Abstract
Although hormonal regulation of ovarian follicle development has been extensively investigated, most studies concentrate on the development of early antral follicles to the preovulatory stage, leading to the successful use of exogenous FSH for infertility treatment. Accumulating data indicate that preantral follicles are under stringent regulation by FSH and local intraovarian factors, thus providing the possibility to develop new therapeutic approaches. Granulosa cell-derived C-type natriuretic factor not only suppresses the final maturation of oocytes to undergo germinal vesicle breakdown before ovulation but also promotes preantral and antral follicle growth. In addition, several oocyte- and granulosa cell-derived factors stimulate preantral follicle growth by acting through wingless, receptor tyrosine kinase, receptor serine kinase, and other signaling pathways. In contrast, the ovarian Hippo signaling pathway constrains follicle growth and disruption of Hippo signaling promotes the secretion of downstream CCN growth factors capable of promoting follicle growth. Although the exact hormonal factors involved in primordial follicle activation has yet to be elucidated, the protein kinase B (AKT) and mammalian target of rapamycin signaling pathways are important for the activation of dormant primordial follicles. Hippo signaling disruption after ovarian fragmentation, combined with treating ovarian fragments with phosphatase and tensin homolog (PTEN) inhibitors and phosphoinositide-3-kinase stimulators to augment AKT signaling, promote the growth of preantral follicles in patients with primary ovarian insufficiency, leading to a new infertility intervention for such patients. Elucidation of intraovarian mechanisms underlying early folliculogenesis may allow the development of novel therapeutic strategies for patients diagnosed with primary ovarian insufficiency, polycystic ovary syndrome, and poor ovarian response to FSH stimulation, as well as for infertile women of advanced reproductive age.
Introduction
FSH Promotes Preantral Follicle Development, Whereas C-Type Natriuretic Peptide Is a Follicle-Stimulating Factor and an Oocyte Maturation Inhibitor
Diverse Oocyte and Granulosa Cell Factors Regulate Preantral Follicle Growth
Ovarian Hippo Signaling Constrains Follicle Development and Promotion of Preantral Follicle Growth by CCN Growth Factors
Hippo Signaling in Ovarian Physiology and Pathophysiology
Regulation of Primordial Follicle Activation by AKT and Mammalian Target of Rapamycin Signaling Pathways
IVA of Preantral Follicles After Hippo Signaling Disruption and AKT Stimulation
Etiologies of POI and Promotion of Preantral Follicle Growth in POI Patients
Predicting Follicle Reserve and Promotion of Preantral Follicle Growth in Women With Diminished Ovarian Reserve
Does a Subgroup of PCOS Patients Have Ovarian Structural Defects?
Future Treatment Options
I. Introduction
Mammalian ovaries consist of follicles as basic functional units. Follicle development starts during fetal (for humans) or neonatal (for rodents) life when primordial follicles are formed. Once initiated to grow, the activated primordial follicles with a single layer of flattened granulosa cells surrounding the primordial oocytes develop into primary, secondary, and eventually antral follicles (1). Most early antral follicles undergo atretic degeneration (2), whereas a few of them, under cyclic gonadotropin stimulation occurring after puberty, reach the preovulatory stage (Figure 1) (3, 4). In women of reproductive age, these preovulatory/Graafian follicles are the major source of the cyclic secretion of ovarian estrogens. In response to the preovulatory gonadotropin surge during each reproductive cycle, the dominant Graafian follicle ovulates to release the mature oocyte for fertilization, whereas the remaining theca and granulosa cells undergo transformation to become the corpus luteum that contributes to circulating progesterone. Menstrual discharges are induced in primates after cyclic changes in the uterine endometrium regulated by circulating estrogen and progesterone secreted by large antral and preovulatory follicles (5).
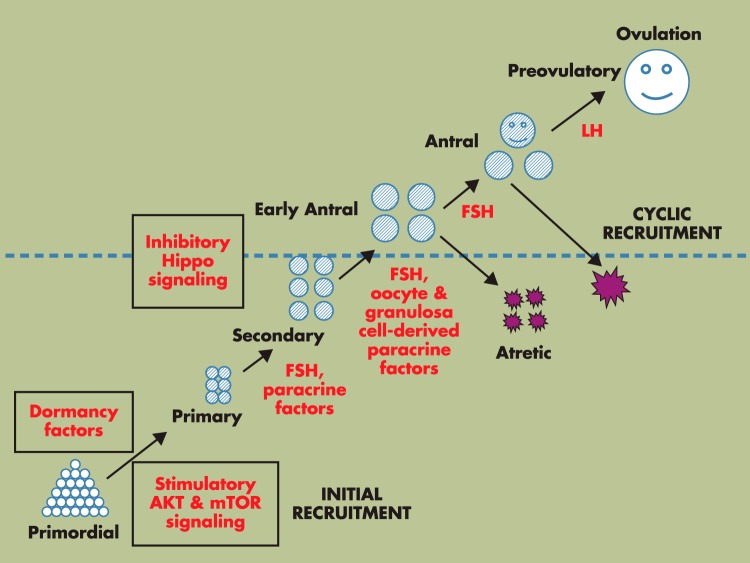
Figure 1.
Hormonal regulation of preantral follicle growth. Selective primordial follicles develop to the primary stage under the control of AKT and mTOR signaling (initial recruitment), whereas most primordial follicles remain arrested by dormancy factors. Once initiated to growth, primordial follicles develop through primary and secondary stages before acquiring an antral cavity. Although most early antral follicles undergo atresia, select antral follicles supported by cyclic changes in pituitary FSH and LH reach the preovulatory stage, capable of releasing mature oocytes after ovulation for fertilization (cyclic recruitment) (1). In addition to the well-studied role of FSH on antral follicle growth (above the dashed line), FSH also regulates preantral follicle growth, together with a large number of oocyte- and granulosa cell-derived paracrine factors (below the dashed line). Furthermore, development of preantral and antral follicle is restrained by the inhibitory Hippo signaling pathway.
The total number of ovarian follicles is determined early in life, and depletion of this pool leads to reproductive senescence. The fate of each follicle is distinct and controlled by endocrine and, more importantly, by diverse paracrine factors. Most investigations on follicle development have focused on follicles at early antral to preovulatory stages and their regulation by gonadotropins (3, 4, 6, 7). During this final stage of follicle development, FSH is the major stimulator of follicle development, and the clinical use of FSH as a therapeutic agent concentrates on the development of early antral to preovulatory follicles for infertility treatment (8). Fewer studies deal with hormonal regulation of the growth of preantral follicles, including activation of dormant primordial follicles (9) and the development of primary and secondary follicles to the early antral stage.
The present review focuses on hormonal regulation and development of the early (primordial, primary, and secondary) stages of folliculogenesis. In addition to summarizing data on the roles of endocrine hormones (LH and FSH) on preantral follicle growth (Figure 1), emphasis is placed upon diverse paracrine and autocrine factors of oocyte and granulosa cell origins to promote or suppress preantral follicle development. We also discuss the importance of the ovarian Hippo signaling system in constraining the growth of preantral and antral follicles as well as the role of the AKT signaling pathway in promoting primordial follicle activation and secondary follicle growth (Figure 1). Mechanisms underlying the activation and growth of primordial follicles will also be addressed. Although there are clear differences in the number of follicles selected for final maturation and ovulation in different species, it remains unclear whether major differences exist in the regulation of early folliculogenesis. Although most findings discussed here are based on murine models, species-specific differences are indicated when known.
In addition to understanding ovarian physiology, we discuss potential etiologies and treatments for the 2 major human ovarian diseases, primary ovarian insufficiency (POI) and polycystic ovary syndrome (PCOS). We will also discuss potential treatments for infertile patients with poor ovarian responses to stimulation by exogenous FSH, those with a low ovarian reserve as well as cancer and other patients with cryopreserved ovarian tissues (10).
II. FSH Promotes Preantral Follicle Development, Whereas C-Type Natriuretic Peptide Is a Follicle-Stimulating Factor and an Oocyte Maturation Inhibitor
Most studies analyze the growth-promoting (11) and antiatretic actions (2) of FSH in antral follicles, whereas FSH regulation of preantral follicle development has received less attention. Earlier studies have demonstrated that FSH receptors are expressed in follicles from primary to later stages (12) and treatment with FSH and LH promotes preantral follicle growth (13). Although follicles in FSH receptor-null mice (14) and hypophysectomized women (15) can still develop to the preantral stage, in vitro and in vivo studies indicate that the growth of preantral follicles could be enhanced by endogenous and exogenous gonadotropins.
Reduction of the high circulating levels of gonadotropins in juvenile rats after either hypophysectomy or GnRH antagonist treatment results in decreased ovarian weight at day 19 of age that is associated with a reduced number of developing follicles and increased atresia of remaining follicles (13). In contrast, FSH treatment of prepubertal intact, hypophysectomized, or GnRH antagonist-treated rats containing preantral and smaller follicles results in increased ovarian weight and follicle development up to the antral stage. Thus, the development of follicles can be divided into gonadotropin-dependent and gonadotropin-responsive stages (Figure 1, above and below the dashed line). Although development to the antral stage is not dependent on FSH as shown in FSH-null mice (14), preantral follicles are responsive to FSH treatment. In addition to in vivo studies, findings using cultured preantral follicles demonstrated important roles of FSH and other factors in the promotion of preantral follicle growth in diverse species (16–19).
These findings raise an interesting question regarding the routine monitoring of antral follicle growth to the preovulatory stage during a 10- to 14-day window in women. It may be possible that preantral follicles could respond to prolonged (>2 weeks) FSH stimulation in patients presenting with only preantral follicles, leading to antral and preovulatory follicle development.
In addition to FSH, C-type natriuretic peptide (CNP) has recently been found to be a follicle-stimulating factor. Natriuretic peptides comprise a family of 3 structurally related molecules: atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and CNP (20). CNP is encoded by the NPPC gene which is expressed in diverse cell types in which the precursor natriuretic peptide precursor C (NPPC) protein is cleaved into the 22-amino-acid peptide CNP (21). CNP activates its cognate receptor natriuretic peptide receptor-B (NPRB), also known as NPR2 or guanylyl cyclase B, whereas ANP and BNP stimulate NPRA, also known as NPR1 or guanylyl cyclase A (22, 23). Both receptors are membrane-anchored guanylyl cyclase enzymes that signal via the production of the second messenger cGMP and undergo both homologous and heterologous desensitization, reflected by dephosphorylation of specific sites in the kinase-homology domain (24). ANP and BNP act as endocrine hormones to regulate blood pressure and volume and inhibit cardiac hypertrophy (25). In contrast, CNP acts in an autocrine/paracrine fashion to induce bone growth (26) and to increase vasorelaxation (27).
Earlier studies have reported ovarian expression of NPPC and NPRB and their regulation by gonadotropins (28, 29). Transcripts for both NPPC and the NPRB receptor are expressed in granulosa and cumulus cells of antral and preovulatory follicles. Treatment of cumulus-oocyte complexes with CNP stimulates cGMP production in cumulus cells and inhibits meiotic resumption of oocytes (30). Thus, CNP of granulosa and cumulus origins stimulates cGMP production by acting on its receptor in cumulus cells. Due to the existence of tight junctions between cumulus and oocytes, cGMP of cumulus origin diffuses into oocytes to suppress phosphodiesterase 3 activity, leading to the elevation of intra-oocyte cAMP levels and oocyte maturation arrest (31) (Figure 2). Before the LH surge, high levels of intraovarian CNP prevent premature maturation of oocytes, whereas the ovulatory LH surge decreases CNP levels in murine ovaries and human follicular fluid to allow germinal vesicle breakdown of oocytes (32). These findings are consistent with earlier identification of a low-molecular-weight oocyte maturation inhibitor in follicular fluid and granulosa cell extracts (33).
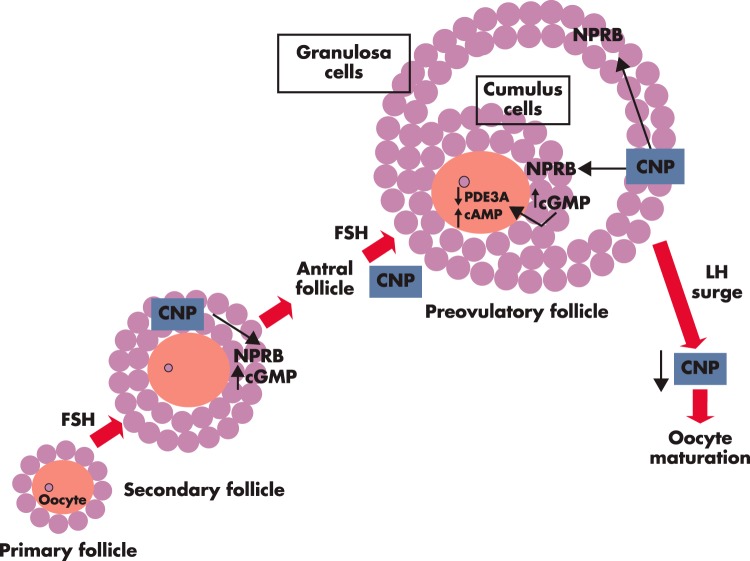
Figure 2.
CNP is an intraovarian factor important for preantral and antral follicle growth as well as for oocyte maturation inhibition. Based on murine studies, CNP is secreted by granulosa cells of secondary and antral follicles in response to FSH stimulation. CNP acts through its receptor NPRB, expressed in granulosa cells of secondary follicles, to increase cGMP production and to stimulate follicle development (38). In addition, CNP acts through its receptor, expressed in cumulus cells of antral and preovulatory follicles, to increase cGMP production. Cumulus cell-produced cGMP, after transporting to oocytes through gap junctions, inhibits the activity of the phosphodiesterase 3A (PDE3A) enzyme, leading to increased intra-oocyte cAMP levels, thus suppressing oocyte maturation (30). The preovulatory LH surge decreases CNP levels in the preovulatory follicles and allows meiotic maturation of preovulatory oocytes (32).
