Abstract
Currently available data on prognostic implication of additional neoplasms in GIST miss comprehensive information on patient outcome with regard to overall or disease specific and disease free survival. Registry data of GIST patients with and without additional neoplasm were compared in retrospective case series. We investigated a total of 836 patients from the multi-center Ulmer GIST registry. Additionally, a second cohort encompassing 143 consecutively recruited patients of a single oncology center were analyzed. The frequency of additional malignant neoplasms in GIST patients was 31.9% and 42.0% in both cohorts with a mean follow-up time of 54 and 65 months (median 48 and 60 months), respectively. The spectrum of additional neoplasms in both cohorts encompasses gastrointestinal tumors (43.5%), uro-genital and breast cancers (34.1%), hematological malignancies (7.3%), skin cancer (7.3%) and others. Additional neoplasms have had a significant impact on patient outcome. The five year overall survival in GIST with additional malignant neoplasms (n = 267) was 62.8% compared to 83.4% in patients without other tumors (n = 569) (P < .001, HR=0.397, 95% CI: 0.298-0.530). Five-year disease specific survival was not different between both groups (90.8% versus 90.9%). 34.2% of all deaths (n = 66 of n = 193) were GIST-related. The presented data suggest a close association between the duration of follow-up and the rate of additional malignancies in GIST patients. Moreover the data indicate a strong impact of additional malignant neoplasms in GIST on patient outcome. A comprehensive follow-up strategy of GIST patients appears to be warranted.
Introduction
Gastrointestinal stromal tumors (GIST) are the most common primary mesenchymal neoplasms of the gastrointestinal tract with an incidence of 7 to 20 per million [1], [2], [3], [4], [5], [6]. Predominant locations are the stomach (60% to -70%) and small intestine (25% to 30%), less common sites are the large bowel (up to 5%) and the omentum/mesentery (up to 5%) [7], [8]. Key event of pathogenesis in GIST are gain of function mutations in the genes encoding the receptor tyrosine kinase KIT (80% to 90%) or platelet derived growth factor receptor α (PDGFRα) (5% to 10%) [9], [10], [11]. Prognosis of GIST and patient outcome is based on tumor size, mitotic rate and tumor location [12], [13], [14], [15], [16]. Complete surgical resection is the primary and the only curative treatment for local GIST. Adjuvant therapy of the tyrosine kinase inhibitor (TKI) imatinib is recommended in high and intermediate risk GIST [17]. TKI application remains the treatment of choice for non-resectable GIST or metastatic disease either in the neo-adjuvant or primary setting [18], [19]. Clinical presentation of GIST is variable. Abdominal pain or discomfort, palpable abdominal mass or gastrointestinal hemorrhage are the mainly reported symptoms, mostly related to tumorsite. About 20% to 30% of GISTs are asymptomatic and therefore diagnosed incidentally (e.g. during surgical procedures for other diseases [8]).
GIST are reported to occur in association with other secondary neoplasms at rates of 14% to 25% [20], [21], [22], [23], [24], [25]. The spectrum of additional malignancies varies among the most common cancers, such as colorectal cancer, prostate cancer, breast cancer and gastric cancer. However, there are also reports on additional neoplasms like hematological germ cell and neuroendocrine tumors [20], [21], [22], [23], [24], [25]. So far data are limited on the impact of secondary neoplasms on GIST prognosis and/or patient outcome. The largest study including 783 patients of one institutional tumor registry reported a rate of secondary neoplasms in 20% [22]. In contrast, very recently 43% of secondary neoplasms have been reported of a GIST cohort including only 86 patients [26]. Systematic work is missing regarding patient outcome and the assessment of overall survival (OS), disease specific survival (DSS) and disease free survival.
Therefore, the aim of the present study was to elucidate comprehensively the frequency and the type of additional neoplasms in a large-scale cohort of patients with GIST with focus on disease prognosis and patient outcome.
Patients and Methods
Patients
This study included 836 patients with histologically confirmed diagnosis of GIST from the multi-center Ulmer GIST Registry from 2006 to 2011. These patients were registered in a multi-center network encompassing 18 oncological centers in South Germany, according to the User’s Guide to Registries Evaluating Patient Outcomes and to the Strengthening (of) the Reporting of Observational Studies in Epidemiology (STROBE) Statement [27], [28], [29], [30], [31]. All cooperating centers are non restricted, open hospitals implicating neither demographic nor social or clinical selection bias. Patients diagnosed before 2006 were registered retrospectively. Since 2006 registration and follow up was intended to be prospectively. After obtaining patients’ consent descriptive and clinical data were collected from medical records, by a personal patient contact and/or contact with the treating physicians. Patients with a multineoplastic syndrome (i.e. Carney triad, type 1 neurofibromatosis/NF-1, familial GIST etc.) were not included.
The following clinicopathological parameters have been assessed: age, gender, clinical manifestation, tumor localization, primary size, mitotic rate, risk classification according to Fletcher et al. [12], Miettinen et al. [16] and Joensuu et al. [15], histological subtype, immunohistochemical staining (KIT in all cases; other markers if available), treatment (surgical resection, extent of resection, TKI application). Main attention was focused on the occurrence of additional neoplasms (benign and/or malign) i.e. GIST patients with multiple tumors as well as secondary malignancies. Outcome measurements were metastases, tumor recurrence and death (GIST related or not). The patients were divided into two main groups: GIST patients with and without additional neoplasms. In order to ascertain center specific differences as selection bias, data from the single oncology center of the University Hospital of Ulm were compared with data from the multicenter Ulmer GIST registry.
