF response of the small bistratified cell arises by ON versus OFF pathway excitation and is entirely consistent with the distinctive anatomy of this circuit (Fig. 8). The voltage clamp data however also show clearly that synaptic inhibition is present and that there is a dramatic asymmetry in the deployment of this inhibition whereby all of the inhibition, both feedforward and crossover, is associated with the L + M side of the opponent equation. The intriguing question remains then as to why the synaptic inhibition is so clearly asymmetrical. Indeed, it appears to be an exception to the standard pattern of retinal circuitry that the excitatory S cone bipolar input to the ganglion cell dendritic tree is not associated with feedforward amacrine cell synaptic architecture. One clue comes from the effects of blockade of all inhibition. The S cone-mediated response is unaltered as would be predicted from the voltage clamp reversal potentials; however, the L + M evoked current amplitude increases. Thus one function of the asymmetrical inhibition may be to contribute to the L + M versus S opponent balance under conditions when a weaker input from the sparse S cones opposes a very strong input from the more numerous L and M cones. While the L, M, and S cones show comparable sensitivity to the spectral distribution in natural scenes, the actual S-cone quantal catch is greatly reduced relative to L and M cones due to optical blurring and protective filtering by the lens and macular pigment of short wavelength light (Garrigan et al., 2010). Thus, this unusual synaptic asymmetry may reflect a balance between the optical constraints, protection from retinal damage, and the need to extract and transmit a chromatic signal.
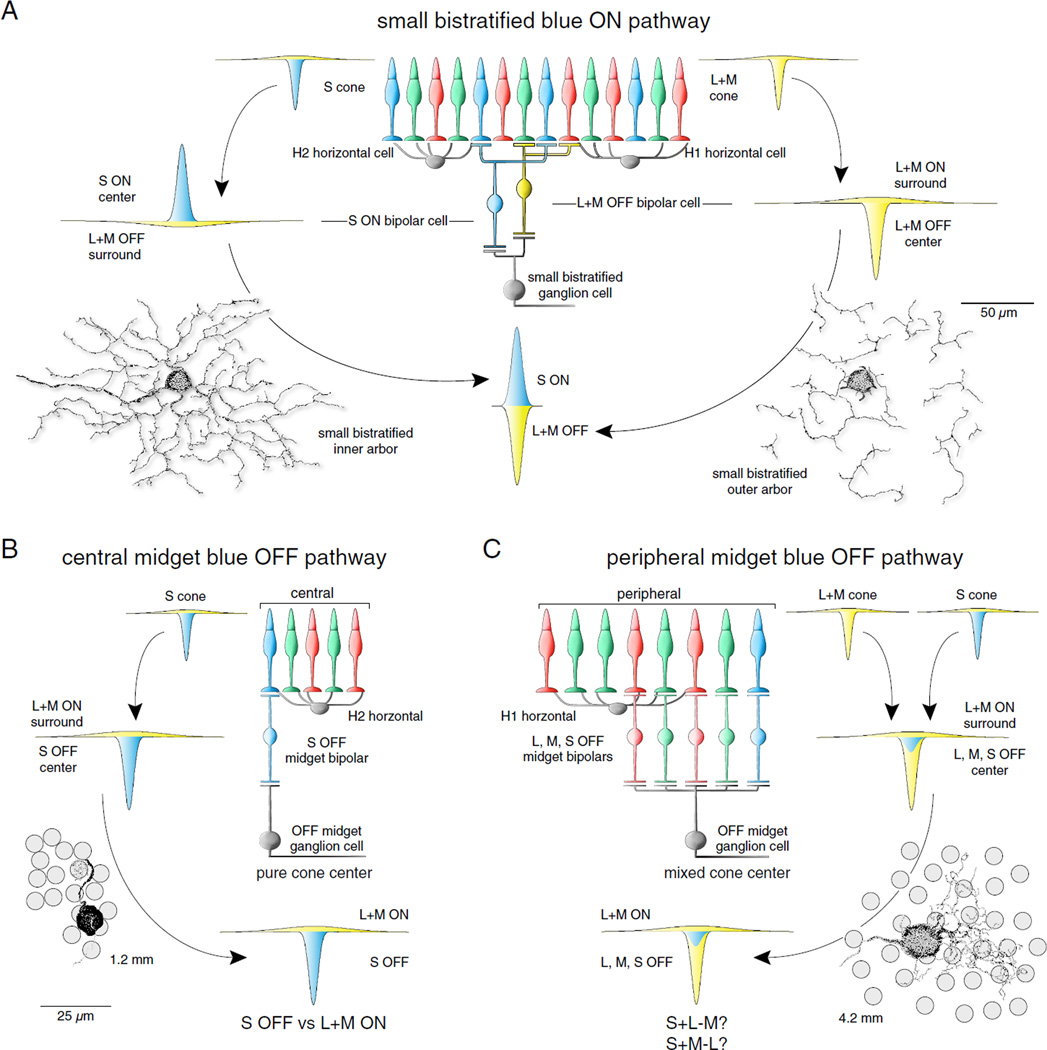
Fig. 8.
