Abstract
Error-monitoring, or the ability to recognize one's mistakes and implement behavioral changes to prevent further mistakes, may be impaired in individuals with Autism Spectrum Disorder (ASD). Children and adolescents (ages 9-19) with ASD (n = 42) and typical development (n = 42) completed two face processing tasks that required discrimination of either the gender or affect of standardized face stimuli. Post-error slowing and the difference in Error-Related Negativity amplitude between correct and incorrect responses (ERNdiff) were used to index error-monitoring ability. Overall, ERNdiff increased with age. On the Gender Task, individuals with ASD had a smaller ERNdiff than individuals with typical development; however, on the Affect Task, there were no significant diagnostic group differences on ERNdiff. Individuals with ASD may have ERN amplitudes similar to those observed in individuals with typical development in more social contexts compared to less social contexts due to greater consequences for errors, more effortful processing, and/or reduced processing efficiency in these contexts. Across all participants, more post-error slowing on the Affect Task was associated with better social cognitive skills.
Keywords: Error-Related Negativity (ERN), post-error slowing, error-monitoring, face processing, autism, development, individual differences
INTRODUCTION
Error-monitoring is an executive functioning skill characterized by the ability to monitor one's actions, recognize when one has made a mistake, and implement behavioral changes to prevent further mistakes from occurring. The ERN is an electrophysiological indicator of error-monitoring ability; it is a negative Event-Related Potential (ERP) that generally appears within 100 milliseconds after a person incorrectly responds to a stimulus (Holroyd & Coles, 2002). The literature suggests that the ERN is generated within the caudal region of the anterior cingulate cortex (ACC; O'Connell et al., 2007) and is produced whenever there is a mismatch between the participant's enacted (erroneous) response and the intended correct response (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000). Research on the relation between the ERN and error awareness has yielded mixed results (Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001; Scheffers & Coles, 2000), with a recent review suggests that the ERN may serve as a feed-forward signal that provides input to the process of emerging error-awareness (Wessel, 2012). Post-error slowing is a behavioral indicator of error-monitoring ability. Post-error slowing occurs when a participant slows down on subsequent trials after having made an error; it is thought to be a compensatory mechanism by which the participant avoids making errors on future trials (Rabbitt, 1966).
Error-Monitoring in Autism Spectrum Disorder
Rueda, Posner, and Rothbart (2005) suggest that the development of attentional control and monitoring is critical for the emergence of self-regulation. In Autism Spectrum Disorder (ASD), error-monitoring ability may be important for regulating autistic symptomology. Thakkar et al. (2008), for example, show that functional and structural abnormalities of the ACC may contribute to restricted and repetitive behaviors in ASD. In addition, self-monitoring interventions, such as those that teach participants to use a checklist to monitor their behavior, are often associated with decreases in stereotypic behaviors and increases in social skills (e.g., Koegel, Koegel, Hurley, & Frea, 1992; Morrison, Kamps, Garcia, & Parker, 2001; Parker & Kamps, 2011). Given that error-monitoring impairments may exacerbate and error-monitoring improvements may ameliorate autistic symptomology, error-monitoring ability may be a key construct to consider in ASD.
Overall, individuals with ASD show poor error-monitoring compared to individuals with typical development. Individuals with ASD tend to display smaller ERN amplitudes and show less post-error slowing (Bogte, Flamma, van der Meere, & van Engeland, 2007; Santesso et al., 2011; Sokhadze et al., 2010, 2012; South, Larson, Krauskopf, & Clawson, 2010; Vlamings, Jonkman, Hoeksma, van Engeland, & Kemner, 2008). Thus, individuals with ASD seem to have more difficulty recognizing errors and are less likely to engage in compensatory mechanisms following error commission.
Since individuals with ASD have more difficulty processing information in social versus nonsocial contexts (e.g., Dichter & Belger, 2007; Ozonoff, 1995), individuals with ASD may also have more difficulty monitoring errors in social versus nonsocial contexts. In the current literature, error-monitoring ability in individuals with ASD has only been examined using standard, nonsocial lab tasks, such as the Flanker task. A better understanding of error-monitoring ability in social contexts may be critical for developing interventions to improve social awareness and behavior in ASD.
Error-Monitoring across Development
Changes in brain structure, function, and connectivity occur with age (see Casey, Galvan, & Hare, 2005 for a review). Correspondingly, executive functioning skills also mature with age (e.g., Brocki & Bohlin, 2004; Huizinga, Dolan, & van der Molen, 2006). In error-monitoring, the amplitude of the ERN increases with age in individuals with typical development (e.g., Davies, Segalowitz, & Gavin, 2004; Hogan, Vargha-Khadem, Kirkham, & Baldeweg, 2005; Ladouceur, Dahl, & Carter, 2007; Santesso & Segalowitz, 2008; Segalowitz & Davies, 2004; Wiersema, van der Meere, & Roeyers, 2007). This age-related increase may be associated with greater awareness of errors and/or maturation of the ACC. The effect of age on post-error slowing in typical development is still unclear, with some studies suggesting that post-error slowing increases with age (Hogan et al., 2005; Santesso & Segalowitz, 2008), but others suggesting that post-error slowing is not influenced by age (Davies et al., 2004; Ladouceur et al., 2007; Segalowitz & Davies, 2004; Wiersema et al., 2007).
Given that individuals with ASD show improvements in some executive functions with age (Robinson, Goddard, Dritschel, Wisley, & Howlin, 2009) and given that age is known to influence error-monitoring ability in children and adolescents with typical development, age may also influence error-monitoring ability in children and adolescents with ASD. In their study on error-monitoring in ASD, Santesso et al. (2011) briefly note that age does not attenuate diagnostic group differences in error-monitoring, but they do not directly examine the effects of age on error-monitoring in ASD. Thus, in the current literature, it is not clear whether error-monitoring ability in individuals with ASD changes with age. If error-monitoring ability in individuals with ASD improves with age, it offers further support that this ability is malleable and may be amenable to intervention (e.g., Koegel et al., 1992; Morrison et al., 2001; Parker & Kamps, 2011).
Individual Differences in Error-Monitoring
Autistic Symptomology
Autistic symptomology is defined by impairments in social interaction and communication and restricted and repetitive behaviors, interests, and/or activities (American Psychiatric Association, 2013). Individuals with ASD who are able to recognize and compensate for errors may show reductions in autistic symptomology (e.g., Koegel et al., 1992; Morrison et al., 2001; Parker & Kamps, 2011). In the literature, the relation between error-monitoring ability and autistic symptomology is unclear: greater error-monitoring ability, indexed by larger ERN amplitudes, has been associated with less autistic symptomology (Henderson et al., 2006; Santesso et al., 2011), more autistic symptomology (South et al., 2010), and has shown no relation with autistic symptomology (Vlamings et al., 2008). A limitation of these prior studies is the broad age range examined, without controlling for the effects of age. Across development, error-monitoring ability tends to increase and autistic symptomology tends to decrease (e.g., Esbensen, Seltzer, Lam, & Bodfish, 2009; Seltzer et al., 2003); thus, it may be important to consider and control for the effects of age when evaluating the relation between error-monitoring ability and autistic symptomology.
