Abstract
Background
Anecdotal observation suggests that older patients in medical intensive care units receive higher doses of psychoactive medications during evening shifts than day and night shifts.
Objectives
To determine the dosing patterns and total doses of fentanyl, lorazepam, and haloperidol according to nursing shift in a cohort of older patients in a medical intensive care unit.
Methods
The sample consisted of 309 patients 60 years and older admitted to the medical intensive care unit at Yale-New Haven Hospital, New Haven, Connecticut. Data on time, dosage, and route of administration of the drugs were collected. Data were analyzed by using a Bayesian random effects Poisson model adjusted for individual heterogeneity, excess zero doses, and important clinical covariates.
Results
Mean age of the patients was 75 years; 58% received fentanyl, 55% received lorazepam, and 32% received haloperidol. Although dosing with fentanyl did not differ according to shift, doses of both lorazepam and haloperidol were higher during the evening shifts (4 pm to midnight) than during the day or night shifts. Compared with women, men received higher doses of both haloperidol and lorazepam and variability between shifts was greater.
Conclusions
In this longitudinal, observational sample of older patients, data indicated a positive association between dose levels of lorazepam and haloperidol during the evening nursing shifts relative to other shifts. Further investigation is needed to determine potential causes and to evaluate the impact on outcomes of sleep deprivation and delirium.
Delirium is an acute change in mental status with alteration in cognition and attention that generally fluctuates over time. Delirium is especially common in the medical intensive care unit (MICU), where the estimated prevalence is 50% to 90% in older patients.1 Delirium in the MICU has been independently associated with increased mortality,2,3 length of hospital stay,4 and increased hospitalization costs.5 Many risk factors have been identified for delirium, including advanced age, preexisting dementia, greater severity of illness, hypertension, active tobacco use, and metabolic disturbances.6,7
Medications have also been associated with delirium in older MICU patients. In one study,8 one of the strongest risk factors for delirium in older MICU patients was prescription of benzodiazepines before MICU admission. In a study9 of adults admitted to MICUs or coronary ICUs who were treated with mechanical ventilation, lorazepam was an independent risk factor for development of delirium, even after adjustments were made for other medications and risk factors. In addition, benzodiazepine and opioid administration, after corrections were made for other factors, was independently associated with prolonged episodes of delirium in older patients.10 Prolonged delirium leads to longer MICU stays and to complications, such as greater risk for nosocomial infections, that contribute to longer lengths and higher costs of stay.
Because administration of medications is a potentially modifiable factor for delirium, we quantitatively examined patterns of drug administration in a large cohort of older MICU patients. We were interested in determining the dosing patterns of psychoactive medications for several reasons. First, delirium may occur more frequently during evening or nighttime hours than during the day and thus lead to more drug usage. Second, some psychoactive medications can shorten the REM stage of sleep and can potentially worsen delirium and cognitive impairment. Finally, increased doses of medications given overnight may affect a patient’s ability to be weaned from mechanical ventilation during the day. This study was the first step in documenting whether patients received different amounts of medication according to the time of day.
Patients and Methods
Study Design
The study sample consisted of a prospective cohort of 309 patients 60 years and older who were admitted to the MICU of Yale-New Haven Hospital, New Haven, Connecticut. At the time of enrollment, the MICU was a 24-bed unit staffed during the day by a board-certified critical care attending physician, pulmonary and critical care fellows, and internal medicine house staff. During the evening and night shifts, medicine house staff provided care with backup from attending physicians and fellows who were on call. The nurse to patient ratio was 1 to 2 except for circumstances, such as continuous venovenous hemofiltration, that required a 1 to 1 ratio. The number of nurses was the same during the day, evening, and night shifts. The majority of the nurses worked 12-hour shifts, from 7 am to 7 pm and from 7 pm to 7 am.
The prevalence of delirium in older medical intensive care patients is about 50% to 90%.
During the period of study enrollment, no sedation protocol was followed, but the drugs available to the MICU staff for treating pain, sedation, and delirium included fentanyl, morphine, lorazepam, midazolam, propofol, and haloperidol. The choice of medication and dosage was determined daily by the medical team and was addressed on work rounds. During this period, no protocols were in place for daily awakening, spontaneous breathing trials, screening for delirium, or early ambulation. Nurses assessed patients’ need for sedation by using the Richmond Agitation-Sedation Scale.11 Patients receiving continuous infusions of sedatives were assessed hourly; other patients were assessed every 4 hours or as needed. Although the scale was used on an hourly basis, documentation did not include the exact times of assessment, a prerequisite for synchronizing scores on the scale with drug dosing. For this reason, the scale was not used to guide sedation during the study period.
