Abstract
Background
Abnormal regulation of extracellular signal-regulated kinases 1 and 2 (ERK) has been implicated in L-DOPA-induced dyskinesia (LID), a motor complication affecting Parkinson’s disease (PD) patients subjected to standard pharmacotherapy. We examined the involvement in LID of the mitogen- and stress-activated kinase 1 (MSK1), a downstream target of ERK and an important regulator of transcription.
Methods
MSK1 knockout (MSK1 KO) mice and ΔFosB- or ΔcJun-overexpressing transgenic mice were lesioned with 6-hydroxydopamine to produce a model of PD and assessed for LID following chronic L-DOPA administration. Biochemical processes were evaluated by Western blotting or immunoflourescence. Histone H3 phosphorylation was analyzed by chromatin immunoprecipitation (ChIP) followed by promotor-specific quantitative PCR.
Results
Genetic inactivation of MSK1 attenuated LID and reduced the phosphorylation of histone H3 at Ser10 in the striatum. ChIP analysis showed that this reduction occurred at the level of the fosB gene promoter. In line with this observation, the accumulation of ΔFosB produced by chronic L-DOPA was reduced in MSK1 KO. Moreover, inducible overexpression of ΔFosB in striatonigral medium spiny neurons exacerbated dyskinetic behavior, whereas overexpression of ΔcJun, which reduces ΔFosB-dependent transcriptional activation, counteracted LID.
Conclusions
These results indicate that abnormal regulation of MSK1 contributes to the development of LID and to the concomitant increase in striatal ΔFosB, which may occur via increased histone H3 phosphorylation at the fosB promoter. They also show that accumulation of ΔFosB in striatonigral neurons is causally related to the development of dyskinesia.
Keywords: Parkinson’s disease, dopamine D1 receptor, histone, mouse, striatum, medium spiny neurons
Introduction
Dyskinesia is a frequent and debilitating motor side effect produced in Parkinson’s disease (PD) patients by prolonged administration of L-DOPA (1). Several lines of evidence indicate that L-DOPA-induced dyskinesia (LID) is caused by long-term modifications of signaling in striatal neurons. These changes occur in concert with the loss of dopamine innervation and include a pronounced sensitization of dopamine D1 receptors (D1Rs) (2–4). In rodent and non-human primate models of PD, such sensitization confers to L-DOPA the ability to promote cAMP signaling and to activate the extracellular signal-regulated protein kinases 1 and 2 (ERK), which have been implicated in the development of dyskinetic behavior (5–11).
L-DOPA-induced activation of ERK is accompanied by increased phosphorylation of the mitogen- and stress-activated kinase 1 (MSK1) (8, 10). This effect occurs selectively in the GABAergic medium spiny neurons (MSNs) of the striatonigral pathway, which express D1Rs (8). The activation of MSK1 associated with LID leads to phosphorylation of histone H3 at Ser10 (5, 8, 10), which has been proposed to play a permissive role in gene expression (12–16). While it is possible that alterations in gene transcription produced by MSK1-mediated phosphorylation of histone H3 may contribute to the long-term changes involved in dyskinesia, there has to date been no investigation of the involvement of MSK1 in LID.
LID is accompanied by increased expression of several immediate early genes, including fosB and its truncated splice product, ΔFosB (17–20). The latter is a highly stable transcription factor, which in combination with JunD induces long-lasting effects by promoting the expression of several late response genes through binding to their activator protein-1 (AP1) consensus sites (21). Accumulation of ΔFosB has been linked to the development of dyskinetic behavior in animal models (17, 22, 23). Interestingly, inhibition of ERK signaling decreases the accumulation of ΔFosB induced by L-DOPA (24). However, the exact mechanism underlying this effect remains to be established.
In this study, we tested the hypothesis of an involvement of MSK1 in the accumulation of ΔFosB in response to repeated L-DOPA and in the concomitant development of dyskinesia.
Methods and Materials
Animals
Male MSK1 knock out (MSK1 KO) mice (25), mice overexpressing ΔFosB, mice overexpressing ΔcJun (see below) and wild type littermates were maintained in a 12 hrs light-dark cycle at a stable temperature of 22°C with food and water ad libitum. Male bitransgenic mice derived from NSE-tTA (line A) x TetOp-ΔFosB (line 11) and NSE-tTA (line A) x TetOp-FLAG-ΔcJun (line E) mice (26–28) were conceived and raised on 100 μg/ml doxycycline (Dox) to suppress ΔFosB or ΔcJun expression during development. Importantly, in line A, tTA expression is driven by the NSE promoter specifically in striatonigral MSNs, thereby generating mice in which ΔFosB and ΔcJun are selectively overexpressed in these neurons (26, 29). Littermates were divided at weaning: half remained on Dox and half were switched to water, and the animals were used 8 to 11 weeks later when transcriptional effects of ΔFosB and ΔcJun are maximal (29, 30). MSK1 KO mice and line 11A mice were fully backcrossed on C57BL/6N and C57BL/6J backgrounds, respectively. Line EA is a roughly 50:50 mixture of FVB and 129 backgrounds. These differences in background most likely explain some of the variation in dyskinetic behavior observed between the controls of the three transgenic lines. Therefore, for every experiment, littermate wild type controls were used to avoid any effects of genetic background. Heterozygous bacterial artificial chromosome transgenic mice expressing EGFP under the control of the promoter for the dopamine D2 receptor (Drd2-EGFP) or the dopamine D1R (Drd1a-EGFP) were generated by the GENSAT (Gene Expression Nervous System Atlas) program at the Rockefeller University (31) and were crossed on a C57BL/6 background for more than 10 generations. Experiments were carried out during the light phase, in accordance with the guidelines of Research Ethics Committee of Karolinska Institutet, the Swedish Animal Welfare Agency, the Society for Neuroscience and the Institutional Animal Care and Use Committee (IACUC) at Icahn School of Medicine at Mount Sinai.
Drugs
All drugs were purchased from Sigma-Aldrich (St. Louis, MO). 6-hydroxydopamine-HCl (6-OHDA) was dissolved in saline containing 0.02% ascorbic acid. L-DOPA (3,4-dihydroxy-L-phenylalanine) was dissolved in saline and injected in combination with the peripheral DOPA decarboxylase inhibitor benserazide hydrochloride in a volume of 10 mL/kg body weight.
6-OHDA lesion
Mice were lesioned with 6-OHDA using a well-established protocol (5, 8, 10) described in detail in Supplement 1.
Cylinder test
The cylinder test was conducted as previously described (Santini, 2009) at the end of the 3 weeks recovery period to assess limb akinesia. The effect of L-DOPA was assessed the following day, which corresponded to the first day of treatment. This time point was chosen due to the low levels of observable dyskinesia, which would otherwise interfere with test performance.
