Abstract
Aims
To investigate the pharmacokinetics (PK) of sertraline in overdose and the effect of single dose activated charcoal (SDAC).
Methods
Patients presenting to a toxicology unit with sertraline overdoses had demographic and clinical information recorded, and serial serum collected for measurement of sertraline concentrations. Monolix® version 4.2 was used to develop a population PK model of sertraline overdose and the effect of SDAC. Uncertainty in dose time was accounted for by shifting dose time using lag time with between subject variability (BSV). BSV on relative fraction absorbed was used to model uncertainty in dose.
Results
There were 77 timed sertraline concentrations measured in 28 patients with sertraline overdoses with a median dose of 1550 mg (250–5000 mg). SDAC was given to seven patients between 1.5 and 4 h post-overdose. A one compartment model with lag time of 1 h and first order input and elimination adequately described the data. Including BSV on both lag time and relative fraction absorbed improved the model. The population PK parameter estimates for absorption rate constant, volume of distribution and clearance were 0.895 h−1, 5340 l and 130 l h−1, respectively. The calculated half-life of sertraline following overdose was 28 h (IQR 19.4−30.6h). When given up to 4 h post-overdose, SDAC significantly increased the clearance of sertraline by a factor of 1.9, decreased the area under the curve and decreased the maximum plasma concentration (Cmax).
Conclusions
Sertraline had linear kinetics in overdose with parameter values similar to those in therapeutic use. SDAC is effective in increasing clearance when given 1.5 to 4 h post-overdose.
Keywords: activated charcoal, overdose, pharmacokinetics, poisoning, poisoning, sertraline
What is Already Known about this Subject
There are no data available to describe the pharmacokinetics of sertraline in overdose.
Currently, single dose activated charcoal is recommended up to 1.h post-drug ingestion.
What this Study Adds
The pharmacokinetics of sertraline in overdose are similar to those in therapeutic use.
Activated charcoal given up to 4 h post-overdose of sertraline increases the clearance of sertraline and reduces the AUC.
Introduction
Antidepressant drugs are one of the major groups of drugs taken in overdose for deliberate self-poisoning. The types of antidepressants prescribed, and therefore available and taken in overdose, have changed over the last 20 years. The selective serotonin re-uptake inhibitors (SSRIs), citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine and sertraline, are now one of the most commonly prescribed groups of drugs for psychiatric disorders, particularly depression 1–3. They are also one of the most common groups of drugs seen in deliberate self-poisoning 4. The major toxic effect of these drugs is serotonin toxicity with approximately 15% of SSRI overdoses resulting in serotonin toxicity requiring hospital admission and medical treatment 5. Serotonin toxicity, often termed ‘serotonin syndrome’, is potentially life-threatening and presents as neuromuscular excitation (clonus, myoclonus, hyper-reflexia and/or tremor), autonomic hyperactivity (tachycardia, fever, diaphoresis and/or mydriasis) and altered mental status (confusion, agitation and/or excitement). Severe serotonin toxicity may require urgent medical intervention and intensive monitoring 5–7.
Despite the importance of antidepressant overdose there are limited studies investigating the pharmacokinetics (PK) and pharmacodynamics (PD) of antidepressant drugs in overdose. An understanding of the PK and PD of drugs in overdose is important to quantify and understand the likely effects of these drugs in overdose over time and guide risk assessment and treatment 8. There is no information on the PK of sertraline in overdose, despite being one of the most commonly taken SSRIs in overdose 5.
In recent years, there has been significant debate over the use of single dose activated charcoal (SDAC) in the management of overdose patients. Recommendations suggest that administration is ineffective in improving clinical outcomes, particularly if given more than 1 h post-overdose 9,10. However, recent PK and PD studies investigating decontamination procedures in antidepressant and antipsychotic overdose have found that the use of SDAC may have benefit when administered more than 1 h post-ingestion 11–14. In citalopram overdose the fraction absorbed was reduced by 22% and the clearance increased by 72% when SDAC was administered between 1 and 4 h post-ingestion 11. Similarly, in quetiapine overdose, the fraction of drug absorbed was reduced by 35% following the administration of SDAC more than 1 h following overdose 12. In the case of citalopram overdose, this translated into a meaningful clinical effect with the administration of SDAC up to 4 h post-overdose reducing the risk of QT prolongation 13. Similar beneficial effects have been shown in studies of escitalopram and venlafaxine 14–16.
