Abstract
Objective
The objective of this study was to use a well-established monkey model of atherosclerosis to determine how life stage and preexisting atherosclerosis influences the effectiveness of high isoflavone soy diet to inhibit the progression of atherosclerosis.
Methods
Premenopausal monkeys were fed for 34 months an atherogenic diet deriving its protein primarily from either animal sources (casein/lactalbumin (CL), n=37) or high isoflavone soy beans (Soy, n=34). Animals were then ovariectomized (OVX) and randomized to groups consuming the same diet (groups CL-CL, n=20 and Soy-Soy, n=17) or the alternate diet for an additional 34 months (groups CL-Soy, n=17 and Soy-CL=17). At ovariectomy, the left common iliac artery was removed to determine the amount of premenopausal atherosclerosis. At necropsy, the right common iliac (RCI) and coronary arteries were collected and atherosclerosis extent was quantified. The CL-CL condition was considered ‘control’.
Results
Modeling Asian women that remain in Asia, monkeys consuming soy protein both pre and postmenopausally had markedly reduced extent of coronary artery atherosclerosis relative to CL controls (p=0.008). The subset of animals intended to model Asian women that migrate to a Western country (consuming soy premenopausally and CL postmenopausally) had increased progression of postmenopausal iliac artery atherosclerosis (p=0.003), and were not protected against the development of coronary artery atherosclerosis relative to controls. Relevant to the administration of soy diets to postmenopausal Western women, the monkeys fed CL premenopausally and changed postmenopausally to soy, derived atheroprotective benefits only if they began the postmenopausal treatment period with relatively small (below the median) plaques. Relative to controls, this group (with small plaques at ovariectomy) had reduced progression of iliac atherosclerosis (p=0.038) and smaller coronary artery plaques (p=0.0001) that were less complicated (p=0.05) relative to controls.
Conclusions
The results suggest that significant atheroprotective benefits of dietary soy follow from treatment that begins premenopausally and continues postmenopausally, or if started during the early postmenopause among individuals whose plaques are still small.
Keywords: Soy diets, isoflavones, atherosclerosis, menopause
INTRODUCTION
Morbidity and mortality rates due to cardiovascular disease (CVD) in women are lower in Asian compared to Western countries. It has been proposed that the consumption of diets high in soy protein characteristic of traditional foods in Japan and China, may contribute to such cardioprotection 1;2. Additionally, epidemiological studies have demonstrated that Asian women migrating to a Western country develop a CVD burden similar to that of Western women, perhaps due to changes in diet that include increased consumption of animal protein and decreased consumption of soy/soy isoflavones 3;4. Isolated soy protein differs from animal proteins in terms of peptides, proteins, the overall amino acid composition, the elevated content of isoflavones, as well as other constituents, each of which may have biologic effects in vivo. The atheroprotective effects of dietary soy appear to be only minimally attributable to effects on plasma lipids (5) and we have evidence from studies of isoflavone-rich soy vs. isoflavone-poor soy (alcohol extracted) that soy isoflavones have atheroprotective effects 6. However, there are no data describing the atherosclerosis burden of women that consume soy premenopausally and continue to do so postmenopausally in comparison to those who may have consumed animal protein during premenopause and then were supplemented with soy postmenopausally. Results from the Women’s Isoflavone Soy Health (WISH) randomized clinical trial on the effects of soy supplementation on the progression of subclinical atherosclerosis (carotid intima media thickness; CIMT) in postmenopausal women 45 to 92 years of age7 suggest that soy supplementation did not significantly reduce atherosclerosis progression across the all individuals, but was effective in a subset of women who were <5 years postmenopausal. In addition, a small group of Asian women treated with soy had a borderline significant reduction of atherosclerosis progression.
There are several unanswered questions concerning the effect of life stage on the cardiovascular effects of soy supplementation. In the present study we attempted to model those questions by evaluating the progression of atherosclerosis in pre-and surgically postmenopausal cynomolgus monkeys consuming a moderately atherogenic diet that derived the majority of its protein from either soy or a casein-lactalbumin (CL) mixture. Specifically, we addressed the following questions: 1) To what extent does life stage/menopausal status influence atheroprotective effects of dietary soy? 2) What are the effects on atherosclerosis progression of consuming soy premenopausally and changing to a more Western diet (animal protein) postmenopausally? 3) Does postmenopausal soy supplementation provide atheroprotective effects for subjects that have consumed a Western diet (animal protein) premenopausally?
METHODS
Monkeys, diets and study design
One hundred adult female cynomolgus macaques (Macaca fascicularis) were imported from Indonesia (Institude Pertanian Bogor). Ninety-five monkeys began the study and were randomized to eat either an atherogenic control diet containing proteins derived from casein-lactalbumin (CL, n=43) or an atherogenic diet containing soy protein isolate (Soy, n=42) as the primary source of protein for 34 months. Details about the animals and diets have been published previously8. Briefly, both diets were formulated to be identical in caloric content of protein, fat, carbohydrates and cholesterol (0.28 mg cholesterol/Cal) and to mimic a typical diet consumed by women in the United States. After 34 premenopausal months diet, animals were ovariectomized and immediately randomized either to continue to be fed CL o Soy, or fed the other diet (CL or Soy) for the subsequent 34 months (Figure 1). At the time of ovariectomy, a segment of the left common iliac artery (LCI) was removed surgically in order to determine the amount of atherosclerosis present at the end of the premenopausal phase (postmenopausal baseline, PBL)8. The designations of the dietary regimens are: Control group consuming casein-lactalbumin pre- and post- menopausally (CL-CL, n=20), a group consuming Soy pre- and post- menopausally (Soy-Soy, n=17); a group consuming soy premenopausally and CL postmenopausally (Soy-CL, n=17); and a group consuming CL premenopausally and Soy postmenopausally (CL-Soy, n=17). The subjects for the subset analyses were fed CL-Soy and were divided at the median of the premenopausal baseline plaque size. Only those monkeys which completed the study and from which we have complete data, were considered eligible for the analysis.
