Abstract
Extraocular muscles (EOMs) are highly specialized skeletal muscles that originate from the head mesoderm and control eye movements. EOMs are uniquely spared in Duchenne muscular dystrophy and animal models of dystrophin deficiency. Specific traits of myogenic progenitors may be determinants of this preferential sparing, but very little is known about the myogenic cells in this muscle group. While satellite cells (SCs) have long been recognized as the main source of myogenic cells in adult muscle, most of the knowledge about these cells comes from the prototypic limb muscles. In this study, we show that EOMs, regardless of their distinctive Pax3-negative lineage origin, harbor SCs that share a common signature (Pax7+, Ki67−, Nestin-GFP+, Myf5nLacZ+, MyoD-positive lineage origin) with their limb and diaphragm somite-derived counterparts, but are remarkably endowed with a high proliferative potential as revealed in cell culture assays. Specifically, we demonstrate that in adult as well as in aging mice, EOM SCs possess a superior expansion capacity, contributing significantly more proliferating, differentiating and renewal progeny than their limb and diaphragm counterparts. These robust growth and renewal properties are maintained by EOM SCs isolated from dystrophin-null (mdx) mice, while SCs from muscles affected by dystrophin deficiency (i.e., limb and diaphragm) expand poorly in vitro. EOM SCs also retain higher performance in cell transplantation assays in which donor cells were engrafted into host mdx limb muscle. Collectively, our study provides a comprehensive picture of EOM myogenic progenitors, showing that while these cells share common hallmarks with the prototypic SCs in somite-derived muscles, they distinctively feature robust growth and renewal capacities that warrant the title of high performance myo-engines and promote consideration of their properties for developing new approaches in cell-based therapy to combat skeletal muscle wasting.
Keywords: Extraocular muscles, Retractor bulbi, Satellite cells, FACS, Clonal growth, Renewal, Engraftment, Cre/loxP, Mdx4cv, Pax3, Pax7, Myf5, MyoD, Nestin-GFP, Myosin light chain 3F-nLacZ, Duchenne muscular dystrophy
Introduction
Extraocular muscles (EOMs) comprise a group of highly specialized skeletal muscles controlling eye movements (Demer, 2007). The EOMs represent a unique skeletal muscle phenotype based on a range of properties, including specialized patterns of innervation and diversity of expressed sarcomeric myosin isoforms (Spencer and Porter, 2006). The developmental origin of EOMs adds another distinct facet to this muscle group. While body and limb muscles develop from the somites, EOMs are descended from prechordal and paraxial head mesoderm (Couly et al., 1992; Noden and Francis-West, 2006). Accordingly, the progenitors establishing the EOM primordia are of Pax3-negative origin, in contrast to the Pax3-positive lineage origin of limb and body muscles (Goulding et al., 1994; Horst et al., 2006; Tajbakhsh et al., 1997). Nevertheless, EOM development is orchestrated by the same members of the bHLH transcription factor family (MyoD, Myf5, MRF4, myogenin) that are involved in the specification and differentiation of body and limb muscles (Kassar-Duchossoy et al., 2004; Noden and Francis-West, 2006; Sambasivan et al., 2009).
The EOMs are also distinct from other skeletal muscles in their differential response to disease, being preferentially involved or spared in a variety of metabolic, mitochondrial and neuromuscular disorders (Kaminski et al., 2002; Schoser and Pongratz, 2006; Valdez et al., 2012; Yu Wai Man et al., 2005). Especially intriguing for muscular dystrophy research is the sparing of this muscle group in Duchenne muscular dystrophy. EOMs remain anatomically and functionally spared even at the late stages of the disease despite the severe pathology observed in other skeletal muscles (Kaminski et al., 1992; Khurana et al., 1995). Likewise, EOMs are spared in animal models of muscular dystrophy resulting from the absence of dystrophin or other dystroglycan complex-related proteins (Khurana et al., 1995; Porter and Karathanasis, 1998; Porter et al., 2001). The mechanism behind EOM sparing has remained unclear (Pacheco-Pinedo et al., 2009; Porter, 1998; Zeiger et al., 2010), but specific properties of EOM myogenic progenitors have been proposed as possible contributory factors (Kallestad et al., 2011; Porter et al., 2006).
Satellite cells (SCs), Pax7+ myogenic progenitors situated between the basal lamina and sarcolemma of the myofibers, have long been recognized as the major source of myonuclei during muscle growth and repair (Mauro, 1961; Seale et al., 2000; Yablonka-Reuveni, 2011). SCs are proliferative during the postnatal growth phase, adding nuclei to the enlarging myofibers (Moss and Leblond, 1971; White et al., 2010). In adult muscles, SCs are typically quiescent, but can be activated in response to muscle injury (Montarras et al., 2013; Schultz et al., 1978). Depending on the magnitude of tissue trauma, SCs may divide minimally to repair subtle damage within individual myofibers or produce a larger progeny pool that forms new myofibers in cases of overt muscle trauma (Grounds and Yablonka-Reuveni, 1993; Hawke and Garry, 2001). In addition to generating differentiated myogenic progeny, at least some SCs can self-renew, thereby meeting the defining criteria of bona fide resident stem cells (Collins et al., 2005; Kuang et al., 2007; Sacco et al., 2008).
Most SC studies, whether performed in vivo or with isolated cells, have focused on the easily accessible limb muscles. Such studies have provided significant insights into SC molecular/cellular signatures during growth, aging and repair (Chang and Rudnicki, 2014; Zammit et al., 2006). Limb muscles have also been the main focus in studies on the potential of SCs as donor cells to alleviate muscle wasting, especially in dystrophinopathy. Despite their myogenic aptitude, enthusiasm for using limb SC-derived myoblasts has been weakened by their limited expandability and engraftment outcomes (Konieczny et al., 2013; Miller et al., 1997; Tremblay et al., 1993; Wilschut et al., 2012). This drawback may be related to heterogeneity in limb SC “stemness” with only a minority of cells appearing capable of both progeny production and self-renewal (Day et al., 2010; Kuang et al., 2007). Indeed, in mouse transplantation studies only a very small fraction of donor SCs was able to contribute to myofiber formation and to enter the satellite cell niche within the host muscle (Beauchamp et al., 1999; Collins et al., 2005; Sacco et al., 2008). Subsequently, alternative (non-SC) sources of donor cells for muscle therapy have been explored (Negroni et al., 2011; Tedesco and Cossu, 2012), while bona fide SCs from non-limb muscle groups have received only limited attention. Significantly, insight into SCs in muscle groups that are spared from the impact of Duchenne muscular dystrophy could unveil new cellular and molecular players that may be beneficial for treating muscle wasting disorders.
Although the unique anatomical and physiological features of EOMs have drawn much interest (Andrade et al., 2000; da Silva Costa et al., 2012; Spencer and Porter, 2006; Zhou et al., 2010), knowledge about the EOM myogenic progenitors is currently limited. One study that has used transgenic Pax7-nGFP mice for isolating and characterizing myogenic progenitors, placed the Pax7+ SCs as the main myogenic population in adult EOM as in the other muscles (Sambasivan et al., 2009). Additionally, the isolation of a number of EOM populations (SP, MP, EECD34) containing myogenic cells has been described (Kallestad et al., 2011; Kallestad and McLoon, 2010; McDonald et al., 2014). Our interest in EOM SCs originated from an earlier study where we established EOM-derived myogenic cultures as a control for analysis of the myogenic potential of retina pericytes (Kirillova et al., 2007). Using our standard approach for isolating myogenic cells (Danoviz and Yablonka-Reuveni, 2012), we observed that EOM cultures produced numerous myogenic progeny despite the very small initial number of cells obtained from these tiny muscles (unpublished).
In the current study we aimed to specifically characterize EOM SCs and their regenerative capacity. We focus on their in-situ signature, in-vitro expansion, and in-vivo engraftment aptitude. To identify potential unique properties of EOM SCs, most aspects were analyzed in comparison to SCs from the limb and diaphragm somite-derived muscles.
Materials and methods
Mice
All mice were on C57BL/6 background and from colonies we have maintained long-term at the University of Washington. Mice were housed in a pathogen-free facility under 12:12-h light/dark cycle and were fed ad libitum Lab Diet 5053 (Purina Mills). Animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington. Most studies were performed with adult mice (4–6 month old) but as indicated in the Results, some studies were done with younger (3-week old) or older (12–24 month old) mice, and unless otherwise noted, only males were used. The following strains (all described in our previous studies) were used for tissue and cell isolation: (i) Knockin heterozygous Cre males MyoDCre [Myod1tm2.1(icre)Glh, (Kanisicak et al., 2009)], and Pax3Cre [(Pax3tm1(cre)Joe/J, (Engleka et al., 2005)] were crossed with knockin reporter females R26mTmG [Gt(ROSA)26Sortm4(ACTB-tdTomato,–EGFP)Luo/J, (Muzumdar et al., 2007)] to generate F1 animals (Stuelsatz et al., 2012; Stuelsatz et al., 2014). (ii) Transgenic, heterozygous Nestin-GFP (Mignone et al., 2004) and MLC3F-nLacZ [MLC3F=muscle-specific myosin light chain 3F; AKA 3F-nlacZ-2E and Tg(Myl1-lacZ) 1Ibdml/J, (Beauchamp et al., 2000; Kelly et al., 1995)]. (iii) Myf5nLacZ, knockin heterozygous (Tajbakhsh et al., 1996; Tajbakhsh et al., 1997). When indicated, reporter mice were crossed to generate double heterozygous Nestin-GFP/MLC3F-nLacZ and Nestin-GFP/Myf5nLacZ (Day et al., 2010). (iv) Dystrophin-null mdx4cv (Chapman et al., 1989; Im et al., 1996) were crossed with Nestin-GFP mice to produce mdx4cv/Nestin-GFP experimental mice.
Additionally, Rag1−/−/mdx5cv double-null mice (8–9 week old) were used as hosts in cell transplantation studies. This dystrophin-null strain that also lacks mature T and B cells (Mombaerts et al., 1992) was initially developed at the Jackson Lab for studies of Dr. Emanuela Gussoni (Boston Children’s Hospital, unpublished). Notably, the mdx4cv and mdx5cv alleles were generated by point mutations upon mutagen-treatment of male mice (Banks et al., 2010; Chapman et al., 1989; Im et al., 1996). These mdx “cv” strains have been preferred by some laboratories due to the reduced occurrence of revertants (i.e., spontaneously appearing dystrophin+ myofibers, Lu et al., 2000) compared to the “standard” mdx mice (spontaneous mutation) (Arpke et al., 2013; Danko et al., 1992; Decrouy et al., 1997; Im et al., 1996; Judge et al., 2006).
Tissue harvesting
Hindlimb muscles (LIMB; consisting of tibialis anterior [TA], extensor digitorum longus [EDL] and gastrocnemius) and whole diaphragm (DIA; includes the costal and crural muscles and the central tendon) were harvested according to our routine procedures for cell isolation or tissue sectioning (Danoviz and Yablonka-Reuveni, 2012; Day et al., 2007; Stuelsatz et al., 2012). For the EOM we processed two types of preparations, one for tissue sections and one for cell isolation as detailed in our recent publication (Stuelsatz et al., 2014). A typical EOM preparation for histology comprised the EOMs, the retractor bulbi muscle (an ocular accessory muscle involved in retracting the eye into the orbit forcing the mouse third eyelid across the cornea), the optic nerve and the associated periocular connective tissues, all attached to the eyeball (LifeMap, 2013), while for cell isolation only the EOMs were collected. In both types of EOM preparations, all 4 rectus muscles were included in their entirety, but the 2 oblique muscles typically tear during isolation (due to their association with the trochlea or the orbit wall), resulting in loss of a portion of these muscles. Tissues harvested for histology or cell culture were kept at room temperature in PBS or DMEM solution, respectively, for subsequent processing. The DMEM solution consists of Dulbecco’s modified Eagle’s medium (high glucose, with L-glutamine, 110 mg/l sodium pyruvate, and pyridoxine hydrochloride, Hyclone) supplemented with antibiotics (50 U/ml penicillin and 50 mg/ml streptomycin, Gibco-Invitrogen) and was used throughout the various procedures described below.
