Abstract
Objective
The purpose of this study was to observe left ventricular function during acute high-altitude exposure in a large group of healthy young males.
Methods
A prospective trial was conducted in Szechwan and Tibet from June to August, 2012. By Doppler echocardiography, left ventricular function was examined in 139 healthy young Chinese men at sea level; within 24 hours after arrival in Lhasa, Tibet, at 3700 m; and on day 7 following an ascent to Yangbajing at 4400 m after 7 days of acclimatization at 3700 m. The resting oxygen saturation (SaO2), heart rate (HR) and blood pressure (BP) were also measured at the above mentioned three time points.
Results
Within 24 hours of arrival at 3700 m, the HR, ejection fraction (EF), fractional shortening (FS), stroke volume (SV), cardiac output (CO), and left ventricular (LV) Tei index were significantly increased, but the LV end-systolic dimension (ESD), end-systolic volume (ESV), SaO2, E/A ratio, and ejection time (ET) were significantly decreased compared to the baseline levels in all subjects. On day 7 at 4400 m, the SV and CO were significantly decreased; the EF and FS Tei were not decreased compared with the values at 3700 m; the HR was further elevated; and the SaO2, ESV, ESD, and ET were further reduced. Additionally, the E/A ratio was significantly increased on day 7 but was still lower than it was at low altitude.
Conclusion
Upon acute high-altitude exposure, left ventricular systolic function was elevated with increased stroke volume, but diastolic function was decreased in healthy young males. With higher altitude exposure and prolonged acclimatization, the left ventricular systolic function was preserved with reduced stroke volume and improved diastolic function.
Introduction
In recent years, increasing numbers of individuals have been exposed to high-altitude environments through entertainment, physical training, work, or habitation. Atmospheric pressure falls with ascending altitudes, and with it, there are decreases in the partial pressure of inspired oxygen, arterial pressure of oxygen and arterial oxygen saturation. Therefore, exposure to hypobaric hypoxia is associated with a series of adaptive responses, such as cardiovascular compensation, to combat the diminished arterial oxygen content. The initial cardiac response to hypoxia is characterized by an increase in cardiac output with tachycardia [1–3], which helps maintain oxygen delivery. Accurate delineation of the cardiovascular alterations after subjects have been at high altitudes has interested investigators since the early years of the last century [4,5]. However, hostile environmental conditions, sample size limitations and taking place solely in simulative altitude chambers [6,7], where the environment and activity are continuously controlled, lacking of cold, wind field environments have been the main deterrents, so the effect of acute high-altitude exposure upon left ventricular stroke volume is a controversial problem. Previous reports show stroke volume adaptations ranging from a decrease to a distinct increase [6,8–10].
In addition, the convenience of current transportation methods allows for rapid ascent to altitudes that can compromise acclimatization and expose inexperienced climbers to the hazards of high altitude. Thus, it is urgent to identify the pattern of left ventricular function changes in high-altitude fields. We therefore assessed left ventricular function by conventional Doppler method analysis in a large group of healthy non-acclimatized young males at 500 m, within 24 h after rapid ascent by train to 3700 m and on the 7th day of acclimatization at 4400 m.
Materials and Methods
Subjects
In total, 139 healthy young Chinese males who lived in the low lands (500 m) were recruited for the study. The average age, height, weight, body mass index (BMI) and body surface area (BSA) at the time of present study were 22.2±3.3 years old (y)(ranged from 18 to 33 years old), 171.4±4.5 centimeters, 63.4±7.1 kilograms, 21.4±2.0 kilograms/meters2(Kg/m2) and 1.7±0.1 square meters(m2), respectively. Subjects with any one of the following conditions were excluded: respiratory diseases, cardiovascular diseases, malignant tumors, liver and kidney dysfunction and diseases of the immune system, as well as people with psychiatric disorders who could not complete the questionnaires. Thirty-two of the 139 subjects had altitude exposure history from six months ago, but no high-altitude cerebral edema or pulmonary edema had occurred. Seventy-five of them were cigarette smokers. In addition, the subjects did not take medication or receive any intervention before ascending.
The study was approved by the Ethics Committee of Xinqiao Hospital, the Second Clinic Medical College of the Third Military Medical University of the PRC. Written informed consent was obtained from all participants after being informed of the study’s purpose, procedures, and inherent risks and benefits.
Study protocol
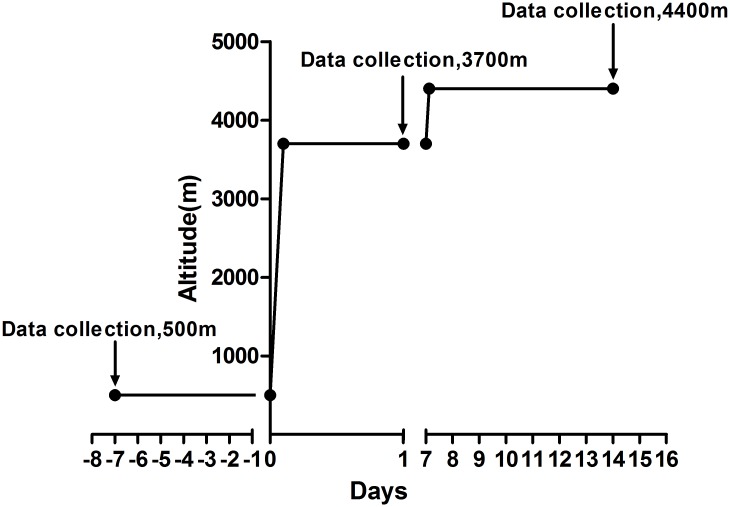
All subjects ascended to 3700 m (Lhasa in Tibet) in 2.5 hours by plane from 500 m (Chengdu in Sichuan province). The subjects traveled by motorcar and arrived in Yangbajing (in Tibet, at 4400 m) within 3 hours, where they then stayed for 7 days after they had acclimatized at 3700 m for a week (Fig. 1). Structured case report form (CRF) questionnaires were used to record demographic data (age, height, weight, and ethnicity), physiological data (BP, SaO2 and HR), previous high-altitude exposure (>2500 m) before this travel, and symptoms related to AMS (headache, dizziness/lightheadedness, gastrointestinal symptoms, insomnia and fatigue/weakness).