Data Collection
Patients were enrolled between September 5, 2002, and September 30, 2004. All patients admitted during that time were screened for eligibility, Patients were eligible if they were more than 60 years old, were able to communicate before admission, spoke English, were in the MICU more than 24 hours, were not admitted from another MICU, and had an identifiable proxy able to give consent and provide background information. Of the 318 eligible patients, 309 patients were enrolled. The study sample was restricted to those patients, among all patients receiving the drug being modeled, who survived in the MICU for 3 days or longer. The study was approved by the institutional review board of Yale University School of Medicine.
Patient Characteristics
Upon enrollment, each patient had baseline data collected via his or her proxy. Baseline characteristics included demographic information, medical and psychiatric history, alcohol use, and smoking history. Also, during the proxy interviews, data were collected to complete the Informant Questionnaire on Cognitive Decline in the Elderly12 to screen for preexisting dementia, the Katz Activities of Daily Living Scale,13 and the Lawton Instrumental Activities of Daily Living Scale.14 Patients’ charts were reviewed for the Charlson Comorbidity Index,15 scores on the Acute Physiology and Chronic Health Examination (APACHE) II,16 scores on the Richmond Agitation- Sedation Scale,11 the presence of metabolic abnormalities (liver and renal function), and the need for mechanical ventilation. Patients were assessed daily for delirium by a trained research nurse who used the Confusion Assessment Method for the ICU, and the findings were supplemented with a daily validated chart review.17 Patients were weighed upon admission to the ICU.
Detailed records of administration of medications were collected for all patients in the study by chart review and through an automated pharmacy medication system. Transdermal and intravenous drug delivery were included for fentanyl. Intravenous and oral routes of drug delivery were included for lorazepam and haloperidol. Although morphine, midazolam, and propofol were available in the formulary, too few patients received these medications to feasibly model the temporal patterns of the drugs’ dosing. For this reason, morphine, midazolam, and propofol were not included in the analysis. Data were collected on the time, dose, and route of administration of all medications, starting at MICU admission. All routes of drug administration were included in the dosing calculation. The medication data were then grouped to represent time of day by nursing shift: day (8 am to 4 pm), evening (4 pm to midnight), and night (midnight to 8 am). Data collection began at the time of admission to the MICU.
Statistical Analysis
Characteristics of the study sample were summarized with means and standard deviations or medians and interquartile ranges for continuous variables and with counts and percentages for dichotomous variables. The mean dose of each drug was calculated for each of the first 15 eight-hour shifts (the first 5 days of MICU admission). Complete data from patients taking a particular medication were used to create longitudinal plots of dosage by shift by plotting mean dosage levels by number of shifts spent in the MICU. Mean dose of drug was also stratified and plotted according to the patient’s sex. These plots indicated the need to adjust for linear trend and periodicity, a situation that mandated use of a model that incorporated shift number (linear trend) and evening shift (periodicity).
A Bayesian model was used for examining the association between evening shift and dosing level of each drug. The model had 95% credible intervals, assumed a Poisson distribution of the outcome, adjusted for the number of shifts each patient was in the MICU, and included random effects adjusted for each patient and nursing shift. This Bayesian model was chosen because it flexibly accommodated the substantive statistical challenges involved in the analysis of data from an MICU. These challenges include a large number of zero doses per patient among the various shifts, periodicity potentially introduced by shift-based dosing trends, and the autocorrelation of each patient’s multiple shifts. The Bayesian approach is more powerful than other approaches for a small sample size and allows for a large number of random effects that can effectively account for dose variability both between and within patient- or shift-specific sets of shifts and doses. Two additional advantages of this Bayesian approach are the automatic imputation of missing data and the ability to conduct posterior predictive simulations to test model fit and related statistics difficult to model directly. This analytical approach is reported in detail elsewhere.18
Each drug model was also adjusted for the following clinically important covariates: age in years, the APACHE II score for severity of illness, occurrence of death after the third day of the MICU stay, dementia as indicated by a score of 3.3 or greater on the Informant Questionnaire on Cognitive Decline in the Elderly, treatment with mechanical ventilation, male sex, race, and weight in pounds. These covariates were chosen a priori on the basis of factors that clinically most likely had an impact on sedative and narcotic dosing in the ICU. Although none of the doses of the 3 drugs were based on weight, weight was included for 2 reasons. First, some of the drugs can accumulate in fat stores, and second, clinicians often start out with lower doses of these medications for smaller, older patients.
The doses per shift for each patient’s first day in the MICU were temporally aligned as follows. For patients admitted during the night shift of the day of admission, data were complete through death or discharge. For patients admitted during the day or evening shifts of their first calendar day in the MICU, values were assigned for missing data for the previous shifts (ie, night or day shifts) of that calendar day only. This step was necessary to synchronize the shift numbers according to their proper designations as night, day, or evening shifts within each calendar day. Because sensitivity analysis for either day admission or evening admission in preliminary models showed these effects were non-significant, these adjustments were not included in the final model. An additional advantage of the Bayesian approach is that missing values anywhere in the sequence of doses per shift, which are assumed to be missing at random, are imputed during the modeling process.