There is limited information available on the PK of sertraline following therapeutic dose studies. Following oral administration the drug is slowly absorbed with the maximum concentration (Cmax) occurring 4 to 6 h after oral administration 17. It undergoes extensive first pass metabolism via a number of cytochrome P450 (CYP) isoenzymes (CYP2B6, CYP2C9, CYP2C19, CYP2D6 and CYP3A4), to produce a weakly active metabolite for serotonin re-uptake, N-desmethylsertraline 17–20. The half-life of the parent compound, sertraline, is approximately 26 h and it is approximately 98% protein bound 21,22. Sertraline appears to follow linear kinetics in therapeutic use, with the measured plasma sertraline concentration proportional to dose over the range of 50–200 mg day−1 23.
This study aims to describe the pharmacokinetics of sertraline in overdose using a population analysis approach to quantify the relationship between dose and the time course of drug concentrations, and to investigate the effect of SDAC administration on the PK of sertraline overdose.
Methods
Study design and setting
This was a population pharmacokinetic study of sertraline using drug concentration data collected prospectively from patients who had taken an overdose of sertraline from February 2001 to February 2010. Written informed consent was obtained from patients for collection of blood samples for analysis of sertraline. The study was approved by the Hunter Area Research Ethics Committee and the University of Newcastle Human Research Ethics Committee. The study was conducted in a regional toxicology unit which accepts referrals and admissions for all poisoning and overdose patients from a population of over half a million people.
Patients
Demographic and clinical information is collected for all patients admitted to the toxicology unit during their admissions and is maintained in a clinical database. Details include age, sex and information relating to the drug overdose (timing of the overdose event, quantity [strength and amount] of medication), co-ingestants, clinical effects and any treatments administered (single dose activated charcoal [SDAC]). Treatment is determined by the admitting clinical toxicologist. A proprietary SDAC product was used which was formulated as a suspension of 50 g activated charcoal in 250 ml of purified water, with sucrose, propylene glycol, glycerol, citric acid and sodium hydroxide as excipients (Carbosorb®, Phebra Pty Ltd, NSW, Australia). Blood samples were collected from patients on admission and during their hospital stay. The number of samples collected was dependent upon the duration of the hospital stay and the willingness of the patient to provide blood samples. The exact times were recorded.
Dosing history was obtained from the patient on admission and then on subsequent occasions when reviewed by the attending toxicologist. This was also corroborated where possible by friends/relatives, the ambulance officer record and by tablet counts when available. In this study drug overdose was defined as any dose greater than the maximum daily dose where the intention was self-harm. Uncertainty in the reported dose was assessed based on the reliability of the history using a previously described veracity score 11. The veracity was scored using a five point categorical scale ranging from an accurate history (score = 0; exact dosing) to a very poor history (score = 4; large error in reported dose allowed). There were no patients with an accurate history in this study, so a score of 1 was taken as if it were an exact dosing history. Uncertainty in the overdose time (termed mod-time) was also included in the analysis and was based on allowing a range in the time of ingestion using the earliest possible ingestion time (last contact with family or friends) and the latest possible ingestion time (e.g. ambulance call time). The range in mod-time in this study was 5 min to 2 h.
Drug analysis
Samples collected were centrifuged and the serum taken from the tube was frozen at −20°C prior to analysis. Measurement of sertraline concentration was performed using high performance liquid chromatography (HPLC) using the method described by Kristensen et al. with the limit of detection of sertraline of 0.005 mg l−1 24. The measured concentrations of sertraline in our study were between 0.016 and 0.896 mg l−1.
Pharmacokinetic analysis
Population pharmacokinetic analysis was performed using the non-linear mixed effect modelling software Monolix® version 4.2 (Lixoft, Orsay, France, http://www.lixoft.com) which utilizes the Stochastic Approximation Expectation Maximization algorithm (SAEM) and a Markov chain Monte-Carlo (MCMC) procedure to compute the maximum likelihood estimates of the population means and between subject variances for the PK parameters 25. The number of chains was fixed to 15 for all computations with convergence demonstrated.
One and two compartment models, with zero and first order oral absorption kinetics and models with and without Michaelis−Menten elimination were assessed and compared to determine the best structural model. For the residual unexplained variability, additive and proportional models and a combination of the two were evaluated. Between subject variability (BSV) included in the model was assumed to have log-normal distribution. Initial parameters estimated using the model were the absorption rate coefficient (Ka), bioavailability (F), shifted lag time (ts,lag), volume of distribution (V) and clearance (CL). Shifted lag time with BSV was used to shift the dose time to allow for uncertainty in dose time both before and after the reported dose time, rather than estimating a true lag time.
