Abstract
Aims
In order to exert its pharmacodynamic effect, the diabetes drug metformin needs to be taken up into hepatocytes by the organic cation transporter (OCT) system. A recent in vitro study found that proton pump inhibitors (PPIs) inhibit OCT1, OCT2 and OCT3, suggesting that PPIs might reduce metformin's effectiveness. This pharmacoepidemiologic study looked for evidence of a clinical effect of such an interaction.
Methods
This was an observational cohort study examining changes in glycosylated haemoglobin (HbA1c) with exposure to metformin and to PPIs as single agents and in combination. The aim was to assess evidence of a deleterious drug−drug interaction.
Results
PPIs did not reduce the effectiveness of metformin, and indeed were associated with a minimally better glycaemic response by − 0.06 HbA1c percentage points (95% confidence interval, −0.10, −0.01) in metformin initiators.
Conclusions
Despite a mechanistic basis for a potential drug–drug interaction, we found no evidence of a deleterious interaction between PPIs and metformin.
Keywords: diabetes, drug–drug interaction, glucose, HbA1c, metformin, proton pump inhibitor
What is Already Known About This Subject
A potentially important deleterious pharmacologic interaction between metformin and proton pump inhibitors has been identified, but it is unknown whether this interaction is clinically significant.
What This Study Adds
This study found no evidence of a clinically significant interaction. Proton pump inhibitors do not appear to blunt the effectiveness of metformin to any clinically significant degree.
This finding relieves clinicians of the need to avoid proton pump inhibitor use in patients with diabetes because of fears of a potential interaction with metformin.
Introduction
A critical step in the pharmacokinetics of metformin is its uptake into target tissues by the organic cation transporter (OCT) system. A recent in vitro study found that proton pump inhibitors (PPIs) at therapeutic concentrations can inhibit uptake of metformin into cells via the OCT1, OCT2 and OCT3 transporters 1. Since such inhibition could prevent metformin from reaching key target cells including hepatocytes, those authors hypothesized that PPIs may impair the glucose-lowering effect of metformin.
Reviews of the potential for PPIs to interact with other drugs have outlined many potential mechanisms. The possibilities include alteration of drug absorption through changes in gastric pH and alteration of hepatic drug metabolism through CYP2C19 and other enzymes 2. Interactions with other CYP isoforms have been documented, and vary across different PPIs, making it possible that drug–drug interactions (DDIs) may be specific to different PPIs 3.
Metformin is not metabolized but rather excreted unchanged in the urine. While this eliminates the potential for DDIs involving hepatic metabolism as a mechanism, the discovery that PPIs interact with the OCT transporter system 1 raises concerns about DDIs through several other plausible mechanisms, as this system appears to be involved in intestinal absorption, hepatic uptake and renal excretion of metformin 4. The best-characterized of these effects is the OCT-1 transporter's role in hepatic uptake of metformin. Impairment of its activity is associated with reduced distribution of metformin to the liver in both human and animal models 5,6. The liver is believed to be metformin's principal site of action, and both knockout of OCT1 in mice and reduced-function genetic OCT1 variants in human volunteers are associated with significantly reduced effects of metformin on blood glucose 7.
Two recent short term randomized crossover studies in healthy subjects found that co-administration of metformin with PPIs did not appear to alter metformin's effect on glucose homeostasis, but did increase the area under the curve (AUC) of metformin's plasma concentration by approximately 15% 8,9. The authors hypothesized that the modest increase in metformin plasma concentration might actually be due to inhibition of OCT transporters, which could reduce uptake into the liver and leave more drug in the plasma. While this study provided initial evidence that OCT transporter interaction with PPIs might not render metformin ineffective, the authors pointed out that further study was needed because these short term results in healthy volunteers did not necessarily apply to patients with diabetes mellitus.