Cell isolation
For each preparation, cells were typically isolated from 2–3 mice. Harvested tissues were dissociated according to our two established methods that rely either on Pronase digestion (Danoviz and Yablonka-Reuveni, 2012; Yablonka-Reuveni, 2004) or collagenase/dispase digestion (Ieronimakis et al., 2010; Stuelsatz et al., 2014). When digested with Pronase, the isolated muscles were thoroughly cleaned while gently tearing the muscles apart into small pieces with forceps. Tissue digestion was then carried out for 1 h at 37 °C (with gentle swirling every 15 min) followed by vigorous trituration to release cells from the muscle bulk. When digested with collagenase/dispase, the isolated muscles were superficially cleaned from fascia and associated connective tissue, then cut into small pieces using scissors and digested for 45 min at 37 °C with intermittent triturations every 15 min. For both tissue digestion approaches, the isolated cell suspensions were filtered through cell strainers (100 μm and 40 μm) and cells were then harvested by centrifugation at 1000 μg for 10 min.
Cell sorting by flow cytometry
Cell pellets obtained from digested muscles were suspended in DMEM containing 10% horse serum and 10 μM Hoechst 33342 and incubated for 30 min at 37 °C, after which preparations were kept on ice. From this point, two different protocols, detailed in our previous publications, were used to isolate SCs by flow cytometry (Day et al., 2007; Stuelsatz et al., 2014). When working with Pronase-digested preparations from Nestin-GFP mice, the Hoechst-labeled cells were directly sorted based on GFP expression (Day et al., 2007). For cell preparations harvested by collagenase/dispase, the Hoechst-labeled cells were pelleted, resuspended in a solution of fluorescent antibodies and incubated on ice for 1 h (Stuelsatz et al., 2014). The antibody cocktail consisted of anti-Sca1 (APC, clone D7), anti-CD31 (APC, clone 390) and anti-CD45 (PE-Cy7, clone 30-F11), all from eBioscience, diluted in ice-cold PBS containing 0.3% bovine serum albumin (BSA, Omnipur, fractionV; Calbiochem) to a final concentration of 600 ng/106 cells for each antibody except for the anti-Sca1, used at 300 ng/106 cells (Stuelsatz et al., 2014). The cells were then rinsed with and resuspended in ice-cold PBS-0.3%BSA before sorting. This second approach that relies on collagenase/dispase digestion and sorting based on both Nestin-GFP and cell surface antigen, allows measuring CD45+ cells when desired (see Results). Additionally, removal of endothelial cells (CD31+), which also express the Nestin-GFP transgene (Day et al., 2007), is achieved by the triple antigen CD31/CD45/Sca1 negative selection, in agreement with the published signature of LIMB SCs (Ieronimakis et al., 2010; Sacco et al., 2008). Differently, our Pronase-based protocol used for muscle dissociation, minimizes the contribution of non-myogenic cells even without cell sorting (Danoviz and Yablonka-Reuveni, 2012; Shefer et al., 2006), yielding pure myogenic cultures from the Nestin-GFPhigh sorted populations (Day et al., 2007).
An Influx Cell Sorter (BD Biosciences, equipped with 350, 488 and 638 nm lasers) was used and sorted cells were collected within the G0–G1 population defined based on Hoechst staining, thereby eliminating residual cell aggregates and cellular debris. In some experiments, we also labeled the cells with 7-amino-actinomycin D (7AAD, 1:200; Invitrogen, added just before sorting) for dead cell exclusion. The 7AAD analyses demonstrated that our sorted cell fractions of interest were free of dead cells; hence, selecting for G0–G1 cells based on Hoechst dye was sufficient. Sort gates were determined by comparing fluorophore signal intensities between the unstained control and each single antibody/fluorophore control. Positive events were set to at least 10 times the fluorescence intensity of negative events. Data was acquired at 20,000–100,000 events per sample and sorted cells were collected in standard growth medium. Post-hoc analysis and flow cytometry plots were generated using FlowJo (TreeStar).
Analysis of sorted cells in primary culture and clonal assays
Growth medium consisted of DMEM containing 20% fetal bovine serum (FBS, Gibco-Invitrogen), 10% horse serum (HS, Gibco-Invitrogen), and 1% chicken embryo extract prepared from whole 10-day-old embryos (Shefer and Yablonka-Reuveni, 2005). This growth medium supports both proliferation and differentiation of cultured SCs (Shefer et al., 2006; Yablonka-Reuveni, 2004). Tissue culture wells were coated with Matrigel diluted in DMEM (1 mg/ml final concentration, BD Biosciences) (Danoviz and Yablonka-Reuveni, 2012). Cultures were incubated at 37 °C, 5%CO2 in a humidified tissue culture incubator and growth medium and was replaced every third day after initial plating.
Primary cultures were initiated in 24-well trays at a density of 1000 cells per well. For each preparation (per each muscle group) 3–5 wells were typically seeded for analyses. Depending on overall cell density at the time point of interest, 10–25 arbitrary fields were quantified per well using a 20 × objective. Quantifications were based on counting DAPI-stained cell nuclei (and when applicable immunolabeled cells as described later).
Clones were established in 48-well trays using a seeding density of 4 cells per well for LIMB and 2 cells per well for DIA and EOM preparations. This clonal plating density was based on our pilot studies that indicated a lower clonal recovery of LIMB SCs. Following our earlier studies (Day et al., 2010; Shefer et al., 2004), clonal cultures were left undisturbed during the initial 4 days, then individual colonies were identified and their positions were marked to facilitate monitoring progress of each clone at later time points. Cultures were maintained for up to 3 weeks to monitor growth, formation of myotubes, and the emergence of Nestin-GFP positive cells. Clone size was determined at day 10 by counting the number of DAPI-stained nuclei in fixed cultures. For each clone, a manual cell counting of individual nuclei (in single cells and myotubes) was performed using ImageJ software after merging in Photoshop all low magnification images (taken with 4 × objective). Alternatively, clone size was estimated according to the size of the microscopic field the clone occupied on days 7 and 21 (i.e. clones were categorized as small, medium, large or X-large depending on whether the clone occupied a 20 ×, 10 ×, 4 × or several 4 × objective fields, respectively).
Immunofluorescence analysis of freshly isolated cells, cultured cells, and single myofibers
All preparations were fixed with paraformaldehyde (PFA, 2% final concentration) for 10 min at room temperature, then permeabilized with Triton X-100, blocked with normal goat serum, immunolabeled, DAPI stained and mounted with Vectashield, all according to our established protocols (Danoviz and Yablonka-Reuveni, 2012; Shefer et al., 2006). Freshly isolated cells were obtained from Pronase digested muscles following the method described above (except that here, muscles did not require to be as thoroughly cleaned from connective tissues) and pellets were resuspended in 150 μl of PBS and then deposited onto a Superfrost Plus slides (Thermo Fisher Scientific) by cytocentrifugation (1000 rpm for 5 min, Wescor cytopro cytocentrifuge). Notably, the use of cell preparations that were not subjected to cell sorting allowed for a higher cell number yield, achieving optimal pelleting during cytocentrifugation. Cultured cells were prepared by Pronase or collagenase/dispase digestion of the muscles as described above. EOM myofibers were isolated according to our protocol (Keire et al., 2013, Note 18d) and further processed as in our EDL myofiber preparations (Keire et al., 2013; Shefer et al., 2006).
The following primary antibodies were used: anti-Pax7 (mouse IgG1, 1:100, bioreactor supernatant, Developmental Studies Hybridoma Bank [DSHB]); anti-sarcomeric myosin heavy chain (mouse IgG2b, clone MF20, hybridoma supernatant, DSHB, 1:2); anti-Ki67 (rabbit IgG, 1:300, Abcam, ab66155). Secondary antibodies used were all produced in goat and conjugated with AlexaFluor (Invitrogen, 1:1000 dilution); these included anti-mouse IgG1-568; anti-mouse IgG2b-647; anti-rabbit IgG-568. GFP reporter detection in preparations from Nestin-GFP mice was typically done based on direct fluorescence, alternatively, as the fixation for immunostaning can reduce the intensity of direct GFP fluorescence, an anti-GFP antibody was used if necessary for signal amplification (rabbit IgG, 1:20,000 Abcam, ab6556, Day et al., 2010).
Analysis of tissue sections (immunofluorescence, H&E staining)
Freshly harvested tissues were embedded in OCT (Tissue-Tek), flash frozen in isopentane cooled with liquid nitrogen and stored at −80 °C until ready to be cut into 10-μm thick transverse sections using a Leica CM1850 cryostat. Sections were placed on Superfrost Plus slides and allowed to air dry for about 30 min before being processed immediately or stored at −80 °C.
For Pax7/laminin double immunostaining, sections were fixed for 10 min in 2% PFA and antigen retrieval (required for optimal Pax7 detection) was performed by incubating slides in a solution consisting of 10 mM Tris (pH 9), 1 mM EDTA, 0.05% Tween 20 for 30 min at 97 °C and cooling slides in the same buffer at room temperature for 10 min (adapted from He et al., 2010). Slides were removed from retrieval buffer, allowed to air dry briefly, rinsed in Tris buffered saline (TBS) for 5 min and allowed to air dry briefly again. Sections were then blocked overnight with normal goat serum, reacted with antibodies, counterstained with DAPI and mounted, all following our routine immunostaining protocol (Danoviz and Yablonka-Reuveni, 2012; Stuelsatz et al., 2014). Primary antibodies included the anti-Pax7 detailed above and an anti-laminin (1:100; rabbit IgG; ab2034, Chemicon/Millipore). Secondary antibodies were AlexaFluor-conjugated goat anti-rabbit IgG-568 and goat anti-mouse IgG2a-488 (1:1000; Invitrogen).
For hematoxylin and eosin (H&E) staining, sections of unfixed tissues were hydrated in water for 5 min and then stained in Harris hematoxylin for 3–5 min. Slides were then rinsed in tap water, dipped in Scott’s bluing reagent, rinsed in tap water, incubated in 70% ethanol for 2 min, stained in eosin Y (0.25% solution) for 2 min, dehydrated through graded alcohols and cleared in Xylene substitute before being mounted with Xylene substitute mounting medium (Thermo Fisher Scientific).
X-gal staining
β-galactosidase (β-gal) activity in cells expressing lacZ reporter was detected by X-gal staining in cultured cells or whole TA muscles, as previously described (Day et al., 2010; Stuelsatz et al., 2012). Cell cultures were fixed in 2% PFA for 10 min at room temperature as described above for cell culture immunostaining. When staining cells immediately after plating, the seeded cells were allowed to settle down for ~30 min and then fixed as above. Whole TAs were cut longitudinally into 2 halves and then fixed in the same manner as the cell cultures, except that fixation was done for 15 min. Fixed specimens were incubated in X-gal staining solution for 4–6 h or overnight, for cultured cells or whole muscle, respectively.
SC transplantation to host TA muscles and engraftment analysis
Host mice (Rag1−/−/mdx5cv, 8–9 week old) were anesthetized with isoflurane. Analgesic (ketoprofen) was then administrated subcutaneously (5 mg/kg) and the site of injection was shaved and prepped with ethanol and betadine. A small incision was made through the skin to expose the base of the TA from the distal tendon to mid-TA. The intramuscular injection was done with a Hamilton syringe equipped with a 31 G needle (0.85″ length, point style 4 at 12° bevel) entering in the lower end of the muscle and traveling to about 3/4 up before being slowly pulled back while releasing cells in order to disperse cells evenly along the needle path. For each host animal, the TA from one leg was injected with EOM donor SCs, while the TA from the contralateral leg was injected with LIMB donor SCs (15 μl per injection). Following cell injection the skin was closed with a surgical adhesive and a supplemental isotonic saline solution was administrated via intra-peritoneal injection.