Fig 1. Altitude ascent profile of participants from plain to plateau.
All subjects ascended to 3700 m (Lhasa in Tibet) in 2.5 hours by plane from 500 m (Chengdu in Sichuan province). After they acclimatized at 3700 m for a week, the subjects traveled by motorcar and arrived in Yangbajing (in Tibet, at 4400 m) within 3 hours; they in Yangbajing for 7 days. For all participants, the starting data collection point was in Chengdu (500 m), the second data collection point was in Lhasa (3700 m) within 24 h of arrival, and the third data collection was on the 7th day in Yangbajing (4400 m).
All of the above mentioned procedures were performed at 500 m within one week before ascension to Chengdu, within 24 h after arrival at 3700 m in Lhasa at approximately 13:00 pm June 23rd–25th, 2012 (examinations were performed at approximately 8:00 am–12:00 pm the next morning upon arrival), and on the 7th day after ascending to 4400 m (Yangbajing, July 7th–9th, 2012, following 7 days of acclimatization at altitudes of 3700 m in Lhasa). Heart rate, oxygen saturation, and conventional transthoracic Doppler echocardiographic measurements were performed at the above-mentioned three time points. All of the questionnaires and measurements were non-invasive.
AMS
Acute mountain sickness (AMS) is commonly experienced by people traveling to high altitudes, typically those above 2500 m. The AMS diagnoses were based on the Lake Louise score (LLS), an international standard scoring system for AMS [11]. This defines the syndrome of AMS as characterized by five self-reported symptoms: headache, dizziness or lightheadedness, gastrointestinal symptoms (anorexia, nausea or vomiting), difficulty sleeping, and fatigue or weakness. Every item is measured on a 4-point Likert scale, range from 0 to 3, with 0 indicating none, 1 for slight, 2 for moderate, and 3 for severe. The AMS, defined as the total score of the 5 items, must amount to a 3 or higher, but headache must be present in the context of these symptoms within several hours or days of arrival at altitude. A total score of 3–4 is defined as mild AMS, and a total score of 5 or higher is defined as severe AMS [12,13].
Physiological data
The oxygen saturation (SaO2) was measured with a pocket pulse oximeter (NONIN-9550, Nonin Onyx, America). The blood pressure (BP) and heart rate (HR) were determined with a sphygmomanometer (HEM-6200, Omron, China). These data were measured in triplicate after the subjects had rested in a seated position for 20 minutes. The mean arterial pressure (MAP) was calculated according to the systolic blood pressure (SBP) and diastolic blood pressure (DBP): MAP = DBP+ (SBP—DBP)/3.
Measurement of left ventricular systolic function
A color Doppler echocardiography instrument (CX 50, Philips Ultrasound System, Andover, MA, USA) with a probe of 2 to 4 MHz was used to measure the left ventricular end-diastolic dimension (LVEDD), end-systolic dimension (LVESD) and HR, where the left parasternal long-axis and the short-axis views at the mid-left ventricular level were obtained when the heart rate was in a steady state. The left ventricular end-systolic volume (LVESV) and left ventricular end-diastolic volume (LVEDV) were obtained by Teichholz’s formula [14]. The stroke volume (SV), cardiac output (CO), ejection fraction (EF), and fractional shortening (FS) were also calculated. The stroke volume index (SVI) and cardiac index (CI) were the SV and CO, adjusted by BSA, respectively.
Left ventricular diastolic function and Tei index
In the Doppler examinations, the mitral inflow velocity pattern was recorded from the apical long-axis view, and the pulsed wave Doppler sample volume was positioned at the tip of the mitral leaflet during LV diastole to measure the early (E) and late (A) diastolic wave peak velocities; the E/A ratio was obtained through dividing the E wave peak velocity by the A wave peak velocity. The LV outflow velocity pattern was recorded from the apical long-axis view, with the pulsed wave Doppler sample volume positioned just below the aortic valve. Then, the time intervals for calculating the LV Tei index were measured from the mitral inflow and the LV outflow recordings [15] (Fig. 2). Three consecutive beats were measured and averaged for each parameter. In Fig. 2, the time from the cessation to the onset of mitral inflow (a) represents the interval between the mitral valve closure and opening, which is equal to the sum of the isovolumic contraction time (ICT), ejection time (ET) and isovolumic relaxation time (IRT). The ET (b) of the LV was measured in the LV outflow velocity pattern. The Tei index was defined as the sum of the ICT and IRT divided by the ET; thus, the LV Tei index was determined using (a-b)/b [16]. A single observer performed offline analysis of these parameters after returning to a low altitude.