The study was approved by the institutional ethics committee and all patients gave written informed consent (Study No.: 90 + 91/2006).
Statistical Analyses
Patient characteristics between the groups were compared by χ2 or Fishers exact test. Estimates for disease-free-survival (DFS), disease-specific-survival (DSS) and overall-survival (OS) were obtained by the Kaplan-Meier method [32] and differences between Kaplan-Meier curves were investigated by the log-rank test (after Mantel-Cox or Breslow-test, respectively). For analyses of disease-specific survival, non GIST-related deaths were censored. If applicable, the Hazard Ratio (HR) and 95% confidence interval (95% CI) were calculated regarding tumor-related death and tumor recurrence and/or metastasis by applying univariate Cox proportional hazards regression models. To prove the most relevant findings of the univariate model and Kaplan-Meier analyses, an additional multivariate Cox proportional hazards regression model has been established considering the variables age, gender, mitotic rate, tumor size and location. Statistical analysis was performed using SPSS V19.0 (SPSS Inc., USA). A value of α < .05 was considered to be significant.
Results
Descriptive and clinical data for both cohorts are given in Table 1. 836 GIST patients with a median age of 67.9 years (range 24.5-94.8 years) and a female to male ratio of 1:1 were included in all analyses. Most tumors were localized in the stomach (63.7%) followed by the small bowel (28.8%), and other tissues (7.5%) including colorectal (4.7%) and extra-gastrointestinal (1.5%) GIST. The most common clinical manifestation was abdominal pain (21.9%) followed by gastro-intestinal bleeding (21.2%) and weight loss (5.7%). 39.2% had no clinical symptoms and the GISTs were detected incidentally. Surgical resection was performed in 98% with R0 resection in 92.9% of patients. TKI treatment was used in 20.9% of GIST. Major cell morphology was spindle cell (83.2%), 16.8% showed a mixed or epithelioid pattern. Mean and median tumor size was 5.4 cm (± SD 4.7 cm) and 4.1 cm (0.3-40 cm). 16.8% and 70.3% of tumors showed a mitotic rate of ≥ 10/50 high power fields (HPF) and < 5/50HPF, respectively. KIT staining was positive in 97.8%. Risk classifications according to Fletcher et al. [12] and Joensuu [15] (modified NIH-classification) resulted in high risk patients in 26.5% and 32.3%, respectively and in intermediate/low risk patients in 73.4% and 67.7%, respectively.
Table 1.
Demographic and Clinicopathological Data of 836 GIST Patients with Additional Neoplasms
| Parameter | Single center(n = 143) | Multi center(n = 836) | ||
|---|---|---|---|---|
| Epidemiology | (N) | % | (N) | % |
| Gender (male vs. female) | (70/73) | 49.0/51.0 | (423/413) | 50.6/49.6 |
| Mean age at diagnosis (year ± S.D.) | 64.7 (12.7) | 66.3 (12.3) | ||
| Median age at diagnosis (year, range) | 66.7 (24.5; 94.1) | 67.9 (24.5; 94.8) | ||
| Localisation of GIST | ||||
| Gaster/small bowel/other | (84/48/9) | 59.6/34.0/6.4 | (519/235/61) | 63.7/28.8/7.5 |
| Pathological parameters | ||||
| Cell morphology (spindle vs. epithelioid/mixed) | (116/13) | 89.9/10.1 | (618/125) | 83.2/16.8 |
| Mean tumor size (cm) | 6.0 (5.6) | 5.4 (4.7) | ||
| Median tumor size (cm) | 4.4 (0.4; 40.0) | 4.1 (0.3; 40) | ||
| Mitotic rate (< 5 vs. ≥ 5/50 HPF) | (73/39) | 65.2/34.8 | (421/178) | 70.3/29.7 |
| Mitotic rate (< 10 vs. ≥ 10/50 HPF) | (92/20) | 82.1/17.9 | (497/100) | 83.2/16.8 |
| IHC KIT (pos vs. neg) | (128/2) | 97.7/2.3 | (743/17) | 97.8/2.2 |
| IHC CD34 (pos vs. neg) | (98/21) | 82.4/17.6 | (544/73) | 88.2/11.8 |
| Risk classification | ||||
| NIH (high/intermediate/low/very low) | (38/21/39/25) | 30.9/17.1/31.7/20.3 | (173/145/219/115) | 26.5/22.2/33.6/17.6 |
| AFIP (high/intermediate/low/very low) | (32/8/50/27) | 27.4/6.8/42.7/23.1 | (135/83/269/140) | 21.5/13.2/42.9/22.3 |
