Abstract
The evolution of human immunodeficiency virus (HIV) treatment has improved our understanding and management of complex pharmacological issues that have driven improved outcomes and quality of life of the HIV-infected patient. These issues include adherence, long- and short-term toxicities, pharmacoenhancement, pharmacogenomics, therapeutic drug monitoring, differential penetration of drugs into sanctuary sites, such as the central nervous system, genital tract and small bowel, and drug–drug and drug–food interactions related to cytochrome P450 drug-metabolizing enzymes, uridine diphosphate glucuronyltransferases and drug transporters, to name a few. There is future promise, as an increased understanding of the immunopathogenesis of HIV and global public health initiatives are driving novel treatment approaches with goals to prevent, control and, ultimately, eradicate HIV.
Keywords: adherence, antiretroviral, drug interactions, human immunodeficiency virus, pharmacogenomics, therapeutic drug monitoring
Introduction: the evolution of human immunodeficiency virus treatments
In <30 years of antiretroviral therapy (ART), there have been more than 25 drugs developed (Figure 1). In 1987, the first antiretroviral agent, zidovudine (AZT), a nucleoside reverse transcriptase inhibitor (NRTI), was shown to have a positive impact on clinical progression and death 1. The challenges of early NRTI regimens included high pill burdens, inconvenient dosing, treatment-limiting toxicities and incomplete virological suppression. Sequential monotherapy and incomplete virological suppression resulted in the emergence of multiple resistance mutations, with long-term treatment consequences. Human immunodeficiency virus (HIV) protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs), introduced in the mid-1990s, revolutionized the management of HIV infection. Highly active antiretroviral therapy (HAART) regimens, consisting of two NRTIs plus a PI or NNRTI, were capable of virological suppression (<400 copies ml−1), and widespread uptake quickly led to dramatic reductions in morbidity and mortality in the developed world 2. The strategy of using two NRTIs plus a potent third agent still forms the cornerstone of current treatment principles, and is now referred to as combination antiretroviral therapy (cART; Table 1).
Figure 1.
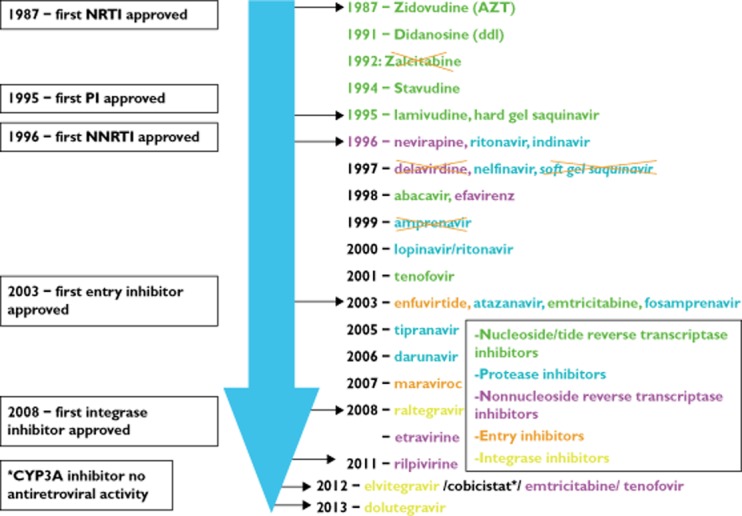
Time lines of antiretroviral development. The time course of development of >25 drugs across five different classes over the last 27 years is highlighted according to the US Food and Drug Administration (FDA) approval date. Those that are no longer in use or available are illustrated with an ‘X’ through them
Table 1.
What to start: specific agents recommended as first-line treatment and differences between the guidelines are highlighted, showing the eventual convergence of first-line recommendations between the developing and developed world
| DHHS | EACS | WHO | |
|---|---|---|---|
| Year/month | Preferred combination | ART regimen | Recommended ART regimen |
| 1998/06 | AZT/3TC (fixed-dose combination), AZT/ddI, AZT/ddC, AZT/ddI, D4T/ddI, D4T/3TC + IDV, SQV, NFV, RTV or SQV/RTV | ||
| 1998/12 | As above + addition of EFV as the third agent | ||
| 1999 | As above with addition of ddI/3TC as NRTI combination | ||
| 2000 | As above but high-dose RTV removed as third agent and ddI/3TC removed as NRTI combination | ||
| 2001 | As above with addition of RTV-boosted PI regimens as third agents [IDV/r, SQV/r, LPV/r (fixed-dose combination PI)] | ||
| 2002 | AZT/3TC + EFV, PI/r, NFV or ABC+ | ||
| 2003/07 | 3TC + (d4T, AZT or TDF) + EFV or 3TC + (d4T or AZT) + LPV/r | ||
| 2003/11 | As above but addition of FTC as alternative to 3TC in combination with (d4T, AZT or TDF) + EFV | Weighed pros and cons of NNRTI based vs. PI based with triple NRTI (AZT-3TC-ABC+) regimens | AZT or d4T + 3TC + NVP or EFV |
| 2005 | As above with deletion of d4T from first-line treatment and addition of FTC as alternative to 3TC in combination with AZT, LPV/r | ||
| 2006 | AZT/3TC or TDF/FTC (both fixed-dose combination) + EFV, ATV/r, FPV/r or LPV/r | ||
| 2008/01 | ABC+/3TC or TDF/FTC (both once daily fixed-dose combination) + EFV, ATV/r, FPV/r or LPV/r | ||
| 2008/11 | TDF/FTC + EFV, ATV/r, FPV/r, LPV/r, DRV/r | ABC+/3TC or TDF/FTC + EFV or NVP or a PI/r (FPV/r, LPV/r, SQV/r) | |
| 2009 | TDF/FTC + EFV or ATV/r or DRV/r or RAL | AZT or TDF + 3TC or FTC + EFV or NVP | |
| 2012 | As above | ||
| 2013/02 | TDF/FTC + EFV or ATV/r or DRV/r or RAL | ABC+/3TC or TDF/FTC + EFV or RPV (VL <100 000 copies ml−1) or ATV/r or DRV/r or RAL | TDF + 3TC or FTC + EFV |
| 2013/10 | As above, added TDF/FTC/EVG/co (if creatinine clearance >70 ml min−1) and DTG plus TDF/FTC or ABC+/3TC as preferred regimens |
ABC is used only in HLA-B&57:01-negative individuals. Abbreviations are as follows: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; ATV, atazanavir; AZT, zidovudine; co, cobicistat; d4T, stavudine; ddC, zalcitabine; ddI, didanosine; DHHS, Department of Health and Human Services; DRV, darunavir; DTG, dolutegravir; EACS, European AIDS Clinical Society; EFV, efavirenz; EVG, elvitegravir; FPV, fosamprenavir; FTC, emtricitabine; IDV, indinavir; LPV, lopinavir; NFV, nelfinavir; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; r, boosting dose ritonavir 100–200 mg daily; RAL, raltegravir; RPV, rilpivirine; RTV, high-dose ritonavir; SQV, saquinavir; TDF, tenofovir; VL, HIV viral load; WHO, World Health Organization.
Fluctuations in HIV treatment guidelines over the last two decades reflect the evolving challenges in this field (Figure 2). In 1998, with new treatment optimism, the mantra was ‘hit hard, hit early’ 3 (Figure 2). However, the challenges of HAART were soon realized, i.e. high pill burdens, inconvenient dosing, stringent food requirements, treatment-limiting toxicities and numerous drug interactions. Unrealistic levels of adherence (≥95%) were required to maintain adequate ART exposure and maintain viral suppression 4,5. Protease inhibitors were limited by unfavourable pharmacokinetic (PK) characteristics, including limited oral bioavailability, short half-lives, significant inter- and intrapatient variability, propensity for drug interactions, risk of resistance, toxicity and storage/stability issues. The NNRTIs had the advantage of long half-lives, but disadvantages of toxicities, drug interactions and single mutation conferring high-level class resistance.
