Abstract
Objectives:
The aim of this was to determine survival after starting neoadjuvant therapy for patients who became ineligible for orthotopic liver transplantation (OLT).
Methods and Materials:
Since January 1993, 215 patients with unresectable cholangiocarcinoma began treatment with planned OLT. Treatment included external-beam radiation therapy (EBRT) with fluorouracil, bile duct brachytherapy, and postradiotherapy fluorouracil or capecitabine before OLT. Adverse findings at the staging operation, death, and other factors precluded OLT in 63 patients (29%), of whom 61 completed neoadjuvant chemoradiation.
Results:
By October 2012, 56 (89%) of the 63 patients unable to undergo OLT had died. Twenty-two patients (35%) became ineligible for OLT before the staging operation, 38 (60%) at the staging operation, and 3 (5%) after staging. From the date of diagnosis, median overall survival was 12.3 months. Survival was 17% at 18 months and 7% at 24 months. Median survival after fallout was 6.8 months. Median survival after the staging operation was 6 months. Two patients lived for 3.7 and 8.7 years before dying of cancer or liver failure caused by persistent biliary stricture at the site of the original cancer, respectively. Univariate analysis showed that time from diagnosis to fallout correlated with overall survival (P=0.04).
Conclusions:
In highly selected patients initially suitable for OLT, the mortality rate for cholangiocarcinoma was high in patients who became ineligible for OLT. Their survival, however, was comparable to expected survival for patients with locally advanced or metastatic disease treated with nontransplant therapies. The most common reason for patient fallout was adverse findings at the staging operation.
Key Words: cholangiocarcinoma, liver transplantation, neoadjuvant chemoradiation
Perihilar cholangiocarcinoma is a rare malignancy that is uniformly fatal if left untreated. At Mayo Clinic, a clinical regimen with strict patient selection criteria was developed in 1993 for unresectable hilar cholangiocarcinoma.1,2 The regimen combines neoadjuvant chemoradiation therapy, operative staging, and orthotopic liver transplantation (OLT).3 Neoadjuvant therapy includes external-beam radiation therapy (EBRT), intrabiliary duct brachytherapy,4 and postbrachytherapy chemotherapy (initially fluorouracil and later oral capecitabine). Five-year survival is 54% for all patients who begin the treatment program and 73% for those able to complete the regimen with OLT.1 Approximately 50% of patients have no detectable residual cholangiocarcinoma in their explanted livers.1,2 Portal vein encasement is the only pretreatment prognostic factor that predicts for residual disease in explanted livers.5
Not all patients who begin the regimen complete all the intended therapies. Adverse findings at the staging operation, death, and other factors preclude patients from undergoing OLT. This study was performed to report the outcome and characteristics of patients who began treatment with curative intent on the transplant regimen but subsequently became ineligible for OLT. The outcome of this group of patients who underwent neoadjuvant therapies has not been reported previously.
METHODS AND MATERIALS
The Mayo Clinic Institutional Review Board approved this study. A prospective database has been maintained for the cholangiocarcinoma transplant regimen since its inception in January 1993. Medical records from local and outside hospitals were reviewed. Criteria for enrollment in the transplant regimen have previously been described in detail.1 Briefly, patients with extrahepatic cholangiocarcinoma not amenable to conventional surgical resection and no lymph node metastases were considered as candidates for OLT. Both tissue diagnosis and clinical criteria were used for diagnosing hilar cholangiocarcinoma at our institution. The clinical criteria required the presence of a malignant-appearing stricture on percutaneous or endoscopic retrograde cholangiopancreatography, combined with 1 of the 4 following criteria: positive findings on brushing or biopsy; polysomy on fluorescence in situ hybridization testing; cancer antigen (CA) 19-9 level higher than 100 U/mL in the absence of cholangitis; or mass on axial imaging at the level of the stricture.6 Clinicopathologic factors such as age, sex, confirmed pathology prior to treatment, the presence of primary sclerosing cholangitis (PSC) or inflammatory bowel disease, high CA 19-9 levels, and the presence of a visible mass on cross-sectional imaging were examined for potential correlation with survival.