Abnormal involuntary movements (AIMs)
6-OHDA-lesioned mice were treated for 9 days (MSK1 KO mice and wild-type littermates) or 14 days (mice overexpressing ΔFosB or ΔcJun and controls) with one injection per day of 10 mg/kg of L-DOPA plus 7.5 mg/kg of benserazide (MSK1 KO mice and wild-type littermates) or 20 mg/kg of L-DOPA plus 12 mg/kg of benserazide (mice overexpressing ΔFosB or ΔcJun and respective controls). These doses of L-DOPA and benserazide were selected within a range employed in previous studies (10, 20, 24, 32, 33). AIMs were assessed after the last injection of L-DOPA (day 9 or day 14) by an observer blind to mouse genotype or treatment, using a pharmacologically validated mouse model of LID (32). These durations of treatment with L-DOPA were chosen in order to ensure a sufficient expression of ΔFosB, which has been previously shown to occur in response to chronic drug administration (20, 34). Briefly, 20 min after L-DOPA administration, mice were placed in separate cages and individual dyskinetic behaviors were assessed for 1 min (monitoring period) every 20 min, over a period of 2 hr. Purposeless movements, clearly distinguished from natural stereotyped behaviors (such as grooming, sniffing, rearing, and gnawing), were classified into three different subtypes: axial AIMs (contralateral dystonic posture of the neck and upper body toward the side contralateral to the lesion), limb AIMs (jerky and fluttering movements of the limb contralateral to the side of the lesion), and orolingual AIMs (vacuous jaw movements and tongue protrusions). Axial and limb AIMs were scored on a severity scale from 0 to 4: 0, absent; 1, occasional; 2, frequent; 3, continuous; 4, continuous and not interruptible by external stimuli. Orofacial AIMs were assigned a score of 0, absent, or 1, present.
Western blotting
Mice with a unilateral 6-OHDA lesion were treated with L-DOPA (10 mg/kg) plus benserazide (7.5 mg/kg) and killed 30 min post-injection by decapitation. Striatal tissue punches (1 mm thickness, 2 mm diameter; 3 punches per hemisphere) were taken using a stainless steel mouse brain matrix, sonicated in 1% SDS and boiled for 10 min. Immunoreactivity corresponding to total or phosphorylated histone H3 dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32), ERK and tyrosine hydroxylase (TH) was determined as described in in detail in Supplement 1.
Immunofluorescence
Mice were rapidly anaesthetized with pentobarbital (500 mg/kg, i.p., Sanofi-Aventis; France) and transcardially perfused with 4% (weight/vol) paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.5) (35) either 1hr (anti-p-MSK1) or 24 hr (anti-FosB) after the last L-DOPA administration. Brains were post-fixed overnight in the same solution and stored at 4°C. Thirty μm-thick sections were cut with a vibratome (Leica; France) and stored at −20°C in a solution containing 30% (vol/vol) ethylene glycol, 30% (vol/vol) glycerol and 0.1 M sodium phosphate buffer until they were processed for immunofluorescence. Brain regions corresponding to the dorsal striatum were identified using a mouse brain atlas (36) and sections 1.10 mm from bregma were taken. Free-floating sections were rinsed three times for 10 min each in Tris-buffered saline (TBS; 0.25 M Tris and 0.5 M NaCl, pH 7.5). After 20 min incubation in 0.2% Triton X-100 in TBS, sections were rinsed three times in TBS again. Immunoreactivity was analyzed by incubating sections overnight with primary antibodies against FosB (1:200, Santa Cruz Biotechnology; Dallas, TX) or phospho-Thr581-MSK1 (1:500, Cell Signaling Technology; Danvers, MA). Although the anti-FosB antibody recognizes both ΔFosB and full-length FosB, at the time point studied all FosB-immunoreactive protein represents ΔFosB (18). Sections were then rinsed three times for 10 min in TBS and incubated for 45 minutes with goat Cy3-coupled (1:400, Jackson Laboratory; Bar Harbor, ME) secondary antibody. Sections were rinsed for 10 min twice in TBS and twice in TB (0.25 M Tris) before mounting in 1,4-diazabicyclo--[2. 2. 2]-octane (DABCO, Sigma-Aldrich; St. Louis, MO). Images from the dorsal lateral striatum were obtained bilaterally using sequential laser scanning confocal microscopy (Zeiss LSM510; Oberkochen, Germany). Neuronal quantification for FosB immunostaining was performed in 375×375 μm images by counting Cy3-immunofluorescent nuclei for two slices per animal, using the average as the dependent variable. Cell counting was done by an observer blind to genotype and experimental group.
Chromatin preparation and immunoprecipitation
Unilaterally 6-OHDA lesioned mice were injected with 10 mg/kg body weight of L-DOPA with the peripheral DOPA decarboxylase inhibitor benserazide hydrochloride (7.5 mg/kg) and sacrificed by decapitation 1hr after the last injection. The heads of the animals were immediately immersed in liquid nitrogen for 6 sec. The brains were dissected out, and 3 striatal punches (cf. above) from each hemisphere were fixed for 12 min in cold 1% formaldehyde/PBS followed by glycine incubation. The fixed punches were then washed 3 times with cold phosphate buffered saline (PBS) containing phosphatase inhibitors and snap-frozen. Chromatin preparation and immunoprecipitation were perforemd as described in Supplement 1.