Social Cognition
Social cognition is the ability to cognitively process social information and includes skill sets such as thinking about someone else's thoughts (theory of mind) and processing facial affect (e.g., Baron-Cohen, Wheelwright, Hill, Raste, & Plumb, 2001; Happe, 1994). The ability to monitor one's actions may be critical for the development of higher-order social cognitive skills (e.g., Mundy & Newell, 2007). Larson, Fair, Good, and Baldwin (2010) and Santesso and Segalowitz (2009) reported that larger ERN amplitudes were associated with increased empathy in individuals with typical development. Larson et al. (2010) suggest that error-monitoring ability and empathy are both rooted in vigilance: error-monitoring requires vigilance to one's own actions whereas empathy requires vigilance to the actions and feelings of other people. Hoffmann, Wascher, and Falkenstein (2012) recently noted that larger ERN amplitudes were associated with greater social orientation (e.g., taking social responsibility, being helpful). In the ASD literature, Santesso et al. (2011) reported that larger ERN amplitudes related to better social skills in individuals with ASD. Again, the effects of age were not controlled for in this analysis, and given that both error-monitoring ability and social skills increase with age (e.g., Hu, Chan, & McAlonan, 2010; Zajdel, Bloom, Fireman, & Larsen, 2013), age may be an important confounding variable to consider and control for in such analyses.
The Current Study
In the current study, we examined error-monitoring ability, specifically post-error slowing and the ERN, in individuals with and without ASD ranging in age from 9-19 years old. The first goal of this study was to examine the influence of social context on error-monitoring ability in ASD. To our knowledge, the current paper is the first to examine post-error slowing and the ERN in response to social stimuli in individuals with ASD; it is also the first to compare post-error slowing and the ERN across more social (affect processing) versus less social (gender processing) contexts in individuals with ASD. On the basis of the extant literature, we predicted that individuals with ASD would show poor error-monitoring (less post-error slowing and smaller ERN amplitudes) compared to individuals with typical development. We also predicted that individuals with ASD would have more difficulty monitoring errors on the Affect Task compared to the Gender Task while individuals with typical development would monitor errors similarly across both tasks.
The second goal of this study was to evaluate the effect of age on error-monitoring ability in ASD. To our knowledge, the current paper is the first to directly examine age effects on post-error slowing and the ERN in individuals with ASD. The previous literature on error-monitoring led us to predict that older participants, regardless of diagnostic group, would show more error-monitoring (more post-error slowing and larger ERN amplitudes) than younger participants.
The final goal of this study was to examine associations between error-monitoring ability and individual differences in autistic symptomology and social cognition. To our knowledge, the current paper is the first to examine these associations in individuals with ASD while controlling for the effects of age, a potential confounding variable. We hypothesized that more error-monitoring (more post-error slowing and larger ERN amplitudes) would be associated with less autistic symptomology and better social cognitive skills.
METHOD
Participants
Participants were part of a larger ongoing study of social and emotional development in children with ASD. Participants with ASD (n = 60) were recruited from the University of Miami/Nova Southeastern University Center for Autism and Related Disabilities. Participants with typical development (n = 54) were recruited from local schools. In the initial screening, participants were excluded from participation if they had a history of seizures, a genetic condition (e.g., Fragile X Syndrome), a reading level below the second grade, psychotic symptoms, a previously abnormal EEG, or if they were nonverbal. In addition, participants with ASD had to have an ASD diagnosis from a community mental health professional.
After informed consent was obtained, participants underwent a second screening. Participants with ASD were required to meet 2 of the following 3 diagnostic criteria: ≥ 7 on the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, Dilavore, & Risi, 2002), ≥ 13 on the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003), and ≥ 13 on the Autism Spectrum Screening Questionnaire (ASSQ; Ehlers, Gillberg, & Wing, 1999). Participants with typical development were excluded from the sample if they met any of these cutoff scores.1 Additionally, all participants had to have a verbal IQ ≥ 70, as assessed by the Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV; Wechsler, 2003) or Wechsler Adult Intelligence Scale – Fourth Edition (WAIS-IV; Wechsler, 2008).
Eighteen participants with ASD were excluded from the final sample: 6 participants did not complete the experimental protocol, 6 participants did not meet the diagnostic criteria, 1 participant had not officially received an ASD diagnosis from a community mental health professional, 1 participant did not respond within the timing parameters of the Affect Task, and 4 participants with the lowest verbal IQ were excluded for matching purposes. Twelve participants with typical development were excluded from the final sample: 8 participants met the diagnostic cutoff for autism on one or more measures and 4 participants with the highest verbal IQ were excluded for matching purposes. The final sample for this study was 42 participants with ASD (38 males) and 42 participants with typical development (36 males). The diagnostic groups did not significantly differ on age, t(82) = -0.02, p = 0.98, performance IQ, t(82) = 1.26, p = 0.21, or gender distribution, χ(1, N = 84) = 0.45, p = 0.50. Since verbal IQ approached significance, t(82) = 1.99, p = 0.05, its effects were considered in the analyses. See Table 1.
Table 1.
Participant characteristics
| ASD |
TD |
|||||
|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | |
| Age (years) | 15.17 | 2.68 | 9.59 - 19.28 | 15.16 | 2.90 | 9.48 - 19.21 |
| Verbal Comprehension Index | 103.17 | 15.87 | 77 - 140 | 108.88 | 9.69 | 81 - 128 |
| Perceptual Reasoning Index | 103.19 | 15.03 | 69 - 135 | 107.38 | 15.51 | 75 - 143 |
| Autistic Symptomology Composite | 0.88* | 0.41* | 0.13 - 2.04* | −0.90 | 0.22 | −1.21 - −0.36 |
| Social Cognition Composite | −0.39* | 0.83* | −2.91 - 0.70* | 0.35 | 0.58 | −1.53 - 1.15 |
Include scores from participants who were included in the post-error response time and/or ERN amplitude analyses (n = 41).
Measures
Many participants in the current study had completed assessments in our laboratory as part of a larger ongoing study. The WISC-IV and ADOS show good test-retest reliability over time (Lord et al., 2000; Williams, Weiss, & Rolfhus, 2003); thus, if a participant completed the WISC-IV or ADOS as part of the larger study within the previous two years, scores from the previous assessment were used. In addition, if any of the other assessments had been completed within the previous six months, scores from that previous assessment were used.
Cognitive
Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV; Wechsler, 2003) & Wechsler Adult Intelligence Scale – Fourth Edition (WAIS-IV; Wechsler, 2008)
The majority of participants were assessed using the WISC-IV (n = 65). Participants older than 16 who had not been assessed on the WISC-IV in the previous two years were assessed using the WAIS-IV (n = 19). WISC-IV and WAIS-IV scores are highly correlated, and both assessments have well-established reliability and validity (Wechsler, 2008; Williams et al., 2003). The Vocabulary and Similarities subscales were used to estimate the Verbal Comprehension Index, and the Matrix Reasoning and Block Design subscales were used to estimate the Perceptual Reasoning Index.
Autistic Symptomology
Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2002)
The ADOS is a semi-structured observational assessment that evaluates an individual's language and communication, reciprocal social interaction, imagination, and stereotyped behaviors and restricted interests. The ADOS has high reliability and validity, with an algorithm that is both specific and sensitive in identifying individuals with autism (Lord et al., 2000).
Autism Spectrum Screening Questionnaire (ASSQ; Ehlers et al., 1999)
This 28-item questionnaire asks parents to rate their child's behaviors as being the same, somewhat different, or different from the behaviors of other children. This measure has good reliability and has been validated against other assessments of behavioral disorders (Ehlers et al., 1999).