The preoperative chemoradiation regimen was previously described.7 For EBRT, 45 Gy at 1.5 Gy twice daily using 3-dimensional conformal techniques was typically prescribed, with concurrent fluorouracil, 225 mg/m2 per day administered by continuous venous infusion. The irradiated volume included the primary tumor and regional (periductal and celiac) lymph nodes. Intrabiliary catheter-guided brachytherapy with iridium-192 followed, for which the radiation dose was prescribed to a 1 cm radius. The catheter placement was directed by endoscopic retrograde cholangiopancreatography. Previously, patients received low-dose rate brachytherapy, typically 20 Gy at a 1 cm radius over approximately 24 hours and, more recently, by high-dose rate brachytherapy, given as 16 Gy in 4 fractions at 1 cm over 2 days. After brachytherapy, patients typically received capecitabine, 2000 mg/m2 per day, divided twice daily, for 2 of every 3 weeks, as maintenance chemotherapy until OLT.
Before OLT, a staging operation was performed that included abdominal exploration for lymph nodes or nodules suspicious for tumor and also regular biopsies of perihilar lymph nodes. Before 2002, the laparotomy was performed as the time neared for OLT. Between 2002 and 2009, staging was carried out immediately after brachytherapy per an agreement with the United Network for Organ Sharing Region 7 Regional Review Board. Since 2010, staging has been carried out close to the time of OLT or 1 day before living donor transplantation. After 2006, most staging operations were performed using hand-assisted laparoscopy with a smaller subcostal incision.
The time at which patients became ineligible for OLT, that is, the timing of fallout, in relation to the staging operation was investigated. Survival since diagnosis was calculated according to the Kaplan-Meier method.8 Log-rank tests were used to determine whether individual variables were associated with survival. Cox proportional hazards models were used to obtain multivariate hazard ratios and score P-values. The covariates tested in the multivariate model contained all of the clinicopathologic factors mentioned above (including type of fallout) and the completion status of the individual neoadjuvant treatments by modality. All tests were 2-sided with 5% type I error rates.
RESULTS
Patient and Tumor Characteristics
Since January 1993, 215 patients with unresectable cholangiocarcinoma began neoadjuvant therapy with the intent to proceed with operative staging, then OLT. Adverse findings at the staging operation, death, and other factors precluded 63 patients (29%) from OLT, which is the group that forms the basis for this report.
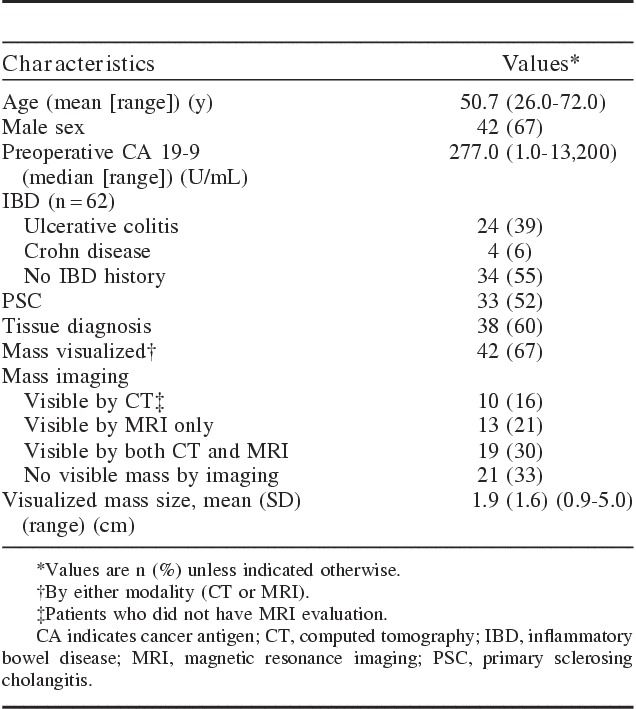
By October 2012, 56 (89%) of the 63 patients unable to undergo an OLT had died. Table 1 lists the patient and tumor characteristics. The mean age at diagnosis was 50.7 years. Thirty-eight patients (60%) had a tissue diagnosis, and clinical criteria provided the diagnosis in 25 (40%). The median preoperative CA 19-9 for the 63 patients was 277.0 U/mL, with a wide range of 1.0 to 13,200 U/mL. Forty-two patients (67%) had a measurable mass visualized at the hilar region by magnetic resonance imaging (MRI) or computed tomography (CT); 33% of patients’ cholangiocarcinomas could not be visualized by either CT or MRI. Thirteen patients (21%) had masses that could be visualized only by MRI. Twenty-four patients (39%) had ulcerative colitis (UC), 33 (52%) had PSC, and 22 (35%) had both.