| mod. NIH (high/intermediate/low/very low) | (40/19/37/27) | 32.5/15.4/30.1/22.0 | (211/107/195/140) | 32.3/16.4/29.9/21.4 |
| Clinical parameters | ||||
| Resection (R0 vs. R1/2) | (106/10) | 91.4/8.6 | (631/48) | 92.9/7.1 |
| Multivisceral resections (yes vs. no) | (31/112) | 21.5/78.5 | (194/642) | 23.2/76.8 |
| TKI-Therapy (yes vs. no) | (23/109) | 19.7/80.3 | (150/568) | 20.9/79.1 |
| Incidental diagnosis (yes vs. no) | (51/79) | 39.2/60.8 | (307/465) | 39.8/60.2 |
| Secondary neoplasms | ||||
| Rate of malignant neoplasms | (60) | 42.0 | (267) | 31.9 |
| Rate of benign neoplasms | (30) | 21.0 | (209) | 25.0 |
| Rate of malignant or benign neoplasms | (81) | 56.6 | (422) | 50.5 |
| Rate of multiple malignant NPL | (11) | 7.6 | (45) | 5.3 |
| Symptoms | ||||
| Abdominal pain | (44) | 46.8 | (172) | 21.9 |
| GI bleeding | (39) | 41.5 | (167) | 21.2 |
| Weight loss | (15) | 16.0 | (45) | 5.7 |
| Survival analysis | ||||
| Mean follow-up time (year ± SD) | 5.4 (3.9) | 4.5 (3.5) | ||
| Median follow-up time (year, range) | 5.0 [0.01; 18.5] | 4.0 [0.01; 23.2] | ||
| Death (total/GIST-related) | (41/15) | 28.7/10.5 | (193/66) | 23.1/7.9 |
| Rate of recurrence or metastases | (30 of 135) | 22.1 | (146 of 761) | 19.2 |
| Overall-survival-rates (1-/3-/5-year) | (121/93/69) | 93.2/84.3/75.4 | (629/454/300) | 92.3/84.0/76.6 |
| Disease-specific survival-rates (1-/3-/5-year) | (121/93/69) | 97.6/93.1/88.7 | (629/454/300) | 97.3/94.3/90.9 |
| Disease-free-survival rates (1-/3-/5-year) | (105/77/57) | 86.4/80.9/78.5 | (526/366/239) | 88.9/84.8/81.9 |
SD, standard deviation; GI, gastrointestinal; TKI, tyrosine kinase inhibitor; IHC, immunohistochemistry.
Outcome Measurements
Metastases or tumor recurrence occurred in 19.2% (n = 146 of 761 patients), while 11.2% (93 of 836 pts) of GIST patients had initial metastases. Median follow up-time until tumor recurrence or metastases in high-risk patients was 2.75 years (range 0.12; 22.6 yrs). 23.1% (n = 193) patients died, 66 of them (34.2%) due to GIST-related causes. 1-, 3- and 5-year OS were 92.3%, 84.0% and 76.6%, respectively. The 1-, 3- and 5-year DSS was 97.3%, 94.3% and 90.9%, respectively. The 1-, 3- and 5-year DFS was 88.9%, 84.8% and 81.9%, respectively. Five-year patient outcome for high risk versus non-high risk patients of the Ulmer multicenter registry was significantly reduced with a DSS of 78.4% vs. 95.8% (HR=7.17, CI 95% 3.56-14.45, P < .001) and a DFS of 50.0% vs. 93.8% (HR = 9.54, 95% CI 5.82-15.62, P < .001). OS was not different between both groups (71.8% vs. 80.8%).
GIST and Additional Malignancies
In the Ulmer GIST registry (n = 836) additional neoplasms associated with GIST were found in 50.5% (422 patients with 579 malign and/or benign tumors; Table 1). A total of 31.9 % of GIST showed malignant tumors (317 tumors in 267 patients). The median follow-up time was 4.0 years (range 0.01-23.2 years). In the single center cohort a total of 56.6% GIST patients showed additional neoplasms. Malignancies in addition to GIST occurred in 42.0% (60 of 143 patients with 73 tumors). The median follow-up time was 5.0 years (range 0.01 to 18.5 yrs). The detailed spectrum of additional secondary malignacies is given in Table 2. Fourty-five GIST patients (5.3%) were affected by two or more tumors (Supplemental Table 1). Three of those patients had three and one patient even four malignant cancers. Detailed information of additional benign tumors are given in the Supplemental Table 2). Comparative analyses between both cohorts (single versus multi-center GIST cohort) showed no significant differences for clinicopathological data (i.e. tumor location, tumor size, mitotic rate, histological subtype, KIT positivity, resection extent and rate of TKI-application; Table 1).
Table 2.