Results
Genetic inactivation of MSK1 attenuates LID
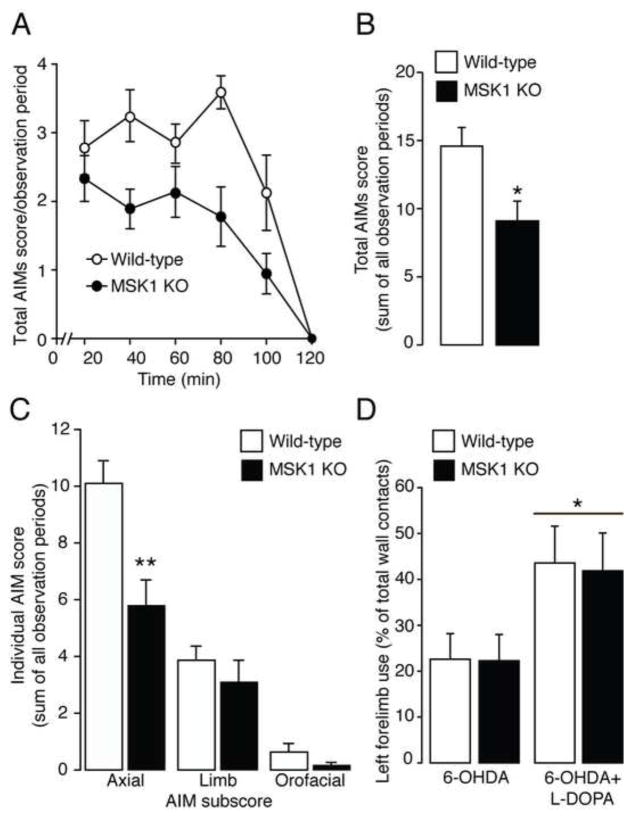
Previous studies indicated that LID is accompanied by a large increase in the phosphorylation of MSK1 in striatonigral MSNs (8, 10). Therefore, we examined the involvement of MSK1 in dyskinetic behavior. Wildtype and MSK1 KO mice were lesioned unilaterally with 6-OHDA and injected for 9 days with 10 mg/kg of L-DOPA (in combination with 7.5 mg/kg of benserazide to limit the peripheral conversion of L-DOPA to dopamine). AIMs were determined for 2 hr immediately after the last administration of L-DOPA. Chronic administration of L-DOPA induced a severe dyskinetic response in wild-type mice, which was reduced in MSK1 KO littermates. Repeated measures ANOVA of total AIMs scores revealed a significant effect of time (F(5,75) = 58.66; p < 0.001) and genotype (F(1,15) = 7.01; p < 0.05), but no time x genotype interaction (F(5,75) = 2.18; p > 0.05) (Fig. 1A). Cumulative AIMs scores summed over the entire two-hour observation were reduced in KO when compared to wild-type mice (two tailed unpaired t-test: t(15) = 2.68; p < 0.05; n = 8–9/experimental group) (Fig. 1B). Analysis of each individual AIM summed over the entire two-hour observation revealed that the effect of MSK1 inactivation was most prominent for axial AIMs (two tailed unpaired t-test: t(15) = 3.54; p < 0.01 for axial AIMs, t(15) = 0.83; p > 0.05 for limb AIMs, t(15) = 1.51; p > 0.05 for orofacial AIMs; n = 8–9/experimental group) (Fig. 1C).
Figure 1. L-DOPA-induced dyskinesia is reduced in mitogen- and stress-activated kinase 1 knock out (MSK1 KO) mice.
Wild-type and MSK1 KO mice received unilateral injections of 6-hydroxydopamine (6-OHDA) and were treated for 9 days with L-DOPA. A, Time profile of total abnormal involuntary movements (AIMs) scored for 1 min every 20 min over a period of 120 min after the last administration of L-DOPA. Repeated measures ANOVA revealed a significant effect of genotype (p < 0.05). B, Sum of total AIMs scored during all observation periods. * p < 0.05 vs. wild-type littermates. C, Sums of AIM subscores (axial, limb and orofacial) scored during all observation periods. ** p < 0.01 vs. wild-type littermates. D, Left forelimb use determined by the cylinder test in 6-OHDA lesioned wild-type and MSK1 KO mice before (left) and after (right) administration of L-DOPA. * p < 0.05 vs. 6-OHDA lesioned without L-DOPA. All data are shown as means ± SEM.
In order to exclude a possible effect of MSK1 inactivation on the anti-Parkinsonian properties of L-DOPA, wild-type and MSK1 KO mice were examined in the cylinder test before and after the first administration of L-DOPA. Lesioning with 6-OHDA resulted in a large decrease in the use of the contralateral forelimb in both wild-type and MSK1 KO mice. Moreover, the ability of 10 mg/kg of L-DOPA to revert forelimb akinesia was indistinguishable in the two groups of mice. Repeated measures ANOVA indicated a significant effect of L-DOPA (F(1,9) = 8.48; p < 0.05) but no effect of genotype (F(1,9) = 0.0002; p > 0.05) nor a L-DOPA x genotype interaction (F(1,9) = 0.07; p > 0.05) (n = 5–6/experimental group; Fig. 1D).
L-DOPA-induced phosphorylation of histone H3 is reduced in the striata of MSK1 KO mice
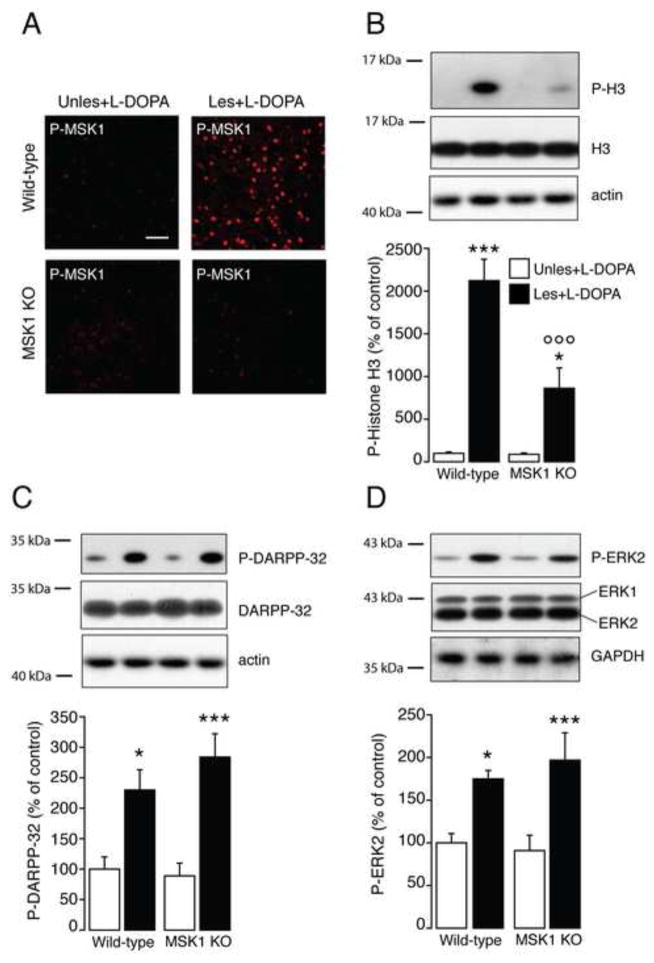
We next examined the involvement of MSK1 in the regulation exerted by L-DOPA on the phosphorylation of histone H3 at Ser10. Wild-type and MSK1 KO mice were lesioned unilaterally with 6-OHDA, treated for 9 days with L-DOPA and killed 30 min after the last drug administration. In wild-type mice, L-DOPA increased the phosphorylation of MSK1 in the dopamine-depleted striatum, but not in the unlesioned striatum (Fig. 2A). These data confirmed previous work indicating that loss of dopamine is a critical factor at the basis of the ability of L-DOPA to activate the ERK/MSK1 cascade (4, 10). The increase in MSK1 phosphorylation observed in wild-type mice was accompanied by enhanced levels of phospho-Ser10-histone H3 as determined by Western blotting (Fig. 2B). Notably, in MSK1 KO mice, the absence of phosphorylated MSK1 (Fig. 2A) was paralleled by a large reduction in histone H3 phosphorylation. Two-way ANOVA followed by Bonferroni-Dunn test indicated a significant lesion x genotype interaction (F(1,28) = 13.02; p < 0.05; n = 8/experimental group) (Fig. 2B). Importantly, in the dopamine depleted striatum, genetic inactivation of MSK1 did not affect the ability of L-DOPA to promote phosphorylation of DARPP-32. Two-way ANOVA followed by Bonferroni-Dunn test indicated a significant effect of lesion (F(1,28) = 30.67, p < 0.0001), no effect of genotype (F(1,28) = 0.53, p > 0.05) and no significant lesion x genotype interaction (F(1,28) = 1.25, p > 0.05) (n = 8/experimental group; Fig. 2C). Similar results were obtained for ERK2. Two-way ANOVA followed by Bonferroni-Dunn test indicated a significant effect of lesion (F(1,28) = 20.93, p < 0.0001), no effect of genotype (F(1,28) = 0.12, p > 0.05) and no significant lesion x genotype interaction (F(1,28) = 0.63; p > 0.05) (n = 8/experimental group; Fig. 2D).