Figure 2.
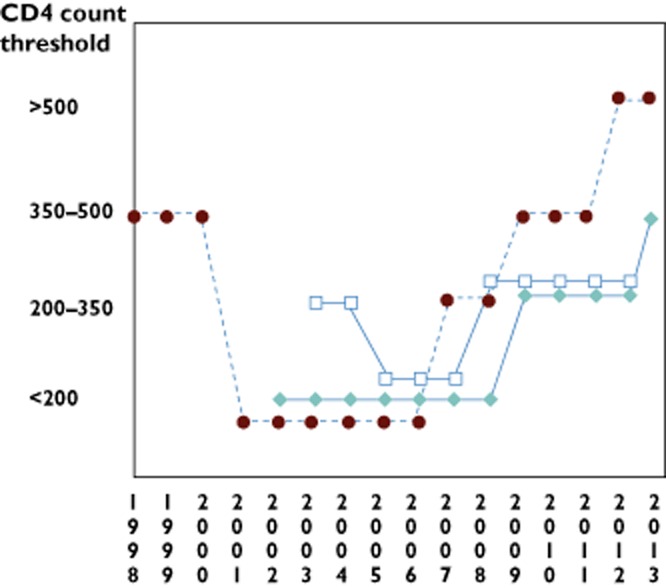
Convergence of human immunodeficiency virus (HIV) treatment guidelines in the developed and developing world. Changes in recommendations on when to start antiretroviral therapy (ART) in asymptomatic HIV-infected individuals based on CD4+ count criteria are shown. The Department of Health and Human Services (DHHS), European and World Health Organization (WHO) guidelines over time showing the fluctuations in recommendations which, in the developed world, represented a change from the initial optimism of ‘hit hard, hit early’ in 1998 to the reality of challenging high pill burden combinations. In the developing world, the trend has moved upwards, largely based on increased availability of ART and HIV care. The DHHS guidelines have now moved to an evidence-based approach, where treatment is recommended in all asymptomatic individuals but with various grades of evidence. The European guidelines have been more conservative, awaiting the results of the START study, a randomized double-blinded study of treatment initiation in treatment-naïve asymptomatic HIV-infected individuals with CD4+ T-cell count ≥500 or <500 mm−3. Although there are differences between guidelines, all agree and recommend earlier ART initiation regardless of CD4+ count in high-risk patient groups, such as those with co-morbidities such as co-infection with hepatitis B or C, HIV-associated nephropathy, rapid decline in CD4+, pregnancy and in sero-discordant partner situations.  , DHHS (US);
, DHHS (US);  , WHO;
, WHO;  , EACS (European). ART, antiretroviral therapy; DHHS, Department of Health and Human Services; EACS, European AIDS Clinical Society; HIV, human immunodeficiency virus; WHO, World Health Organization
, EACS (European). ART, antiretroviral therapy; DHHS, Department of Health and Human Services; EACS, European AIDS Clinical Society; HIV, human immunodeficiency virus; WHO, World Health Organization
The ability to measure lower level viraemia (<40 or 50 copies ml−1) further highlighted the challenge of incomplete virological suppression, particularly in treatment-experienced patients with multiple NRTI mutations. High pill burdens and the emergence and recognition of long-term and sometimes irreversible toxicities, such as thymidine analogue (d4T/AZT)-associated lipoatrophy, led to further treatment fatigue and a backlash against earlier treatment initiation. By 2001, the DHHS guidelines swung back to recommending treatment for asymptomatic patients only with CD4+ <200 mm−3/viral load >100 000 copies ml−1 (Figure 2). The quest for new approaches to treatment included a paradigm-shifting large randomized clinical trial, known as the SMART (‘strategies for management of antiretroviral therapy’) study, which randomized patients either to continuous therapy or a drug-conservation arm, with either planned deferral of initial therapy until CD4+ <250 mm−3 or CD4+-guided discontinuation of treatment 6. The study results showed without question that those patients randomized to drug conservation endured significantly more AIDS and collective non-AIDS morbidity and mortality in comparison to those on continuous therapy, and put to rest the notion of ‘drug holidays’ as a viable solution to pill fatigue and toxicities 6.
More recent cohort studies have also suggested a potential benefit of earlier therapy on survival 7, and an ongoing randomized controlled study, the ‘START study’, examining the impact of earlier treatment in asymptomatic individuals with CD4+ T-cell count >500 mm−3 on HIV and non-HIV events, is expected to be completed by 2016 8.
Treatment success in experienced populations now approaches that seen in treatment-naïve populations, and this relates to development of new classes of drugs, such as integrase strand transfer inhibitors, second-generation NNRTIs, longer-acting PIs with fewer toxicities and heat-stable ritonavir.
The realization that there would be no immediate cure for HIV highlighted the need to scale up preventive approaches. It is estimated that >50% of HIV transmission results from the 25% of individuals who are unaware of their HIV status and that >50% of HIV-diagnosed individuals are not engaged in medical care 9. The HPTN-052 study was a landmark randomized double-blind controlled study that randomized the HIV-positive individual in largely heterosexual sero-discordant couples to immediate vs. deferred ART until CD4+ T-cell count ≤350 mm−3. The results confirmed only one transmission event in the treatment arm (linked to transmission before treatment began) with 27 linked transmission events occurring in the deferred-therapy arm (P < 0.001). Individuals in the immediate-therapy arm showed a significantly longer time to develop AIDS and tuberculosis events 10. This study has been a strong driver of the global homogenization of HIV treatment guidelines, supporting earlier and unselective initiation of ART across the Department of Health and Human Services (DHHS), International AIDS Society and World Health Organization (WHO) guidelines (Figure 2). Early ART approaches in the resource-poor settings were also modelled to be cost effective over the lifetime of serodiscordant couples 11.
The treatment of HIV has presented many pharmacological challenges and triumphs. This review reflects on these and the future of HIV treatment.
Pharmacological challenges and triumphs
Pharmacoenhancement/pharmacokinetic boosting
Early PIs needed to be dosed three times daily, either fasting or with a significant meal and/or liquid intake, amounting to daily pill burdens of up to 22. Even with adherence to these stringent criteria, achievable drug exposures were often perilously close to the minimal concentration required for viral inhibition. Imperfect adherence, malabsorption or undetected drug interactions placed patients at risk for subtherapeutic exposures and risk of virological breakthrough and development of resistance. A significant advance in treatment was achieved with the concept of pharmacoenhancement with ritonavir. The demonstration that low-dose ritonavir (100–200 mg daily) could be used to increase the systemic bioavailability of the accompanying cytochrome P450 (CYP)3A4 substrate PI by increasing total area under the concentration–time curve, half-life and minimal concentrations and decreasing PK variability was a major advance 12. This allowed simplification to once or twice daily administration and led to improved patient adherence and virological outcomes, particularly in treatment-experienced patients, where boosted regimens had the ability to overcome low-level PI resistance 13–16.
