Abstract
Previous research on Staphylococcus aureus in pigs focused on livestock-associated methicillin-resistant S. aureus (MRSA) and had a qualitative cross-sectional design. This study aimed to elucidate the frequency, load, and stability of S. aureus nasal carriage in pigs over time and investigated possible associations between carriage and immune response. Nasal swabs were collected three times weekly from 480 tagged adult pigs in 20 Danish production farms. S. aureus and MRSA were quantified on selective media by the most-probable-number method. The levels of IgG against 10 S. aureus antigens in serum were quantified in selected pigs by a Luminex assay. All the farms were positive for S. aureus and 15 for MRSA, leading to overall prevalences of persistent and intermittent carriers and noncarriers of 24, 52, and 23%, respectively. Carriage frequency and nasal loads were significantly higher on MRSA-positive farms. Logistic-regression modeling revealed the presence of individual pigs characterized by high nasal loads (≥10,000 CFU per swab) and stable carriage regardless of farm- and pen-associated factors. On the other hand, the humoral response was strongly influenced by these environmental factors. The existence of a minority of shedders contributing to maintenance of S. aureus within farms opens up new perspectives on the control of MRSA in pig farming.
INTRODUCTION
Staphylococcus aureus is an opportunistic pathogen that colonizes the skin and mucosae of humans and several animal species, including pigs. In humans, S. aureus infections are more prevalent in carriers than in noncarriers and are usually caused by the colonizing strain (1, 2). Colonization patterns are consistent in the human population, where approximately 20% of healthy people are persistent carriers, 30% are intermittent carriers, and 50% are noncarriers (1). Recent work suggested a new classification into only two groups, carriers and noncarriers, based on the similar carriage dynamics and immune responses in intermittent carriers and noncarriers (3). Various studies have shown that people exposed to animals, such as farm workers and veterinarians, have a significant risk of carrying methicillin-resistant S. aureus (MRSA) (4–8). There is particular concern about the spread of livestock-associated MRSA sequence type 398 (ST398), since the lineage has been associated with colonization (9, 10) and infection (4, 11–14) of humans exposed to livestock, particularly pigs. In addition to the public health concern, spread of MRSA ST398 is an economic burden to the health care system in countries that have adopted a search-and-destroy policy against MRSA (15–17).
Understanding the complex phenomenon of S. aureus colonization is crucial to develop effective measures for MRSA control in livestock. However, very little is known about the prevalence, load, and persistence of S. aureus colonization in livestock, mainly because most studies have investigated MRSA exclusively, had a qualitative cross-sectional design, and used enrichment isolation methods that are very sensitive but do not allow quantification. As a consequence, none of the previous studies was able to discriminate between truly colonized and contaminated pigs. The objectives of this longitudinal study were (i) to elucidate the frequency, load, and stability of S. aureus carriage in the nasal cavity of pigs and (ii) to investigate possible associations between carriage and immune response. The latter objective was included in the study because high levels of IgG against S. aureus-specific antigens have been associated with persistent carriage in humans (3, 18).
MATERIALS AND METHODS
Sampling sites and times.
A longitudinal quantitative study was performed in 20 Danish production farms situated in the Central Jutland Region. The farms were selected by convenience, as they were served by the veterinary practice involved in the study (Odder Dyreklinik). All the farms except one (farm 12) had integrated production and purchased 1,000 to 2,000 30-kg pigs per production cycle. Samples were collected between May and October 2013, during which time the farms were included on a continuous basis in groups of four at a time. Within each farm, 24 adult pigs (6 pigs per pen) in the finishing sections were ear tagged for individual identification and sampled three times at a weekly frequency (samplings 1, 2, and 3). Nasal swabs (Dryswab; MWE, United Kingdom) were collected by trained veterinarians by swabbing the nasal mucosa of each nostril four times and were processed in the laboratory within 24 h after collection. All the swabs were assigned by the investigator to one of three categories based on the degree of cleanliness observed by visual examination: no evident debris (category 1), regular amount of debris (category 2), and large amount of debris (category 3).