Social Communication Questionnaire (SCQ; Rutter et al., 2003)
This parent questionnaire was developed from the 40 critical items of the Autism Diagnostic Interview (ADI; Lord, Rutter, & Le Couteur, 1994). As with the ADI, this questionnaire focuses on reciprocal social interaction, communication, and repetitive and stereotyped patterns and behaviors. The SCQ has good reliability and has been validated by high correlations with the ADI (Berument, Rutter, Lord, Pickles, & Bailey, 1999).
Social Responsiveness Scale (SRS; Constantino & Gruber, 2005)
This 65-item parent-report questionnaire focuses on children's social awareness, cognition, communication, motivation, and mannerisms. The questionnaire has high inter-rater reliability and has been validated against the ADI (Constantino et al., 2003).
Social Cognition
Reading the Mind in the Eyes Test (Baron-Cohen et al., 2001)
In this task, participants are presented with 28 photographs of a person's eyes and four emotion words. Participants choose the emotion that best describes what each person is thinking or feeling. This task has acceptable reliability (Voracek & Dressler, 2006). Individuals with ASD show impaired performance on this task, but not on a gender recognition control task (Baron-Cohen et al., 2001).
Strange Stories Task (Happe, 1994)
Participants read a series of 12 stories that feature social situations designed to assess mentalizing ability, such as a lie, sarcasm, or figure of speech. Participants answer questions about these stories; their answers are first coded as correct or incorrect and second coded as providing physical or mental explanations of the story. This measure has been validated by associations with theory-of-mind tasks and is sensitive to diagnostic group differences in social cognition (Happe, 1994).
Composite Scores
Total scores from the ASSQ, SCQ, and SRS were highly correlated (Intraclass Correlation Coefficient (ICC) = 0.91) and formed a composite measure of Autistic Symptomology. The Reading the Mind in the Eyes Test Total, the number of questions answered correctly on the Strange Stories Task, and the number of mental explanations given on the Strange Stories Task were substantially correlated (ICC = 0.65) and formed a composite measure of Social Cognition. To form the composite measures, scores from the contributing assessments were standardized and averaged together. If data were missing on one or more of the contributing assessments, scores on the remaining assessment(s) were used. See Table 1.
EEG Procedure and Task
Participants wore a 128-lead Geodesic sensor net, and EEG data were continuously recorded. When possible, impedances were kept below 40 kΩ; data were edited after data collection to remove any electrode channels that exceeded the noise threshold (see section on EEG Data Editing and Reduction). The EEG signal was amplified (x1000) and filtered (0.1 Hz high-pass filter and 100 Hz elliptical low-pass filter). The conditioned signal was multiplexed and digitized at 250 Hz using an analog-to-digital converter and a Macintosh computer. A Dell computer, interfaced and synchronized via serial port, generated the stimuli using E-Prime software. Data were collected referenced to VRef (Cz).
Participants were alternately assigned to complete either the Gender or Affect Task as their first task. On both tasks, participants viewed a series of color face photographs from the NimStim Face Stimulus Set (Tottenham et al., 2009) and used a button box to indicate whether each face was male or female (Gender Task), or angry or happy (Affect Task). Participants completed 15 practice trials and 30 timing trials in which face photographs were presented for 750 ms and participants had to make a decision about the gender/affect of the face within this period of time. During the test trials, participants were presented with 3 sets of 104 face stimuli (312 total face stimuli). The timing parameters for the test trials were individually titrated for the Gender Task (M = 361.36, SD = 90.25) and Affect Task (M = 402.69, SD = 111.10), based on each participant's reaction time and accuracy in the timing trials. Automated feedback was presented following the participant's response: correct, not correct, or too slow to respond.
EEG Data Editing and Reduction
NetStation (Luu et al., 2010), EEGLAB (Delorme & Makeig, 2004), and ERPLAB (Lopez-Calderon & Luck, 2014) were used to process the EEG data. Data were re-filtered at a lowpass of 30 Hz. Data were segmented into four categories: correct responses to happy faces, incorrect responses to happy faces, correct responses to angry faces, and incorrect responses to angry faces. A channel with more than 200 μV between its minimum and maximum amplitude values (performing a moving average of 80 ms) for a given segment was identified as a bad channel for that segment. A channel was marked as a bad channel throughout the whole recording if it was marked bad for more than 25% of the segments. Segments with more than 15 bad channels were rejected. If a segment had 15 or fewer bad channels, the data in the bad channels were replaced with data interpolated from nearby good channels.
Data were baseline corrected to the interval -100 to 0 ms. Independent components analysis was run on all channels, and components that were indicative of eye blinks were removed from the data (Delorme, Fernsler, Serby, & Makeig, 2006). Artifact detection was performed on electrode sites of interest in order to detect and remove portions of data with a step-like change in voltage greater than 100 μV. In addition, all segments were manually inspected, and any remaining noisy segments were removed. Data were re-referenced to an average reference, and segments were grand-averaged within participants.
Data Analyses
Trials in which the participant responded in less than 100 ms were removed from further analysis. Accuracy was operationalized as the proportion of correct responses out of the total number of recorded responses. Reaction time was operationalized as the reaction time for correct responses.
Participants were included in the data analyses for post-error response time if they had at least 10 instances each of error trials directly followed by correct trials (i.e., error-correct trials) and correct trials directly followed by correct trials (i.e., correct-correct trials) on both the Gender and Affect Tasks. Five participants with ASD did not meet this criterion. Post-error response time was calculated as the mean reaction time of error-correct trials minus the mean reaction time of correct-correct trials. Thus, a positive post-error response time is indicative of slowing down on trials following an error (i.e., post-error slowing).
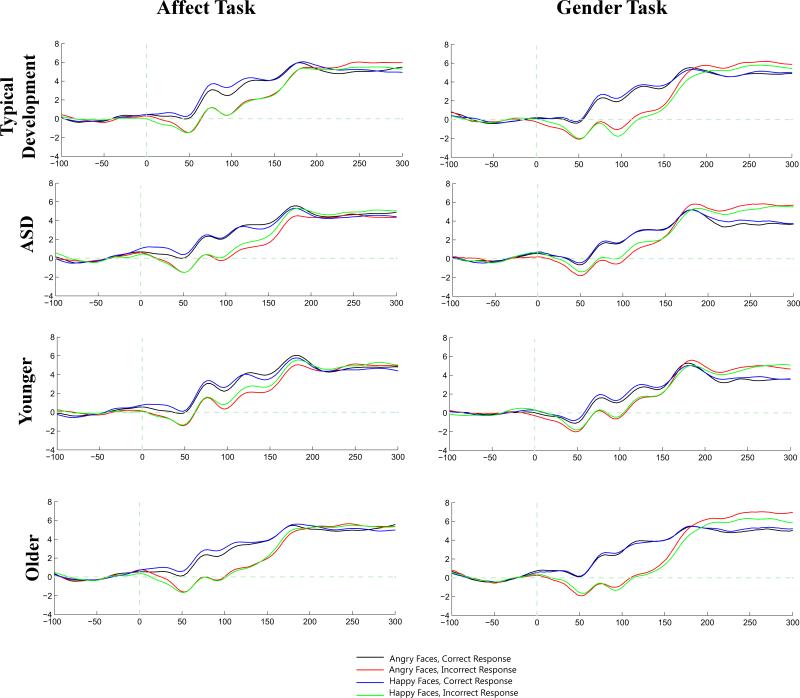
Olvet and Hajcak (2009) and Pontifex et al. (2010) show that the ERN can be reliably quantified using 6-8 error trials. Thus, in the present study, participants were included in the data analyses for ERN amplitude if they had at least 10 artifact-free trials for all 4 conditions on both the Gender and Affect Tasks. Four participants with ASD and three participants with typical development did not meet this criterion.2 ERN amplitude was calculated as the mean amplitude between 25-75 ms following the participant's response and was examined across the following midline electrode sites: 11 (Fz), 6 (Fcz), VRef (Cz), 55 (Cpz), and 62 (Pz). See Figure 1.