Initial estimates of parameters were taken from previous pharmacokinetic studies of sertraline 17,22,26. An initial value of Ka of 0.5 h−1 was based on multiple dose administration of the therapeutic use of sertraline from the published literature 22. Similarly, the initial estimates used in the model for CL (100 l h−1) and V (3500 L) were based on a 70 kg adult 22.
Uncertainty in overdose history
We attempted to include both uncertainty in the reported dose (veracity score) and the reported time of ingestion (mod-time), similar to previous analyses 11,12,27,28. However, previously veracity was included in the model by allowing the uncertainty in dose (Δdose) to be drawn from a normal distribution with a mean of zero and precision that incorporated an additive and proportional component based on veracity 11. This was not possible in Monolix and uncertainty in dose amount was instead modelled by allowing between subject variability (BSV) in the relative bioavailability (F) which could then be modified by the veracity score. Firstly the relative bioavailability was fixed to 1 and the BSV estimated for each patient to account for uncertainty in dose. The BSV on F for each veracity score was then plotted to determine if veracity provided any additional information (i.e. the BSV was smaller for smaller veracity scores). The BSV for patients with the lowest veracity score (1 in this study) was then set to zero. The BSV was then constrained less for increasing veracity scores to account for increasing uncertainty. Models were compared with and without the veracity score.
Similarly, the uncertainty in time of dose time (mod-time) could not be incorporated in the model using a distribution form as previously reported 12 because it caused instability during modelling. Uncertainty in the dose time was therefore incorporated by using a shifted lag time parameter (ts,lag) in the model and using BSV on this ts,lag parameter to represent uncertainty in dose time. To do this all dose times and times of drug concentration measurements were advanced by 1 h to allow a ‘negative’ time effect (i.e. drug taken prior to the reported ingestion time) because the traditional lag time parameter can only be positive. It should be noted that the ts,lag parameter was not used as the traditional lag time parameter (i.e. to represent a lag in gastrointestinal absorption) but as uncertainty in timing of the overdose.
Effect of covariates
The effect of covariates, including age and sex, were explored by visual inspection of the individual parameter estimates vs. the covariate of interest. Age and sex were not included in final model evaluation due to the absence of an association visually. Weight was not considered because it was not available for the majority of patients. Weighing overdose patients during a hospital admission is not performed routinely and therefore not possible to include in the model.
The administration of SDAC was included after considering uncertainty in dose and dose time. The effect of SDAC was evaluated as a fractional effect on relative bioavailability (fF-char) and a fractional effect on CL (fCL-char). This factor was set to 1 for patients who were not administered charcoal i.e. no effect on bioavailability or clearance.
Thus in the model the estimation of the effect of charcoal on clearance is:
where θCL, is the typical value of clearance. Similar reasoning was used to estimate the effect of charcoal on the relative fraction absorbed (F).
Final model selection and evaluation was based on a number of criteria including goodness of fit plots which included observations vs. predictions, plots of the weighted residuals and the visual predictive check (VPC). The log likelihood was computed using importance sampling and was used to discriminate between models through the difference in log likelihood (−2 LL). A P value of 0.05 was considered statistically significant. For the inclusion of covariates in the model a change of 20% in the parameter (F or CL) was regarded as clinically significant change, as per previous studies 11. We also examined the between subject variability estimates, deviance distribution errors and the reduction in the log likelihood ratio test.
Derived pharmacokinetic parameters, including half-life (t1/2), maximum serum concentration (Cmax), time to maximum serum concentration (tmax) and area under the curve (AUC), were calculated for each patient from their individual predicted patient parameters in the final model. The derived parameters were then presented as a median and range for the whole group. A typical concentration time plot for the median dose taken in the study was created by simulating 1000 patients and plotting the median concentration vs. time.
Results
Patient data
There were 28 patients recruited during the study period, 21 were female and the median age was 32 years (15−55 years). The median reported dose was 1550 mg (250−5000 mg). Patient demographic information is provided in Table 1. Twenty-one patients co-ingested other drugs and/or alcohol as part of their overdose. None of the co-ingested drugs was known to either inhibit or induce the metabolism of sertraline, so co-ingested medications were not considered further. Seven of the 28 patients (25%) developed serotonin toxicity. Four patients (14%) had a Glasgow coma score (GCS) <15, but only one had a GCS <10. One patient was admitted to intensive care and required intubation and ventilation for a sedative co-ingested drug. There were no deaths or other major complications including seizures, arrhythmias and hypotension.
Table 1.