S. aureus and MRSA quantification.
Viable S. aureus and MRSA bacteria were quantified by the most-probable-number (MPN) method (19). Briefly, cotton swabs were suspended in 1 ml saline, and four serial dilutions (10−1 to 10−4) were enriched in Mueller-Hinton broth with 6.5% NaCl. Following 18 h of incubation, the enrichment cultures were plated onto SaSelect agar (Bio-Rad, Hercules, CA, USA) and MRSA2 Brilliance agar (Oxoid, United Kingdom) for quantification of S. aureus and MRSA bacteria, respectively. Presumptive S. aureus/MRSA colonies from the highest dilution displaying growth were confirmed by PCR amplification of nuc (20) and mecA (21) using a porcine MRSA ST398 strain as a positive control. If S. aureus/MRSA was not detected, colonies from the previous dilution were subjected to PCR for validation of the counts. The most probable number was estimated from the highest dilution showing growth of S. aureus/MRSA confirmed by PCR.
Serological profiling.
Levels of IgG were analyzed in blood samples taken in sampling 3 from pigs that were positive or negative in both previous samplings. The sera were isolated by centrifugation and frozen at −20°C within 24 h after blood collection. Levels of IgG against 10 S. aureus-specific antigens were simultaneously measured using a Luminex assay (18, 22). The 10 S. aureus-specific antigens (alpha-toxin, clumping factor A [ClfA], gamma-hemolysin B [HlgB], iron surface determinant A [IsdA], peptidoglycan hydrolase [LytM], staphylococcal enterotoxin I [SEI], SD repeat-containing protein D [SdrD], staphylococcal superantigen-like proteins 1 and 5 [SSL-1 and SSL-5], and toxic shock syndrome toxin 1 [TSST-1]) were selected based on humoral responses observed in experimentally colonized piglets (W. Van Wamel, unpublished data). Tests were performed in independent duplicates, and the median fluorescence intensity (MFI) values, reflecting semiquantitative antibody levels, were averaged. Duplicates with a coefficient of variance (CV) higher than 25% were considered not reproducible and deleted from the data set (22).
Statistical analysis.
The MPN method generated categorical data on five levels of S. aureus/MRSA load, namely, 0 (<100 CFU), A (≥100 and <1,000 CFU), B (≥1,000 and <10,000 CFU), C (≥10,000 and <100,000 CFU), and D (≥100,000 CFU). Four logistic-regression models were investigated, dichotomizing the outcomes as 0/ABCD, 0A/BCD, 0AB/CD, and 0ABC/D. Farm, pen, and pig were included as random effects and swab cleanliness and week as fixed effects. The S. aureus load obtained from the same pig in the previous sampling was included as an explanatory variable. This variable had only two observations per pig, since no data were available prior to sampling 1, and generated odds ratios (OR), which are easier to interpret than the random effects.
To identify possible associations between carriage and immune response, we studied the correlation between the IgG levels for each antigen and the variables farm, pen, and pig in three random-effect models. The first model included all three variables and generated three random effects: farm, pen, and pig. The second model included only pen and pig, generating two random effects, farmpen (the farm effect incorporated into the pen effect) and pig. The third model included only pig, generating the random effect totalpig (the farm and pen effects incorporated into the pig effect). The correlations of IgG levels and these random effects were studied for each S. aureus antigen. When correlations were significant, a linear-regression model was run.
RESULTS
In-herd carriage frequency.
All the farms were S. aureus positive, and 15 of the 20 farms were MRSA positive. The average in-herd frequencies of S. aureus-positive pigs in samplings 1, 2, and 3 were 51, 53, and 44%, respectively. The overall in-herd carriage frequency was significantly lower (19%; range, 0 to 50%) in the 5 MRSA-negative farms than in the 15 MRSA-positive farms (59%; range, 8 to 100%) (P < 0.0001). The in-herd frequencies of pigs that were positive in at least one of the three samplings were 76% (12 to 100%) for S. aureus and 62% (4 to 100%) for MRSA in MRSA-positive farms.
Stability of carriage.
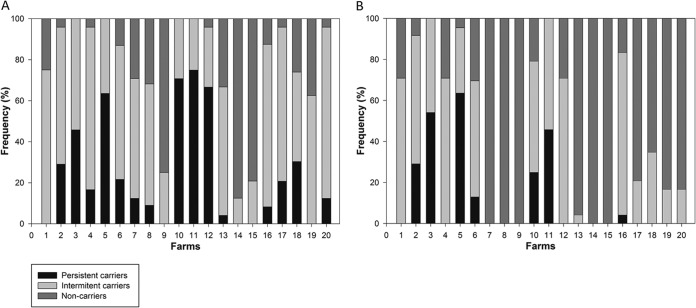
Among the 480 pigs tested, 24% (farm range, 0 to 75%) were positive for S. aureus at all sampling times (persistent carriers), 52% (12 to 83%) were positive at at least one sampling time (intermittent carriers), and 23% (0 to 87%) were negative at all sampling times (noncarriers). The remaining 1% (n = 6) died during the study period. The frequencies of S. aureus and MRSA persistent and intermittent carriers and noncarriers on each farm are shown in Fig. 1.