As indicated in Table 1, data on the dementia covariate were missing for 1 patient; data for that patient were excluded from the analysis. No data were missing for the outcome or the other covariates used in the model. Exploratory analysis was performed by using SAS, version 9.2,19 models were evaluated by using WinBUGS 1.4,20 and posterior predictive simulations were run in the R language by using the arm package.21 Significance for all parameters was interpreted as a 95% credible interval that excludes the null value of 1.
Table 1.
Characteristics of patients on admission to the intensive care unit who received fentanyl, lorazepam, or haloperidol and stayed at least 3 days
| Characteristic | Entire cohort (N= 309)a |
Fentanyl (n = 159)b |
Lorazepam (n = 148)c |
Haloperidol (n = 91)d |
|---|---|---|---|---|
| Age, mean (SD), y | 74.7 (8.5) | 74.1 (8.4) | 72.9 (7.9) | 74.5 (7.8) |
| Male sex, No. (%) | 145 (47) | 80 (50) | 75 (51) | 40 (44) |
| Education, mean (SD), y | 12.5 (2.8) | 12.6 (2.8) | 12.5 (2.8) | 12.5 (2.9) |
| Nonwhite race, No. (%) | 51 (17) | 21 (13) | 18 (12) | 15 (16) |
| Score on Acute Physiology and Chronic Health Evaluation II, mean (SD) | 23.5 (6.4) | 24.3 (6.6) | 24.4 (6.4) | 24.2 (5.9) |
| Charlson Comorbidity Index, mean (SD) | 1.8 (1.9) | 1.6 (1.8) | 1.8 (1.8) | 1.5 (1.6) |
| Admitted from home,e No. (%) | 241 (78) | 127 (80) | 123 (83) | 74 (81) |
| Baseline medical status | ||||
| Evidence of depression,f No. (%) | 85 (28) | 49 (31) | 47 (32) | 34 (37) |
| Dementia, No. (%) | 95 (31) | 47 (30) | 43 (29) | 37 (41) |
| Any impairment in activities of daily living, No. (%) | 111 (36) | 56 (35) | 51 (34) | 38 (42) |
| Admitting diagnosis, No. (%) | ||||
| Sepsis | 51 (17) | 20 (13) | 18 (12) | 14 (15) |
| Respiratory problem | 156 (50) | 94 (59) | 102 (69) | 55 (60) |
| Neurological problem | 5 (2) | 2 (1) | 0 (0) | 2 (2) |
| Gastrointestinal hemorrhage | 52 (17) | 28 (18) | 15 (10) | 8 (9) |
| Other | 45 (15) | 15 (9) | 1 (1) | 12 (13) |
| Intensive care unit factors | ||||
| Delirium during stay,g No. (%) | 239 (77) | 144 (91) | 137 (93) | 91 (100) |
| Intubated, No. (%) | 167 (54) | 131 (82) | 122 (82) | 71 (78) |
| Days of mechanical ventilation, median (IQR) | 6 (3–11) | 6 (3–13) | 6.5 (4–12) | 8 (4–18) |
| Length of stay, median (IQR), d | 4 (3–8) | 7 (4–13) | 7 (5–13) | 8 (5–17) |
Abbreviation: IQR, interquartile range.
For Charlson comorbidity index, data are missing for 1 patient; for dementia, data are missing for 3 patients.
For Charlson comorbidity index, data are missing for 1 patient; for dementia, data are missing for 1 patient.
For Charlson comorbidity index, data are missing for 1 patient; for dementia, data are missing for 2 patients.
For Charlson comorbidity index, data are missing for 1 patient.
Admitted from home, rather than from a skilled nursing facility or rehabilitation center.
Evidence provided by surrogate or chart.
Delirium assessed by interview (Confusion Assessment Method for the Intensive Care Unit) or chart review during entire stay in intensive care unit.
Results
A total of 159 patients qualified for the drug model for fentanyl, 91 patients for haloperidol, and 148 for lorazepam. The mean age was 75 years. A total of 44 patients (14%) were taking opioids before hospital admission, 44 patients (14%) were taking benzodiazepines, and 2 patients (0.6%) were taking haloperidol. During the MICU stay, 58% received fentanyl, 55% received lorazepam, and 32% received haloperidol. A fentanyl patch was used for 7 patients, and 30 patients received oral doses of lorazepam. Among the patients who received haloperidol, 83 received intravenous doses and 7 received oral doses. Table 1 lists patients’ baseline characteristics. A total of 9 patients received an atypical antipsychotic, and 30 received vecuronium.
The rate of delirium in the entire sample (N=309 patients) was 77%. Delirium rates varied between patients receiving fentanyl (91%), lorazepam (93%), and haloperidol (100%). In the entire cohort, more patients with baseline dementia (40%) received haloperidol than did patients without baseline dementia (26%).
