Abstract
OBJECTIVE AND SUMMARY BACKGROUND DATA
The decision regarding elective cholecystectomy in older patients with symptomatic cholelithiasis is complicated. We developed and validated a prognostic nomogram to guide shared decision-making for these patients.
METHODS
We used Medicare claims (1996–2005) to identify the first episode of symptomatic cholelithiasis in patients >65 who did not undergo hospitalization or elective cholecystectomy within 2.5 months of the episode. We described current patterns of care and modeled their risk of emergent gallstone-related hospitalization or cholecystectomy at two years. Model discrimination and calibration were assessed using a random split sample of patients.
RESULTS
We identified 92,436 patients presenting to the emergency department (ED, 8.3%) or physician’s office (91.7%) and who were not immediately admitted. The diagnosis for the initial episode was biliary colic/dyskinesia (65.3%), acute cholecystitis (26.6%), choledocholithiasis (5.7%), or gallstone pancreatitis (2.4%). The 2-year emergent gallstone-related hospitalization rate was 11.1%, with associated in-hospital morbidity and mortality rates of 56.5% and 6.5%. Factors associated with gallstone-related acute hospitalization included male gender, increased age, fewer comorbid conditions, complicated biliary disease on initial presentation, and initial presentation to the ED. Our model was well-calibrated and identified 51% of patients with a <10% risk of 2-year complications and 5.4% with >40% risk (C-statistic 0.69, 95% CI 0.63–0.75).
CONCLUSIONS
Surgeons can use this prognostic nomogram to accurately provide patients with their 2-year risk of developing gallstone-related complications, allowing patients and physicians to make informed decisions in the context of their symptom severity and its impact on their quality of life.
INTRODUCTION
Gallstone disease is a leading cause for inpatient admissions for gastrointestinal disease and is the most costly digestive disease in the United States. The cost of treating symptoms and complications related to gallstone disease in the United States is currently estimated to exceed $6.5 billion annually.1 After an initial episode of biliary colic, 20–40% of patients will experience recurrent episodes.2–4 Within one year, approximately 14% will develop acute cholecystitis, 5% will develop gallstone pancreatitis, and 5% will develop common bile duct stones.5–7
A 2009 Cochrane meta-analysis of randomized controlled trials indicated that early cholecystectomy for symptomatic cholelithiasis is associated with decreased risk for conversion, decreased operative time, and decreased length of stay in the hospital when compared to delayed intervention.8 Based on these data, the current standard of care for patients presenting with symptomatic cholelithiasis is early, elective cholecystectomy in an effort to avoid gallstone-related complications and costs. However, in older patients the decision to perform elective cholecystectomy is complicated by multiple competing risks. Associated chronic illness increases the morbidity and mortality of elective cholecystectomy. At the same time, older patients are at an increased risk of developing gallstone-related complications.9–14 Once complications occur, the treatment-related morbidity and mortality increase significantly in this vulnerable population.
The management of older patients who present with symptomatic cholelithiasis has not been well described. Our first objective was to use Medicare claims data to comprehensively describe the trajectory of older patients who are managed nonoperatively after an incident episode of symptomatic cholelithiasis. Our second objective was to develop and validate a risk prediction model that would identify older patients who are at highest risk for recurrent episodes. The ability to provide patients with their individualized risk of developing gallstone-related complications can improve the shared decision-making process in the management of these patients.
METHODS
This study was determined to be exempt from review by the Institutional Review Board at the University of Texas Medical Branch.
Data Source
We used a 5% national sample of Medicare claims data from 1995–2007. Medicare claims data include patient demographic information, outpatient visits, physician services, and hospital admissions.15 Data from Medicare Part A inpatient billing claims (MEDPAR) and Medicare Part B claims, including the Carrier claims and Outpatient Standard Analytic File (SAF) were used.16
Cohort Selection
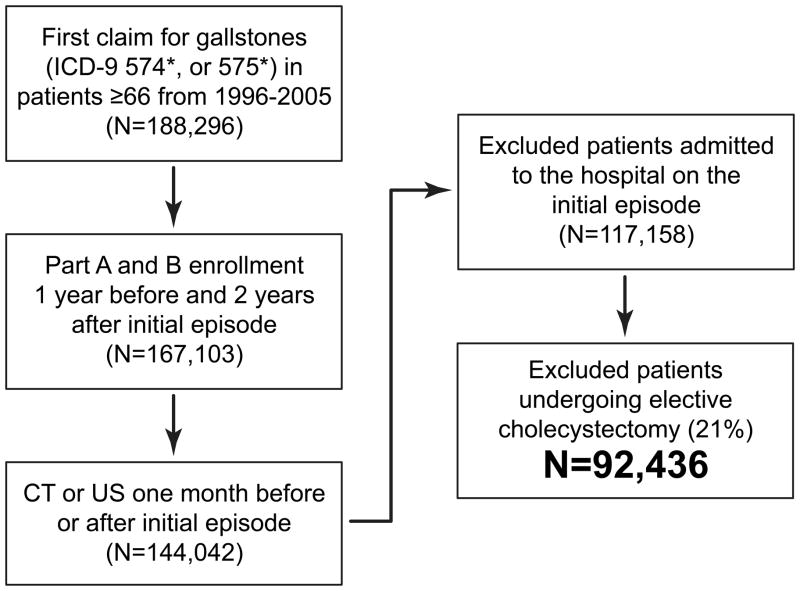
Figure 1 illustrates the cohort selection for the study. We identified all MEDPAR, Outpatient SAF, and Carrier claims for hospital, emergency department, and physician visits with 1) an International Classification of Diseases, 9th Edition, Clinical Modification (ICD-9-CM) primary diagnosis of 574* or 575* or 2) primary diagnosis of acute pancreatitis (ICD-9-CM 577.0) and a secondary diagnosis of ICD-9-CM 574* or 575* as has been done previously.17 While Medicare data from 1995–2007 were available, we only included incident gallstone cases from 1996–2005. This was done in order to: 1) identify patient comorbidities from the claims data the year prior to the incident diagnosis and 2) to enable us to “follow” all patients via their claims data for at least two years after the date of initial diagnosis. The first claim for symptomatic cholelithiasis was identified for each patient and defined as the incident episode. Patients were included if they were aged 66 years or older and had Medicare Parts A and B fee-for-service and no HMO for one year prior (to identify incident cases) and two years following the incident claim, or until death. Patients who were admitted to a hospital or underwent cholecystectomy at the time of the incident episode of symptomatic cholelithiasis were excluded. To ensure we were capturing patients with confirmed symptomatic cholelithiasis, patients were excluded if the diagnosis of cholelithiasis was not accompanied by computed tomography (CT) and/or ultrasound (US) in the one month before or after the claim. CT and US were identified in the carrier and outpatient SAF claims files using Current Procedural Terminology codes (Supplemental Table 1).