Type and Frequency of Secondary Malignancies in Patients With GIST
| Present study 2014 |
Pandungeran et al. 2010 |
Agaimy et al. 2006 |
|||||
|---|---|---|---|---|---|---|---|
| n | (N) | % | (N) | % | (N) | % | |
| GI-cancers | (138) | 43.5 | (48) | 25.8 | (228) | 46.9 | |
| Colorectal | (68) | 21.5 | (18) | 9.7 | (109) | 22.4 | |
| Rectal cancer | 22 | ||||||
| Colon cancer | 46 | ||||||
| HBP NPL | (20) | 6.3 | (8) | 4.3 | (20) | 4.1 | |
| Pancreas Cancer | 15 | ||||||
| Gallbladder | 2 | ||||||
| IPMN | 1 | ||||||
| Cholangio carcinoma | 1 | ||||||
| Ampullary cancer | 1 | ||||||
| Esophagus and stomach | (44) | 13.9 | (14) | 7.5 | (106) | 21.8 | |
| Stomach cancer | 33 | ||||||
| Esophagus cancer | 11 | ||||||
| Other GI Ca | (6) | ||||||
| Neuroendocrine-cancer | 4 | ||||||
| Duodenal cancer | 1 | ||||||
| Jejunal cancer | 1 | ||||||
| Urological, gynaecologoical and breast cancer | (108) | 34.1 | (77) | 41 | (129) | 29 | |
| Gynaecological tumor | (61) | 19.2 | |||||
| Prostate cancer | 38 | ||||||
| Uterus cancer | 12 | ||||||
| Cervix cancer | 4 | ||||||
| Ovarian cancer | 4 | ||||||
| Uterus leiomyosarcoma | 2 | ||||||
| Seminoma | 1 | ||||||
| Urological cancer | (21) | 6.6 | (18) | 9.7 | (37) | 7.6 | |
| Renal cell cancer | 12 | ||||||
| Urothelial cancer | 9 | ||||||
| Breast cancer | (26) | 8.2 | (15) | 8.1 | (34) | 7.0 | |
| Blood-NPL | (23) | 7.3 | (12) | 6.5 | (37) | 7.6 | |
| Multiple myeloma | 7 | ||||||
| Lymphoma | 7 | ||||||
| Chronic lymphatic leucemia | 5 | ||||||
| Acute myeloid leucemia | 1 | ||||||
| Poly cythemia vera | 2 | ||||||
| Hairy cell leukemia | 1 | ||||||
| Skin tumors | (23) | 7.3 | (9) | 4.8 | (13) | 2.7 | |
| Squamous carcinoma | 10 | ||||||
| Malignant melanoma | 8 | ||||||
| others | 5 | ||||||
| Lung tumors | (8) | 2.5 | (10) | 5.4 | (26) | 5.4 | |
| Lung cancer | 7 | ||||||
| Carcinoid | 1 | ||||||
| others | (17) | 5.4 | (31) | 16.6 | (49) | 10.1 | |
| Pheochromocytoma | 3 | ||||||
| Thyroid cancer | 3 | ||||||
| CUP | 2 | ||||||
| Larynx cancer | 2 | ||||||
| Oral cancer | 2 | ||||||
| Malignant paraganglioma | 1 | ||||||
| Osteosarcoma | 1 | ||||||
| Leiomyosarcoma | 1 | ||||||
| Glomus tumor (malignant) | 1 | ||||||
| Uvea melanoma | 1 | ||||||
| (n = 295 own pts.) (n = 191 literature) |
|||||||
| Total | (n = 317) | 100% | (n = 186) | 100% | (n = 486) | 100% | |
IPMN, Intraductal papillary mucinous neoplasm; CUP, Carcinoma of unknown primary.
Outcome of GIST With and Without Additional Malignancies
The OS after 5 years in GIST patients with and without secondary malignancies was 62.8% and 83.4%, respectively indicating a significant difference (P < .001 log-rank; P < .001 Cox model, HR 0.397, 95% CI: 0.298-0.530; Figure 1, Table 3, Table 4). Data regarding 5 year DSS were not different between both groups (90.8% and 90.9%, Figure 2, Table 3, Table 4). The median survival time in GIST patients with and without secondary malignancies was 7.4 years (range 5.9-8.8) and 14.8 years (range 10.3-19.3), respectively. Detailed results are shown in Table 3.
Figure 1.
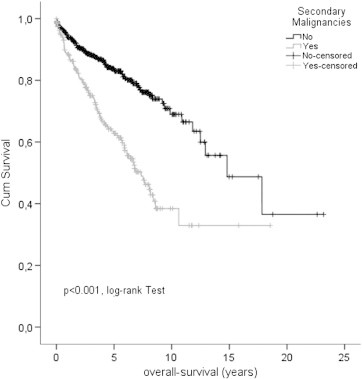
Overall survival in comparison of GIST patients with and without secondary malignancies.
Table 3.
Comparison of GIST Patients With and Without Second Malignancies
| Parameter | (n = 267) With 2nd malignancies |
(n = 569) without 2nd malignancy |
P value, (− test) |
||
|---|---|---|---|---|---|
| (N) | % | (N) | % | ||
| Epidemiology | |||||
| Gender (male vs. female) | (148/119) | 55.4/44.6 | n = 294/n = 275 | 48.3/51.7% | P = .0561) |
| Mean age at diagnosis (year, (± SD) | 69.3a (10.6) | 64.9 (12.9) | P < .0012) | ||