Figure 2. Histone H3 phosphorylation at Ser10 is decreased in mitogen- and stress-activated kinase 1 knock out (MSK1 KO) mice.
Wild-type and MSK1 KO mice received unilateral striatal injections of 6-hydroxydopamine (Les) while the contralateral striatum remained intact (Unles) and were treated for 9 days with L-DOPA. A, Representative confocal micrographs of phospho-MSK1 (Thr581) (P-MSK1) determined by immunofluorescence in the striata of wild-type and MSK1 KO mice 30 min after the last administration of L-DOPA. Note the absence of P-MSK1 in MSK1 KO mice. Scale bar = 40 μm. B-D, Phospho-Ser10-histone H3 (P-H3; B), phospho-Thr34-DARPP-32 (P-DARPP-32; C) and phospho-Thr202/Tyr204-ERK2 (P-ERK2; D) were determined 30 min after the last administration of L-DOPA by Western blotting. Top: representative autoradiograms obtained using antibodies against phosphorylated proteins, total proteins, and β-actin or GAPDH as loading controls. Bottom: summary of data calculated as percent of control (wild-type unlesioned hemisphere) and shown as means ± SEM. * p < 0.05, *** p < 0.001 vs. unlesioned hemisphere of the same genotype; ○○○ p < 0.001 vs. wild-type lesioned hemisphere.
MSK1 is involved in L-DOPA-induced expression of ΔFosB
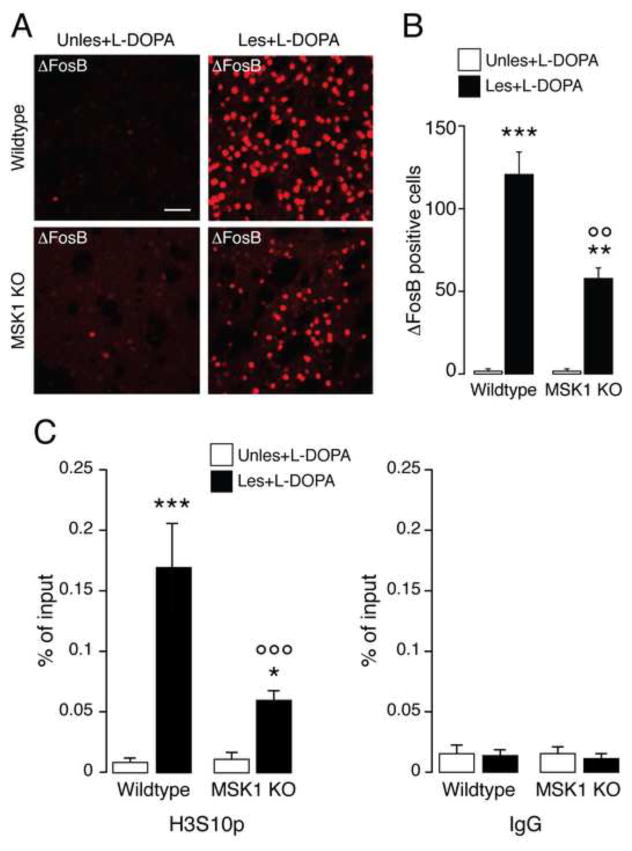
The results described above indicated that MSK1 is involved in L-DOPA-induced phosphorylation of histone H3 at Ser10 and suggested that MSK1 might also mediate some of the changes in gene expression associated to dyskinesia. Therefore, we compared the ability of L-DOPA to induce ΔFosB accumulation in the striata of wild-type and MSK1 KO mice. Following unilateral 6-OHDA lesion, mice were treated for 9 days with L-DOPA and perfused 24 hr after the last injection. Administration of L-DOPA did not produce any ΔFosB accumulation in the unlesioned striata (Fig. 3A). In contrast, we observed a large number of ΔFosB immunoreactive cells in the dopamine depleted striata of wild-type mice. This number was reduced by about 50% in MSK1 KO mice. Two-way ANOVA followed by Bonferroni-Dunn test indicated a significant lesion x genotype interaction (F(1,8) = 17.99, p < 0.01; n = 3/experimental group) (Fig. 3A and B).
Figure 3. Expression of ΔFosB and phosphorylation of histone H3 at the fosB gene promoter is reduced in mitogen- and stress-activated kinase 1 knock out (MSK1 KO) mice.
Wild-type and MSK1 KO mice received unilateral striatal injections of 6-hydroxydopamine (Les) while the contralateral striatum remained intact (Unles). ΔFosB was determined by immunofluorescence 24 hr after the last drug administration. A, Representative confocal micrographs showing ΔFosB immunoreactivity in the striata of a wild-type and a MSK1 KO mice. Scale bar = 40 μm. B, Quantification of ΔFosB positive-neurons. Data are shown as means ± SEM. ** p < 0.01 and *** p < 0.001 vs. unlesioned hemisphere of the same genotype; ○○ p < 0.01 vs. wild-type lesioned hemisphere. C, Wild-type and MSK1 KO mice with unilateral injections of 6-hydroxydopamine were treated with L-DOPA, killed after 60 min and striatal tissue was subjected to chromatin immunoprecipitation using an antibody against phospho-Ser10-H3 histone (H3S10p) (left) or control IgG (right). Epitope enrichment on chromatin was assessed by quantitative PCR using primers for the fosB promoter region and normalized to levels of histone H3 (see Materials and Methods). Data are shown as means ± SEM. * p < 0.05 and *** p < 0.001 vs. unlesioned hemisphere of the same genotype; ○○○ p < 0.001 vs. wild-type lesioned hemisphere.