FIG 1.
Frequencies of S. aureus (A) and MRSA (B) persistent and intermittent carriers and noncarriers in 20 Danish pig production farms. Farms 7, 8, 9, 14, and 15 were MRSA negative.
Nasal bacterial loads.
Nasal S. aureus loads were significantly higher in MRSA-positive farms than in MRSA-negative farms (P = 0.004). In the five MRSA-negative farms, the S. aureus load was 0 (<100 CFU/swab) in 80% (n = 288) of the samples, A (≥100 and <1,000 CFU/swab) in 14% (n = 49), B (≥1,000 and <10,000 CFU/swab) in 3.5% (n = 12), C (≥10,000 and <100,000 CFU/swab) in 1.5% (n = 5), and D (≥100,000 CFU/swab) in 1% (n = 3). On MRSA-positive farms, the S. aureus load was 0 in 40% (n = 428) of the samples, A in 27% (n = 290), B in 22% (n = 237), C in 8% (n = 89), and D in 3% (n = 31) (see Table S1 in the supplemental material).
Modeling of S. aureus carriage in individual pigs.
The data were clustered by farm and pen, which significantly influenced the S. aureus in-herd prevalence. Swab cleanliness did not show any significant trend in the results. Stability of carriage was analyzed as the risk of having an S. aureus carriage status based on the result from the previous week. While the outcomes of the first two logistic-regression models were strongly dependent on farm and pen, the third model showed a lower influence of these variables and a significant influence of pig as a random effect (Table 1 and Fig. 2). In the fourth model, the pig effect was not significant, probably due to the low number of pigs categorized as D. The S. aureus nasal load in the previous week was a significant predictor of carriage in the last two models (P < 0.0001) but not in the first two (P = 0.18), meaning that pigs displaying high loads in the previous week had a significant risk of having high S. aureus loads (C and D) in the following week (Table 2).
TABLE 1.
Influence of farm, pen, and pig on S. aureus carriage in 480 pigs as determined by four logistic-regression models
| Modela | Variable level (P value)b |
||
|---|---|---|---|
| Pig | Pen | Farm | |
| 1 | 0 (1) | 0.47 (<0.0001) | 2.11 (<0.0001) |
| 2 | 0.11 (0.20) | 0.34 (0.0002) | 1.31 (<0.0001) |
| 3 | 0.47 (0.02) | 0.30 (0.04) | 0.84 (<0.0001) |
| 4 | 0.62 (0.12) | 0.39 (0.12) | 0.85 (0.01) |
Each model analyzed the data using a different cutoff value to define carriers. Carriers were defined as ≥100 CFU/swab (model 1), ≥1,000 CFU/swab (model 2), ≥10,000 CFU/swab (model 3), and ≥100,000 CFU/swab (model 4).
The data are shown as random effects at the farm, pen, and pig levels and indicate that model 3 was the only one showing a significant effect of the individual pigs and that it was less influenced by pen and farm effects.
FIG 2.
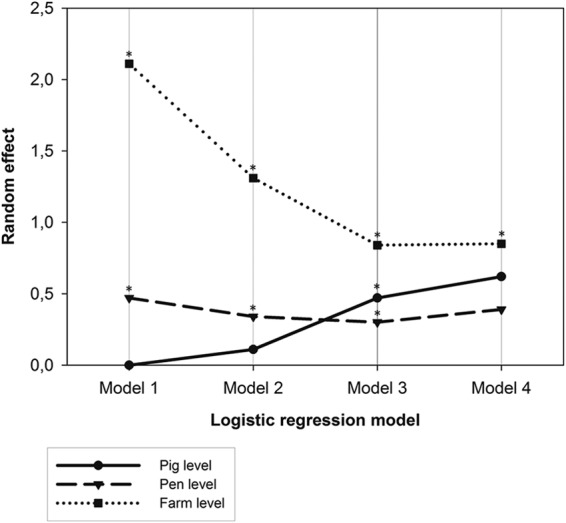
Influence of farm, pen, and pig on Staphylococcus aureus carriage in 480 pigs as determined by four logistic-regression models. Each model analyzed the data using a different cutoff value to define carriers. The data are shown as random effects at the farm, pen, and pig levels and indicate that model 3 was the only one showing a significant effect of the individual pigs and that it was less influenced by pen and farm effects. Asterisks indicate statistical significance. Carriers were defined as ≥100 CFU/swab (model 1), ≥1,000 CFU/swab (model 2), ≥10,000 CFU/swab (model 3), and ≥100,000 CFU/swab (model 4).