TABLE 1.
Patient and Tumor Characteristics (N=63)
Treatment Characteristics
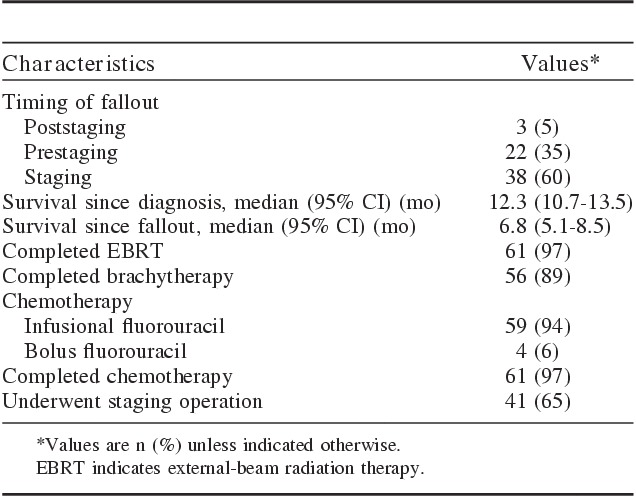
Treatment characteristics for all 63 patients are summarized in Table 2. Sixty-one patients (97%) completed neoadjuvant chemoradiation therapy with EBRT (45 Gy twice daily in 30 fractions); 60 (95%) received brachytherapy or an external-beam boost (4 of 60 patients). Two patients died, while receiving EBRT, of biliary sepsis and extensive abdominal carcinomatosis. Fifty-nine patients (94%) received infusion fluorouracil. Twenty-two patients (35%) became transplant-ineligible before surgical staging, 38 (60%) at the staging operation, and 3 (5%) after staging. Before staging, disease progression (15), failure to thrive (2), other medical events (2, sepsis and renal failure), and death (3, myocardial infarction and pulmonary embolism) disqualified patients for OLT. For the 15 patients who had disease progression before surgical staging, the reasons were liver metastases (4), malignant ascites (3), other distant metastases (3), local tumor progression (1), percutaneous transhepatic cholangiography site recurrence (1), celiac lymph node metastasis (1), and others (2). Except for the patients who dropped out before the planned staging operation (prestaging fallout), 41 patients (65%) underwent the planned staging procedure, which included thorough intra-abdominal inspection, liver palpation, assessment of local disease extent, and regular sampling of hepatic arterial and pericholedochal lymph nodes. Findings at staging included positive lymph nodes (19), peritoneal metastases (14), and local tumor extension (11). These patients became ineligible for OLT as a result of adverse findings during the staging operation. Intrahepatic metastases developed in 2 patients after the staging operation, and 1 patient died of a bleeding ulcer.
TABLE 2.