Figure 1.
Cohort Selection. Symptomatic cholelithiasis defined by 1) Primary diagnosis of ICD-9-CM code 574 of 575 or 2) Primary diagnosis of acute pancreatitis (577.0) and a secondary diagnosis of 574 or 575. Only patients ≥66 who underwent CT and/or US in the month before or after diagnosis were included. Patients who underwent cholecystectomy at initial presentation were excluded. CT=computed tomography, US=ultrasound.
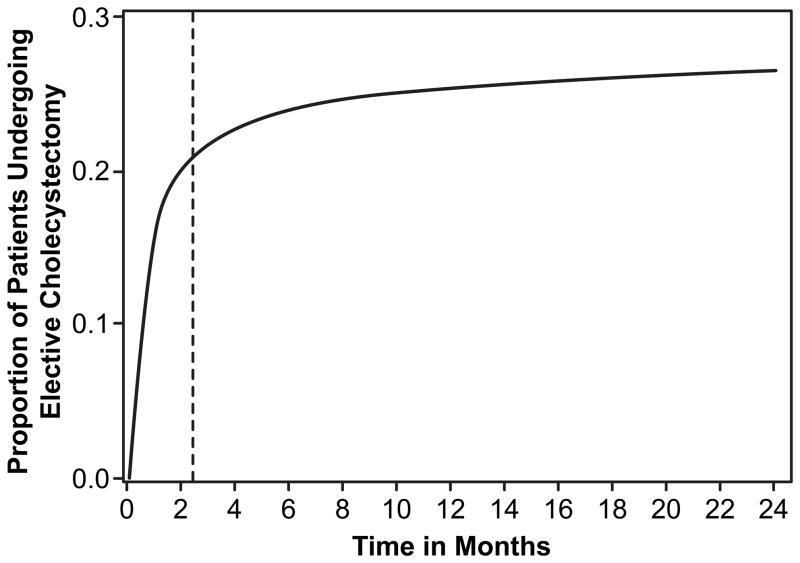
We excluded patients who underwent elective cholecystectomy after the initial episode. To define elective cholecystectomy, we searched for any claim for cholecystectomy (see Supplemental Table 1 for ICD-9-CM and CPT codes) following the incident episode that was coded as “elective” in the Medicare type of admission variable. Then we calculated a cumulative incidence curve for elective cholecystectomy. Patients were censored for death and emergency cholecystectomy. A piecewise regression model was used to identify the time point when the cumulative incidence plateaued (inflection point). The location of this inflection point (or joint point) was estimated by nonlinear least squares regression. For elective cholecystectomy, the inflection point was 2.44 months (Fig. 2, 95% CI 2.39, 2.48). We used this inflection point to define elective cholecystectomy for the cohort, and excluded any patient undergoing a cholecystectomy coded as elective within 2.5 months after the incident episode. The final study cohort included 92,436 patients.
Figure 2.
Cumulative incidence curve for elective cholecystectomy for the study cohort using a piecewise regression model (least squares non-linear regression). Patients were censored for death or emergency cholecystectomy. Of these patients undergoing elective cholecystectomy, 79.6% underwent cholecystectomy at three months and 88.9% underwent cholecystectomy at twelve months. For elective cholecystectomy, the joint point/inflection point (represented by the dotted vertical line) was 2.44 months (95% CI 2.39, 2.48).
Covariates
We recorded patient demographic characteristics including age, sex, race/ethnicity, Elixhauser comorbidity index, and diagnosis at initial claim for symptomatic cholelithiasis (biliary colic/biliary dyskinesia, acute cholecystitis, common bile duct stones, and gallstone pancreatitis; Table 1). Elixhauser comorbidity index was used as a summary measure for patient comorbidity, as described previously.18 Type of initial visit (emergency department versus physician office) was also recorded.