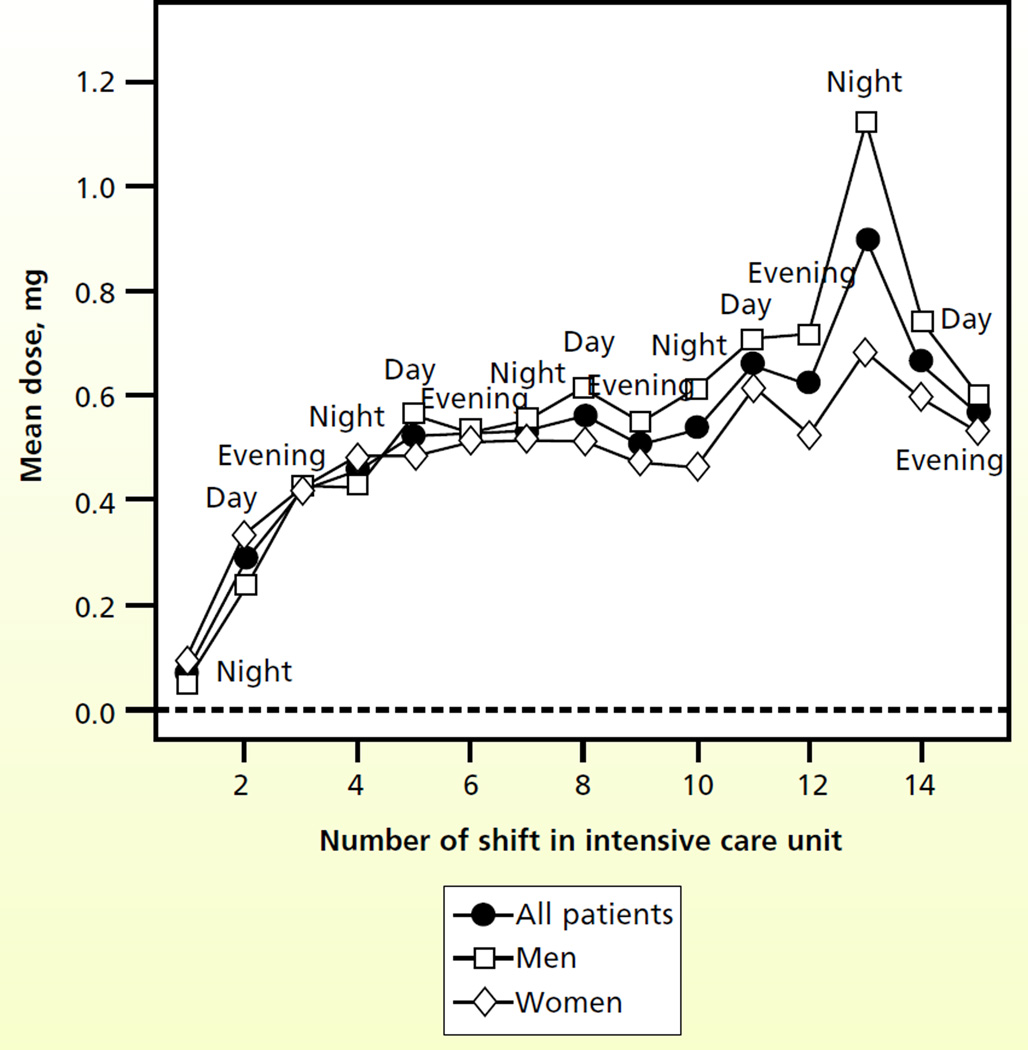
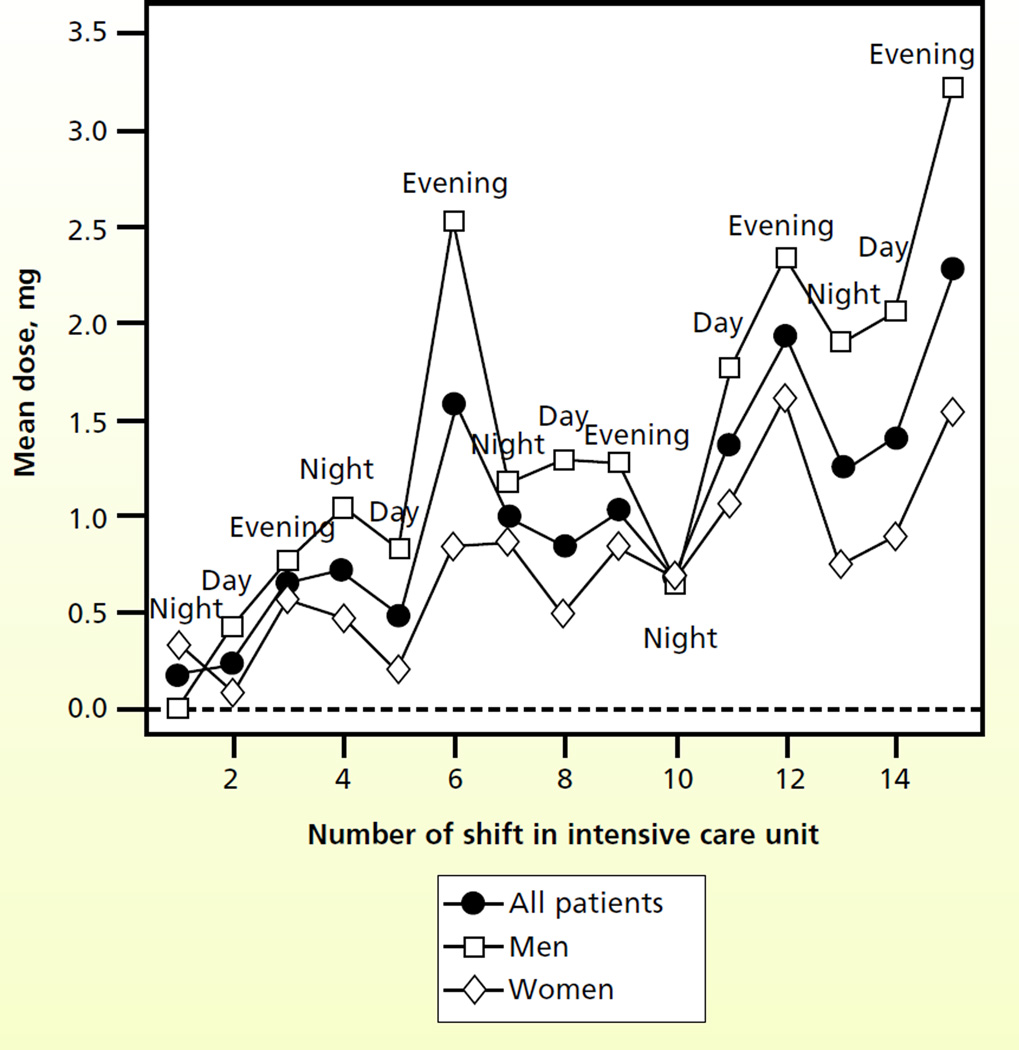
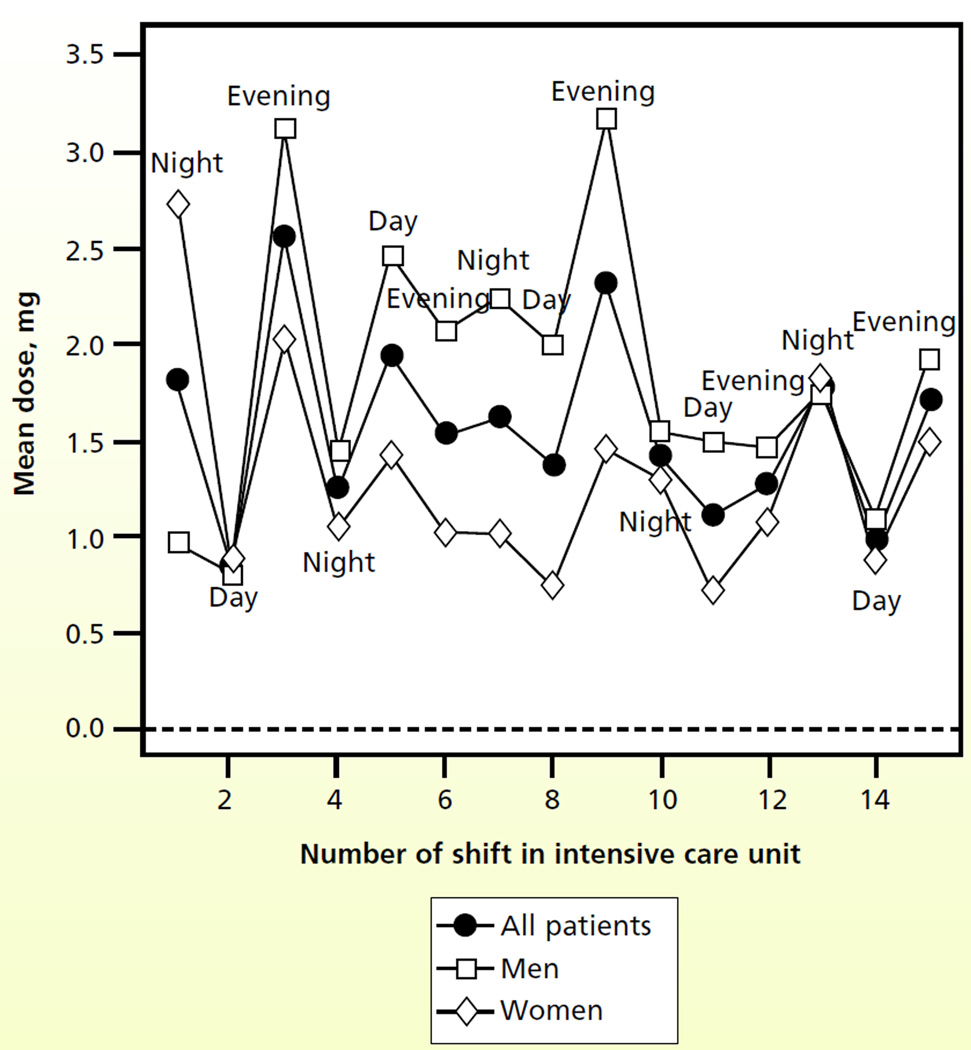
Figure 1 shows the patterns for fentanyl dosing. Whereas the dose of fentanyl gradually increased during the MICU stay, no clear temporal relationship was attributable to time of day. During the first 7 shifts, dosing according to the patient’s sex appeared to be similar, with small differences in mean dose in subsequent shifts. Figure 2 shows a temporal relationship: the dose of haloperidol was higher for evening shifts than for either the day shift or the night shift. The dose of haloperidol also appeared to increase progressively through the MICU stay, and men received higher doses than women did. Figure 3 shows a clear trend of higher doses of lorazepam during the night and evening shifts; the highest doses seemed to be given during the evening shift. Men appeared to receive higher doses of lorazepam than women did, and differences were most apparent during the middle of the MICU course.