Although dwarfism and early death was found in NPPC-null mice lacking CNP (34), NPRB-null mice show not only skeletal defects but also the arrest of ovarian follicles at the secondary follicle stage (35). In NPPC or NPRB hypomorphic mutant mice, meiotic arrest of oocytes is not sustained in most preovulatory follicles, and meiosis resumed precociously (30, 36). Because earlier studies demonstrated the ability of cGMP analogs to promote the development of cultured preantral follicles in rats (37), we tested the ability of CNP to promote follicle growth (38). RT-PCR analyses indicated increases in NPPC and NPRB transcripts during early folliculogenesis in mice, associated with increases in ovarian CNP peptides (38). In cultured somatic cells obtained from infantile mouse ovaries and granulosa cells from prepubertal animals, treatment with CNP stimulates cGMP, but not cAMP, production. Also, treatment of cultured preantral follicles with CNP stimulates follicle growth, whereas treatment of cultured ovarian explants from infantile mice with CNP, similar to FSH, increases ovarian weight gain that is associated with the development of primary and early secondary follicles to the late secondary stage (38).
Importantly, treatment with FSH increases levels of NPPC, but not NPRB, transcripts in ovarian explants, suggesting CNP is downstream of FSH in the regulation of follicle development (38). The more severe defects in follicle development in NPRB-null mice (35) as compared with those of FSH receptor-null mice (14) underscore the essential role of the CNP-NPRB signaling system in preantral follicle growth. It is likely that basal CNP-NPRB signaling is sufficient for suboptimal follicle growth in the absence of FSH receptor signaling. Although an activating mutation in the kinase homology domain of NPRB causes extremely tall stature in a male patient (39), no studies on females are available.
FSH actions are predominantly mediated by cAMP signaling, whereas CNP actions are exclusively mediated by cGMP but not cAMP signaling (38). Although the role of cGMP in follicle growth remains to be elucidated, it is interesting to note that NPRB hypomorphic mice showed altered meiotic capability but retained near normal follicle development, suggesting FSH and CNP could have overlapping roles on follicle growth. It is therefore of interest to examine differences in downstream genes regulated by FSH (predominant cAMP signaling) and CNP (exclusive cGMP signaling) in ovarian follicles.
In vivo studies confirmed the ability of CNP to promote preantral follicle growth by showing that daily injections of infantile mice with CNP promote ovarian growth, allowing successful ovulation induction by gonadotropins (38). Consistent with the role of CNP as an intraovarian factor downstream of FSH, CNP treatment alone in prepubertal mice (without exogenous FSH) promotes early antral follicle growth to the preovulatory stage, leading to efficient ovulation induction by LH/human chorionic gonadotropin (38). Mature oocytes retrieved after CNP pretreatment are fertilizable and could develop into blastocysts in vitro, allowing the delivery of viable offspring. Thus, CNP secreted by growing follicles is capable of stimulating preantral and antral follicle growth (Figure 2). These findings raise the possibility that CNP could be effective in treating FSH poor responders in the clinic because tissue expression of NPRB is limited (brain areas, adrenal, endothelial cells, lung, and kidney) and short-term CNP treatment is unlikely to induce cardiac and renal changes (40) or skeletal overgrowth.
III. Diverse Oocyte and Granulosa Cell Factors Regulate Preantral Follicle Growth
Only a tiny fraction of the follicular pool reaches the final stage for ovulation and the most important function of ovarian follicles is the growth and maturation of a functional oocyte for fertilization and the propagation of the species. It appears that mechanisms have evolved to insure that follicles with a healthy oocyte have a better chance of reaching the final stage of development. One mechanism is the secretion of oocyte-derived paracrine factors capable of promoting the proliferation and differentiation of surrounding somatic cells. At least 3 oocyte-derived factors have been shown to promote granulosa cell growth, including R-spondin2 (41), growth differentiation factor-9 (GDF9), and bone morphogenetic protein-15 (BMP15) (42).
Wingless (WNT) proteins are conserved secreted signaling molecules acting locally to control diverse developmental processes (43). A large family of WNT ligands activates several 7-transmembrane Frizzled receptors, and this ligand-receptor interaction requires the participation of several coreceptors including low-density lipoprotein-related receptor-5 or -6 as well as Kremens. After binding to Frizzled receptors, WNT ligands activate the canonical pathway mediated by disheveled, glycogen synthase kinase-3, and ß-catenin, leading to transcriptional activation of T-cell factor/lymphoid enhancer factor-regulated genes (44). There are 4 paralogous R-spondin proteins. They are coligands for the WNT signaling pathway and stimulate WNT signaling by activating their cognate receptors leucine-rich repeat-containing G-protein coupled receptor (LGR)4, -5, and -6 (45, 46) and by preventing Dickkopf-related protein1-mediated low-density lipoprotein-related receptor-6 and Kremen1 association and internalization (47). R-spondin1 induces intestinal stem cell proliferation by activating the WNT signaling pathway (48).
A transgene mouse insertional mutant, Footless, with a hypomorph of R-spondin2 function, is associated with asymmetric limb malformation and premature ovarian failure (49). The Footless heterozygous females are fertile only until 4 months of age. Likewise, heterozygous mutant R-spondin2+/− female mice show fertility decline beginning at 4 months of age (50). Thus, diminished levels of R-spondin2 could lead to the failure of follicle development during late reproductive life, resembling POI in patients. Mutations of oocyte-specific homeobox gene NOBOX are also associated with premature ovarian failure in mutant mice (51) and patients (52). Of interest, ovarian R-spondin2 expression is substantially decreased in NOBOX-null mice (53).
R-spondin2 transcripts are present exclusively in oocytes of primary and larger follicles but not in primordial follicles (41). In cultured somatic cells isolated from preantral follicles, R-spondin2 treatment synergizes with a WNT ligand to stimulate WNT signaling. In cultured ovarian explants from prepubertal mice containing preantral and smaller follicles, treatment with R-spondin2, similar to FSH, promotes the development of primary follicles to the secondary stage (41). In vivo administration of an R-spondin agonist also stimulates the development of primary follicles to the antral stage in both immature and adult mice. Subsequent treatment with gonadotropins allows the generation of mature oocytes capable of undergoing fertilization, early embryonic development, and successful pregnancy. Furthermore, R-spondin agonist treatment of immune-deficient mice grafted with human cortical fragments stimulates the development of primary follicles to the secondary stage (41). Thus, oocyte-derived R-spondin2 is a paracrine factor essential for preantral follicle development. If comparable R-spondin2 actions are identified in the human, R-spondin agonists could provide a new therapy for infertile women with low responses to the traditional gonadotropin therapy.
In addition to R-spondin2, GDF9 and BMP15 are local factors produced by oocytes, capable of stimulating follicle development. They belong to the TGF-β superfamily of cystine-knot proteins (54) and bind to receptor serine kinases (RSKs) to stimulate downstream signaling (55). Both factors bind to type II RSK BMP receptor II (56) and recruit type I RSK [activin-like kinase (ALK)5 for GDF9 (57) and ALK6 for BMP15 (42)] to regulate downstream SMAD proteins in granulosa cells. Studies using GDF9 null mice demonstrated that GDF9 is important for growth of follicles beyond the primary stage (58). Subsequent studies indicated that GDF9 treatment enhances growth and differentiation of preantral follicles in culture (59) and promotes theca cell androgen biosynthesis (60) and proliferation (61). In vivo, treatment with GDF9 promotes the development of primordial follicles to primary and small preantral stages (62). GDF9 also has antiapoptotic actions during early antral follicle development (63).
BMP15 is a paralogous gene for GDF9. BMP15, like GDF9, is expressed in oocytes throughout folliculogenesis and a potent stimulator of granulosa cell proliferation (64). Although BMP15-null mutation in sheep is associated with infertility (65), there are species-specific differences in the role of BMP15 (66). BMP15-null mice only show a decrease in ovulation rate but not sterility (67). Furthermore, natural mutations of BMP15 in sheep can cause both increased ovulation rate and infertility in a dosage-sensitive manner. In the Inverdale (FecXI) sheep, heterozygous BMP15 mutants exhibit increased ovulation rate, but homozygous mutants are associated with primary ovarian failure (65, 68). Sheep with mutations in both GDF9 and BMP15 have a greater ovulation rate than those with either of the mutations separately (69).
In POI patients, different missense mutations in the BMP15 gene have been identified (70–72), but no clear mutation has been found for GDF9 (73). In contrast, loss-of-function GDF9 mutations found in mothers of dizygotic twins are presumably associated with increases in ovulation rate and fecundity (74). Overall, it is difficult to develop follicle-stimulating agents based on GDF9 and BMP15 because 1) the RSKs (BMP receptor II, ALK5, and ALK6) responsible for GDF9 and BMP15 actions are expressed in multiple tissues, 2) actions of GDF9 and BMP15 overlap with other TGF-β family members, and 3) there are major species-specific differences in the mechanisms of action of GDF9/BMP15 homo- and heterodimers, making preclinical testing difficult (75).
In addition to oocyte factors, a large group of peptide/protein ligands are secreted by granulosa cells and found to modulate follicle growth. These ligands act through receptor tyrosine kinases (RTKs), RSKs, and G protein-coupled receptors (GPCRs) (Table 1). RTK-mediated signaling will be discussed in Section VI, whereas the ovarian roles of RSK ligands (activin, BMP6, and anti-Mullerian hormone [AMH]) have been extensively reviewed (76–79) and will not be discussed further. The physiological roles of most of the above-mentioned ligands in follicle development are less clear because 1) most studies rely upon in vitro culture systems, 2) the ligand-receptor pairs could exert overlapping actions, and 3) few ovary-specific mutant mice have been generated. The roles of GPCR ligands (vasoactive intestinal peptide [VIP] and(pituitary adenylate cyclase-activating polypeptide [PACAP]) will also not be discussed further because both VIP and PACAP, like FSH, act through the cAMP pathway (80).
Table 1.
Intraovarian Paracrine Hormones Act Through RTKs, RSKs, GPCRs, Guanylyl Cyclase Receptor NPRB, and Integrins to Regulate Preantral Follicle Growtha
| Ligands | Receptors |
|---|---|
| IGF-1, KGF, VEGF, FGF2, FGF10 | RTKs |
| Activins, BMP6, AMH | RSKs (types I and II) |
| PACAP, VIP | GPCRs |
| CNP | Guanylyl cyclase (NPRB) |
| CCN2/CTGF | Integrins |
Abbreviations: CTGF, connective tissue growth factor.
Diverse paracrine growth factors are secreted by granulosa cells; they act through several distinct intracellular signaling pathways to promote follicle development. IGF-1, KGF, VEGF, FGF2, and FGF10 act through their respective RTKs in granulosa cells to regulate follicle development. In contrast, activins, AMH, and BMP6 synthesized by granulosa cells act though type I and type II RSKs in granulosa cells to regulate follicle development. Also, both PACAP and VIP produced by granulosa cells increase cAMP production by granulosa cells to regulate follicular functions. CNP secreted by granulosa cells binds to the guanylyl cyclase NPRB to increase cGMP production and promote follicle development. In contrast, CCN2/CTGF, produced by granulosa cells in response to Hippo signaling disruption, interacts with membrane-bound integrins in granulosa cells to promote follicle growth.
In addition to local peptide hormones, ovarian steroids (estrogens, androgens, and progesterone) also play important roles in the intraovarian regulation of folliculogenesis by acting on specific ovarian receptors. In patients, estrogen receptor-α variants have been associated with POI (81), whereas shorter alleles of the CAG repeat in exon 1 of the androgen receptor might be related to enhanced susceptibility to PCOS (82, 83). Due to receptor-mediated actions of ovarian steroids at hypothalamic, pituitary, and other extraovarian sites, ovarian cell-specific receptor gene deletion in mice is more informative to reveal ovarian actions of these steroids. Theca cell-specific deletion of estrogen receptor-α in mice leads to premature ovarian failure (84), whereas deletion of androgen receptors in granulosa cells of mice results in subfertility (85, 86). In addition to defective ovulatory responses found in mice with global deletion of the progesterone receptor (87), detailed studies indicated that progesterone receptors in preovulatory follicles play essential roles in the luteinization process (88).
Although local estrogens promote follicle growth and contribute to the dominance of selected follicles during each reproductive cycle (89), studies on local actions of androgens are complicated by their conversion to estrogens. Studies using cultured granulosa cells demonstrated the ability of a nonaromatizable androgen, DHT, to increase FSH receptor content (90) and to stimulate estrogen (91) and progesterone (92) biosynthesis. However, higher ratios of androgens to estrogens are associated with atresia of human follicles (93). Of interest, isolated preantral murine follicles grown in concentrations of FSH that are marginal for follicle development develop faster in the presence of DHT, indicating the ability of androgens to enhance follicle development (94).
In vivo studies on androgen actions in the ovary are further complicated due to the ability of androgens and estrogens to regulate pituitary gonadotropin secretion. Treatment with androgens augments follicular FSH receptor expression in primate follicles (95), whereas administration of a weak androgen, dehydroepiandrosterone, has been found to be valuable in treating patients with diminished ovarian reserve (96–98) and to improve oocyte and embryo quality in poor responders (99). Although cellular mechanisms still remain to be elucidated, these findings suggest the potential use of weak androgens for infertility treatment.
IV. Ovarian Hippo Signaling Constrains Follicle Development and Promotion of Preantral Follicle Growth by CCN Growth Factors
As early as the 1930s, ovarian wedge resection (100) was used for treating patients with PCOS to induce follicle growth. This is followed by recent success based on ovarian drilling by diathermy or laser (101). In addition, ovarian cortices are routinely fragmented to allow better freezing and grafting for fertility preservation in cancer patients who underwent sterilizing treatment (102). Subsequent autotransplantation of ovarian fragments is associated with follicle growth. Based on the hypothesis that damage to ovaries could promote follicle growth, we cut ovaries from juvenile mice containing secondary and smaller follicles into 3 pieces, followed by allotransplantation under kidney capsules of adult hosts for 5 days. Of interest, major increases in graft sizes were evident in fragmented ovaries as compared with paired intact ovaries (103). This is due to the promotion of preantral follicle development to late secondary and antral/preovulatory stages. Furthermore, cutting of ovaries from day 23 mice containing early antral and smaller follicles also promotes follicle growth. The observed damage-induced follicle growth was also found using rat ovaries, suggesting the findings are not species-specific.