Table 1.
Patient Characteristics, Overall Cohort (N=92,436) and for Model Training Sample (N=46,218) and Model Validation Sample (N=46,218)
| Characteristic | Overall Cohort N (%) |
Training Sample N (%) |
Validation Sample N (%) |
|---|---|---|---|
| Age Group | |||
| 66–69 | 16,138 (17.5) | 8,040 (17.4) | 8,098 (17.5) |
| 70–74 | 21,795 (23.6) | 10,957 (23.7) | 10,838 (23.5) |
| 75–79 | 21,668 (23.4) | 10,848 (23.5) | 10,820 (23.4) |
| 80+ | 32,835 (35.5) | 16,373 (35.4) | 16,462 (35.6) |
| Sex | |||
| Female | 56,510 (61.1) | 28,258 (61.1) | 28, 252 (61.1) |
| Race | |||
| White | 80,884 (87.5) | 40, 473 (87.6) | 40,411 (87.4) |
| Black | 6,964 (7.5) | 3,434 (7.4) | 3,530 (7.6) |
| Hispanic | 2,252 (2.4) | 1,139 (2.5) | 1,113 (2.4) |
| Other | 2,336 (2.5) | 1,172 (2.5) | 1,164 (2.5) |
| Elixhauser Comorbidity Index | |||
| 0 | 15,581 (16.9) | 7,817 (16.9) | 7,764 (16.8) |
| 1 | 19,691 (21.3) | 9,843 (21.3) | 9,848 (21.3) |
| 2 | 17,199 (18.6) | 8,501 (18.4) | 8,698 (18.8) |
| ≥ 3 | 39,965 (43.2) | 20,057 (43.4) | 19,908 (43.1) |
| Initial Diagnosis | |||
| Biliary Colic | 60,385 (65.3) | 30,260 (65.5) | 30,125 (65.2) |
| Acute Cholecystitis | 24,633 (26.7) | 12,195 (26.4) | 12,438 (26.9) |
| Common Bile Duct Stones | 5,224 (5.7) | 2,633 (5.7) | 2,591 (5.6) |
| Gallstone Pancreatitis | 2,194 (2.4) | 1,130 (2.4) | 1,064 (2.3) |
| Location of Initial Visit | |||
| Physician Visit | 84,755 (91.7) | 42,325 (91.6) | 42,430 (91.8) |
| Emergency Room Visit | 7,681 (8.3) | 3,893 (8.4) | 3,788 (8.2) |
Description of Trajectory
Patients were followed from the date of initial episode to any gallstone-related event. Potential gallstone-related events included emergent hospitalization, emergent cholecystectomy, delayed elective cholecystectomy (>2.5 months after initial episode), or emergency department/outpatient physician visits with a gallstone-related diagnosis. Emergent admission was identified using MEDPAR inpatient billing claims and defined as any gallbladder-related claim coded as “emergency” or “urgent” in the type of admission variable. Overall in-hospital morbidity and mortality, perioperative complications, and 30-day operative mortality were calculated for patients who underwent emergent cholecystectomy. Codes used to identify perioperative complications within thirty days of the date of surgery are included in Supplemental Table 1. Operative mortality was defined as death from any cause occurring within thirty days from the date of surgery or a discharge status coded as “Discharged dead.” Patients who did not experience any of the above outcomes and did not die were presumed not to have any gallstone-related problems over the two-year period following the initial episode.
Trajectory of Care
Descriptive statistics were used to summarize the sample characteristics at initial presentation and to describe patients within each trajectory of care: 1) patients without further problems, 2) patients who presented with multiple emergency department or physician visits but were not admitted, and 3) patients who required emergent hospitalization.
Risk Prediction Model Creation and Validation
For the purpose of model identification and validation, we randomly split the overall cohort of patients into a training sample (N=46,218) and a validation sample (N=46,218). We used the training sample for model selection and then applied the obtained model parameters to check the performance of the selected model in the validation sample. We developed a Cox proportional hazards regression model to identify factors independently associated with emergent care (admission and/or emergent cholecystectomy) two years after the initial episode, with patients censored for death or elective cholecystectomy after 2.5 months and prior to any emergent hospitalization. Covariates that were included in this model included age group, race/ethnicity, gender, Elixhauser comorbidity index, initial diagnosis (biliary colic, gallstone pancreatitis, common bile duct stones, and acute cholecystitis), and initial visit (emergency vs. outpatient physician). A Cox proportional hazards model was chosen so that patients could be censored for elective cholecystectomy and death.
The findings from this Cox proportional hazards model were used to formulate a prognostic nomogram in R version 3.0.2 (The R Foundation for Statistical Computing, Department of Biostatistics, Vanderbilt University, Nashville, TN) using the package rms.19 We named this model “Predicting Risk of Complications in Older Patients with Gallstones,” or the PREOP-Gallstones model. To analyze the discrimination power of the model and correctly distinguish the patients who required emergent care from those who did not need emergent care within two years, we estimated overall C-statistics as described by Uno et al.20 C-statistics in the setting of a survival analysis can be considered as an expansion of the receiver operating characteristics (ROC) curve.21 Using regression parameters from our Cox proportional hazards model used in training sample, we estimated the expected probability of requiring emergent care at two years for each patient in the model validation sample. We used modified Hosmer-Lemeshow χ2 with 9 df to analyze the agreement of predicted probabilities of our risk prediction model to the actual observed outcomes (model calibration).22 For this purpose, predicted probabilities of requiring emergent care were categorized into deciles and the mean of each decile was compared with the actual observed incidence of emergent care as estimated by Kaplan-Meier analysis. The predicted mean of each decile in the training model sample was compared to the mean observed proportion of patients in the model validation sample who actually required emergent care in a Kaplan-Meier time-to-event analysis.