To further elucidate a possible involvement of MSK1-mediated Ser10 phosphorylation of histone H3 in the induction of ΔFosB expression, we performed ChIP experiments in striatal tissue from 6-OHDA-lesioned wild-type and MSK1 KO mice 60 min after administration of L-DOPA. As shown in Fig. 3C, phosphorylation of histone H3 at Ser10 was increased at the promoter region of the fosB gene in the dopamine depleted hemisphere of wild-type mice; moreover, this effect was strongly reduced in MSK1 KO mice. Two-way ANOVA followed by Bonferroni-Dunn test indicated a significant lesion x genotype interaction (F(1,8) = 29.40, p < 0.001; n = 3/experimental group).
Changes in the expression and function of ΔFosB affect the dyskinetic response to L-DOPA
The involvement of MSK1 in LID and its participation to the regulation of ΔFosB suggested that changes affecting the expression or activity of ΔFosB might also contribute to the emergence of dyskinetic behavior.
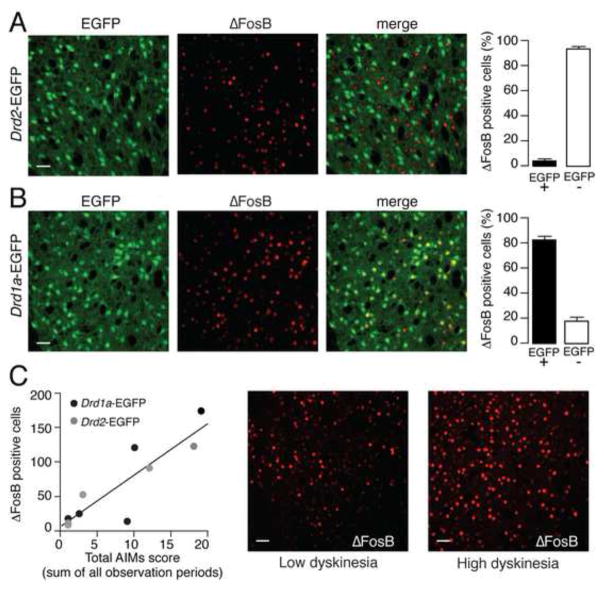
Previous work showed that FosB mRNA and FosB-like immunoreactive proteins are increased by L-DOPA in prodynorphin expressing MSNs, which correspond to the direct, striatonigral pathway (17, 20). Therefore we examined the cellular localization of ΔFosB in dyskinetic mice. Drd2- and Drd1a-EGFP mice were treated for 9 days with 10 mg/kg of L-DOPA and perfused 24 hr after the last drug administration. We found that, in Drd2-EGFP mice, ΔFosB immunoreactivity was localized to EGFP negative cells, with only a minimal percentage of cells showing double labeling for EGFP and ΔFosB (Fig. 4A). Conversely, in Drd1a-EGFP mice, ΔFosB immunoreactivity was for the most part observed in EGFP positive cells (Fig. 4B). These results indicate that the increase in ΔFosB produced by chronic administration of L-DOPA occurs in striatonigral MSNs. We also observed a significant correlation between the increase in ΔFosB observed in Drd1a- and Drd2-EGFP mice and the severity of AIMs (R2 = 0.78, p < 0.001, n = 5/strain) (Fig. 4C).
Figure 4. L-DOPA-induced increased in ΔFosB expression is localized to striatonigral MSNs and correlates with the severity of AIMs.
Drd2-EGFP (A) and Drd1a-EGFP (B) mice were lesioned with 6-OHDA, treated chronically with L-DOPA (10 mg/kg) for 9 days and perfused 24 hr after the last drug administration. A, B, Immunofluorescence for EGFP and ΔFosB in the striata of Drd2-EGFP (A) and Drd1a-EGFP (B) mice. Scale bar = 40 μm. Right panels show quantification of ΔFosB positive cells among EGFP-positive (EGFP+) and EGFP-negative (EGFP−) neurons. C, AIMs were examined in Drd2- and Drd1a-EGFP mice immediately after the last administration of L-DOPA (day 9). Left panel shows linear regression analysis indicating a significant correlation between severity of AIMs and levels of ΔFosB (p < 0.001). Right panels show immunofluorescence for ΔFosB in the striatum of a mouse with low dyskinesia compared with a mouse with high dyskinesia. Scale bar = 40 μm.