Figure 1.
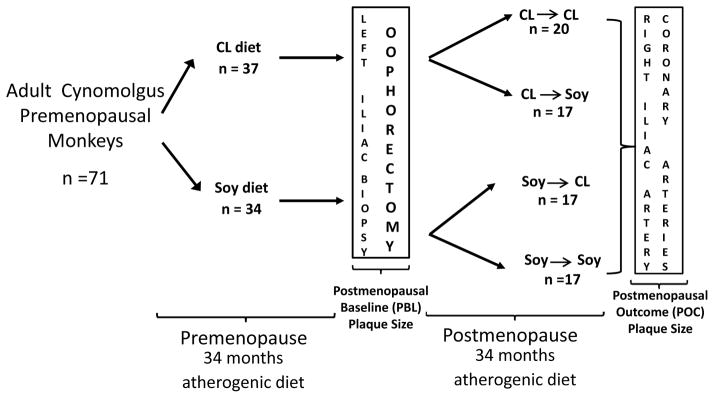
Randomized monkey trial study design. CL diet (n=37), Casein-Lactalbumin (CL) protein based atherogenic diet (0.28 mg cholesterol/Cal); Soy diet (n=34), soy protein based atherogenic diet (0.28 mg Cholesterol/Cal) (treatment diet). CL-CL (Control Group, n=20), animals fed CL diet pre and postmenopausally; Soy-Soy (n=17), animals fed Soy diet pre and postmenopausally; Soy-CL (n=17), animals fed Soy diet premenopausally and were switched to eat CL diet postmenopausally; CL-Soy (n=17), animals fed CL diet premenopausally and were switched to eat Soy diet postmenopausally.
All procedures involving animals were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services and guidelines established by the Wake Forest University Animal Care and Use Committee.
Plasma lipid measurements
Total plasma cholesterol (TPC), high density lipoprotein cholesterol (HDL-c), very low density and low density lipoprotein cholesterol (VLDL-c + LDL-c) and triglycerides (TG) concentrations were determined at 10, 21, 30 months after ovariectomy at the Comparative Medicine Research Center Clinical Chemistry Laboratory. An average of the three measurements is reported. Details about the methods used to evaluate plasma lipid and lipoprotein concentrations as well as lipid profiles during the premenopausal period have been published previously5.
Serum isoflavone measurements
During the postmenopausal period, monkeys were fed in the morning and then were sedated 4 hours after feeding for blood collection. Serum isoflavone concentrations were determined at 12 and 23 months postmenopause by liquid chromatographic-photodiode array mass spectrometric analysis9. Plasma concentrations during the premenopausal stage have been published previously8.
Measurements of plaque progression
To estimate changes in plaque size and severity from the postmenopausal baseline (PBL) for individual monkeys, we used a paired artery, the left iliac artery (LCI), shown previously to have essentially the same plaque sizes in both the left and right arteries and to be highly associated with coronary artery plaque extent6;10. Briefly, monkeys were anesthetized with an intramuscular injection of ketamine (10 mg/kg) followed by isoflurane gas. The LCI was approached by abdominal incision and blunt dissected from the vein. After proximal and distal ligatures were placed in the LCI, a ~0.5 cm sections were excised from each animal. The artery sections were cleaned from adventitial tissue, opened longitudinally, and a portion was fixed in 4% paraformaldehyde and embedded in a paraffin block. Atherosclerotic plaque size PBL was determined in the LCI removed at the time of ovariectomy using the methods described below. Atherosclerosis and gene expression outcomes from this baseline iliac artery have previously published8.
Necropsy procedure
After 34 months of postmenopausal diet, monkeys were euthanized using sodium pentobarbital (100mg/kg, IV), a method consistent with the recommendations of the panel on euthanasia of the American Veterinary Medical Association. The circulatory system was flushed with lactated Ringers. The heart was removed and perfusion fixed for 1 h at 100 mm/Hg pressure using 10% neutral buffered formalin. The right common iliac artery (RCI) (postmenopausal outcome [POC]) and the coronary arteries were collected for further histopathologic evaluation. The RCI was opened longitudinally, laid flat on cardboard, and a portion was immersion fixed in 10% neutral buffered formalin. Each coronary artery (left anterior descending [LAD], left circumflex artery [LCA], right coronary artery [RCA]) was sectioned in 5 blocks (3 mm each in length) and cut perpendicular to the long axis of the arteries. Five-μm sections were made from each block and stained with Verhoeff-van Gieson’s stain. Each section was captured digitally using a Nikon DS Fi1 camera mounted on an Olympus BH-2 microscope equipped with a mechanical stage. Morphometric measurements were made using Image-Pro Plus version 7.0 imaging software (Media Cybernetics, Inc., Bethesda, MD).