C-statistics were estimated by using R package ‘survC1’20 and all other statistical analyses were performed using SAS version 9.3 (SAS Inc., Cary, NC, USA). Statistical significance was accepted at the p<0.05 level.
RESULTS
Overall Sample Characteristics (Table 1)
We identified 117,158 patients presenting with an initial episode of symptomatic cholelithiasis; 24,722 (21.1%) underwent elective cholecystectomy within 2.5 months, leaving 92,436 patients who met our inclusion criteria. The mean age was 77.0 ± 7.2 years. The majority of patients were white (87.5%) and female (61.1%). Outpatient evaluation was initially performed in 91.7% of patients, while the remaining 8.3% first presented to the emergency department. The primary visit diagnosis for the incident episode was biliary colic in 65.3%, acute cholecystitis in 26.7%, common bile duct stones in 5.7%, and gallstone pancreatitis in 2.4%.
Description of Trajectory
Figure 2 shows the cumulative incidence of elective cholecystectomy a one year. As described in the methods, elective cholecystectomy was defined as cholecystectomy within 2.5 months of diagnosis based on the inflection point. 77.2% of elective cholecystectomies were performed in the first 2.5 months after the incident episode of symptomatic gallstones.
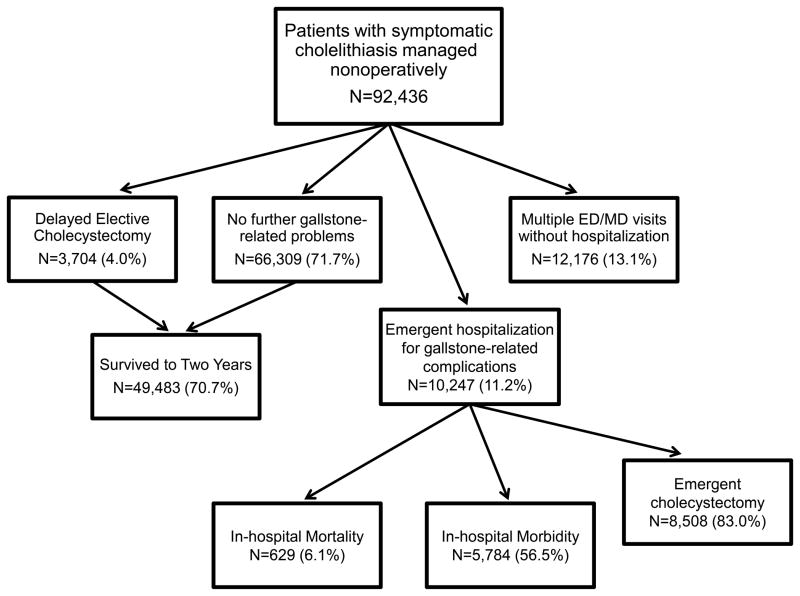
Of the 92,436 patients who did not undergo early elective cholecystectomy, 10,247 (11.1%) eventually required emergency hospitalization for gallstone-related complications, and 8,508, or 83.0%, of these admitted patients underwent emergency cholecystectomy (Fig. 3). An additional 12,176 patients (13.1%) re-presented to the emergency department or to an outpatient physician at least once over the two years for biliary symptoms but did not require hospitalization or emergent cholecystectomy. 3,704 patients underwent delayed elective cholecystectomy more than 2.5 months after the initial episode. Of these patients, 3.4% had postoperative complications, and perioperative mortality was 0%. Finally, 69,309 patients (71.7%) did not require any emergent gallstone-related intervention or additional visits. 49,483 (53.5%) survived to two years and the remainder died of unrelated causes during the two year follow-up period.
Figure 3.
Trajectory of care for Medicare patients with symptomatic cholelithiasis initially managed nonoperatively (N=92,436). 10,247 (11.1%) subsequently required emergent care, 12,176 (13.1%) had recurrent symptoms but did not require hospitalization or surgery, and 70,013 did not require any further intervention. Of the patients who did not require intervention, 70.7% survived to two years. ED=emergency department.
In-hospital morbidity and mortality were high for the 10,247 patients who presented with emergent hospitalization for gallstone-related complications; 56.5% and 6.1%, respectively. Perioperative morbidity (26.8%) and mortality (1.2%) for the 8,508 patients who underwent emergency cholecystectomy were also elevated.