Distinctive circuitry and synaptic bases for S-ON and S-OFF opponent pathways in the primate retina. (A) Key elements of the small bistratified cell circuit illustrating the synaptic basis of the spatially coextensive S ON versus L + M OFF or “blue-yellow” receptive field structure. On the left: an L + M cone-surround appears in the S-cone (left) from H2 horizontal cell negative feedback (Packer et al., 2010). This receptive field is presumably transmitted to the blue cone bipolar and in turn to the small bistratified cell “inner” dendrites (curved arrows on left; see also Fig. 3). On the right: an L + M cone-mediated surround arising from H1 horizontal cell negative feedback also appears in L and M cones (Verweij et al., 2003) and is presumably transmitted to OFF-center diffuse bipolar cells and in turn to the small bistratified cell “outer” dendrites (curved arrows on the right) (e.g., Percival et al., 2009). No net surround results at the ganglion cell, leaving spatially restricted S ON and L + M OFF center mechanisms as the basis for the cone-opponent receptive field (see Crook et al., 2009 for details). (B, C) Key elements of the OFF midget circuit illustrating a synaptic basis for S-OFF opponent pathways in central (0–10° eccentricity) and peripheral (10–20° eccentricity) retina. (B) Formation of S versus L + M receptive field in the S cone and S-cone bipolar as described in (A) above. In central retina, each S-cone receives triad associated flat contacts from a flat midget bipolar creating an S OFF center, L + M ON surround that is transmitted via a “private-line” pathway to an OFF-midget ganglion cell (curved arrows). These midget ganglion cells should therefore display center-surround organization and S OFF versus L + M ON color opponency. (C) In the retinal periphery, midget ganglion cell dendritic trees enlarge and collect input from 3–8 cones via single cone-connecting midget bipolar cells (see Crook et al., 2013). Converging S, L, and M cone inputs are predicted to create a receptive field in the midget ganglion cell with more complex chromatic properties such as S + L versus M and S + M versus L opponency which would depend on the relative strength of L versus M cone inputs to center versus surround for any given midget cell (see Crook et al., 2011). Cell tracings in (B) and (C) are shown in conjunction with the approximate positions of the overlying cone inner segments and pedicles indicated by the shaded circles.
Transfer of S-OFF signals from outer to inner retina
As reviewed above, experimental characterization of an OFF pathway counterpart to the small bistratified blue-ON circuit has taken a more tortuous and contentious path at each synaptic step from the S-cone to the ganglion cell. Taken together, both the ultrastructural (Klug et al., 2003) and recent physiological (Field et al., 2010) results converge on the identification of an OFF-midget pathway as a major conveyor of S-OFF signals via the primary visual pathway. On the other hand, as will be considered further below, recent measurements of S-OFF LGN relay cells also suggest an additional nonmidget pathway origin for this pathway (Tailby et al., 2008a,b). In correspondence, S-OFF responses have been observed in melanopsin-expressing ganglion cells with very large receptive fields (Dacey et al., 2005). The major and crucial piece of information missing from this story is physiological measurement from identified midget cells in the central and near peripheral retina that clearly show color opponent S-OFF/L + M-ON center-surround receptive field organization.