The Hippo signaling pathway is essential for organ size control, and components of this pathway are conserved in all metazoan animals (104–106). Hippo signaling consists of several negative growth regulators acting in a serine/threonine kinase cascade that ultimately phosphorylates and inactivates key transcriptional coactivators, yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) (Figure 3). When Hippo signaling is disrupted, nuclear YAP or TAZ interacts with TEAD (transcription factors containing the transcriptional enhancer activator [TEA] DNA binding domain) proteins to increase the expression of downstream CCN growth factors and baculoviral inhibitors of apoptosis repeats-containing proteins (BIRC) apoptosis inhibitors (104). The name CCN is derived from major family members including cysteine-rich angiogenic protein 61 (CYR61 or CCN1), connective tissue growth factor (CTGF or CCN2), and NOV (nephroblastoma overexpressed or CCN3). These CCN and BIRC proteins, in turn, stimulate cell growth, survival, and proliferation (107). The Hippo signaling pathway regulates liver growth and is a suppressor of liver tumor formation. Specific deletion of SAV1 (Salvador homolog 1) or MST1/2 (macrophage-stimulating protein 1/2) gene in hepatocytes results in enlarged livers in mice (108, 109). Likewise, conditional deletion of SAV1 leads to enlarged hearts (110). YAP is a candidate oncogene, and several other components of the Hippo pathway are tumor suppressors, thus connecting the regulation of organ size and tumorigenesis (111). In murine and human ovaries, key Hippo signaling genes (YAP, TAZ, MST1/2, SAV1, and LATS1/2) are expressed in follicles at different stages (103).
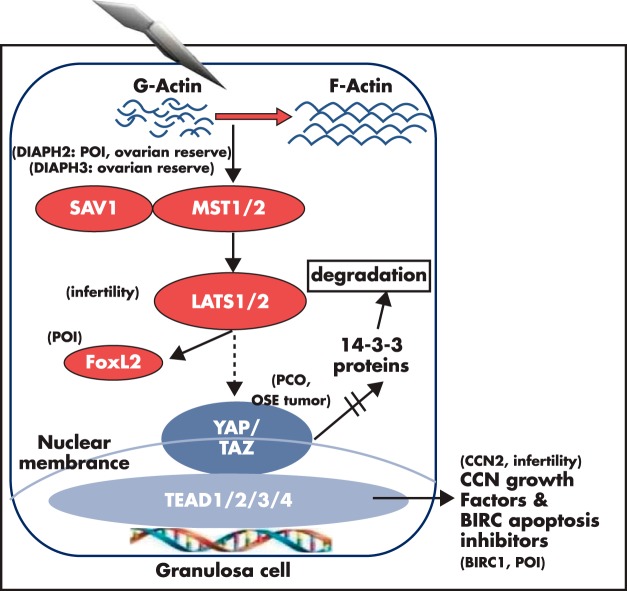
Figure 3.
Actin polymerization disrupts ovarian Hippo signaling and promotes nuclear YAP actions to increase downstream CCN growth factors and apoptosis inhibitors involvement in POI, PCOS, follicle reserve, and infertility. The Hippo serine/threonine kinase signaling cascade is conserved from flies to mammals to restrict tissue growth. Mammalian MST1 and MST2 are orthologous to the fly Hpo (Hippo) gene. MST1/2, acting in concert with Sav1, phosphorylates downstream LATS1/2 to suppress YAP and TAZ actions by phosphorylating YAP and TAZ, leading to the degradation of phospho-YAP/TAZ after binding to cytoplasmic 14–3-3 proteins. Ovarian fragmentation induces actin polymerization (conversion of G-actin to F-actin) and disruption of the Hippo signaling pathway, leading to decreases in YAP phosphorylation and increased nuclear levels of YAP (103). Nuclear YAP interacts with cotranscriptional factors TEAD1/2/3/4 to stimulate the expression of downstream CCN growth factors and BIRC apoptosis inhibitors, leading to cell proliferation. Genomic and genetic studies underscore the important roles of the ovarian Hippo signaling pathway. DIAPH genes are important for actin polymerization. Disruption of DIAPH2 has been found in a familiar case of POI (126), whereas this gene is also important for ovarian reserve in a genome-wide association study (127). Likewise, DIAPH3 has been implicated in regulating ovarian reserve in a genome-wide association study (230). For Hippo pathway genes, LATS1 deletion in mice leads to infertility and ovarian tumorigenesis (129), whereas LATS1 regulates the activity of FOXL2 (130), a gene defective in some POI patients. Genome-wide association studies also implicate the Hippo effector gene YAP in PCO patients (131), whereas overexpression of YAP was found in ovarian surface epithelium (OSE) tumors (132). For downstream genes, conditional deletion of CCN2 in granulosa cells leads to subfertility (133), whereas gene copy changes for the BIRC1 apoptosis inhibitor gene is associated with POI in patients (134).
Unlike many extracellular ligand-induced signaling pathways including WNT, RTKs, and RSKs, the Hippo pathway does not have dedicated extracellular ligands and receptors but is instead regulated mainly by a network of upstream components involved in regulating cell adhesion, shape, and polarity (112). Actin comprises up to 10% of total soluble proteins in eukaryotic cells. Rapid changes in the polymerization of globular actin (G-actin) to the filamentous actin (F-actin) mediate cell adhesion, shape maintenance, and locomotion. Taking advantage of the ease to perform genome-wide RNA interference screening in insect cells, cells expressing a reporter gene driven by the promoter of Yki (an ortholog of vertebrate YAP) was used to identify genes capable of disrupting Hippo signaling. Of interest, most genes identified are upstream of actin polymerization, and deletion of them induces extra F-actin formation and disrupts Hippo signaling. Furthermore, overexpression of an activated version of the actin-polymerizing formin diaphanous (DIAPH) induces overgrowth in Drosophila imaginal discs. The role of actin polymerization in the disruption of Hippo signaling is also conserved in human HeLa cells (113, 114).
We found that fragmentation of murine ovaries induces a transient increase in the polymerization of G-actin to F-actin, disrupts Hippo signaling, decreases YAP phosphorylation, and increases nuclear YAP levels, leading to increased expression of downstream CCN growth factors and BIRC inhibitors (Figure 3) (103). The essential role of Hippo signaling effector YAP in mediating fragmentation-induced follicle growth is demonstrated by the inhibitory effect of verteporfin (103), a low-molecular-weight compound capable of blocking interactions between YAP and downstream TEAD transcriptional factors (115). Transcripts for the 4 CCN growth factors induced after ovarian fragmentation in mice were also found to increase after cutting thawed human cortical strips to small cubes. In addition, treatment of murine ovarian explants with recombinant CCN2, -3, -5, and -6 promotes the development of preantral follicles (103), presumably by binding to their cell surface integrin receptors (116). These findings demonstrate the important role of CCN proteins as ovarian growth factors.
Earlier studies on ovarian transplantation suggested that ovarian damage could promote follicle growth. Although initial success of orthotopic whole ovary transplantation in mice was reported in 1940 (117, 118), this procedure was substantially improved by cutting ovaries in half before transplantation (119). For patients, ovarian cryo-preservation and auto-transplantation can restore fertility in women who underwent sterilizing treatments for cancer (102), with a few dozen successful deliveries reported so far. In these studies, ovarian cortices are routinely fragmented to allow better cryo-preservation and grafting (120). These observations can now be explained by ovarian fragmentation-induced disruption of Hippo signaling that promotes follicle growth (103).
V. Hippo Signaling in Ovarian Physiology and Pathophysiology
Findings of ovarian Hippo signaling suggest that ovarian follicles, after activation from dormancy, could have different growth trajectories determined by local Hippo signals that suppress follicle growth. Of interest, 3 cohorts of secondary follicles with different growth trajectories were identified in nonhuman primates using encapsulated 3-dimensional cultures (121). Because the constraint exerted by local Hippo signaling in situ could vary among individual follicles at the same stage of development, one can explain the large variation of follicle lifespans found after tracing of follicles in transgenic mice (122). The first wave of follicle growth was monitored in transgenic mice expressing an inducible forkhead box L2-directed fluorescence marker protein in granulosa cells. Although the transgenic marker was induced at a fixed time point during the initiation of primordial follicle activation, the duration required for the development of primordial follicles to the preovulatory stage varied from 23 to 90 days. These findings explain earlier futile attempts to determine the exact lifespan of follicles (123). It is possible that local Hippo signaling is regulated based on the location of individual follicles inside the ovary. Hippo signaling could also be involved in interfollicle communications (124); larger follicles could reinforce Hippo signaling in neighboring smaller follicles to suppress their growth. During each ovulation, large structural changes associated with follicle rupture could also disrupt local Hippo signaling due to rupture-induced changes in actin polymerization near the surface epithelium. Monthly disruption of Hippo signaling and resultant overproliferation of surface epithelial cells could increase the susceptibility to ovarian surface epithelial cancer (125).
Genomic and genetic studies provide further support for the essential roles of ovarian Hippo signaling and actin polymerization in regulating follicle development. Defects in Hippo signaling genes are associated with POI, PCOS, ovarian follicle reserve, and ovarian tumorigenesis in patients as well as ovarian infertility in mice (Figure 3). The upstream DIAPH proteins suppress actin depolymerization. Among 3 mammalian DIAPH genes, the coding region of DIAPH2 is disrupted in both mother and daughter of a POI family due to chromosomal translocation (126). DIAPH2 is also a candidate gene for menopausal age in women based on a genome-wide association study (127). Likewise, DIAPH3 is a marker of follicle reserve and menopause in women of multiple ethnicities (128). Among Hippo pathway genes, LATS1-null mice are subfertile with 60% showing complete sterility; some of them also develop ovarian stromal cell tumors (129). LATS1 phosphorylates FOXL2, a POI susceptibility gene, and regulates FOXL2 transcriptional activity (130). For the Hippo effector YAP, genome-wide association analyses of PCO patients identified 2 single-nucleotide polymorphism candidates in the third and seventh introns of YAP (131). YAP was also found to be a tumor suppressor for ovarian surface epithelial cancers (132). For downstream genes, granulosa cell-specific deletion of CCN2 disrupts follicle development and ovulation (133). The mildly subfertile and late-onset phenotypes of CCN2 mutant mice could be due to compensatory actions of paralogous CCN (3, 5, and 6) growth factors also expressed in the ovary. Furthermore, BIRC1 shows gene copy variations in POI patients analyzed by array comparative genomic hybridization profiling (134).
The Hippo signaling pathway is regulated by the physical and mechanical microenvironment of cells (135). Mechanical cues from the extracellular matrix, cell adhesion sites, cell shape, and the actomyosin cytoskeleton converge on the regulation of the Hippo signaling pathway to affect cell fates. Culturing mesenchymal stem cells in a stiff extracellular matrix increases YAP activity and promotes osteogenesis, whereas culturing them in a soft matrix decreases YAP activity and promotes adipogenesis (136). In mammalian early embryos, F-actin bundles are abundant in the trophectoderm (137), associated with increased YAP activity and cell proliferation. In contrast, F-actin staining is low in the inner cell mass, showing lower YAP activity and cell differentiation (138). Mammary epithelial cells embedded in a soft matrix grow into spheric epithelial monolayers with a central lumen. These cells undergo growth arrest, polarization, and eventually, differentiation (139). In contrast, culturing mammary epithelial cells in a stiff matrix increases cell proliferation and promotes cell invasiveness into the surrounding matrix. Many tumors have a rigid structure because they have a stiff stroma and high cytoskeletal tension that enhances cell proliferation (140). Although Hippo signaling changes have not been investigated in great detail, these findings are consistent with increased YAP activity and proliferation of cells situated in a stiff niche, whereas a softer niche is associated with differentiated cells with lower YAP activity.
In the ovary, primordial follicles are all situated in the cortical region, which is more rigid than the inner medullar region. Unknown mechanisms maintain most primordial follicles in the dormant state. Once primordial follicles are activated to grow, Hippo signaling of cells in follicles near the cortical area could be disrupted due to the stiff niche, leading to increased YAP activity and CCN growth factor secretion followed by cell proliferation and follicle growth into the secondary stage. As follicles grow larger, they move into the softer medullar region and subsequent resumption of Hippo signaling could slow down follicle growth.
Although it is difficult to compare follicles inside ovaries with those isolated for culture, recent studies indicated that a stiff culture environment favors high progesterone and androgen secretion, whereas decreasing alginate stiffness results in estrogen production that exceeds progesterone and androgen accumulation, suggesting a link between the biomechanical environment and follicle function (141). Based on follicle culture studies, Woodruff and Shea (142) advanced a hypothesis suggesting that follicle activation is dependent on the physical environment of the ovary in addition to well-established hormonal cues.
VI. Regulation of Primordial Follicle Activation by AKT and Mammalian Target of Rapamycin Signaling Pathways
Earlier studies demonstrated that oocytes in dormant primordial follicles are metabolically active and transcribe genes essential for oocyte growth (143). Thus, primordial oocytes in these follicles are preloaded with gene transcripts for further follicle growth, suggesting the existence of factors important for maintaining follicle dormancy by preventing the translation of these transcripts in most primordial follicles (Figure 1). Using in vitro cultures, mutant animals, specific inhibitors, and passive immunoneutralization approaches, a number of factors have been found to be important for primordial follicle growth. These include kit ligand (144, 145), neurotrophins (146), vascular endothelial growth factor (VEGF) (147), BMP4 (148), BMP7 (149), leukemia inhibitory factor (150), basic fibroblast growth factor (FGF) (151), keratinocyte growth factor (KGF) (152), and others. Although these findings suggest the involvement of an overlapping and redundant group of extracellular intraovarian factors in primordial follicle activation, the exact factors involved in the activation of a select few primordial follicles at a given time under physiological states is still poorly understood.
Because diverse local factors involved in initial follicle recruitment are likely to converge on common intracellular signaling pathways, it is easier to manipulate the functions of intracellular signaling pathways by pharmacologically activate dormant primordial follicles. Recent studies provide insights into the intracellular signaling mechanisms important for primordial follicle activation from the dormant state (153). Studies using c-kit ligand that activates c-kit (an RTK) in mouse and rat oocytes demonstrated the stimulation of AKT activity and the suppression of a downstream transcriptional factor forkhead box O3 (FOXO3) (154). After Kit ligand activates its cognate RTK, phosphorylation of the intracellular region of the RTK stimulates phosphatidylinositol 3-kinase (PI3K) activity, leading to the conversion of the lipid second messenger phosphatidylinositol-4,5-bisphosphate (PIP2) into phosphatidylinositol-3,4,5-triphosphate (PIP3). PIP3, in turn, recruits and activates phosphatidylinositol-dependent kinase 1, followed by AKT activation (Figure 4). Translocation of AKT to the nucleus suppresses the transcriptional activity of FOXO3. This pathway is also regulated by the inhibitory tumor-suppressor phosphatase and tensin homolog (PTEN) enzyme that negatively regulates PI3K signaling by dephosphorylating PIP3 and converting it back to PIP2.