Patient demographic and overdose information
| Median (range) | Number of patients (%) | |
|---|---|---|
| Age (years) | 32 (15–55) | 28 (100) |
| Male | 7 (25) | |
| Prescribed therapeutic dose (mg) for patients on sertraline prior to the overdose event | 100 (25–200) | 24 (86) |
| Overdose reported (mg) | 1550 (250–5000) | 28 (100) |
| Co-ingested medications/substances: | ||
| Alcohol | 10 (36) | |
| Analgesics (paracetamol, codeine, ibuprofen) | 6 (21) | |
| Anticoagulants (warfarin) | 1 (4) | |
| Antiepileptics (sodium valproate) | 1 (4) | |
| Antihistamines (dexchlorpheniramine, doxylamine) | 3 (11) | |
| Antihypertensives (atenolol, candesartan) | 2 (7) | |
| Antipsychotics (olanzapine, quetiapine, risperidone) | 3 (11) | |
| Benzodiazepines | 4 (14) | |
| Decongestants (phenylephrine, pseudoephedrine) | 2 (7) | |
| Herbal preparations (valerian) | 1 (4) | |
| Unknown substances | 1 (4) | |
| Nil | 9 (32) | |
| Measured sertraline concentration range (mg l−1) | 0.016–0.896 | |
| Veracity score | ||
| 1 | 14 (50) | |
| 2 | 11 (39) | |
| 3 | 3 (11) | |
| 4 | 0 (0) | |
| Single dose activated charcoal (SDAC) | 7 (25)* | |
| Median overdose in patients treated with SDAC (mg) | 1900(250–2800) | |
| Median overdose in patients not treated with SDAC (mg) | 1500 (300–5000) |
One patient received a partial dose of SDAC of approximately 12.5 g.
Seven patients were administered SDAC, although one of these received approximately a quarter of the dose only. The administration of charcoal for these seven patients was timed between 1.5 and 4 h (median time 3 h) after the reported time of overdose. The median overdose of sertraline ingested by these seven patients was 1900 mg (250–2800 mg).
There were a total of 77 blood samples collected from the 28 patients with a median of two samples per patient (range 1−6). The times of blood sampling for analysis of sertraline concentration ranged from 1.17−68 h after the reported time of the drug ingestion. Each patient presented following an overdose on one occasion only.
Pharmacokinetic analysis
The dose normalized plasma concentration−time profiles for the 28 patients are shown in Figure 1 one compartment model with first order absorption and linear elimination kinetics adequately described the data. A proportional error model provided the best error model, as a combined additive and proportional error model produced negligible additive error or improvement.
Figure 1.
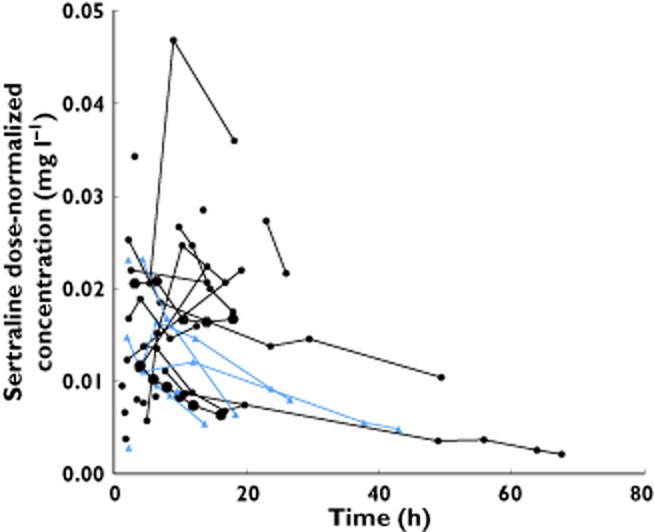
Observed dose-normalized sertraline concentration (to the median therapeutic dose of 100 mg) vs. time post-overdose.  , Patient administered activated charcoal;
, Patient administered activated charcoal;  , Patient not administered activated charcoal
, Patient not administered activated charcoal
The inclusion of BSV on F improved the model. Plots of the BSV on F vs. the veracity score showed no relationship between veracity and BSV on F (Figure S1) and attempts to incorporate veracity did not improve the model. The inclusion of ts,lag improved the model and ts,lag was initially estimated to be close to 1 (i.e. confirming that the actual dosing time was shifted by 1 h), ts,lag was then fixed to 1 in subsequent models and BSV on ts,lag estimated. The effect of SDAC on CL and F was considered separately. Administration of SDAC was estimated to increase the CL by a factor of 1.9 (P < 0.05). In contrast, the administration of SDAC decreased F by 27% (P > 0.05) which was not statistically significance.