Donor SCs were isolated from Pronase-digested muscles of adult (4–5 month old) Nestin-GFP/MLC3F-nLacZ mice by GFP-based flow cytometry as described above. As the MLC3F-nLacZ transgene produces a specific LacZ expression in myofiber nuclei (Beauchamp et al., 2000; Day et al., 2010; Kirillova et al., 2007; Zammit et al., 2006), it has been used to detect donor SC-derived differentiated progeny in engraftment studies (Collins et al., 2005). Freshly sorted cells were collected in PBS and their concentration adjusted to 1000 cells per 15 μl and kept on ice until the injection. Parallel aliquots were followed-up in primary culture conditions (as detailed above) for quality control of the injected preparations. Both males and females were used as host and donor mice but sexes were always matched between the donor and the corresponding host mouse.
Transplanted muscles were collected 21 days post-transplantation to evaluate engraftment by X-gal staining and genomic analysis of donor-derived cells based on levels of LacZ and GFP genes (detailed below). For both engraftment analysis approaches, controls with vehicle only injected TAs were also performed to ensure reliability of detection. For the genomic analysis, DNA from whole engrafted TA muscles was isolated using DNeasy kit (Qiagen) and used for analyzing by PCR the level of GFP and MLC3F-nLacZ genes (donor-derived) vs. H-ras and Cyp1A1 (contributed by both host and donor cells). PCR was performed using HotStarTaq DNA polymerase (Qiagen) in a 25 μl total volume using 10 pmol of forward and reverse primers and 2 μl or 4 μl of DNA (100 ng/μl stock solution) per reaction for GFP and LacZ or H-ras and Cyp1A1, respectively. The PCR program consisted of incubation at 95 °C for 10 min, followed by 28–32 cycles of 94 °C for 30 s, 62 °C for 45 s, 72 °C for 1 min, and a final extension step at 72 °C for 10 min. The number of amplification cycles was 28 for H-ras and Cyp1A1, and 32 for LacZ and GFP. PCR products were separated on 1.5% agarose gels containing 1:10,000 dilution of SYBR Green I (Molecular Probes). Gels were imaged using Gel Logic 212 Pro (Carestream). Quantification of the resulting bands for each PCR product was done using ImageJ software. Each donor gene product (LacZ and GFP) was first normalized with each of the reference gene product (Cyp1A1 and H-ras). The EOM/LIMB ratio was then calculated for each of these normalized values. Given that within the same mouse these ratios are comparable regardless of the donor or reference gene used, final results are presented per each mouse as the average of these ratios. Primer set sequences (and product size):
LacZ, GCTTCATCCACCACATACAG/CGATGAGCGTGGTGGTTATG (402 bp).
GFP, CGACGTAAACGGCCACAAG/GTAGTTGCCGTCGTCCTTG (259 bp).
H-ras, ATGACAGAATACAAGCTTGTGGTG/GGCAAATACACAGAGGAAGCCCTC (459 bp, 249 bp if used for cDNA analysis) (Kastner et al., 2000; Smith et al., 1994).
Cyp1A1, GACACAGTGATTGGCAGAGA/AACGGATCTATGGTCT-GACC (144 bp) (Dai et al., 2009).
Microscopy and imaging
Observations were made with an inverted fluorescent microscope (Eclipse TE2000-S, Nikon), except for TA engraftment studies by X-gal staining where a Nikon Stereoscopic Zoom Microscope (SMZ1500) was used. Images were typically acquired using CoolSNAP ES monochrome CCD camera (Photometrics) controlled with MetaVue Imaging System (Universal Imaging Corporation). For acquiring real color images of X-gal staining, images were taken with a Digital Sight DS-Ri1 color camera controlled by NIS-Elements F software (Nikon). Digitized images were assembled using Adobe Photoshop software.
Data analysis and statistics
Graphs were generated and data were analyzed by one-way ANOVA with Bonferroni–Holm or Tukey post-hoc analysis using Excel with Daniel’s XL Toolbox Add-In (by Daniel Kraus, Würzburg, Germany). For all studies, unless otherwise noted, p-values of less than 0.05 were considered to be statistically significant and 3–5 experimental repeats were performed.
Results and discussion
Although derived from a Pax3-negative lineage, EOM myogenic progenitors display classic SC features in common with their limb and diaphragm counterparts
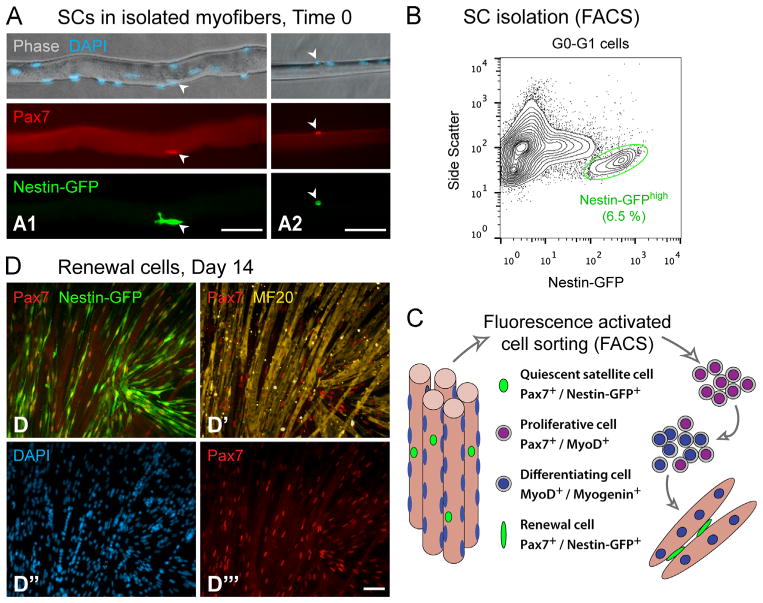
In accordance with EOM development from a Pax3-negative lineage (Harel et al., 2009; Sambasivan et al., 2009; Stuelsatz et al., 2014), myogenic progenitors in adult EOMs do not demonstrate ancestral, nor current, expression of Pax3 (Fig. S1). Regardless of this distinct linage origin, in common with all muscles examined to date, myogenic progenitors in adult EOMs display classic SC features based on being of MyoD-positive lineage origin (Fig. S1; in accordance with Kanisicak et al., 2009) and expressing Pax7 (Fig. S2; as previously reported for postnatal (P10) EOM in Harel et al., 2009). The presence of Pax7+ SCs is evident in isolated EOM myofibers along with co-expression of the Nestin-GFP transgene (Fig. 1A), previously reported by us as a characteristic of SCs in LIMB and DIA (Day et al., 2007; Day et al., 2010; Fig. S1 in Stuelsatz et al., 2012). Pax7/Nestin-GFP co-expression was additionally established in freshly isolated SC preparations from EOM, LIMB and DIA (Fig. S2). Also, in common with SCs in all muscle groups examined to date (Beauchamp et al., 2000; Day et al., 2010; Ono et al., 2010; Stuelsatz et al., 2012), myogenic progenitors in adult EOM are β-gal positive when analyzed using the Myf5nLacZ knockin reporter mouse (Fig. S3).
Fig. 1.
In-situ detection, isolation and cell culture marker signature of EOM SCs from adult mice. (A) Isolated EOM myofibers from adult Nestin-GFP transgenic mice demonstrating co-expression of the SC marker Pax7 and transgenic Nestin-GFP along with DAPI+ nuclei. EOM myofibers vary in their dimensions and shown images illustrate “thick” (A1) and “narrow” (A2) specimens. Arrowheads point to examples of SCs defined based on triple-labeling for Pax7, Nestin-GFP and DAPI. (B) A representative flow cytometry profile of Nestin-GFPhigh cells isolated from EOM by Pronase digestion. The plot shows GFP fluorescence (X-axis) vs. the side scatter parameter (Y-axis) among all G0–G1 cells. The % value indicates the frequency of the highlighted Nestin-GFPhigh population out of the parent G0–G1 population analyzed. The side-scatter parameter, measuring cell granularity (internal complexity, Yablonka-Reuveni, 1988), has been used here to better distinguish the SC population. Additional cell sorting plots of SCs isolated from EOM vs. LIMB and DIA are shown in Fig. S3A and B. (C) Schematic of myogenic marker expression by EOM SCs and their progeny, showing the typical progression through proliferation, differentiation and renewal stages in primary culture (modified from Yablonka-Reuveni, 2011). SCs were sorted as shown in panel B and the expression of the characteristic myogenic markers was analyzed by immunostaining with commonly used antibodies (Danoviz and Yablonka-Reuveni, 2012; Shefer et al., 2006). (D–D‴) Pax7/sarcomeric myosin (MF20) immunostaining of day 14 cultures demonstrating Pax7+ renewal cells expressing the Nestin-GFP transgene and located in between the myotubes (MF20+). Nuclei of immunostained cultures are highlighted by DAPI counterstaining. Scale bars, 50 μm.
Next, we have extended our approach for SC isolation from LIMB and DIA SCs (Day et al., 2007; Day et al., 2010), and utilized the Nestin-GFP transgene fluorescence to sort EOM SCs by flow cytometry (Figs. 1 and S3). Antigen-based cell sorting further demonstrated that the bulk of EOM SCs (Nestin-GFPhigh) are CD31−/CD45−/Sca1− (Fig. S3B and G–G‴), in agreement with the published signature of LIMB SCs (Ieronimakis et al., 2010; Sacco et al., 2008). Our characterization of the sorted Nestin-GFPhigh cells according to myogenic marker expression (by immunostaining) in primary culture, shows a typical progression through proliferation, differentiation and renewal, as summarized schematically in Fig. 1C. During the initial days in culture (days 3–4), essentially all cells were positive for Pax7 and MyoD, a common signature of proliferating progeny of SCs (Halevy et al., 2004; Shefer et al., 2006; Zammit et al., 2004), followed by the appearance of differentiating, Myogenin+ single cells, while formation of myotubes, that just had begun at day 6, was evident throughout the culture by day 7. EOM cultures were also monitored for the dynamics of Nestin-GFP transgene expression. As we have previously demonstrated for LIMB, SC proliferating progeny do not retain Nestin-GFP expression, but once cultures increase in cell density and develop myotubes, Nestin-GFP expression re-emerges in quiescent Pax7+/MyoD− cells (i.e. renewal cells) (Day et al., 2007; Day et al., 2010). Likewise, in the present EOM study, while proliferating progeny of cultured SCs no longer exhibit Nestin-GFP fluorescence, later, the transgene is re-expressed in numerous renewal cells (i.e., Nestin-GFP+/Pax7+) that appear among the myotubes (detected morphologically and by immunostaining for sarcomeric myosin) (Figs. 1C and D and S3G–G‴) and are distinct from the differentiating MyoD+/Myogenin+ single cells (Day et al., 2007). Notably, while the detection of renewal cells in SC cultures according to the emergence of Pax7+/MyoD− cells has been reported (Day et al., 2009; Halevy et al., 2004; Ono et al., 2010; Shefer et al., 2006; Zammit et al., 2004), the expression of Nestin-GFP in renewal cells provides a visual means to monitor the dynamics of SC renewal in live cultures.