Figure 1.
Mean doses of fentanyl by shift number in the intensive care unit and sex of the patient (n = 159 patients).
Figure 2.
Mean doses of haloperidol by shift number in the intensive care unit and sex of the patient (n = 91 patients).
Figure 3.
Mean doses of lorazepam by shift number in the intensive care unit and sex of the patient (n = 148 patients).
After adjustment for clinical covariates in the Bayesian model, patients taking haloperidol received a mean evening dose that was 75% higher than the mean doses received during the day and night shifts combined (Table 2). Likewise patients taking lorazepam received a mean evening dose that was 55% higher than the mean doses given during the day and night shifts combined. We found no association between evening shift and dosage of fentanyl. Examination of the linear trends over shifts indicated a small (7%) significant increase in fentanyl dosing relative to dosing during the previous shift. Haloperidol dosing increased 41% from the previous shift. We found no significant linear trend in the dosing of lorazepam.
Table 2.
Associations between evening shift and medication dosages
| Main results | Relative risk (95% credible interval) | ||
|---|---|---|---|
| Fentanyla (n = 159)b | Lorazepama (n = 148)b | Haloperidola (n = 91)b | |
| Evening shift (4 pm-midnight)c | 1.06 (0.93, 1.22) | 1.55 (1.29, 1.85)d | 1.75 (1.14, 2.68)d |
| Linear trend over shifts | 1.07 (1.05, 1.09)d | 1.02 (0.99, 1.05) | 1.41 (1.32, 1.52)d |
Each drug model included adjustment for age, score on Acute Physiology and Chronic Health Evaluation II, death in the intensive care unit, dementia (score on short form of the Informant Questionnaire on Cognitive Decline in the Elderly >3.3), length of stay <5 days, mechanical ventilation, sex, race, and weight in pounds.
Number of persons taking drug who survived at least 3 days in the intensive care unit.
Comparison of mean drug dose given on evening shift relative to mean doses on day and night shifts combined.
Statistical significance from 95% credible interval exclusive of zero.
Compared with patients who did not receive mechanical ventilation, those who required mechanical ventilation received significantly higher doses of fentanyl (relative risk [RR], 6.8; 95% credible interval [95%CI], 2.73–15.94) and lorazepam (RR, 3.4; 95% CI, 1.69–6.49) Men received higher doses of haloperidol than did women (RR, 2.3; 95% CI, 1.11–5.71), and patients who died in the MICU received lower doses of haloperidol than did patients who survived (RR, 0.33; 95% CI, 0.14–0.79).
Discussion
This study is the first one in which changes in patients’ doses of psychoactive medications were quantitatively evaluated over time and diurnal variation. The results indicate that, regardless of a patient’s sex, higher doses of haloperidol and lorazepam were given during the evening shift than during the day shift and the night shift. The mean dose of fentanyl increased over time and showed the least temporal variation according to nursing shifts.
Although we expected a diurnal trend in medication dosing, reasons for the diurnal changes are unclear. Both patient and medical staff factors may have contributed to the trends observed. For example, patients might have been more agitated or delirious during evening shifts and the agitation or delirium might account for the use of higher doses of medication. Studies from mental health units have similarly indicated increased dosing of medications in the evening to address increased agitation.22 Alterations in circadian rhythms associated with the MICU environment, such as excessive noise and bright lights and critical illness itself, could likewise contribute to patients’ increased wakefulness during the evening and night hours and sleep during the daytime hours.23,24 Although we did not examine circadian rhythm or sleep in this study, the disparity in doses in the evening shift increased with time in the MICU, suggesting that disruption in circadian rhythm might have played a role. Studies of sleep patterns in the MICU and of diurnal variations in cortisol or melatonin levels over time in older MICU patients would be informative.