A new pharmacokinetic enhancer, cobicistat, was designed to inhibit the CYP3A enzyme without antiretroviral activity. At a dose of 150 mg, it was found to exhibit similar CYP3A4 activity to ritonavir 100 mg. Similar to ritonavir, cobicistat also inhibits CYP2D6 and the transporter P-glycoprotein, but not CYP1A2, CYP2C9 or CYP2C19. The integrase inhibitor elvitegravir is a CYP3A4 substrate and is coformulated with cobicistat in combination with tenofovir and emtricitabine (FTC) to allow once daily dosing as a single pill 17,18. Cobicistat has also been shown to be an effective booster of atazanavir, and a co-formulated product with darunavir is currently in development. Studies have suggested that cobicistat shares gastrotinestinal and metabolic toxicities with ritonavir.
The concept of pharmacokinetic enhancement has also been applied to hepatitis C (HCV) antiviral therapy, where ritonavir is being co-administered with ABT-450 and danoprevir in phase 2 and late phase 3 studies to optimize PK and simplify dosing 19,20. Drawbacks to the use of pharmacokinetic boosters include increased risk of undesirable interactions with concomitant medications.
Therapeutic drug monitoring
Measurement of antiretroviral plasma concentrations allows individualization of drug dosing to optimize exposure and decrease the risk of toxicity or treatment failure. Protease inhibitors and NNRTIs are considered especially ideal candidates for therapeutic drug monitoring (TDM) owing to high inter- and intrapatient variability, susceptibility to drug interactions and identification of minimal concentrations for efficacy. Other drugs are less suited for TDM due to varying drug distribution/site of action, and plasma concentration measurements may not be as well correlated with response. For instance, TDM of NRTIs is limited by the practical difficulties of measuring intracellular concentrations. Other barriers to TDM include cost, accessibility and variable inter- and intralaboratory standardization.
Early prospective, randomized studies in treatment-naïve populations suggested benefit with indinavir and nelfinavir TDM 21,22. The clinical benefit of TDM was shown in a retrospective study in 137 patients. Improved clinical outcome was demonstrated in 16 of 20 (80%) patients who underwent TDM-guided dose adjustment; 10 patients experienced resolution of drug-related toxic effects, and six patients had improved virological response, with viral load reductions of >1 log 23. Subsequent negative studies in treatment-experienced patients were limited by the inclusion of patients already virologically suppressed at baseline, use of target concentrations that were inadequate for drug-resistant virus, delayed implementation of TDM, limited follow-up, low rates of adherence to TDM recommendations and lack of statistical power 24,25. Treatment guidelines for HIV have endorsed the use of ART TDM in the management of special populations, such as those with PK variation or at high risk for drug interactions, including patients with concomitant tuberculosis, opportunistic infections, hepatitis B or C co-infection, children, pregnancy, ageing, cancer, organ transplant and suspected non-adherence or virological failure 26–28.
Understanding and avoiding drug–drug and food–drug interactions
Drug interactions continue to be a significant challenge in the management of patients with HIV. In the early days, NRTIs in use were associated with many side-effects and overlapping toxicities with drugs used to treat opportunistic infections (Table 2). With the arrival of PIs and NNRTIs and the advent of pharmacokinetic boosting, drug interactions became more complex due to the numerous effects on CYP450 enzymes and transporters, thus making these antiretrovirals frequent perpetrators and victims of drug–drug interactions (Table 2).
Table 2.
Actual and potential drug interactions in HIV infection
| Pre- and early HAART era | Contemporary cART era | ||||||
|---|---|---|---|---|---|---|---|
| Effect | HIV drug(s) | Drugs for concomitant OIs, etc. | Effect | HIV drug(s) | Concomitant drugs/diseases | ||
| Pharmacodynamic | Bone marrow suppression | AZT | Sulfa drugs, ganciclovir, foscarnet, amphotericin B | Bone turnover | Tenofovir | Osteoporosis | |
| Hepatotoxicity | PIs, NNRTIs | Antimycobacterials | Hepatotoxicity | PIs, NNRTIs | Hepatitis B/C | ||
| Pancreatitis | ddI, ddC, d4T | Pentamidine, foscarnet | Metabolic disorders | PIs, some NNRTIs/NRTIs | Cardiovascular disease, diabetes | ||
| Peripheral neuropathy | Isoniazid, vinca alkaloids | Nephrotoxicity | Tenofovir | Nonsteroidal anti-inflammatory drugs, nephrotoxins, renal impairment | |||
| QT prolongation | Rilpivirine, some PIs (e.g. saquinavir, fosamprenavir) | Antiarrhythmics, antipsychotics, citalopram/escitalopram, macrolides, methadone, moxifloxacin, pentamidine, telaprevir, congenital long QT syndrome | |||||
| Pharmacokinetic | Absorption | Absorption | |||||
| • Gastric pH | ddI (basic) | Ketoconazole, itraconazole (acidic) | • Gastric pH | Atazanavir, rilpivirine (acidic) | Antacids, H2RA, proton-pump inhibitors | ||
| Indinavir, delavirdine (acidic) | Antacids, H2RA, proton-pump inhibitors | ||||||
| • Chelation | ddI | Quinolones | • Chelation | Integrase inhibitors | Antacids, oral calcium or iron supplements and buffered medications | ||
| • Food | Indinavir, ddI, efavirenz (empty stomach); PIs (high-fat meal) | • Food | Efavirenz (empty stomach), PIs, rilpivirine, etravirine, elvitegravir (with a meal) | ||||
| Metabolism | PIs, NNRTIs | Rifamycins Anticonvulsants | Metabolism | PIs, elvitegravir/cobicistat, NNRTIs, (perpetrators) | • Anti-infectives: directly acting agents for hepatitis C, rifamycins, azole antifungals | ||
| • Antineoplastics: etoposide, vinca alkaloids, tyrosine kinase inhibitors, paclitaxel, docetaxel, others | |||||||
| • Cardiovascular agents: statins, calcium channel blockers | |||||||
| • Corticosteroids (oral, inhaled, topical, injected), hormonal contraceptives | |||||||
| • Psychotropics: benzodiazepines, antidepressants, anticonvulsants | |||||||
| • Recreational drugs/narcotics: MDMA (ecstasy), methadone, PDE5 inhibitors | |||||||
| • Transplant drugs: ciclosporin, tacrolimus, sirolimus | |||||||
| • Miscellaneous: ergot derivatives | |||||||
| Metabolism | PIs, NNRTIs, cobicistat, elvitegravir/cobicistat, maraviroc (victims) | • Anti-infectives: directly acting agents for hepatitis C, rifamycins, azole antifungals | |||||
| • Anticonvulsants | |||||||
| • Complementary and alternative medicine: St John's wort, ginko biloba, activated charcoal | |||||||
Abbreviations are as follows: AZT, zidovudine; d4T, stavudine; ddC, zalcitabine; ddI, didanosine; H2RA, H2 receptor antagonists; MDMA, 3-4 methylenedioxymethamphetamine; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PDE5, phosphodiesterase 5; PI, protease inhibitor.
Older HIV patients have more co-morbidities and are at risk to be on several medications, heightening the risk of drug–drug interactions 29,30 and complicating the treatment of HIV and concurrent non-AIDS conditions. In addition, clinically significant interactions have been reported between ART and non-oral medications, such as inhaled, topical or injectable corticosteroids 31–33. Complementary and alternative medicine, over-the-counter products and recreational agents may further place patients at risk for unidentified drug interactions 34–36. These challenges should not be overlooked in resource-poor settings, where increased access to protease inhibitors is becoming more common. A recent report highlighting the risk of ergotism secondary to PI therapy and concomitant over-the-counter ergotamine use in Thailand illustrates this concern 37. Identification of drug interactions requires routine medication reconciliation, ideally at each patient clinic visit or at least once a year, and at any interface of care, such as hospital admission or discharge, or consultations with other specialists or healthcare professionals. Patients should use a single pharmacy to ensure comprehensive and current medication profiles. Drug interactions can be managed through adjustment of drug dose(s) or frequency of administration, substitution with an alternative agent, heightened clinical, laboratory and drug-level monitoring, or temporary or permanent discontinuation of noncritical therapies.