The final model was a one compartment first order input model with ts,lag and proportional residual error model. The final model incorporated BSV on the F, which was fixed to 1 and BSV on ts,lag, which was also fixed to 1, to allow variability between patients in dose and dose time, respectively. Finally the model incorporated SDAC as a covariate with a fractional effect on relative clearance (fCL-char). Figure 2 shows the goodness-of-fit plots for the final model. The typical mean population PK parameter estimates from the base and final models with modelling decisions and final model parameters are described in Table 2. The derived pharmacokinetic parameters for sertraline are included in Table 3. The median half-life for patients given SDAC was 15 h (IQR 10.7–22.2 h) compared with 29.4 h (IQR 26.4–30.8 h) in those not given SDAC. The median AUC for patients given SDAC was 5.63 mg l−1 h (IQR 3.86−8.82 mg l−1 h) compared with 15.13 mg l−1 h (IQR 7.63–20.84 mg l−1 h) in those not given SDAC. Plots showing the effects of SDAC on the PK parameters from the final model are shown in Figure 3.
Figure 2.
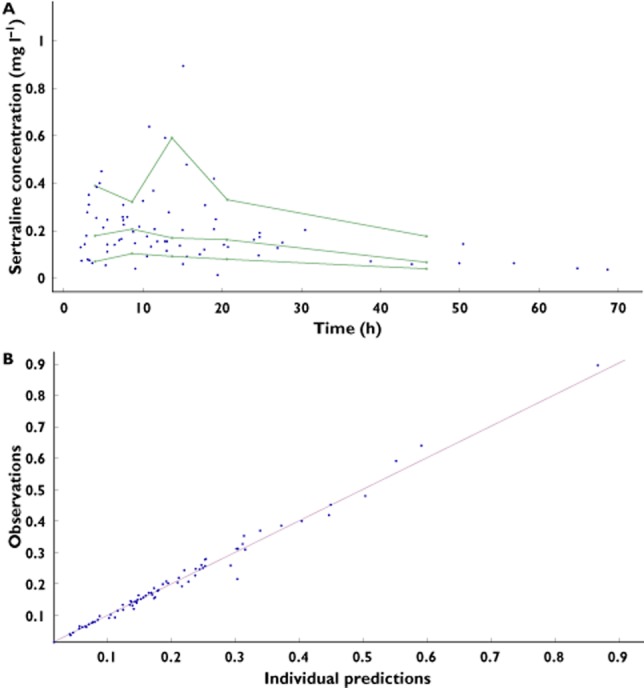
Goodness-of-fit plots including (A) a visual predictive check (VPC) showing the observed data with the 10th, 50th and 90th percentiles and (B) a plot of observations vs. individual predictions from the final model
Table 2.
Parameter estimates using Monolix® version 4.2 and the effect of single dose activated charcoal on bioavailability and clearance
| Base model | Model 1 – SDAC fF-char | Model 2 – Final SDAC fCL-char | |
|---|---|---|---|
| Mean value (RSE%) | Mean value (RSE%) | Mean value (RSE%) | |
| Structural model (θ) | |||
| ts,lag (h) | 1 (fixed) | 1 (fixed) | 1 (fixed) |
| Ka (h−1) | 1.06 (83) | 1.02 (95) | 0.895 (42) |
| F | 1 (fixed) | – | 1 (fixed) |
| V (l) | 5130 (15) | 4730 (14) | 5340 (18) |
| CL (l h−1) | 151 (20) | 144 (19) | – |
| fF-char | – | 0.731 (178) | – |
| θF | – | 1 (fixed) | – |
| fCL-char | – | – | 1.92 (70) |
| θCL | – | – | 130 (34) |
| Between subject variance (ω) | |||
| ts,lag | 0.849 (45) | 0.794 (51) | 0.922 (52) |
| Ka | 1.51 (139) | 1.63 (104) | 1.02 (77) |
| F | 0.321 (40) | 0.275 (43) | 0.303 (48) |
| V | 0.087 (95) | 0.146 (80) | 0.085 (219) |
| CL | 0.18 (77) | 0.109 (134) | 0.126 (103) |
| Coefficient of variation for proportional residual error (CV%) | 11.8 | 11.9 | 11.7 |
| Objective function (−2 log-likelihood value) | −207.10 | −208.09 | −212.04 |
ts,lag = shifted dose time (estimated time plus 1 h), Ka = absorption rate constant, F = relative bioavailability, V = volume of distribution, CL = clearance, fF-char = fractional effect of SDAC on bioavailability, fCL-char = fractional effect of SDAC on clearance. CL = θCL × fCL-char. F = θF × fF-char.