Figure 1.
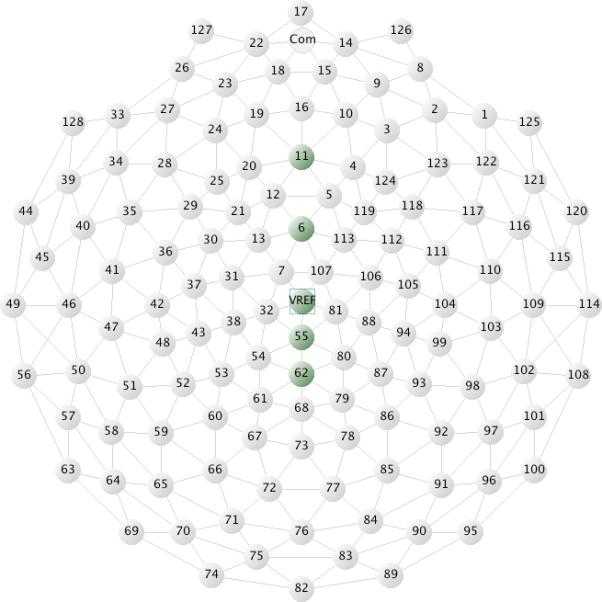
Electrode sites used to evaluate the ERN.
Of the segments eligible to be included in the data analyses for ERN amplitude (response time ≥ 100 ms, participant responded to the stimulus), an average of 3.3% / 2.7% of segments were removed from the Affect Task and an average of 4.6% / 4.1% segments were removed from the Gender Task for participants with typical development / ASD due to bad channels or artifact detection. On average, 57 / 53 segments per category for the Affect Task and 54 / 51 segments per category for the Gender Task were included in the analyses for participants with typical development / ASD.
RESULTS
Preliminary Analyses
A preliminary ANOVA analysis with ERN amplitude as the dependent variable was performed to determine which electrode sites showed the strongest ERN response (i.e., the most discrimination between correct and incorrect trials). Task (Gender vs. Affect), Electrode Site (6, 11, VRef, 55, 62), and Accuracy (correct vs. incorrect response) were the within-subjects factors. The analysis yielded a main effect of Electrode Site, F(4, 304) = 94.07, p < 0.01, η2p = 0.55, and a main effect of Accuracy, F(1, 76) = 74.32, p < 0.01, η2p = 0.49, which were qualified by a significant interaction between Electrode Site and Accuracy, F(4, 304) = 20.96, p < 0.01, η2p = 0.22. Post hoc probing showed that incorrect trials had a more negative amplitude than correct trials across all electrode sites; however, the difference between correct and incorrect trials was particularly salient for electrode sites 6, t(76) = 9.55, p < 0.01, and VRef, t(76) = 8.00, p < 0.01. Therefore, electrode sites 6 (Fcz) and VRef (Cz) were the focus of further analyses.
In the remaining ERN analyses, the difference score between correct and incorrect trials was analyzed, with the amplitude of incorrect trials subtracted from the amplitude for correct trials (i.e., ERNDiff). Thus, a positive ERNDiff value is indicative of a more negative amplitude for incorrect trials than correct trials. The ERNDiff was calculated as the average of the difference scores for electrode sites 6 (Fcz) and VRef (Cz).
Task Results
ANCOVAs were used to evaluate the effects of Diagnostic Group (ASD vs. typical development) and Task (Gender vs. Affect) on accuracy, reaction time, post-error response time, and ERNDiff. Facial expression (happy vs. angry) was also included as a factor in the accuracy, reaction time, and ERNDiff analyses, as there is some evidence to suggest that the ERN is enhanced for happy/positive stimuli compared to angry/negative stimuli (Compton et al., 2007; Larson, Perlstein, Stigge-Kaufman, Kelly, & Dotson, 2006). Facial expression was not included as a factor in the post-error response time analyses, as consecutive trials did not necessarily share the same facial expression. In order to examine the effects of age and verbal IQ as continuous variables, they were initially included as covariates in the analyses.
The ANCOVA assumption of parallel regression slopes was examined by testing for an interaction between diagnostic group and age and an interaction between diagnostic group and verbal IQ. The interaction terms were not significant for any of the ANCOVA analyses, suggesting that the effects of age and verbal IQ were similar across diagnostic groups. Thus, the assumption of parallel regression slopes was met, and the interaction terms were removed from further analyses. As verbal IQ did not have a significant effect in any of the subsequent analyses, it was removed as a covariate. See Table 2 for the marginal means for accuracy, reaction time, post-error response time, and ERNDiff.
Table 2.
Estimated marginal means for accuracy (%) and reaction time (ms), evaluated at a mean age of 15.17 years; post-error response time (ms), evaluated at a mean age of 15.16 years; and ERNDiff (μV), evaluated at a mean age of 15.22 years
| Affect Task |
Gender Task |
|||
|---|---|---|---|---|
| Angry Faces | Happy Faces | Angry Faces | Happy Faces | |
| Accuracy | ||||
| ASD | 58.2 (2.0) | 60.1 (2.0) | 61.0 (1.8) | 60.5 (1.8) |
| Typical Development | 59.5 (2.0) | 62.0 (2.0) | 63.7 (1.8) | 64.1 (1.8) |
|
Reaction Time | ||||
| ASD | 286.38 (13.93) | 285.75 (13.45) | 265.09 (10.52) | 266.51 (10.44) |
| Typical Development | 291.83 (13.93) | 288.81 (13.45) | 266.43 (10.52) | 265.62 (10.44) |
|
Post-Error Response Time* | ||||
| ASD | −0.21 (3.16) | −0.93 (2.68) | ||
| Typical Development | −0.52 (2.96) | −0.29 (2.52) | ||
|
ERNDiff | ||||
| ASD | 1.49 (0.35) | 1.48 (0.32) | 0.98 (0.31) | 0.70 (0.34) |
| Typical Development | 1.28 (0.34) | 1.74 (0.32) | 1.66 (0.30) | 1.82 (0.33) |
Note. Standard errors are in brackets. Age is evaluated at slightly different ages in the models due to small differences in sample size.
Facial expression (angry vs. happy) was not included as a factor in the post-error response time analyses.
Accuracy and Reaction Time
There was a main effect of age on accuracy, F(1, 81) = 9.73, p < 0.01, η2p = 0.11, such that accuracy increased with age. Across both the Affect and Gender Tasks, there was a main effect of facial expression on reaction time, F(1, 81) = 4.57, p = 0.04, η2p = 0.05, which was qualified by an interaction between facial expression and age, F(1, 81) = 4.14, p = 0.05, η2p = 0.05. To follow-up, the marginal means for facial expression were compared at one standard deviation below the mean age (12.39 years), the mean age (15.17 years), and one standard deviation above the mean age (17.94 years), as recommended by Aiken and West (1991). In order to control for multiple comparisons in the post hoc analyses, we applied a Bonferroni correction such that p = 0.017 (α = 0.05/3) was used as the cut-off for significant results and p = 0.033 (α = 0.10/3) was used as the cut-off for marginally significant results. While the effect of facial expression was not significant for participants at the younger age, F(1, 81) = 3.92, p = 0.051, η2p = 0.05, the mean age, F(1, 81) = 0.58, p = 0.449, η2p = 0.01, or the older age, F(1, 81) = 0.82, p = 0.367, η2p = 0.01, younger participants tended to respond more quickly to happy faces whereas older participants tended to respond more quickly to angry faces.