Fig 2. Illustration of a pulsed Doppler diagram showing the measurement of each parameter for calculating the Tei index.
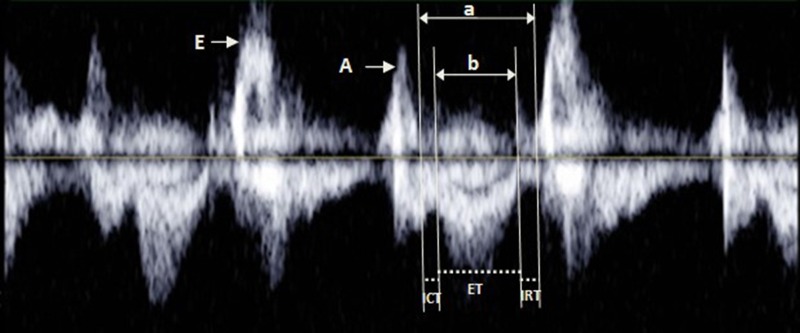
a = isovolumetric contraction time (ICT) + ejection time (ET) + isovolumetric relaxation time (IRT); b = ET. Tei index = (ICT+IRT)/ET = (a-b)/b.
Statistical analysis
The continuous data are expressed as the mean ± SD. The baseline and the two high-altitude measurements in the lowlanders were analyzed using a one-way analysis of variance for repeated measures, followed by Newman-Keuls post hoc comparisons where appropriate. All data and calculations were performed with SPSS 19.0 software (Chicago, IL). A two-sided ±-level of significance of 0.05 was used for all tests, and a probability value less than 0.05 was considered to be statistically significant. Before analysis, all data were reviewed by the principal investigator for completeness.
Results
AMS
Within 24 hours after arrival at 3700 m, 75 of 139 subjects (53.96%) met the criteria for AMS; there were 43 mild AMS cases and 32 severe AMS cases. There were still 27 subjects (19.42%) who conformed to the Lake Louise score criteria after 7 days of acclimatization at an altitude of 4400 m, ten of whom had severe AMS, comparatively, and seventeen of whom had mild AMS. Fortunately, there was no one experienced high-altitude cerebral edema or pulmonary edema during this trip.
Physiological parameters
As shown in Table 1, the SaO2 value was significantly reduced in all subjects from 98.42±x0.94% to 88.92±2.67% (P < 0.001) at resting state upon acute high-altitude exposure. However, the HR was significantly elevated from 62.47±8.98 beats/min to 82.91±11.56 beats/min (P < 0.001) under hypoxic conditions. However, the BP showed almost no change. After 7 days acclimatization at an altitude of 4400 m, the SaO2 value was further reduced from 88.92±2.67% to 87.96±2.57% (P < 0.01). However, the HR was further elevated from 82.91±11.56 beats/min to 86.85±15.55 beats/min (P < 0.01). The BP was increased after 7 days acclimatization at an altitude of 4400 m compared with the BP within 24 hours upon arrival at 3700 m, and the diastolic blood pressure (DBP) and mean arterial pressure (MAP) were significantly higher than those at sea level.
Table 1. Physiologic parameters at low and high altitude.
| Physiologic parameters | 500 m | 3700 m, 24 h | 4400 m, 7 d |
|---|---|---|---|
| SaO2 (%) | 98.42±0.94 | 88.92±2.67** | 87.96±2.57** ‡ |
| HR (beats/min) | 62.47±8.98 | 82.91±11.56** | 86.85±15.55** ‡ |
| SBP (mmHg) | 116.14±11.67 | 114.56±10.64 | 117.45±11.44 † |
| DBP (mmHg) | 74.57±10.90 | 74.96±9.57 | 78.40±10.09** ‡ |
| MAP (mmHg) | 88.42±10.65 | 88.16±9.31 | 91.41±9.74* ‡ |
Normally distributed data are presented as the means ± standard deviations.
*P<0.05,
**P<0.01, compared with 500 m;
†P < 0.05,
‡P < 0.01, compared with 3700 m. SaO2, oxygen saturation; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure.
Left ventricular size
Comparisons were made between measurements at three different altitudes with respect to global left ventricular size (Table 2). Within 24 hours after arrival at 3700 m, the LVESD and LVESV were significantly decreased (P < 0.01), with the ESV especially reduced from 38.51±9.18 ml to 33.80±6.34 ml (P < 0.001). Furthermore, the LVEDD and LVEDV did not show significant alterations. After 7 days of acclimatization at an altitude of 4400 m, the LVESD and LVESV were further reduced compared with the corresponding values at 3700 m and were far lower than at sea level (P < 0.001). In addition, the LVEDD and LVEDV were also significantly decreased, and the LVEDV was reduced from 101.89±11.92 ml to 96.06±9.98 ml (P < 0.001).