Recent results from voltage clamp recordings of L versus M cone opponency in midget ganglion cells show that center-surround receptive field organization provides the basis for color opponency in these cells (Crook et al., 2011; Crook et al., 2013). If this receptive field structure is extended to the instance of an S-cone connection, the known anatomy and physiology of the midget pathway makes several clear predictions, represented schematically in Figure 8 (Fig. 8B and 8C). First, given the relative S-cone density in non-human primates (Martin & Grünert, 1999; Martin et al., 2000), only ~ 1–4% of the single cone connecting midget ganglion cells (central ~ 10°) should display S-OFF center receptive fields. Second, assuming an L + M surround is transmitted from the S-cone to the S-OFF midget bipolar, then these ganglion cells should further display S-OFF versus L + M-ON cone opponency if center-surround antagonism is engaged with suitable visual stimuli (Fig. 8B; Crook et al., 2011; Martin et al., 2011). Third, at greater retinal eccentricities (10–20°), the midget ganglion cell would receive additional nonselective input from ~ 2–8 L and/or M cones (Crook et al., 2013). Assuming approximately linear summation of the cone inputs to the receptive field (Verweij et al., 1999b; Diller et al., 2004), the predicted result would be more complex cone opponency including S + L versus M and S + M versus L cones (Fig. 8C) and a relatively weaker contribution of the S-cone input to the OFF-center mechanism. Such receptive fields have been observed consistently from early studies of primate ganglion cell physiology (de Monasterio et al., 1975) to a most recent and more quantitative analysis at the LGN level in macaque monkey (Tailby et al., 2008a) None of these studies, however, have directly linked the S-OFF receptive field to the anatomical midget pathway. Tailby et al. (2008a) noted that the S-OFF cells had higher achromatic spatial resolution than the S-ON cells, which would be consistent with a midget pathway origin, however other features of the cells’ responsivity led these authors to question this conclusion. In addition, a second study characterizing the properties of S-OFF relay cells in the LGN of a New World Marmoset monkey found that S-OFF cells showed larger receptive fields than either S-ON cells or other presumed midget pathway relay cells lacking S cone input (Tailby et al., 2008b). On the other hand, the chromatic properties expected when an S-OFF input is added into a midget receptive field that is already constructing L versus M cone opponency due to the differential weighting of cone inputs to center versus surround are exactly what was found by Tailby et al. (2008a) and also found in psychophysical studies that attempt to isolate the human S-OFF channel (Shinomori et al., 1999; McLellan & Eskew, 2000). Moreover, such a channel in which S + L versus M and S + M versus L cone opponency appear is an expected component in multistage models of human color vision (Stockman & Brainard, 2010). It is critical therefore that future experiments could test the hypotheses laid out in Fig. 8B and 8C by directly linking the S-cone signal to the midget ganglion cell in central retina and characterizing receptive field structure, cone opponency, and underlying synaptic mechanisms.
Summary
In the primate retina, the H2 horizontal cells uniquely receive input from S, L, and M cones with selective and abundant input from the S cones. Negative feedback to S cones from L and M cones via the H2 horizontal cell network gives rise to center-surround receptive field structure and “blue-yellow” color oppo-nency in the S-cone.
Among the diverse cone bipolar cell populations, the S-cone selective blue-cone bipolar is the major conduit for S-ON signals to the inner retina of primates and other nonprimate mammals. It is notable that the high density ON-midget bipolar pathway totally or largely avoids any connection with the S-cone. Sparse S-cone connectivity is present for other presumed ON-cone bipolar cell types that also receive abundant input from L and M cones, but the functional significance of these minor S-cone pathways remains to be established.
An S-OFF bipolar partner comparable to the blue-cone ON bipolar cell is lacking in both primate and nonprimate mammals. However, in the retina of macaque monkey, the OFF-midget bipolar contacts the S-cone and is the presumed basis for an S-OFF pathway to the inner retina. As for the ON-pathway bipolar cells, other OFF-bipolar types, in primate and nonprimate mammals receive combined input from S and nonS cones.
The distinctive bipolar connectivity for S-ON and S-OFF pathways in the primate suggests that different synaptic mechanisms underlie the appearance of cone opponency at the ganglion cell level. In the S-ON pathway, output of the S-ON blue cone bipolar converges with output of an L + M cone connecting OFF-bipolar at the dendritic tree of the small bistratified cell. S versus L + M opponency thus piggy-backs on the ON– OFF pathway dichotomy to create a spatially coextensive and color-coding receptive field structure. Common L + M surrounds in these bipolar cells established at the level of the cones by horizontal cell negative feedback appear to be synaptically canceled at the ganglion cell level.
By contrast, the known anatomy and physiology of the OFF-midget pathway suggests that in central retina, S-OFF versus LM-ON opponency will be associated with and arise via center-surround receptive field antagonism. In addition, as midget ganglion cell dendritic trees enlarge in the near retinal periphery to collect input from multiple L and M cones in addition to an S-cone-connected midget bipolar cell, S-OFF based opponency will become chromatically more complex giving rise to a relatively weaker S-OFF signal combined with L- and/or M-cone OFF signals. These clear predictions from known morphology will require physiological testing from identified OFF-midget ganglion cells.
Acknowledgment
We thank the anonymous reviewers for helpful comments on the manuscript and Beth Peterson for technical assistance and for preparation of the figures. This work was supported by National Institutes of Health grants RR00166 to the Tissue Distribution Program of the National Primate Research Center at the University of Washington, EY06678 (DMD) and EY01730 (Vision Research Core) and NIH Vision Research Training Grant, 5 T32 EY 007031 (J.C.).