Post-Error Response Time
There was a main effect of task on post-error response time, F(1, 76) = 4.07, p = 0.05, η2p = 0.05, which was qualified by an interaction between task and age, F(1, 76) = 4.27, p = 0.04, η2p = 0.05. To follow-up, the marginal means for task were compared at one standard deviation below the mean age (12.35 years), the mean age (15.16 years), and one standard deviation above the mean age (17.97 years), as recommended by Aiken and West (1991). In order to control for multiple comparisons in the post hoc analyses, we applied a Bonferroni correction such that p = 0.017 (α = 0.05/3) was used as the cutoff for significant results and p = 0.033 (α = 0.10/3) was used as the cut-off for marginally significant results. While the effect of task was not significant for participants at the younger age, F(1, 76) = 1.97, p = 0.164, η2p = 0.03, the mean age, F(1, 76) = 0.01, p = 0.933, η2p < 0.01, or the older age, F(1, 76) = 2.32, p = 0.132, η2p = 0.03, younger participants tended to show more post-error slowing on the Gender Task and older participants tended to show more post-error slowing on the Affect Task.
ERN Amplitude
There was a main effect of age on ERNDiff, F(1, 74) = 15.53, p < 0.01, η2p = 0.17, such that participants showed greater differentiation between correct and incorrect responses with age. There was also a marginal main effect of diagnostic group on ERNDiff, F(1, 74) = 2.85, p = 0.10, η2p = 0.04, which was qualified by a marginal interaction between diagnostic group and task, F(1, 74) = 3.37, p = 0.07, η2p = 0.04. In order to control for multiple comparisons in the post hoc analyses, we applied a Bonferroni correction such that p = 0.013 (α = 0.05/4) was used as the cut-off for significant results and p = 0.025 (α = 0.10/4) was used as the cut-off for marginally significant results. Follow-up ANCOVAs showed that individuals with typical development had a marginally larger ERNDiff than individuals with ASD on the Gender Task, F(1, 74) = 5.77, p = 0.019, η2p = 0.07. In contrast, diagnostic group was unrelated to ERNDiff on the Affect Task, F(1, 74) = 0.01, p = 0.934, η2p < 0.01. The effect of task was not significant for individuals with ASD, F(1, 36) = 1.89, p = 0.178, η2p = 0.05, or individuals with typical development, F(1, 37) = 0.23, p = 0.631, η2p = 0.01. See Figures 2-3.
Figure 2.
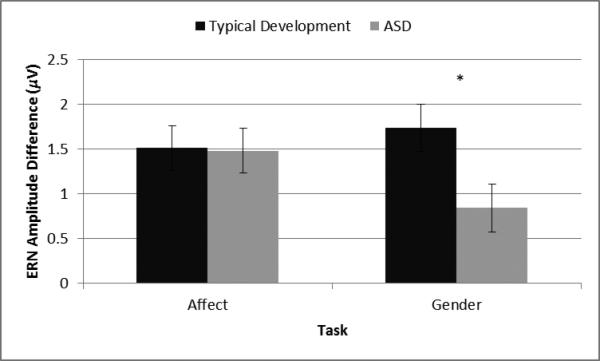
The interaction between task and diagnostic group on ERNDiff. * p < 0.025
Figure 3.
Grand averaged waveforms on the Affect and Gender Tasks for participants with typical development and ASD, regardless of age, and younger (< 15.8 years) and older (> 15.8 years) participants, regardless of diagnostic group. Electrode 6 (Fcz) is shown.
Individual Differences in Error-Monitoring
Four hierarchical linear regressions were used to examine the associations between error-monitoring (post-error response time or ERNDiff) and individual differences in autistic symptomology and social cognition. In each regression, diagnostic group (0 = typical development, 1 = ASD), age (centered), and verbal IQ (centered) were entered in the first block of predictors; the designated error-monitoring variables (post-error response time or ERNDiff, centered) for both Affect and Gender Tasks were entered in the second block of predictors; and the interactions between those designated error-monitoring variables and diagnostic group were entered in the third block of predictors. Note that the results for all of the predictors are reported in Table 3, but only the results for predictors of theoretical interests (i.e., variables in the second and third blocks) are presented in the text.
Table 3.
Hierarchical regression models
| Autistic Symptomology |
Social Cognition |
|||
|---|---|---|---|---|
| B | β | B | β | |
| Post-Error Response Time | ||||
| Diagnostic Group | 1.75 (0.08)** | 0.93 | −0.62 (0.13)** | −0.38 |
| Age | 0.01 (0.01) | 0.03 | 0.10 (0.02)** | 0.34 |
| Verbal IQ | 0.00 (0.00) | −0.03 | 0.02 (0.01)** | 0.37 |
| Affect Post-Error Response Time | 0.01 (0.00)* | 0.16 | ||
|
ERNDiff | ||||
| Diagnostic Group | 1.75 (0.08)** | 0.93 | −0.57 (0.13)** | −0.39 |
| Age | 0.01 (0.01) | 0.02 | 0.10 (0.02)** | 0.37 |
| Verbal IQ | 0.00 (0.00) | −0.03 | 0.02 (0.01)** | 0.36 |
Note. Standard errors are in brackets.
p < 0.05
p < 0.10.
Autistic Symptomology
Post-Error Response Time
The first block significantly predicted autistic symptomology, F(3, 75) = 188.52, p < 0.01. The second block, ΔF(2, 73) = 0.24, p = 0.79, did not significantly predict autistic symptomology above and beyond the first block. Thus, the first block was retained as the final model and predicted 88.3% of the variance in autistic symptomology.
ERNDiff
The first block significantly predicted autistic symptomology, F(3, 73) = 193.61, p < 0.01. The second block, ΔF(2, 71) = 0.65, p = 0.52, did not significantly predict autistic symptomology above and beyond the first block. Thus, the first block was retained as the final model and predicted 88.8% of the variance in autistic symptomology.
Social Cognition
Post-Error Response Time
The first block significantly predicted social cognition, F(3, 75) = 24.49, p < 0.01. The second block, ΔF(2, 73) = 2.10, p = 0.13, did not significantly predict social cognition above and beyond the first block. However, post-error response time on the Affect Task marginally predicted social cognition, t(73) = 1.95, p = 0.06. Thus, a separate model was run in which post-error response time on the Affect Task was the only predictor in the second block. In this separate model, the second block marginally predicted social cognition above and beyond the first block, ΔF(1, 74) = 3.73, p = 0.06. Thus, the second block (containing only post-error response time on the Affect Task) was retained as the final model and predicted 51.9% of the variance in social cognition. In this model, more post-error slowing on the Affect Task was marginally associated with better social cognitive skills, t(74) = 1.93, p = 0.06.
ERNDiff
The first block significantly predicted social cognition, F(3, 73) = 19.35, p < 0.01. The second block, ΔF(2, 71) = 0.71, p = 0.49, did not significantly predict social cognition above and beyond the first block. Thus, the first block was retained as the final model and predicted 44.3% of the variance in social cognition.