TABLE 2.
Probability of a pig being an S. aureus carrier based on the nasal load in the previous week according to four logistic-regression models
| S. aureus nasal load in the previous week (CFU/swab)a | OR (CI)b |
|||
|---|---|---|---|---|
| Model 1 (negative/positive) | Model 2 (0A/BCD) | Model 3 (0AB/CD) | Model 4 (0ABC/D) | |
| D | 2.18 (0.71–6.74) | 3.63c (1.35–9.75) | 7.07c (2.57–19.47) | 31.65c (2.87–349) |
| C | 1.34 (0.67–2.68) | 0.88 (0.45–1.72) | 2.01 (0.82–4.91) | 34.69c (3.82–315) |
| B | 1.07 (0.68–1.69) | 0.87 (0.54–1.41) | 2.19c (1.08–4.46) | 19.20c (2.31–160) |
| A | 0.96 (0.64–1.48) | 0.81 (0.50–1.30) | 0.78 (0.34–1.79) | 7.45 0.80–69.0) |
| 0 | 1 | 1 | 1 | 1 |
Results for the S. aureus load were classified into 0 (<100 CFU), A (≥100 and <1,000), B (≥1,000 and <10,000), C (≥10,000 and <100,000), and D (≥100,000).
The data were analyzed as a binomial distribution dichotomizing the outcome (S. aureus nasal load), as indicated in parentheses. The results were controlled for farm, pen, and pig effects. CI, confidence interval.
Statistically significant.
Serological profiles.
A total of 209 blood samples were collected based on the results of the first two samplings. These samples accounted for 57 persistent carriers, 81 intermittent carriers, and 71 noncarriers, considering the carriage status in the three samplings. The average levels of IgG against each antigen are shown in Table 3 for persistent and intermittent carriers and noncarriers. Although IgG levels against two adhesion factors (ClfA and IsdA) and four toxins (alpha-toxin, HlgB, SSL-1, and TSST-1) correlated with one or more random effects, linear-regression analysis revealed that only environmental random effects (attributable to farm and pen) were significant. No significant correlations were found between IgG levels and individual random effects (attributable to pig) (see Table S2 in the supplemental material).
TABLE 3.
Average, maximum, and minimum levels of IgG against 10 S. aureus-specific antigens observed in 209 pigs classified as permanent (n = 57) or intermittent (n = 70) carriers or noncarriers (n = 71)
| Pig and IgG level | Value (MFI) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adhesion factors |
Toxins |
|||||||||
| ClfAa | IsdAb | SdrD | Alpha-toxina | HlgBb | LytM | SEI | SSL-1a | SSL-5 | TSST-1b | |
| Noncarriers | ||||||||||
| Avg | 1,940 | 812 | 168 | 3,319 | 1,523 | 453 | 161 | 1,194 | 251 | 946 |
| Maximum | 4,698 | 2,334 | 682 | 6,770 | 4,558 | 2,222 | 776 | 4,935 | 632 | 3,371 |
| Minimum | 381 | 69 | 17 | 1,145 | 438 | 78 | 23 | 188 | 49 | 300 |
| Intermittent carriers | ||||||||||
| Avg | 1,831 | 955 | 218 | 3,564 | 1,659 | 614 | 201 | 1,260 | 247 | 1,096 |
| Maximum | 4,663 | 2,660 | 822 | 7,411 | 4,180 | 3,712 | 1,158 | 4,532 | 659 | 4,052 |
| Minimum | 366 | 315 | 8 | 1,680 | 477 | 199 | 83 | 205 | 38 | 360 |
| Permanent carriers | ||||||||||
| Avg | 2,821 | 1,091 | 257 | 3,998 | 1,850 | 547 | 186 | 1,818 | 294 | 1,366 |
| Maximum | 7,166 | 2,846 | 758 | 7,279 | 5,533 | 1,984 | 736 | 5,385 | 713 | 4,603 |
| Minimum | 608 | 184 | 10 | 1,463 | 229 | 101 | 40 | 200 | 42 | 270 |
Humoral response correlating with the random effect farmpen.