Prognostic Nomogram Creation and Validation (Figure 4)
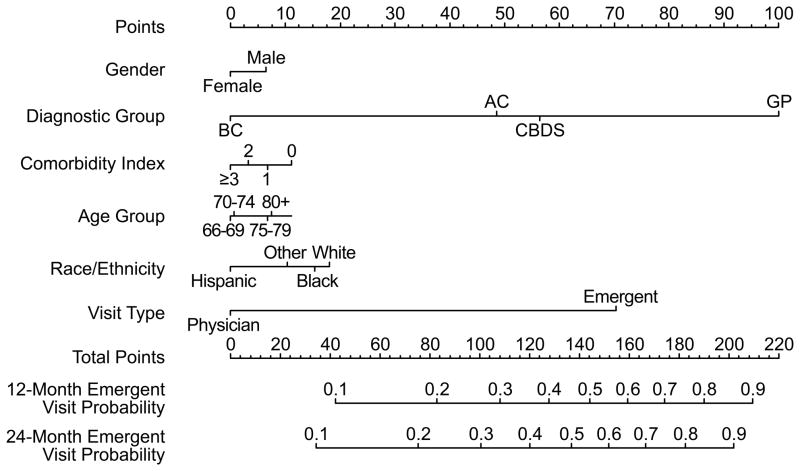
Figure 4.
“Predicting Risk of Complications in Older Patients with Gallstones,” PREOP-Gallstones model nomogram. To use the nomogram, a vertical line is drawn from each factor to the corresponding position on the “Points” line. Once the points are tabulated for each factor, the points are then added. A line is drawn from this position on the “Points” line to the “Emergent Visit Probability” lines to determine a patient’s 12-month and 24-month risk for developing emergent gallstone-related complications.
The overall cohort was randomly split into equal-sized training and validation samples; sample characteristics and 2-year rates of emergent events were similar for the two samples (Table 1). The training sample was used to generate a Cox proportional hazards model for the development of emergent gallstone-related events (Table 2). Factors independently associated with need for emergency care were older age, white race, male sex, initial visit to emergency room department, and a diagnosis of complicated gallstone disease (gallstone pancreatitis, common bile duct stones, and acute cholecystitis) at initial presentation. Patients with the least comorbidities were also more likely to require emergency care.
Table 2.
Cox Proportional Hazards Model of Factors Associated with an Emergent Event, Training Sample of Patients*
| N= 46,218 | Emergent Event | |
|---|---|---|
| Factor (Reference Group) | HR | 95% CI |
| Age 70–74 (Age 66–69) | 1.02 | 0.94–1.10 |
| Age 75–80 (Age 66–69) | 1.10 | 1.02–1.18 |
| Age 80+ (Age 66–69) | 1.15 | 1.07–1.24 |
| Male (Female) | 1.13 | 1.07–1.18 |
| Race (White) | ||
| Black | 0.99 | 0.90–1.08 |
| Hispanic | 0.73 | 0.62–0.87 |
| Other | 0.86 | 0.74–1.01 |
| Elixhauser score 0 (≥ 3) | 1.20 | 1.12–1.28 |
| Elixhauser score 1 (≥ 3) | 1.06 | 0.99–1.12 |
| Elixhauser score 2 (≥ 3) | 1.00 | 0.94–1.07 |
| Acute Cholecystitis (BC) | 2.48 | 2.36–2.62 |
| Common Bile Duct Stones (BC) | 2.92 | 2.69–3.18 |
| Gallstone Pancreatitis (BC) | 6.03 | 5.52–6.58 |
| ED visit (physician visit) | 3.48 | 3.28–3.69 |
Emergent Event defined as emergency hospitalization and/or cholecystectomy. HR=hazard ratio, CI= confidence interval, BC= biliary colic, ED= emergency department
Based on this multivariable model, we developed our model, the PREOP-Gallstones model, which can be used to promote shared decision making (Fig. 4). A prognostic nomogram can be used by clinicians to visually calculate probabilities of an outcome using data based on regression modeling. In using this nomogram, a line is drawn vertically from each factor to the corresponding point. Once all factors and points are tabulated, these points can be added, and then another vertical line is used to determine a patient’s 12-month and 24-month risk for developing emergent gallstone-related complications.
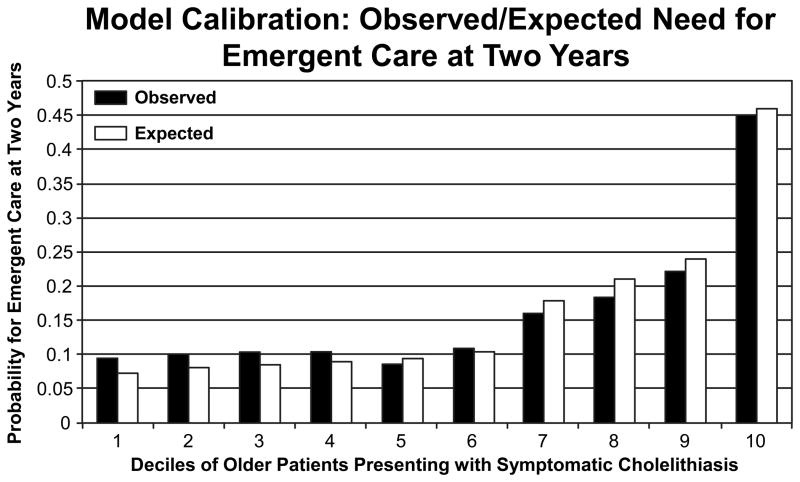
When applied to the validation sample, the C statistic for our model was 0.69 (95% confidence interval, 0.63 to 0.75). Figure 5 shows the calibration plot comparing predicted deciles of risk of emergent events at 2 years and actual observed risk for the training sample. Our model estimates closely approximated the observed mean frequencies of emergent care. Our model was able to accurately identify the 10% of the older population who had a greater than 45% risk of developing gallstone-related complications and 50% of the population whose risk was <10%. Model specificity and sensitivity were 71.2% and 91%, respectively.