Summary of Results
Accuracy in identifying the gender and emotional expression of faces increased with age for both participants with typical development and ASD. Across the Affect and Gender Tasks, younger participants tended to respond more quickly to happy faces whereas older participants tended to respond more quickly to angry faces; additionally, younger participants showed more evidence of post-error slowing on the Gender Task whereas older participants showed more evidence of post-error slowing on the Affect Task. ERNDiff increased with age for both participants with typical development and ASD. On the Gender Task only, participants with typical development showed a marginally greater ERNDiff than participants with ASD. Finally, above and beyond the effects of diagnosis, age, and verbal IQ, more post-error slowing on the Affect Task was marginally associated with better social cognitive skills.
DISCUSSION
Developmental Effects
In the current study, age had a stronger and more ubiquitous effect on both face processing and error-monitoring ability than diagnosis. This result is consistent with recent literature suggesting that development may be a stronger predictor of face processing ability than diagnosis in higher-functioning children and adolescents with ASD (Hileman, Henderson, Mundy, Newell, & Jaime, 2011). This result is also consistent with the error-monitoring literature, in which participants show larger ERN amplitudes with age (e.g., Davies et al., 2004; Hogan et al., 2005; Ladouceur et al., 2007; Santesso & Segalowitz, 2008; Segalowitz & Davies, 2004; Wiersema et al., 2007), and our general hypothesis of improved error-monitoring ability with age. The current study suggests that children and adolescents, regardless of diagnostic group, experience maturational changes in the neural systems underpinning error-monitoring and become better at error-monitoring with age. As both face processing accuracy and error-monitoring ability increased with age, greater error-monitoring ability may have led to more accurate face processing, or conversely, greater skill in face processing may have facilitated error-monitoring.
Diagnostic Group Effects
Participants with ASD and typical development did not show any differences in accuracy or reaction time on the Affect and Gender Tasks, suggesting that these tasks were of similar difficulty across diagnostic groups. This lack of diagnostic group differences in task performance allows for an unbiased comparison of error-monitoring ability across groups.
Contrary to our hypotheses of 1) robust deficits in both behavioral and electrophysiological indices of error-monitoring and 2) greater error-monitoring deficits on the Affect Task compared to the Gender Task, individuals with ASD and typical development only showed differential error-monitoring on ERN amplitude for the Gender Task. Individuals with ASD and typical development showed comparable error-monitoring on post-error response time for the Affect and Gender Tasks and ERN amplitude for the Affect Task.
These results are contrary to much of the existing literature (Bogte et al., 2007; Santesso et al., 2011; Sokhadze et al., 2010, 2012; South et al., 2010; Vlamings et al., 2008) and may suggest that error-monitoring is not consistently impaired in individuals with ASD. An important difference between the current study and the existing literature is the context in which error-monitoring occurred: In the current study, error-monitoring is evaluated in a social context (face stimuli), and in the existing literature, error-monitoring is evaluated in a nonsocial context (e.g., arrow, letter, and shape stimuli). These results are consistent with a recent study (Sabatino et al., 2013) in which individuals with ASD showed greater neural activation in response to face stimuli compared to nonsocial stimuli on a cognitive control fMRI task.
Taking into account the existing literature and the results of the current study, individuals with ASD may have ERN amplitudes similar to those observed in individuals with typical development in more social contexts compared to less social or nonsocial contexts (Affect Task vs. Gender Task, face stimuli in the current study vs. nonsocial stimuli in the extant literature). There are at least two potential explanations for this finding. First, ERN amplitude may be enhanced in conditions with greater consequences for errors, such as conditions in which errors are punished, motivational incentives for correct performance are increased, or participants’ performance is evaluated (e.g., Groom et al., 2013; Hajcak, Moser, Yeung, & Simons, 2005; Riesel, Weinberg, Endrass, Kathmann, & Hajcak, 2012). For individuals with ASD, errors in social contexts may be particularly consequential (e.g., bullying, teasing, loneliness), potentially leading to an enhanced ERN amplitude in social contexts, similar to the ERN amplitude observed in individuals with typical development. Second, ERN amplitude may be enhanced in conditions that require greater processing effort or conditions that yield decreased processing efficiency (Sabatino et al., 2013). For individuals with ASD, errors in social contexts may be more effortful to detect and may be processed less efficiently in the ACC, again potentially leading to an enhanced ERN amplitude in social contexts, similar to the ERN amplitude observed in individuals with typical development.
Individual Differences
Autistic Symptomology
Contrary to our hypothesis, error-monitoring ability was not associated with autistic symptomology. Vlamings et al. (2008) also failed to find a relation between error-monitoring ability and autistic symptomology. The results of the present study suggest that error-monitoring ability is relatively independent of autism severity per se, but may be associated with individual differences in presentation, including co-occurring social cognitive impairments.
Social Cognition
In accordance with our hypothesis, post-error slowing on the Affect Task was related to better social cognition. This result is consistent with the literature, which shows that individuals who engage in more error-monitoring have better social skills or a more social orientation (Hoffmann et al., 2012; Larson et al., 2010; Santesso & Segalowitz, 2009). Interestingly, error-monitoring on the Gender Task was not related to better social cognition. Since a person's affect changes frequently, but a person's gender is constant, error-monitoring of affect may be more closely associated with and more important for social cognition than error-monitoring of gender. Error-monitoring of affect may facilitate the development of social cognitive skills, or conversely, social cognitive skills may lead to increased error-monitoring of affect.
Limitations and Future Directions
There are several limitations of the present study that may have affected the results and their interpretation. First, many eye blinks were observed in the current EEG data set. Although these eye blinks were removed using independent components analysis, certain eye blinks may not have been identified or may have been incompletely or inaccurately regressed from the data (Luck, 2005). Second, error rates for this task were relatively high for both participants with ASD and typical development. Vocat, Pourtois, and Vuilleumier (2008), however, showed that the ERN is reliably elicited by errors, even when errors are frequent. Therefore, it is unlikely that the high error rate in the current study significantly impacted the ERN. Third, the typical development group may not have had enough variability in autistic symptomology and social cognition for individual differences to be adequately analyzed. Fourth, this study was not a longitudinal study. Thus, it was not possible to determine direction of effects: error-monitoring ability may have influenced social cognition, or individual differences in social cognition may have affected error-monitoring ability. Also, a longitudinal sample is necessary for definitively establishing developmental, as opposed to age-related, changes in error-monitoring ability in ASD. Finally, although this study assessed error-monitoring ability across a more social context (Affect Task) and a less social context (Gender Task), this study did not directly assess or compare error-monitoring ability across social and nonsocial contexts.
This study was the first to examine post-error slowing and the ERN in response to social stimuli in individuals with ASD. The results of this study suggest that individuals with ASD may have ERN amplitudes similar to those in individuals with typical development in more social contexts compared to less social contexts; social contexts may have greater consequences for errors or social contexts may require increased processing effort and yield decreased processing efficiency. Future studies should directly compare ERN amplitude and other indices of error-monitoring across social versus nonsocial contexts, conditions with high versus low consequences for errors, and tasks that require greater versus less processing effort. Future studies should also examine the Pe, a positive peak following the ERN that is more closely linked to error-awareness (Wessel, 2012), in response to social versus nonsocial stimuli in individuals with ASD.
In addition, this was the first study to examine developmental influences on post-error slowing and the ERN in ASD. Given the strong influence of development in this study, we recommend that future studies on error-monitoring in children and adolescents with ASD include age as a predictor of error-monitoring ability.