Plaque extent and AHA severity grades
Plaque extent was expressed as the cross-sectional intimal area (IA, mm2). For the coronary arteries, a mean of the intimal areas of the 15 blocks (mean IA) was reported as previously described11. Because plaque complications occur independently from plaque extent, lesions were also scored for severity using the American Heart Association guidelines12 for severity on a 0 to V scale: a) ‘0’ = arteries with no lesion; b) ‘I’ = Intimal lesions that consisted of smooth muscle adaptive thickening; c) ‘II’ = smooth muscle adaptive thickening containing macrophage foam cells; d) ‘III’ = necrosis of macrophages and the accumulation of small pools of extracellular lipid; e) ‘IV’ = lesions in which the small pools of extracellular lipid had coalesced into a core of extracellular lipid; and f)’V’ = the core of extracellular lipid was separated from the lumen by a definitive fibromuscular cap. Grades were determined blind to treatment by three independent observers.
Statistical Analyses
General
Measurements of plaque extent of LCI, RCI and coronaries were square root transformed so that the distribution of each measure would be closely conformed to a normal distribution. The Δ plaque extent of common iliac arteries was calculated subtracting the square rooted right common iliac plaque extent [outcome] from the squared rooted left common iliac plaque extent [baseline]. The averages of the change (Δ) values for each condition are presented as mean ± SEM on the original measurement scale based on back transformation. One-way analysis of variance (ANOVA) was used to compare Δ plaque extent of iliac arteries (mm2) and coronary plaque extent (mm2) of each condition to the control group (CL-CL group). Fisher’s exact test was used to evaluate the frequency of AHA grades (plaque severity), comparing each condition to control (CL-CL group) in terms of the distribution of minimally affected (0, I, II) vs. more severely affected (≥III) lesions. The p-values were adjusted for multiple comparisons using a Bonferroni correction.
Subgroup Analyses
The finding in the WISH Trial that women less than 5 years postmenopausal experienced reduced rates of progression of carotid artery intima-media thickness suggested to us that their favorable outcome may have been because their atherosclerosis was less extensive (plaques smaller) when the soy supplement was administered. Consequently, we conducted subset analyses on monkeys that were fed CL diet premenopausally. Here, monkeys were divided at the median based on premenopausal plaque extent (PBL); in each such subgroup, coronary artery atherosclerosis extent and severity and the Δ common iliac atherosclerosis in monkeys fed the CL diet were compared to those supplemented with soy diet postmenopausally. Outcome conditions were compared using T-test analysis. A p-value of <0.05 was considered statistically significant. Both general and subgroup analyses were performed using SPSS 11.5 software (SPSS Inc., Chicago, IL).
RESULTS
Plasma lipid and lipoprotein concentrations
The mean plasma lipid and lipoprotein concentrations for the four groups over the postmenopausal period are presented in Table 1. For all variables, except for plasma TG, beneficial effects (lowered TPC and LDL+VLDLc and increased HDLc) were observed in the CL-Soy and Soy-Soy conditions compared to CL-CL group. The Soy-CL condition was not statistically different from the CL-CL condition.
Table 1.
Effect of diet on postmenopausal cardiovascular risk factors
| Study Group | n | TPC | p-value | HDL-c | p-value | VLDL+LDL-c | p-value | TPC:HDL | p-value | TG | p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CL-CL | 20 | 358 ± 23 | - | 39 ± 3 | - | 319 ± 24 | - | 10.5 ± 1.1 | - | 30 ± 2 | - |
| Soy-Soy | 17 | 279 ± 16 | 0.038 | 69 ± 5 | <0.0001 | 211 ± 18 | <0.0001 | 3.71 ± 0.3 | <0.0001 | 43 ± 4 | 0.91 |
| Soy-CL | 17 | 345 ± 26 | 0.846 | 38 ± 3 | 0.700 | 306 ± 28 | 1.000 | 7.5 ± 0.8 | 0.701 | 35 ± 5 | 0.693 |
| CL-Soy | 17 | 255 ± 20 | 0.004 | 68 ± 6 | <0.0001 | 184 ± 23 | <0.0001 | 4.5 ± 0.6 | <0.0001 | 46 ± 11 | 0.112 |
Data are presented as mean ± SEM. p-values represent group differences relative to CL-CL (Control group). CL-CL (n=20), animals fed CL diet pre and postmenopausally; Soy-Soy (n=17), animals fed Soy diet pre and postmenopausally; Soy-CL (n=17), animals fed Soy diet premenopausally and were switched to eat CL diet postmenopausally; CL-Soy (n-17), animals fed CL diet premenopausally and were switched to eat Soy diet postmenopausally. TPC, total plasma cholesterol, HDL-c, high-density lipoprotein cholesterol; VLDL + LDL-c, very low-density lipoprotein cholesterol + low-density lipoprotein cholesterol; TPC:HDL, ratio of TPC to HDL; TG, triglycerides
Serum isoflavone concentrations
Monkeys that were fed soy diet (CL-Soy and Soy-Soy conditions) had significantly elevated serum concentrations of the two primary isoflavones: genistein CL-Soy, 167 ± 20 nM, and Soy-Soy, 169 ± 23 nM; Daidzein CL-Soy, 183 ± 22 nM, and Soy-Soy, 206 ± 22 nM. Similarly, the serum concentration of the daidzein metabolite, Equol was elevated, CL-Soy 515 ± 108 nM, and Soy-Soy 516 ± 115 nM. There were no significant associations between the serum concentrations of the two primary isoflavones or equol on the progression of iliac artery atherosclerosis, or coronary artery atherosclerosis.