Clinicians are encouraged to consult an HIV pharmacist and to use specialized resources that are regularly updated to aid in management of potential drug interactions, because product monographs and standard references may not always contain newly identified data. Recommended websites include those run by the University of Liverpool Pharmacology Group 38, The University of California, San Francisco 39 and Toronto General Hospital 40.
Adherence/new drug formulations
While taking >80% of medication is considered excellent adherence for most chronic diseases, it became clear in the early days of HAART that >95% adherence to ART was required in order to achieve virological suppression reliably 5,41 The first agent to be approved for treatment of HIV was AZT, which was originally dosed at 200 mg every 4 h, based on its short plasma half-life. However, after realization that intracellular concentrations were more relevant than plasma concentrations for NRTIs, AZT dosing was later modified to 300 mg twice daily, and most drugs in this class are now dosed once daily. Recent progress in overcoming ART adherence challenges has included the development of improved drug formulations, which lack food and storage considerations, lower pill burden, and more favourable short- and long-term toxicity profiles. For instance, development of an enteric-coated didanosine capsule formulation removes the risk for cation/buffer interactions, while extended-release formulations for stavudine and nevirapine allow once daily dosing. Fosamprenavir, a prodrug of amprenavir, is associated with significantly reduced daily pill burden, dosing schedule and pill size compared with its predecessor. More recently, development of a heat-stable version of ritonavir has allowed for increased convenience and patient acceptability.
Fixed-dose combination products are available for preferred NRTI backbone regimens, the boosted PI combination of lopinavir/ritonavir, and a soon-to-be-released combination of darunavir/cobicistat. Adherence and ART success rates have improved 42,43. In 2006, the approval of co-formulated tenofovir/FTC/efavirenz allowed for the first ‘one pill, once a day’ regimen. There are currently three US Food and Drug Administration (FDA)-approved single-tablet regimens (STRs), which allow for single-tablet once daily dosing, namely FTC–tenofovir–efavirenz, FTC–tenofovir–rilpivirine and FTC–tenofovir–elvitegravir–cobicistat. A fourth STR of abacavir, 3TC and dolutegravir is pending approval.
Fixed-dose combination products and STRs do not allow the flexibility to individualize dose, and an additional caveat is the genericization of antiretrovirals, which may provide financial incentives to move away from the more expensive STRs in the developed world where generic STRs are not available.
Treatment toxicity and regimen simplification
With the success of modern cART and the trend towards earlier initiation of treatment, long-term treatment toxicities, including psychiatric, metabolic, renal, bone and cardiovascular issues, are increasingly being recognized as important contributors to discontinuation of treatment and/or increased morbidity, especially in the ageing HIV population. The search for safer and better-tolerated ART options continues. One agent in late-stage development is tenofovir alafenamide, which is a prodrug of tenofovir disoproxil fumarate. Tenofovir alafenamide at a dose of 25 mg provides enhanced delivery of tenofovir to lymphatic tissues, which results in significantly enhanced concentrations of tenofovir diphosphate in peripheral blood mononuclear cells (∼5-fold increase) and ∼90% lower circulating tenofovir compared with administration of a standard 300 mg dose of tenofovir disoproxil 44. When single-tablet regimens of elvitegravir, cobicistat, emtricitabine and either tenofovir disoproxil or tenofovir alafenamide were compared in treatment-naïve subjects, the tenofovir alafenamide treatment arm showed similar rates of viral suppression at week 48 and significantly lower bone mineral density change and lesser increase in serum creatinine in comparison to the tenofovir disoproxil-containing STR arm 45.
More recently, interest has focused on strategies of switching virologically suppressed patients to better-tolerated or simplified regimens in order to enhance long-term adherence and perseverance with treatment. Switches from efavirenz or boosted PI-based regimens to a rilpivirine–tenofovir–emtricitabine single-tablet regimen have led to improvements in toxicities, including central nervous system (CNS) and fasting lipids, respectively, while maintaining virological suppression 46,47.
Switching from boosted PIs or NNRTIs to an Integrase strand transfer inhibitor-based regimen in virologically suppressed patients has been similarly successful 48–50. However, as illustrated by the results of the SWITCHMRK study 46,51, inclusion of agents with a high barrier to resistance is critical to maintaining continued viral suppression, particularly in patients with pre-existing treatment-resistance mutations.
Sanctuary sites
Increased knowledge regarding the distribution of antiretrovirals into various compartments and sanctuary sites has helped to individualize clinical treatment. In the pre-HAART era, AIDS-associated dementia was a frequent and devastating condition, in which high-dose and poorly tolerated oral or intrathecal AZT was the only available strategy to control HIV replication in the CNS. AIDS-associated dementia has significantly decreased with cART, but there is increasing concern regarding more subtle HIV-associated neurocognitive disorders, even in patients without detectable HIV in plasma. The development of HIV-associated neurocognitive disorders may be correlated with how efficiently various antiretrovirals penetrate the CNS. Early studies yielded inconsistent data regarding the association between cumulative CNS penetration effectiveness scores and neurocognitive outcome 52. A recent study suggests that this approach may have benefit in protecting against cognitive deterioration 53. Further research in this area is needed.
Physiological changes associated with pregnancy may affect PI PK, and TDM and/or dose adjustment is recommended in the second or third trimester to ensure therapeutic antiretroviral concentrations, particularly at the time of delivery, in order to minimize the risk of vertical HIV transmission. Maternal genital tract viral burden has been shown to be an independent factor associated with HIV transmission, and adequate genital tract antiretroviral exposures may play a role in prevention. This concept was illustrated in an unfortunate case of transmission of multidrug-resistant virus to a newborn in a treatment-experienced but virologically suppressed woman on salvage ART where enfuvirtide was the only active agent, which penetrated the genital tract poorly 54.
The strategy of using antiretroviral therapy in individuals at high risk of acquiring HIV (i.e. pre-exposure prophylaxis or PrEP) represents another significant milestone in HIV therapeutics. In 2012, tenofovir/emtricitabine became the first regimen to receive an FDA indication for PrEP. Eight PrRP studies have been conducted, employing topical and oral formulations of ART 5001–5008. The study populations for these studies mainly consisted of African women at high risk of heterosexual HIV acquisition (CAPRISA 004, FEM-PrEP, Partners PrEP, TDF2, VOICE/MTN 003 and FACTS 001). However, they also involved African heterosexual men in two studies (Partners PrEP, TDF2), men who have sex with men (from the Americas, Thailand and South Africa) in the iPrEx study and Thai male injecting drug users in the Bangkok Tenofovir Study. The effectiveness from these studies ranged from 75% reduction in HIV-1 incidence in the Partners PrEP study with FTC/tenofovir (TDF) prophylaxis to no reduction seen in the FEM-PrEP study (also using FTC/TDF). Notably, the success with the prophylactic therapies is dependent on the degree of adherence to the study regimens. The iPrEx study demonstrated that participants having detectable drug concentrations were strongly associated with a significantly lower risk for HIV-1 acquisition (73% efficacy with ≥90% adherence) 5001.
Efficacy results have varied across these PrEP trials and have been significantly lower in those involving women. This also highlights the potential differences in antiretroviral PK/pharmacodynamics in the male and female genital tracts alongside the significant influence of adherence to therapy.
Localized delivery systems, such as intravaginal rings 5002,5003, show some promise; however, more insight into pharmacological limitations is needed to develop effective PrEP options.