Further complicating the picture, PPIs have been proposed to have intrinsic glucose lowering properties of their own 10. This hypothesis was based on a small, cross-sectional observational study of patients with diabetes, in which patients who were taking a PPI had lower glycosylated haemoglobin (HbA1c) than those not taking one. In this instance, the epidemiological finding preceded any mechanistic investigation, but the authors proposed that PPIs may have insulin sensitizing properties.
PPIs are among the most commonly used drugs and are taken by many patients with diabetes 11,12. Metformin is the first line drug for type 2 diabetes and is one of the most widely prescribed drugs in the world 13. If PPIs were to blunt the effectiveness of metformin, it could have a considerable impact on the care of diabetes worldwide. If PPIs actually were to have direct glucose lowering effects, that could also have clinical relevance, although with opposite implications. In this study, we aimed to conduct a pharmacoepidemiologic study of whether there is any evidence that an interaction of PPIs with metformin affects the most clinically relevant outcome, long-term glycaemic control, in patients with type 2 diabetes.
Methods
Study design
We conducted a retrospective cohort study to test the primary hypothesis that there is an interaction between PPI exposure and metformin effectiveness as measured by HbA1c. A secondary goal was to assess whether PPIs had any direct effect on HbA1c. This was tested first by assessing whether there was any change in HbA1c when PPIs were initiated in patients who were either not on antidiabetic drug therapy or who were on metformin monotherapy. We then examined whether metformin was less effective when initiated in patients receiving PPI therapy than when given to patients not receiving a PPI. Effectiveness was defined as the absolute reduction in HbA1c from baseline to the average of measurements taken 3–9 months later. HbA1c was chosen as the outcome because it is the most regularly monitored marker of glycaemic control and is the standard measure for effectiveness of diabetes drugs 14,15. Random blood glucoses were also used as an outcome, but only in post hoc exploratory analyses.
Study population
This study used the Health Improvement Network (THIN) database, a primary care electronic medical record database in the UK. THIN contains over 9 million individuals acceptable for research who have contributed person-time from over 500 different general practices from 1986 to 2012. The database includes demographic information on patients, as well as records of prescribed drugs, medical diagnoses, as well as vital signs and laboratory values on a subset of patients. THIN is a representative subset of the UK's general population 16. The cohort for this study was restricted to 2003 and later because HbA1c was more regularly reported after that year 14.
Cohort definition
Cohort entry for the primary analysis occurred when patients met criteria for one of the following exposure categories: 1a) initiation of metformin monotherapy without current or past use of PPIs, 1b) initiation of metformin monotherapy after at least 180 days of PPI therapy, 2a) initiation of PPI therapy without current or past use of metformin or 2b) initiation of PPI therapy after at least 180 days of treatment with metformin. For all of these groups, patients were excluded if they took any other diabetes drugs or a histamine H2-receptor antagonist (H2RA) within 180 days prior to or 7 days after cohort entry. For exclusion criteria, a single prescription during the relevant time window was sufficient.
For ongoing exposure, patients were considered exposed after receipt of a prescription, for the number of days the prescription was intended to last based on dosage instructions and the number of pills dispensed (imputed as 30 days if this information was not available), plus 90 days. A gap greater than 90 days between periods of time covered by prescriptions ended a period of exposure.
Patients were eligible to contribute person-time to the study only if they had a measured HbA1c during the month prior to cohort entry up to 7 days after cohort entry. HbA1c was considered baseline for up to a week after cohort entry because HbA1c takes several months to change in response to diabetes treatment 15.
Outcome
The outcome for this study was change in HbA1c from baseline (as measured in the month prior to cohort entry up to 7 days after cohort entry) to the average HbA1c measured 3–9 months after drug exposure. The rationale for this time window was that HbA1c does not fully reflect changes in glycaemic control for 3 months. As a secondary outcome, the percentage of patients requiring additional diabetes drugs other than metformin at 1 year was assessed.
Secondary and exploratory analyses
In planned secondary analyses, the study was repeated with sulfonylurea exposure in place of metformin and with H2RA exposure in place of PPI exposure. In the event of any positive evidence for a PPI−metformin interaction or for a main effect of PPIs on HbA1c, this was intended as a negative control, since H2RAs and sulfonylureas have comparable indications to PPIs and metformin, respectively, but would not be expected to interact in the same way.