Postmenopausal Iliac artery atherosclerosis progression-all monkeys in the trial
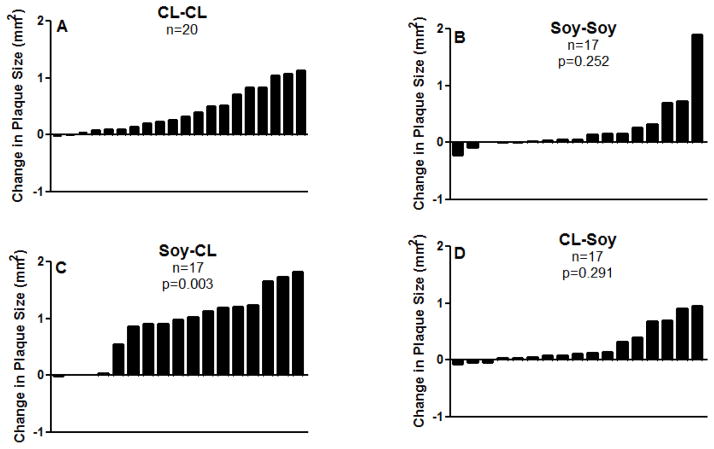
Postmenopausal atherosclerosis progression was determined as the difference in iliac artery plaque size from PBL to the end of the 34 month trail (i.e., Δ iliac artery atherosclerosis). Figure 2 illustrates the change in plaque size of all the monkeys in the trial. Changes in plaque size in the experimental conditions were compared to the CL-CL group. Monkeys consuming soy both pre and postmenopausally (Soy-Soy, Question 1) tended to have less plaque progression (Soy-Soy: 0.24 ± 0.11 vs. CL-CL: 0.42 ± 0.08 mm2) but the difference was not statistically significant (p=0.25). It is noteworthy, however, that among the Soy-Soy monkeys most animals had only trivial increases in plaque sizes. There was a clear increase in plaque progression of the Soy-CL fed monkeys (Question 2) (0.89 ± 0.14 vs. 0.42 ± 0.08 mm2, p=0.003). Although the average changes in plaque size of the monkeys in the CL- Soy (Question 3) condition was about half that of the CL-CL monkeys (CL-Soy: 0.26 ± 0.08 vs. CL-CL: 0.42 ± 0.08 mm2), the difference was not statistically significant (p=0.29).
Figure 2.
Change in common iliac plaque size (right common iliac [outcome] – left common iliac [baseline] plaque sizes in mm2) of individual animals in each treatment condition. A) CL-CL (n=20), animals fed CL diet pre and postmenopausally and served as ‘Control group”; B) Soy-Soy (n=17), animals fed Soy diet pre and postmenopausally; C) Soy-CL (n=17), animals fed Soy diet premenopausally and were switched to eat CL diet postmenopausally; D) CL-Soy(n=17), animals fed CL diet premenopausally and were switched to eat Soy diet postmenopausally. Variability is expressed as SEM. p-values compare each condition to the control condition (CL-CL).
Coronary artery atherosclerosis extent and severity-all monkeys in the trial
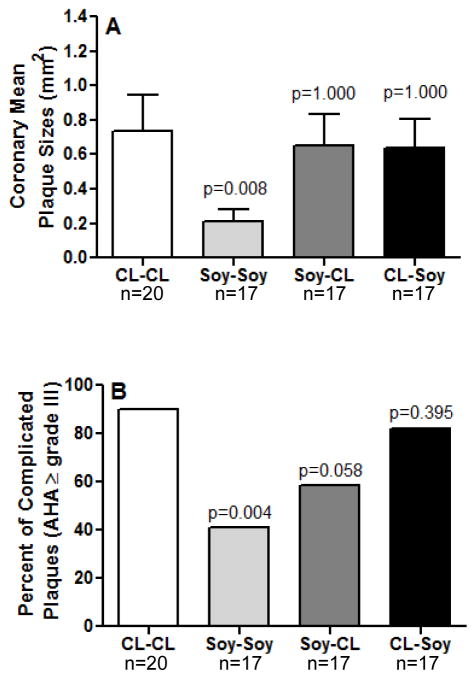
The extent of coronary artery atherosclerosis in the different treatment conditions is presented in Figure 3A. Overall, only monkeys consuming soy both pre- and postmenopausally (Soy-Soy) had significantly smaller mean plaque size (0.209 ± 0.071 mm2) compared to CL-CL controls (0.739 ± 0.204 mm2) (p= 0.008). Coronary plaque extent of monkeys in the Soy-CL (0.650 ± 0.186 mm2) and the CL-Soy (0.636 ± 0.167 mm2) groups were not significantly different from the CL-CL control group (0.739 ± 0.204 mm2).
Figure 3.
Analysis of coronary mean plaque sizes (A) and distribution of complicated plaques (AHA grade ≥ III) (B) by groups. CL-CL (control group, n=20), animals fed CL diet pre and postmenopausally; Soy-Soy (n=17), animals fed Soy diet pre and postmenopausally; Soy-CL (n=17), animals fed Soy diet premenopausally and were switched to eat CL diet postmenopausally; CL-Soy (n=17), animals fed CL diet premenopausally and were switched to eat soy diet postmenopausally. Variability is expressed as SEM. p-values compare each treatment group to the control condition (CL-CL).