Increased use of lorazepam and haloperidol might be justified for medical reasons if patients are more agitated in the evening. However, in a study25 of medical and surgical ICU patients, 77% of agitated patients were agitated both day and night, 16% were agitated only during the day, and the other 6% were agitated only at night. However, only results for day vs night were described, and the day-night shifts included 50% of the evening shifts. In our cohort, patients who had preexisting dementia also received more haloperidol than did patients without preexisting dementia. We do not know why haloperidol was given, but because dementia is one of the strongest risk factors for delirium,8 the patients might have been agitated and delirious.
Patients may be more agitated or delirious during evening shifts.
Increased use of lorazepam and haloperidol during the evening hours may also be associated with variations in staffing during the evening and night shifts, such as more junior doctors and less ancillary support for nurses. In our MICU the nurses work 12-hour shifts, and the increase in doses on the evening shift (4 pm to midnight) might have been due to doses given at change of shift. We do not have the data to confirm this possibility. In a survey26 of nurses in the United States, 28% reported their use of sedation was affected by staffing ratios, and 38% reported that the need to perform competing tasks played a role in their decision to sedate a patient. These results raise concerns that increased sedation in the evening does not represent an optimal response to patients’ needs but rather is a result of factors such as staffing and experience of the care providers. Systematic quality improvement efforts might result in enhancement of medication dosing variation caused by differing levels of staff experience or competing patient care tasks.
After adjustment for weight, men still received higher doses of haloperidol.
In our cohort, men received higher doses of fentanyl, lorazepam, and haloperidol than did women. This difference is often attributed to men’s higher body mass, more frequent perceptions that men’s behavior is aggressive, and the need for greater sedation because of metabolic differences between men and women. However, after adjustments were made for covariates, including weight, men were still more likely than women to receive higher doses of haloperidol. Whether or not these higher doses of medications are detrimental to older men admitted to the MICU is unclear.
Compared with the entire cohort, patients who were not intubated were given higher doses of haloperidol, and those who were intubated received higher doses of lorazepam. Haloperidol causes less respiratory suppression than lorazepam does, a difference that might explain why haloperidol was chosen more frequently for patients who did not receive mechanical ventilation. Preferential use of lorazepam in intubated patients may also reflect historical practices of sedating patients receiving mechanical ventilation to prevent agitation and ventilator dysynchrony and to provide amnesia. However, the need for sedation for effective delivery of mechanical ventilation has been challenged, and drug-induced amnesia can increase rather than decrease the psychiatric sequelae of critical illness such as posttraumatic stress disorder.27–29 Because of the association of sedation with delirium, posttraumatic stress disorder, delusional memories, and other adverse outcomes, future work is needed to ensure that sedation is reserved for patients who will benefit from its use, such as patients who are unable to synchronize with the ventilator or patients with alcohol use disorders who are at risk for dangerous agitation.
Study Limitations
Our analysis has several limitations. Although we used the Richmond Agitation-Sedation Scale11 to assess patients for level of consciousness, we did not have the assessments synchronized with the times medications were administered. We also do not have information on the specific level of training of the physicians and nurses presiding over specific shifts, data that most likely are a source of substantive variability in dosing levels (interphysician and internurse variability). Like much research conducted in the ICU, our study was observational. Observational studies lack the balance of covariates, both measured and unmeasured, theoretically afforded by randomized trials. Finally, the study was done in a single medical center with older MICU patients, characteristics that limit the generalizability of the results. The data were collected between September 2002 and September 2004, before sedation holidays, sedation protocols, and early mobilization of patients in the ICU became standard treatment. Although overall doses of sedation may have decreased with the advent of sedation protocols and sedation holidays, no data suggest that the diurnal patterns of increased lorazepam and haloperidol in the evening shift would differ from those presented here. Furthermore, in many ICUs, the implementation of sedation protocols, sedation holidays, and early mobilization has not been accomplished.30,31 Further research will be needed to evaluate how each of the drug dosing patterns we observed may affect patient-centered outcomes such as sleep and posttraumatic stress disorder and ICU-specific outcomes such as duration of mechanical ventilation, length of stay, and occurrence of delirium.
Conclusions
We found that older MICU patients received increased doses of lorazepam and haloperidol during evening shifts. Whether patients’ needs or factors related to the health care system are promoting the trends we observed is unclear, and further investigation into potential causes is needed. Meanwhile, critical care practitioners should implement best practices based on evidence, including sedation protocols and daily awakening trials.32
Acknowledgments
FINANCIAL DISCLOSURES
This work was supported in part by the American Lung Association and Connecticut Thoracic Society (CG-002-N), Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (2P30AG021342-06), the T. Franklin Williams Geriatric Development Initiative through The CHEST Foundation, ASP, Hartford Foundation, grants from the National Institute on Aging (K23AG 23023 and 1R21NR011066, Dr Pisani; 1R21AG033130-01A2, Dr Murphy), and CTSA UL1 RR024139 from the National Center for Research Resources (Dr Murphy).