| Median age at diagnosis (year, [range]) | 69.7 [36.8; 94.8] | 66.5 [24.5; 93.3] | - | ||
| Localisation of GIST | |||||
| Gaster/Small bowel/Other | (172/72/21) | 64.9/27.2/7.9 | (347/163/40) | 63.1/29.6/7.3 | - |
| Pathological Parameters | |||||
| Cell morphology (spindle vs. epithelioid/mixed) | (198/31) | 86.5/13.5 | (420/94) | 81.7/18.3 | P = .1101) |
| Mean tumor size (cm, ± SD) | 4.1 (3.8) | 5.9 (5.0) | P < .0012) | ||
| Median tumor size (cm, [range]) | 3.0 [0.03; 20.0] | 4.9 [0.2; 40.0] | - | ||
| Mitotic rate (< 5 vs. ≥ 5/50 HPF) | (136/45) | 75.1/24.9 | (285/133) | 68.2/31.8 | P = .0871) |
| Mitotic rate (< 10 vs. ≥ 10/50 HPF) | (153/27) | 85.0/15.0 | (344/73) | 82.5/17.5 | P = .4521) |
| IHC KIT (pos vs. neg) | (237/3) | 98.8/1.3 | (506/14) | 97.3/2.70 | P = .2111) |
| IHC CD34 (pos vs. neg) | (175/19) | 90.2/9.8 | (369/54) | 87.2/12.8 | P = .2891) |
| Risk Classification | |||||
| NIH (high vs. Non-high risk) | (41/162) | 20.2/79.8 | (132/449) | 29.4/70.6 | P = .0141) |
| AFIP (high vs. Non-high risk) | (32/163) | 16.4/83.6 | (103/329) | 23.8/76.2 | P = .0141) |
| mod. NIH (high vs. Non-high risk) | (52/151) | 25.6/74.4 | (159/291) | 35,3/64.7 | P = .0141) |
| Clinical parameters | |||||
| Resection (R0 vs. R1/2) | (198/13) | 93.8/6.2 | (433/35) | 92.5/7.5 | P = .5351) |
| TKI-Therapy (yes vs. no) | (34/189) | 15.2/84.8 | (116/379) | 23.4/76.6 | P = .0131) |
| Incidental diagnosis (yes vs. no) | (147/98) | 60.0/40.0 | (160/367) | 30.4/69.6 | P < .0011) |
| Survival analysis | |||||
| Mean follow-up time (year, (± SD) | 4.0 (± 3.2) | 4.7 (± 3.6) | P = .0162) | ||
| Median follow-up time (year, [range]) | 3.5 [0.01; 18.5] | 4.2 [0.01; 23.2] | - | ||
| Death (GIST-related vs. not) | (19/98) | 7.1/36.7 | (47/95) | 8.3/16.7 | - |
| Rate of recurrence or metastases | (38) | 16.8 | (99) | 20,2 | P = .2781) |
| Overall-survival-rates (1-/3-/5-year) | (194/135/88) | 88.5/75.0/62.8 | (435/319/212) | 94.0/88.3/83.4 | P < .0013) |
| Disease-specific survival (1-/3-/5-year) | (194/135/88) | 99.0/96.5/90.8 | (435/319/212) | 96.6/93.4/90.9 | P = .6033) |
| Disease-free survival (1-/3-/5-year) | (159/108/66) | 90.7/88.1/82.7 | (367/258/173) | 88.1/83.4/81.5 | P = .5863) |
1) χ2-test, 2)t test, 3) log-rank test.
Table 4.
Comparative Survival Analyses of GIST Patients With and Without Secondary Malignancies
| (Ntotal= 267/569) |
With 2nd NPL |
Without 2nd NPL |
Univariate Cox model |
Multivariate Cox model |
||
|---|---|---|---|---|---|---|
| % | (N) | % | (N) | P, HR (95% CI) | P, HR (95% CI) | |
| Disease-specific-survival | ||||||
| 1-year DSS | 99.0 | (194) | 96.6 | (435) | P = .603, HR 1.164 (0.657;2.061) | P = .880, HR 1.053 (0.536;2.070) |
| 3-year DSS | 96.5 | (135) | 93.4 | (319) | ||
| 5-year DSS | 90.8 | (88) | 90.9 | (212) | ||
| Overall-survival | ||||||
| 1-year OS | 88.5 | (194) | 94.0 | (435) | P < .001 HR 0.397 (0.298;0.530) | P < .001 HR 0.402 (0.282;0.575) |
| 3-year OS | 75.0 | (135) | 88.3 | (319) | ||
| 5-year OS | 62.8 | (88) | 83.4 | (212) | ||
| Disease-free survival | ||||||
| 1-year DFS | 90.7 | (159) | 88.1 | (367) | P = .586 HR 1.118 (0.748;1.672) | P = .616 HR 0.883 (0.544;1.435) |
| 3-year DFS | 88.1 | (108) | 83.4 | (258) | ||
| 5-year DFS | 82.7 | (66) | 81.5 | (173) | ||
DSS, disease specific survival; DSF, disease free survival; OS, overall survival; HR, hazard ratio.
Multivariate Cox proportional hazards regression model has been adjusted considering the variables age, gender, mitotic rate, tumor size and localization (GIST).
Figure 2.
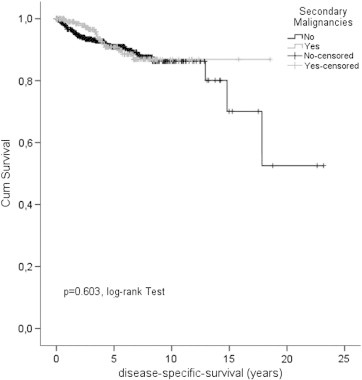
Disease specific survival in GIST patients with and without secondary malignancy.