The effects of soy on plaque severity are presented in figure 3B. Monkeys that consumed soy both pre- and postmenopausally (Soy-Soy) had a significantly lower proportion of complicated plaques (41.1%, 7/17) than did the CL-CL group (90%, 18/20) (p=0.004). Plaque severity scores of the CL-Soy and the Soy-CL groups were not statistically different from scores in the CL-CL group.
Subgroup analysis of iliac artery atherosclerosis progression
Figure 4 illustrates the differences in premenopausal and postmenopausal iliac artery atherosclerosis of the monkeys that consumed CL premenopausally and divided into subgroups based on having either small or large plaques at PBL. Among the monkeys with small plaques at ovariectomy, soy markedly reduced plaque progression (mean differences in plaque progression of 0.03 ± 0.02 vs. 0.30 ± 0.10 mm2, p=0.038); in contrast, among the monkeys with large plaques at PBL, soy did not significantly inhibit plaque progression (0.41 ± 0.11 vs. 0.56 ± 0.12 mm2, p=0.396). Among the monkeys fed CL postmenopausally, there was a non-significant tendency for the plaques to have progressed somewhat less if the plaques were small at PBL (0.30 ± 0.10 vs. 0.56 ± 0.12 mm2; p < 0.10).
Figure 4.
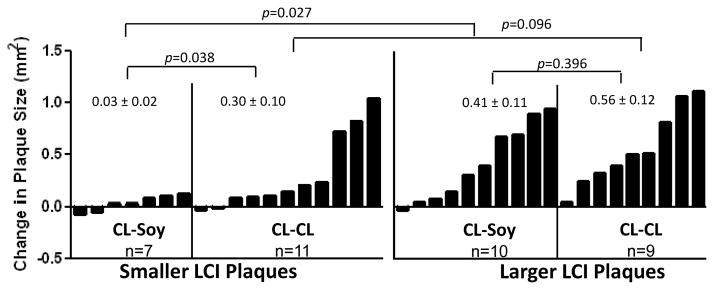
Subgroup analysis the change of iliac artery atherosclerosis (right common iliac [outcome] – left common iliac [baseline] plaque sizes in mm2). Animals were divided by the median based on pretreatment plaque size (LCI, left common iliac) into smaller or larger plaques and the change in atherosclerotic plaque size among animals fed CL and Soy were compared. Variability is expressed as SEM.
Subgroup analyses of coronary artery atherosclerosis extent and severity
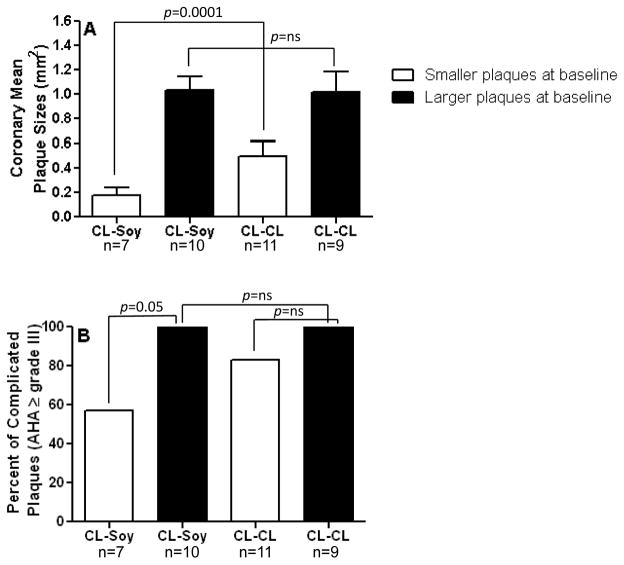
The effect of the CL-SOY diet treatment on coronary artery atherosclerosis in relation to pre-menopausal iliac artery plaque size is depicted in Figure 5A. Animals above and below the median in premenopausal plaque size differed substantially in coronary artery extent despite having the same pre- and post-menopausal diet treatment (CL-SOY: mean plaque sizes of 0.17 ± 0.06 vs. 1.03 ± 0.16 mm2). Notably, while monkeys below the median for iliac artery atherosclerosis had relatively small coronary plaques irrespective of postmenopausal consumption, the lesions in monkeys consuming the CL-SOY were significantly smaller than those consuming the CL-CL diet (mean plaque sizes of 0.49 ± 0.12 CL-small vs. 0.17 ± 0.06 Soy-small, p=0.0001). In contrast to the beneficial effects of soy on the monkeys with small pretreatment plaques, no beneficial effect was seen among those with large pretreatment plaques (mean plaque sizes for the soy group of 1.03 ± 0.10 mm2 and for the CL group, 1.01 ± 0.16 mm2).
Figure 5. Effect of soy on plaque progression is dependent on baseline (pre-ovariectomy) plaque extent.
Subgroup analysis of coronary mean plaque extent (A) and distribution of complicated plaques (AHA grade ≥ III) (B) are depicted. Animals from the CL-Soy group were divided by the median based on pretreatment (prior to ovariectomy) common iliac plaque size into animals with smaller (open bars) or larger plaques (solid bars). Coronary artery atherosclerosis extent is expressed as mean ± SEM and plaques AHA grade ≥ III were expressed a percent.