In exploratory analyses, change in average random serum glucose was assessed, to test the hypothesis that any such findings would be consistent with the primary findings on HbA1c.
Definition of covariates
Baseline covariates consisted of baseline HbA1c, body mass index (BMI), gender, age, year of treatment and concomitant use of antihypertensive medications, corticosteroids, statins, fibrates and antipsychotics.
Statistical analysis
Baseline covariates were described using dichotomous or continuous measures as appropriate, and differences between exposure groups were described using standardized differences 17. The outcomes were expressed primarily as change from baseline to follow-up. The statistical significance of the change was assessed using a paired t-test. Linear models were then used to estimate the change after adjusting for baseline covariates and to test for the significance of effect modification. Specifically, one linear model was used to describe changes in HbA1c for individuals starting metformin, and the presence or absence of baseline PPI use was included as a covariate. The same was done for individuals starting a PPI, with baseline metformin use as a covariate. Analysis was done using SAS software (SAS Institute, Cary, NC, USA) and R software (R Foundation for Statistical Computing, Vienna, Austria).
Informed consent and ethics statement
Approval for this study was obtained from the University of Pennsylvania Institutional Review Board and from THIN's Scientific Review Committee. The requirement for informed consent was waived by the University of Pennsylvania Institutional Review Board because these were anonymized data.
Results
After restriction to individuals with adequate baseline data and follow-up, 30 954 patients started metformin monotherapy without PPI exposure, while 3618 started metformin in the setting of at least 180 days of continuous PPI therapy. One thousand three hundred ninety-six eligible patients started a PPI without being on any diabetes drugs and 801 started a PPI in the setting of at least 180 days of continuous metformin monotherapy. Patients starting metformin had similar baseline values for BMI (∼32 kg m−2) and HbA1c (∼8.8%) regardless of whether PPIs were also used, while patients starting a PPI alone had an average baseline HbA1c of 6.8%. Rates of background medication use varied appreciably between groups and of note PPI use was associated with higher rates of baseline corticosteroid use (Table 1).
Table 1.
Basic demographics, restricted to individuals with HbA1c measured within 1 month prior to drug initiation. Due to large cohort size, most differences between groups are statistically significant
| Metformin (SD) | Metformin on PPI (SD) | S.Diff | PPI (SD) | S. Diff. | PPI on Metformin (SD) | S. Diff. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 30954 | 3618 | 1396 | 801 | |||||||
| Age (years) | 60 | 13 | 65 | 12 | 0.37 | 67 | 13 | 0.51 | 66 | 12 | 0.42 |
| Female gender | 41% | 49% | 0.15 | 47% | 0.11 | 51% | 0.19 | ||||
| Year | 2007 | 2.42 | 2008 | 2.32 | 0.23 | 2008 | 2.31 | 0.11 | 2008 | 2.15 | 0.35 |
| Baseline BMI (kg m−2) | 32.2 | 6.40 | 32.6 | 6.07 | 0.06 | 30.2 | 6.28 | 0.32 | 30.9 | 6.27 | 0.20 |
| Baseline HbA1c (%) | 8.94 | 2.05 | 8.70 | 1.88 | 0.12 | 6.83 | 1.35 | 1.03 | 7.10 | 1.09 | 0.90 |
| Statin | 46% | 67% | 0.42 | 46% | 0.00 | 80% | 0.69 | ||||
| Calcium channel blocker | 21% | 32% | 0.27 | 25% | 0.09 | 35% | 0.33 | ||||
| β-adrenoceptor blocker | 21% | 35% | 0.34 | 23% | 0.05 | 27% | 0.15 | ||||
| Angiotensin converting enzyme inhibitor-angiotensin receptor blocker | 39% | 55% | 0.31 | 42% | 0.04 | 69% | 0.60 | ||||
| Thiazide | 20% | 24% | 0.12 | 18% | 0.05 | 24% | 0.11 | ||||
| Steroid | 4% | 13% | 0.40 | 7% | 0.15 | 8% | 0.18 | ||||
| Antipsychotic | 2% | 4% | 0.16 | 2% | 0.02 | 2% | 0.01 | ||||
SD, standard deviation; S.Diff, standardized difference relative to metformin (difference in means between groups divided by pooled SD).