Table 3.
Pharmacokinetic data for sertraline following overdose in 28 patients and the effect of SDAC, based on the median overdose of 1500 mg
| Total (n = 28) | No SDAC (n = 21) | SDAC (n = 7) | |
|---|---|---|---|
| median [range] | median [range] | median [range] | |
| t1/2 (h) | 27.6 [19.3–30.6] | 29.4 [26.4–30.8] | 15 [10.7–22.2] |
| Cmax (mg l−1) | 0.25 [0.18–0.42] | 0.33 [0.18–0.43] | 0.22 [0.18–0.42] |
| tmax (h) | 2.8 [2.1–3.6] | 2.9 [2.3–3.6] | 2.5 [1.9–3.6] |
| AUC (mg l−1 h) | 8.96 [6.45–19.08] | 15.13 [7.63–20.84] | 5.63 [3.86–8.82] |
Figure 3.
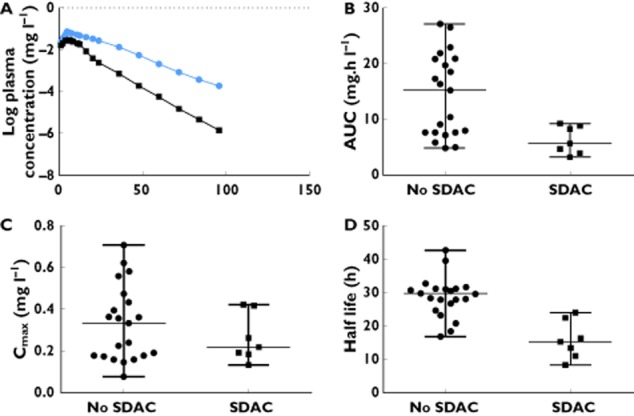
Plots showing the effect of single dose activated charcoal decontamination, on (A) log median values of the plasma concentration–time course for an overdose of 1500 mg, (B) the area under the curve (AUC) (showing median and range), (C) the maximum plasma concentration (Cmax) (showing median and range) and (D) half- life (showing median and range) following sertraline overdose.  , No SDAC;
, No SDAC;  , SDAC
, SDAC
Discussion
The study has defined the pharmacokinetics of sertraline in overdose and the effect of SDAC. The PK profile of sertraline in overdose was best described by a one compartment model with linear elimination using Monolix®, and was similar to previous pharmacokinetic studies of sertraline in therapeutic doses. Decontamination with SDAC had a beneficial effect by increasing the clearance of sertraline by a factor of 1.9 which reduced the AUC and the median value of Cmax (Figure 2). This suggests there may be benefit in administering SDAC but further study is required to determine if this effect on the pharmacokinetics translates to clinical benefit.
Following oral administration, sertraline reaches a peak plasma concentration 4−6 h after administration 17. The drug undergoes extensive first pass metabolism via several metabolic pathways 17–19. Previous studies of the pharmacokinetics of drugs in overdose have found that SDAC has a variable effect on CL and F. SDAC is able to bind unabsorbed drug and thus can affect relative bioavailability. In addition it is possible that activated charcoal is able to remove some drugs from the circulation via gastric dialysis. In citalopram overdose SDAC increased CL and decreased F 11. In contrast, in quetiapine overdose SDAC was observed to decrease F but had no significant effect on CL 12. The reasons for these differences are not easily explained. It has been previously suggested that SDAC is more likely to have an effect on CL for drugs with longer half-lives, such as citalopram 11 compared with drugs with shorter half-lives such as quetiapine 12. We found a significant effect of SDAC on CL of sertraline in overdose, and no effect on relative bioavailability (F), consistent with the fact that sertraline has a long half-life. It remains probable that SDAC affects both F and CL in most cases but that the influence varies based on the PK properties of the drug in question and the study designs and sparseness of the data may in many cases be insufficient to disentangle these effects.
SDAC was administered between 1 and 4 h post-overdose, which is similar to other pharmacokinetic studies of drugs in overdose where SDAC is rarely given within 1 h, and usually between 2 and 4 h post-overdose 11,14,15. Previous studies found that the incidence of toxic effects, such as seizures following venlafaxine overdose 29 or prolongation of the QT interval with citalopram or escitalopram 13,16, can be reduced if charcoal is administered more than 1 h following the overdose. Similarly, in quetiapine overdose there is a potential for benefit when SDAC is given up to 6 h post-overdose 12. Although current treatment guidelines only recommend the use of SDAC within 1 h of overdose 9,10, our findings add further support for the administration of SDAC beyond this time. However, it will be important to demonstrate that the use of SDAC also results in a significant effect on the pharmacodynamics of sertraline, such as a reduction in serotonin toxicity.