Table 2. Left ventricular size and function at low and high altitude.
| Item | 500 m | 3700 m, 24 h | 4400 m, 7 d |
|---|---|---|---|
| Left ventricular size | |||
| EDD (mm) | 46.82±3.03 | 46.78±2.50 | 45.82±2.00** ‡ |
| ESD (mm) | 30.29±2.91 | 29.36±2.32** | 28.75±1.81** † |
| EDV (ml) | 103.16±16.70 | 101.89±11.92 | 96.06±9.98** ‡ |
| ESV (ml) | 38.51±9.18 | 33.80±6.34** | 31.80±4.77** † |
| Left ventricular function | |||
| EF (%) | 62.87±5.61 | 66.90±4.18** | 66.96±3.07** |
| FS (%) | 35.41±4.72 | 37.26±3.55** | 37.27±2.45** |
| SV (ml) | 64.65±10.95 | 68.09±8.38** | 64.27±6.86 ‡ |
| SVI | 38.06±6.54 | 40.27±5.61** | 38.06±5.28 ‡ |
| CO | 4.04±0.84 | 5.38±1.07** | 4.81±0.94** ‡ |
| CI | 2.38±0.50 | 3.17±0.66** | 2.85±0.61** ‡ |
Normally distributed data are presented as the means ± standard deviations.
**P<0.01, compared with 500 m;
†P < 0.05,
‡P < 0.01, compared with 3700 m. EDD, end-diastolic dimension; ESD, end-systolic dimension; EDV, end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction; FS, fractional shortening; SV, stroke volume; SVI, stroke volume index; CO, cardiac output; and CI, cardiac index.
Left ventricular systolic function
Regarding the global LV systolic function, as expected, the EF, FS, CO, and CI increased significantly under hypoxic stress compared to the corresponding baseline values in all subjects (Table 2). However, in our study, the SV and SVI were significantly increased during hypoxic conditions compared to the same measurements under normoxia (P = 0.001).
After 7 days of acclimatization at a higher altitude (Yangbajing, 4400 m), the SV, SI, CO and CI decreased significantly compared to the corresponding measurements in Lhasa (3700 m) in all subjects (P < 0.01) (Table 2), but the CO and CI were still higher than those parameters at 500 m, and the SV and SI returned to baseline levels. There were no significant alterations after lower hypoxic stress in terms of EF and FS compared to the corresponding measurements in Lhasa (3700 m), but the EF and FS were significantly higher than the same measurements at sea level.
Left ventricular diastolic function
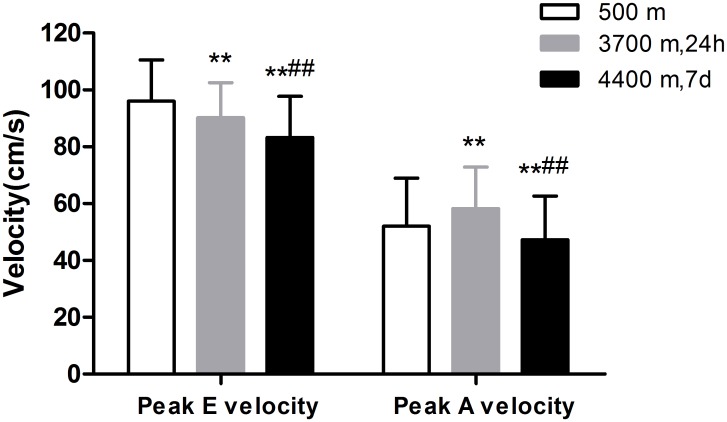
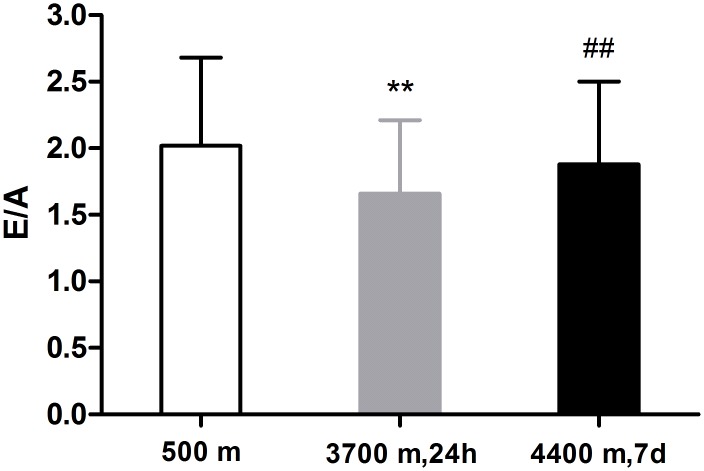
As shown in Fig. 3, within 24 h after acute high-altitude exposure, the mitral peak E velocity (early LV filling) decreased (96.03±14.55 cm/s vs. 90.26±12.28 cm/s, P = 0.001), the peak A velocity (filling due to auricular contraction) increased from 52.00±16.94 cm/s to 58.23±14.64 cm/s and the E/A ratio (an index of early—late LV filling) was significantly decreased (P < 0.001)(Fig. 4). At day 7 after acclimatization at 4400 m, both the mitral inflow peak E-velocity and peak A-velocity were significantly decreased compared with those values in Lhasa and in the lowlands, resulting in a slightly increased E/A ratio of the participants compared with that in Lhasa, which was still lower than that at sea level.
Fig 3. Comparison of mitral peak early diastolic filling velocity and peak late diastolic filling velocity in 139 lowlanders at the altitudes of 500 m, 3700 m and 4400 m.
At the elevated altitudes, the peak E velocity decreased, initially increasing within 24 h at 3700 m but decreasing by day 7 at 4400 m. (Note: **P<0.01, compared with 500 m; ##P<0.01, compared with 3700 m.)