It was common for other medications to be started at the same time as metformin and these prescribing patterns also varied between exposure groups. For example, for patients not already on a PPI, 19% started statin therapy at the same time they started metformin. For patients already on a PPI, 6% started statin therapy at the same time as metformin. Similarly, 8% of patients not on a PPI started angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (ACE/ARB) therapy at the same time as metformin, while 2% of patients already on a PPI started an ACE/ARB at the same time as metformin.
Table 2 shows the monthly frequency of HbA1c monitoring before and following cohort entry. In the month before starting metformin, there were an average of 0.61 HbA1c measurements in those not receiving a PPI and 0.66 measurements in those receiving a PPI. In the year following cohort entry, the mean number of monthly HbA1C measurements in those receiving metformin ranged from 0.12 to 0.25 depending on whether a PPI was used concomitantly (Figure 1) and whether metformin was added to the PPI or vice versa.
Table 2.
Percentage of all patients (including those ineligible due to lack of baseline data) with an HbA1c measured in the defined time periods relative to cohort entry date
| Baseline 30 days before to 7 days after new drug exposure | 8–90 days after new drug exposure | Follow-up 91–270 days after new drug exposure | |
|---|---|---|---|
| Metformin | 61% | 46% | 72% |
| Metformin on PPI | 66% | 48% | 74% |
| PPI | 8% | 15% | 26% |
| PPI on metformin | 23% | 36% | 63% |
Figure 1.
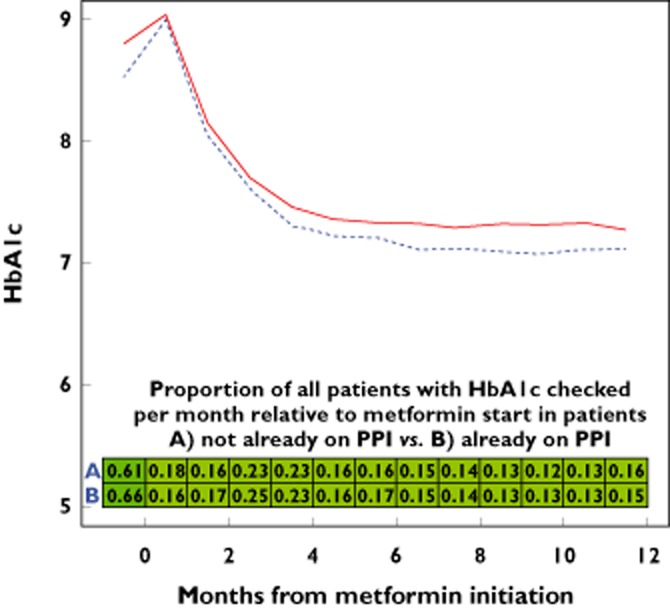
Unadjusted HbA1c over time with metformin exposure with background PPI exposure (dotted blue line) and without (solid red line). Shading of boxes is proportional to frequency of HbA1c checks per month
The unadjusted change in HbA1c from baseline to the average between 3 and 9 months later was −1.63% [95% confidence interval (CI) −1.65, −1.61] for metformin alone and −1.53% (95% CI −1.59, −1.47) when metformin was added to ongoing PPI therapy (Table 3). When PPIs were started, there was no detectable crude change in HbA1c whether the patient was already on metformin (−0.02%, 95% CI −0.08, 0.04) or not (0.02%, 95% CI −0.04, 0.08).
Table 3.