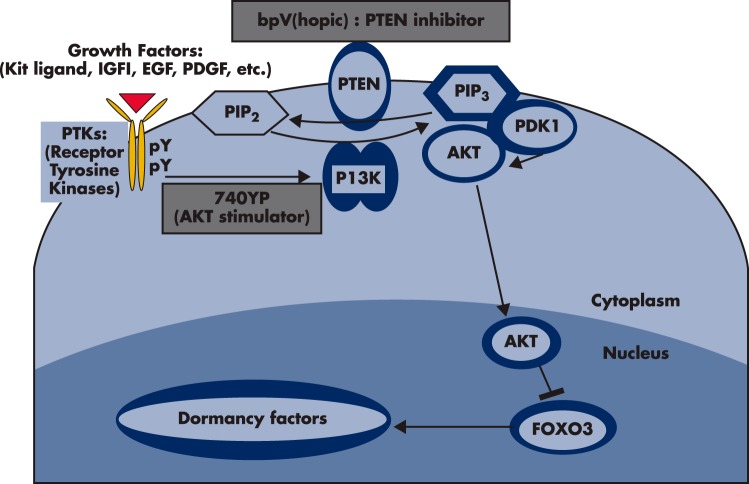
Figure 4.
The PTEN-PI3K-AKT pathway in oocytes regulates primordial follicle activation. Mouse models were used to investigate the regulation of primordial follicle dormancy. The FOXO3 gene in primordial oocytes serves as a break to prevent the initiation of follicle growth. Activation of upstream RTKs by their cognate ligands (kit ligand, IGF-1, EGF, platelet-derived growth factor [PDGF], VEGF, etc) stimulates the autophosphorylation of intracellular regions of these receptors. Activated receptors then stimulate PI3K activity, leading to increases in PIP3 levels and AKT stimulation. Activated AKT then migrates to the cell nucleus and suppresses FOXO3 actions to promote primordial follicle growth. The PTEN gene encodes an enzyme that converts PIP3 to PIP2, thus damping the actions of PI3K. Oocyte-specific deletion of the PTEN gene leads to global activation of primordial follicles (156) whereas treatment with PTEN inhibitors, bpV(hopic) [(5-hydroxy-2-pyridinecarboxylato-kN1, kO2)oxodiperoxy-vanadate (2-), dipotassium], promotes primordial follicle activation (165). Although the exact ligand-receptor pairs responsible for the activation of primordial follicles during physiological conditions are unknown, treatment of ovaries with a cell membrane-permeable peptide (740Y-P, designed based on the phosphorylated intracellular region of the PDGF receptor) stimulates AKT signaling and promotes the activation of primordial follicles (165).
The important roles of the PI3K-PTEN-AKT-FOXO3 pathway in primordial follicle activation are highlighted by transgenic mouse studies. FOXO3-null mice show global activation of all dormant follicles at the neonatal stage, resulting in a premature ovarian failure phenotype during later life (155). PTEN converts PIP3 to PIP2 and dampens actions of endogenous follicle-activating RTK ligands. Mutant mice with oocyte-specific PTEN deletion also showed activation of all primordial follicles during neonatal life (156). Furthermore, inducible deletion of PTEN in the oocytes of adult mice also increased AKT phosphorylation and nuclear export of FOXO3 proteins, leading to primordial follicle activation (157). Although the role of PI3K-PTEN-AKT-FOXO3 signaling in oocytes is well-established based on murine models, it is important to note that the downstream FOXO gene is likely to be FOXO1 in women (158). In the mouse ovary, FOXO1 is highly expressed in somatic cells (but not in oocytes) and is also downstream of PTEN-AKT signaling (159). Although the role of FOXO proteins in the Hippo signaling pathway is unclear, it is interesting to note that CCN2 downstream of Hippo signaling is positively regulated by FOXO1/3 and activin in granulosa cells (98).
Mammalian target of rapamycin (mTOR) is a serine/threonine kinase that regulates cell growth and proliferation. The rapamycin-sensitive mTOR complex 1 positively regulates cell growth and proliferation by promoting biosynthesis of proteins, lipids, and organelles and by limiting catabolic processes such as autophagy (160). In addition to the PTEN-AKT-FOXO3 signaling pathway, suppression of mTOR complex 1 activity by the tuberous sclerosis 1-tuberous sclerosis 2 complex in oocytes has also been shown to be a prerequisite for maintaining the dormancy of primordial follicles based on extensive studies using mice with oocyte-specific deletion of Tsc1 and Tsc2 genes (161, 162). Of interest, double deletion of Tsc1 and PTEN leads to synergistically enhanced oocyte growth and follicle activation when compared with singly mutated mice (162). These findings demonstrate the essential and coordinate roles of AKT and mTOR signaling pathways in regulating primordial follicle dormancy and in preserving the length of female reproductive life (163). In addition to primordial follicles, mTOR signaling is also important for granulosa cell growth in antral follicles (164).
Based on the importance of the PI3K-PTEN-AKT-FOXO3 pathway in primordial follicle activation, an in vitro activation (IVA) approach was used to promote the growth of dormant primordial follicles in neonatal mouse ovaries. Short-term in vitro treatment of ovaries with a PTEN inhibitor and a PI3K-stimulating phosphopeptide (designed based on the intracellular region of activated platelet-derived growth factor receptor) increases AKT activity, leading to nuclear exclusion of FOXO3 in primordial oocytes (165). After transplantation of ovaries under kidney capsules of ovariectomized hosts, primordial follicles develop to the preovulatory stage with mature eggs displaying normal epigenetic modification of imprinted genes. After in vitro fertilization (IVF) of these oocytes and subsequent embryo transfer, healthy progeny with proven fertility could be delivered.
Similar in vitro treatment of human primordial follicles in ovarian cortical fragments also leads to the activation of dormant follicles. After xenotransplantation to immune-deficient mice for 6 months, preovulatory follicles containing oocytes capable of undergoing nuclear maturation could be generated (165).
VII. IVA of Preantral Follicles After Hippo Signaling Disruption and AKT Stimulation
The PI3K-PTEN-AKT pathway not only regulates primordial follicle dormancy at the oocyte level but also plays an obligatory role in the FSH stimulation of granulosa cell differentiation of antral follicles (166) as well as in oocyte maturation of preovulatory follicles (167). Although in vitro culture studies demonstrated stimulatory effects of IGF-1 (168), KGF (169), VEGF (170), FGF2 (171), and FGF10 (172) to modulate granulosa cell functions and promote preantral follicle growth (Table 1), the most convincing study showing the importance of these RTK ligands derives from mutant mice with specific deletion of the PTEN gene in granulosa cells of secondary and early antral follicles (159). These mice showed increases in granulosa cell proliferation, decreases in follicle atresia, and increases in ovulatory efficacy (159), underscoring the importance of the AKT signaling pathway in granulosa cells.
When isolated secondary follicles from infantile mice were cultured with PTEN inhibitors and PI3K stimulators to stimulate AKT signaling, follicle growth was evident (103). Furthermore, ovaries from day 10 mice containing secondary and smaller follicles also respond to AKT signaling stimulation after short-term treatment with IVA drugs (PTEN inhibitor and PI3K-stimulating peptide), leading to increased preantral follicle development after grafting (103). Of interest, IVA drug treatment, when combined with fragmentation-induced Hippo signaling disruption, leads to additive increases in preantral follicle development. Using human cortical pieces containing secondary and smaller follicles, ovarian fragmentation, followed by IVA drug treatment, also facilitates rapid follicle growth in xenografts of immune-deficient mice with the generation of preovulatory follicles within 4 weeks (103).
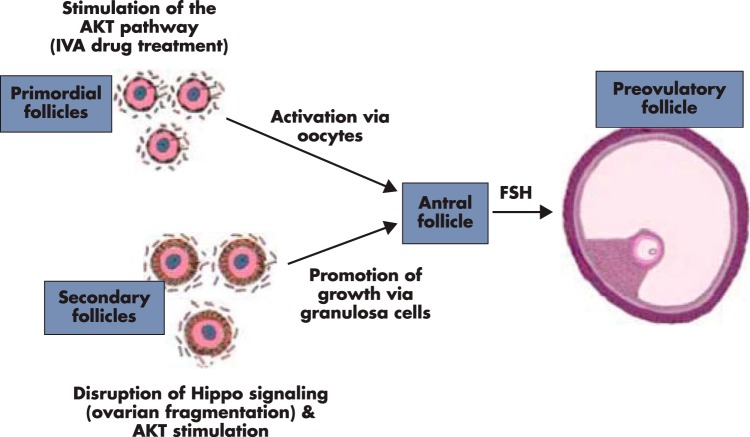
It is clear that both primordial and secondary follicles can be promoted to grow likely through different mechanisms (Figure 5). Treatment with IVA drugs activates dormant primordial follicles by stimulating the PTEN-PI3K-AKT-FOXO3 pathway in oocytes. In contrast, ovarian fragmentation (Hippo signaling) followed by IVA drug treatment (AKT stimulation) act through granulosa cells to promote secondary follicle growth. These findings provide the basis to activate dormant primordial and restrained secondary/preantral follicles to start/resume growth in patients with low ovarian reserve.
Figure 5.
Ovarian fragmentation and IVA drug treatment promote follicle growth via different mechanisms. Incubation of ovaries with PTEN inhibitors and PI3K stimulators activate dormant primordial follicles by increasing oocyte AKT activity to promote nuclear exclusion of FOXO3, thus preventing its suppression of primordial follicle growth. For secondary follicles, ovarian fragmentation disrupts Hippo signaling to increase downstream CCN growth factors and BIRC apoptosis inhibitors, leading to follicle growth. In addition, concomitant treatment with PTEN inhibitor and PI3K stimulators leads to additive promotion of follicle growth by increasing AKT activity in granulosa cells of secondary follicles.
VIII. Etiologies of POI and Promotion of Preantral Follicle Growth in POI Patients
POI, also known as premature ovarian failure, occurs in 1% of women (173, 174). The known causes for POI include genetic aberrations involving the X-chromosome or autosomes as well as autoimmune ovarian damages (175). However, the specificity of antiovarian antibodies and their pathogenic roles are questionable (176, 177). Furthermore, surgery, radiation, and chemotherapeutic interventions as well as exposure to certain environmental factors (eg, viral infection or toxins) could also lead to POI. Presently, the only proven means for infertility treatment in POI patients involve assisted conception with the use of donated oocytes (178, 179). For cancer patients before chemo- or radiation therapy, cryopreservation of ovarian tissues, mature oocytes, and embryos are potential options (178).
Due to heterogeneity of POI etiologies, varying amounts of residual preantral follicles are still present in ovaries of some POI patients. Routine diagnosis of POI is based on the monitoring of amenorrhea along with elevated serum FSH concentrations above 40 IU/L and low serum estradiol levels in women below 40 years of age. Although monitoring of serum AMH secreted by secondary follicles is gaining popularity in evaluating ovarian reserve (10), routine diagnosis is based on cessation of menses. However, a menses-based diagnosis could preclude the detection of preantral follicles in POI patients because sloughing off of endometrial lining during menstrual bleeding is induced by variations in ovarian sex steroids secreted by antral and larger follicles.
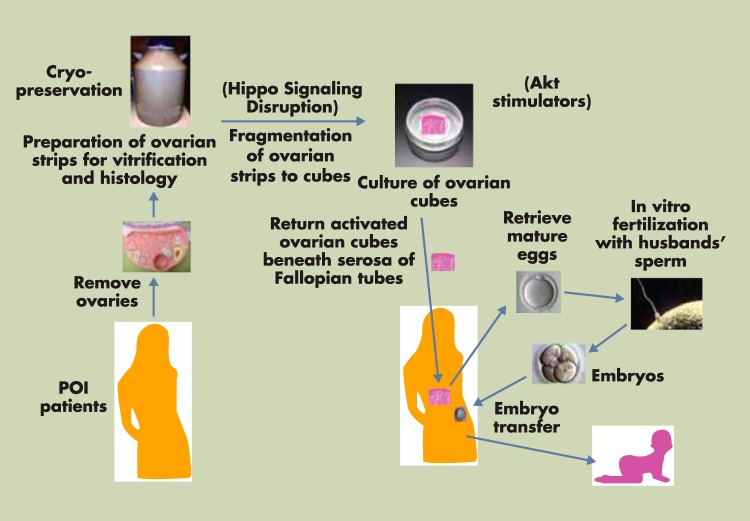
Because treatment with PTEN inhibitors/PI3K stimulators activates dormant primordial follicles, whereas ovarian fragmentation and IVA drug treatment lead to additive stimulation of secondary follicle growth, ovarian fragmentation and IVA drug treatment were combined to activate residual preantral follicles in ovaries of 27 POI patients (Figure 6). One or both ovaries from POI patients were removed using laparoscopic surgery. Ovaries were cut into strips (1–2 mm thickness and 1 × 1 cm) before vitrification (180). Frozen ovarian strips were then thawed and fragmented into ∼100 cubes of 1 to 2 mm2, followed by IVA drug treatment for 2 days. Forty to 80 ovarian cubes each were then autotransplanted beneath the serosa of the 2 Fallopian tubes. After transvaginal ultrasound monitoring, together with serum estrogen measurement, follicle growth was found in about 50% of patients showing residual follicles. In most POI patients responding to IVA treatment, preovulatory follicles were found within weeks or a few months, suggesting the growth of preantral follicles. In contrast, some preovulatory follicles were detected only after 6 months or longer, likely due to the activation of dormant primordial follicles (165). After IVF and embryo transfer, 2 patients became pregnant (103) (Figure 6). Consistent with the reported safety of the IVA procedure in mice (181), 2 healthy IVA babies have been born with the first one being more than 1 year of age. The IVA approach leads to a new infertility treatment strategy for POI patients with a very poor prognosis for achieving a pregnancy otherwise.