Fig 4. Comparison of the ratio of the early diastolic filling velocity to the late diastolic filling velocity (E/A ratio) in 139 lowlanders at the altitudes of 500 m, 3700 m and 4400 m.
At the elevated altitudes, the E/A ratio decreased, though it increased on day 7 at 4400 m compared with the ratio observed at 3700 m. (Note: **P<0.01, compared with 500 m; ##P<0.01, compared with 3700 m.)
LV Tei index
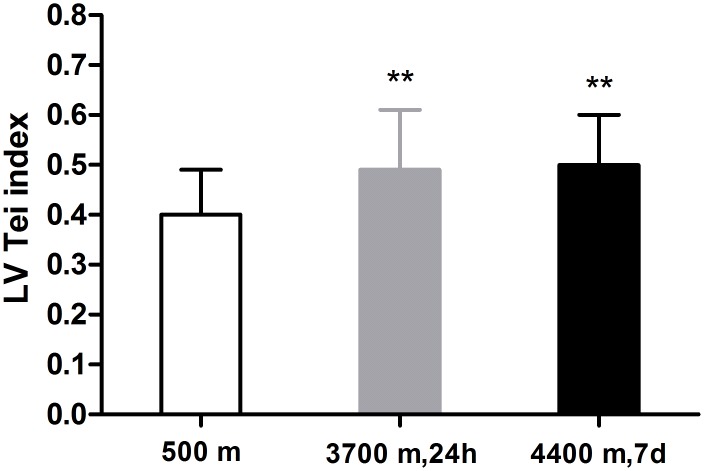
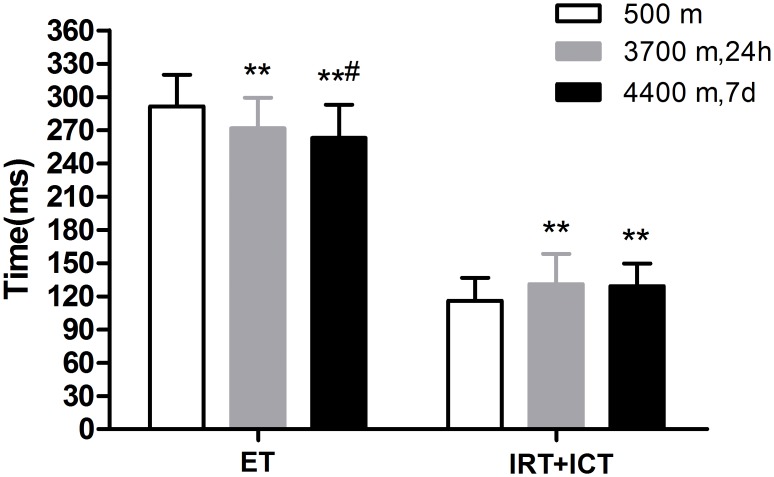
Fig. 6 shows the changing LV Tei index pattern at 500 m, within 24 h after acute 3700 m high-altitude exposure and day 7 after acclimatization at 4400 m. Within 24 h after acute high-altitude exposure, due to a significant prolongation of the sum of the ICT and IRT from 115.85±20.68 ms to 130.84±27.37 ms (P < 0.01) (Fig. 5) and a significant shortening of the ET (291.53±28.54 ms vs. 271.74±27.46 ms, P < 0.001), the LV Tei index was significantly higher than that in the lowlands (0.40±0.09 vs. 0.49±0.12, P < 0.001). In addition, on day 7 after acclimatization at 4400 m, the LV Tei index was further increased, though not significantly (0.49±0.12 vs. 0.50±0.10, P > 0.05), along with a further decreased ET (from 271.74±27.46 ms to 262.94±30.01 ms, P < 0.05) and a preserved ICT and IRT sum (130.84±27.37 ms vs. 129.16±20.34 ms, P > 0.05).
Fig 6. Comparison of the left ventricular Tei index (LV Tei index) in 139 lowlanders at the altitudes of 500 m, 3700 m and 4400 m.
The LV Tei index was significantly increased at high altitude, but there was no significant difference between the values at 3700 m and 4400 m. (Note: **P<0.01, compared with 500 m.)
Fig 5. Comparison of the ejection time (ET) and the sum of isovolumic contraction time (ICT) plus isovolumic relaxation time (IRT) in 139 lowlanders at the altitudes of 500 m, 3700 m and 4400 m.
At the elevated altitudes, the ET decreased, and it further decreased on day 7 at 4400 m compared with that at 3700 m. However, the sum of ICT and IRT was significantly prolonged at a plateau. (Note: **P<0.01, compared with 500 m; #P<0.05, compared with 3700 m.)
Discussion
Most investigations have reported that altitude-related hypoxia induces tachycardia [10,17], which is caused by an increase in sympathetic activity [18], as evidenced by elevated plasma and urinary norepinephrine and epinephrine concentrations [19,20]. We also confirmed this finding, and even with prolonged acclimatization, the HR is higher in the present study following high-altitude exposure. In addition, the blood pressure was unchanged upon acute high-altitude exposure, which is in keeping with previous observations [21]. With higher altitude exposure, the systolic blood pressure and diastolic blood pressure were elevated, which may also suggest that the activation of neurohumoral factors is stronger than the effects of hypoxia-induced vasodilatation.