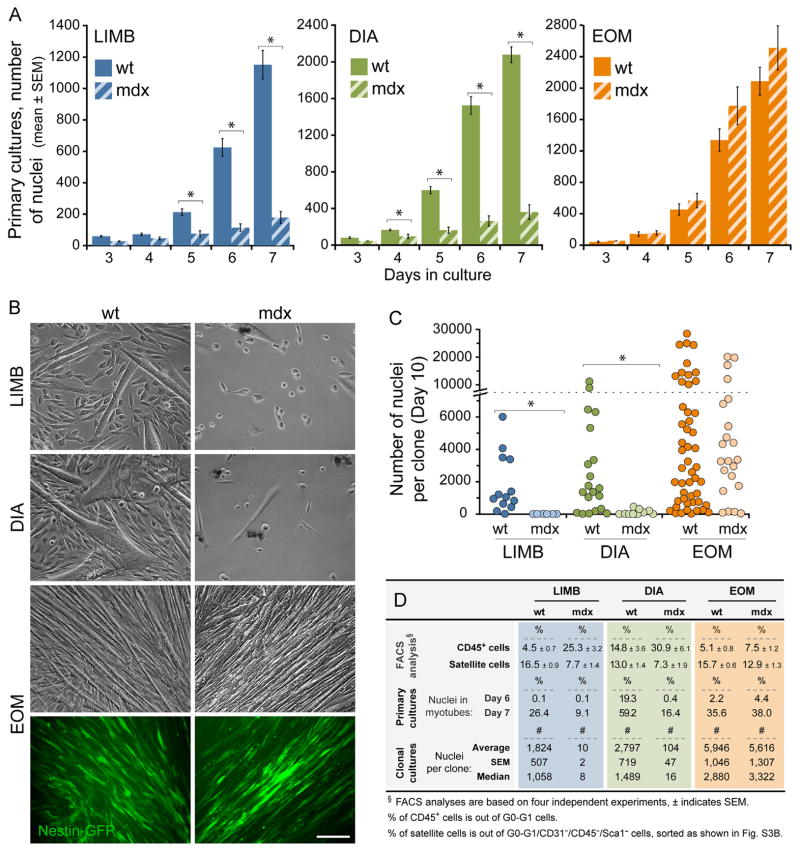
Progeny of EOM SCs outperform their LIMB and DIA counterparts in primary culture and clonal analyses – evidence for cell intrinsic distinctions
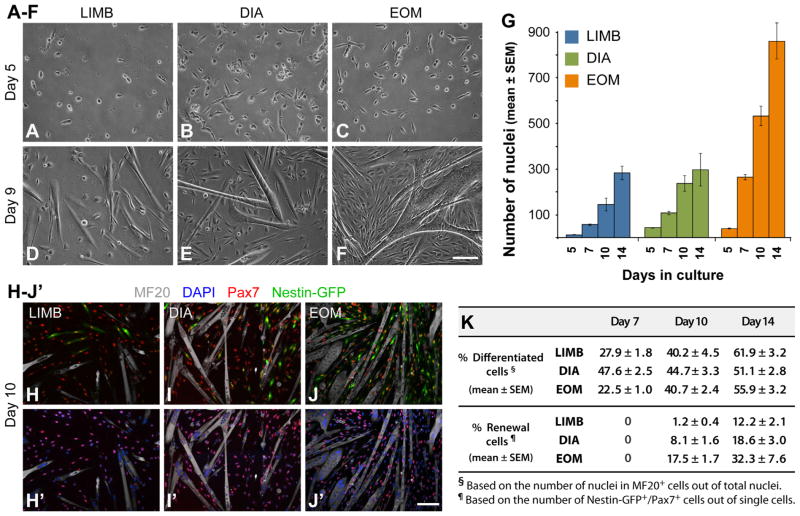
SCs from LIMB, DIA and EOM muscles (isolated based on Nestin-GFP fluorescence as in Figs. 1B and S3A) were plated at the same density across muscle groups (1000 cells per well in 24-well trays) to follow up the dynamics of cell growth, differentiation and renewal (Fig. 2). Representative phase images are shown in Fig. 2A–F and overall growth patterns based on the total number of nuclei (DAPI+ nuclei in single cells and myotubes) are illustrated in Fig. 2G. For all three muscle groups, there was little if any growth before the 3rd culture day, followed by a proliferative phase between days 3 and 5, and formation of myotubes that became evident throughout the cultures by day 7 along with continuous cell expansion that was more robust in EOM cultures as clearly discerned by culture days 10 and 14. Growth dynamics were further investigated by double-immunostaining for Pax7 and sarcomeric myosin (MF20) together with DAPI staining (Fig. 2H–K). While at late time points the percentage of nuclei in differentiated (MF20+) cells among all three muscle groups appeared to be conserved with a value of ~40% on day 10 and ~50–60% on day 14 (Fig. 2K), DIA cultures persistently exhibited an earlier onset of differentiation, already reaching their maximum fusion index by day 7 (Fig. 2K). The quantification of Nestin-GFP+/Pax7+ cells (Fig. 2K) demonstrates a higher presence of renewal cells in EOM cultures. This enhanced renewal could possibly be related to the overall increased cell density in EOM primary cultures that, based on SC clonal analyses shown next, appears to reflect an intrinsic property of individual EOM SCs.
Fig. 2.
Growth analysis of LIMB, DIA and EOM SC primary cultures. SCs were isolated from adult Nestin-GFP mice as described in Figs. 1B and S3A and plated at 1000 cells per well in 24-well plates. (A–F) Representative phase images of day 5 and day 9 cultures. (G) The number of total nuclei per microscopic field (20 × objective) was counted based on DAPI staining of cultures fixed on days 5, 7, 10 and 14. Data are expressed as mean ± SEM. Per each culture day, one-way ANOVA analysis reveals a statistical difference (p<0.01) between the three muscle groups, except for DIA vs. EOM cultures on day 5 (p=0.36), and LIMB vs. DIA cultures on day 10 (p=0.05) and on day 14 (p=0.86). (H–J′) Representative fluorescent images of day 10 LIMB, DIA and EOM cultures double-immunostained for Pax7 and sarcomeric myosin (MF20), demonstrating the presence of Pax7+/Nestin-GFP+ renewal cells. (K) Quantification of differentiated and renewal cells on days 7, 10 and 14 of LIMB, DIA and EOM cultures immunostained for Pax7 and sarcomeric myosin (MF20). Scale bars, 100 μm (A–F) and (H–J′).
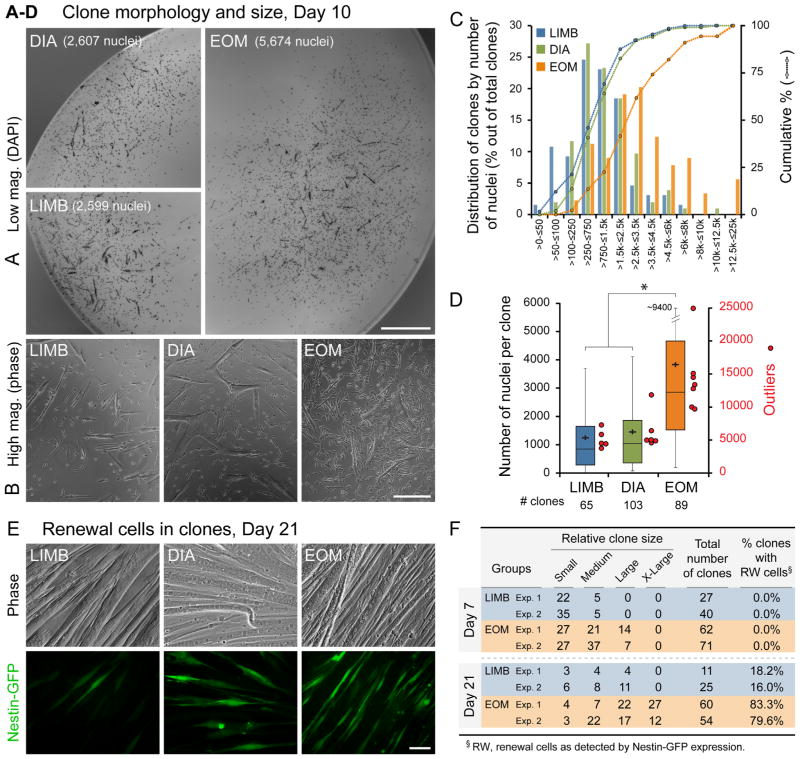
Next, we were interested to determine if the distinct growth and renewal dynamics of EOM primary cultures reflect intrinsic properties of individual SCs. For this, SCs from the three muscle groups, isolated as in Fig. 1B, were analyzed clonally (Fig. 3). In one set of experiments, clone size was quantified at day 10 based on the total number of DAPI+ nuclei in single cells and myotubes (Fig. 3A–D). A striking distinction of the EOM clones, evident even before cell counting, was the increased presence of larger and denser clones as illustrated in the lower and higher magnification images (Fig. 3A and B). The distribution of clones according to the number of nuclei and cumulative percentile curves are depicted in Fig. 3C. This graph clearly displays greater numbers of clones with higher numbers of nuclei in EOM compared to LIMB and DIA. Only EOM depict clones with more than 12,500 nuclei (6%, 5 out of 89 total clones), while at the low end, LIMB and DIA have respectively 12 (18%) and 11 (11%) clones with less than 200 nuclei, whereas EOM has none. Descriptive statistics of the clonal data are further summarized in a boxplot format (Fig. 3D), demonstrating a higher median, a higher average and a higher range of “maximum outliers” for EOM clones. Notably, whereas about 50% (44 out of 89 total) of EOM clones display more than 2900 nuclei, only 8% (5 out of 65 total) of LIMB clones and 13% (13 out of 103 total) of DIA clones harbor such cell number.
Fig. 3.
Clonal analysis of LIMB, DIA and EOM SCs. SCs were isolated from adult Nestin-GFP mice as described in Figs. 1B and S3A. (A–D) Clonal cultures were fixed on day 10 and stained with DAPI for quantifying clone size according to the number of nuclei. (A and B) Examples of (A) low-magnification whole clone images (DAPI stained), and (B) high-magnification phase images demonstrating the typical morphology and cell density of average size LIMB, DIA and EOM clones. (C) Distribution of LIMB, DIA and EOM clones according to the number of nuclei per clone. Clones are clustered in bins in ascending order according to the number of nuclei per clone (x-axis) vs. the percentage of clones in each bin out of total clones analyzed (histograms, left y-axis). In the cumulative curves, each data point (round-shaped, right y-axis) shows the percentage of clones out of total clones analyzed that contain less than, or equal to (≤) the corresponding number of nuclei per clone size range indicated on the x-axis bins. (D) Clonal data analyzed in (C) are also illustrated as boxplots, depicting the quartile distribution, mean (black crosses) and outliers (red circles, right y-axis) for the number of nuclei per clone; whisker ranges and outliers were calculated as previously detailed by us in (Shefer et al., 2013). Asterisk denotes statistically significant difference in clone size between EOM vs. LIMB and DIA clones (p<0.01). (E) Representative phase and fluorescence images of day 21 clones established from LIMB, DIA and EOM SCs showing Nestin-GFP+ renewal cells with a typical higher manifestation in EOM clones. (F) Quantification of SC clones that harbor Nestin-GFP+ cells demonstrates that renewal cells are consistently more frequent in EOM clones. The relative clone size was also estimated by categorizing each clone as small, medium, large or X-large depending on whether the clone occupied a 20×, 10 ×, 4 × or several 4 × objective fields, respectively. On day 7, only EOM cultures displayed clones in the “Large” category. Then, by day 21, solely EOM harbored “X-Large” clones, while clones falling in the two smaller categories represented only 30% of total clones for EOM vs. 60% for LIMB cultures. Scale bars, 1 mm (A), 250 μm (B), 50 μm (E).
In a second set of clonal experiments, we monitored the extent of renewal cells within clones, based on the re-appearance of Nestin-GFP+ cells (Fig. 3E and F). Representative images of day 21 clones (Fig. 3E) illustrate the typical presence of a higher number of renewal cells within EOM clones compared to LIMB and DIA clones. Furthermore, the frequency of clones containing renewal cells was strikingly higher in EOM preparations (Fig. 3F). At 3 weeks, renewal cells were well pronounced in about 80% of the EOM clones while only about 17% of the LIMB clones contained such cells. DIA clones were not quantified here for renewal cells, but values were found to be in the mid-range between LIMB and EOM based on a separate study where LIMB and DIA were compared (unpublished). Note that the appearance and quantity of renewal cells within clones was in accordance with clone size (Fig. 3F), with both cell density and presence of myotubes within clones being critical for the development of renewal cells. Indeed, previous studies have noted a relationship between density of myogenic cultures and extent of renewal cells (Day et al., 2010; Ono et al., 2010).