Figure 6.
Ovarian fragmentation/AKT stimulation followed by autografting promotes follicle growth in POI patients to generate mature oocytes for IVF embryo transfer, pregnancy, and delivery. Under laparoscopic surgery, one or both ovaries from POI patients were removed and cut into strips before vitrification. After thawing, strips were fragmented into 1- to 2-mm2 cubes, before treatment with AKT stimulators (PTEN inhibitors and PI3K stimulator). Two days later, cubes were autografted under laparoscopic surgery beneath the serosa of Fallopian tubes. Follicle growth was monitored weekly or biweekly via transvaginal ultrasound and based on serum estrogen levels. After detection of antral follicles, patients were treated with FSH followed by human chorionic gonadotropin when preovulatory follicles were found. Mature oocytes were then retrieved and fertilized with husbands' sperm in vitro before cyropreservation of 4-cell-stage embryos. Patients then received hormonal treatments to prepare the endometrium for implantation followed by transferring of thawed embryos and pregnancy. [Modified from K. Kawamura et al: Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474 (103), with permission. © National Academy of Sciences of the United States of America.]
Although these observations may have profound clinical implications, it has to be emphasized that the above-described findings involve uncontrolled cases in a small population of women diagnosed with POI. Spontaneous recovery of menstrual cycles and subsequent pregnancies has been described in POI patients (182). Therefore, controlled studies should be undertaken and preliminary results confirmed before such approaches can be advocated for more widespread clinical use. To avoid residual effects of IVA drugs in vivo and to minimize unwanted retardation of human follicle growth found after prolonged culture with PTEN inhibitors (183), ovarian fragments were treated with IVA drugs for only 2 days and rinsed extensively before grafting back to patients.
IX. Predicting Follicle Reserve and Promotion of Preantral Follicle Growth in Women With Diminished Ovarian Reserve
For POI patients and other patients with low ovarian reserve as well as women suffering from decreased fertility of ovarian origins, it is important to identify biomarkers to predict ovarian reserve to estimate the chances for patients to generate mature oocytes spontaneously or in response to assisted reproductive technology (ART). Due to an age-dependent decline in both quality and quantity of oocytes, patient age is the most important parameter in determining the chances that a women will conceive (10). In the 2011 ART report by the U.S. Centers for Disease Control and Prevention, canceled ART cycles increase from 6.4% in female patients less than 35 years of age to 26.8% for patients greater than 44 years of age. In addition to age, a large number of clinical parameters might predict poor responses to gonadotropin stimulation, including serum levels of basal FSH and inhibin B, antral follicle count, ovarian volume, and serum AMH levels (184–187).
AMH expression is negligible in primordial follicles, low in granulosa cells of primary follicles, and highest in granulosa cells of secondary and small antral follicles. In larger antral follicles, AMH expression gradually declines (188). These findings are consistent with the elevation of serum AMH levels in PCOS patients showing excess early antral follicles (189, 190). Because serum AMH levels decrease over time in young normo-ovulatory women and are strongly correlated with the number of antral follicles, serum AMH levels have been used to monitor ovarian reserve (191) for predicting outcomes from ovarian stimulation and the timing of menopause (184). Because AMH is produced by granulosa cells from primary to small antral but not primordial follicles (192), the use of serum AMH levels as an ovarian reserve marker could neglect the presence of primordial follicles (193). However, a correlation between serum AMH levels and primordial follicle counts has been reported (194), suggesting that additional studies are needed to elucidate the relationship between primordial and larger follicles.
Future searches for markers of preantral follicles could include different oocyte-derived factors due to the large size of oocytes as compared with small numbers of surrounding somatic cells in primordial and primary follicles. GDF9 and BMP15 are predominantly secreted by oocytes of primary and larger human follicles (195, 196), whereas oocyte-specific R-spondin2 is expressed in primary and larger follicles in rodents (41). For these oocyte factors, development of ultrasensitive assays is needed to detect the presumably low leakage of these secreted factors from ovaries into the systemic circulation.
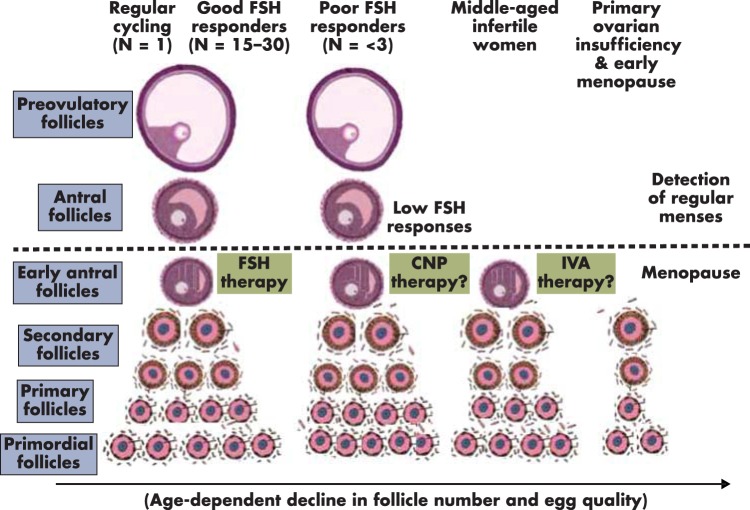
In women with regular menstrual cyclicity, ∼20 early antral follicles are present during the early follicular phase (197), producing low amounts of sex steroids (198). During the late follicular phase of the menstrual cycle, a single antral follicle develops to the preovulatory stage and secrets sufficient estrogens to induce uterine cell proliferation (Figure 7). After ovulation, the newly formed corpus luteum secretes progesterone and estrogens to induce uterine cell differentiation, followed by menstrual bleeding when levels of sex steroids decline during luteolysis. In these regularly cycling women, treatment with exogenous gonadotropins usually promotes the development of a large number (15–30) of preovulatory follicles (8) (Figure 7). A subgroup of these women develops fewer (<3) preovulatory follicles after exogenous FSH stimulation (199). These FSH poor responders have early antral follicles but may have insufficient expression of functional FSH receptors or other defects (Figure 7). As discussed earlier, these patients may benefit from CNP therapies to promote preantral and antral follicle growth.
Figure 7.
Diagrammatic representation of hypothesized follicle dynamics under different clinical conditions. In women with regular menstrual cyclicity (left panel), ∼20 early antral follicles are present during the early follicular phase and produce low amounts of sex steroids. During the first half of the menstrual cycle, development of a single antral and then preovulatory follicle (n = 1) confers the secretion of sufficient estrogens and progesterone to induce uterine changes, resulting in regular menses. Treatment of these women with exogenous gonadotropins promotes development of a large number [15 to 30] of preovulatory follicles (good FSH responders). Ovarian activity above the dashed line denotes sufficient ovarian sex steroid secretion to affect uterine functions, leading to regular menstrual cycles. For poor FSH responders, early antral follicles may be present, but insufficient expression of functional FSH receptors could lead to the development of fewer (<3) preovulatory follicles after exogenous FSH stimulation. These patients could benefit from CNP therapies. For middle-aged infertile women with irregular menstrual cycles, few early antral follicles are present, but many preantral follicles still exist. These patients could benefit from IVA therapy or treatment with ovarian paracrine factors, including CNP. For POI patients and infertile women showing early menopause before 51 years of age, residual preantral follicles could still be present in the ovary. The IVA procedure promotes primordial follicle activation as well as stimulates secondary follicle growth by combining Hippo signaling disruption and AKT stimulation. The arrow at the bottom emphasizes the age-dependent decline in follicle number and egg quality under both physiological and pathophysiological conditions.
Due to the delay of childbearing age in the modern society, many middle-aged women beyond 40 years of age are experiencing infertility because of diminishing ovarian reserve. Decreases in egg quantity, as well as quality, progress exponentially after 35 years of age. Based on the 2008 report by the U.S. Centers for Disease Control and Prevention, the likelihood of conception per cycle for a woman at 40 and 45 years of age is approximately 10% and 1%, respectively (200). Although middle-aged women experience irregular menstrual cyclicity, their ovaries still contain preantral follicles (10) (Figure 7). However, many of these patients do not respond to the traditional gonadotropin therapy. For these patients and others with diminished ovarian reserve (201), the IVA approach or treatment with ovarian paracrine factors, including CNP, may be effective in stimulating preantral follicle growth.
On average, menopause occurs at 51 years of age. For POI patients and infertile women showing early menopause before 51 years of age, residual preantral follicles could still be present in the ovary (Figure 7). The IVA procedure promotes primordial follicle activation as well as stimulates secondary follicle growth by combining Hippo signaling disruption and AKT stimulation.
X. Does a Subgroup of PCOS Patients Have Ovarian Structural Defects?
Between 5% and 10% of reproductive-age women are infertile due to the PCOS (202). PCOS patients have enlarged ovaries with a thickened sclerotic capsule. PCOS ovaries contain a high number of small antral follicles but no preovulatory follicles (203). In their original paper published in 1935, Stein and Leventhal (100) reported 7 cases of amenorrhea associated with polycystic ovaries. After wedge resection of ovaries, normal menses reappeared in all 7 patients. Subsequently, surgical ovarian wedge resection was largely abandoned due to the risk of postsurgical adhesions and introduction of medical ovulation induction with clomiphene citrate and exogenous gonadotropins. Based on the wedge resection principle, laparoscopic ovarian drilling (LOD) (204, 205) can now be performed on an outpatient basis with less trauma and fewer postoperative adhesions than the traditional wedge resection approaches. Of interest, there was no difference in the live birth rate and miscarriage rate in women with clomiphene-resistant PCOS undergoing LOD compared with gonadotropin treatment. As compared with the risk of ovarian hyperstimulation after FSH treatment, the reduction in multiple pregnancy rates in women undergoing LOD makes this option attractive (206). However, there are ongoing concerns regarding long-term effects of LOD on ovarian reserve.
Although the factors that mediate ovarian tissue responses to these damaging procedures (wedge resection and LOD) have not been elucidated, changes in actin polymerization and Hippo signaling in specific regions of the ovary could play an important role. In PCOS patients after wedge resection, there is a marked but temporary reduction of ovarian androstenedione secretion and a persistent decrease in testosterone secretion. Because these changes had no discernible effect on circulating gonadotropins, it was proposed that intraovarian mechanisms are involved (207, 208).
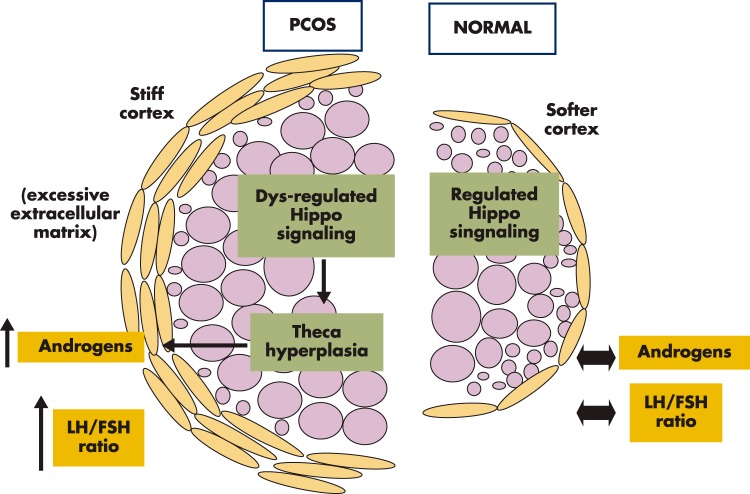
Multiple genome-wide association (209–212) and candidate gene studies (213–217) implicated up to 100 PCOS susceptibility genes in the ovary (listed in the Ovarian Kaleidoscope Database at ovary.stanford.edu) (218). However, the exact cellular signaling pathways underlying the PCOS phenotypes remain elusive. As discussed in Section V, more rigid cortical niche disrupts Hippo signaling and promotes follicle growth. As follicles grow larger and move into the softer medullary region, subsequent resumption of Hippo signaling slows down their growth (Figure 8, right). For the classical PCOS cases reported by Stein and Leventhal (100), extra-rigid and sclerotic cortex is formed due to increased cortical collagen and accompanying stromal hypertrophy (219). This phenotype could be related to defects in actin polymerization, leading to higher F-actin content and/or aberrant biosynthesis of intercellular matrix proteins. The hardened cortical layer contains more polymerized actin with increased tensile strength (higher F-actin) of the stromal tissue could disrupt local Hippo signaling, leading to YAP overactivity in stromal and follicular cells (Figure 8, left). This is followed by increased secretion of CCN growth factors to promote stromal, thecal, and granulosa cell proliferation, resulting in the growth of multiple early antral follicles. Theca cell hyperplasia is a hallmark of PCOS, and increased androgen production by thecal cells could lead to secondary increases in pituitary LH secretion (220). The mechanical cue for thecal and stromal cell hyperplasia could establish a vicious cycle for excessive androgen biosynthesis, increases in LH to FSH ratios, arrest of early antral follicles, and enlarged ovaries characteristic of PCOS.
Figure 8.
Hypothesized ovarian structural abnormality in PCOS. Aberrant extracellular matrix and Hippo signaling defects lead to thecal hyperplasia and the PCO phenotype. Normal ovaries have softer cortexes, and Hippo signaling restrains most follicles from overgrowth, leading to physiological levels of ovarian androgen secretion and serum LH to FSH ratios. A subgroup of PCOs could have defects in extracellular matrix regulation, leading to rigid and sclerotic cortexes. The stiff cortex of these ovaries could lead to dysregulation of Hippo signaling and excessive proliferation of stromal, theca, and granulosa cells. Thecal cell hyperplasia could increase androgen production and elevate ratios of serum LH to FSH levels, followed by the arrest of a high number of early antral follicles and enlarged ovaries characteristic of PCOS.