Discussion
In 2000, at the first time Maiorana et al. reported on a small series of epithelioid gastric GIST associated with additional neoplasms [33]. Kalmár et al. concluded a significantly higher secondary neoplasia rate in GIST in comparison to a Hungarian control population (21.7% vs 4%; P < .001) based on 5 from 23 GIST patients with secondary malignancies and a follow-up time of 1–157 month. Mean age as well as the male to female ratio was similar in both groups [34]. Ruka et al. reported on 18 patients (10.0%) with a history of other malignancies among 180 GIST patients with a median age of 60 years whereby two GIST patients suffered from more than one other tumor [35]. In the same year Kövér et al. emphasized the high association of GIST with additional neoplasms, reporting of 7 from 43 GIST patients (16.3%) with secondary malignancies [36]. Agaimy et al. described 18.6% of GIST occurring secondary neoplasms (18/97 cases) with a female to male ratio of 2:1. GIST showed a trend towards small size (only 2 vs 18 large GIST), the majority of associated malignancies (50%) were classified as GI-cancers [37]. In 2006, Wronski et al. described a 14% rate of secondary neoplasms in GIST in 4/28 GIST patients [38]. A review of the literature and own cases by Agaimy et al. revealed 518 cancers in 486 GIST patients among a total of 4813 patients, accounting for secondary neoplasias and multiple neoplasias in 10.1% and 0.6% of GIST patients, respectively [20]. In the literature the reported frequency of additional neoplasms in GIST patients varies by 4.5% to 33% (mean 13%), and about half of them (47%) accounted for GI-cancers, followed by urogenital tumors. In the series of Liszka et al. 2007, 17 of 82 patients presented with secondary malignant neoplasia (20.7%) and a trend towards a small intestinal manifestation was found [21]. Focusing on immune-histochemical and mutational analysis, Arnogiannaki et al. 2010 reported no differences between GIST patients with (6 of 20 GIST) or without additional malignancy [39]. The largest clinical study, including 783 GIST patients, reports on 153 patients (20.3%) with additional malignancies [22]. Of interest 15.7% (n = 24/153) presented with synchronous and 84.3% (n = 129/153) with metachronous malignant neoplasms. A total of 20.9% GIST (n = 32/153) developed secondary malignancies (after the diagnosis of GIST) and 63.4% (n = 97/153) have had a history of previous malignancies however, without impact on the prognosis of GIST.
Taken together, the currently available data on additional neoplasms in GIST miss comprehensive information on patient outcome with regard to overall or disease specific and disease free survival. Therefore the present study, focused on the prognostic impact of additional neoplasms in GIST, investigating a large-scale multicenter cohort of 836 patients with GIST and in addition a single-center cohort of 143 GIST patients.
We observed 31.9% and 42.0% of GIST associated with additional malignancies by mean follow-up times of 4.5 years and 5.4 years, respectively. As expected the duration of the follow-up time was closely linked to the occurrence of secondary malignancies in GIST. In accordance with other studies, type of secondary malignancies encompasses the most common cancer entities such as GI cancers (43.5%) and urogenital and breast cancers (34.1%). Of note lung cancer (2.5%) was remarkable underrepresented [20], [21], [22], [26]. Within the three largest studies, the majority (75% to 80%) of GIST-associated tumors are gastrointestinal (25.8% to 46.6%) as well as urogenital and breast cancer (29% to 41%) [20], [22] (Table 2).
The impact on prognosis of secondary malignancies is indicated by a significantly reduced five year OS rate of 62.8% compared to 83.4% in GIST patients without associated malignancies (P < .001 log rank; P < .001 Cox model, HR 0.397, 95% CI: 0.298; 0.530; Figure 1, Table 3, Table 4). Five year DSS rates were identical (90.8% and 90.9%), demonstrating the importance of comprehensive outcome measures (e.g. OS vs DSS) in cancer patients.
Most GISTs associated additional malignancies occurred in patients who are 5 to 10 years older (P < .001, t test) and they tend to fall into the low or very low risk category (P = .002, χ2-test), which is in accordance with the published data [20], [21], [22], [26]. The rate and spectrum of additional neoplasms (5% to 43%), the male to female ratio (58.3% males: 41.6% females to 42.1% males: 59.9% females) as well as the impact of second malignancies on the prognosis of GIST (none to significant) varies among several studies according to study size, duration of follow-up and comprehensiveness of survival analysis [20], [21], [22], [23], [26], [33], [36], [38]. These differences may also arise due to referral bias, different durations of follow up, study designs and comprehensiveness of survival analysis or simply due to different populations.
In accordance with Vassos et al. [26], the presented data confirm the major impact of GIST-associated malignancies on the prognosis of GIST and underpins the relevance of comprehensive outcome measurements. With regard to the 10 year survival probability, indicating an almost 50% difference between OS (38.4%) and DSS (86.8%) in the present study, we suggest a regular and continuous follow up of GIST patients at least for 10 years. Currently, pathophysiological mechanisms explaining the high risk of GIST patients for secondary malignancies are unknown which needs systematic basic as well as clinical research activities for the future. Moreover treatment strategies like the adjuvant TKI use in GIST may benefit from such kind of knowledge both for the selection of right patient and clinical outcome.
Acknowledgement
We do thank Annette Blatz (University of Ulm, Germany) for editorial assistance and Annemarie and Karl Schmieder (Schwäbisch Gmünd, Germany) for data management.
KK and MiSc had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Key message: About 40% of GIST patients of the Ulmer GIST registry (n = 836 GIST) revealed additional neoplasms resulting significantly in disease prognosis and patient outcome. Comprehensive follow-up strategies of GIST appear to be justified in order to improve disease prognosis and patient care.
Authors' Contributions: The contributions of each author to the manuscript are: KK and MiSc conceived and designed the study. KK, SW, and MiSc were involved in the data aquisition. KK, SW, MaSc, AA, DHB, UK, and MiSc contributed to data analysis and interpretation. KK, MaSc, AA and MiSc contributed to the writing of the report. The authors have no conflict of interest or financial disclosure to declare.