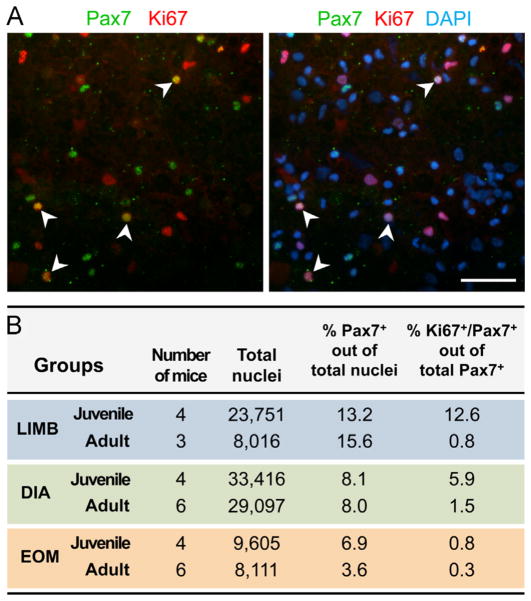
We next investigated the in-vivo proliferative status of EOM SCs compared to their LIMB and DIA counterparts. An a priori increased activity of the EOM cells in vivo, could potentially account for their enhanced in-vitro performance. Our pilot histological studies of muscles from adult mice (4–6 month old) injected with BrdU for 3 days (according to Ieronimakis et al., 2010) only detected extremely rare proliferating cells when examined with anti-BrdU or anti-Ki67 antibodies, while many nuclei in the periocular glandular tissue were positive (data not shown). This rarity of proliferating cells combined with the paucity of SCs in adult muscles (Fig. S2A–C′), prompted us to analyze SC proliferative status in freshly isolated cells released from whole muscle, which permits looking at populations large enough for a meaningful comparison between EOM, LIMB and DIA muscles (Fig. 4A and B). We opted here to look at unsorted populations, allowing us to gain insight into both the SC population (Pax7+ cells) and the non-myogenic cells, and to obtain cell numbers high enough for effective cell pelleting by cytocentrifugation (especially important for the EOM with its smaller size and SC numbers). In all three muscle groups from adult mice (4–6 month old), the percentage of proliferating SCs (Ki67+/Pax7+ cells out of total Pax7+ cells) was low (0.8%, 1.5% and 0.3% for LIMB, DIA and EOM, respectively, Fig. 4B). Although an earlier study reported that mouse EOMs distinctively retain a pool of activated SCs in adult life (McLoon and Wirtschafter, 2002), the proliferative index was also relatively low (~1%) and was expressed as frequency of myofibers harboring BrdU+ SCs in cross sections, while in our current study, values were obtained in reference to total number of Pax7+ cells. For an additional control, Pax7/Ki67 immunostaining analyses were also performed on cells dissociated from muscles of juvenile mice (3-week old) in which SCs are known to be more proliferative. While LIMB and DIA preparations showed an increase in the frequency of proliferating SCs with younger age, only a minimal change was identified for EOM SCs (Fig. 4B), suggesting that in-vivo proliferation of EOM SCs ceases earlier in juvenile life. It is conceivable that the smaller diameter of EOM myofibers indeed entails a shorter period of myofiber enlargement by myonuclear accretion. Human studies have shown that EOM myofibers attain maximal diameter by ~12 year of age, while limb and body muscles reach their maximal myofiber diameter ~20 years of age (Grounds and Shavlakadze, 2011). Myofiber growth by nuclear accretion is known to cease earlier than the subsequent growth by cytoplasmic enlargement; e.g., in the mouse LIMB (EDL muscle) myonuclear addition occurs until 3 weeks of age while growth by volume continues even at 8 weeks of age (White et al., 2010). Intriguingly, this hypothesis of an earlier cession of EOM SC proliferation during the juvenile growth phase could theoretically be an underlying factor for the increased performance of EOM SCs in the in-vitro analyses summarized above.
Fig. 4.
Assessment of in-vivo proliferative activity of SCs. Unsorted cell preparations obtained after Pronase digestion of LIMB, DIA and EOM of adult (4–6 month old) and juvenile (3-week old) mice were subjected to cytocentrifugation, followed by Pax7/Ki67 double immunostaining combined with DAPI counter-staining. (A) Image shown is of a preparation from LIMB of juvenile mice, which contains the highest number of Ki67+/Pax7+ double-labeled cells. (B) SCs were identified based on Pax7 immunostaining and their proliferative activity was determined by calculating the percent of cells double-immunolabelled for Pax7 and Ki67 (Ki67+/Pax7+ cells) out of all Pax7+ cells. Preparations of spleen cells were used as a highly proliferative control for Ki67 immunolabeling (Ki67+ cells: 48.1% out of 7998 and 18.0% out of 4324 total nuclei, in juvenile and adult mice, respectively); these cells were also found negative for Pax7. Scale bar, 50 μm.
Collectively, we have demonstrated that adult EOMs harbor Pax7+ SCs that are quiescent, typical to SCs in prototypic adult muscles, but are uniquely endowed with a high proliferative potential as revealed in cell culture assays. This comprehensive study of SC performance is the first to demonstrate the superiority of EOM SCs not only in primary culture but also at the clonal level, showing a superior growth potential and renewal capacity compared to SCs from “traditional” muscle groups. The superiority of EOM SCs is also maintained in aging according to our primary culture and clonal studies with cells isolated from 22–24 month old mice (Fig. S4A–C).
EOM SCs from dystrophin-null mdx4cv mice retain performance superiority
As detailed in Introduction, the EOMs are spared in Duchenne muscular dystrophy and in animal models lacking dystrophin. The status of SCs in EOMs from dystrophin-null models however, has been scarcely investigated. Although comparisons of EOM vs. LIMB CD34+ populations from mdx mice were published, these studies only focused on relative proportions of freshly sorted cells, but did not analyze the myogenic nature/performance of these populations (Hebert et al., 2013; Kallestad et al., 2011). The prevailing view that SC proliferative potential is exhausted over time in Duchenne muscular dystrophy (due to ongoing demand for SC contribution during the repeated rounds of myofiber degeneration and regeneration, Webster and Blau, 1990) would suggest that EOM SCs are not affected in the dystrophin-null muscle as they are not exposed to ongoing regenerative demand in the in-vivo state. If dystrophin is however required for actual SC performance, once the EOM SCs from dystrophin-null muscles are prompted to enter a regenerative activity, they may exhibit a declining performance compared to wildtype EOM SCs.
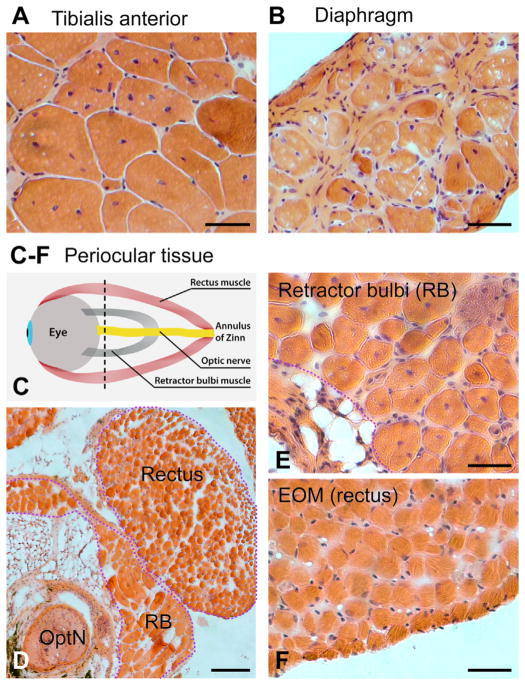
To bypass the distinctive in-vivo milieu of the cells in affected (LIMB and DIA) or unaffected (EOM) environments of dystrophin-null muscles we again compared SCs in culture, using the mdx4cv mice as the source for dystrophin-null SCs. Fig. 5 depicts images of H&E stained muscle sections from LIMB, DIA and EOM of adult mdx4cv mice. The series of histological images illustrates that while LIMB and DIA display typical signs of muscle pathology associated with the absence of dystrophin (central myonuclei, split myofibers and fibrosis, with the latter more pronounced in DIA), EOM show no such pathology. Noticeably, the retractor bulbi (RB), an ocular accessory muscle, although residing adjacent to the EOM (Fig. 5E) and of the same Pax3-negative embryonic origin (Stuelsatz et al., 2014), is affected by dystrophin absence as demonstrated by the extensive presence of central myonuclei similar to LIMB (Fig. 5A). EOM sparing vs. RB pathology is in accord with earlier findings in the “standard” mdx mouse (Andrade et al., 2000; Marques et al., 2007).
Fig. 5.
H&E stained cross sections of tissue preparations from mdx4cv mice. (A) TA, (B) DIA, and (C–F) periocular tissues from 1 year old mice. (C) Schematic of a periocular tissue preparation that comprises the EOMs, the retractor bulbi (RB) and the optic nerve (OptN), harvested together with the eyeball. The black dotted line indicates the level of the sections shown in: (D) periocular tissue (low magnification), (E) RB and (F) EOM (high magnification). Scale bars, 50 μm (A and B) and (E and F), 200 μm (D).
To enable sorting of SCs from mdx4cv muscles, we generated a colony of mdx4cv/Nestin-GFP mice. We first confirmed that the agreement between Pax7 and Nestin-GFP was retained in the dystrophin-null mouse (Fig. S5A–D″). In this set of experiments, the inclusion of antigen-based sorting (Fig. S3B) has enabled us to assay the frequency of CD45+ hematopoietic cells (Fischer et al., 1991), which are involved in inflammatory and fibrosis processes that occur in mdx pathology (Fischer et al., 1991; Morrison et al., 2000; Spencer et al., 2001; Villalta et al., 2009). As done above with wildtype cultures, myogenic homogeneity of the sorted populations was validated according to myogenic marker expression by antibody staining of the resulting primary cultures (data not shown). To analyze SC performance, primary and clonal cultures of sorted SCs (mdx4cv vs. wildtype) were initiated at 1000 cells per well of 24-well trays or as 2–4 cells in 48-well trays, respectively, in the same manner as in Figs. 2 and 3.
Collectively, the comparison between mdx4cv and wildtype adult mice yielded the following key observations (Fig. 6): (i) In accordance with the muscle histopathology evidence (Fig. 5), the frequency of CD45+ cells (out of G0-G1 cells) increased significantly (p<0.03) in mdx4cv LIMB and DIA (Fig. 6D). In mdx4cv EOM preparations, there was only a trend (but not a statistical difference, p=0.06) of CD45+ cell increase that could likely be explained by the overall increase in inflammatory cell production shown for mdx mice (Lagrota-Candido et al., 2002). Conversely, there was a decline in SC frequency in LIMB and DIA (p<0.03) isolated preparations from mdx4cv mice, while EOM preparations were unaffected (p=0.09) when examining sorted populations (Fig. 6D). (ii) Primary cultures from LIMB and DIA of mdx4cv mice demonstrated only a meager growth (and no appearance of Nestin-GFP+ renewal cells), while mdx4cv EOM cultures retained robust growth and renewal (Fig. 6A and B). In our additional studies of LIMB cultures from juvenile mdx4cv mice, SC growth impairment was first observed after 6 weeks of age, while cultures appeared normal beforehand. (iii) Clonal assays of SCs further demonstrated the inability of individual SCs from mdx4cv LIMB and DIA preparations to establish clones (according to clone number and size), while EOM clones retained robust growth when prepared from mdx4cv mice (Fig. 6C and D). Noticeably, a severely compromised myogenic growth was also observed in isolated EDL myofibers and in cultures of unsorted cell preparations from LIMB and DIA muscles of adult mdx4cv mice (data not shown), which argues against the possible presence of better performing myogenic progenitors that could have been excluded from our mdx4cv cell culture assays summarized in Fig. 6.
Fig. 6.