Fibrillins are extracellular matrix molecules that assemble into microfibrils in many connective tissues. Family-based association studies identified fibrillin-3 (FNB3) as a PCOS susceptibility gene (214, 221). Fibrillin-3 is present in the stromal compartment of fetal ovaries and highly expressed in developing human fetal ovaries when stromal tissue is expanding and follicles are forming (222). Overexpression of FNB3 in the extracellular matrix in some PCOS patients could lead to thickened ovarian capsules, associated with increased tensile strength (higher F-actin) of stromal tissues surrounding the follicles. The stiff cortical region could decrease local Hippo signaling, leading to overactivity of YAP, increased CCN growth factor secretion, stromal cell proliferation, and thecal cell hyperplasia (Figure 8). Of interest, genome-wide association studies identified 2 single-nucleotide polymorphism alleles in the YAP gene of PCOS patients (131). This finding is consistent with the hypothesis that YAP overactivity could lead to excessive stromal cell proliferation, theca cell hyperplasia, and the PCOS phenotypes. Patients with YAP overactivity could have polycystic follicles but without defects in actin polymerization. Future genome-wide association studies on a subgroup of PCOS patients could identify additional gene variants involved in actin polymerization and extracellular matrix formation responsible for the sclerotic cortical phenotype.
XI. Future Treatment Options
For patients with ovaries containing only preantral follicles and those with poor responses to the traditional FSH therapy, current clinical approaches involve predominantly the use of donor oocytes, and its global use is growing year by year (223, 224). Success of the IVA approach highlights the possibility to promote the growth of preantral follicles. Because gonadotropin stimulation of early antral follicles to escape from atresia and continue growth into preovulatory follicles is well-established, the next challenge for ovarian infertility treatment involves the generation of early antral follicles from preantral follicles to allow exogenous gonadotropin therapies. In the IVA protocol, promotion of secondary follicles to the preovulatory stage requires only a few weeks (103). If mature oocytes can be generated from preovulatory follicles derived from arrested preantral follicles, patients can have their own genetic children. Because grafting of activated follicles in the IVA protocol can be performed at any time after successful vitrification of tissues derived from one or portions of one ovary, future options for women include cryopreservation of ovarian tissues at an appropriate younger age to minimize age-related deterioration of egg quality.
In the future, infertile patients with secondary follicles could be treated with FSH, CNP, and Hippo signaling disrupters as well as AKT and mTOR stimulators to generate early antral follicles (Figure 1). To avoid potential systematic side effects of CNP, paracrine factors, and signaling pathway-regulating drugs, one can take advantage of the established ultrasound and laparoscopic procedures to administer drugs directly into the ovary to promote preantral follicle growth. This approach could include the administration of long-acting CNP analogs, R-spondin agonists, AKT-stimulating drugs, and actin polymerization-promoting drugs. It is important to note that all these potential therapies only increase the number of mature oocytes generated by patients but do not alter the age-associated decline in oocyte quality (Figure 7).
It is clear that PCOS represents a heterogeneous disease with multiple origins (225). In addition to ovarian structural alterations and associated endocrine changes, some patients are at high metabolic risk, and metabolic changes could exacerbate ovarian dysfunctions. It is of interest to investigate the relationship between ovarian structural changes and metabolic disturbances in PCOS. Although multiple early antral follicles are arrested in PCOS, increases in endogenous or exogenous FSH levels lead to the development of preovulatory follicles. New follicle-tracing approaches are needed to investigate whether these early antral follicles are indeed arrested or undergoing slow turnover. With a better understanding of the ovarian Hippo signaling system, PCOS patients may be treated locally with actin-polymerization drugs to disrupt ovarian Hippo signaling (226) instead of wedge resection, ovarian drilling, or even FSH administration. Because sphingosine-1-phosphate, lysophosphatidic acid, and thrombin have been shown to promote actin polymerization, disrupt Hippo signaling, and increase YAP nuclear localization (227–229), these compounds could also be useful in promoting follicle growth. After transient disruption of local Hippo signaling, one follicle becomes dominant and grows into the preovulatory follicle. Regular cyclicity could ensue, followed by normal conception and pregnancy. The use of locally administered drugs minimizes follicle loss associated with ovarian damaging procedures. Furthermore, local administration of CCN growth factors downstream of ovarian Hippo signaling could be effective in promoting follicle growth.
In addition to new treatments for PCOS, further investigation of intraovarian control of early folliculogenesis could provide opportunities to design new therapies for infertile patients still possessing preantral follicles but are not responding to the traditional gonadotropin therapy.
Acknowledgments
This work was supported by National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (U54 HD068158 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, to A.J.H.) and Grant-in-aid for Scientific Research Japan Society for the Promotion of Science (Research B: 24390376, and Innovative Areas, Mechanisms regulating gamete formation in animals: 26114510, to K.K.) and by research funds from the Smoking Research Foundation (to K. K.) and the Takeda Science Foundation (to K. K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALK
- activin-like kinase
- AMH
- anti-Mullerian hormone
- ANP
- atrial natriuretic peptide
- ART
- assisted reproductive technology
- BMP15
- bone morphogenetic protein-15
- BNP
- brain natriuretic peptide
- CNP
- C-type natriuretic peptide
- DIAPH
- actin-polymerizing formin diaphanous
- F-actin
- filamentous actin
- FOXO3
- forkhead box O3
- G-actin
- globular actin
- GDF9
- growth differentiation factor-9
- GPCR
- G protein-coupled receptor
- IVA
- in vitro activation
- IVF
- in vitro fertilization
- KGF
- keratinocyte growth factor
- LGR
- leucine-rich repeat-containing G-protein coupled receptor
- LOD
- laparoscopic ovarian drilling
- mTOR
- mammalian target of rapamycin
- NPPC
- natriuretic peptide precursor C
- NPRB
- natriuretic peptide receptor-B
- PACAP
- pituitary adenylate cyclase-activating polypeptide
- PCOS
- polycystic ovary syndrome
- PI3K
- phosphatidylinositol 3-kinase
- PIP2
- phosphatidylinositol-4,5-bisphosphate
- PIP3
- phosphatidylinositol-3,4,5-triphosphate
- POI
- primary ovarian insufficiency
- PTEN
- phosphatase and tensin homolog
- RSK
- receptor serine kinase
- RTK
- receptor tyrosine kinase
- TAZ
- transcriptional coactivator with PDZ-binding motif
- TEA
- transcriptional enhancer activator
- TEAD
- transcription factors containing the TEA/ATTS DNA binding domain
- VEGF
- vascular endothelial growth factor
- VIP
- vasoactive intestinal peptide
- WNT
- wingless
- YAP
- yes-associated protein.
References
- 1. McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. [DOI] [PubMed] [Google Scholar]
- 2. Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev. 1994;15:707–724. [DOI] [PubMed] [Google Scholar]
- 3. Zeleznik AJ. Follicle selection in primates: “many are called but few are chosen”. Biol Reprod. 2001;65:655–659. [DOI] [PubMed] [Google Scholar]
- 4. Richards JS. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev. 1980;60:51–89. [DOI] [PubMed] [Google Scholar]
- 5. Goodman A, Hodgen G. The ovarian triad of the primate menstrual cycle. Recent Prog Horm Res. 1983;39:1. [DOI] [PubMed] [Google Scholar]
- 6. Hsueh AJ, Adashi EY, Jones PB, Welsh TH., Jr Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984;5:76–127. [DOI] [PubMed] [Google Scholar]
- 7. Fauser BC, Van Heusden AM. Manipulation of human ovarian function: physiological concepts and clinical consequences. Endocr Rev. 1997;18:71–106. [DOI] [PubMed] [Google Scholar]
- 8. Macklon NS, Stouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27:170–207. [DOI] [PubMed] [Google Scholar]
- 9. Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab. 2010;21:96–103. [DOI] [PubMed] [Google Scholar]
- 10. Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–493. [DOI] [PubMed] [Google Scholar]
- 11. Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15:725–751. [DOI] [PubMed] [Google Scholar]
- 12. Oktay K, Briggs D, Gosden RG. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J Clin Endocrinol Metab. 1997;82:3748–3751. [DOI] [PubMed] [Google Scholar]
- 13. McGee EA, Perlas E, LaPolt PS, Tsafriri A, Hsueh AJ. Follicle-stimulating hormone enhances the development of preantral follicles in juvenile rats. Biol Reprod. 1997;57:990–998. [DOI] [PubMed] [Google Scholar]
- 14. Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141:1795–1803. [DOI] [PubMed] [Google Scholar]
- 15. Schoot DC, Harlin J, Shoham Z, et al. Recombinant human follicle-stimulating hormone and ovarian response in gonadotrophin-deficient women. Hum Reprod. 1994;9:1237–1242. [DOI] [PubMed] [Google Scholar]
- 16. Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73:942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cortvrindt R, Smitz J, Van Steirteghem A. Assessment of the need for follicle stimulating hormone in early preantral mouse follicle culture in vitro. Hum Reprod. 1997;12:759–768. [DOI] [PubMed] [Google Scholar]
- 19. Wright CS, Hovatta O, Margara R, et al. Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum Reprod. 1999;14:1555–1562. [DOI] [PubMed] [Google Scholar]
- 20. Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990;70:665–699. [DOI] [PubMed] [Google Scholar]
- 21. Sudoh T, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990;168:863–870. [DOI] [PubMed] [Google Scholar]
- 22. Koller KJ, Lowe DG, Bennett GL, et al. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science. 1991;252:120–123. [DOI] [PubMed] [Google Scholar]
- 23. Tremblay J, Desjardins R, Hum D, Gutkowska J, Hamet P. Biochemistry and physiology of the natriuretic peptide receptor guanylyl cyclases. Mol Cell Biochem. 2002;230:31–47. [PubMed] [Google Scholar]
- 24. Schulz S. C-type natriuretic peptide and guanylyl cyclase B receptor. Peptides. 2005;26:1024–1034. [DOI] [PubMed] [Google Scholar]
- 25. Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355:1126–1130. [DOI] [PubMed] [Google Scholar]
- 26. Suga S, Nakao K, Itoh H, et al. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of “vascular natriuretic peptide system”. J Clin Invest. 1992;90:1145–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hobbs A, Foster P, Prescott C, Scotland R, Ahluwalia A. Natriuretic peptide receptor-C regulates coronary blood flow and prevents myocardial ischemia/reperfusion injury: novel cardioprotective role for endothelium-derived C-type natriuretic peptide. Circulation. 2004;110:1231–1235. [DOI] [PubMed] [Google Scholar]
- 28. Jankowski M, Reis AM, Mukaddam-Daher S, Dam TV, Farookhi R, Gutkowska J. C-type natriuretic peptide and the guanylyl cyclase receptors in the rat ovary are modulated by the estrous cycle. Biol Reprod. 1997;56:59–66. [DOI] [PubMed] [Google Scholar]
- 29. Gutkowska J, Jankowski M, Sairam MR, et al. Hormonal regulation of natriuretic peptide system during induced ovarian follicular development in the rat. Biol Reprod. 1999;61:162–170. [DOI] [PubMed] [Google Scholar]
- 30. Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Norris RP, Ratzan WJ, Freudzon M, et al. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawamura K, Cheng Y, Kawamura N, et al. Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum Reprod. 2011;26:3094–3101. [DOI] [PubMed] [Google Scholar]
- 33. Tsafriri A, Pomerantz SH. Oocyte maturation inhibitor. Clin Endocrinol Metab. 1986;15:157–170. [DOI] [PubMed] [Google Scholar]
- 34. Chusho H, Tamura N, Ogawa Y, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98:4016–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tamura N, Doolittle LK, Hammer RE, Shelton JM, Richardson JA, Garbers DL. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci U S A. 2004;101:17300–17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kiyosu C, Tsuji T, Yamada K, Kajita S, Kunieda T. NPPC/NPR2 signaling is essential for oocyte meiotic arrest and cumulus oophorus formation during follicular development in the mouse ovary. Reproduction. 2012;144:187–193. [DOI] [PubMed] [Google Scholar]
- 37. McGee E, Spears N, Minami S, et al. Preantral ovarian follicles in serum-free culture: suppression of apoptosis after activation of the cyclic guanosine 3′,5′-monophosphate pathway and stimulation of growth and differentiation by follicle-stimulating hormone. Endocrinology. 1997;138:2417–2424. [DOI] [PubMed] [Google Scholar]
- 38. Sato Y, Cheng Y, Kawamura K, Takae S, Hsueh AJ. C-type natriuretic peptide stimulates ovarian follicle development. Mol Endocrinol. 2012;26:1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hannema SE, van Duyvenvoorde HA, Premsler T, et al. An activating mutation in the kinase homology domain of the natriuretic peptide receptor-2 causes extremely tall stature without skeletal deformities. J Clin Endocrinol Metab. 2013;98:E1988–E1998. [DOI] [PubMed] [Google Scholar]
- 40. Barletta G, Lazzeri C, Vecchiarino S, et al. Low-dose C-type natriuretic peptide does not affect cardiac and renal function in humans. Hypertension. 1998;31:802–808. [DOI] [PubMed] [Google Scholar]
- 41. Cheng Y, Kawamura K, Takae S, et al. Oocyte-derived R-spondin2 promotes ovarian follicle development. FASEB J. 2013;27:2175–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Juengel JL, Bodensteiner KJ, Heath DA, et al. Physiology of GDF9 and BMP15 signalling molecules. Anim Reprod Sci. 2004;82:447–460. [DOI] [PubMed] [Google Scholar]
- 43. Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. [DOI] [PubMed] [Google Scholar]
- 44. Mao J, Wang J, Liu B, et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. [DOI] [PubMed] [Google Scholar]
- 45. de Lau W, Barker N, Low TY, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. [DOI] [PubMed] [Google Scholar]
- 46. Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. 2011 R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc Natl Acad Sci U S A. 2011;108:11452–11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim KA, Wagle M, Tran K, et al. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell. 2008;19:2588–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim KA, Kakitani M, Zhao J, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. [DOI] [PubMed] [Google Scholar]