Funding: MS was in part supported by the Robert Bosch Stiftung, Stuttgart, Germany and the IZEPHA grant Tübingen-Stuttgart [#8-0-0].
Disclosure: The authors have no conflict of interest or financial disclosure to declare.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neo.2014.12.001.
Contributor Information
K. Kramer, Email: klaus.kramer@uniklinik-ulm.de, kramer.k@web.de.
S. Wolf, Email: sebe_wolf@yahoo.de.
B. Mayer, Email: benjamin.mayer@uni-ulm.de.
S.A. Schmidt, Email: stefan.schmidt@uniklinik-ulm.de.
A. Agaimy, Email: abbas.agaimy@uk-erlangen.de.
D. Henne-Bruns, Email: doris.henne-bruns@uniklinik-ulm.de.
U. Knippschild, Email: uwe.knippschild@uniklinik-ulm.de.
M. Schwab, Email: matthias.schwab@ikp-stuttgart.de.
M. Schmieder, Email: GISTUlm@gmail.com.
Appendix A. Supplementary Data
Supplemental Table 1. 41 GIST patients with two additional malignancies (out of 836 GIST patients of the Ulmer GIST registry).
Supplemental Table 2. Benign secondary neoplasms associated with GIST in 207 of 836 patients.
References
- 1.Tran T., Davila J.A., El-Serag H.B. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100:162–168. doi: 10.1111/j.1572-0241.2005.40709.x. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson B., Bümming P., Meis-Kindblom J.M., Odén A., Dortok A., Gustavsson B. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era – a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 3.Tryggvason G., Kristmundsson T., Orvar K., Jónasson J.G., Magnússon M.K., Gíslason H.G. Clinical study on gastrointestinal stromal tumors (GIST) in Iceland, 1990–2003. Dig Dis Sci. 2007;52:2249–2253. doi: 10.1007/s10620-006-9248-4. [DOI] [PubMed] [Google Scholar]
- 4.Steigen S.E., Bjerkehagen B., Haugland H.K., Nordrum I.S., Løberg E.M., Isaksen V. Diagnostic and prognostic markers for gastrointestinal stromal tumors in Norway. Mod Pathol. 2008;21:46–53. doi: 10.1038/modpathol.3800976. [DOI] [PubMed] [Google Scholar]
- 5.Cassier P.A., Ducimetière F., Lurkin A., Ranchère-Vince D., Scoazec J.-Y., Bringuier P.-P. A prospective epidemiological study of new incident GISTs during two consecutive years in Rhône Alpes region: incidence and molecular distribution of GIST in a European region. Br J Cancer. 2010;103:165–170. doi: 10.1038/sj.bjc.6605743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzen C.-Y., Wang J.-H., Huang Y.-J., Wang M.-N., Lin P.-C., Lai G.-L. Incidence of gastrointestinal stromal tumor: a retrospective study based on immunohistochemical and mutational analyses. Dig Dis Sci. 2007;52:792–797. doi: 10.1007/s10620-006-9480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodall C.E., Brock G.N., Fan J., Byam J.a, Scoggins C.R., McMasters K.M. An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system. Arch Surg. 2009;144:670–678. doi: 10.1001/archsurg.2009.108. [DOI] [PubMed] [Google Scholar]
- 8.Steigen S.E., Eide T.J. Gastrointestinal stromal tumors (GISTs): a review. APMIS. 2009;117:73–86. doi: 10.1111/j.1600-0463.2008.00020.x. [DOI] [PubMed] [Google Scholar]
- 9.Joensuu H., Vehtari A., Riihimäki J., Nishida T., Steigen S.E., Brabec P. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265–274. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 10.Rubin B.P., Antonescu C.R., Scott-Browne J.P., Comstock M.L., Gu Y., Tanas M.R. A knock-in mouse model of gastrointestinal stromal tumor harboring kit K641E. Cancer Res. 2005;65:6631–6639. doi: 10.1158/0008-5472.CAN-05-0891. [DOI] [PubMed] [Google Scholar]
- 11.Hirota S., Isozaki K., Moriyama Y., Hashimoto K., Nishida T., Ishiguro S. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [80-] [DOI] [PubMed] [Google Scholar]
- 12.Fletcher C.D.M., Berman J.J., Corless C., Gorstein F., Lasota J., Longley B.J. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 13.Hornick J.L., Fletcher C.D.M. The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol. 2007;38:679–687. doi: 10.1016/j.humpath.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Huang H.-Y., Li C.-F., Huang W.-W., Hu T.-H., Lin C.-N., Uen Y.-H. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007;141:748–756. doi: 10.1016/j.surg.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Miettinen M., Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Blay J.-Y., Wardelmann E. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl. 7):vii49–vii55. doi: 10.1093/annonc/mds252. [DOI] [PubMed] [Google Scholar]
- 18.Grignol V.P., Termuhlen P.M. Gastrointestinal stromal tumor surgery and adjuvant therapy. Surg Clin North Am. 2011;91:1079–1087. doi: 10.1016/j.suc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Reichardt P., Reichardt A. Gastrointestinal stromal tumour (GIST): current standards in multimodal management. Zentralbl Chir. 2011;136:359–363. doi: 10.1055/s-0031-1271596. [DOI] [PubMed] [Google Scholar]