References
- Ahnelt P, Keri C, Kolb H. Identification of pedicles of putative blue-sensitive cones in the human retina. The Journal of Comparative Neurology. 1990;293:39–53. doi: 10.1002/cne.902930104. [DOI] [PubMed] [Google Scholar]
- Ahnelt P, Kolb H. Horizontal cells and cone photoreceptors in human retina: A golgi-electron microscopic study of spectral connectivity. The Journal of Comparative Neurology. 1994a;343:406–427. doi: 10.1002/cne.903430306. [DOI] [PubMed] [Google Scholar]
- Ahnelt P, Kolb H. Horizontal cells and cone photoreceptors in primate retina: A golgi-light microscopic study of spectral connectivity. The Journal of Comparative Neurology. 1994b;343:387–405. doi: 10.1002/cne.903430305. [DOI] [PubMed] [Google Scholar]
- Ahnelt PK, Kolb H, Pflug R. Identification of a subtype of cone photoreceptor, likely to be blue sensitive, in the human retina. The Journal of Comparative Neurology. 1987;255:18–34. doi: 10.1002/cne.902550103. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. Morphological classification of bipolar cells of the primate retina. The European Journal of Neuroscience. 1991;3:1069–1088. doi: 10.1111/j.1460-9568.1991.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Breuninger T, Puller C, Haverkamp S, Euler T. Chromatic bipolar cell pathways in the mouse retina. The Journal of Neuroscience. 2011;31:6504–6517. doi: 10.1523/JNEUROSCI.0616-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins DJ. Representation of cone signals in the primate retina. Journal of the Optical Society of America. A, Optics, Image Science, and Vision. 2000;17:597–606. doi: 10.1364/josaa.17.000597. [DOI] [PubMed] [Google Scholar]
- Calkins DJ. Seeing with S cones. Progress in Retina and Eye Research. 2001;20:255–287. doi: 10.1016/s1350-9462(00)00026-4. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Tsukamoto Y, Sterling P. Microcircuitry and mosaic of a blue-yellow ganglion cell in the primate retina. The Journal of Neuroscience. 1998;18:3373–3385. doi: 10.1523/JNEUROSCI.18-09-03373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TL, Goodchild AK, Martin PR. The morphology and distribution of horizontal cells in the retina of a new world monkey, the marmoset Callithrix jacchus : A comparison with macaque monkey. Visual Neuroscience. 1997;14:125–140. doi: 10.1017/s0952523800008828. [DOI] [PubMed] [Google Scholar]
- Chan TL, Grünert U. Horizontal cell connections with short wavelength-sensitive cones in the retina: A comparison between New World and Old World primates. The Journal of Comparative Neurology. 1998;393:196–209. [PubMed] [Google Scholar]
- Chan TL, Martin PR, Clunas N, Grunert U. Bipolar cell diversity in the primate retina: Morphologic and immunocytochemical analysis of a new world monkey, the marmoset Callithrix jacchus. The Journal of Comparative Neurology. 2001;437:219–239. doi: 10.1002/cne.1280. [DOI] [PubMed] [Google Scholar]
- Crook JD, Davenport CM, Peterson BB, Packer OS, Detwiler PB, Dacey DM. Parallel ON and OFF cone bipolar inputs establish spatially coextensive receptive field structure of blue-yellow ganglion cells in primate retina. The Journal of Neuroscience. 2009;29:8372–8387. doi: 10.1523/JNEUROSCI.1218-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JD, Manookin MB, Packer OS, Dacey DM. Excitatory synaptic conductances mediate ‘blue-yellow’ and ‘red-green’ opponency in macaque monkey retinal ganglion cells. ARVO e-abstracts. 2010:5178. [Google Scholar]
- Crook JD, Manookin MB, Packer OS, Dacey DM. Horizontal cell feedback without cone type-selective inhibition mediates “red-green” color opponency in midget ganglion cells of the primate retina. The Journal of Neuroscience. 2011;31:1762–1772. doi: 10.1523/JNEUROSCI.4385-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JD, Packer OS, Troy JB, Dacey DM. Synaptic mechanisms of color and luminance coding: rediscovering the X-Y dichotomy in primate retinal ganglion cells. In: Chalupa M, Werner JS, editors. The New Visual Neuro-sciences. Cambridge, MA: MIT press; 2013. [Google Scholar]
- Curcio CA, Allen KA, Sloan KR, Lerea CL, Hurley JB, Klock IB, Milam AH. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. The Journal of Comparative Neurology. 1991;312:610–624. doi: 10.1002/cne.903120411. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Morphology of a small-field bistratified ganglion cell type in the macaque and human retina. Visual Neuroscience. 1993;10:1081–1098. doi: 10.1017/s0952523800010191. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Circuitry for color coding in the primate retina. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:582–588. doi: 10.1073/pnas.93.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Crook JD, Manookin MB, Packer OS. Absence of synaptic inhibition associated with S-cone ON excitatory input to the small bistratified, blue-yellow opponent ganglion cell of the macaque monkey retina. ARVO e-abstracts. 2011:4571. [Google Scholar]