EOM SCs from dystrophin-null mdx4cv mice retain performance superiority. LIMB, DIA and EOM SCs were isolated from Nestin-GFP (wt) and mdx4cv/Nestin-GFP (mdx) 6 month old mice by flow cytometry according to Nestin-GFP expression combined with an antigen-based sorting approach (Fig. S3B), which permitted assaying the level of CD45+ cells as a reference for the inflammatory process associated with dystrophinopathy. (A) For each muscle group, the number of total nuclei was counted based on DAPI staining of cultures fixed on days 3–7. Data are expressed as mean (±SEM) number of nuclei per 10 microscopic fields (20 × objective) and not per single field as in Fig. 2G due to the low number of cells in mdx LIMB and DIA cultures. Within each muscle group, asterisks denote statistically significant differences (p<0.03, one-way ANOVA) for wt vs. mdx cultures per each culture day; notably, there are no statistical differences between EOM wt vs. mdx at any of the culture days. (B) Representative images of day 14 cultures of LIMB, DIA and EOM depict the meager growth of mdx cultures from LIMB and DIA SCs, while also highlighting the outperformance of EOM SCs within the wt groups. Among the mdx cultures, only those from EOM developed Nestin-GFP+ renewal cells. Scale bar, 100 μm. (C) Clonal cultures of LIMB, DIA and EOM SCs isolated from wt and mdx mice were quantified on day 10 in the same manner as in Fig. 3A–D. Clones are plotted individually based on total number of DAPI+ nuclei per clone. One-way ANOVA analysis reveals a statistical difference (p<0.03, asterisks) between wt and mdx clones within LIMB and DIA, but not within EOM (p=0.13). A statistical difference (p<0.03) was also noted between EOM clones vs. LIMB and DIA regardless of mdx or wt origin. (D) Summary of additional relevant data from the analysis of the sort profiles, and the primary and clonal cultures. The percentage of CD45+ cells was determined by using a distinctive fluorochrome (PE-Cy7 for anti-CD45 vs. APC for anti-CD31 and anti-Sca1) when gating out the CD31+, CD45+, and Sca1+ cells. For all panels, asterisks denote statistically significant differences (p<0.03).
The impairment of LIMB and DIA SCs from adult mdx4cv mice that we have persistently observed in cell culture assays seems to be in contrast with the in-vivo regenerative activity observed in affected mdx muscles and presumed to reflect the presence of performing SCs (Heslop et al., 2000; McGeachie et al., 1993; Yokota et al., 2006). One possible explanation to this discrepancy could be that the residual SC activity detected in the current LIMB and DIA cell cultures from the dystrophin-deficient mice is sufficient for supporting the reported in-vivo mdx regenerative activity. Moreover, our unpublished observation that the extent of attrition in primary cell cultures from LIMB and DIA of mdx4cv mice was somewhat alleviated when initiating cultures at a much higher density further supports the possibility of a beneficial “community effect”. Notably, a recent study of mdx mice corroborates our cell culture results with mdx4cv mice, pointing out to a decline in satellite cell activity in older dystrophin-null mice compared to wildtype (Jiang et al., 2014). It remains however possible that limb and body muscles are more affected in the mdx4cv model, as this strain has a lower number of revertants (dystrophin+) myofibers than the “standard” mdx mice (Danko et al., 1992). Indeed, while we observed extensive abnormal morphologies (branching, internal splitting, bifurcation) in most EDL myofibers by 12–16 weeks of age in mdx4cv mice (Fig. S5E–H; see also Banks et al., 2014 for similar conclusions), such level of malformations was observed in “standard” mdx mice only when reaching 26 weeks of age, while less than 5% of the myofibers displayed abnormalities by 15 weeks of age (Head et al., 1992; Williams, 1993). However, a side by side comparison between mdx4cv, mdx and wildtype mice, looking at SC clonal growth, myofiber abnormalities and whole muscle regenerative capacity is yet to be performed.
Overall, our cell culture experiments where SCs are “freed” from their in-vivo dystrophic context have permitted comparison of SC populations in a common “growth promoting” environment. Using this in-vitro approach, and especially analyzing clonal growth of single cells, we have clearly demonstrated that SCs from LIMB and DIA muscles perform poorly when isolated from dystrophin-null mice, whereas SCs isolated from mdx4cv EOM were unaffected and performed at the same level as their wildtype counterparts. This comparable (and robust) performance of EOM SCs from mdx4cv and wildtype mice further indicates that dystrophin is not essential for EOM SC activity, while the poor performance of mdx4cv LIMB and DIA SCs could possibly be associated with the accelerated myogenic differentiation observed previously in primary cultures from “standard” mdx mice (Yablonka-Reuveni and Anderson, 2006). Future studies are needed to establish the mechanism behind the preserved or impaired performance of SCs from the different muscle groups of dystrophin-null mice.
EOM SCs demonstrate higher engraftment efficiency after intramuscular transplantation
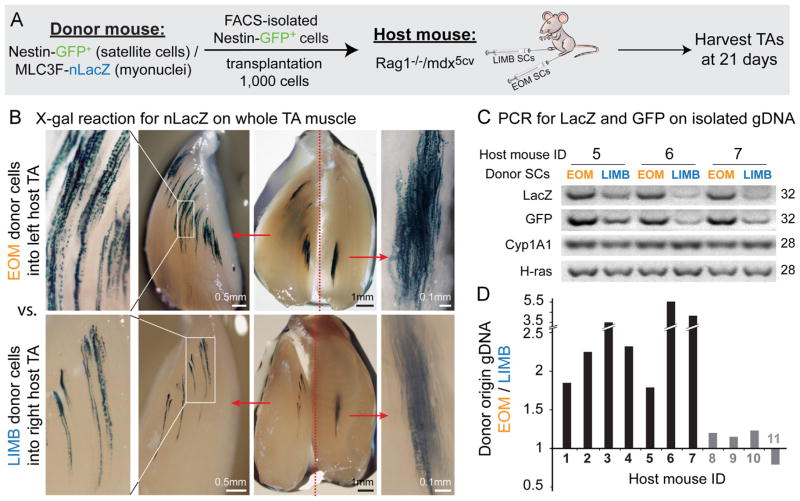
In the studies described above we evaluated SC performance in cell culture, thereby providing an identical environment in which to study all SCs populations from wildtype and mdx4cv mice for LIMB, DIA and EOM. The goal of this engraftment study was to evaluate whether the regenerative superiority of EOM SCs revealed in cell culture assays is maintained in vivo when SCs are again exposed to a common environment. For this, donor SCs from EOM and LIMB were transplanted into tibialis anterior (TA) muscles of Rag1−/−/mdx5cv mice. The dystrophin-null context has provided a muscle environment that promotes SC myonuclear contribution due to ongoing muscle repair, while the Rag1-null context minimizes possible rejection of donor cells as the mutant lacks mature B and T cells (see Materials and methods). Given the distinct lineage origin of donor EOM SCs and host TA muscles, we first validated that EOM and LIMB SC progeny could fuse with each other. Using a co-culture approach with reporter-specific marking per each lineage, we demonstrated that progeny of EOM SCs frequently generated hybrid myotubes with their LIMB counterparts (Fig. S6).
For the engraftment study, donor SCs were isolated by flow cytometry based on Nestin-GFP expression (Figs. 1B and S3A) from Nestin-GFP/MLC3F-nLacZ double transgenic mice. As detailed in Materials and methods, the muscle-specific MLC3F-nLacZ trans-gene drives β-gal expression in myofiber nuclei, providing a histological means to trace contribution of differentiated progeny of donor SCs based on X-gal staining. Additionally, both Nestin-GFP and MLC3F-nLacZ transgenes were used here to quantify donor contribution based on a genomic analysis (PCR on isolated gDNA). To minimize engraftment variations due to potential mouse to mouse differences, comparisons between EOM and LIMB donor SCs were done within individual host mice, with EOM SCs being injected into one TA muscle and LIMB SCs into the contralateral TA muscle. For each independent experiment (n=4), 3–4 host mice were engrafted with EOM and LIMB SCs populations isolated from the same cohort of donor mice. Injected TAs were harvested 3 weeks after transplantation and for each experiment 1–2 mice were processed for X-gal staining and the rest for genomic analysis.
Inspection of X-gal staining in whole TAs demonstrated that donor EOM SCs contributed nLacZ+ myonuclei at a greater level than LIMB SCs (Fig. 7B). The detection of the nuclear reporter nLacZ in myonuclei upon donor cell engraftment is not necessarily an exact reflection of the number of donor-derived nuclei as the reporter protein, translated in the myofiber cytoplasm, may also enter host myonuclei within neighboring domains of the same myofiber (Kirillova et al., 2007; Yang et al., 1997). However, the degree of MLC3F-nLacZ nuclei in donor muscle is certainly related to the degree of engraftment and provides an efficient qualitative measure. Donor cell contribution was further quantified by genomic analysis according to the donor-specific LacZ and GFP levels each normalized to the level of two reference genes (Cyp1A1 and H-ras), which permitted calculating the ratio between EOM and LIMB engraftment per individual mice as detailed in Fig. 7C and D. The majority of mice analyzed demonstrated a higher engraftment of EOM SCs with EOM/LIMB engraftment ratios at ~1.8–5.6 (n=7), while no mice harbored a significant higher engraftment of LIMB SCs. A pilot engraftment analysis of EOM vs. DIA SCs performed as above also indicated a better efficiency of EOM SCs. This genomic analysis detects donor contribution, regardless of whether cells contributed to myofiber nuclei or just engrafted into the muscle tissue. Hence, the use of both the histological and PCR approaches has provided a more comprehensive insight into the engraftment potential of each SC population, demonstrating altogether the regenerative superiority of EOM SCs in terms of in-vivo engraftment potency.
Fig. 7.
X-gal staining and PCR analysis demonstrating higher engraftment efficiency of EOM vs. LIMB SCs following intra-muscular transplantation into the TA muscles of host Rag1−/−/mdx5cv mice. (A) EOM and LIMB SCs were isolated from double transgenic Nestin-GFP/MLC3F-nLacZ mice (4–5 month old, 3 donors per experiment) as described in Figs. 1B and S3A and injected into the TAs of Rag1−/−/mdx5cv host mice (8–9 week old, 3–4 hosts per experiment). For each host animal, the TA from one leg was injected with EOM donor SCs, while the TA from the contralateral leg was injected with LIMB donor SCs. The level of donor-derived contribution in host muscles was then determined 3-weeks post transplantation. (B) Images of X-gal stained host TAs from one representative mouse, demonstrating the expression of the MLC3F-nLacZ reporter (from transplanted donor SCs) in myofiber nuclei. TAs were first cut longitudinally into 2 halves before fixation and were further trimmed after X-gal reaction to obtain more in-depth view and better imaging of the sites containing LacZ positive nuclei. Scale bars, for each injected TA from left to right, 0.5 mm, 1 mm and 0.1 mm. (C) Examples of PCR products used to quantify the level of donor-derived genomic material (LacZ and GFP) in comparison to the level of the reference genes contributed by host and donor material (Cyp1A1 and H-ras). (D) Each donor gene product (LacZ and GFP) was first normalized with each of the reference gene products (Cyp1A1 and H-ras), resulting in a total of 4 normalized values (2 per each donor gene). The EOM/LIMB ratio was then calculated for each of these normalized values. Given that within the same mouse these ratios are comparable regardless of the donor or reference gene used, final results are presented per each mouse as the average of these ratios. Black histograms denote mice demonstrating a higher engraftment of EOM SCs (~1.8–5.6 fold over LIMB SCs engraftment), while gray histograms denote mice with no apparent distinctions between EOM and LIMB SC engraftment. For both X-gal staining and PCR analysis, controls with vehicle only injected TAs were found negative for donor-derived signal, ensuring the reliability of detection.