Humoral response correlating with the random effect farm.
DISCUSSION
The present study is the first attempt to generate quantitative longitudinal data on nasal S. aureus carriage in pigs. When modeling S. aureus carriage, grouping of the low nasal-load scores (0, A, and B) resulted in a better estimate of the individual-pig effect, indicating that variations between 0 and 10,000 CFU/swab were not related to individual factors but to unknown factors at the farm and pen level. In contrast, individual factors were shown to have a significant effect in pigs with high nasal loads (C and D). Moreover, the nasal load was associated with stability of carriage, as it was possible to predict the nasal loads of pigs showing high loads (C and D) in the previous week (Table 2), meaning that these pigs likely carried constantly high S. aureus numbers in consecutive samplings. Altogether, these results indicate the existence of individual pigs characterized by high nasal loads (>10,000 CFU/swab) and stable carriage. As these presumptive truly colonized animals represent a minority of the pig population under study and were likely to contribute to maintenance of S. aureus within farms, our findings open up new perspectives for controlling MRSA in pig farming. We are currently investigating whether S. aureus colonization of these individuals is predisposed by host genetic factors. Additional research is warranted to determine if MRSA occurrence in pig farms can be controlled by removing these pigs from the farm environment.
The proportion of S. aureus persistent carriers observed in pigs (24%) resembles that reported in humans (20%) (1). However, this observation should not be generalized, since carriage was highly dependent on farm- and pen-specific factors and may also depend on geographical factors. The relatively high proportion of intermittent carriers (52%) is not surprising considering the environmental conditions at pig farms, which are characterized by heavy S. aureus contamination and a high density of animals (23, 24). According to colonization studies in humans, intermittent carriers may simply reflect exposure to S. aureus, as they have the same elimination kinetics, immune response, and bacterial load as noncarriers (3). Our data suggest that the classification into carriers and noncarriers proposed by van Belkum et al. (3) for humans is also valid for pigs.
The nasal S. aureus loads observed in pigs are in line with those reported in human persistent (3.6 log CFU; range, 1.9 to 3.9) and intermittent (1.4 log CFU; range, 0.3 to 3.3) carriers (25). Verhoeven et al. (26) showed that persistent carriers have higher nasal loads (>102 CFU/swab) than intermittent carriers (<102 CFU/swab). Pig farmers with high nasal MRSA loads have also been shown to be persistent carriers more often than farmers with low MRSA loads (27). It should be noted that counts determined by the MPN method, as was done in the present study, are semiquantitative, and their level of approximation is particularly high for pigs displaying high nasal loads (e.g., between 10,000 and 100,000). This does not affect the model but does not allow the estimation of average or median counts without assuming error. Despite this limitation, the method was proven to be reliable in a pilot study using spiked samples with known S. aureus concentrations (data not shown). As shown in Table S1 in the supplemental material, in 60% of the cases, the loads of S. aureus and MRSA matched. In 36% of the samples, the level of S. aureus was higher than the level of MRSA, suggesting coexistence of methicillin-susceptible and methicillin-resistant strains in the nose of the same individual. In the remaining 4% of the samples (n = 46), the level of MRSA was inexplicably higher than the level of total S. aureus. We believe this is due to higher sensitivity of the MRSA isolation method, as the lack of antimicrobials in the agar medium used for S. aureus quantification allows the growth of other bacteria that may compete with S. aureus and complicate visual detection on the plates.
Large differences were observed between farms with regard to in-herd carriage frequency (Fig. 1). Interestingly, MRSA-positive farms had significantly higher S. aureus carriage frequencies (60%) and nasal loads than MRSA-negative farms (less than 20%). The higher S. aureus prevalence in MRSA-positive farms indicates that certain MRSA clones may be able to spread at higher rates, driven either by selective pressure or by adaptation of these clones to pig farming (28). Others have attempted to compare the colonization kinetics of different lineages in pigs under experimental conditions, showing MRSA ST398 as the most successful (29). The present study did not analyze S. aureus strain diversity, as it was not designed to assess whether differences in S. aureus prevalence between farms were due to specific lineages.