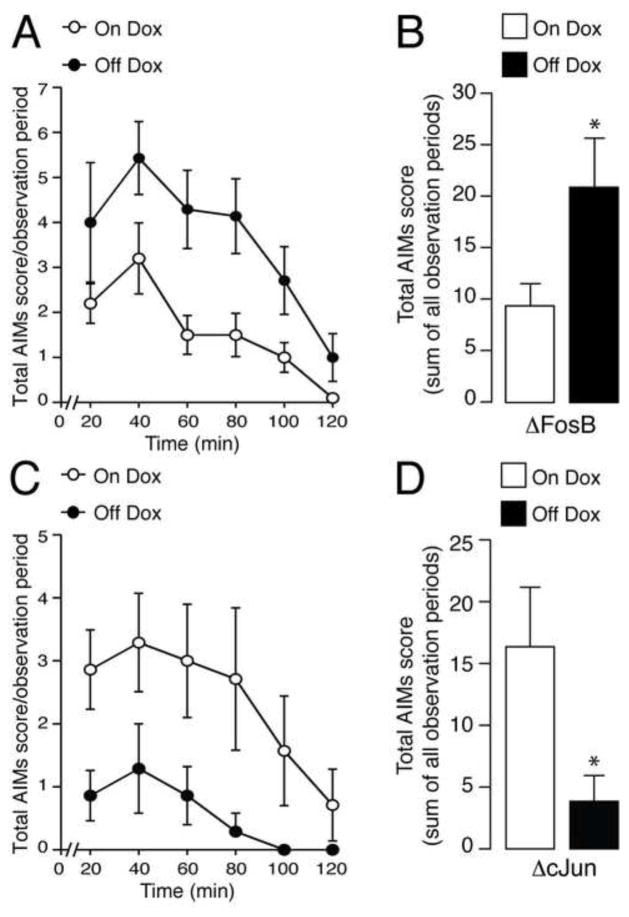
Based on these findings we examined the impact on LID produced by increased expression of ΔFosB in the MSNs of the direct pathway. To this end we used bitransgenic mice that inducibly overexpress, specifically in the striatonigral MSNs, ΔFosB (29) or ΔcJun, a transcriptionally inactive truncated cJun mutant that antagonizes ΔFosB activity (28). Mice were lesioned with 6-OHDA and treated for 14 days with 20 mg/kg of L-DOPA (in combination with 12.5 mg/kg of benserazide). As shown in Suppl. Fig. 1, discontinuation of Dox increased dramatically the effect of L-DOPA on the expression of ΔFosB mRNA in NSE-tTA x TetOp-ΔFosB mice. Notably, this exacerbation of ΔFosB expression resulted in the development of more severe AIMs. Analysis of the time-course of total AIMs scores with repeated measures ANOVA revealed a significant effect of time (F(5,75) = 7.91; p < 0.01) and genotype (F(1,15) = 7.59; p < 0.05), but no time x genotype interaction (F(5,75) = 1.50; p > 0.05) (Fig. 5A). Cumulative AIMs scores summed over the entire two-hour observation were increased in mice overexpressing ΔFosB when compared to wild-type mice (two tailed unpaired t-test: t(15)=1.70, p < 0.05, n = 7–10/experimental group) (Fig. 5B). In contrast, the dyskinetic response to L-DOPA was significantly attenuated in mice in which the transcriptional activity of ΔFosB was blocked through overexpression of ΔcJun. Analysis of the time-course of total AIMs scores with repeated measures ANOVA revealed a significant effect of time (F(5,80) = 4.38; p < 0.05) and genotype (F(1,16) = 4.96; p < 0.05), but no time x genotype interaction (F(5,80) = 2.78; p > 0.05) (Fig. 5C). Cumulative AIMs scores summed over the entire two-hour observation were decreased in mice overexpressing ΔcJun when compared to wild-type mice (two tailed unpaired t-test: t(16)=2.55, p < 0.05, n = 8–10/experimental group) (Fig. 5D).
Figure 5. Effects of doxycycline (Dox) inducible overexpression of ΔFosB or ΔcJun on L-DOPA-induced dyskinesia.
Mice that inducibly overexpress ΔFosB (A, B, Off Dox) or ΔcJun (C, D, Off Dox) and control littermates (A–D, On Dox) were lesioned with 6-OHDA and treated for 14 days with 20 mg/kg of L-DOPA (in combination with 12.5 mg/kg of benserazide). A, C, Time profile of total abnormal involuntary movements (AIMs) in mice scored for 1 min every 20 min over a period of 2 hr after the last administration of L-DOPA. Repeated measures ANOVA revealed a significant effect of genotype for mice overexpressing ΔFosB and ΔcJun (p < 0.05 vs. respective control On Dox littermates). B, Sum of total abnormal involuntary movements (AIMs) scored for a period of 2 hr immediately following the last administration of L-DOPA. * p < 0.05 vs. control (On Dox) littermates for the FosB group and for the ΔcJun group. Data are shown as means ± SEM.
Discussion
In this study we show that aberrant activation of the histone kinase MSK1 is implicated in LID and in the accumulation of the transcription factor ΔFosB associated with this condition. Moreover, we show that administration of L-DOPA results in the accumulation of phospho-Ser10-histone H3 at the fosB gene promoter and that this effect is dramatically reduced by genetic inactivation of MSK1.
Extensive work indicates that ERK activation in the striatum plays a critical role in the emergence of LID (10, 11, 20, 24, 37). Studies performed in rodents and non-human primates showed that pharmacological and genetic interventions aimed at reducing ERK activation during chronic administration of L-DOPA decrease dyskinetic behavior (10, 24, 37). One critical question related to these findings concerns the identification of downstream effectors targeted by ERK and implicated in the dyskinetic response to L-DOPA. The important role played by ERK in the regulation of synaptic plasticity and transcriptional activity (38) suggests that at least some of these effectors may be involved in the control of gene expression and ultimately in the emergence of maladaptive mechanisms at the basis of dyskinesia. In this regard, MSK1 represents an interesting subject of study because of its involvement in the regulation of the cAMP response element-binding protein and chromatin rearrangement via histone phosphorylation (39, 40).
Administration of drugs promoting dopamine transmission, such as cocaine and amphetamine, activates the ERK/MSK1 signaling cascade in striatal MSNs (12, 35). As in the case of L-DOPA (8), this effect involves activation of D1Rs and occurs specifically in the MSNs of the striatonigral pathway (41). Interestingly, it has been shown that the progressive enhancement of the motor stimulant response produced by repeated administration of cocaine is blunted in MSK1 KO mice (12). This observation is in line with the idea of a similar involvement of MSK1 in LID, which is also manifested as a progressive and uncontrolled exacerbation of motor activity.
MSK1 has been implicated in cocaine-mediated expression of c-Fos (12). In this study, we show that activation of MSK1 participates to the accumulation of ΔFosB, which is produced by chronic L-DOPA and is associated to the emergence of dyskinesia. Importantly, we provide evidence indicating the relevance of aberrant MSK1 and ΔFosB regulation for the development of LID. First, genetic inactivation of MSK1 reduces dyskinesia. Second, LID is also reduced by functional antagonism of ΔFosB exerted by inducible overexpression of the dominant negative ΔcJun specifically in striatonigral MSNs. Finally, inducible overexpression of ΔFosB in the same neuronal population exacerbates dyskinesia.
In striatonigral MSNs, the activation of ERK/MSK1 signaling produced by L-DOPA results in increased phosphorylation of histone H3 at Ser10 (5, 8, 10). This modification has been shown to occur in association with Lys14 acetylation at the promoters of c-Fos and c-Jun (13, 42), where it induces transcriptional activity (12–16). Interestingly, studies performed in mouse fibroblasts indicate that MSK1-dependent phosphorylation of histone H3 at Ser10 is per se sufficient to induce the expression of c-Jun (16). The immediate early genes c-Fos and c-Jun form AP-1 complexes, which in turn can control transcription at specific promoter sites, including the fosB promoter (43). Thus, it is possible that the reduction in ΔFosB observed in MSK1 KO mice is caused by decreased formation of AP1 complex.
