Abstract
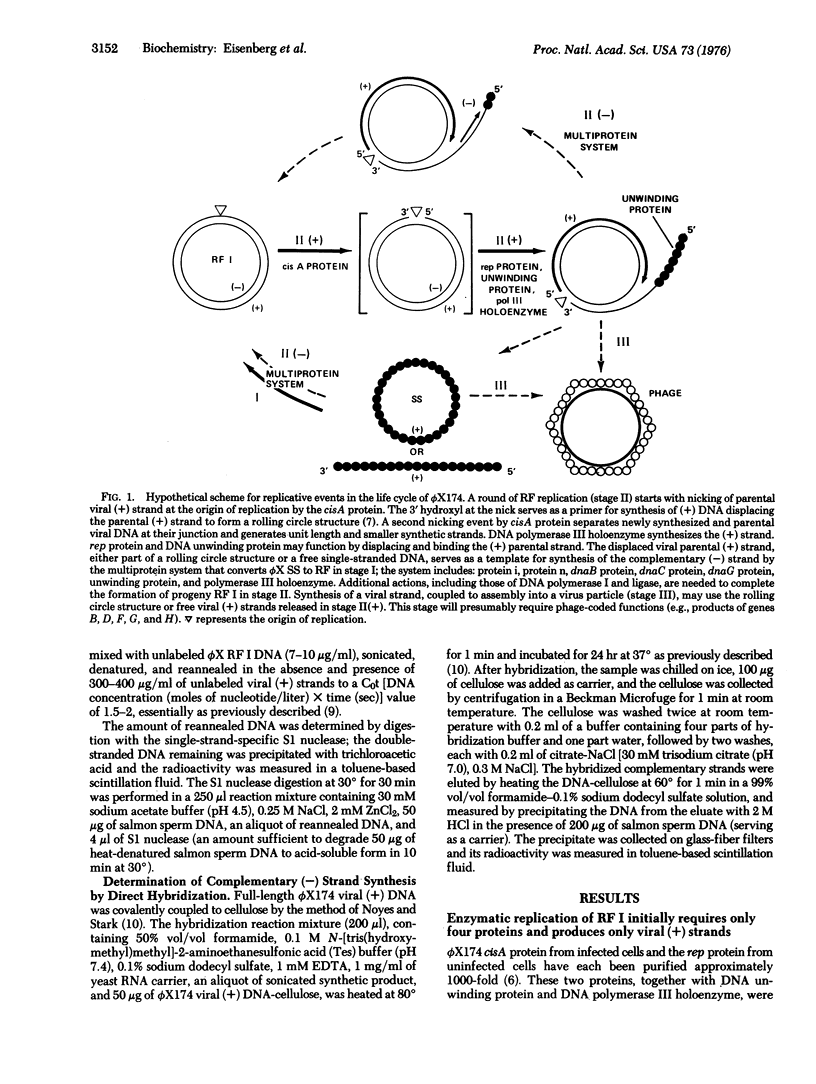
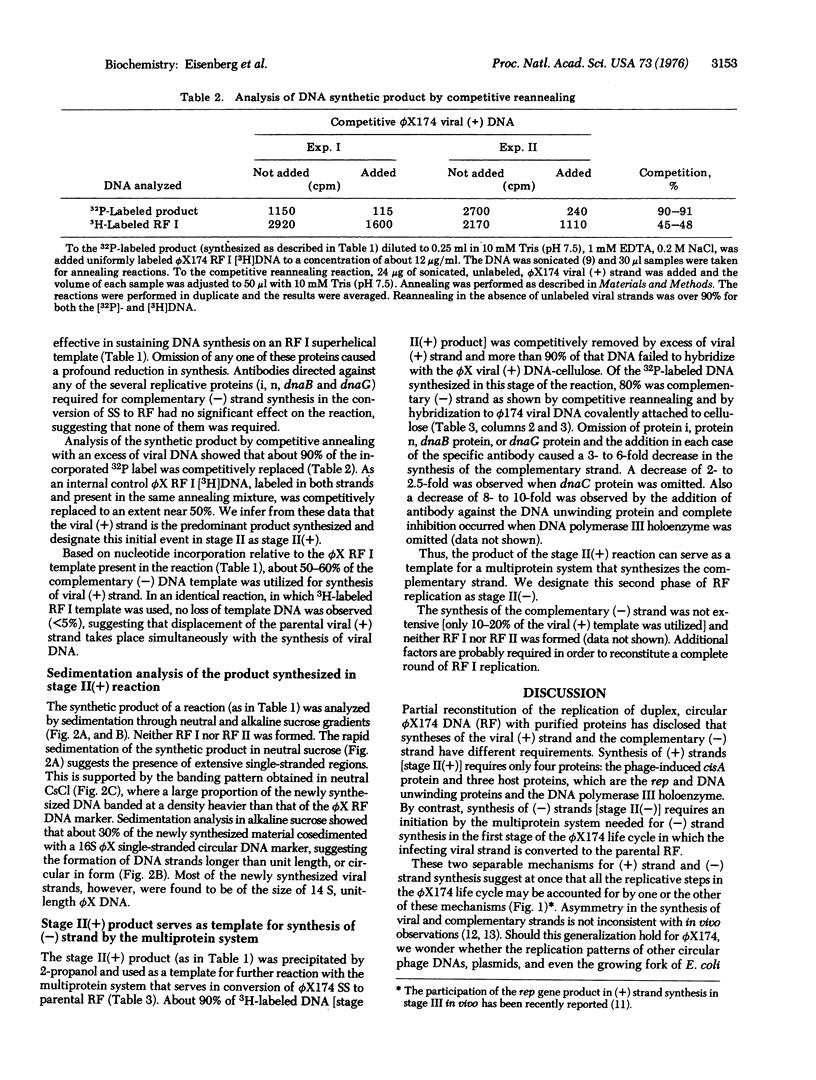
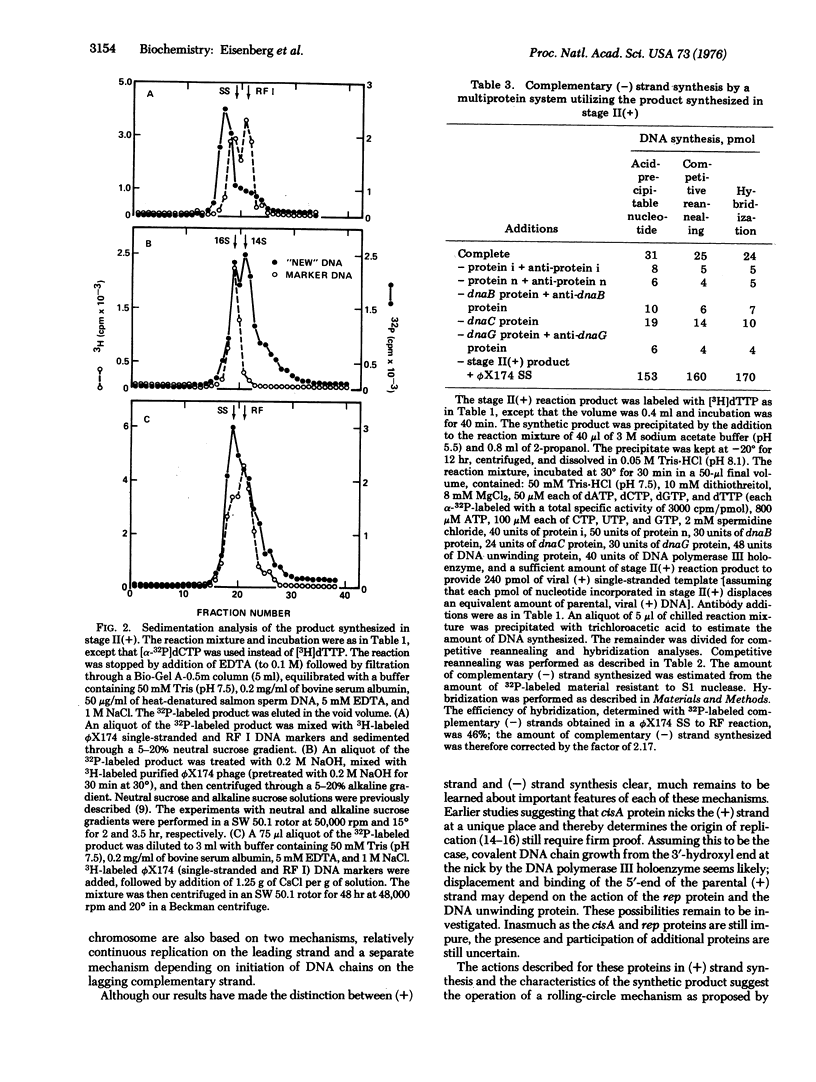
Multiplication of the duplex, circular, phage phiX174DNA (replicative form, RF) in stage II of the replicative life cycle has been observed with a crude enzyme preparation [Eisenberg et al. (1976) Proc, Natl. Acad, Sci. USA 73, 1594-1597]. This stage has now been partially reconstituted with purified proteins and subdivided into two stages: II(+) and II(-). In stage II(+), viral (+) strand synthesis is carried out by four proteins: the phage-induced, cistron A-dependent protein, rep-dependent protein, DNA unwinding protein, and DNA polymerase III holenzyme. In stage II(-), complementary (-) strand synthesis utilizes the product of stage II(+) as template and the multiprotein system previously identified in the stage I synthesis of a complementary strand on the viral DNA template to produce RF. The multiprotein system includes DNA unwinding protein, proteins i and n, dnaB protein, dnaC protein, dnaG protein, and DNA polymerase III holoenzyme. A discussion of these two separate mechanism for synthesis of (+) and (-) strands suggests that they may account for essentially all the replicative stages in the life cycle of phiX174.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas P. D., Jansz H. S. Bacteriophage phiX174 DNA synthesis in a replication-deficient host: determination of the origin of phiX DNA replication. J Mol Biol. 1976 Apr 15;102(3):633–656. doi: 10.1016/0022-2836(76)90339-9. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Iwaya M., Larison L. L. The rep mutation. II. Its effect on Escherichia coli and on the replication of bacteriophage phi X174. Virology. 1972 Aug;49(2):486–496. doi: 10.1016/0042-6822(72)90500-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Denhardt D. T. Structure of nascent phiX174 replicative form: evidence for discontinuous DNA replication. Proc Natl Acad Sci U S A. 1974 Mar;71(3):984–988. doi: 10.1073/pnas.71.3.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Harbers B., Hours C., Denhardt D. T. The mechanism of replication of phiX174 DNA. XII. Non-random location of gaps in nascent phiX174 RF II DNA. J Mol Biol. 1975 Nov 25;99(1):107–123. doi: 10.1016/s0022-2836(75)80162-8. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Scott J. F., Kornberg A. An enzyme system for replication of duplex circular DNA: the replicative form of phage phi X174. Proc Natl Acad Sci U S A. 1976 May;73(5):1594–1597. doi: 10.1073/pnas.73.5.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Formation of the parental replicative form DNA of bacteriophage phi-X174 and initial events in its replication. J Mol Biol. 1971 Nov 14;61(3):565–586. doi: 10.1016/0022-2836(71)90065-9. [DOI] [PubMed] [Google Scholar]
- Fukuda A., Sinsheimer R. L. Process of infection with bacteriophage phi X 174 XXXVIII. Replication of phi chi 174 replicative form in vivo. J Virol. 1976 Mar;17(3):776–787. doi: 10.1128/jvi.17.3.776-787.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Henry T. J., Knippers R. Isolation and function of the gene A initiator of bacteriophage phi-chi 174, a highly specific DNA endonuclease. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1549–1553. doi: 10.1073/pnas.71.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes B. E., Stark G. R. Nucleic acid hybridization using DNA covalently coupled to cellulose. Cell. 1975 Jul;5(3):301–310. doi: 10.1016/0092-8674(75)90105-1. [DOI] [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Sinsheimer R. L. Bacteriophage phi-X174 and related viruses. Prog Nucleic Acid Res Mol Biol. 1968;8:115–169. [PubMed] [Google Scholar]
- Tessman E. S. Mutants of bacteriophage S13 blocked in infectious DNA synthesis. J Mol Biol. 1966 May;17(1):218–236. doi: 10.1016/s0022-2836(66)80104-3. [DOI] [PubMed] [Google Scholar]
- Weiner J. H., McMacken R., Kornberg A. Isolation of an intermediate which precedes dnaG RNA polymerase participation in enzymatic replication of bacteriophage phi X174 DNA. Proc Natl Acad Sci U S A. 1976 Mar;73(3):752–756. doi: 10.1073/pnas.73.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Conversion of phiX174 viral DNA to double-stranded form by purified Escherichia coli proteins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4120–4124. doi: 10.1073/pnas.71.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]