Figure 5.
Model calibration results. The observed-to-expected probabilities for patients requiring emergent care (hospitalization and/or cholecystectomy) at two years. The graph illustrates that there was little difference between the expected calculated probability for emergent care and the mean observed probabilities.
DISCUSSION
We used a large population-based cohort to describe the trajectory of care in older patients presenting with an initial episode of symptomatic cholelithiasis who did not undergo elective cholecystectomy. While cholecystectomy is the standard of care for patients presenting with symptomatic cholelithiasis, we observed that many older patients did not undergo elective cholecystectomy. As in prior studies,2,23,24 the majority of older patients with symptomatic cholelithiasis in our study who did not undergo elective cholecystectomy did not ultimately experience complications that required hospitalization or surgery. However, nearly one-third required delayed elective cholecystectomy, had ongoing symptoms, or required emergency gallstone-related hospitalization, and morbidity and in-hospital mortality high for these patients who eventually required emergent care. Based on these data, we developed the PREOP-Gallstones model, a nomogram that reliably predicted patients with an over 40% 2-year risk of developing gallstone-related complications (approximately 10% of the cohort) and an additional 50% of patients with less than 10% 2-year risk.
Our study is the first to incorporate easily measurable characteristics into a risk prediction model to identify the likelihood for emergent biliary-related hospitalization for older patients with biliary disease. A prognostic nomogram visually depicts the findings of multivariate modeling, has been previously implemented into decision modeling for surgical patients,25,26 and has been suggested to enhance communication between patients and practitioners.27–29 Unlike complex statistical models, nomograms can be easily integrated into the patient-practitioner encounter and thereby improve the shared decision-making process. The PREOP-Gallstones model included variables such as age, race/ethnicity, gender, number of comorbidities, initial diagnosis, and location for initial visit, and was able to identify a group of patients at high-risk (>40%) for subsequent gallstone-related emergent hospitalizations and a group at low risk. Practitioners can use these readily-available characteristics to counsel each individual patient on their expected risks for requiring emergency care.
The characteristics associated with emergent biliary complications were not unanticipated. Initial presentation to the emergency department and complicated biliary disease, factors that are likely proxies for disease severity, were also independent risk factors for subsequent hospitalization. Older age has been previously suggested to be a risk factor for persistent biliary symptoms after an initial attack.23 Increased comorbidity, which for these older patients is a threat for a competing risk of early death, was a protective factor for subsequent biliary complications. While this finding appears counterintuitive, the number of comorbidities in this vulnerable cohort of older patients likely limited their life expectancy. Many of these patients with multiple comorbidities may have died from their chronic medical conditions such as heart disease, before they could develop symptoms of gallstone disease. This is further supported by the relatively high 22.9% mortality rate at two years we observed for the overall cohort, and the dramatically elevated 2-year mortality rate of 40.4% for those patients with 3 or more Charlson comorbidities. Finally, we observed, as others have, that male sex is an indicator for increased biliary disease severity.30–36 The male predominance in emergent biliary surgery has been suggested to be related to social factors (men may be less likely follow medical advice),31 biochemical factors (female hormones may sensitize women to the inflammation of cholecystitis), anatomic factors, or less frequent use of medical services amongst men.37
The relative contributions for these factors should also be placed in the appropriate context. The two factors most strongly associated with an emergent presentation were initial patient presentation to the emergency department and a diagnosis of complicated biliary disease. As the nomogram illustrates, a patient presenting with either of these factors has a dramatically elevated risk for emergent biliary complications at two years (15–25%). In contrast, a patient presenting with the cumulative burden of all the other factors combined but without complicated biliary disease or emergent presentation would have only an approximate 10% risk at two years. As a result, the PREOP-Gallstones model could be validated and the practical utility demonstrated by future studies using electronic medical records in clinical practice settings with additional pertinent clinical findings, such as ultrasound characteristics or patient laboratory values.
Our findings also suggest that elective cholecystectomy is underused in a cohort of older patients with symptomatic cholelithiasis who are more likely to experience disease progression than younger patients. The reasons for this disparity are multi-factorial, but one contributing factor is the absence of a solid evidence-base on which to counsel patients. The PREOP-Gallstones model fills this knowledge gap for both patients and practitioners.
Our study has several limitations. Our goal was to review current practice patterns and identify risk factors for developing complications related to gallstones, to determine if all older patients do in fact need cholecystectomy after an initial episode, and if they do not, who would most benefit. As such, we excluded patients admitted to the hospital on the initial episode, as these were patients with severe enough symptoms to require admission. We also excluded patients who underwent an early elective cholecystectomy in the initial 2.5 months after diagnosis (N=24,722), representing only 21% of the study population. For these patients, the decision to perform early cholecystectomy was likely dictated by patient disease severity, patient or practitioner preference, or any number of reasons that our analysis cannot capture. We suspect that the rates of emergent admission would be higher if these 21% did not undergo cholecystectomy. As a result, our model findings only apply to a marginal population of patients in whom the decision to perform cholecystectomy can be difficult.
