Abstract
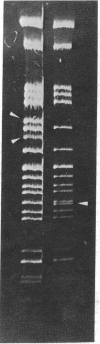
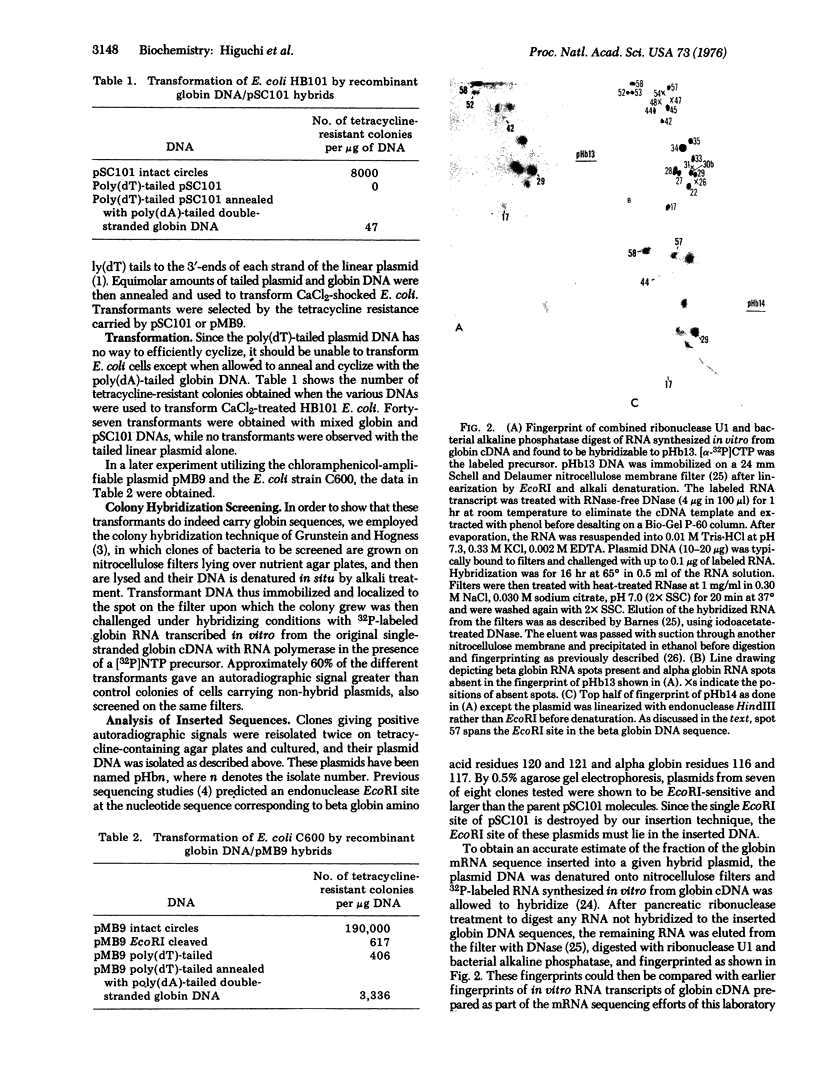
Complementary DNA, transcribed in vitro from purified rabbit globin messenger RNA and made double-stranded, has been inserted into Escherichia coli plasmids pSC101 and pMB9 by the poly(dT)/poly(dA) "tailing" and annealing technique. E. coli transformants given by this DNA preparation have been shown to contain globin sequences by the hybridization of globin RNA to DNA from clones grown and lysed in situ on nitrocellulose filters. An estimate of the amount of inserted globin sequences has been provided by fingerprint analysis of globin mRNA sequences hybridized to the purified plasmid chimeras. Inserted sequences so far subjected to detailed analysis have been ascribed to the rabbit beta globin chain. The susceptibility of inserted beta globin, sequences to the restriction endonuclease EcoRI confirms the existence of a site already found through previous nucleotide sequence analysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Enzymatic synthesis of oligodeoxynucleotides. Biochemistry. 1971 Feb 2;10(3):536–542. doi: 10.1021/bi00779a029. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos R., Gillis E., Fiers W. The enzymic addition of poly(A) to the 3'-end of RNA using bacteriophage MS 2 RNA as a model system. Eur J Biochem. 1976 Feb 16;62(2):401–410. doi: 10.1111/j.1432-1033.1976.tb10172.x. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Maniatis T., Kafatos F. C., Jeffrey A., Vournakis J. N. Full length and discrete partial reverse transcripts of globin and chorion mRNAs. Cell. 1975 Apr;4(4):367–378. doi: 10.1016/0092-8674(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Gilbert J. M., Anderson W. F. Cell-free hemoglobin synthesis. II. Characteristics of the transfer ribonucleic acid-dependent assay system. J Biol Chem. 1970 May 10;245(9):2342–2349. [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K. I., Gonçalves J. M., Houts G. E., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. II. Properties of the terminal deoxynucleotidyltransferase. J Biol Chem. 1967 Jun 10;242(11):2780–2789. [PubMed] [Google Scholar]
- Lobban P. E., Kaiser A. D. Enzymatic end-to end joining of DNA molecules. J Mol Biol. 1973 Aug 15;78(3):453–471. doi: 10.1016/0022-2836(73)90468-3. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Cohen S. N., Chang A. C., Boyer H. W., Goodman H. M., Helling R. B. Replication and transcription of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1974 May;71(5):1743–1747. doi: 10.1073/pnas.71.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Poon R., Paddock G. V., Heindell H., Whitcome P., Salser W., Kacian D., Bank A., Gambino R., Ramirez F. Nucleotide sequence analysis of RNA synthesized from rabbit globin complementary DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3502–3506. doi: 10.1073/pnas.71.9.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- Rabbitts T. H. Bacterial cloning of plasmids carrying copies of rabbit globin messenger RNA. Nature. 1976 Mar 18;260(5548):221–225. doi: 10.1038/260221a0. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Kourilsky P., Mach B. Insertion of a rabbit beta-globin gene sequence into an E. coli plasmid. Nucleic Acids Res. 1975 Dec;2(12):2365–2378. doi: 10.1093/nar/2.12.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W. A. DNA sequencing techniques. Annu Rev Biochem. 1974;43(0):923–965. doi: 10.1146/annurev.bi.43.070174.004423. [DOI] [PubMed] [Google Scholar]
- Salser W., Bowen S., Browne D., el-Adli F., Fedoroff N., Fry K., Heindell H., Paddock G., Poon R., Wallace B. Investigation of the organization of mammalian chromosomes at the DNA sequence level. Fed Proc. 1976 Jan;35(1):23–35. [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wade Nicholas. Recombinant DNA: NIH sets strict rules to launch new technology. Science. 1975 Dec 19;190(4220):1175–1179. doi: 10.1126/science.11643293. [DOI] [PubMed] [Google Scholar]