This study suggests that individuals who engage in error-monitoring have better social cognitive skills. In addition, the literature shows that self-monitoring interventions in ASD can reduce stereotypic and disruptive behaviors and increase task engagement, social interaction, and peer-directed verbalizations (e.g., Koegel et al., 1992; Morrison et al., 2001; Parker & Kamps, 2011). Thus, a better understanding of error-monitoring ability in individuals with ASD, particularly in a social context, may be integral to the development of successful interventions in the future.
Research Highlights.
Regardless of diagnostic group (Autism Spectrum Disorder (ASD) vs. typical development), face processing ability (accuracy in identifying the affect and gender of a face) and error-monitoring ability (difference in Error-Related Negativity amplitude between correct and incorrect responses; ERNdiff) were greater in older participants compared to younger participants, suggesting that these skills may improve with age.
When identifying the gender of a face, participants with ASD had a smaller ERNdiff (less differentiation between correct and incorrect responses) than participants with typical development. However, when identifying the affect of a face, participants with and without ASD did not differ on ERNdiff. Individuals with ASD may have ERN amplitudes similar to those observed in individuals with typical development in more social contexts compared to less social contexts due to greater consequences for errors, more effortful processing, and/or reduced processing efficiency in these contexts.
For all participants, more post-error slowing when processing the affect of a face was associated with better social cognitive skills.
ACKNOWLEDGEMENTS
This research was supported by the National Institute of Health (NIH), through Grants R01MH071273 and T32MH073124, and the Institute of Education Sciences, U.S. Department of Education, through Grant DED-R305C050052. The opinions expressed are those of the authors and do not represent views of NIH or the U.S. Department of Education. The authors thank Michael Alessandri, Jessica Alvarez, Alexis Bitting, Diana Borrero, Jeffrey Brosco, Elektra Burgos, Jessica Cardenas, Raphaelle Cuenod, Irene Daboin, Cristina De Armas, Stephanie Dickinson, Michaela Gaffley, Bridget Gamber, Allyson Hodgkins, Mark Jaime, Nicole Kojkowski, Krystal Lago, Peggy Laguerre, Javier Lopez-Calderon, Adam McMahon, Daniel Messinger, Leena Mohapatra, Lisa Newell, Kimie Ono, Jessica Smolarz, and Marygrace Yale-Kaiser for their contributions.
Footnotes
Due to time constraints, one participant with typical development did not complete the ADOS. This participant did not meet diagnostic cutoff scores on the SCQ or ASSQ and was therefore retained in the sample.
For one participant, all EEG data segments were rejected due to number of bad channels during the Gender Task, and for another participant, all EEG data segments were rejected due to number of bad channels during both the Affect and Gender Tasks. For the remaining five participants, one or two conditions in either the Affect or Gender Task did not meet the criterion of 10 artifact-free trials.
REFERENCES
- Aiken L, West S. Multiple Regression: Testing and Interpreting Interactions. Sage Publications, Inc.; Newbury Park, CA: 1991. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Author; Washington, D.C.: 2013. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “reading the mind in the eyes” test revised version: A study with normal adults, and adults with Asperger Syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry. 2001;42(2):241–251. doi:10.1111/1469-7610.00715. [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: Diagnostic validity. British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. doi:10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Bogte H, Flamma B, van der Meere J, van Engeland H. Post-error adaptation in adults with high functioning autism. Neuropsychologia. 2007;45(8):1707–1714. doi: 10.1016/j.neuropsychologia.2006.12.020. doi:10.1016/j.neuropsychologia.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Brocki KC, Bohlin G. Executive functions in children aged 6 to 13: A dimensional and developmental study. Developmental Neuropsychology. 2004;26(2):571–593. doi: 10.1207/s15326942dn2602_3. doi:10.1207/s15326942dn2602_3. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Current Opinion in Neurobiology. 2005;15(2):239–244. doi: 10.1016/j.conb.2005.03.012. doi:10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Carp J, Chaddock L, Fineman SL, Quandt LC, Ratliff JB. Anxiety and error monitoring: Increased error sensitivity or altered expectations? Brain and Cognition. 2007;64(3):247–256. doi: 10.1016/j.bandc.2007.03.006. doi:10.1016/j.bandc.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Reich W. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. doi:10.1023/A:1025014929212. [DOI] [PubMed] [Google Scholar]
- Constantino J, Gruber C. The Social Responsiveness Scale (SRS) Manual. Western Psychological Services; Los Angeles, CA: 2005. [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004;25(3):355–376. doi: 10.1207/s15326942dn2503_6. doi:10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Delorme A, Fernsler T, Serby H, Makeig S. EEGLAB Tutorial. University of California – San Diego; San Diego: 2006. [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. doi:10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. NeuroImage. 2007;35:1219–1230. doi: 10.1016/j.neuroimage.2006.12.038. doi:10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S, Gillberg C, Wing L. A screening questionnaire for Asperger Syndrome and other high-functioning autism spectrum disorders in school age children. Journal of Autism and Developmental Disorders. 1999;29(2):129–141. doi: 10.1023/a:1023040610384. doi:10.1023/A:1023040610384. [DOI] [PubMed] [Google Scholar]
- Esbensen AJ, Seltzer MM, Lam KSL, Bodfish JW. Age-related differences in restricted repetitive behaviors in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(1):57–66. doi: 10.1007/s10803-008-0599-x. doi:10.1007/s10803-008-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: A tutorial. Biological Psychology. Special Issue: Error Processing and Adaptive Responding. 2000;51(2-3):87–107. doi: 10.1016/s0301-0511(99)00031-9. doi:10.1016/S0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Groom MJ, Liddle EB, Scerif G, Liddle PF, Batty MJ, Liotti M, Hollis CP. Motivational incentives and methylphenidate enhance electrophysiological correlates of error monitoring in children with attention deficit/hyperactivity disorder. Journal of Child Psychology and Psychiatry. 2013;54(8):836–845. doi: 10.1111/jcpp.12069. doi: 10.1111/jcpp.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42(2):151–160. doi: 10.1111/j.1469-8986.2005.00270.x. doi:10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Happé FGE. An advanced test of theory of mind: Understanding of story characters' thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. Journal of Autism and Developmental Disorders. 1994;24(2):129–154. doi: 10.1007/BF02172093. doi:10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- Henderson H, Schwartz C, Mundy P, Burnette C, Sutton S, Zahka N, Pradella A. Response monitoring, the error-related negativity, and differences in social behavior in autism. Brain and Cognition. 2006;61(1):96–109. doi: 10.1016/j.bandc.2005.12.009. doi:10.1016/j.bandc.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hileman CM, Henderson H, Mundy P, Newell L, Jaime M. Developmental and individual differences on the P1 and N170 ERP components in children with and without autism. Developmental Neuropsychology. 2011;36(2):214–236. doi: 10.1080/87565641.2010.549870. doi:10.1080/87565641.2010.549870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S, Wascher E, Falkenstein M. Personality and error monitoring: an update. Frontiers in Human Neuroscience. 2012;6:171. doi: 10.3389/fnhum.2012.00171. doi:10.3389/fnhum.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan AM, Vargha-Khadem F, Kirkham FJ, Baldeweg T. Maturation of action monitoring from adolescence to adulthood: An ERP study. Developmental Science. 