- Dacey DM, Diller LC, Verweij J, Williams DR. Physiology of L- and M-cone inputs to H1 horizontal cells in the primate retina. Journal of the Optical Society of America. A, Optics, Image Science, and Vision. 2000a;17:589–596. doi: 10.1364/josaa.17.000589. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB. The blue-ON opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB, Stafford DK, Pokorny J, Smith VC. Horizontal cells of the primate retina: Cone specificity without spectral opponency. Science. 1996;271:656–659. doi: 10.1126/science.271.5249.656. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao H-W, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau K-W, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Packer OS. Colour coding in the primate retina: Diverse cell types and cone-specific circuitry. Current Opinion in Neurobiology. 2003;13:421–427. doi: 10.1016/s0959-4388(03)00103-x. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Packer OS, Diller LC, Brainard DH, Peterson BB, Lee BB. Center surround receptive field structure of cone bipolar cells in primate retina. Vision Research. 2000b;40:1801–1811. doi: 10.1016/s0042-6989(00)00039-0. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Peterson BB, Liao H-W, Yau K-W. Two types of melanopsin-containing ganglion cell in the primate retina: Links to dopaminergic amacrine and DB6 cone bipolar cells. Investigative Ophthalmology & Visual Science. 2006;47:3111. [Google Scholar]
- Dacey DM, Peterson BB, Robinson FR, Gamlin PD. Fireworks in the primate retina: In vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37:15–27. doi: 10.1016/s0896-6273(02)01143-1. [DOI] [PubMed] [Google Scholar]
- Davenport CM, Detwiler PB, Dacey DM. Effects of pH buffering on horizontal and ganglion cell light responses in primate retina: Evidence for the proton hypothesis of surround formation. The Journal of Neuroscience. 2008;28:456–464. doi: 10.1523/JNEUROSCI.2735-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Monasterio FM. Asymmetry of On-and OFF-pathways of blue-sensitive cones of the retina of macaques. Brain Research. 1979;166:39–48. doi: 10.1016/0006-8993(79)90647-4. [DOI] [PubMed] [Google Scholar]
- de Monasterio FM, Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. The Journal of Physiology. 1975;251:167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Monasterio FM, Gouras P, Tolhurst DJ. Trichromatic color opponency in ganglion cells of the rhesus monkey retina. The Journal of Physiology. 1975;251:197–216. doi: 10.1113/jphysiol.1975.sp011087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Monasterio FM, Schein SJ, McCrane EP. Staining of blue-sensitive cones of the macaque retina by a fluorescent dye. Science. 1981;213:1278–1281. doi: 10.1126/science.7268439. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Krauskopf J, Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. The Journal of Physiology. 1984;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller L, Packer OS, Verweij J, McMahon MJ, Williams DR, Dacey DM. L and M cone contributions to the midget and parasol ganglion cell receptive fields of macaque monkey retina. The Journal of Neuroscience. 2004;24:1079–1088. doi: 10.1523/JNEUROSCI.3828-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers HU, Gouras P. Three cone mechanisms in the primate electroretinogram: Two with, one without off-center bipolar responses. Vision Research. 1986;26:245–254. doi: 10.1016/0042-6989(86)90019-2. [DOI] [PubMed] [Google Scholar]
- Fahey PK, Burkhardt DA. Center-surround organization in bipolar cells: Symmetry for opposing contrasts. Visual Neuroscience. 2003;20:1–10. doi: 10.1017/s0952523803201012. [DOI] [PubMed] [Google Scholar]