The overall average prevalence of MRSA-positive pigs in the three samplings (40%) was lower than that recently reported in Danish pig slaughterhouses (77%) (30). The higher prevalence observed at the slaughterhouse level supports the findings of other studies showing contamination of MRSA-negative pigs during transportation or at the slaughterhouse (31). The prevalence of MRSA-positive farms was markedly higher (75%) than those previously reported in Danish pig farms in 2010 and 2011 (16%) (10). However, the farms tested in our study were not representative of the whole country, as they were concentrated in the Central Jutland Region. Moreover, this difference could also be due to the MRSA detection procedure used in this study, which had a longitudinal design and was based on a larger number of pigs per farm and individual processing of the swabs instead of the pooling technique employed in the previous studies.
Previous studies of human S. aureus colonization (18) showed associations between carriage and humoral immune response against S. aureus antigens. In our study, this association was not significant when controlling environmental factors, such as farm and pen, in the random-effect models. According to our results, the immune status of pigs neither facilitated nor protected from carriage. The serological profiles of pigs could be predicted better by the level of exposure, measured as the positivity of other pigs in the same pen and farm, than by their individual carriage status. When individual factors appeared to be significantly associated with specific humoral responses, they were proven to be insignificant by linear regression. These results indicated a strong influence of the environment on the humoral response of pigs. However, a possible individual influence cannot be ruled out, since there was colinearity between random effects (i.e., the effects of the environment and those of the individual pigs correlated with each other). These are important observations, since for the first time we were able to measure exposure and control for it in the analysis using information on the farm prevalence, pen prevalence, and nasal loads of farm and pen mates, allowing us to compare carriage and exposure as explanatory variables of immune response. This kind of analysis would be very difficult to achieve in human studies.
Conclusions.
Longitudinal quantification of S. aureus and MRSA in nasal samples allowed identification of pigs characterized by high and stable nasal loads. These shedders are likely to contribute to maintenance of S. aureus within herds by contaminating other pigs and the farm environment. Their identification opens new perspectives for MRSA control in pig farming. However, identification is not easy to perform on a large scale, and serological screening was proven to be useless for this purpose. Technological advances are needed for rapid identification of shedders based on nasal loads.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Danish Council for Independent Research, Technology, and Production Science, Project PIG STAPH—the Role of Porcine Immunity and Genetics in Staphylococcus aureus Colonization.
We are grateful to Ann Kirstine Ballebye Lind and others who assisted her in the field work; to Manja Koudal Hanegård for excellent technical assistance; to John Frazier for SSL1 and SSL5; to Gerard Lina for alpha-toxin, HlgB, and SEI; to Timothy Foster for ClfA, IsdA, SasG, and SdrD; to Girbe Buist for LytM; and to Barbara Bröker for TSST-1.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03392-14.
REFERENCES
- 1.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 2.Sakwinska O, Kuhn G, Balmelli C, Francioli P, Giddey M, Perreten V, Riesen A, Zysset F, Blanc DS, Moreillon P. 2009. Genetic diversity and ecological success of Staphylococcus aureus strains colonizing humans. Appl Environ Microbiol 75:175–183. doi: 10.1128/AEM.01860-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Belkum A, Verkaik NJ, de Vogel CP, Boelens HA, Verveer J, Nouwen JL, Verbrugh HA, Wertheim HF. 2009. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis 199:1820–1826. doi: 10.1086/599119. [DOI] [PubMed] [Google Scholar]