Outcome and Treatment Characteristics Among Patients Who Became Ineligible for Liver Transplant (N=63)
Survival Analysis
In this group of 63 patients, the median overall survival was 12.3 months (95% confidence interval [CI], 10.7-13.5 mo) from the date of diagnosis (Fig. 1). Survival was 17% at 18 months and 7% at 24 months. Median survival after fallout was 6.8 months (95% CI, 5.1-8.5 mo). Median survival after the staging operation was 6.0 months (95% CI, 3.8-9.5 mo). Two patients lived for 3.7 and 8.7 years, before dying of cancer and liver failure caused by persistent biliary stricture, either because of persistent cancer or scarring, at the site of the original cancer (autopsy not performed), respectively. From the date of diagnosis, the median time to fallout was 5.5 months (95% CI, 4.6-6.4 mo). The majority of patients fell out of the clinical transplant regimen within 1 year (Fig. 2).
FIGURE 1.
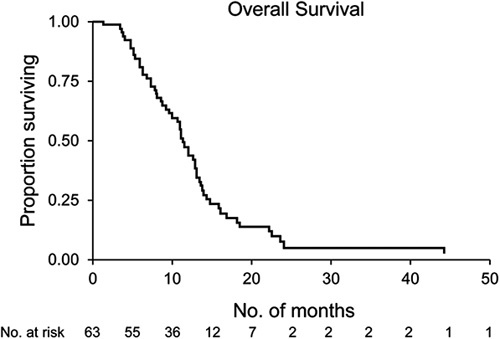
The Kaplan-Meier curve of overall survival since the date of diagnosis of 63 patients who became ineligible for liver transplant.
FIGURE 2.
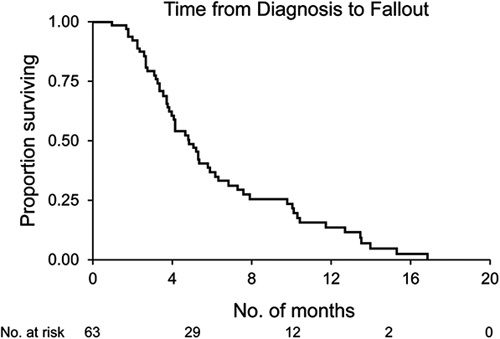
The Kaplan-Meier curve of time from diagnosis to fallout of 63 patients who became ineligible for liver transplant.
Exploratory univariate analyses of factors potentially affecting overall survival are summarized in Table 3. Age, sex, tissue diagnosis, mass visualization on CT or MRI, the presence of inflammatory bowel disease or PSC, completion of chemotherapy, EBRT, or brachytherapy, and types of fallout (prior to or during surgical staging) were analyzed. Only the completion of EBRT was statistically significant for longer median survival on univariate and multivariate analyses. Patients who fell out as a result of findings at surgical staging lived slightly longer than prestaging fallout patients (12.1 vs. 10.8 mo; P=0.14). Additional univariate analysis showed that time from diagnosis to fallout did correlate with overall survival (P=0.04) but not tissue diagnosis, CA 19-9 level, EBRT or brachytherapy dose, age, type of fallout (prestaging or staging), or UC or PSC status (Fig. 3).
TABLE 3.