Concluding remarks
Our findings contribute novel insights into EOM SCs, focusing on their in-situ signature, in-vitro expansion, and in-vivo engraftment aptitude. To identify potential unique properties of EOM SCs, most aspects were analyzed in comparison to SCs from the limb and diaphragm muscles. We have shown that EOM SCs, regardless of their distinctive Pax3-negative origin, display a common in-situ molecular signature with their limb and diaphragm counterparts (Pax7+, Ki67−, Nestin-GFP+, Myf5nLacZ+, MyoD-positive lineage origin). Further comparisons among the three muscle groups have demonstrated that EOM SCs possess a superior expansion capacity, contributing significantly more proliferating and differentiating progeny and impressively, many more renewal cells than their limb and diaphragm counterparts. Interestingly, SCs of the masseter muscle were also shown to have a larger proliferative and renewal potential when compared clonally with EDL SCs (Ono et al., 2010). The masseter is also of a Pax3-negative origin, but is classified within a muscle group distinct from the EOMs (Harel et al., 2009). While the masseter progenitors are derived from an Isl1-positive lineage (and also show ancestral expression of Nkk2.5), the EOM progenitors are not (Harel et al., 2009). Although it is tempting to consider the Pax3-negative origin of SCs in both EOM and masseter as playing a role in the expanded performance capacity of SCs from these muscles, a side by side comparison of SCs from these two lineages is first required. Additionally, we showed that the robust growth and renewal properties of EOM SCs are maintained in preparations isolated from dystrophin-null (mdx4cv) mice, while SCs from muscles affected by dystrophin deficiency (i.e., limb and diaphragm) expand poorly in cell culture assays. EOM SCs also retain higher performance in cell transplantation assays in which donor cells were engrafted into host mdx limb muscle.
Collectively, our study provides a comprehensive picture of EOM myogenic progenitors, showing that while these cells share common hallmarks with the prototypic SCs in somite-derived muscles, they distinctively feature robust growth and renewal capacities that warrant the title of high performance myoengines and promote consideration of their properties for developing new approaches in cell-based therapy to combat skeletal muscle wasting. Insights into the specific traits that underlie the EOM SCs’ robust performance can potentially establish new molecular avenues for manipulating donor myogenic progenitors from the easily accessible limb muscles. Furthermore, if proven relevant for clinical use (i.e., expandable in vitro with resulting progeny retaining high regenerative potency in vivo), human EOM SCs could serve directly as an effective source for cell-based therapy. Indeed, a protocol for retrieving human EOMs could ultimately be developed based on the already existing Eye Bank programs, allowing for the availability of a wide range of allotypes of human donor EOM cells.
Supplementary Material
Acknowledgments
We are grateful to Donna Prunkard and Dr. Peter Rabinovitch for their valuable assistance with cell sorting (performed at the core facility of the University of Washington Nathan Shock Center of Excellence). This work was supported by a grant to Z.Y.R from the National Institutes of Health (R01 AG035377). Z.Y.R. acknowledges additional support during the course of this study from the National Institutes of Health (R01 AG021566 and R21 AR057794) and from the Muscular Dystrophy Association (award #135908). N.I. was supported by the Genetic Approaches to Aging Training Program (T32 AG000057).
References
- Andrade FH, Porter JD, Kaminski HJ. Eye muscle sparing by the muscular dystrophies: lessons to be learned? Microsc Res Tech. 2000;48:192–203. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<192::AID-JEMT7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Arpke RW, Darabi R, Mader TL, Zhang Y, Toyama A, Lonetree CL, Nash N, Lowe DA, Perlingeiro RC, Kyba M. A new immuno-, dystrophin-deficient model, the NSG-mdx(4Cv) mouse, provides evidence for functional improvement following allogeneic satellite cell transplantation. Stem Cells. 2013;31:1611–1620. doi: 10.1002/stem.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks GB, Combs AC, Chamberlain JS. Sequencing protocols to genotype mdx, mdx(4cv), and mdx(5cv) mice. Muscle Nerve. 2010;42:268–270. doi: 10.1002/mus.21700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks GB, Combs AC, Odom GL, Bloch RJ, Chamberlain JS. Muscle structure influences utrophin expression in mdx mice. PLoS Genet. 2014;10:e1004431. doi: 10.1371/journal.pgen.1004431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang NC, Rudnicki MA. Satellite cells: the architects of skeletal muscle. Curr Top Dev Biol. 2014;107:161–181. doi: 10.1016/B978-0-12-416022-4.00006-8. [DOI] [PubMed] [Google Scholar]
- Chapman VM, Miller DR, Armstrong D, Caskey CT. Recovery of induced mutations for X chromosome-linked muscular dystrophy in mice. Proc Natl Acad Sci USA. 1989;86:1292–1296. doi: 10.1073/pnas.86.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- da Silva Costa RM, Kung J, Poukens V, Demer JL. Nonclassical innervation patterns in mammalian extraocular muscles. Curr Eye Res. 2012;37:761–769. doi: 10.3109/02713683.2012.676699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danko I, Chapman V, Wolff JA. The frequency of revertants in mdx mouse genetic models for Duchenne muscular dystrophy. Pediatr Res. 1992;32:128–131. doi: 10.1203/00006450-199207000-00025. [DOI] [PubMed] [Google Scholar]
- Danoviz ME, Yablonka-Reuveni Z. Skeletal muscle satellite cells: background and methods for isolation and analysis in a primary culture system. Methods Mol Biol. 2012;798:21–52. doi: 10.1007/978-1-61779-343-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K, Paterson B, Yablonka-Reuveni Z. A distinct profile of myogenic regulatory factor detection within Pax7+ cells at S phase supports a unique role of Myf5 during posthatch chicken myogenesis. Dev Dyn. 2009;238:1001–1009. doi: 10.1002/dvdy.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol. 2007;304:246–259. doi: 10.1016/j.ydbio.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K, Shefer G, Shearer A, Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol. 2010;340:330–343. doi: 10.1016/j.ydbio.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decrouy A, Renaud JM, Davis HL, Lunde JA, Dickson G, Jasmin BJ. Mini-dystrophin gene transfer in mdx4cv diaphragm muscle fibers increases sarcolemmal stability. Gene Ther. 1997;4:401–408. doi: 10.1038/sj.gt.3300407. [DOI] [PubMed] [Google Scholar]
- Demer JL. Mechanics of the orbita. Dev Ophthalmol. 2007;40:132–157. doi: 10.1159/0000100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleka KA, Gitler AD, Zhang M, Zhou DD, High FA, Epstein JA. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol. 2005;280:396–406. doi: 10.1016/j.ydbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Fischer EH, Charbonneau H, Tonks NK. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991;253:401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Goulding M, Lumsden A, Paquette AJ. Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development. 1994;120:957–971. doi: 10.1242/dev.120.4.957. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Shavlakadze T. Growing muscle has different sarcolemmal properties from adult muscle: a proposal with scientific and clinical implications: reasons to reassess skeletal muscle molecular dynamics, cellular responses and suitability of experimental models of muscle disorders. Bioessays. 2011;33:458–468. doi: 10.1002/bies.201000136. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Yablonka-Reuveni Z. Molecular and cell biology of skeletal muscle regeneration. Mol Cell Biol Hum Dis Ser. 1993;3:210–256. doi: 10.1007/978-94-011-1528-5_9. [DOI] [PubMed] [Google Scholar]
- Halevy O, Piestun Y, Allouh MZ, Rosser BW, Rinkevich Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn. 2004;231:489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- Harel I, Nathan E, Tirosh-Finkel L, Zigdon H, Guimaraes-Camboa N, Evans SM, Tzahor E. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell. 2009;16:822–832. doi: 10.1016/j.devcel.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- He S, Yoon HS, Suh BJ, Eccles MR. PAX3 Is extensively expressed in benign and malignant tissues of the melanocytic lineage in humans. J Investig Dermatol. 2010;130:1465–1468. doi: 10.1038/jid.2009.434. [DOI] [PubMed] [Google Scholar]
- Head SI, Williams DA, Stephenson DG. Abnormalities in structure and function of limb skeletal muscle fibres of dystrophic mdx mice. Proc Biol Sci. 1992;248:163–169. doi: 10.1098/rspb.1992.0058. [DOI] [PubMed] [Google Scholar]
- Hebert SL, Daniel ML, McLoon LK. The role of Pitx2 in maintaining the phenotype of myogenic precursor cells in the extraocular muscles. PLoS One. 2013;8:e58405. doi: 10.1371/journal.pone.0058405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop L, Morgan JE, Partridge TA. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J Cell Sci. 2000;113 (Pt 12):2299–2308. doi: 10.1242/jcs.113.12.2299. [DOI] [PubMed] [Google Scholar]
- Horst D, Ustanina S, Sergi C, Mikuz G, Juergens H, Braun T, Vorobyov E. Comparative expression analysis of Pax3 and Pax7 during mouse myogenesis. Int J Dev Biol. 2006;50:47–54. doi: 10.1387/ijdb.052111dh. [DOI] [PubMed] [Google Scholar]
- Ieronimakis N, Balasundaram G, Rainey S, Srirangam K, Yablonka-Reuveni Z, Reyes M. Absence of CD34 on murine skeletal muscle satellite cells marks a reversible state of activation during acute injury. PloS One. 2010;5:e10920. doi: 10.1371/journal.pone.0010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im WB, Phelps SF, Copen EH, Adams EG, Slightom JL, Chamberlain JS. Differential expression of dystrophin isoforms in strains of mdx mice with different mutations. Hum Mol Genet. 1996;5:1149–1153. doi: 10.1093/hmg/5.8.1149. [DOI] [PubMed] [Google Scholar]
- Jiang C, Wen Y, Kuroda K, Hannon K, Rudnicki MA, Kuang S. Notch signaling deficiency underlies age-dependent depletion of satellite cells in muscular dystrophy. Dis Model Mech. 2014;7:997–1004. doi: 10.1242/dmm.015917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge LM, Haraguchiln M, Chamberlain JS. Dissecting the signaling and mechanical functions of the dystrophin-glycoprotein complex. J Cell Sci. 2006;119:1537–1546. doi: 10.1242/jcs.02857. [DOI] [PubMed] [Google Scholar]
- Kallestad KM, Hebert SL, McDonald AA, Daniel ML, Cu SR, McLoon LK. Sparing of extraocular muscle in aging and muscular dystrophies: a myogenic precursor cell hypothesis. Exp Cell Res. 2011;317:873–885. doi: 10.1016/j.yexcr.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallestad KM, McLoon LK. Defining the heterogeneity of skeletal muscle-derived side and main population cells isolated immediately ex vivo. J Cell Physiol. 2010;222:676–684. doi: 10.1002/jcp.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski HJ, al-Hakim M, Leigh RJ, Katirji MB, Ruff RL. Extraocular muscles are spared in advanced Duchenne dystrophy. Ann Neurol. 1992;32:586–588. doi: 10.1002/ana.410320418. [DOI] [PubMed] [Google Scholar]
- Kaminski HJ, Richmonds CR, Kusner LL, Mitsumoto H. Differential susceptibility of the ocular motor system to disease. Ann N Y Acad Sci. 2002;956:42–54. doi: 10.1111/j.1749-6632.2002.tb02807.x. [DOI] [PubMed] [Google Scholar]
- Kanisicak O, Mendez JJ, Yamamoto S, Yamamoto M, Goldhamer DJ. Progenitors of skeletal muscle satellite cells express the muscle determination gene, MyoD. Dev Biol. 2009;332:131–141. doi: 10.1016/j.ydbio.2009.05.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassar-Duchossoy L, Gayraud-Morel B, Gomes D, Rocancourt D, Buckingham M, Shinin V, Tajbakhsh S. Mrf4 determines skeletal muscle identity in Myf5: Myod double-mutant mice. Nature. 2004;431:466–471. doi: 10.1038/nature02876. [DOI] [PubMed] [Google Scholar]