In addition, the model C-statistic would suggest a marginal discriminatory ability, but our calibration results indicate that these findings are likely due to poor discrimination amongst patients in the lowest deciles of requiring emergent care. However, within each decile, prediction was very accurate, and our model was able to accurately identify the 10% of patients who have a >40% risk of developing emergent biliary complications (high specificity) and over 50% who have a less than 10% 2-year risk.
In addition, the PREOP-Gallstones model is based on administrative data that cannot capture practitioner or patient intent. Some patients may have elected to forego surgery counter to recommendations, or there may have been indications for delaying surgery such as need for anti-platelet therapy for a recent percutaneous coronary intervention. In other cases, patients may have been scheduled for elective cholecystectomy but their disease progressed in the interim. Given the model limitations, physicians should consider the results in the context of each individual patient’s symptom severity, presentation, and preferences. For instance, while one patient may view a 10% risk for emergent care as prohibitive, another may view this as minute, and as a result surgeons should work with patients to develop the optimal individualized treatment plan for them. In this manner our model can be used in uncertain cases to counsel patients that are at high-risk for disease progression and who may benefit most from early elective cholecystectomy. Conversely, our model was able to generally identify certain patients that have low risk (<10%) for subsequent biliary complications, and elective cholecystectomy may not be necessary for this group. Further validation of our model with other samples may be needed to illustrate its utility in this regard.
The PREOP-Gallstones model will enable internists, hospitalists, primary care physicians, and surgeons to use readily identifiable patient characteristics to quantify an individualized risk score of requiring emergent biliary care. Practitioners can use these data to accurately provide patients with their 2-year risk of developing gallstone-related complications at the point of care, allowing patients to make informed decisions in the context of their symptom severity and its impact on their quality of life. This approach has the potential to mitigate the morbidity and mortality of emergency care and reduce unnecessary cholecystectomy for patients who are unlikely to benefit.
Supplementary Material
Acknowledgments
Funding: Supported by grants from the UTMB Clinical and Translational Science Award #UL1TR000071, NIH T-32 Grant # 5T32DK007639, and AHRQ Grant # 1R24HS022134
References
- 1.Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7:132–140. doi: 10.1007/s11894-005-0051-8. [DOI] [PubMed] [Google Scholar]
- 2.Thistle JL, Cleary PA, Lachin JM, et al. The natural history of cholelithiasis: the National Cooperative Gallstone Study. Ann Intern Med. 1984;101:171–175. doi: 10.7326/0003-4819-101-2-171. [DOI] [PubMed] [Google Scholar]
- 3.Epari KP, Mukhtar AS, Fletcher DR, et al. The outcome of patients on the cholecystectomy waiting list in Western Australia 1999–2005. ANZ J Surg. 2010;80:703–709. doi: 10.1111/j.1445-2197.2010.05428.x. [DOI] [PubMed] [Google Scholar]
- 4.Rutledge D, Jones D, Rege R. Consequences of delay in surgical treatment of biliary disease. Am J Surg. 2000;180:466–469. doi: 10.1016/s0002-9610(00)00520-1. [DOI] [PubMed] [Google Scholar]
- 5.Gurusamy KS, Davidson BR. Surgical treatment of gallstones. Gastroenterol Clin North Am. 2010;39:229–244. viii. doi: 10.1016/j.gtc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 6.McSherry CK, Ferstenberg H, Calhoun WF, et al. The natural history of diagnosed gallstone disease in symptomatic and asymptomatic patients. Ann Surg. 1985;202:59–63. doi: 10.1097/00000658-198507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somasekar K, Shankar PJ, Foster ME, et al. Costs of waiting for gall bladder surgery. Postgrad Med J. 2002;78:668–669. doi: 10.1136/pmj.78.925.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurusamy KS, Samraj K, Fusai G, et al. Early versus delayed laparoscopic cholecystectomy for biliary colic. Cochrane Database Syst Rev. 2008:CD007196. doi: 10.1002/14651858.CD007196.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Arthur JD, Edwards PR, Chagla LS. Management of gallstone disease in the elderly. Ann R Coll Surg Engl. 2003;85:91–96. doi: 10.1308/003588403321219849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubecz A, Langer M, Stadlhuber RJ, et al. Cholecystectomy in the very elderly--is 90 the new 70? J Gastrointest Surg. 2012;16:282–285. doi: 10.1007/s11605-011-1708-2. [DOI] [PubMed] [Google Scholar]
- 11.Hazzan D, Geron N, Golijanin D, et al. Laparoscopic cholecystectomy in octogenarians. Surg Endosc. 2003;17:773–776. doi: 10.1007/s00464-002-8529-z. [DOI] [PubMed] [Google Scholar]
- 12.Lo CM, Lai EC, Fan ST, et al. Laparoscopic cholecystectomy for acute cholecystitis in the elderly. World J Surg. 1996;20:983–986. doi: 10.1007/s002689900148. discussion 987. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell JG, Tyler BA, Rutledge R, et al. Cholecystectomy in patients aged 80 and older. Am J Surg. 1998;176:627–631. doi: 10.1016/s0002-9610(98)00282-7. [DOI] [PubMed] [Google Scholar]
- 14.Pavlidis TE, Marakis GN, Symeonidis N, et al. Considerations concerning laparoscopic cholecystectomy in the extremely elderly. J Laparoendosc Adv Surg Tech A. 2008;18:56–60. doi: 10.1089/lap.2007.0037. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed December, 2012];Research, Statistics, Data & Systems. Available at: http://www.cms.gov/Research-Statistics-Data-and-Systems/Research-Statistics-Data-and-Systems.html.