Univariate Results for Overall Survival Since Diagnosis
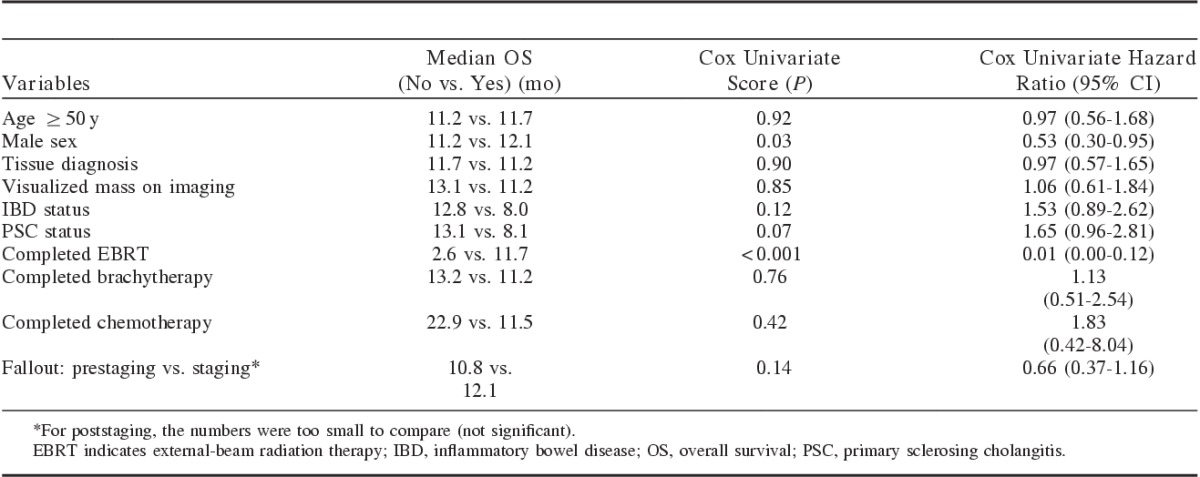
FIGURE 3.
Covariate plot for overall survival versus time from diagnosis to fallout, in months. Univariate analysis showed time from diagnosis to fallout correlated with overall survival (P=0.04) but not tissue diagnosis, cancer antigen 19-9 level, external-beam radiation therapy or brachytherapy dose, age, blood type, ulcerative colitis or primary sclerosing cholangitis status, or type of fallout (prestaging or staging).
DISCUSSION
At present, OLT is the only potentially curative treatment available for patients with hilar cholangiocarcinoma, which is deemed unresectable by conventional surgery. In a group of 48 patients with unresectable cholangiocarcinoma treated with a combination of fluorouracil-based chemotherapy, EBRT, and intraluminal brachytherapy, the 2-year survival rate was 18%.9 In another retrospective study,10 52 patients with unresectable, locally advanced cholangiocarcinoma were treated with definitive chemoradiation therapy. The median survival rate was 10 months. The combination of cisplatin and gemcitabine, evaluated in a randomized phase 3 trial, improved survival for patients with locally advanced or metastatic cholangiocarcinoma,11 although the treatment was not curative. In a surgical series of 373 patients without transplant, the 1- and 5-year survival rates were 33% and 4%, respectively.12 Curative resection was achieved in 36.2% of the patients. Early-stage liver resection and use of adjuvant chemotherapy were favorable characteristics for longer overall survival.12
The patients who are eligible for the liver transplant regimen at our institution are carefully selected.1 The patients have either locally advanced cholangiocarcinoma that is deemed unresectable by a hepatobiliary surgeon or cholangiocarcinoma arising in the background of PSC. At diagnosis, absence of intrahepatic or extrahepatic metastases must be confirmed by imaging, and the patient must be medically fit for both neoadjuvant chemoradiation therapy and subsequent OLT. The surgical staging procedure is mandatory before OLT. Careful selection of patients with cholangiocarcinoma for the liver transplant regimen is critical for the potential chance of long-term cure.
Neoadjuvant treatments, including EBRT, brachytherapy, and chemotherapy, are important components of the transplant regimen, as results are poor with transplant alone.13,14 In the University of Cincinnati experience,13 the 5-year survival was only 23% with transplant alone, and 51% of patients had tumor recurrence after transplant. The majority (84%) of the recurrences was within 2 years, and 76% of the deaths occurred within 6 months of transplant. The Spanish group used OLT alone,14 which similarly yielded a limited 5-year survival rate of 30%. Tumor recurred in 53% of the patients with hilar cholangiocarcinoma, which was the main reason for patient death. In 10 patients who underwent OLT and were found to have incidental cholangiocarcinoma in their explants, the median overall survival was 2.5 years.15 None of them received neoadjuvant chemotherapy or radiotherapy.