- 49. Bell SM, Schreiner CM, Hess KA, Anderson KP, Scott WJ. Asymmetric limb malformations in a new transgene insertional mutant, footless. Mech Dev. 2003;120:597–605. [DOI] [PubMed] [Google Scholar]
- 50. Nam JS, Park E, Turcotte TJ, et al. Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb. Dev Biol. 2007;311:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–1159. [DOI] [PubMed] [Google Scholar]
- 52. Qin Y, Choi Y, Zhao H, Simpson JL, Chen ZJ, Rajkovic A. NOBOX homeobox mutation causes premature ovarian failure. Am J Hum Genet. 2007;81:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Choi Y, Qin Y, Berger MF, Ballow DJ, Bulyk ML, Rajkovic A. Microarray analyses of newborn mouse ovaries lacking Nobox. Biol Reprod. 2007;77:312–319. [DOI] [PubMed] [Google Scholar]
- 54. Vitt UA, Hsu SY, Hsueh AJ. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol Endocrinol. 2001;15:681–694. [DOI] [PubMed] [Google Scholar]
- 55. Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. [DOI] [PubMed] [Google Scholar]
- 56. Vitt UA, Mazerbourg S, Klein C, Hsueh AJ. Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol Reprod. 2002;67:473–480. [DOI] [PubMed] [Google Scholar]
- 57. Mazerbourg S, Klein C, Roh J, et al. Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5. Mol Endocrinol. 2004;18:653–665. [DOI] [PubMed] [Google Scholar]
- 58. Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. [DOI] [PubMed] [Google Scholar]
- 59. Vitt UA, Hayashi M, Klein C, Hsueh AJ. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod. 2000;62:370–377. [DOI] [PubMed] [Google Scholar]
- 60. Solovyeva EV, Hayashi M, Margi K, et al. Growth differentiation factor-9 stimulates rat theca-interstitial cell androgen biosynthesis. Biol Reprod. 2000;63:1214–1218. [DOI] [PubMed] [Google Scholar]
- 61. Spicer LJ, Aad PY, Allen DT, Mazerbourg S, Payne AH, Hsueh AJ. Growth differentiation factor 9 (GDF9) stimulates proliferation and inhibits steroidogenesis by bovine theca cells: influence of follicle size on responses to GDF9. Biol Reprod. 2008;78:243–253. [DOI] [PubMed] [Google Scholar]
- 62. Vitt UA, McGee EA, Hayashi M, Hsueh AJ. In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats. Endocrinology. 2000;141:3814–3820. [DOI] [PubMed] [Google Scholar]
- 63. Orisaka M, Orisaka S, Jiang JY, et al. Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Mol Endocrinol. 2006;20:2456–2468. [DOI] [PubMed] [Google Scholar]
- 64. Otsuka F, Yao Z, Lee T, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15. Identification of target cells and biological functions. J Biol Chem. 2000;275:39523–39528. [DOI] [PubMed] [Google Scholar]
- 65. Galloway SM, McNatty KP, Cambridge LM, et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25:279–283. [DOI] [PubMed] [Google Scholar]
- 66. Persani L, Rossetti R, Di Pasquale E, Cacciatore C, Fabre S. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum Reprod Update. 2014;pii:dmu036. [DOI] [PubMed] [Google Scholar]
- 67. Yan C, Wang P, DeMayo J, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. [DOI] [PubMed] [Google Scholar]
- 68. Davis GH, McEwan JC, Fennessy PF, et al. Infertility due to bilateral ovarian hypoplasia in sheep homozygous (FecXI FecXI) for the Inverdale prolificacy gene located on the X chromosome. Biol Reprod. 1992;46:636–640. [DOI] [PubMed] [Google Scholar]
- 69. Hanrahan JP, Gregan SM, Mulsant P, et al. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol Reprod. 2004;70:900–909. [DOI] [PubMed] [Google Scholar]
- 70. Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet. 2004;75:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dixit H, Rao LK, Padmalatha VV, et al. Missense mutations in the BMP15 gene are associated with ovarian failure. Hum Genet. 2006;119:408–415. [DOI] [PubMed] [Google Scholar]
- 72. Tiotiu D, Alvaro Mercadal B, Imbert R, et al. Variants of the BMP15 gene in a cohort of patients with premature ovarian failure. Hum Reprod. 2010;25:1581–1587. [DOI] [PubMed] [Google Scholar]
- 73. Laissue P, Christin-Maitre S, Touraine P, et al. Mutations and sequence variants in GDF9 and BMP15 in patients with premature ovarian failure. Eur J Endocrinol. 2006;154:739–744. [DOI] [PubMed] [Google Scholar]
- 74. Simpson CM, Robertson DM, Al-Musawi SL, et al. Aberrant GDF9 expression and activation are associated with common human ovarian disorders. J Clin Endocrinol Metab. 2014;99:E615—E624. [DOI] [PubMed] [Google Scholar]
- 75. Peng J, Li Q, Wigglesworth K, et al. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci U S A. 2013;110:E776–E785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Durlinger AL, Gruijters MJ, Kramer P, et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–4899. [DOI] [PubMed] [Google Scholar]
- 77. Durlinger AL, Kramer P, Karels B, et al. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. [DOI] [PubMed] [Google Scholar]
- 78. Campbell BK, Clinton M, Webb R. The role of anti-Müllerian hormone (AMH) during follicle development in a monovulatory species (sheep). Endocrinology. 2012;153:4533–4543. [DOI] [PubMed] [Google Scholar]
- 79. Richards JS, Russell DL, Ochsner S, et al. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res. 2002;57:195–220. [DOI] [PubMed] [Google Scholar]
- 80. Lee J, Park HJ, Choi HS, et al. Gonadotropin stimulation of pituitary adenylate cyclase-activating polypeptide (PACAP) messenger ribonucleic acid in the rat ovary and the role of PACAP as a follicle survival factor. Endocrinology. 1999;140:818–826. [DOI] [PubMed] [Google Scholar]
- 81. Qin Y, Sun M, You L, et al. ESR1, HK3 and BRSK1 gene variants are associated with both age at natural menopause and premature ovarian failure. Orphanet J Rare Dis. 2012;7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xia Y, Che Y, Zhang X, et al. Polymorphic CAG repeat in the androgen receptor gene in polycystic ovary syndrome patients. Mol Med Report. 2012;5:1330–1334. [DOI] [PubMed] [Google Scholar]
- 83. Zhang T, Liang W, Fang M, Yu J, Ni Y, Li Z. Association of the CAG repeat polymorphisms in androgen receptor gene with polycystic ovary syndrome: a systemic review and meta-analysis. Gene. 2013;524:161–167. [DOI] [PubMed] [Google Scholar]
- 84. Lee S, Kang DW, Hudgins-Spivey S, et al. Theca-specific estrogen receptor-α knockout mice lose fertility prematurely. Endocrinology. 2009;150:3855–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24:1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Walters KA, Middleton LJ, Joseph SR, et al. Targeted loss of androgen receptor signaling in murine granulosa cells of preantral and antral follicles causes female subfertility. Biol Reprod. 2012;87:151. [DOI] [PubMed] [Google Scholar]
- 87. Lydon JP, DeMayo FJ, Funk CR, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. [DOI] [PubMed] [Google Scholar]
- 88. Natraj U, Richards JS. Hormonal regulation, localization, and functional activity of the progesterone receptor in granulosa cells of rat preovulatory follicles. Endocrinology. 1993;133:761–769. [DOI] [PubMed] [Google Scholar]
- 89. Zeleznik AJ. The physiology of follicle selection. Reprod Biol Endocrinol. 2004;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sen A, Prizant H, Light A, et al. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci U S A. 2014;111:3008–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hillier SG, De Zwart FA. Evidence that granulosa cell aromatase induction/activation by follicle-stimulating hormone is an androgen receptor-regulated process in-vitro. Endocrinology. 1981;109:1303–1305. [DOI] [PubMed] [Google Scholar]
- 92. Armstrong DT, Dorrington JH. Androgens augment FSH-induced progesterone secretion by cultured rat granulosa cells. Endocrinology. 1976;99:1411–1414. [DOI] [PubMed] [Google Scholar]
- 93. McNatty KP, Baird DT, Bolton A, Chambers P, Corker C, McLean H. Concentration of oestrogens and androgens in human ovarian venous plasma and follicular fluid throughout the menstrual cycle. J Endocrinol. 1976;71:77–85. [DOI] [PubMed] [Google Scholar]
- 94. Murray AA, Gosden RG, Allison V, Spears N. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil. 1998;113:27–33. [DOI] [PubMed] [Google Scholar]
- 95. Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951–2956. [DOI] [PubMed] [Google Scholar]
- 96. Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15:2129–2132. [DOI] [PubMed] [Google Scholar]
- 97. Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR). Reprod Biol Endocrinol. 2011;9:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sunkara SK, Coomarasamy A, Arlt W, Bhattacharya S. Should androgen supplementation be used for poor ovarian response in IVF? Hum Reprod. 2012;27:637–640. [DOI] [PubMed] [Google Scholar]
- 99. Zangmo R, Singh N, Kumar S, Vanamail P, Tiwari A. Role of dehydroepiandrosterone in improving oocyte and embryo quality in IVF cycles. Reprod Biomed Online. 2014;28:743–747. [DOI] [PubMed] [Google Scholar]
- 100. Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181. [Google Scholar]
- 101. Farquhar C, Brown J, Marjoribanks J. Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2012;6:CD001122. [DOI] [PubMed] [Google Scholar]
- 102. Donnez J, Silber S, Andersen CY, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43:437–450. [DOI] [PubMed] [Google Scholar]
- 103. Kawamura K, Cheng Y, Suzuki N, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. [DOI] [PubMed] [Google Scholar]
- 105. Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hergovich A. Mammalian Hippo signalling: a kinase network regulated by protein-protein interactions. Biochem Soc Trans. 2012;40:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci. 2008;33:461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lu L, Li Y, Kim SM, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lee KP, Lee JH, Kim TS, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Heallen T, Zhang M, Wang J, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13:63–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sansores-Garcia L, Bossuyt W, Wada K, et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30:2325–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fernández BG, Gaspar P, Brás-Pereira C, Jezowska B, Rebelo SR, Janody F. Actin-capping protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138:2337–2346. [DOI] [PubMed] [Google Scholar]
- 115. Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Brigstock D. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. [DOI] [PubMed] [Google Scholar]
- 117. Robertson GA. Ovarian transplantation in the house mouse. Proc Soc Exp Biol Med. 1940;44:302–304. [Google Scholar]
- 118. Russell WL, Hurst JG. Pure strain mice born to hybrid mothers following ovarian transplantation. Proc Natl Acad Sci U S A. 1945;31:267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Stevens LC. A modification of Robertson's technique of homoiotopic ovarian transplantation in mice. Transplant Bull. 1957;4:106–107. [PubMed] [Google Scholar]
- 120. Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 C. Endocrinology. 1999;140:462–471. [DOI] [PubMed] [Google Scholar]
- 121. Xu J, Bernuci MP, Lawson MS, et al. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zheng W, Zhang H, Gorre N, Risal S, Shen Y, Liu K. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum Mol Genet. 2014;23:920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. [DOI] [PubMed] [Google Scholar]
- 124. Baker SJ, Spears N. The role of intra-ovarian interactions in the regulation of follicle dominance. Hum Reprod Update. 1999;5:153–165. [DOI] [PubMed] [Google Scholar]
- 125. Xia Y, Chang T, Wang Y, et al. YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS One. 2014;9:e91770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bione S, Sala C, Manzini C, et al. A human homologue of the Drosophila melanogaster diaphanous gene is disrupted in a patient with premature ovarian failure: evidence for conserved function in oogenesis and implications for human sterility. Am J Hum Genet. 1998;62:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. He C, Kraft P, Chasman DI, et al. A large-scale candidate gene association study of age at menarche and age at natural menopause. Hum Genet. 2010;128:515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Schuh-Huerta SM, Johnson NA, Rosen MP, Sternfeld B, Cedars MI, Reijo Pera RA. Genetic markers of ovarian follicle number and menopause in women of multiple ethnicities. Hum Genet. 2012;131:1709–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. St John MA, Tao W, Fei X, et al. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet. 1999;21:182–186. [DOI] [PubMed] [Google Scholar]
- 130. Pisarska MD, Kuo FT, Bentsi-Barnes IK, Khan S, Barlow GM. LATS1 phosphorylates forkhead L2 and regulates its transcriptional activity. Am J Physiol Endocrinol Metab. 2010;299:E101–E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Li T, Zhao H, Zhao X, et al. Identification of YAP1 as a novel susceptibility gene for polycystic ovary syndrome. J Med Genet. 2012;49:254–257. [DOI] [PubMed] [Google Scholar]
- 132. Hall CA, Wang R, Miao J, et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010;70:8517–8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nagashima T, Kim J, Li Q, et al. Connective tissue growth factor is required for normal follicle development and ovulation. Mol Endocrinol. 2011;25:1740–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Aboura A, Dupas C, Tachdjian G, et al. Array comparative genomic hybridization profiling analysis reveals deoxyribonucleic acid copy number variations associated with premature ovarian failure. J Clin Endocrinol Metab. 2009;94:4540–4546. [DOI] [PubMed] [Google Scholar]
- 135. Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. [DOI] [PubMed] [Google Scholar]
- 136. Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. [DOI] [PubMed] [Google Scholar]
- 137. Slager HG, Good MJ, Schaart G, Groenewoud JS, Mummery CL. Organization of non-muscle myosin during early murine embryonic differentiation. Differentiation. 1992;50:47–56. [DOI] [PubMed] [Google Scholar]
- 138. Nishioka N, Inoue K, Adachi K, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. [DOI] [PubMed] [Google Scholar]
- 139. Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin Cancer Biol. 2008;311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. [DOI] [PubMed] [Google Scholar]
- 141. West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gallardo TD, John GB, Shirley L, et al. Genomewide discovery and classification of candidate ovarian fertility genes in the mouse. Genetics. 2007;177:179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hutt KJ, McLaughlin EA, Holland MK. KIT/KIT ligand in mammalian oogenesis and folliculogenesis: roles in rabbit and murine ovarian follicle activation and oocyte growth. Biol Reprod. 2006;75:421–433. [DOI] [PubMed] [Google Scholar]