In our study, the LV contractility was elevated during exposure to high altitude in all subjects. This is in keeping with previous findings that suggested that LV systolic function was improved in hypoxic healthy volunteers [1–3,22,23] and even in studies referring to adults and children exposed to high altitude [21,24]. The improved cardiac systolic function in hypoxic environments allows for the output of the maximum amount of blood to transfer a sufficient amount of oxygen to support the metabolic demands of a body challenged by hypoxic stress [9]. As expected, the present data did show an improvement in cardiac output in all subjects, due to their elevated HR and SV. Our findings revealed that the stroke volume was increased with unaltered left ventricular preload but marked LV ejection fraction and fractional shortening, leading to a significant decrease in the end-systolic volume. The reduction of stroke volume at high altitude has been reported many times before [7,10,25]. Unchanged stroke volumes at rest were observed between measurements from a hypobaric chamber to those in high-altitude fields [6,26]. The differences between these studies may be related to the limitations of the small sample size and due to their taking place solely in simulative chambers without true environment stimulation. Furthermore, hypoxia was found to be associated with a decreased early diastolic mitral filling velocity annuli E/A ratio, resulting from an increase in left atrial contraction. These results are in keeping with previous reports of hypoxic exposure-induced LV diastolic dysfunction according to transmitral inflow [2,3,27–29]. Cardiac contraction is an energetic process, and cardiac relaxation is far more energy consuming [30]. Thus, the cardiac diastolic dysfunction observed prior to systolic function in response to hypoxia in a hypobaric chamber [1,6] results from a rapid decrease in high-energy phosphate metabolism in the cardiac left ventricle in humans [31].
With acclimatization to higher altitudes, cardiac output tended to decrease with increased hemoglobin and a greater capacity for carrying oxygen to tissues. Furthermore, the stroke volume was obviously reduced because of significant decrease in LV preload in all subjects. There are two possible explanations for the decreased LV preload. The first would be LV relaxation dysfunction because of a reduced energy supply [32,33]. The second explanation relates to the decrease in plasma volume that has been observed in lowlanders at altitude in many studies [34–37]. Hypoxic exposure at a higher altitude has been associated with decreased mitral early and late inflow velocity and an unchanged annuli E/A ratio compared with sea level in all subjects. These changes in the LV filling patterns consistent with impaired LV relaxation at high altitude have been observed in previous echocardiographic studies [3,27,28].
The LV Tei index has been proven to be a sensitive indicator of the global left ventricle function [38]. In the present study, the LV Tei index was significantly increased due to ICT and IRT prolongation and ET shortening upon acute exposure to high altitude. ET shortening may be related to an increase in HR. After 7 days of acclimatization at a higher altitude, the LV Tei index was still obviously higher than at sea level, in accordance with a previous study [39] in which the LV Tei index was also significantly elevated after 50 d of high-altitude exposure. Yakabe et al. [40] discovered that the Tei index is preload-dependent, and Cheung et al. [41] revealed that the afterload increase, preload reduction, and HR [42] are associated with significant increases in the Tei index. In our study, the elevated LV Tei index may be attributed to a reduced left ventricular preload, higher SBP and higher HR in all subjects after 7 days of acclimatization at 4400 m.
Limitations
Several limitations exist in our study. First, in this study, all of the subjects were young adult males, which makes it difficult to extend our findings to other populations. Second, tissue Doppler imaging (TDI) was not used in this study; instead, we performed M-mode and pulse Doppler echocardiographic examinations that are commonly used in the clinic. In future studies, TDI will be used, as it will provide a more comprehensive evaluation of cardiac function. Lastly, we did not examine changes in the blood volume, plasma volume, erythrocyte volume or urine volume in this study, and thus, the reason for the preload reduction is not clear.
Conclusions
In conclusion, left ventricular contractility is elevated, but diastolic function was decreased during exposure to high altitude, which is associated with an improvement in the LV ejection fraction, fractional shortening and stroke volume in healthy young males. With higher altitude exposure and prolonged acclimatization, the left ventricular systolic function was preserved, and there was improved diastolic function, which is associated with a preserved LV ejection fraction and fractional shortening and an elevated E/A ratio but a decreased stroke volume in all subjects.
Acknowledgments
The authors are grateful to the 139 volunteers for their patience and cooperation throughout this exceptional experience. We are also grateful to Leyna D. and April F. of American Journal Experts for editing the manuscript.