- Field GD, Gauthier JL, Sher A, Greschner M, Machado TA, Jepson LH, Shlens J, Gunning DE, Mathieson K, Dabrowski W, Paninski L, Litke AM, Chichilnisky EJ. Functional connectivity in the retina at the resolution of photo-receptors. Nature. 2010;467:673–677. doi: 10.1038/nature09424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field GD, Sher A, Gauthier JL, Greschner M, Shlens J, Litke AM, Chichilnisky EJ. Spatial properties and functional organization of small bistratified ganglion cells in primate retina. The Journal of Neuroscience. 2007;27:13261–13272. doi: 10.1523/JNEUROSCI.3437-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigan P, Ratliff CP, Klein JM, Sterling P, Brainard DH, Balasubramanian V. Design of a trichromatic cone array. PLoS Computational Biology. 2010;6:e1000677. doi: 10.1371/journal.pcbi.1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Grunert U. Synaptic input to small bistratified (blue-ON) ganglion cells in the retina of a new world monkey, the marmoset Callithrix jacchus. The Journal of Comparative Neurology. 1999;413:417–428. doi: 10.1002/(sici)1096-9861(19991025)413:3<417::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Goodchild AK, Chan TL, Grünert U. Horizontal cell connections with short-wavelength-sensitive cones in macaque monkey retina. Visual Neuroscience. 1996;13:833–845. doi: 10.1017/s0952523800009093. [DOI] [PubMed] [Google Scholar]
- Grunert U, Jusuf PR, Lee SC, Nguyen DT. Bipolar input to melanopsin containing ganglion cells in primate retina. Visual Neuroscience. 2011;28:39–50. doi: 10.1017/S095252381000026X. [DOI] [PubMed] [Google Scholar]
- Hack I, Peichl L. Horizontal cells of the rabbit retina are non-selectively connected to the cones. The European Journal of Neuroscience. 1999;11:2261–2274. doi: 10.1046/j.1460-9568.1999.00647.x. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Grunert U, Wassle H. The synaptic architecture of AMPA receptors at the cone pedicle of the primate retina. The Journal of Neuroscience. 2001;21:2488–2500. doi: 10.1523/JNEUROSCI.21-07-02488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Möckel W, Ammermüller J. Different types of synapses with different spectral types of cones underlie color opponency in a bipolar cell of the turtle retina. Visual Neuroscience. 1999;16:801–809. doi: 10.1017/s0952523899164186. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. The Journal of Neuroscience. 2005;25:5438–5445. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr S, Klug K, Sterling P, Schein S. Inner S-cone bipolar cells provide all of the central elements for S cones in macaque retina. The Journal of Comparative Neurology. 2003;457:185–201. doi: 10.1002/cne.10553. [DOI] [PubMed] [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating syn-aptic cleft mediate feedback from horizontal cells to cone photorecep-tors by modulating Ca2+ channels. The Journal of General Physiology. 2003;122:657–671. doi: 10.1085/jgp.200308863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstein EP, Verweij J, Schnapf JL. Electrical coupling between red and green cones in primate retina. Nature Neuroscience. 2004;7:745–750. doi: 10.1038/nn1274. [DOI] [PubMed] [Google Scholar]
- Jacoby RA, Wiechmann AF, Amara SG, Leighton BH, Marshak DW. Diffuse bipolar cells provide input to OFF parasol ganglion cells in the macaque retina. The Journal of Comparative Neurology. 2000;416:6–18. doi: 10.1002/(sici)1096-9861(20000103)416:1<6::aid-cne2>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HR, Peterson BB, Haun TJ, Dacey DM. Characterization of a novel large-field cone bipolar cell type in the primate retina: Evidence for selective cone connections. Visual Neuroscience. 2011;28:29–37. doi: 10.1017/S0952523810000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug K, Herr S, Ngo IT, Sterling P, Schein S. Macaque retina contains an S-cone OFF midget pathway. The Journal of Neuroscience. 2003;23:9881–9887. doi: 10.1523/JNEUROSCI.23-30-09881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Goede P, Roberts S, McDermott R, Gouras P. Uniqueness of the S-cone pedicle in the human retina and consequences for color processing. The Journal of Comparative Neurology. 1997;386:443–460. doi: 10.1002/(sici)1096-9861(19970929)386:3<443::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Kouyama N, Marshak DW. Bipolar cells specific for blue cones in the macaque retina. The Journal of Neuroscience. 1992;12:1233–1252. doi: 10.1523/JNEUROSCI.12-04-01233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Grunert U. Retinal connectivity and primate vision. Progress in Retina and Eye Research. 2010;29:622–639. doi: 10.1016/j.preteyeres.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. The physiological basis of heterochromatic flicker photometry demonstrated in the ganglion cells of the macaque retina. The Journal of Physiology. 1988;404:323–347. doi: 10.1113/jphysiol.1988.sp017292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. The Journal of Physiology. 1989;414:223–243. doi: 10.1113/jphysiol.1989.sp017685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Grunert U. Connections of diffuse bipolar cells in primate retina are biased against S-cones. The Journal of Comparative Neurology. 2007;502:126–140. doi: 10.1002/cne.21284. [DOI] [PubMed] [Google Scholar]