Pharmacogenomics to identify/minimize toxicity
A hypersensitivity syndrome associated with the NRTI abacavir was first described in the premarketing phase of drug development and was characterized by fever, malaise, gastrointestinal symptoms and later rash on first exposure, with severe hypotension and shock on rechallenge 5004,5005. The discovery of an association between abacavir hypersensitivity and the human leukocyte antigen (HLA) class I allele HLA-B&57:01 and its translation into routine HIV clinical practice as a guideline-endorsed preventive screening strategy has created a roadmap for pharmacogenomic translation 5004,5006. Since the introduction of routine HLA-B&57:01 screening prior to abacavir prescription, true immunologically mediated abacavir hypersensitivity has disappeared 5007. Other ART toxicity–pharmacogenomic relationships that have been explored in detail and reproduced amongst different groups include CYP2B6 and uridine diphosphate glucuronyltransferases polymorphisms and efavirenz exposure/pharmacokinetics and atazanavir-associated unconjugated hyperbilirubinaemia, respectively 5008. Some studies have suggested relationships between genetic polymorphisms and discontinuation related to ART-related adverse events 63. One small study successfully reduced the dose of efavirenz in patients with the CYP2B6 516 G→T polymorphism using adjunctive TDM 64. The CYP2B6 516 G→T polymorphism is particularly prevalent in Black African and African American populations (up to 20%), and PK studies have demonstrated higher exposure in these populations 65.
Future promises
Throughout the progress of the last three decades, the primary goals of ART remain largely unchanged, i.e. to suppress HIV viral load, to restore immune function, to preserve future treatment options and to improve quality and quantity of life. Newer considerations have included the control of HIV transmission (‘treatment as prevention’), consideration for managing and preventing non-AIDS as well as AIDS morbidity and the ultimate goal of HIV eradication or functional cure.
Emerging data have suggested that treatment-as-prevention approaches are cost effective 11; however, there is a massive demand for scale-up, and continued improvements in cost efficiency will be necessary to achieve broader access to antiretroviral therapy. In 2012, 9.7 million people with HIV were accessing ART, and this was a 1.6 million increase from 2011 66. It has been estimated that broader access to treatment following current HIV treatment guidelines would avoid 19 million new HIV infections by 2025. The immediate goal by the Joint United Nations Programme on HIV/AIDS is to achieve coverage of 15 million by 2015. There have been significant efforts and achievements in decreasing the cost of ART and HIV testing. This has included process chemistry improvement to ART drug manufacturing by generic companies, reformulation, dose optimization and extension of shelf-life 67. For example, process chemistry was able to reduce the number of manufacturing steps for efavirenz from four to two, resulting in a 75% price decrease from $240 per patient year−1 in 2006 to $60 per patient year−1 in 2011 68. Similar process chemistry improvements in manufacturing have been applied to tenofovir disoproxil fumarate and are in development for darunavir.
An exciting new pharmacological development has been that of nanotechnology to develop long-acting injections of antiretroviral drugs that could be administered once monthly 69,70. Current injectable nanoformulations that are under study include the second-generation NNRTI rilpivirine and a new long-acting integrase inhibitor (GSK-1265744). GSK-1265744, a dolutegravir analogue, is detectable in plasma up to 48 weeks following a single injection. These two drugs have been studied in combination in Phase I studies examining PK and safety. Forty-eight week results from a Phase IIb study of rilpivirine plus oral GSK-1265744 as maintenance therapy showed similar virological suppression rates in comparison to standard efavirenz-based treatment 71. These promising results support the development of using rilpivirine and GSK-1265744 as a monthly injectable regimen, which may help to combat adherence challenges. Use of these injectable drugs is also being explored in the developing world as a preventive strategy.
Although ART controls actively replicating HIV, latent HIV persists in resting memory CD4+ T cells, and this remains the major barrier to HIV eradication or cure. Treatment intensification studies with multiple antiretroviral agents, including CCR5 and integrase inhibitors, have not resulted in a decreased size of the HIV reservoir or prevented recurrent HIV viraemia off ART 72. Short-course ART during primary infection has been shown to have immunological and virological benefit and may reduce the size of the latent reservoir of HIV, protect HIV-specific cellular and humoral responses and restrict the diversification of HIV, but is unlikely to effect HIV cure 73. The promise of HIV eradication was fuelled by the ‘Berlin patient’, who received an allogeneic stem cell transplantation from an HLA-matched donor homozygous for a 32 bp deletion in the CCR5 allele 6 years ago and has remained free of recrudescent HIV replication off cART 74. The definition and quantification of HIV eradication has not been precisely defined; however, 5 years after cART withdrawal there has been significant waning of HIV-specific T-cell responses and no evidence of active HIV replication in this patient 75. Experimental pharmacological strategies to bring HIV out of latency have been examined in early phase studies in humans. This has included the use of inhibitors of histone deacetylase, such as vorinostat, which has shown some promise in increasing cell-associated HIV RNA, although cells do not die on reactivation of latent HIV, meaning that it is likely that a combination of pharmacological, gene and therapeutic vaccine approaches will be necessary to effect eradication 76,77.
Conclusions
Treatment of HIV has evolved from gruelling regimens with high pill burden, inconvenient dosing, treatment-limiting toxicities, food and drug interactions, incomplete viral suppression and emergence of drug resistance to manageable one or two pill once daily regimens that can be initiated in early HIV disease and continued with control of viral replication over much of an individual's lifespan. Life expectancies of those who have achieved immune reconstitution and remain virologically suppressed should be close to normal. Pharmacological advances in the study and treatment of HIV have been at the frontier of science. Examples include pharmacogenomic discovery and translation (HLA-B&57:01 screening to prevent abacavir hypersensitivity), drug class and formulation discovery (fixed-dose combination, long-acting injectable nano-formulations), pharmacokinetic enhancement and the management and provision of decision support for complex drug–drug and food–drug interactions. Current and future challenges include the continued globalization of ART through scale-up and continued improvements in cost efficiency, engagement and retention of patients in care, new immunological and pharmacological approaches to prevention and the science and translation of HIV eradication strategies.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work. EJP and AT have received educational grants and honoraria from ViiV Healthcare, Janssen, Merck Pty Ltd and Gilead over the last 3 years. AT has received educational grants and honoraria from AbbVie and Bristol Myers Squibb over the last 3 years. None of the authors has any relationships or activities that could appear to have influenced the submitted work.