Unadjusted and adjusted changes in HbA1c (absolute % units) by cohort (for age, gender, baseline HbA1c, baseline BMI, year of treatment initiation, and both incident and baseline use of calcium channel blockers, β-adrenoceptor blockers, ACE inhibitors and ARBs, thiazides, β-adrenoceptor blockers, antipsychotics and steroids)
| Baseline | 95% CI | 3–9 month post | 95% CI | Crude difference | 95% CI | P value | Adjusted difference | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metformin | 8.94 | 8.92 | 8.97 | 7.31 | 7.30 | 7.33 | −1.63 | −1.65 | −1.61 | <0.001 | −1.63 | −1.66 | −1.60 |
| Metformin on PPI | 8.70 | 8.64 | 8.76 | 7.17 | 7.14 | 7.21 | −1.53 | −1.59 | −1.47 | <0.001 | −1.69 | −1.74 | −1.64 |
| PPI | 6.83 | 6.76 | 6.90 | 6.81 | 6.74 | 6.88 | −0.02 | −0.08 | 0.04 | 0.47 | 0.02 | −0.11 | 0.14 |
| PPI on metformin | 7.10 | 7.02 | 7.17 | 7.12 | 7.04 | 7.20 | 0.02 | −0.04 | 0.08 | 0.43 | 0.13 | −0.01 | 0.28 |
A multivariable model was used to assess the change in HbA1c with metformin exposure, adjusting for baseline BMI, baseline HbA1c, start date, gender, age and concomitant medications, including PPI use. When there was no baseline PPI use, the adjusted change in HbA1c with metformin was −1.63%, 95% CI −1.66, −1.60. When there was baseline PPI use, the adjusted change was −1.69%, 95% CI −1.74, −1.64. These estimates differed significantly: when background PPI use was present vs. absent, metformin was associated with a greater HbA1c reduction by 0.06 HbA1c percentage points (95% CI, −0.10, −0.01, P = 0.01). A model assessing the association between incident PPI exposure and change in HbA1c showed no evidence of any interaction or direct effect from PPI (Table 3).
Rates of addition of new diabetes drugs once metformin was started were the same regardless of whether or not metformin was added to PPI therapy. At 9 months, 20% of patients who had started metformin had also been prescribed additional diabetes medication, regardless of whether or not they were also exposed to a PPI.
As planned, the analysis was repeated with H2RAs in place of PPIs and with sulfonylureas in place of metformin. There was no independent association between H2RA use and change in HbA1c, nor any effect of PPI exposure on sulfonylurea effectiveness (data not shown).
In a post hoc analysis, changes in random glucose measurements were assessed. Results were consistent with the changes seen in HbA1c (Table 4). In a second post hoc analysis, patients with baseline or incident steroid exposure were excluded from the cohort and results were not materially affected (data not shown). In a third post hoc analysis, exposure was stratified by the specific PPI used (esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole) and there was no evidence of heterogeneity of effect by PPI (http://Table S1). Finally, there was no evidence of a dose−response effect related either to metformin or to PPI dose (data not shown).
Table 4.
Unadjusted and adjusted changes in serum glucose (mg dl–1) by cohort (for age, gender, baseline glucose, baseline BMI, year of treatment initiation, and both incident and baseline use of calcium channel blockers, β-adrenoceptor blockers, ACE inhibitors and ARBs, thiazides, β-adrenoceptor blockers, antipsychotics, and steroids)
| Baseline | 95% CI | 3–9 month post | 95% CI | Crude difference | 95% CI | P value | Adjusted difference | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metformin | 248 | 246 | 250 | 180 | 179 | 182 | −68 | −69 | −66 | <0.001 | −67 | −69 | −64 |
| Metformin on PPI | 241 | 236 | 247 | 174 | 171 | 178 | −67 | −72 | −62 | <0.001 | −69 | −73 | −64 |
| PPI | 138 | 135 | 141 | 134 | 131 | 137 | −4 | −7 | −1 | 0.004 | 1 | −7 | 8 |
| PPI on metformin | 155 | 145 | 166 | 159 | 147 | 172 | 4 | −9 | 16 | 0.16 | 18 | −8 | 45 |
The finding that other medications, including antihypertensives and statins, were started along with metformin at generally higher rates in patients not already on PPI therapy prompted us to examine the sensitivity of these results to this type of potentially unmeasured confounding. As noted above, we found that concomitant PPI use was associated with a 0.06 HbA1c percentage unit better response to metformin. If PPIs were actually associated with a reduction of metformin's effectiveness by 0.5 HbA1c percentage units, an unmeasured exposure would have to cause a net improvement of 0.06 + 0.5 = 0.56 HbA1c percentage units in the PPI/metformin group to result in the association seen here. If such an exposure – for example, a drug for another indication that happened also to affect HbA1c – were present in 50% of the PPI/metformin cohort and 0% of the metformin only cohort, it would have to result in a 1.12% change in HbA1c.