- Lee SC, Jusuf PR, Grunert U. S-cone connections of the diffuse bipolar cell type DB6 in macaque monkey retina. The Journal of Comparative Neurology. 2004;474:353–363. doi: 10.1002/cne.20139. [DOI] [PubMed] [Google Scholar]
- Lee SC, Telkes I, Grunert U. S-cones do not contribute to the OFF-midget pathway in the retina of the marmoset, Callithrix jacchus. The European Journal of Neuroscience. 2005;22:437–447. doi: 10.1111/j.1460-9568.2005.04231.x. [DOI] [PubMed] [Google Scholar]
- Lennie P, Movshon JA. Coding of color and form in the genicu-lostriate visual pathway (invited review). Journal of the Optical Society of America. A, Optics, Image Science, and Vision. 2005;22:2013–2033. doi: 10.1364/josaa.22.002013. [DOI] [PubMed] [Google Scholar]
- Lennie P, Pokorny J, Smith VC. Luminance. Journal of the Optical Society of America. A, Optics, Image Science, and Vision. 1993;10:1283–1193. doi: 10.1364/josaa.10.001283. [DOI] [PubMed] [Google Scholar]
- Li W, DeVries SH. Separate blue and green cone networks in the mammalian retina. Nature Neuroscience. 2004;7:751–756. doi: 10.1038/nn1275. [DOI] [PubMed] [Google Scholar]
- Li W, DeVries SH. Bipolar cell pathways for color and luminance vision in a dichromatic mammalian retina. Nature Neuroscience. 2006;9:669–675. doi: 10.1038/nn1686. [DOI] [PubMed] [Google Scholar]
- Marc RE, Sperling HG. Chromatic organization of primate cones. Science. 1977;196:454–456. doi: 10.1126/science.403607. [DOI] [PubMed] [Google Scholar]
- Mariani AP. Bipolar cells in monkey retina selective for the cones likely to be blue-sensitive. Nature. 1984;308:184–186. doi: 10.1038/308184a0. [DOI] [PubMed] [Google Scholar]
- Marshak DW, Yamada ES, Bordt AS, Perryman WC. Synaptic input to an ON parasol ganglion cell in the macaque retina: A serial section analysis. Visual Neuroscience. 2002;19:299–305. doi: 10.1017/s0952523802192078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, Blessing EM, Buzas P, Szmajda BA, Forte JD. Transmission of colour and acuity signals by parvocel-lular cells in marmoset monkeys. The Journal of Physiology. 2011;589:2795–2812. doi: 10.1113/jphysiol.2010.194076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, Grünert U. Analysis of the short wavelength-sensitive (“blue”) cone mosaic in the primate retina: Comparison of New World and Old World monkeys. The Journal of Comparative Neurology. 1999;406:1–14. doi: 10.1002/(sici)1096-9861(19990329)406:1<1::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Martin PR, Grunert U, Chan TL, Bumsted K. Spatial order in short-wavelength-sensitive cone photoreceptors: A comparative study of the primate retina. Journal of the Optical Society of America. A, Optics, Image Science, and Vision. 2000;17:557–567. doi: 10.1364/josaa.17.000557. [DOI] [PubMed] [Google Scholar]
- McLellan JS, Eskew RT. ON and OFF S-cone pathways have different long-wave cone inputs. Vision Research. 2000;40:2449–2465. doi: 10.1016/s0042-6989(00)00107-3. [DOI] [PubMed] [Google Scholar]
- O’Brien JJ, Chen X, Macleish PR, O’Brien J, Massey SC. Photoreceptor coupling mediated by connexin36 in the primate retina. The Journal of Neuroscience. 2012;32:4675–4687. doi: 10.1523/JNEUROSCI.4749-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer OS, Verweij J, Li PH, Schnapf JL, Dacey DM. Blue-yellow opponency in primate S cone photoreceptors. The Journal of Neuroscience. 2010;30:568–572. doi: 10.1523/JNEUROSCI.4738-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L, Sandmann D, Boycott BB. Comparative anatomy and function of mammalian horizontal cells. In: Chalupa LM, Finlay BL, editors. Development and Organization of the Retina. New York: Plenum Press; 1998. pp. 147–172. [Google Scholar]
- Percival KA, Jusuf PR, Martin PR, Grunert U. Synaptic inputs onto small bistratified (blue-ON/yellow-OFF) ganglion cells in marmoset retina. The Journal of Comparative Neurology. 2009;517:655–669. doi: 10.1002/cne.22183. [DOI] [PubMed] [Google Scholar]
- Puller C, Haverkamp S, Grunert U. OFF midget bipolar cells in the retina of the marmoset, Callithrix jacchus, express AMPA receptors. The Journal of Comparative Neurology. 2007;502:442–454. doi: 10.1002/cne.21315. [DOI] [PubMed] [Google Scholar]