The effect of pretreatment iliac artery plaque size on the frequency of occurrence of complicated plaques is presented in Figure 5B. The soy diet significantly reduced the percent of coronary artery plaques that were complicated among those monkeys with small pretreatment plaques; whereas, no such beneficial effect was seen among those monkeys with small pretreatment plaques and that continued to be fed CL. Notably, the occurrence of complicated plaques was the same for soy and CL fed monkeys with large plaques at PBL.
DISCUSSION
The results of our study addressed three critical questions: 1) to what extent is pre- and post-menopausal soy consumption atheroprotective? Answer – coronary artery atherosclerosis extent and severity were significantly inhibited in those monkeys that were fed soy both pre and postmenopausally; 2) what are the effects on atherosclerosis progression of consuming soy premenopausally and changing to a more Western diet postmenopausally? Answer – atherosclerosis progression in the iliac arteries and coronary artery atherosclerosis in animals initiating CL diet postmenopausally were not different from that of those consuming the CL diet pre- and postmenopausally; 3) does postmenopausal soy supplementation provide atheroprotective effects for subjects that have consumed a Western diet premenopausally? Answer- postmenopausal soy consumption inhibited atherosclerosis in both the common iliac and the coronary arteries only in those monkeys with relatively small plaques at the start of the postmenopausal treatment phase (i.e., those that were below the median).
Among other results, postmenopausal soy treatment, relative to CL exposure, had a robust beneficial effect on plasma lipid profiles. The favorable outcome on plasma lipid profiles was observed irrespective of the premenopausal diet 5. The effects of soy intervention on the plasma lipid profiles in this study were larger than reported to occur when soy supplements are given to women. Several well controlled studies have concluded that soy supplementation results in limited reductions in TPC concentrations, primarily in the LDL-c fraction, and no changes in HDL-c concentrations13–15. That same result was noted in the WISH trial7. One potential explanation for the difference in the responsiveness to soy between our monkey model and women is the amount of soy consumed, as over 80% of the protein in the soy diet was derived from soy. The 25g/day received by the women would only account for about 54% or so of their daily protein requirements of 46 g/day (CDC, http://www.cdc.gov/nutrition/everyone/basics/protein.html). Much attention has been focused on the fact that cynomolgus monkeys are efficient at converting daidzein to equol, whereas only a fraction (25–40%) of women are significant equol producers 16. However, we previously observed no effect of dietary equol on plasma lipids in ovariectomized monkeys, suggesting that the differing effects of soy on plasma lipids in women and monkeys are not dependent upon equol17. Further, we found no association between equol production among the monkeys consuming soy in this postmenopausal phase and any of the outcome measures. Additionally, the WISH trial found that the effects of soy supplementation on CIMT were the similar in equol producers and their non-converting counterparts7.
The WISH trial focused on the effects of dietary soy on carotid artery intima-medial thickness. The current results extend the WISH trial by including measures of atherosclerosis extent in coronary arteries as well as at two time points in the iliac arteries, a surrogate measure shown in prior studies to be highly correlated with coronary atherosclerosis extent5;10;18. The present study also controlled soy protein exposure (in the context of an atherogenic diet that remained relatively high in saturated fat and cholesterol) and in premenopausal as well as surgically-postmenopausal conditions, allowing atherosclerosis progression under different soy regimens to be compared. The translational relevance of atherosclerosis in the four treatment conditions seems clear: 1) the CL-CL (control) condition represents women who habitually consume a typical American diet for their entire lives; 2) the Soy-Soy condition represents women who habitually consume a typical Asian diet across their lifespan; 3) the CL-Soy condition represents women who begin consuming soy at menopause, and 4) the Soy-CL potentially reflects the experience of women who migrate from an Asian diet to a typical American diet.
In view of the beneficial effects on the plasma lipid profiles of postmenopausal soy diets in this trial, it was unexpected that there would be no overall inhibition of iliac artery atherosclerosis progression regardless of their premenopausal diet. Again, considering the overall analysis, only the monkeys that received soy both pre and postmenopausally had less extensive and severe coronary artery atherosclerosis.
While the measures of iliac artery atherosclerosis at the beginning and end of the postmenopausal phase allow one to estimate change in atherosclerosis over time, the interpretation of the coronary outcome is limited by the absence of a direct measure of coronary atherosclerosis at surgical menopause. Considering all treatment conditions, the only significant effect on the coronary arteries (in both extent and severity) was in those animals that consumed soy both pre and postmenopausally (i.e., perhaps comparable to Asian women who continue consumption of a diet high in soy). It is noteworthy that the coronary outcome is discordant with the beneficial postmenopausal effect of Soy on the lipids of the monkeys in the CL-Soy condition (marked increases in HDL-c and reductions in VLDL+LDL-c). The lack of benefit of post-menopausal soy on lesion size could be explained by previous findings that atherosclerotic lesion size does not necessarily decrease in response to plasma lipid lowering19, although changes in lesion lipid and connective tissue content can occur20.