Discussion
Recent mechanistic studies have raised the possibility that PPIs might interfere with the effectiveness of metformin 1, which, if true, could have major clinical and public health implications. Reassuringly, we found that metformin was at least as effective in reducing HbA1c in patients who were receiving chronic PPI therapy as it was in patients who were not.
Contrary to our expectation based on mechanistic data, metformin was actually associated with a 0.06% significantly larger absolute HbA1c decline (i.e. −1.69% vs. −1.63%) when it was used in patients already on chronic PPI therapy. While this difference is very small and unlikely to have much clinical significance, we speculate that it may be due in part to amelioration by PPIs of metformin's gastrointestinal side effects, improving adherence. Alternatively, it may be due to the increase in plasma metformin concentrations with PPI co-administration described previously in healthy volunteers 8,9.
This study has limitations. Ascertainment bias, confounding and concomitant use of other medications could mask an association. We assessed for the probability of ascertainment bias by assessing the frequency of HbA1c checks. Concomitant use of PPI inhibitors and metformin was not associated with a change in the frequency of HbA1c checks vs. metformin alone. In addition, the different exposure categories were not identical and confounding by baseline factors, such as diabetes severity, could affect these results. The use of change in HbA1c from a measured baseline amelioriates this concern and multivariable adjustment was undertaken to take measurable differences between groups into account.
Concomitant use of other medications is another potential source of bias, as steroids, antipsychotics, statins and some antihypertensives have been reported to affect serum glucose concentrations and HbA1c 18–21. However, when we controlled for baseline use of these medication classes, our results were not affected. Additional bias from other treatments that were not measured or controlled for is a possibility, but would have to be very strong to conceal a clinically meaningful deleterious effect from the combination of metformin with PPIs.
In conclusion, in contrast to expectations based on mechanistic data, PPI use did not impair metformin effectiveness, nor did PPIs have clinically significant effects on glycaemic control in their own right.
Competing Interests
S. Hennessy has consulted for Abbott Laboratories, Hoffmann La-Roche Ltd, Novartis Pharmaceuticals, Bayer Healthcare LLC, AstraZeneca and Bristol-Myers Squibb, received research support from AstraZeneca and Bristol-Myers Squibb, and received institutional support from Pfizer Inc. and Sanofi to support pharmacoepidemiology training. This research was funded in part by NIH grants R01AG025152 and R01DK102694.
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work except for the NIH grants noted in the paragraph above, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years except for SH as noted in the paragraph above and no other relationships or activities that could appear to have influenced the submitted work.
Author contributions
All authors were involved in the research design. JHF analysed the data. All authors were involved in writing the manuscript.