- Puthussery T, Gayet-Primo J, Taylor WR, Haverkamp S. Immunohistochemical identification and synaptic inputs to the diffuse bipolar cell type DB1 in macaque retina. The Journal of Comparative Neurology. 2011;519:3640–3656. doi: 10.1002/cne.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A, DeVries SH. A non-canonical pathway for mammalian blue-green color vision. Nature Neuroscience. 2012;15:952–953. doi: 10.1038/nn.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomori K, Spillmann L, Werner JS. S-cone signals to temporal OFF-channels: Asymmetrical connections to postreceptoral chromatic mechanisms. Vision Research. 1999;39:39–49. doi: 10.1016/s0042-6989(97)00460-4. [DOI] [PubMed] [Google Scholar]
- Shinomori K, Werner JS. The impulse response of S-cone pathways in detection of increments and decrements. Visual Neuroscience. 2008;25:341–347. doi: 10.1017/S0952523808080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira LC, Lee BB, Yamada ES, Kremers J, Hunt DM, Martin PR, Gomes FL. Ganglion cells of a short-wavelength-sensitive cone pathway in New World monkeys: Morphology and physiology. Visual Neuroscience. 1999;16:333–343. doi: 10.1017/s0952523899162138. [DOI] [PubMed] [Google Scholar]
- Solomon SG, Lee BB, White AJ, Ruttiger L, Martin PR. Chromatic organization of ganglion cell receptive fields in the peripheral retina. The Journal of Neuroscience. 2005;25:4527–4539. doi: 10.1523/JNEUROSCI.3921-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman A, Brainard DH. Color Vision Mechanisms. New York: McGraw-Hill; 2010. [Google Scholar]
- Sun H, Smithson HE, Zaidi Q, Lee BB. Do magnocellular and parvocellular ganglion cells avoid short-wavelength cone input? Visual Neuroscience. 2006a;23:441–446. doi: 10.1017/S0952523806233042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Smithson HE, Zaidi Q, Lee BB. Specificity of cone inputs to macaque retinal ganglion cells. Journal of Neurophysiology. 2006b;95:837–849. doi: 10.1152/jn.00714.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmajda BA, Buzas P, Fitzgibbon T, Martin PR. Geniculocortical relay of blue-off signals in the primate visual system. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19512–19517. doi: 10.1073/pnas.0606970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailby C, Solomon SG, Lennie P. Functional asymmetries in visual pathways carrying S-cone signals in macaque. The Journal of Neuroscience. 2008a;28:4078–4087. doi: 10.1523/JNEUROSCI.5338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailby C, Szmajda BA, Buzas P, Lee BB, Martin PR. Transmission of blue (S) cone signals through the primate lateral geniculate nucleus. The Journal of Physiology. 2008b;586:5947–5967. doi: 10.1113/jphysiol.2008.161893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Progress in Retina and Eye Research. 2012;31:407–441. doi: 10.1016/j.preteyeres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valberg A, Lee BB, Tigwell DA. Neurones with strong inhibitory s-cone inputs in the macaque lateral geniculate nucleus. Vision Research. 1986;26:1061–1064. doi: 10.1016/0042-6989(86)90040-4. [DOI] [PubMed] [Google Scholar]
- Verweij J, Dacey DM, Peterson BB, Buck SL. Sensitivity and dynamics of rod signals in H1 horizontal cells of the macaque monkey retina. Vision Research. 1999a;39:3662–3672. doi: 10.1016/s0042-6989(99)00093-0. [DOI] [PubMed] [Google Scholar]
- Verweij J, Diller LC, Williams DR, Dacey DM. The relative strength of L and M cone inputs to H1 horizontal cells in primate retina. Investigative Ophthalmology & Visual Science. 1999b;40:S240. [Google Scholar]
- Verweij J, Hornstein EP, Schnapf JL. Surround antagonism in macaque cone photoreceptors. The Journal of Neuroscience. 2003;23:10249–10257. doi: 10.1523/JNEUROSCI.23-32-10249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Research. 1996;36:3943–3953. doi: 10.1016/s0042-6989(96)00142-3. [DOI] [PubMed] [Google Scholar]
- Wassle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. The Journal of Neuroscience. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. Journal of Neurophysiology. 1966;29:1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Williams DR, MacLeod DIA, Hayhoe MM. Punctate sensitivity of the blue-sensitive mechanism. Vision Research. 1981;21:1357–1375. doi: 10.1016/0042-6989(81)90242-x. [DOI] [PubMed] [Google Scholar]
- Wong KY, Dowling JE. Retinal bipolar cell input mechanisms in giant Danio: III. ON-OFF bipolar cells and their color-opponent mechanisms. Journal of Neurophysiology. 2005;94:265–272. doi: 10.1152/jn.00271.2004. [DOI] [PubMed] [Google Scholar]
- Yin L, Smith RG, Sterling P, Brainard DH. Physiology and morphology of color-opponent ganglion cells in a retina expressing a dual gradient of S and M opsins. The Journal of Neuroscience. 2009;29:2706–2724. doi: 10.1523/JNEUROSCI.5471-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]