- Kastner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z. Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem. 2000;48:1079–1096. doi: 10.1177/002215540004800805. [DOI] [PubMed] [Google Scholar]
- Keire P, Shearer A, Shefer G, Yablonka-Reuveni Z. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol Biol. 2013;946:431–468. doi: 10.1007/978-1-62703-128-8_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R, Alonso S, Tajbakhsh S, Cossu G, Buckingham M. Myosin light chain 3F regulatory sequences confer regionalized cardiac and skeletal muscle expression in transgenic mice. J Cell Biol. 1995;129:383–396. doi: 10.1083/jcb.129.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana TS, Prendergast RA, Alameddine HS, Tome FM, Fardeau M, Arahata K, Sugita H, Kunkel LM. Absence of extraocular muscle pathology in Duchenne’s muscular dystrophy: role for calcium homeostasis in extraocular muscle sparing. J Exp Med. 1995;182:467–475. doi: 10.1084/jem.182.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirillova I, Gussoni E, Goldhamer DJ, Yablonka-Reuveni Z. Myogenic reprogramming of retina-derived cells following their spontaneous fusion with myotubes. Dev Biol. 2007;311:449–463. doi: 10.1016/j.ydbio.2007.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny P, Swiderski K, Chamberlain JS. Gene and cell-mediated therapies for muscular dystrophy. Muscle Nerve. 2013;47:649–663. doi: 10.1002/mus.23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrota-Candido J, Vasconcellos R, Cavalcanti M, Bozza M, Savino W, Quirico-Santos T. Resolution of skeletal muscle inflammation in mdx dystrophic mouse is accompanied by increased immunoglobulin and interferon-gamma production. Int J Exp Pathol. 2002;83:121–132. doi: 10.1046/j.1365-2613.2002.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LifeMap. LifeMap Sciences, Inc; 2013. < http://discovery.lifemapsc.com/library/images/extraocular-skeletal-muscle-anatomy>. [Google Scholar]
- Lu QL, Morris GE, Wilton SD, Ly T, Artem’yeva OV, Strong P, Partridge TA. Massive idiosyncratic exon skipping corrects the nonsense mutation in dystrophic mouse muscle and produces functional revertant fibers by clonal expansion. J Cell Biol. 2000;148:985–996. doi: 10.1083/jcb.148.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques MJ, Pertille A, Carvalho CL, Santo Neto H. Acetylcholine receptor organization at the dystrophic extraocular muscle neuromuscular junction. Anat Rec. 2007;290:846–854. doi: 10.1002/ar.20525. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AA, Kunz MD, McLoon LK. Dystrophic changes in extraocular muscles after gamma irradiation in mdx:utrophin(+/−) mice. PLoS One. 2014;9:e86424. doi: 10.1371/journal.pone.0086424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachie JK, Grounds MD, Partridge TA, Morgan JE. Age-related changes in replication of myogenic cells in mdx mice: quantitative autoradiographic studies. J Neurol Sci. 1993;119:169–179. doi: 10.1016/0022-510x(93)90130-q. [DOI] [PubMed] [Google Scholar]
- McLoon LK, Wirtschafter J. Activated satellite cells are present in uninjured extraocular muscles of mature mice. Trans Am Ophthalmol Soc. 2002;100:119–123. discussion 123–114. [PMC free article] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Miller RG, Sharma KR, Pavlath GK, Gussoni E, Mynhier M, Lanctot AM, Greco CM, Steinman L, Blau HM. Myoblast implantation in Duchenne muscular dystrophy: the San Francisco study. Muscle Nerve. 1997;20:469–478. doi: 10.1002/(sici)1097-4598(199704)20:4<469::aid-mus10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Montarras D, L’Honore A, Buckingham M. Lying low but ready for action: the quiescent muscle satellite cell. FEBS J. 2013;280:4036–4050. doi: 10.1111/febs.12372. [DOI] [PubMed] [Google Scholar]
- Morrison J, Lu QL, Pastoret C, Partridge T, Bou-Gharios G. T-cell-dependent fibrosis in the mdx dystrophic mouse. Lab Investig. 2000;80:881–891. doi: 10.1038/labinvest.3780092. [DOI] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Negroni E, Vallese D, Vilquin JT, Butler-Browne G, Mouly V, Trollet C. Current advances in cell therapy strategies for muscular dystrophies. Expert Opin Biol Ther. 2011;11:157–176. doi: 10.1517/14712598.2011.542748. [DOI] [PubMed] [Google Scholar]
- Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- Ono Y, Boldrin L, Knopp P, Morgan JE, Zammit PS. Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev Biol. 2010;337:29–41. doi: 10.1016/j.ydbio.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Pinedo EC, Budak MT, Zeiger U, Jorgensen LH, Bogdanovich S, Schroder HD, Rubinstein NA, Khurana TS. Transcriptional and functional differences in stem cell populations isolated from extraocular and limb muscles. Physiol Genomics. 2009;37:35–42. doi: 10.1152/physiolgenomics.00051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JD. Commentary: extraocular muscle sparing in muscular dystrophy: a critical evaluation of potential protective mechanisms. Neuromuscul Disord. 1998;8:198–203. doi: 10.1016/s0960-8966(98)00015-7. [DOI] [PubMed] [Google Scholar]
- Porter JD, Israel S, Gong B, Merriam AP, Feuerman J, Khanna S, Kaminski HJ. Distinctive morphological and gene/protein expression signatures during myogenesis in novel cell lines from extraocular and hindlimb muscle. Physiol Genomics. 2006;24:264–275. doi: 10.1152/physiolgenomics.00234.2004. [DOI] [PubMed] [Google Scholar]
- Porter JD, Karathanasis P. Extraocular muscle in merosin-deficient muscular dystrophy: cation homeostasis is maintained but is not mechanistic in muscle sparing. Cell Tissue Res. 1998;292:495–501. doi: 10.1007/s004410051078. [DOI] [PubMed] [Google Scholar]
- Porter JD, Merriam AP, Hack AA, Andrade FH, McNally EM. Extrao-cular muscle is spared despite the absence of an intact sarcoglycan complex in gamma- or delta-sarcoglycan-deficient mice. Neuromuscul Disord. 2001;11:197–207. doi: 10.1016/s0960-8966(00)00171-1. [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambasivan R, Gayraud-Morel B, Dumas G, Cimper C, Paisant S, Kelly RG, Tajbakhsh S. Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Dev Cell. 2009;16:810–821. doi: 10.1016/j.devcel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Schoser BG, Pongratz D. Extraocular mitochondrial myopathies and their differential diagnoses. Strabismus. 2006;14:107–113. doi: 10.1080/09273970600701218. [DOI] [PubMed] [Google Scholar]
- Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J Exp Zool. 1978;206:451–456. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Shefer G, Rauner G, Stuelsatz P, Benayahu D, Yablonka-Reuveni Z. Moderate-intensity treadmill running promotes expansion of the satellite cell pool in young and old mice. FEBS J. 2013;280:4063–4073. doi: 10.1111/febs.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci. 2004;117:5393–5404. doi: 10.1242/jcs.01419. [DOI] [PubMed] [Google Scholar]
- Shefer G, Yablonka-Reuveni Z. Basic Cell Culture Protocols. Vol. 290. Humana Press; Totowa, NJ: 2005. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells; pp. 281–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CK, 2nd, Janney MJ, Allen RE. Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J Cell Physiol. 1994;159:379–385. doi: 10.1002/jcp.1041590222. [DOI] [PubMed] [Google Scholar]
- Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol. 2001;98:235–243. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- Spencer RF, Porter JD. Biological organization of the extraocular muscles. Prog Brain Res. 2006;151:43–80. doi: 10.1016/S0079-6123(05)51002-1. [DOI] [PubMed] [Google Scholar]
- Stuelsatz P, Keire P, Almuly R, Yablonka-Reuveni Z. A contemporary atlas of the mouse diaphragm: myogenicity, vascularity, and the Pax3 connection. J Histochem Cytochem. 2012;60:638–657. doi: 10.1369/0022155412452417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuelsatz P, Shearer A, Yablonka-Reuveni Z. Ancestral Myf5 gene activity in periocular connective tissue identifies a subset of fibro/adipogenic progenitors but does not connote a myogenic origin. Dev Biol. 2014;385:366–379. doi: 10.1016/j.ydbio.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Buckingham M. Muscle progenitor cells failing to respond to positional cues adopt non-myogenic fates in myf-5 null mice. Nature. 1996;384:266–270. doi: 10.1038/384266a0. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- Tedesco FS, Cossu G. Stem cell therapies for muscle disorders. Curr Opin Neurol. 2012;25:597–603. doi: 10.1097/WCO.0b013e328357f288. [DOI] [PubMed] [Google Scholar]
- Tremblay JP, Malouin F, Roy R, Huard J, Bouchard JP, Satoh A, Richards CL. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant. 1993;2:99–112. doi: 10.1177/096368979300200203. [DOI] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PloS One. 2012;7:e34640. doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet. 2009;18:482–496. doi: 10.1093/hmg/ddn376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster C, Blau HM. Accelerated age-related decline in replicative lifespan of Duchenne muscular dystrophy myoblasts: implications for cell and gene therapy. Somat Cell Mol Genet. 1990;16:557–565. doi: 10.1007/BF01233096. [DOI] [PubMed] [Google Scholar]
- White RB, Bierinx AS, Gnocchi VF, Zammit PS. Dynamics of muscle fibre growth during postnatal mouse development. BMC Dev Biol. 2010;10:21. doi: 10.1186/1471-213X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA, Head SI, Lynch GS, Stephenson DG. Contractile properties of skinned muscle fibres from young and adult normal and dystrophic (mdx) mice. J Physiol. 1993;460:51–67. doi: 10.1113/jphysiol.1993.sp019458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilschut KJ, Ling VB, Bernstein HS. Concise review: stem cell therapy for muscular dystrophies. Stem Cells Transl Med. 2012;1:833–842. doi: 10.5966/sctm.2012-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. Discrimination of myogenic and nonmyogenic cells from embryonic skeletal muscle by 90 degrees light scattering. Cytometry. 1988;9:121–125. doi: 10.1002/cyto.990090204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. Isolation and culture of myogenic stem cells. In: Lanza RR, Blau H, Melton D, Moore M, Thomas ED, Verfaillie C, Weissman IL, WM, editors. Handbook of Stem Cells. Vol. 2. Elsevier: Academic Press; San Diego: 2004. pp. 571–580. [Google Scholar]
- Yablonka-Reuveni Z. The skeletal muscle satellite cell: still young and fascinating at 50. J Histochem Cytochem. 2011;59:1041–1059. doi: 10.1369/0022155411426780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Anderson JE. Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev Dyn. 2006;235:203–212. doi: 10.1002/dvdy.20602. [DOI] [PubMed] [Google Scholar]
- Yang J, Ontell MP, Kelly R, Watkins SC, Ontell M. Limitations of nls beta-galactosidase as a marker for studying myogenic lineage or the efficacy of myoblast transfer. Anat Rec. 1997;248:40–50. doi: 10.1002/(SICI)1097-0185(199705)248:1<40::AID-AR5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Yokota T, Lu QL, Morgan JE, Davies KE, Fisher R, Takeda S, Partridge TA. Expansion of revertant fibers in dystrophic mdx muscles reflects activity of muscle precursor cells and serves as an index of muscle regeneration. J Cell Sci. 2006;119:2679–2687. doi: 10.1242/jcs.03000. [DOI] [PubMed] [Google Scholar]
- Yu Wai Man CY, Chinnery PF, Griffiths PG. Extraocular muscles have fundamentally distinct properties that make them selectively vulnerable to certain disorders. Neuromuscul Disord: NMD. 2005;15:17–23. doi: 10.1016/j.nmd.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- Zeiger U, Mitchell CH, Khurana TS. Superior calcium homeostasis of extraocular muscles. Exp Eye Res. 2010;91:613–622. doi: 10.1016/j.exer.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liu D, Kaminski HJ. Myosin heavy chain expression in mouse extraocular muscle: more complex than expected. Investig Ophthalmol Vis Sci. 2010;51:6355–6363. doi: 10.1167/iovs.10-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.