We determined whether there was a subset of surgically postmenopausal monkeys that derived cardiovascular benefits from consuming the soy diet. As indicated, we conducted a subgroup analysis that focused only on animals that – like many American women – consumed the CL diet premenopausally. Among those individuals, postmenopausal Soy treatment significantly inhibited the progression of both iliac artery and coronary artery atherosclerosis, but only in animals that started with relatively small lesions (below the median as indicated by iliac artery atherosclerosis determined at surgical menopause). The results of our subgroup analyses suggest clearly that the stage of plaque progression premenopausally is a major determinant of the potential cardiovascular benefits of postmenopausal soy supplementation; a situation that may be comparable to the loss of beneficial effects from estradiol when plaques progressed to the complicated stage. We have described in great detail this relative loss of the beneficial effects of estradiol in the presence of established lesions 21, now commonly referred to as the “Timing Hypothesis”. Interestingly, Hodis et al.7 speculated recently that the timing hypothesis may relate to phytoestrogens (isoflavones) in the same way as the estradiol effect (loss of estrogen receptors with plaque progression). Relative to the finding of an atheroprotective effect of soy diets fed to premenopausal monkeys, Walker et al. observed that arterial ER alpha expression was correlated with serum genistein concentrations (r=0.45, p=0.005) and ER beta was correlated with serum concentrations of daidzein (r=0.39, p=0.015)8. Their study suggested that the premenopausal atheroprotective effects of soy may relate to interactions occurring between the isoflavones, the estrogen receptors and NF-kB. That the lack of effect of soy diets on monkeys with relatively large (above the median) plaques may likely relate to the loss of arterial estrogen receptors, as ER protein has been found to be reduced in atherosclerotic human arteries, to be negatively correlated with atherosclerosis in postmenopausal women 22 and most importantly, ER expression is inversely associated with plaque size in both male and female monkeys5;8.
Postmenopausal serum isoflavones were not associated with atherosclerosis. It is important to point out some aspects of the isoflavone measures. Serum concentrations of the isoflavones were measured in blood samples collected 4 hours post-feeding at 2 time points during the menopausal phase. We do not therefore have total exposure, or area under the curve of isoflavone exposure, with which to do these analyses. We also do not have data on tissue levels of isoflavones which could differ significantly from the blood levels and play a role in the biologic effects. Lastly, the proteins and peptides in soy also have bioactivity, and could have atheroprotective effects 24. More research needs to be done to address these possibilities.
Conclusions and potential clinical value
The results of this preclinical trial are consistent with the results of the WISH trial and extend the observations of that trial to include the coronary arteries. Overall, the extent and severity of the atherosclerotic lesions among these monkeys at the initiation of the postmenopausal phase was generally comparable to the extent and severity of atherosclerosis that has been reported in women 45 to 92 years. Considering the group as a whole, protective effects of soy were observed premenopausally 8 as well as postmenopausally in those subjects that continued to consume soy postmenopausally.
The finding of most clinical/public health significance in this preclinical trial was that pretreatment atherosclerosis stage is a determinant of the potential atheroprotective effect of soy treatment, a finding that echoed the outcome of the WISH trial. In considering the potential cardiovascular benefits of soy isoflavones, the healthcare provider must weight carefully the potential benefits as opposed to potential adverse effects, such as the recent findings pertaining to general intelligence indices23. These data suggest that cardiovascular benefits of soy supplements are greatest when treatment is initiated premenopausally and continues postmenopausally, and may be beneficial in the very early postmenopause while plaques are usually still small.
Acknowledgments
Source of Funding: This project was supported by National Institutes of Health Grants HL45666 and HL079421 (Jay R. Kaplan)
Footnotes
Conflicts of Interest/Financial Disclosure: G.C.M. received support as a Wake-Merck Cardiovascular Research Fellow supported by an education grant from Merck Pharmaceuticals.
Contributor Information
Giselle C. Meléndez, Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
Thomas C. Register, Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
Susan E. Appt, Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
Thomas B. Clarkson, Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
Adrian A. Franke, University of Hawaii Cancer Center, Honolulu, Hawaii
Jay R. Kaplan, Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
References
- 1.Zhang X, Shu XO, Gao YT, Yang G, Li Q, Li H, Jin F, Zheng W. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J Nutr. 2003 Sep;133(9):2874–8. doi: 10.1093/jn/133.9.2874. [DOI] [PubMed] [Google Scholar]
- 2.Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation. 2007 Nov 27;116(22):2553–62. doi: 10.1161/CIRCULATIONAHA.106.683755. [DOI] [PubMed] [Google Scholar]
- 3.Moriguchi EH, Moriguchi Y, Yamori Y. Impact of diet on the cardiovascular risk profile of Japanese immigrants living in Brazil: contributions of World Health Organization CARDIAC and MONALISA studies. Clin Exp Pharmacol Physiol. 2004 Dec;31(Suppl 2):S5–S7. doi: 10.1111/j.1440-1681.2004.04119.x. [DOI] [PubMed] [Google Scholar]