- 4.Huijsdens XW, van Dijke BJ, Spalburg E, van Santen-Verheuvel MG, Heck ME, Pluister GN, Voss A, Wannet WJ, de Neeling AJ. 2006. Community-acquired MRSA and pig-farming. Ann Clin Microbiol Antimicrob 5:26. doi: 10.1186/1476-0711-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moodley A, Stegger M, Bagcigil AF, Baptiste KE, Loeffler A, Lloyd DH, Williams NJ, Leonard N, Abbott Y, Skov R, Guardabassi L. 2006. spa typing of methicillin-resistant Staphylococcus aureus isolated from domestic animals and veterinary staff in the UK and Ireland. J Antimicrob Chemother 58:1118–1123. doi: 10.1093/jac/dkl394. [DOI] [PubMed] [Google Scholar]
- 6.Weese JS, Rousseau J, Traub-Dargatz JL, Willey BM, McGeer AJ, Low DE. 2005. Community-associated methicillin-resistant Staphylococcus aureus in horses and humans who work with horses. J Am Vet Med Assoc 226:580–583. doi: 10.2460/javma.2005.226.580. [DOI] [PubMed] [Google Scholar]
- 7.van Duijkeren E, Ten Horn L, Wagenaar JA, de Bruijn M, Laarhoven L, Verstappen K, de Weerd W, Meessen N, Duim B. 2011. Suspected horse-to-human transmission of MRSA ST398. Emerg Infect Dis 17:1137–1139. doi: 10.3201/eid1706.101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oppliger A, Moreillon P, Charriere N, Giddey M, Morisset D, Sakwinska O. 2012. Antimicrobial resistance of Staphylococcus aureus strains acquired by pig farmers from pigs. Appl Environ Microbiol 78:8010–8014. doi: 10.1128/AEM.01902-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kock R, Harlizius J, Bressan N, Laerberg R, Wieler LH, Witte W, Deurenberg RH, Voss A, Becker K, Friedrich AW. 2009. Prevalence and molecular characteristics of methicillin-resistant Staphylococcus aureus (MRSA) among pigs on German farms and import of livestock-related MRSA into hospitals. Eur J Clin Microbiol Infect Dis 28:1375–1382. doi: 10.1007/s10096-009-0795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DANMAP 2011. 2012. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. http://www.danmap.org/∼/media/Projekt%20sites/Danmap/DANMAP%20reports/Danmap_2011.ashx.
- 11.Ruhlmann CH, Kolmos HJ, Kristiansen JE, Skov R. 2008. Pigs as an infection source for methicillin resistant Staphylococcus aureus infections in humans. Ugeskr Laeger 170:3436 (In Danish.) [PubMed] [Google Scholar]
- 12.Grisold AJ, Zarfel G, Hoenigl M, Krziwanek K, Feierl G, Masoud L, Leitner E, Wagner-Eibel U, Badura A, Marth E. 2010. Occurrence and genotyping using automated repetitive-sequence-based PCR of methicillin-resistant Staphylococcus aureus ST398 in Southeast Austria. Diagn Microbiol Infect Dis 66:217–221. doi: 10.1016/j.diagmicrobio.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Hartmeyer GN, Gahrn-Hansen B, Skov RL, Kolmos HJ. 2010. Pig-associated methicillin-resistant Staphylococcus aureus: family transmission and severe pneumonia in a newborn. Scand J Infect Dis 42:318–320. doi: 10.3109/00365540903510708. [DOI] [PubMed] [Google Scholar]
- 14.Lozano C, Aspiroz C, Ezpeleta AI, Gómez-Sanz E, Zarazaga M, Torres C. 2011. Empyema caused by MRSA ST398 with atypical resistance profile, Spain. Emerg Infect Dis 17:138–140. doi: 10.3201/eid1701.100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kock R, Siam K, Al-Malat S, Christmann J, Schaumburg F, Becker K, Friedrich AW. 2011. Characteristics of hospital patients colonized with livestock-associated meticillin-resistant Staphylococcus aureus (MRSA) CC398 versus other MRSA clones. J Hosp Infect 79:292–296. doi: 10.1016/j.jhin.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Kock R, Becker K, Cookson B, van Gemert-Pijnen JE, Harbarth S, Kluytmans J, Mielke M, Peters G, Skov RL, Struelens MJ, Tacconelli E, Navarro Torne A, Witte W, Friedrich AW. 2010. Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill 15:19688. [DOI] [PubMed] [Google Scholar]