Funding Statement
This work was supported by grants from the Special Health Research Project, Ministry of Health of PR China (no. 201002012) and was conducted by Professor Huang Lan and team members, whose work on the Tibet trek allowed the authors’ research to be conducted. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Boussuges A, Molenat F, Burnet H, Cauchy E, Gardette B, et al. (2000) Operation Everest III (Comex’97): modifications of cardiac function secondary to altitude-induced hypoxia. An echocardiographic and Doppler study. Am J Respir Crit Care Med 161: 264–270. [DOI] [PubMed] [Google Scholar]
- 2. Mason NP, Petersen M, Melot C, Imanow B, Matveykine O, et al. (2003) Serial changes in nasal potential difference and lung electrical impedance tomography at high altitude. J Appl Physiol (1985) 94: 2043–2050. [DOI] [PubMed] [Google Scholar]
- 3. Allemann Y, Rotter M, Hutter D, Lipp E, Sartori C, et al. (2004) Impact of acute hypoxic pulmonary hypertension on LV diastolic function in healthy mountaineers at high altitude. Am J Physiol Heart Circ Physiol 286: H856–862. [DOI] [PubMed] [Google Scholar]
- 4. Grollman A (1930) Physiological variations of the cardiac output of man. VIT. The effect of high altitude on the cardiac output and its related functions; an account of experiments conducted on the summit of Pike’s Peak, Colorado. American Journal of Physiology 93: 19–39. [Google Scholar]
- 5. Asmussen E, Consolazio F (1941) The circulation in rest and work on Mount Evans (4300 m). American Journal of Physiology 132: 555–563. [Google Scholar]
- 6. Kjaergaard J, Snyder EM, Hassager C, Olson TP, Oh JK, et al. (2006) The effect of 18 h of simulated high altitude on left ventricular function. Eur J Appl Physiol 98: 411–418. [DOI] [PubMed] [Google Scholar]
- 7. Reeves JT, Groves BM, Sutton JR, Wagner PD, Cymerman A, et al. (1987) Operation Everest II: preservation of cardiac function at extreme altitude. J Appl Physiol (1985) 63: 531–539. [DOI] [PubMed] [Google Scholar]
- 8. Vallecilla C, Khiabani RH, Sandoval N, Fogel M, Briceno JC, et al. (2014) Effect of high altitude exposure on the hemodynamics of the bidirectional Glenn physiology: Modeling incremented pulmonary vascular resistance and heart rate. J Biomech. 10.1016/j.jbiomech.2014.12.037 [DOI] [PubMed] [Google Scholar]
- 9. Hanaoka M, Kogashi K, Droma Y, Urushihata K, Kubo K (2011) Myocardial performance index in subjects susceptible to high-altitude pulmonary edema. Intern Med 50: 2967–2973. [DOI] [PubMed] [Google Scholar]
- 10. Fowles RE, Hultgren HN (1983) Left ventricular function at high altitude examined by systolic time intervals and M-mode echocardiography. Am J Cardiol 52: 862–866. [DOI] [PubMed] [Google Scholar]
- 11. Roach RC BP, Hackett PH, Oelz O (1993) The Lake Louise Acute Mountain Sickness scoring system In: Sutton JR, Houston CS, Coates G, eds. Burlington, VT: Queen City Press; Hypoxia and Molecular Medicine 272–274. [Google Scholar]
- 12. Riepl RL, Fischer R, Hautmann H, Hartmann G, Muller TD, et al. (2012) Influence of acute exposure to high altitude on basal and postprandial plasma levels of gastroenteropancreatic peptides. PLoS One 7: e44445 10.1371/journal.pone.0044445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu TY, Ding SQ, Liu JL, Jia JH, Chai ZC, et al. (2012) Smoking, acute mountain sickness and altitude acclimatisation: a cohort study. Thorax 67: 914–919. 10.1136/thoraxjnl-2011-200623 [DOI] [PubMed] [Google Scholar]
- 14. Teichholz LE, Kreulen T, Herman MV, Gorlin R (1976) Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol 37: 7–11. [DOI] [PubMed] [Google Scholar]
- 15. Tei C (1995) New non-invasive index for combined systolic and diastolic ventricular function. J Cardiol 26: 135–136. [PubMed] [Google Scholar]
- 16. Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, et al. (1996) Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr 9: 838–847. [DOI] [PubMed] [Google Scholar]
- 17. Balasubramanian V, Mathew OP, Tiwari SC, Behl A, Sharma SC, et al. (1978) Alterations in left ventricular function in normal man on exposure to high altitude (3658 m). Br Heart J 40: 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duplain H, Vollenweider L, Delabays A, Nicod P, Bartsch P, et al. (1999) Augmented sympathetic activation during short-term hypoxia and high-altitude exposure in subjects susceptible to high-altitude pulmonary edema. Circulation 99: 1713–1718. [DOI] [PubMed] [Google Scholar]
- 19. Strobel G, Neureither M, Bartsch P (1996) Effect of acute mild hypoxia during exercise on plasma free and sulphoconjugated catecholamines. Eur J Appl Physiol Occup Physiol 73: 82–87. [DOI] [PubMed] [Google Scholar]
- 20. Olsen NV, Kanstrup IL, Richalet JP, Hansen JM, Plazen G, et al. (1992) Effects of acute hypoxia on renal and endocrine function at rest and during graded exercise in hydrated subjects. J Appl Physiol (1985) 73: 2036–2043. [DOI] [PubMed] [Google Scholar]