In our study, survival was poor among patients who were initially eligible for the transplant regimen and began neoadjuvant therapy but later became ineligible for OLT. Although male patients had significantly better survival in this group (P=0.03, Table 3), median survival was improved by only 0.9 months. The reason for this very modest improvement in outcome is unknown; it is possible that it is a chance observation in the context of an exploratory subset analysis. Most commonly, the patients became ineligible for OLT as a result of adverse findings at surgical staging. This highlights the critical importance of staging laparotomy for detection of patients unlikely to benefit from transplant. It seems likely that patients found to be unsuitable for transplant following staging laparotomy have either more rapidly progressive disease or are in a later phase in the natural history of their disease, compared with patients who go on to transplant. In this group of transplant-ineligible patients, the median overall survival was 12.3 months, with a median survival after fallout of only 6.8 months. Two patients were long-term survivors despite adverse findings at the staging operation (one had positive pericholedochal lymph nodes and omental metastases, and the other had positive hepatic artery lymph nodes). Patients who fell out as a result of adverse findings at operative staging lived a median period of 12.1 months, which was comparable or slightly better than the survival of patients with locally advanced or metastatic cholangiocarcinoma documented in prospective trials.11,16,17 This is an important observation, as the use of neoadjuvant therapy in this group of patients who were destined to fall out from the OLT regimen did not appear to shorten their expected survival. Palliative treatment after fallout was at the discretion of the treating physician. Until recently, there has been no established standard of care in this regard. In 2010, a phase 3 clinical trial showed that a combination of gemcitabine and cisplatin improves survival among selected patients with incurable bile duct cancer.11 This regimen should be strongly considered for patients with incurable bile duct cancer as it is the only regimen supported by level 1 evidence.
At the time of enrollment to the clinical OLT regimen, is there any prognosticator that may predict which patients will become ineligible for liver transplant? Since 2003, we have used endoscopic ultrasound with aspiration of regional lymph nodes prior to neoadjuvant therapy, which has reduced the positive staging exploration rate to 15%. In addition, our recent experience showed that pretreatment pathologic confirmation of cholangiocarcinoma is associated with a higher rate of positive findings at the staging operation and transplant ineligibility; however, for patients who could successfully complete transplant, the recurrence rate was not different.6 In our group of transplant-fallout patients with or without tissue diagnosis, no difference was found in overall survival (P=0.90). For all our patients undergoing the transplant regimen, older age, high CA 19-9 level, residual tumor >2 cm in the explant, and tumor grade predicted a higher chance of recurrence.18 Advanced stage of disease was an independent poor prognosticator for both intrahepatic and extrahepatic cholangiocarcinoma.19
Surgical staging remains a critical part of our clinical transplant regimen. Eleven (92%) of 12 patients with local tumor progression were diagnosed at surgical staging, and 38 patients (60%) who fell out from the transplant regimen were due to adverse findings discovered at the staging operation. The finding at operative staging of nodal involvement was the most common reason for exclusion from OLT after surgical staging, occurring in 19 (50%) of 38 patients.
CONCLUSIONS
In highly selected patients with cholangiocarcinoma initially suitable for liver transplant, the mortality rate was high, as expected for patients who subsequently became ineligible for transplant. The most common reason for patient fallout was adverse findings at the staging operation, which highlights the importance of the surgical staging before liver transplant to optimize the allocation of limited organ resources to those who would most likely benefit from this combined-modality approach, as well as the need to improve the efficacy of neoadjuvant therapy. The survival for patients who fell out prior to OLT was comparable to other patients with locally advanced and metastatic cholangiocarcinoma treated with best nontransplant-based therapies. As neoadjuvant therapy prior to transplant did not appear to hurt patients who were destined to fall out at operative staging, we should consider being more inclusive in choosing patients (ie, less selective) for the Mayo Clinic transplant regimen in the future.
Footnotes
Portions of this work were presented in abstract form at the 54th annual meeting of the American Society for Therapeutic Radiology and Oncology, Boston, MA, October 28 to 31, 2012.