The present study shows that knockout of MSK1 decreases histone H3 phosphorylation at Ser10 in striatal extracts and reduces histone H3 phosphorylation at the fosB promoter. This suggests that MSK1-dependent phosphorylation of histone H3 at Ser10 may contribute to the accumulation of ΔFosB implicated in dyskinesia. The partial reduction of histone H3 phosphorylation observed in MSK1 null mice indicates that additional kinases participate to chromatin modifications in response to L-DOPA. For instance, cAMP-dependent protein kinase (PKA) has been proposed to regulate Ser10 phosphorylation in response to administration of antipsychotic drugs (44, 45).
The ability of L-DOPA to increase ERK phosphorylation depends in large part on intact cAMP signaling (7, 10, 33); but see (46). Thus, inhibition of PKA (7), or genetic inactivation of DARPP-32 (10, 33), a critical mediator of cAMP signaling (47), reduces LID and the associated increase in ERK phosphorylation. Our results show that MSK1 deficiency attenuates dyskinesia in spite of persistent activation of PKA/DARPP-32 and ERK. It is likely that this persistent activation is responsible for the proportion of LID still observed in MSK1 KO mice. Indeed, MSK1 is one of many downstream targets controlled by dysregulated cAMP and ERK signaling and concurring to the full expression of dyskinetic behavior. For instance, cAMP- and ERK-dependent activation of the mammalian target of rapamycin pathway has been implicated in LID (33, 48). Moreover, both PKA and ERK may act on additional transcription factors (e.g., cAMP response element binding protein and Elk-1), potentially involved in dyskinesia.
MSK1 inactivation affect specifically axial AIMs, which indicates a prominent role played by MSK1 in the dystonic component of L-DOPA-induced motor complications. Previous work showed that partial inhibition of ERK and ΔFosB expression produced a similar selective effect on axial and orofacial AIMs, without a reduction of limb AIMs (37). Taken together, these observations suggest that dystonic AIMs are particularly sensitive to reduced accumulation of ΔFosB. In contrast, a reduction in the choreic symptoms may require a more prominent decrease in the expression of ΔFosB, or alternatively may be achieved by acting on parallel downstream targets affected by cAMP or ERK signaling.
In conclusion, this study shows that MSK1 is implicated in the development of LID in a mouse model of PD. Abnormal activation of MSK1 leads to increased Ser10 phosphorylation of histone H3 at the fosB gene promoter and to the accumulation of ΔFosB in striatonigral MSNs. Future studies will be necessary to identify specific genes regulated by ΔFosB that promote dyskinesia. This study also shows that specific components of LID can be controlled by targeting selected signaling pathways downstream of major signaling cascades involved in basic physiological processes (e.g., cAMP/PKA and ERK), thereby reducing the likelihood of producing major negative side effects.
Supplementary Material
Administration of L-DOPA to Parkinson’s disease (PD) patients is accompanied by choreic and dystonic motor complications, called dyskinesia. In a mouse model of PD, genetic inactivation of the mitogen- and stress-activated kinase 1 (MSK1) reduces the dystonic component of dyskinesia and the expression of the transcription factor, ΔFosB. Overexpression of ΔFosB in the medium spiny neurons of the striatum exacerbates dyskinesia, whereas overexpression of ΔcJun, which reduces ΔFosB-dependent transcriptional activation, counteracts dyskinesia. These results indicate the involvement of MSK1-mediated gene expression in the motor complications caused by prolonged administration of L-DOPA.
Acknowledgments
This work was supported by grants from the Swedish Research Council (13482 to GF), Stiftelsen Olle Engkvist Byggmästare (to GF), the Parkinson Foundation in Sweden (to GF), the Karolinska Institutet/National Institute of Health Graduate Partnership Program (to GF and MF), ”C.M. Lerici” Foundation (to GS), the Lundbeck Foundation (to KH) and the Danish National Research Foundation (to KH).
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Obeso JA, Olanow CW, Nutt JG. Levodopa motor complications in Parkinson’s disease. Trends Neurosci. 2000;23:S2–7. doi: 10.1016/s1471-1931(00)00031-8. [DOI] [PubMed] [Google Scholar]
- 2.Guigoni C, Aubert I, Li Q, Gurevich VV, Benovic JL, Ferry S, et al. Pathogenesis of levodopa-induced dyskinesia: focus on D1 and D3 dopamine receptors. Parkinsonism Relat Disord. 2005;11(Suppl 1):S25–29. doi: 10.1016/j.parkreldis.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Aubert I, Guigoni C, Hakansson K, Li Q, Dovero S, Barthe N, et al. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol. 2005;57:17–26. doi: 10.1002/ana.20296. [DOI] [PubMed] [Google Scholar]
- 4.Feyder M, Bonito-Oliva A, Fisone G. L-DOPA-induced dyskinesia and abnormal signaling in striatal medium spiny neurons: focus on dopamine D1 receptor-mediated transmission. Front Behav Neurosci. 2011;5:71. doi: 10.3389/fnbeh.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darmopil S, Martin AB, De Diego IR, Ares S, Moratalla R. Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biol Psychiatry. 2009;66:603–613. doi: 10.1016/j.biopsych.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebel M, Chagniel L, Bureau G, Cyr M. Striatal inhibition of PKA prevents levodopa-induced behavioural and molecular changes in the hemiparkinsonian rat. Neurobiol Dis. 2010;38:59–67. doi: 10.1016/j.nbd.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Santini E, Alcacer C, Cacciatore S, Heiman M, Herve D, Greengard P, et al. L-DOPA activates ERK signaling and phosphorylates histone H3 in the striatonigral medium spiny neurons of hemiparkinsonian mice. J Neurochem. 2009;108:621–633. doi: 10.1111/j.1471-4159.2008.05831.x. [DOI] [PubMed] [Google Scholar]
- 9.Santini E, Sgambato-Faure V, Li Q, Savasta M, Dovero S, Fisone G, et al. Distinct changes in cAMP and extracellular signal-regulated protein kinase signalling in L-DOPA-induced dyskinesia. PLoS One. 2010;5:e12322. doi: 10.1371/journal.pone.0012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, et al. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA. Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry. 2007;62:800–810. doi: 10.1016/j.biopsych.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brami-Cherrier K, Valjent E, Herve D, Darragh J, Corvol JC, Pages C, et al. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25:11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell. 2000;5:905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- 14.Clayton AL, Rose S, Barratt MJ, Mahadevan LC. Phosphoacetylation of histone H3 on c-fos- and c-jun-associated nucleosomes upon gene activation. EMBO J. 2000;19:3714–3726. doi: 10.1093/emboj/19.14.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crosio C, Heitz E, Allis CD, Borrelli E, Sassone-Corsi P. Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons. J Cell Sci. 2003;116:4905–4914. doi: 10.1242/jcs.00804. [DOI] [PubMed] [Google Scholar]