- 145. Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–4271. [DOI] [PubMed] [Google Scholar]
- 146. Ojeda SR, Romero C, Tapia V, Dissen GA. Neurotrophic and cell-cell dependent control of early follicular development. Mol Cell Endocrinol. 2000;163:67–71. [DOI] [PubMed] [Google Scholar]
- 147. Roberts AE, Arbogast LK, Friedman CI, Cohn DE, Kaumaya PT, Danforth DR. Neutralization of endogenous vascular endothelial growth factor depletes primordial follicles in the mouse ovary. Biol Reprod. 2007;76:218–223. [DOI] [PubMed] [Google Scholar]
- 148. Tanwar PS, O'Shea T, McFarlane JR. In vivo evidence of role of bone morphogenetic protein-4 in the mouse ovary. Anim Reprod Sci. 2008;106:232–240. [DOI] [PubMed] [Google Scholar]
- 149. Lee WS, Otsuka F, Moore RK, Shimasaki S. Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod. 2001;65:994–999. [DOI] [PubMed] [Google Scholar]
- 150. Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol. 2002;188:65–73. [DOI] [PubMed] [Google Scholar]
- 151. Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol. 2001;175:123–130. [DOI] [PubMed] [Google Scholar]
- 152. Kezele P, Nilsson EE, Skinner MK. Keratinocyte growth factor acts as a mesenchymal factor that promotes ovarian primordial to primary follicle transition. Biol Reprod. 2005;73:967–973. [DOI] [PubMed] [Google Scholar]
- 153. Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438–464. [DOI] [PubMed] [Google Scholar]
- 154. Reddy P, Shen L, Ren C, et al. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol. 2005;281:160–170. [DOI] [PubMed] [Google Scholar]
- 155. Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. [DOI] [PubMed] [Google Scholar]
- 156. Reddy P, Liu L, Adhikari D, J, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. [DOI] [PubMed] [Google Scholar]
- 157. John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Tarnawa ED, Baker MD, Aloisio GM, Carr BR, Castrillon DH. Gonadal expression of foxo1, but not foxo3, is conserved in diverse mammalian species. Biol Reprod. 2013;88:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Fan HY, Liu Z, Cahill N, Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol. 2008;22:2128–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Adhikari D, Flohr G, Gorre N, et al. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod. 2009;15:765–770. [DOI] [PubMed] [Google Scholar]
- 162. Adhikari D, Zheng W, Shen Y, Gorre N, et al. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19:397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Adhikari D, Liu K. mTOR signaling in the control of activation of primordial follicles. Cell Cycle. 2010;9:1673–1674. [DOI] [PubMed] [Google Scholar]
- 164. Huang L, Wang ZB, Jiang ZZ, et al. Specific disruption of Tsc1 in ovarian granulosa cells promotes ovulation and causes progressive accumulation of corpora lutea. PLoS One. 2013;8:e54052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Li J, Kawamura K, Cheng Y, et al. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A. 2010;107:10280–10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Zeleznik AJ, Saxena D, Little-Ihrig L. Protein kinase B is obligatory for follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology. 2003;144:3985–3994. [DOI] [PubMed] [Google Scholar]
- 167. Kalous J, Solc P, Baran V, Kubelka M, Schultz RM, Motlik J. PKB/AKT is involved in resumption of meiosis in mouse oocytes. Biol Cell. 2006;98:111–123. [DOI] [PubMed] [Google Scholar]
- 168. Adashi E. Growth factors and ovarian function: the IGF-I paradigm. Horm Res Paediatr. 1994;42:44–48. [DOI] [PubMed] [Google Scholar]
- 169. McGee EA, Chun SY, Lai S, He Y, Hsueh AJ. Keratinocyte growth factor promotes the survival, growth, and differentiation of preantral ovarian follicles. Fertil Steril. 1999;71:732–738. [DOI] [PubMed] [Google Scholar]
- 170. Danforth DR, Arbogast LK, Ghosh S, Dickerman A, Rofagha R, Friedman CI. Vascular endothelial growth factor stimulates preantral follicle growth in the rat ovary. Biol Reprod. 2003;68:1736–1741. [DOI] [PubMed] [Google Scholar]
- 171. Matos MH, Lima-Verde IB, Bruno JB, et al. Follicle stimulating hormone and fibroblast growth factor-2 interact and promote goat primordial follicle development in vitro. Reprod Fertil Dev. 2007;19:677–684. [DOI] [PubMed] [Google Scholar]
- 172. Chaves RN, Lima-Verde IB, Celestino JJ, et al. Fibroblast growth factor-10 maintains the survival and promotes the growth of cultured goat preantral follicles. Domest Anim Endocrinol. 2010;39:249–258. [DOI] [PubMed] [Google Scholar]
- 173. Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- 174. Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11:391–410. [DOI] [PubMed] [Google Scholar]
- 175. De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376:911–921. [DOI] [PubMed] [Google Scholar]
- 176. Fénichel P, Sosset C, Barbarino-Monnier P, et al. Prevalence, specificity and significance of ovarian antibodies during spontaneous premature ovarian failure. Hum Reprod. 1997;12:2623–2628. [DOI] [PubMed] [Google Scholar]
- 177. Hoek A, Schoemaker J, Drexhage H. Premature ovarian failure and ovarian autoimmunity. Endocr Rev. 1997;18:107–134. [DOI] [PubMed] [Google Scholar]
- 178. Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9:735–749. [DOI] [PubMed] [Google Scholar]
- 179. Sauer MV, Paulson RJ, Lobo RA. A preliminary report on oocyte donation extending reproductive potential to women over 40. N Engl J Med. 1990;323:1157–1160. [DOI] [PubMed] [Google Scholar]
- 180. Suzuki N, Hashimoto S, Igarashi S, et al. Assessment of long-term function of heterotopic transplants of vitrified ovarian tissue in cynomolgus monkeys. Hum Reprod. 2012;27:2420–2429. [DOI] [PubMed] [Google Scholar]
- 181. Adhikari D, Gorre N, Risal S, et al. The safe use of a PTEN inhibitor for the activation of dormant mouse primordial follicles and generation of fertilizable eggs. PLoS One. 2012;7:e39034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Bidet M, Bachelot A, Bissauge E, et al. Resumption of ovarian function and pregnancies in 358 patients with premature ovarian failure. J Clin Endocrinol Metab. 2011;96:3864–3872. [DOI] [PubMed] [Google Scholar]
- 183. McLaughlin M, Kinnell HL, Anderson RA, Telfer EE. Inhibition of phosphatase and tensin homolog (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Mol Hum Reprod. 2014;20:736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Broer SL, Dólleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. 2011;17:46–54. [DOI] [PubMed] [Google Scholar]
- 185. La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update. 2010;16:113–130. [DOI] [PubMed] [Google Scholar]
- 186. Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Müllerian hormone in women. Hum Reprod Update. 2014;20:804. [DOI] [PubMed] [Google Scholar]
- 187. Broer SL, BF, Laven JS, Fauser BC. Anti-Mullerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20:688–701. [DOI] [PubMed] [Google Scholar]
- 188. Weenen C, Laven JS, Von Bergh AR, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. [DOI] [PubMed] [Google Scholar]
- 189. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti-Mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88:5957–5962. [DOI] [PubMed] [Google Scholar]
- 190. Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti-Mullerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab. 2013;98:3332–3340. [DOI] [PubMed] [Google Scholar]
- 191. van Rooij IA, Broekmans FJ, te Velde ER, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–3071. [DOI] [PubMed] [Google Scholar]
- 192. Modi D, Bhartiya D, Puri C. Developmental expression and cellular distribution of Mullerian inhibiting substance in the primate ovary. Reproduction. 2006;132:443–453. [DOI] [PubMed] [Google Scholar]
- 193. Tran ND, Cedars MI, Rosen MP. The role of anti-müllerian hormone (AMH) in assessing ovarian reserve. J Clin Endocrinol Metab. 2011;96:3609–3614. [DOI] [PubMed] [Google Scholar]
- 194. Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95:170–175. [DOI] [PubMed] [Google Scholar]
- 195. McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9:131–136. [DOI] [PubMed] [Google Scholar]
- 196. Aaltonen J, Laitinen MP, Vuojolainen K, et al. Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab. 1999;84:2744–2750. [DOI] [PubMed] [Google Scholar]
- 197. Pache T, Wladimiroff J, De Jong F, Hop W, Fauser B. Growth patterns of nondominant ovarian follicles during the normal menstrual cycle. Fertil Steril. 1990;54:638. [DOI] [PubMed] [Google Scholar]
- 198. van Stanbrink EJ, Hop WC, van Dessel TJ, de Jong FH, Fauser BC. Decremental follicle-stimulating hormone and dominant follicle development during the normal menstrual cycle. Fertil Steril. 1995;64:37–43. [PubMed] [Google Scholar]
- 199. Ferraretti AP, La Marca A, Fauser BC, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26:1616–1624. [DOI] [PubMed] [Google Scholar]
- 200. Ventura SJ, Abma JC, Mosher WD, Henshaw SK. 2008 Estimated pregnancy rates by outcome for the United States, 1990–2004. Natl Vital Stat Rep. 2008;56:1–25, 28. [PubMed] [Google Scholar]
- 201. Santoro N. Update in hyper- and hypogonadotropic amenorrhea. J Clin Endocrinol Metab. 2011;96:3281–3288. [DOI] [PubMed] [Google Scholar]
- 202. Broekmans FJ, Knauff EA, Valkenburg O, Laven JS, Eijkemans MJ, Fauser BC. PCOS according to the Rotterdam consensus criteria: Change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG. 2006;113:1210–1217. [DOI] [PubMed] [Google Scholar]
- 203. Dewailly D, Lujan ME, Carmina E, et al. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2014;20:334–352. [DOI] [PubMed] [Google Scholar]
- 204. Farquhar C, Lilford RJ, Marjoribanks J, Vandekerckhove P. Laparoscopic ‘drilling’ by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;3:CD001122. [DOI] [PubMed] [Google Scholar]
- 205. Abu Hashim H, Al-Inany H, De Vos M, Tournaye H. Three decades after Gjönnaess's laparoscopic ovarian drilling for treatment of PCOS; what do we know? An evidence-based approach. Arch Gynecol Obstet. 2013;288:409–422. [DOI] [PubMed] [Google Scholar]
- 206. Bayram N, Van Wely M, Kaaijk EM, Bossuyt PM, van der Veen F. Using an electrocautery strategy or recombinant follicle stimulating hormone to induce ovulation in polycystic ovary syndrome: randomised controlled trial. BMJ. 2004;328:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Judd HL, Rigg LA, Anderson DC, Yen SS. The effects of ovarian wedge resection on circulating gonadotropin and ovarian steroid levels in patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 1976;43:347–355. [DOI] [PubMed] [Google Scholar]
- 208. Hendriks ML, Ket JC, Hompes PG, Homburg R, Lambalk CB. Why does ovarian surgery in PCOS help? Insight into the endocrine implications of ovarian surgery for ovulation induction in polycystic ovary syndrome. Hum Reprod Update. 2007;13:249–264. [DOI] [PubMed] [Google Scholar]
- 209. Chen ZJ, Zhao H, He L, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43:55–59. [DOI] [PubMed] [Google Scholar]
- 210. Evian Annual Reproduction (EVAR) Workshop Group 2010; Fauser B, Diedrich K, Bouchard P, et al. Contemporary genetic technologies and female reproduction. Hum Reprod Update. 2011;17:829–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Louwers YV, Stolk L, Uitterlinden AG, Laven JS. Cross-ethnic meta-analysis of genetic variants for polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98:E2006–E2012. [DOI] [PubMed] [Google Scholar]
- 212. Zhao H, Chen ZJ. Genetic association studies in female reproduction: from candidate-gene approaches to genome-wide mapping. Mol Hum Reprod. 2013;19:644–654. [DOI] [PubMed] [Google Scholar]
- 213. Urbanek M, Legro RS, Driscoll DA, et al. Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci U S A. 1999;96:8573–8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Ewens KG, Stewart DR, Ankener W, et al. Family-based analysis of candidate genes for polycystic ovary syndrome. J Clin Endocrinol Metab. 2010;95:2306–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Louwers YV, de Jong FH, van Herwaarden NA, et al. Variants in SULT2A1 affect the DHEA sulphate to DHEA ratio in patients with polycystic ovary syndrome but not the hyperandrogenic phenotype. J Clin Endocrinol Metab. 2013;98:3848–3855. [DOI] [PubMed] [Google Scholar]
- 216. Wang P, Zhao H, Li T, et al. Hypomethylation of the LH/choriogonadotropin receptor promoter region is a potential mechanism underlying susceptibility to polycystic ovary syndrome. Endocrinology. 2014;155:1445–1452. [DOI] [PubMed] [Google Scholar]
- 217. McAllister JM, Modi B, Miller BA, et al. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci U S A. 2014;111:E1519–E1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Hsueh AJ, Rauch R. Ovarian Kaleidoscope database: ten years and beyond. Biol Reprod. 2012;86:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Hughesdon P. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv. 1982;37:59–77. [DOI] [PubMed] [Google Scholar]
- 220. Blank SK, McCartney CR, Chhabra S, et al. Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls–implications for regulation of pubertal maturation. J Clin Endocrinol Metab. 2009;94:2360–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Urbanek M, Sam S, Legro RS, Dunaif A. Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab. 2007;92:4191–4198. [DOI] [PubMed] [Google Scholar]
- 222. Hatzirodos N, Bayne RA, Irving-Rodgers HF, et al. Linkage of regulators of TGF-β activity in the fetal ovary to polycystic ovary syndrome. FASEB J. 2011;25:2256–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Garrido N, Bellver J, Remohí J, Simón C, Pellicer A. Cumulative live-birth rates per total number of embryos needed to reach newborn in consecutive in vitro fertilization (IVF) cycles: a new approach to measuring the likelihood of IVF success. Fertil Steril. 2011;96:40–46. [DOI] [PubMed] [Google Scholar]
- 224. Kawwass JF, Monsour M, Crawford S, et al. Trends and outcomes for donor oocyte cycles in the United States, 2000–2010. JAMA. 2013;310:2426–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Dunaif A, Fauser B. Renaming PCOS–a two-state solution. J Clin Endocrinol Metab. 2013;98:4325–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Reddy P, Deguchi M, Cheng Y, Hsueh AJ. Actin cytoskeleton regulates Hippo signaling. PLoS One. 2013;8:e73763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Miller E, Yang J, DeRan M, et al. Identification of Serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol. 2012;19:955–962. [DOI] [PubMed] [Google Scholar]
- 229. Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs). Genes Dev. 2012;26:2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Schuh-Huerta SM, Johnson NA, Rosen MP, Sternfeld B, Cedars MI, Reijo Pera RA. Genetic variants and environmental factors associated with hormonal markers of ovarian reserve in Caucasian and African American women. Hum Reprod. 2012;27:594–608. [DOI] [PMC free article] [PubMed] [Google Scholar]