Published values for the PK estimates for sertraline are available in only limited single dose or short term therapeutic studies. Our estimated V of approximately 76 l kg−1 is similar to values reported in the literature 17, and our estimate for CL of 1.9 l kg−1 h−1 is not dissimilar to that seen in young males (1.41 l h−1 kg−1 [± 0.36]) 22. As sertraline is highly protein bound and V is relatively large, indicating much of the drug is bound to tissue outside of the circulation, it is expected that haemodialysis or haemoperfusion is unlikely to remove significant amounts of drug. However, sertraline is relatively safe in overdose with no significant ECG changes and no deaths have been reported from ingestion of sertraline as a single agent. Serotonin toxicity may require treatment depending on symptom severity. The use of SDAC may assist in increasing the clearance of sertraline and potentially limiting adverse effects of serotonin excess.
Competing Interests
There are no competing interests to declare.
Funding
GKI is supported by an NHMRC Senior Research Fellowship ID1061041.
We thank Peter Hackett for the analysis of serum sertraline concentrations, Joel Iedema for assisting with Monolix coding and Renai Kearney for assisting with medical record retrieval. We thank the medical and nursing staff of the emergency department for assisting in recruiting patients and collection blood samples.
Supporting Information
Figure S1
Plot of between subject variability (BSV) on relative fraction absorbed (f) as a standard deviation (D) vs. the veracity score
References
- Australian Government, Department of Health and Ageing. Australian Statistics on Medicines 2009. Canberra: Commonwealth of Australia; 2011. [Google Scholar]
- National Center for Health Statistics. Health United States 2012: With Special Feature on Emergency Care. Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- Prescriptions Dispensed in the Community, England – 2002–2012. Prescribing and Primary Care Services, Health and Social Care Information Centre. July 2013. Available at http://www.hscic.gov.uk (last accessed 1 February 2014)
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635–642. doi: 10.1093/qjmed/hcg109. [DOI] [PubMed] [Google Scholar]
- Isbister GK, Bowe SJ, Dawson A, Whyte IM. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol. 2004;42:277–285. doi: 10.1081/clt-120037428. [DOI] [PubMed] [Google Scholar]
- Buckley NA, Dawson AH, Isbister GK. Serotonin syndrome. BMJ. 2014;348:g1626. doi: 10.1136/bmj.g1626. [DOI] [PubMed] [Google Scholar]
- Isbister GK, Buckley NA, Whyte IM. Serotonin toxicity: a practical approach to diagnosis and treatment. Med J Aust. 2007;187:361–365. doi: 10.5694/j.1326-5377.2007.tb01282.x. [DOI] [PubMed] [Google Scholar]
- Isbister GK. Pharmacokinetic-pharmacodynamic modeling in overdose patients – is it worth the trouble? Clin Toxicol (Phila) 2010;48:896–897. doi: 10.3109/15563650.2010.533680. [DOI] [PubMed] [Google Scholar]
- Cooper GM, Le Couteur DG, Richardson D, Buckley NA. A randomized clinical trial of activated charcoal for the routine management of oral drug overdose. QJM. 2005;98:655–660. doi: 10.1093/qjmed/hci102. [DOI] [PubMed] [Google Scholar]
- Chyka PA, Seger D, Krenzelok EP, Vale JA. Position paper: single-dose activated charcoal. Clin Toxicol (Phila) 2005;43:61–87. doi: 10.1081/clt-200051867. [DOI] [PubMed] [Google Scholar]
- Friberg LE, Isbister GK, Hackett LP, Duffull SB. The population pharmacokinetics of citalopram after deliberate self-poisoning: a Bayesian approach. J Pharmacokinet Pharmacodyn. 2005;32:571–605. doi: 10.1007/s10928-005-0022-6. [DOI] [PubMed] [Google Scholar]
- Isbister GK, Friberg LE, Hackett LP, Duffull SB. Pharmacokinetics of quetiapine in overdose and the effect of activated charcoal. Clin Pharmacol Ther. 2007;81:821–827. doi: 10.1038/sj.clpt.6100193. [DOI] [PubMed] [Google Scholar]