- 16.Thomson S, Clayton AL, Mahadevan LC. Independent dynamic regulation of histone phosphorylation and acetylation during immediate-early gene induction. Mol Cell. 2001;8:1231–1241. doi: 10.1016/s1097-2765(01)00404-x. [DOI] [PubMed] [Google Scholar]
- 17.Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol Dis. 1999;6:461–474. doi: 10.1006/nbdi.1999.0259. [DOI] [PubMed] [Google Scholar]
- 18.Doucet JP, Nakabeppu Y, Bedard PJ, Hope BT, Nestler EJ, Jasmin BJ, et al. Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of deltaFosB-like protein(s) in both the rodent and primate striatum. Eur J Neurosci. 1996;8:365–381. doi: 10.1111/j.1460-9568.1996.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 19.Lindgren HS, Rylander D, Iderberg H, Andersson M, O’Sullivan SS, Williams DR, et al. Putaminal upregulation of FosB/DeltaFosB-like immunoreactivity in Parkinson’s disease patients with dyskinesia. J Parkinsons Dis. 2011;1:347–357. doi: 10.3233/JPD-2011-11068. [DOI] [PubMed] [Google Scholar]
- 20.Pavon N, Martin AB, Mendialdua A, Moratalla R. ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry. 2006;59:64–74. doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 21.Nestler EJ. Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berton O, Guigoni C, Li Q, Bioulac BH, Aubert I, Gross CE, et al. Striatal overexpression of DeltaJunD resets L-DOPA-induced dyskinesia in a primate model of Parkinson disease. Biol Psychiatry. 2009;66:554–561. doi: 10.1016/j.biopsych.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao X, Yasuda T, Uthayathas S, Watts RL, Mouradian MM, Mochizuki H, et al. Striatal overexpression of DeltaFosB reproduces chronic levodopa-induced involuntary movements. J Neurosci. 2010;30:7335–7343. doi: 10.1523/JNEUROSCI.0252-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fasano S, Bezard E, D’Antoni A, Francardo V, Indrigo M, Qin L, et al. Inhibition of Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) signaling in the striatum reverts motor symptoms associated with L-dopa-induced dyskinesia. Proc Natl Acad Sci U S A. 2010;107:21824–21829. doi: 10.1073/pnas.1012071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiggin GR, Soloaga A, Foster JM, Murray-Tait V, Cohen P, Arthur JS. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol Cell Biol. 2002;22:2871–2881. doi: 10.1128/MCB.22.8.2871-2881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Kelz MB, Zeng G, Sakai N, Steffen C, Shockett PE, et al. Transgenic animals with inducible, targeted gene expression in brain. Mol Pharmacol. 1998;54:495–503. doi: 10.1124/mol.54.3.495. [DOI] [PubMed] [Google Scholar]
- 27.Muschamp JW, Nemeth CL, Robison AJ, Nestler EJ, Carlezon WA., Jr DeltaFosB enhances the rewarding effects of cocaine while reducing the pro-depressive effects of the kappa-opioid receptor agonist U50488. Biol Psychiatry. 2012;71:44–50. doi: 10.1016/j.biopsych.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peakman MC, Colby C, Perrotti LI, Tekumalla P, Carle T, Ulery P, et al. Inducible, brain region-specific expression of a dominant negative mutant of c-Jun in transgenic mice decreases sensitivity to cocaine. Brain Res. 2003;970:73–86. doi: 10.1016/s0006-8993(03)02230-3. [DOI] [PubMed] [Google Scholar]
- 29.Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- 30.McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- 31.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 32.Lundblad M, Usiello A, Carta M, Hakansson K, Fisone G, Cenci MA. Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Exp Neurol. 2005;194:66–75. doi: 10.1016/j.expneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Santini E, Feyder M, Gangarossa G, Bateup HS, Greengard P, Fisone G. Dopamine- and cAMP-regulated Phosphoprotein of 32-kDa (DARPP-32)-dependent Activation of Extracellular Signal-regulated Kinase (ERK) and Mammalian Target of Rapamycin Complex 1 (mTORC1) Signaling in Experimental Parkinsonism. J Biol Chem. 2012;287:27806–27812. doi: 10.1074/jbc.M112.388413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, et al. DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 37.Schuster S, Nadjar A, Guo JT, Li Q, Ittrich C, Hengerer B, et al. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor lovastatin reduces severity of L-DOPA-induced abnormal involuntary movements in experimental Parkinson’s disease. J Neurosci. 2008;28:4311–4316. doi: 10.1523/JNEUROSCI.4720-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 39.Brami-Cherrier K, Roze E, Girault JA, Betuing S, Caboche J. Role of the ERK/MSK1 signalling pathway in chromatin remodelling and brain responses to drugs of abuse. J Neurochem. 2009;108:1323–1335. doi: 10.1111/j.1471-4159.2009.05879.x. [DOI] [PubMed] [Google Scholar]
- 40.Gehani SS, Agrawal-Singh S, Dietrich N, Christophersen NS, Helin K, Hansen K. Polycomb group protein displacement and gene activation through MSK-dependent H3K27me3S28 phosphorylation. Mol Cell. 2010;39:886–900. doi: 10.1016/j.molcel.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clayton AL, Mahadevan LC. MAP kinase-mediated phosphoacetylation of histone H3 and inducible gene regulation. FEBS Lett. 2003;546:51–58. doi: 10.1016/s0014-5793(03)00451-4. [DOI] [PubMed] [Google Scholar]
- 43.Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Guo Y, Schroeder FA, Youngs RM, Schmidt TW, Ferris C, et al. Dopamine D2-like antagonists induce chromatin remodeling in striatal neurons through cyclic AMP-protein kinase A and NMDA receptor signaling. J Neurochem. 2004;90:1117–1131. doi: 10.1111/j.1471-4159.2004.02569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valjent E, Bertran-Gonzalez J, Bowling H, Lopez S, Santini E, Matamales M, et al. Haloperidol regulates the state of phosphorylation of ribosomal protein S6 via activation of PKA and phosphorylation of DARPP-32. Neuropsychopharmacology. 2011;36:2561–2570. doi: 10.1038/npp.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerfen CR, Paletzki R, Worley P. Differences between dorsal and ventral striatum in Drd1a dopamine receptor coupling of dopamine- and cAMP-regulated phosphoprotein-32 to activation of extracellular signal-regulated kinase. J Neurosci. 2008;28:7113–7120. doi: 10.1523/JNEUROSCI.3952-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 48.Santini E, Heiman M, Greengard P, Valjent E, Fisone G. Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Sci Signal. 2009;2:ra36. doi: 10.1126/scisignal.2000308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.