References
- Fischl MA, Richman DD, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, Leedom JM, Groopman JE, Mildvan D, Schooley RT, Jackson G, Durack D, King D the AZT Collaborative Working Group. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317:185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Ho DD. Time to hit HIV, early and hard. N Engl J Med. 1995;333:450–451. doi: 10.1056/NEJM199508173330710. [DOI] [PubMed] [Google Scholar]
- Xu L, Liu H, Hong A, Vivian R, Murray BP, Callebaut C, Choi YC, Lee MS, Chau J, Tsai LK, Stray KM, Strickley RG, Wang J, Tong L, Swaminathan S, Rhodes GR, Desai MC. Structure-activity relationships of diamine inhibitors of cytochrome P450 (CYP) 3A as novel pharmacoenhancers. Part II: P2/P3 region and discovery of cobicistat (GS-9350) Bioorg Med Chem Lett. 2014;24:995–999. doi: 10.1016/j.bmcl.2013.12.057. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagener MM, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, Cohn D, Cooper D, Darbyshire J, Emery S, Fatkenheuer G, Gazzard B, Grund B, Hoy J, Klingman K, Losso M, Markowitz N, Neuhaus J, Phillips A, Rappoport C. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, Hogg RS, Deeks SG, Eron JJ, Brooks JT, Rourke SB, Gill MJ, Bosch RJ, Martin JN, Klein MB, Jacobson LP, Rodriguez B, Sterling TR, Kirk GD, Napravnik S, Rachlis AR, Calzavara LM, Horberg MA, Silverberg MJ, Gebo KA, Goedert JJ, Benson CA, Collier AC, Van Rompaey SE, Crane HM, McKaig RG, Lau B, Freeman AM, Moore RD. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- START Collaboration. Strategic timing of antiretroviral treatment (START) study. Available at http://www.clinicaltrials.gov/ct2/show/NCT00867048?term=hiv+start+study&rank=4 (last accessed 30 March 2014)
- Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis. 2013;57:1164–1171. doi: 10.1093/cid/cit420. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky RP, Ross EL, Kumarasamy N, Wood R, Noubary F, Paltiel AD, Nakamura YM, Godbole SV, Panchia R, Sanne I, Weinstein MC, Losina E, Mayer KH, Chen YQ, Wang L, McCauley M, Gamble T, Seage GR, III, Cohen MS, Freedberg KA. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med. 2013;369:1715–1725. doi: 10.1056/NEJMsa1214720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldin RK, Petruschke RA. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J Antimicrob Chemother. 2004;53:4–9. doi: 10.1093/jac/dkh029. [DOI] [PubMed] [Google Scholar]
- Kempf DJ, King MS, Bernstein B, Cernohous P, Bauer E, Moseley J, Gu K, Hsu A, Brun S, Sun E. Incidence of resistance in a double-blind study comparing lopinavir/ritonavir plus stavudine and lamivudine to nelfinavir plus stavudine and lamivudine. J Infect Dis. 2004;189:51–60. doi: 10.1086/380509. [DOI] [PubMed] [Google Scholar]
- Pennings PS. HIV Drug Resistance: problems and Perspectives. Infect Dis Rep. 2013;5:e5. doi: 10.4081/idr.2013.s1.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensing AM, van Maarseveen NM, Nijhuis M. Fifteen years of HIV Protease Inhibitors: raising the barrier to resistance. Antivir Res. 2010;85:59–74. doi: 10.1016/j.antiviral.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Kim R, Baxter JD. Protease inhibitor resistance update: where are we now? AIDS Patient Care STDs. 2008;22:267–277. doi: 10.1089/apc.2007.0099. [DOI] [PubMed] [Google Scholar]
- Mathias AA, German P, Murray BP, Wei L, Jain A, West S, Warren D, Hui J, Kearney BP. Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. Clin Pharmacol Ther. 2010;87:322–329. doi: 10.1038/clpt.2009.228. [DOI] [PubMed] [Google Scholar]
- Deeks ED. Cobicistat: a review of its use as a pharmacokinetic enhancer of atazanavir and darunavir in patients with HIV-1 infection. Drugs. 2014;74:195–206. doi: 10.1007/s40265-013-0160-x. [DOI] [PubMed] [Google Scholar]
- Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson DR, Zeuzem S, Everson GT, Kwo P, Foster GR, Sulkowski MS, Xie W, Pilot-Matias T, Liossis G, Larsen L, Khatri A, Podsadecki T, Bernstein B. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med. 2014;370:222–232. doi: 10.1056/NEJMoa1306227. [DOI] [PubMed] [Google Scholar]
- Goelzer P, Morcos PN, Tran JQ, Wen B, Shulman NS, Brennan BJ, Hammond J, Singer T, Smith P. 2012. Coadministration of Ritonavir with the HCV Protease Inhibitor Danoprevir Substantially Reduces Reactive Metabolite Formation Both In Vitro and In Vivo. In: 63rd Annual Meeting of the American Association for the Study of Liver Diseases, Boston, MA,
- Burger D, Hugen P, Reiss P, Gyssens I, Schneider M, Kroon F, Schreij G, Brinkman K, Richter C, Prins J, Aarnoutse R, Lange J. Therapeutic drug monitoring of nelfinavir and indinavir in treatment-naive HIV-1-infected individuals. AIDS. 2003;17:1157–1165. doi: 10.1097/00002030-200305230-00007. [DOI] [PubMed] [Google Scholar]
- Fletcher CV, Anderson PL, Kakuda TN, Schacker TW, Henry K, Gross CR, Brundage RC. Concentration-controlled compared with conventional antiretroviral therapy for HIV infection. AIDS. 2002;16:551–560. doi: 10.1097/00002030-200203080-00006. [DOI] [PubMed] [Google Scholar]
- Rendon A, Nunez M, Jimenez-Nacher I, Gonzalez de Requena D, Gonzalez-Lahoz J, Soriano V. Clinical benefit of interventions driven by therapeutic drug monitoring. HIV Med. 2005;6:360–365. doi: 10.1111/j.1468-1293.2005.00321.x. [DOI] [PubMed] [Google Scholar]
- Pretorius E, Klinker H, Rosenkranz B. The role of therapeutic drug monitoring in the management of patients with human immunodeficiency virus infection. Ther Drug Monit. 2011;33:265–274. doi: 10.1097/FTD.0b013e31821b42d1. [DOI] [PubMed] [Google Scholar]
- la Porte CJL, Back DJ, Blaschke T, Boucher CAB, Fletcher CV, Flexner C, Gerber JG, Kashuba ADM, Schapiro J, Burger DM. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev Antivir Ther. 2006;3:4–14. [Google Scholar]
- DHHS Guidelines for the use of antiretroviral agents in HIV-1 infected adult and adolescents. Available at http://aidsinfo.nih.gov/guidelines (last accessed 30 March 2014)
- 2013. European AIDS Clin Soc Guidelines , Version 7.0. October. Available at http://eacsociety.org/Portals/0/Guidelines_Online_131014.pdf (last accessed 30 March 2014)
- 2012. British HIV Association guidelines for the treatment of HIV-1 positive adults with antiretroviral treatment. Available at http://www.bhiva.org/documents/Guidelines/Treatment/2012/hiv1029_2.pdf (last accessed 30 March 2014)
- Marzolini C, Back D, Weber R, Furrer H, Cavassini M, Calmy A, Vernazza P, Bernasconi E, Khoo S, Battegay M, Elzi L. Ageing with HIV: medication use and risk for potential drug-drug interactions. J Antimicrob Chemother. 2011;66:2107–2111. doi: 10.1093/jac/dkr248. [DOI] [PubMed] [Google Scholar]
- Tseng A, Szadkowski L, Walmsley S, Salit I, Raboud J. Association of age with polypharmacy and risk of drug interactions with antiretroviral medications in HIV-positive patients. Ann Pharmacother. 2013;47:1429–1439. doi: 10.1177/1060028013504075. [DOI] [PubMed] [Google Scholar]