- Friberg LE, Isbister GK, Duffull SB. Pharmacokinetic-pharmacodynamic modelling of QT interval prolongation following citalopram overdoses. Br J Clin Pharmacol. 2006;61:177–190. doi: 10.1111/j.1365-2125.2005.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar VV, Oscarsson S, Friberg LE, Isbister GK, Hackett LP, Duffull SB. The effect of decontamination procedures on the pharmacokinetics of venlafaxine in overdose. Clin Pharmacol Ther. 2009;86:403–410. doi: 10.1038/clpt.2009.114. [DOI] [PubMed] [Google Scholar]
- van Gorp F, Duffull S, Hackett LP, Isbister GK. Population pharmacokinetics and pharmacodynamics of escitalopram in overdose and the effect of activated charcoal. Br J Clin Pharmacol. 2012;73:402–410. doi: 10.1111/j.1365-2125.2011.04091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorp F, Whyte IM, Isbister GK. Clinical and ECG effects of escitalopram overdose. Ann Emerg Med. 2009;54:404–408. doi: 10.1016/j.annemergmed.2009.04.016. [DOI] [PubMed] [Google Scholar]
- DeVane CL, Liston HL, Markowitz JS. Clinical pharmacokinetics of sertraline. Clin Pharmacokinet. 2002;41:1247–1266. doi: 10.2165/00003088-200241150-00002. [DOI] [PubMed] [Google Scholar]
- Hiemke C, Hartter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85:11–28. doi: 10.1016/s0163-7258(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Ishizuka T, Shimada N, Yoshimura Y, Kamijima K, Chiba K. Sertraline N-demethylation is catalyzed by multiple isoforms of human cytochrome P-450 in vitro. Drug Metab Dispos. 1999;27:763–766. [PubMed] [Google Scholar]
- Obach RS, Cox LM, Tremaine LM. Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study. Drug Metab Dispos. 2005;33:262–270. doi: 10.1124/dmd.104.002428. [DOI] [PubMed] [Google Scholar]
- Demolis JL, Angebaud P, Grange JD, Coates P, Funck-Brentano C, Jaillon P. Influence of liver cirrhosis on sertraline pharmacokinetics. Br J Clin Pharmacol. 1996;42:394–397. doi: 10.1046/j.1365-2125.1996.42817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronfeld RA, Tremaine LM, Wilner KD. Pharmacokinetics of sertraline and its N-demethyl metabolite in elderly and young male and female volunteers. Clin Pharmacokinet. 1997;32(Suppl. 1):22–30. doi: 10.2165/00003088-199700321-00004. [DOI] [PubMed] [Google Scholar]
- Warrington SJ. Clinical implications of the pharmacology of sertraline. Int Clin Psychopharmacol. 1991;6(Suppl. 2):11–21. doi: 10.1097/00004850-199112002-00004. [DOI] [PubMed] [Google Scholar]
- Kristensen JH, Ilett KF, Dusci LJ, Hackett LP, Yapp P, Wojnar-Horton RE, Roberts MJ, Paech M. Distribution and excretion of sertraline and N-desmethylsertraline in human milk. Br J Clin Pharmacol. 1998;45:453–457. doi: 10.1046/j.1365-2125.1998.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer RJ, Guzy S, Ng C. A survey of population analysis methods and software for complex pharmacokinetic and pharmacodynamic models with examples. AAPS J. 2007;9:E60–83. doi: 10.1208/aapsj0901007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronfeld RA, Wilner KD, Baris BA. Sertraline. Chronopharmacokinetics and the effect of coadministration with food. Clin Pharmacokinet. 1997;32(Suppl. 1):50–55. doi: 10.2165/00003088-199700321-00008. [DOI] [PubMed] [Google Scholar]
- Kumar VV, Duffull SB. Methods to account for inaccuracies in the dosing history when performing population pharmacokinetic analysis. Pharm Res. 2008;25:2740–2749. doi: 10.1007/s11095-008-9638-8. [DOI] [PubMed] [Google Scholar]
- Isbister GK. How do we use drug concentration data to improve the treatment of overdose patients? Ther Drug Monit. 2010;32:300–304. doi: 10.1097/FTD.0b013e3181dca280. [DOI] [PubMed] [Google Scholar]
- Kumar VV, Isbister GK, Duffull SB. The effect of decontamination procedures on the pharmacodynamics of venlafaxine in overdose. Br J Clin Pharmacol. 2011;72:125–132. doi: 10.1111/j.1365-2125.2011.03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Plot of between subject variability (BSV) on relative fraction absorbed (f) as a standard deviation (D) vs. the veracity score