- 21. Allemann Y, Stuber T, de Marchi SF, Rexhaj E, Sartori C, et al. (2012) Pulmonary artery pressure and cardiac function in children and adolescents after rapid ascent to 3,450 m. Am J Physiol Heart Circ Physiol 302: H2646–2653. 10.1152/ajpheart.00053.2012 [DOI] [PubMed] [Google Scholar]
- 22. Oliver RM, Peacock AJ, Challenor VF, Fleming JS, Waller DG (1991) The effect of acute hypoxia on right ventricular function in healthy adults. Int J Cardiol 31: 235–241. [DOI] [PubMed] [Google Scholar]
- 23. Huez S, Retailleau K, Unger P, Pavelescu A, Vachiery JL, et al. (2005) Right and left ventricular adaptation to hypoxia: a tissue Doppler imaging study. Am J Physiol Heart Circ Physiol 289: H1391–1398. [DOI] [PubMed] [Google Scholar]
- 24. Huez S, Faoro V, Guenard H, Martinot JB, Naeije R (2009) Echocardiographic and tissue Doppler imaging of cardiac adaptation to high altitude in native highlanders versus acclimatized lowlanders. Am J Cardiol 103: 1605–1609. 10.1016/j.amjcard.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 25. Suarez J, Alexander JK, Houston CS (1987) Enhanced left ventricular systolic performance at high altitude during Operation Everest II. Am J Cardiol 60: 137–142. [DOI] [PubMed] [Google Scholar]
- 26. Boos CJ, Mellor A, Begley J, Stacey M, Smith C, et al. (2014) The effects of exercise at high altitude on high-sensitivity cardiac troponin release and associated biventricular cardiac function. Clin Res Cardiol 103: 291–299. 10.1007/s00392-013-0654-2 [DOI] [PubMed] [Google Scholar]
- 27. Shave RE, Dawson E, Whyte G, George K, Gaze D, et al. (2004) Effect of prolonged exercise in a hypoxic environment on cardiac function and cardiac troponin T. Br J Sports Med 38: 86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grunig E, Mereles D, Hildebrandt W, Swenson ER, Kubler W, et al. (2000) Stress Doppler echocardiography for identification of susceptibility to high altitude pulmonary edema. J Am Coll Cardiol 35: 980–987. [DOI] [PubMed] [Google Scholar]
- 29. Bouzat P, Walther G, Rupp T, Doucende G, Payen JF, et al. (2013) Time course of asymptomatic interstitial pulmonary oedema at high altitude. Respir Physiol Neurobiol 186: 16–21. 10.1016/j.resp.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 30. Tian R, Nascimben L, Ingwall JS, Lorell BH (1997) Failure to maintain a low ADP concentration impairs diastolic function in hypertrophied rat hearts. Circulation 96: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 31. Holloway CJ, Montgomery HE, Murray AJ, Cochlin LE, Codreanu I, et al. (2011) Cardiac response to hypobaric hypoxia: persistent changes in cardiac mass, function, and energy metabolism after a trek to Mt. Everest Base Camp. FASEB J 25: 792–796. 10.1096/fj.10-172999 [DOI] [PubMed] [Google Scholar]
- 32. Gomez A, Mink S (1992) Interaction between effects of hypoxia and hypercapnia on altering left ventricular relaxation and chamber stiffness in dogs. Am Rev Respir Dis 146: 313–320. [DOI] [PubMed] [Google Scholar]
- 33. Holloway C, Cochlin L, Codreanu I, Bloch E, Fatemian M, et al. (2011) Normobaric hypoxia impairs human cardiac energetics. FASEB J 25: 3130–3135. 10.1096/fj.11-183426 [DOI] [PubMed] [Google Scholar]
- 34. Surks MI, Chinn KS, Matoush LR (1966) Alterations in body composition in man after acute exposure to high altitude. J Appl Physiol 21: 1741–1746. [DOI] [PubMed] [Google Scholar]
- 35. Sawka MN, Young AJ, Rock PB, Lyons TP, Boushel R, et al. (1996) Altitude acclimatization and blood volume: effects of exogenous erythrocyte volume expansion. J Appl Physiol (1985) 81: 636–642. [DOI] [PubMed] [Google Scholar]
- 36. Westerterp KR, Robach P, Wouters L, Richalet JP (1996) Water balance and acute mountain sickness before and after arrival at high altitude of 4,350 m. J Appl Physiol (1985) 80: 1968–1972. [DOI] [PubMed] [Google Scholar]
- 37. Jung RC, Dill DB, Horton R, Horvath SM (1971) Effects of age on plasma aldosterone levels and hemoconcentration at altitude. J Appl Physiol 31: 593–597. [DOI] [PubMed] [Google Scholar]
- 38. Misumi I, Harada E, Doi H, Hayasaki T, Kajihara E, et al. (2002) [Tei index evaluated by M-mode echocardiography in patients with dilated cardiomyopathy]. J Cardiol 39: 85–91. [PubMed] [Google Scholar]
- 39. Zhou Q, Yang S, Luo Y, Qi Y, Yan Z, et al. (2012) A randomly-controlled study on the cardiac function at the early stage of return to the plains after short-term exposure to high altitude. PLoS One 7: e31097 10.1371/journal.pone.0031097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yakabe K, Ikeda S, Urata J, Koga S, Chijiwa R, et al. (2008) Significance of Tei-index alterations induced by acute preload reduction with hemodialysis. Clin Nephrol 70: 41–47. [DOI] [PubMed] [Google Scholar]
- 41. Cheung MM, Smallhorn JF, Redington AN, Vogel M (2004) The effects of changes in loading conditions and modulation of inotropic state on the myocardial performance index: comparison with conductance catheter measurements. Eur Heart J 25: 2238–2242. [DOI] [PubMed] [Google Scholar]
- 42. Poulsen SH, Nielsen JC, Andersen HR (2000) The influence of heart rate on the Doppler-derived myocardial performance index. J Am Soc Echocardiogr 13: 379–384. [DOI] [PubMed] [Google Scholar]