- 17.Guardabassi L, Larsen J, Weese JS, Butaye P, Battisti A, Kluytmans J, Lloyd DH, Skov RL. 2013. Public health impact and antimicrobial selection of meticillin-resistant staphylococci in animals. J Global Antimicrob Resist 1:55–62. doi: 10.1016/j.jgar.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Verkaik NJ, de Vogel CP, Boelens HA, Grumann D, Hoogenboezem T, Vink C, Hooijkaas H, Foster TJ, Verbrugh HA, van Belkum A, van Wamel WJ. 2009. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J Infect Dis 199:625–632. doi: 10.1086/596743. [DOI] [PubMed] [Google Scholar]
- 19.Cochran WG. 1950. Estimation of bacterial densities by means of the “most probable number.” Biometrics 6:105–116. [PubMed] [Google Scholar]
- 20.Sasaki T, Tsubakishita S, Tanaka Y, Sakusabe A, Ohtsuka M, Hirotaki S, Kawakami T, Fukata T, Hiramatsu K. 2010. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J Clin Microbiol 48:765–769. doi: 10.1128/JCM.01232-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira DC, de Lencastre H. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 46:2155–2161. doi: 10.1128/AAC.46.7.2155-2161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crombé F, Vanderhaeghen W, de Vogel CP, Van Wamel WJ, Barbe K, Hermans K, Haesebrouck F, Butaye P. 2013. Serological profiles in nursery piglets colonized with Staphylococcus aureus. Vet Res 44:4. doi: 10.1186/1297-9716-44-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friese A, Schulz J, Hoehle L, Fetsch A, Tenhagen BA, Hartung J, Roesler U. 2012. Occurrence of MRSA in air and housing environment of pig barns. Vet Microbiol 158:129–135. doi: 10.1016/j.vetmic.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Broens EM, Espinosa-Gongora C, Graat EA, Vendrig N, Van Der Wolf PJ, Guardabassi L, Butaye P, Nielsen JP, De Jong MC, Van De Giessen AW. 2012. Longitudinal study on transmission of MRSA CC398 within pig herds. BMC Vet Res 8:58. doi: 10.1186/1746-6148-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nouwen JL, Ott A, Kluytmans-Vandenbergh MF, Boelens HA, Hofman A, van Belkum A, Verbrugh HA. 2004. Predicting the Staphylococcus aureus nasal carrier state: derivation and validation of a “culture rule.” Clin Infect Dis 39:806–811. doi: 10.1086/423376. [DOI] [PubMed] [Google Scholar]
- 26.Verhoeven PO, Grattard F, Carricajo A, Lucht F, Cazorla C, Garraud O, Pozzetto B, Berthelot P. 2012. An algorithm based on one or two nasal samples is accurate to identify persistent nasal carriers of Staphylococcus aureus Clin Microbiol Infect 18:551–557. doi: 10.1111/j.1469-0691.2011.03611.x. [DOI] [PubMed] [Google Scholar]
- 27.van Cleef BA, van Benthem BH, Verkade EJ, van Rijen M, Kluytmans-van den Bergh MF, Schouls LM, Duim B, Wagenaar JA, Graveland H, Bos ME, Heederik D, Kluytmans JA. 2014. Dynamics of methicillin-resistant Staphylococcus aureus and methicillin-susceptible Staphylococcus aureus carriage in pig farmers: a prospective cohort study. Clin Microbiol Infect 20:O764–O771. doi: 10.1111/1469-0691.12582. [DOI] [PubMed] [Google Scholar]
- 28.Price LB, Stegger M, Hasman H, Aziz M, Larsen J, Andersen PS, Pearson T, Waters AE, Foster JT, Schupp J, Gillece J, Driebe E, Liu CM, Springer B, Zdovc I, Battisti A, Franco A, Zmudzki J, Schwarz S, Butaye P, Jouy E, Pomba C, Porrero MC, Ruimy R, Smith TC, Robinson DA, Weese JS, Arriola CS, Yu F, Laurent F, Keim P, Skov R, Aarestrup FM. 2012. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. mBio 3:e00305-11. doi: 10.1128/mBio.00305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabó I, Beck B, Friese A, Fetsch A, Tenhagen BA, Roesler U. 2012. Colonization kinetics of different methicillin-resistant Staphylococcus aureus sequence types in pigs and host susceptibilities. Appl Environ Microbiol 78:541–548. doi: 10.1128/AEM.05327-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DANMAP 2012. 2013. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. http://www.danmap.org/∼/media/Projekt%20sites/Danmap/DANMAP%20reports/DANMAP%202012/Danmap_2012.ashx.
- 31.Broens EM, Graat EA, Van der Wolf PJ, Van de Giessen AW, De Jong MC. 2011. Transmission of methicillin resistant Staphylococcus aureus among pigs during transportation from farm to abattoir. Vet J 189:302–305. doi: 10.1016/j.tvjl.2010.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.