Supporting Information
Table S1 Change in HbA1c with exposure to individual classes of PPI
References
- Nies AT, Hofmann U, Resch C, Schaeffeler E, Rius M, Schwab M. Proton pump inhibitors inhibit metformin uptake by organic cation transporters (OCTs) PLoS One. 2011;6:e22163. doi: 10.1371/journal.pone.0022163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa R, Echizen H. Drug−drug interaction profiles of proton pump inhibitors. Clin Pharmacokinet. 2010;49:509–533. doi: 10.2165/11531320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Blume H, Donath F, Warnke A, Schug BS. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf. 2006;29:769–784. doi: 10.2165/00002018-200629090-00002. [DOI] [PubMed] [Google Scholar]
- Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22:820–827. doi: 10.1097/FPC.0b013e3283559b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DS, Jonker JW, Kato Y, Kusuhara H, Schinkel AH, Sugiyama Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther. 2002;302:510–515. doi: 10.1124/jpet.102.034140. [DOI] [PubMed] [Google Scholar]
- Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, Sheardown SA, Yue L, Burchard EG, Brett CM, Giacomini KM. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2008;83:273–280. doi: 10.1038/sj.clpt.6100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, Ianculescu AG, Yue L, Lo JC, Burchard EG, Brett CM, Giacomini KM. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007;117:1422–1431. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Chung I, Yoon SH, Yu KS, Lim KS, Cho JY, Lee H, Jang IJ, Chung JY. Effects of proton pump inhibitors on metformin pharmacokinetics and pharmacodynamics. Drug Metab Dispos. 2014;42:1174–1179. doi: 10.1124/dmd.113.055616. [DOI] [PubMed] [Google Scholar]
- Ding Y, Jia Y, Song Y, Lu C, Li Y, Chen M, Wang M, Wen A. The effect of lansoprazole, an OCT inhibitor, on metformin pharmacokinetics in healthy subjects. Eur J Clin Pharmacol. 2014;70:141–146. doi: 10.1007/s00228-013-1604-7. [DOI] [PubMed] [Google Scholar]
- Crouch MA, Mefford IN, Wade EU. Proton pump inhibitor therapy associated with lower glycosylated hemoglobin levels in type 2 diabetes. J Am Board Fam Med. 2012;25:50–54. doi: 10.3122/jabfm.2012.01.100161. [DOI] [PubMed] [Google Scholar]
- Ferguson DD, DeVault KR. Medical management of gastroesophageal reflux disease. Expert Opin Pharmacother. 2007;8:39–47. doi: 10.1517/14656566.8.1.39. [DOI] [PubMed] [Google Scholar]
- Krishnan B, Babu S, Walker J, Walker AB, Pappachan JM. Gastrointestinal complications of diabetes mellitus. World J Diabetes. 2013;4:51–63. doi: 10.4239/wjd.v4.i3.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the U.S., 2003-2012. Diabetes Care. 2014;37:1367–1374. doi: 10.2337/dc13-2289. [DOI] [PubMed] [Google Scholar]
- Flory JH, Small DS, Cassano PA, Brillon DJ, Mushlin AI, Hennessy S. Comparative effectiveness of oral diabetes drug combinations in reducing glycosylated hemoglobin. J Comp Eff Res. 2014;3:29–39. doi: 10.2217/cer.13.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275–278. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19:251–255. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DC, Cagliero E, Copeland PM, Borba CP, Evins E, Hayden D, Weber MT, Anderson EJ, Allison DB, Daley TB, Schoenfeld D, Goff DC. Glucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents: a frequently sampled intravenous glucose tolerance test and minimal model analysis. Arch Gen Psychiatry. 2005;62:19–28. doi: 10.1001/archpsyc.62.1.19. [DOI] [PubMed] [Google Scholar]
- Kwon S, Hermayer KL. Glucocorticoid-induced hyperglycemia. Am J Med Sci. 2013;345:274–277. doi: 10.1097/MAJ.0b013e31828a6a01. [DOI] [PubMed] [Google Scholar]
- Ferrari P, Rosman J, Weidmann P. Antihypertensive agents, serum lipoproteins and glucose metabolism. Am J Cardiol. 1991;67:26B–35B. doi: 10.1016/0002-9149(91)90817-5. [DOI] [PubMed] [Google Scholar]
- Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Change in HbA1c with exposure to individual classes of PPI