- 20.Agaimy A., Wünsch P.H., Sobin L.H., Lasota J., Miettinen M. Occurrence of other malignancies in patients with gastrointestinal stromal tumors. Semin Diagn Pathol. 2006;23:120–129. doi: 10.1053/j.semdp.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Liszka Ł., Zielińska-Pajak E., Pajak J., Gołka D., Huszno J. Coexistence of gastrointestinal stromal tumors with other neoplasms. J Gastroenterol. 2007;42:641–649. doi: 10.1007/s00535-007-2082-4. [DOI] [PubMed] [Google Scholar]
- 22.Pandurengan R.K., Dumont a G., Araujo D.M., Ludwig J. a, Ravi V., Patel S. Survival of patients with multiple primary malignancies: a study of 783 patients with gastrointestinal stromal tumor. Ann Oncol. 2010;21:2107–2111. doi: 10.1093/annonc/mdq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira S.S., Werutsky G., Toneto M.G., Alves J.M., Piantá C.D., Breunig R.C. Synchronous gastrointestinal stromal tumors (GIST) and other primary cancers: case series of a single institution experience. Int J Surg. 2010;8:314–317. doi: 10.1016/j.ijsu.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Gonçalves R., Linhares E., Albagli R., Valadão M., Vilhena B., Romano S. Occurrence of other tumors in patients with GIST. Surg Oncol. 2010;19:e140–e143. doi: 10.1016/j.suronc.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Sevinc A., Seker M., Bilici A., Ozdemir N.Y., Yildiz R., Ustaalioglu B.O. Co-existence of gastrointestinal stromal tumors with other primary neoplasms. Hepatogastroenterology. 2011;58:824–830. [PubMed] [Google Scholar]
- 26.Vassos N., Agaimy A., Hohenberger W., Croner R.S. Coexistence of gastrointestinal stromal tumours (GIST) and malignant neoplasms of different origin: Prognostic implications. Int J Surg. 2014 doi: 10.1016/j.ijsu.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Vandenbroucke J.P., von Elm E., Altman D.G., Gøtzsche P.C., Mulrow C.D., Pocock S.J. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18:805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 28.Vandenbroucke J.P., von Elm E., Altman D.G., Gøtzsche P.C., Mulrow C.D., Pocock S.J. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandenbroucke J.P., von Elm E., Altman D.G., Gøtzsche P.C., Mulrow C.D., Pocock S.J. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:W163–W194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 30.Kramer K. University of Ulm; 2012. Establishment of a multi-centric GIST registry and oncologic network. [Google Scholar]
- 31.Gliklich R.E., Dreyer N.A. 2nd ed. vol. 2nd. Agency for Healthcare Research and Quality (US); Rockville (MD): 2010. Registries for Evaluating Patient Outcomes: A User’s Guide. [PubMed] [Google Scholar]
- 32.Kaplan E., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 33.Maiorana A., Fante R., Cesinaro A.M., Fano R.A. Synchronous occurrence of epithelial and stromal tumors in the stomach: a report of 6 cases. Arch Pathol Lab Med. 2000;124:682–686. doi: 10.5858/2000-124-0682-SOOEAS. [DOI] [PubMed] [Google Scholar]
- 34.Kalmár K., Tornóczky T., Pótó L., Illényi L., Kalmár Nagy K., Kassai M. Gastrointestinal stromal tumours in a single institute: is there an association to other gastrointestinal malignancies? Magy Seb. 2004;57:251–256. [PubMed] [Google Scholar]
- 35.Ruka W., Rutkowski P., Nowecki Z., Nasierowska-Guttmejer A., Debiec-Rychter M. Other malignant neoplasms in patients with gastrointestinal stromal tumors (GIST) Med Sci Monit. 2004;10:LE13–LE14. [PubMed] [Google Scholar]
- 36.Kövér E., Faluhelyi Z., Bogner B., Kalmár K., Horváth G., Tornóczky T. Dual tumours in the GI tract: synchronous and metachronous stromal (GIST) and epithelial/neuroendocrine neoplasms. Magy Onkol. 2004;48:315–321. [doi:HUON.2004.48.4.0315] [PubMed] [Google Scholar]
- 37.Agaimy A., Wuensch P.H. Gastrointestinal stromal tumours in patients with other-type cancer: a mere coincidence or an etiological association? A study of 97 GIST cases. Z Gastroenterol. 2005;43:1025–1030. doi: 10.1055/s-2005-858378. [DOI] [PubMed] [Google Scholar]
- 38.Wronski M., Ziarkiewicz-Wroblewska B., Gornicka B., Cebulski W., Slodkowski M., Wasiutynski A. Synchronous occurrence of gastrointestinal stromal tumors and other primary gastrointestinal neoplasms. World J Gastroenterol. 2006;12:5360–5362. doi: 10.3748/wjg.v12.i33.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnogiannaki N., Martzoukou I., Kountourakis P., Dimitriadis E., Papathanasaki A., Nastoulis E. Synchronous presentation of GISTs and other primary neoplasms: a single center experience. In Vivo. 2010;24:109–115. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. 41 GIST patients with two additional malignancies (out of 836 GIST patients of the Ulmer GIST registry).
Supplemental Table 2. Benign secondary neoplasms associated with GIST in 207 of 836 patients.