The authors declare no conflicts of interest.
REFERENCES
- 1.Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl Int. 2010;23:692–697. [DOI] [PubMed] [Google Scholar]
- 2.Rea DJ, Rosen CB, Nagorney DM, et al. Transplantation for cholangiocarcinoma: when and for whom? Surg Oncol Clin N Am. 2009;18:325–337. [DOI] [PubMed] [Google Scholar]
- 3.Heimbach JK, Gores GJ, Nagorney DM, et al. Liver transplantation for perihilar cholangiocarcinoma after aggressive neoadjuvant therapy: a new paradigm for liver and biliary malignancies? Surgery. 2006;140:331–334. [DOI] [PubMed] [Google Scholar]
- 4.Simmons DT, Baron TH, Petersen BT, et al. A novel endoscopic approach to brachytherapy in the management of Hilar cholangiocarcinoma. Am J Gastroenterol. 2006;101:1792–1796. [DOI] [PubMed] [Google Scholar]
- 5.Bhat M, Hathcock M, Kremers WK, et al. Presence of portal vein encasement predicts efficacy of radiation therapy in the liver transplantation for perihilar cholangiocarcinoma protocol. Poster session presented at: the 64th annual meeting of the American Association for the Study of Liver Diseases: the Liver Meeting, 2013 November 1-5; Washington, DC.
- 6.Rosen CB, Darwish Murad S, Heimbach JK, et al. Neoadjuvant therapy and liver transplantation for hilar cholangiocarcinoma: is pretreatment pathological confirmation of diagnosis necessary? J Am Coll Surg. 2012;215:31–38. [DOI] [PubMed] [Google Scholar]
- 7.Gores GJ, Nagorney DM, Rosen CB. Cholangiocarcinoma: is transplantation an option? For whom? J Hepatol. 2007;47:455–459. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 9.Alden ME, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer. Int J Radiat Oncol Biol Phys. 1994;28:945–951. [DOI] [PubMed] [Google Scholar]
- 10.Crane CH, Macdonald KO, Vauthey JN, et al. Limitations of conventional doses of chemoradiation for unresectable biliary cancer. Int J Radiat Oncol Biol Phys. 2002;53:969–974. [DOI] [PubMed] [Google Scholar]
- 11.Valle J, Wasan H, Palmer DH, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. [DOI] [PubMed] [Google Scholar]
- 12.Jan YY, Yeh CN, Yeh TS, et al. Prognostic analysis of surgical treatment of peripheral cholangiocarcinoma: two decades of experience at Chang Gung Memorial Hospital. World J Gastroenterol. 2005;11:1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633–1637. [DOI] [PubMed] [Google Scholar]
- 14.Robles R, Figueras J, Turrion VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghali P, Marotta PJ, Yoshida EM, et al. Liver transplantation for incidental cholangiocarcinoma: analysis of the Canadian experience. Liver Transpl. 2005;11:1412–1416. [DOI] [PubMed] [Google Scholar]
- 16.Goulart BH, Martins RG, Lynch TJ. Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering results. J Clin Oncol. 2001;19:4089. [PubMed] [Google Scholar]
- 17.Gebbia V, Giuliani F, Maiello E, et al. Treatment of inoperable and/or metastatic biliary tree carcinomas with single-agent gemcitabine or in combination with levofolinic acid and infusional fluorouracil: results of a multicenter phase II study. J Clin Oncol. 2001;19:4089–4091. [DOI] [PubMed] [Google Scholar]
- 18.Heimbach JK, Gores GJ, Haddock MG, et al. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703–1707. [DOI] [PubMed] [Google Scholar]
- 19.Singal AG, Rakoski MO, Salgia R, et al. The clinical presentation and prognostic factors for intrahepatic and extrahepatic cholangiocarcinoma in a tertiary care centre. Aliment Pharmacol Ther. 2010;31:625–633. [DOI] [PubMed] [Google Scholar]