- 16.Center for Medicare & Medicaid Services. [Accessed December, 2012]; Available at: http://www.cms.gov/
- 17.Riall TS, Zhang D, Townsend CM, et al. Failure to perform cholecystectomy for acute cholecystitis in elderly patients is associated with increased morbidity, mortality, and cost. J Am Coll Surg. 2010;210:668–677. 677–679. doi: 10.1016/j.jamcollsurg.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 19.Harrell F. rms: Regression Modeling Strategies.: R package version 4.1–1. 2014 [Google Scholar]
- 20.Uno H, Cai T, Pencina MJ, et al. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–1117. doi: 10.1002/sim.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrell F, Lee K, Califf R. Tutorials in Biostatistics: Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.D’Agostino R, Nam B. Evaluation of the performance of survival analysis models: discrimination and calibration measures. Amsterdam, Netherlands: Elsevier; 2004. [Google Scholar]
- 23.Festi D, Reggiani ML, Attili AF, et al. Natural history of gallstone disease: Expectant management or active treatment? Results from a population-based cohort study. J Gastroenterol Hepatol. 2010;25:719–724. doi: 10.1111/j.1440-1746.2009.06146.x. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt M, Søndenaa K, Vetrhus M, et al. A randomized controlled study of uncomplicated gallstone disease with a 14-year follow-up showed that operation was the preferred treatment. Dig Surg. 2011;28:270–276. doi: 10.1159/000329464. [DOI] [PubMed] [Google Scholar]
- 25.Kiran RP, Attaluri V, Hammel J, et al. A novel nomogram accurately quantifies the risk of mortality in elderly patients undergoing colorectal surgery. Ann Surg. 2013;257:905–908. doi: 10.1097/SLA.0b013e318269d337. [DOI] [PubMed] [Google Scholar]
- 26.Tseng WH, Yang X, Wang H, et al. Nomogram to predict risk of 30-day morbidity and mortality for patients with disseminated malignancy undergoing surgical intervention. Ann Surg. 2011;254:333–338. doi: 10.1097/SLA.0b013e31822513ed. [DOI] [PubMed] [Google Scholar]
- 27.Albert JM, Liu DD, Shen Y, et al. Nomogram to predict the benefit of radiation for older patients with breast cancer treated with conservative surgery. J Clin Oncol. 2012;30:2837–2843. doi: 10.1200/JCO.2011.41.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grobman WA, Stamilio DM. Methods of clinical prediction. Am J Obstet Gynecol. 2006;194:888–894. doi: 10.1016/j.ajog.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Kattan MW, Yu C, Salomon L, et al. Development and validation of preoperative nomogram for disease recurrence within 5 years after laparoscopic radical prostatectomy for prostate cancer. Urology. 2011;77:396–401. doi: 10.1016/j.urology.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Russell JC, Walsh SJ, Reed-Fourquet L, et al. Symptomatic cholelithiasis: a different disease in men? Connecticut Laparoscopic Cholesystectomy Registry. Ann Surg. 1998;227:195–200. doi: 10.1097/00000658-199802000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanabria JR, Gallinger S, Croxford R, et al. Risk factors in elective laparoscopic cholecystectomy for conversion to open cholecystectomy. J Am Coll Surg. 1994;179:696–704. [PubMed] [Google Scholar]
- 32.Botaitis S, Polychronidis A, Pitiakoudis M, et al. Does gender affect laparoscopic cholecystectomy? Surg Laparosc Endosc Percutan Tech. 2008;18:157–161. doi: 10.1097/SLE.0b013e318165c899. [DOI] [PubMed] [Google Scholar]
- 33.Waage A, Nilsson M. Iatrogenic bile duct injury: a population-based study of 152 776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg. 2006;141:1207–1213. doi: 10.1001/archsurg.141.12.1207. [DOI] [PubMed] [Google Scholar]
- 34.Sinha S, Hofman D, Stoker DL, et al. Epidemiological study of provision of cholecystectomy in England from 2000 to 2009: retrospective analysis of Hospital Episode Statistics. Surg Endosc. 2013;27:162–175. doi: 10.1007/s00464-012-2415-0. [DOI] [PubMed] [Google Scholar]
- 35.Harboe KM, Bardram L. The quality of cholecystectomy in Denmark: outcome and risk factors for 20,307 patients from the national database. Surg Endosc. 2011;25:1630–1641. doi: 10.1007/s00464-010-1453-8. [DOI] [PubMed] [Google Scholar]
- 36.Giger UF, Michel JM, Opitz I, et al. Risk factors for perioperative complications in patients undergoing laparoscopic cholecystectomy: analysis of 22,953 consecutive cases from the Swiss Association of Laparoscopic and Thoracoscopic Surgery database. J Am Coll Surg. 2006;203:723–728. doi: 10.1016/j.jamcollsurg.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 37.Pinkhasov RM, Wong J, Kashanian J, et al. Are men shortchanged on health? Perspective on health care utilization and health risk behavior in men and women in the United States. Int J Clin Pract. 2010;64:475–487. doi: 10.1111/j.1742-1241.2009.02290.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.