Footnotes
eLetters
Now that you’ve read the article, create or contribute to an online discussion on this topic. Visit www.ajcconline.org and click “Responses” in the second column of either the full-text or PDF view of the article.
To purchase electronic or print reprints, contact American Association of Critical-Care Nurses, 101 Columbia, Aliso Viejo, CA 92656. Phone, (800) 899-1712 or (949) 362-2050 (ext 532); fax, (949) 362-2049; reprints@aacn.org.
Contributor Information
Margaret A. Pisani, Department of Medicine, Yale University School of Medicine, New Haven, Connecticut..
Kyle Bramley, Department of Medicine, Yale University School of Medicine, New Haven, Connecticut..
Michael T. Vest, Yale University School of Medicine, is currently in the Department of Medicine at Christiana Care Health System in Newark, Delaware..
Kathleen M. Akgün, Department of Medicine, Yale University School of Medicine, New Haven, Connecticut..
Katy L.B. Araujo, Department of Medicine, Yale University School of Medicine, New Haven, Connecticut..
Terrence E. Murphy, Department of Medicine, Yale University School of Medicine, New Haven, Connecticut..
REFERENCES
- 1.Stevens RD, Nyquist PA. Coma, delirium, and cognitive dysfunction in critical illness. Crit Care Clin. 2006;22(4):787–804. doi: 10.1016/j.ccc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 3.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32(11):2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 4.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 6.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 7.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27(8):1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 8.Pisani MA, Murphy TE, Van Ness PH, Araujo KL, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007;167(15):1629–1634. doi: 10.1001/archinte.167.15.1629. [DOI] [PubMed] [Google Scholar]
- 9.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 12.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24(1):145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 13.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 14.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 17.Pisani MA, Araujo KL, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10(4):R121. doi: 10.1186/cc5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy TE, Van Ness PH, Araujo KL, Pisani MA. Bayesian time-series analysis of a repeated-measures Poisson outcome with excess zeroes. Am J Epidemiol. 2011;174(11):1230–1237. doi: 10.1093/aje/kwr252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SAS/STAT. 9.2 User’s Manual. Cary, NC: SAS Institute Inc; 2009. [Google Scholar]
- 20.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—A Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10(4):325–337. [Google Scholar]
- 21.Gelman A, Su Y-S, Hill J, Pittau MG, Kerman J, Zheng T. Data Analysis Using Regression and Multilevel/Hierarchical Models. Vienna, Austria: R Foundation for Statistical Computing; 2007. [Google Scholar]
- 22.Baker JA, Lovell K, Harris N. A best-evidence synthesis review of the administration of psychotropic pro re nata (PRN) medication in in-patient mental health settings. J Clin Nurs. 2008;17(9):1122–1131. doi: 10.1111/j.1365-2702.2007.02236.x. [DOI] [PubMed] [Google Scholar]
- 23.Gehlbach BK, Chapotot F, Leproult R, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35(8):1105–1114. doi: 10.5665/sleep.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frisk U, Olsson J, Nylén P, Hahn RG. Low melatonin excretion during mechanical ventilation in the intensive care unit. Clin Sci (Lond) 2004;107(1):47–53. doi: 10.1042/CS20030374. [DOI] [PubMed] [Google Scholar]
- 25.Jaber S, Chanques G, Altairac C, et al. A prospective study of agitation in a medical-surgical ICU: incidence, risk factors, and outcomes. Chest. 2005;128(4):2749–2757. doi: 10.1378/chest.128.4.2749. [DOI] [PubMed] [Google Scholar]
- 26.Guttormson JL, Chlan L, Weinert C, Savik K. Factors influencing nurse sedation practices with mechanically ventilated patients: a US national survey. Intensive Crit Care Nurs. 2010;26(1):44–50. doi: 10.1016/j.iccn.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Kross EK, Gries CJ, Curtis JR. Posttraumatic stress disorder following critical illness. Crit Care Clin. 2008;24(4):875–887. ix–x. doi: 10.1016/j.ccc.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Monger E. Strategies for nursing conscious mechanically ventilated patients in Southampton and Amsterdam. Intensive Crit Care Nurs. 1995;11(3):140–147. doi: 10.1016/s0964-3397(95)80631-8. [DOI] [PubMed] [Google Scholar]
- 29.Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375(9713):475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 30.Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825–832. doi: 10.1097/CCM.0b013e31819b8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill KV, Voils SA, Chenault GA, Brophy GM. Perceived versus actual sedation practices in adult intensive care unit patients receiving mechanical ventilation. Ann Pharmaco ther. 2012;46(10):1331–1339. doi: 10.1345/aph.1R037. [DOI] [PubMed] [Google Scholar]
- 32.Barr J, Fraser GL, Puntillo K, et al. American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]