- 4.Tsugane S, Gotlieb SL, Laurenti R, Souza JM, Watanabe S. Mortality and cause of death among first-generation Japanese in Sao Paulo, Brazil. Int J Epidemiol. 1989 Sep;18(3):647–51. doi: 10.1093/ije/18.3.647. [DOI] [PubMed] [Google Scholar]
- 5.Walker SE, Register TC, Appt SE, Adams MR, Clarkson TB, Chen H, Isom S, Franke AA, Kaplan JR. Plasma lipid-dependent and -independent effects of dietary soy protein and social status on atherogenesis in premenopausal monkeys: implications for postmenopausal atherosclerosis burden. Menopause. 2008 Sep;15(5):950–7. doi: 10.1097/gme.0b013e3181612cef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001 Jan;86(1):41–7. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 7.Hodis HN, Mack WJ, Kono N, Azen SP, Shoupe D, Hwang-Levine J, Petitti D, Whitfield-Maxwell L, Yan M, Franke AA, Selzer RH. Isoflavone soy protein supplementation and atherosclerosis progression in healthy postmenopausal women: a randomized controlled trial. Stroke. 2011 Nov;42(11):3168–75. doi: 10.1161/STROKEAHA.111.620831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker SE, Adams MR, Franke AA, Register TC. Effects of dietary soy protein on iliac and carotid artery atherosclerosis and gene expression in male monkeys. Atherosclerosis. 2008 Jan;196(1):106–13. doi: 10.1016/j.atherosclerosis.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franke AA, Custer LJ, Wilkens LR, Le Marchand LL, Nomura AM, Goodman MT, Kolonel LN. Liquid chromatographic-photodiode array mass spectrometric analysis of dietary phytoestrogens from human urine and blood. J Chromatogr B Analyt Technol Biomed Life Sci. 2002 Sep 25;777(1–2):45–59. doi: 10.1016/s1570-0232(02)00216-7. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan JR, Adams MR, Anthony MS, Morgan TM, Manuck SB, Clarkson TB. Dominant social status and contraceptive hormone treatment inhibit atherogenesis in premenopausal monkeys. Arterioscler Thromb Vasc Biol. 1995 Dec;15(12):2094–100. doi: 10.1161/01.atv.15.12.2094. [DOI] [PubMed] [Google Scholar]
- 11.Clarkson TB, Anthony MS, Mikkola TS, St Clair RW. Comparison of tibolone and conjugated equine estrogens effects on carotid artery atherosclerosis of postmenopausal monkeys. Stroke. 2002 Nov;33(11):2700–3. doi: 10.1161/01.str.0000033130.82164.24. [DOI] [PubMed] [Google Scholar]
- 12.Stary HC. Composition and classification of human atherosclerotic lesions. Virchows Arch A. Pathol Anat Histopathol. 1992;421(4):277–90. doi: 10.1007/BF01660974. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein AH, Jalbert SM, Adlercreutz H, Goldin BR, Rasmussen H, Schaefer EJ, Ausman LM. Lipoprotein response to diets high in soy or animal protein with and without isoflavones in moderately hypercholesterolemic subjects. Arterioscler Thromb Vasc Biol. 2002 Nov 1;22(11):1852–8. doi: 10.1161/01.atv.0000033513.18431.a1. [DOI] [PubMed] [Google Scholar]
- 14.Kreijkamp-Kaspers S, Kok L, Grobbee DE, de Haan EH, Aleman A, Lampe JW, van der Schouw YT. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. 2004 Jul 7;292(1):65–74. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- 15.Dewell A, Hollenbeck PL, Hollenbeck CB. Clinical review: a critical evaluation of the role of soy protein and isoflavone supplementation in the control of plasma cholesterol concentrations. J Clin Endocrinol Metab. 2006 Mar;91(3):772–80. doi: 10.1210/jc.2004-2350. [DOI] [PubMed] [Google Scholar]
- 16.Clarkson TB, Appt SE. Cardiovascular Effects of Dietary Soy. In: Watson Ronald Ross, Preedy Victor R., editors. Nutrition and Heart Disease. 1. Boca Raton, Florida: CRC Press; 2004. pp. 215–36. [Google Scholar]
- 17.Appt SE, Clarkson TB, Register TC, Chen H. Dietary equol does not reproduce effects of dietary soy on cardiovascular disease risk variables; observations from postmenopausal monkeys. The North American Menopause Society. 2005;66 [Google Scholar]
- 18.Kaplan JR, Manuck SB, Anthony MS, Clarkson TB. Premenopausal social status and hormone exposure predict postmenopausal atherosclerosis in female monkeys. Obstet Gynecol. 2002 Mar;99(3):381–8. doi: 10.1016/s0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- 19.Williams JK, Sukhova GK, Herrington DM, Libby P. Pravastatin has cholesterol-lowering independent effects on the artery wall of atherosclerotic monkeys. J Am Coll Cardiol. 1998 Mar 1;31(3):684–91. doi: 10.1016/s0735-1097(97)00537-8. [DOI] [PubMed] [Google Scholar]
- 20.Register TC, Jayo MJ, Jerome CP. Oral contraceptive treatment inhibits the normal acquisition of bone mineral in skeletally immature young adult female monkeys. Osteoporos Int. 1997;7(4):348–53. doi: 10.1007/BF01623776. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson TB, Melendez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause. 2013 Mar;20(3):342–53. doi: 10.1097/GME.0b013e3182843aad. [DOI] [PubMed] [Google Scholar]
- 22.Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 1994 Apr;89(4):1501–10. doi: 10.1161/01.cir.89.4.1501. [DOI] [PubMed] [Google Scholar]
- 23.St John JA, Henderson VW, Hodis HN, Kono N, McCleary CA, Franke AA, Mack WJ. Associations between urine excretion of isoflavonoids and cognition in postmenopausal women in the Women’s Isoflavone Soy Health clinical trial. J Am Geriatr Soc. 2014 Apr;62(4):629–35. doi: 10.1111/jgs.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams MR, Golden DL, Franke AA, Potter SM, Smith HS, Anthony MS. Dietary soy beta-conglycinin (7S globulin) inhibits atherosclerosis in mice. J Nutr. 2004 Mar;134(3):511–6. doi: 10.1093/jn/134.3.511. [DOI] [PubMed] [Google Scholar]