- Foisy MM, Yakiwchuk EM, Chiu I, Singh AE. Adrenal suppression and Cushing's syndrome secondary to an interaction between ritonavir and fluticasone: a review of the literature. HIV Med. 2008;9:389–396. doi: 10.1111/j.1468-1293.2008.00579.x. [DOI] [PubMed] [Google Scholar]
- Tseng A, Foisy M. Important drug-drug interactions in HIV-infected persons on antiretroviral therapy: an update on new interactions between HIV and non-HIV drugs. Curr Infect Dis Rep. 2012;14:67–82. doi: 10.1007/s11908-011-0229-1. [DOI] [PubMed] [Google Scholar]
- Hyle EP, Wood BR, Backman ES, Noubary F, Hwang J, Lu Z, Losina E, Walensky RP, Gandhi RT. High frequency of hypothalamic-pituitary-adrenal axis dysfunction after local corticosteroid injection in HIV-infected patients on protease inhibitor therapy. J Acquir Immune Defic Syndr. 2013;63:602–608. doi: 10.1097/QAI.0b013e31829b662b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegman DJ, Brinkman K, Franssen EJ. Interaction of Ginkgo biloba with efavirenz. AIDS. 2009;23:1184–1185. doi: 10.1097/QAD.0b013e32832c412b. [DOI] [PubMed] [Google Scholar]
- Henry JA, Hill IR. Fatal interaction between ritonavir and MDMA. Lancet. 1998;352:1751–1752. doi: 10.1016/s0140-6736(05)79824-x. [DOI] [PubMed] [Google Scholar]
- Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St John's wort. Lancet. 2000;355:547–548. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- Avihingsanon A, Ramautarsing RA, Suwanpimolkul G, Chetchotisakd P, Bowonwatanuwong C, Jirajariyavej S, Kantipong P, Tantipong H, Ohata JP, Suankratay C, Ruxrungtham K, Burger DM. Ergotism in Thailand caused by increased access to antiretroviral drugs: a global warning. Top Antivir Med. 2013;21:165–168. [PMC free article] [PubMed] [Google Scholar]
- HIV Drug Interactions. Available at http://www.hiv-druginteractions.org/ (last accessed 30 March 2014)
- HIV Insite. Available at http://hivinsite.ucsf.edu/insite?page=ar-00-02 (last accessed 30 March 2014)
- Toronto General Hospital – Immunodeficiency Clinic. Available at http://hivclinic.ca/main/home.html (last accessed 30 March 2014)
- Emamzadeh-Fard S, Fard SE, SeyedAlinaghi S, Paydary K. Adherence to anti-retroviral therapy and its determinants in HIV/AIDS patients: a review. Infect Disord Drug Targets. 2012;12:346–356. doi: 10.2174/187152612804142251. [DOI] [PubMed] [Google Scholar]
- Cortez KJ, Maldarelli F. Clinical management of HIV drug resistance. Viruses. 2011;3:347–378. doi: 10.3390/v3040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachega JB, Parienti JJ, Uthman OA, Gross R, Dowdy DW, Sax PE, Gallant JE, Mugavero MJ, Mills EJ, Giordano TP. Lower Pill Burden and once-daily dosing antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;58:1297–1907. doi: 10.1093/cid/ciu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin F, Fordyce M, Garner W, Ray A, Tanamly S, Lindow J, Kearney BP, Ramanathan S. 2013. Pharmacokinetics, Metabolism and Excretion of Tenofovir Alafenamide (TAF). In: 14th International Workshop on Clinical Pharmacology of HIV Therapy Amsterdam, The Netherlands,: Abstract O-08.
- Sax P, Brar I, Elion R, Zolopa A, Ortiz R, Wang H, Callebaut C, Martin H, Fordyce M, McCallister S. 2013. 48 Week Study of Tenofovir Alafenamide (TAF) vs. Tenofovir Disoproxil Fumarate (TDF), Each in a Single Tablet Regimen (STR) with Elvitegravir, Cobicistat, and Emtricitabine [E/C/F/TAF vs]. E/C/F/TDF] for Initial HIV Treat. In: 53rd ICAAC, Denver, CO, [DOI] [PubMed]
- Nelson M, Winston A, Waters L, Higgs C, Roche M, Mora-Peris B, Milincovic A, Mandalia S, Yapa HM, Fisher M. 2013. Multicentre Open-Label Study of Switching from Atripla to Eviplera for Possible Efavirenz Associated CNS Toxicity. In: ICAAC 2013, Denver, Colorado,: H-672b.
- Pablo Tebas FP, Ruane P, Shamblaw D, Flamm J, Ebrahimi R, Porter D, Williams S, Sparrow T, Fralich T, De-Oerte S. 2013. SPIRIT: simplification to Rilpivirine/Emtricitabine/Tenofovir DF Single-Tablet Regimen from Boosted Protease Inhibitor Maintains HIV-1 Suppression and Improves Fasting Lipids at Week 48. In: IDSA,
- Martinez E, Larrousse M, Llibre JM, Gutierrez F, Saumoy M, Antela A, Knobel H, Murillas J, Berenguer J, Pich J, Perez I, Gatell JM. Substitution of raltegravir for ritonavir-boosted protease inhibitors in HIV-infected patients: the SPIRAL study. AIDS. 2010;24:1697–1707. doi: 10.1097/QAD.0b013e32833a608a. [DOI] [PubMed] [Google Scholar]
- Arribas J, Pialoux G, Gathe J, Di Perri G, Reynes J, Tebas P, Nguyen T, Ebrahimi R, White K, Piontkowsky D. Simplification of PI + RTV + FTC/TDF to E/C/F/TDF Maintains HIV Suppression and is Well-Tolerated. In: 21st Conference on Retroviruses and Opportunistic Infections (CROI), Boston, Massachusetts.
- Pozniak A, Markowitz M, Mills A, Stellbrink H, Antela A, Domingo P, Girard P, Henry K, Garner W, Guyer B. 2014. Switch from NNRTI plus FTC/TDF to E/C/F/TDF Maintains HIV Suppression and is Well-Tolerated. In: 21st Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, [DOI] [PubMed]
- Eron JJ, Young B, Cooper DA, Youle M, Dejesus E, Andrade-Villanueva J, Workman C, Zajdenverg R, Fatkenheuer G, Berger DS, Kumar PN, Rodgers AJ, Shaughnessy MA, Walker ML, Barnard RJ, Miller MD, Dinubile MJ, Nguyen BY, Leavitt R, Xu X, Sklar P. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet. 2010;375:396–407. doi: 10.1016/S0140-6736(09)62041-9. [DOI] [PubMed] [Google Scholar]
- Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: a consensus report of the mind exchange program. Clin Infect Dis. 2013;56:1004–1017. doi: 10.1093/cid/cis975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo M, Durant J, Biscay V, Lebrun-Frenay C, Dunais B, Laffon M, Harvey-Langton A, Cottalorda J, Ticchioni M, Carsenti H, Pradier C, Dellamonica P. Can high central nervous system penetrating antiretroviral regimens protect against the onset of HIV-associated neurocognitive disorders? AIDS. 2014;28:493–501. doi: 10.1097/QAD.0000000000000096. [DOI] [PubMed] [Google Scholar]
- Cohan D, Feakins C, Wara D, Petru A, McNicholl I, Schillinger D, Lu J, Kuritzkes D, Deeks SG. Perinatal transmission of multidrug-resistant HIV-1 despite viral suppression on an enfuvirtide-based treatment regimen. AIDS. 2005;19:989–990. doi: 10.1097/01.aids.0000171417.84162.af. [DOI] [PubMed] [Google Scholar]
- Friend DR, Kiser PF. Assessment of topical microbicides to prevent HIV-1 transmission: concepts, testing, lessons learned. Antiviral Res. 2013;99:391–400. doi: 10.1016/j.antiviral.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Sokal DC, Karim QA, Sibeko S, Yende-Zuma N, Mansoor LE, Baxter C, Grobler A, Frolich J, Kharsany AB, Miya N, Mlisana K, Maarshalk S, Karim SS. Safety of tenofovir gel, a vaginal microbicide, in South African women: results of the CAPRISA 004 Trial. Antivir Ther. 2013;18:301–310. doi: 10.3851/IMP2311. [DOI] [PubMed] [Google Scholar]
- Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. FEM-PrEP Study Group. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT. TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Microbicide Trials Network. 2014. Phase 2B safety and effectiveness study of tenofovir 1% gel, tenofovir, disoproxil fumarate tablet and emtricitabine/tenofovir disoproxil fumarate tablet for the prevention of HIV infection in women. Available at http://www.mtnstopshiv.org/news/studies/mtn003 (last accessed February )
- CONRAD. 2014. Safety and effectiveness of tenofovir gel in the prevention of human immunodeficiency virus (HIV-1) infectious in women and the effects of tenofovir gel on the incidence of herpes simplex virus (HSV-2) infection. Available at http://www.clinicaltrials.gov/ct2/show/NCT01386294?term=FACTS-001&rank=1 (last accessed February )
- NIAID. 2014. Safety and effectiveness of tenofovir 1% gel, tenofovir disproxil fumarate, and emtricitabine/tenofovir disoproxil fumarate tablets in preventing HIV in women. Available at http://www.clinicaltrials.gov/ct2/show/NCT00705679?term=VOICE%2FMTN+003&rank=1 (last accessed February )
- Celum C, Baeten JM. Antiretroviral-based HIV-1 prevention: antiretroviral treatment and pre-exposure prophylaxis. Antivir Ther. 2012;17:1483–1493. doi: 10.3851/IMP2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman AR, Clark MR, Hurlburt JA, Doncel GF. Intravaginal rings as delivery systems for microbicides and multipurpose prevention technologies. Int J Womens Health. 2013;5:695–708. doi: 10.2147/IJWH.S34030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano J, Kashuba A, Becker S, Cummins J, Turpin J, Veronese F. Pharmacokinetics and pharmacodynamics in HIV prevention; current status and future directions: a summary of the DAIDS and BMGF sponsored think tank on pharmacokinetics (PK)/pharmacodynamics (PD) in HIV prevention. AIDS Res Hum Retroviruses. 2013;29:1418–1427. doi: 10.1089/aid.2013.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips EJ, Chung WH, Mockenhaupt M, Roujeau JC, Mallal SA. Drug hypersensitivity: pharmacogenetics and clinical syndromes. J Allergy Clin Immunol. 2011;127:S60–66. doi: 10.1016/j.jaci.2010.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington S, McGuirk S, Powell G, Cutrell A, Naderer O, Spreen B, Lafon S, Pearce G, Steel H. Hypersensitivity reactions during therapy with the nucleoside reverse transcriptase inhibitor abacavir. Clin Ther. 2001;23:1603–1614. doi: 10.1016/s0149-2918(01)80132-6. [DOI] [PubMed] [Google Scholar]
- Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jagel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A. HLA-B&5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- Rauch A, Nolan D, Martin A, McKinnon E, Almeida C, Mallal S. Prospective genetic screening decreases the incidence of abacavir hypersensitivity reactions in the Western Australian HIV cohort study. Clin Infect Dis. 2006;43:99–102. doi: 10.1086/504874. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Novoa S, Martin-Carbonero L, Barreiro P, Gonzalez-Pardo G, Jimenez-Nacher I, Gonzalez-Lahoz J, Soriano V. Genetic factors influencing atazanavir plasma concentrations and the risk of severe hyperbilirubinemia. AIDS. 2007;21:41–46. doi: 10.1097/QAD.0b013e328011d7c1. [DOI] [PubMed] [Google Scholar]
- Lubomirov R, Colombo S, di Iulio J, Ledergerber B, Martinez R, Cavassini M, Hirschel B, Bernasconi E, Elzi L, Vernazza P, Furrer H, Gunthard HF, Telenti A. Association of pharmacogenetic markers with premature discontinuation of first-line anti-HIV therapy: an observational cohort study. J Infect Dis. 2011;203:246–257. doi: 10.1093/infdis/jiq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatanaga H, Hayashida T, Tsuchiya K, Yoshino M, Kuwahara T, Tsukada H, Fujimoto K, Sato I, Ueda M, Horiba M, Hamaguchi M, Yamamoto M, Takata N, Kimura A, Koike T, Gejyo F, Matsushita S, Shirasaka T, Kimura S, Oka S. Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6 &6 and &26. Clin Infect Dis. 2007;45:1230–1237. doi: 10.1086/522175. [DOI] [PubMed] [Google Scholar]
- Mukonzo JK, Owen JS, Ogwal-Okeng J, Kuteesa RB, Nanzigu S, Sewankambo N, Thabane L, Gustafsson LL, Ross C, Aklillu E. Pharmacogenetic-based efavirenz dose modification: suggestions for an African population and the different CYP2B6 genotypes. PLoS One. 2014;9:e86919. doi: 10.1371/journal.pone.0086919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO UNICEF UNAIDS Global update on HIV treatment 2013: results, impact and opportunities. Available at http://www.who.int/hiv/pub/progressreports/update2013/en/ (last accessed 30 March 2014)
- Crawford KW, Ripin DH, Levin AD, Campbell JR, Flexner C. Optimising the manufacture, formulation, and dose of antiretroviral drugs for more cost-efficient delivery in resource-limited settings: a consensus statement. Lancet Infect Dis. 2012;12:550–560. doi: 10.1016/S1473-3099(12)70134-2. [DOI] [PubMed] [Google Scholar]
- Jayaraman K. Finding the right chemistry. Nat Med. 2013;19:1200–1203. doi: 10.1038/nm1013-1200. [DOI] [PubMed] [Google Scholar]
- Spreen WR, Margolis DA, Pottage JC., Jr Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS. 2013;8:565–571. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehendale R, Joshi M, Patravale VB. Nanomedicines for treatment of viral diseases. Crit Rev Ther Drug Carrier Syst. 2013;30:1–49. doi: 10.1615/critrevtherdrugcarriersyst.2013005469. [DOI] [PubMed] [Google Scholar]
- Margolis D, Brinson C, Eron J, Richmond G, LeBlanc R, Griffith S, St. Clair M, Stevens M, Williams P, Spreen W. 744 and rilpvirine as two-drug oral maintenance therapy: LAI116482 (LATTE) Week 48 results. Top Antivir Med. 2014;22:45–46. . [abstract 91LB]. [Google Scholar]
- Gandhi RT, Bosch RJ, Aga E, Albrecht M, Demeter LM, Dykes C, Bastow B, Para M, Lai J, Siliciano RF, Siliciano JD, Eron JJ. No evidence for decay of the latent reservoir in HIV-1-infected patients receiving intensive enfuvirtide-containing antiretroviral therapy. J Infect Dis. 2010;20:293–296. doi: 10.1086/649569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Han X, An M, Liu J, Xu J, Geng W, Ji Y, Shang H. Immunological and virological benefits resulted from short-course treatment during primary HIV infection: a meta-analysis. PLoS One. 2013;8:e82461. doi: 10.1371/journal.pone.0082461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, Blau IW, Hofmann WK, Thiel E. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- Yukl SA, Boritz E, Busch M, Bentsen C, Chun TW, Douek D, Eisele E, Haase A, Ho YC, Hutter G, Justement JS, Keating S, Lee TH, Li P, Murray D, Palmer S, Pilcher C, Pillai S, Price RW, Rothenberger M, Schacker T, Siliciano J, Siliciano R, Sinclair E, Strain M, Wong J, Richman D, Deeks SG. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 2013;9:e1003347. doi: 10.1371/journal.ppat.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent SJ, Reece JC, Petravic J, Martyushev A, Kramski M, De Rose R, Cooper DA, Kelleher AD, Emery S, Cameron PU, Lewin SR, Davenport MP. The search for an HIV cure: tackling latent infection. Lancet Infect Dis. 2013;13:614–621. doi: 10.1016/S1473-3099(13)70043-4. [DOI] [PubMed] [Google Scholar]