2005;8(6):525–534. doi: 10.1111/j.1467-7687.2005.00444.x. doi:10.1111/j.1467-7687.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. doi:10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Hu Z, Chan RCK, McAlonan GM. Maturation of social attribution skills in typically developing children: An investigation using the social attribution task. Behavioral and Brain Functions. 2010;6:10. doi: 10.1186/1744-9081-6-10. doi:10.1186/1744-9081-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44(11):2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. doi:10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Koegel LK, Koegel RL, Hurley C, Frea WD. Improving social skills and disruptive behavior in children with autism through self-management. Journal of Applied Behavior Analysis. 1992;25(2):341–353. doi: 10.1901/jaba.1992.25-341. doi:10.1901/jaba.1992.25-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science. 2007;10(6):874–891. doi: 10.1111/j.1467-7687.2007.00639.x. doi:10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Fair JE, Good DA, Baldwin SA. Empathy and error processing. Psychophysiology. 2010;47(3):415–424. doi: 10.1111/j.1469-8986.2009.00949.x. doi:10.1111/j.1469-8986.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Perlstein WM, Stigge-Kaufman D, Kelly KG, Dotson VM. Affective context-induced modulation of the error-related negativity. NeuroReport: For Rapid Communication of Neuroscience Research. 2006;17(3):329–333. doi: 10.1097/01.wnr.0000199461.01542.db. doi:10.1097/01.wnr.0000199461.01542.db. [DOI] [PubMed] [Google Scholar]
- Lopez-Caledron J, Luck S. ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience. 2014;8:213. doi: 10.3389/fnhum.2014.00213. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. doi:10.1023/A:1005592401947. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Manual: Autism Diagnostic Observation Schedule. Western Psychological Services; Los Angeles, CA: 2002. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic Interview—Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. doi:10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. The MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Luu P, Geyer A, Fidopiastis C, Campbell G, Wheeler T, Cohn J, Tucker DM. Reentrant processing in intuitive perception. PLoS ONE. 2010;5:1–10. doi: 10.1371/journal.pone.0009523. doi:10.1371/journal.pone.0009523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L, Kamps D, Garcia J, Parker D. Peer mediation and monitoring strategies to improve initiations and social skills for students with autism. Journal of Positive Behavior Interventions. 2001;3(4):237–250. doi:10.1177/109830070100300405. [Google Scholar]
- Mundy P, Newell L. Attention, joint attention, and social cognition. Current Directions in Psychological Science. 2007;16(5):269–274. doi: 10.1111/j.1467-8721.2007.00518.x. doi:10.1111/j.1467-8721.2007.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GPH, Kok A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology. 2001:752–760. doi: http://dx.doi.org/10.1017/S0048577201001111. [PubMed]
- O'Connell RG, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, Foxe JJ. The role of cingulate cortex in the detection of errors with and without awareness: A high-density electrical mapping study. European Journal of Neuroscience. 2007;25(8):2571–2579. doi: 10.1111/j.1460-9568.2007.05477.x. doi:10.1111/j.1460-9568.2007.05477.x. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46(5):957–961. doi: 10.1111/j.1469-8986.2009.00848.x. doi:10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Ozonoff S. Reliability and validity of the wisconsin card sorting test in studies of autism. Neuropsychology. 1995;9(4):491–500. doi:10.1037/0894-4105.9.4.491. [Google Scholar]
- Parker D, Kamps D. Effects of task analysis and self-monitoring for children with autism in multiple social settings. Focus on Autism and Other Developmental Disabilities. 2011;26(3):131–142. doi:10.1177/1088357610376945. [Google Scholar]
- Pontifex MB, Scudder MR, Brown ML, O'Leary KC, Wu C, Themanson JR, Hillman CH. On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology. 2010:767–773. doi: 10.1111/j.1469-8986.2010.00974.x. Retrieved from www.csa.com. [DOI] [PubMed]
- Rabbitt PM. Errors and error correction in choice-response tasks. Journal of Experimental Psychology. 1966;71(2):264–272. doi: 10.1037/h0022853. doi:10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Kathmann N, Hajcak G. Punishment has a lasting impact on error-related brain activity. Psychophysiology. 2012;49(2):239–247. doi: 10.1111/j.1469-8986.2011.01298.x. doi:10.1111/j.1469-8986.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- Robinson S, Goddard L, Dritschel B, Wisley M, Howlin P. Executive functions in children with autism spectrum disorders. Brain and Cognition. 2009;71(3):362–368. doi: 10.1016/j.bandc.2009.06.007. doi:10.1016/j.bandc.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK. The development of executive attention: Contributions to the emergence of self-regulation. Developmental Neuropsychology. 2005;28(2):573–594. doi: 10.1207/s15326942dn2802_2. doi:10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Sabatino A, Rittenberg A, Sasson NJ, Turner-Brown L, Bodfish JW, Dichter GS. Functional neuroimaging of social and nonsocial cognitive control in autism. Journal of Autism and Developmental Disorders. 2013:2903–2913. doi: 10.1007/s10803-013-1837-4. doi: http://dx.doi.org/10.1007/s10803-013-1837-4. [DOI] [PMC free article] [PubMed]
- Santesso DL, Drmic IE, Jetha MK, Bryson SE, Goldberg JO, Hall GB, Schmidt LA. An event-related source localization study of response monitoring and social impairments in autism spectrum disorder. Psychophysiology. 2011;48(2):241–251. doi: 10.1111/j.1469-8986.2010.01056.x. doi:10.1111/j.1469-8986.2010.01056.x. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ. Developmental differences in error-related ERPs in middle- to late-adolescent males. Developmental Psychology. 2008;44(1):205–217. doi: 10.1037/0012-1649.44.1.205. doi:10.1037/0012-1649.44.1.205. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ. The error-related negativity is related to risk taking and empathy in young men. Psychophysiology. 2009;46(1):143–152. doi: 10.1111/j.1469-8986.2008.00714.x. doi:10.1111/j.1469-8986.2008.00714.x. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MGH. Performance monitoring in a confusing world: Error-related brain activity, judgments of response accuracy, and types of errors. Journal of Experimental Psychology: Human Perception and Performance. 2000:141–151. doi: 10.1037//0096-1523.26.1.141. doi: http://dx.doi.org/10.1037/0096-1523.26.1.141. [DOI] [PubMed]
- Segalowitz SJ, Davies PL. Charting the maturation of the frontal lobe: An electrophysiological strategy. Brain and Cognition. 2004;55(1):116–133. doi: 10.1016/S0278-2626(03)00283-5. doi:10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Krauss MW, Shattuck PT, Orsmond G, Swe A, Lord C. The symptoms of autism spectrum disorders in adolescence and adulthood. Journal of Autism and Developmental Disorders. 2003;33(6):565–581. doi: 10.1023/b:jadd.0000005995.02453.0b. doi:10.1023/B:JADD.0000005995.02453.0b. [DOI] [PubMed] [Google Scholar]
- Sokhadze E, Baruth J, El-Baz A, Horrell T, Sokhadze G, Carroll T, Casanova MF. Impaired error monitoring and correction function in autism. Journal of Neurotherapy. 2010;14(2):79–95. doi: 10.1080/10874201003771561. doi:10.1080/10874201003771561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze EM, Baruth JM, Sears L, Sokhadze GE, El-Baz AS, Williams EL, Casanova MF. Event-related potential study of attention regulation during illusory figure categorization task in adhd, autism spectrum disorder, and typical children. Journal of Neurotherapy. 2012;16(1):12–31. doi: 10.1080/10874208.2012.650119. doi:10.1080/10874208.2012.650119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, Larson MJ, Krauskopf E, Clawson A. Error processing in high-functioning autism spectrum disorders. Biological Psychology. 2010;85(2):242–251. doi: 10.1016/j.biopsycho.2010.07.009. doi:10.1016/j.biopsycho.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJS, Manoach DS. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD). Brain: A Journal of Neurology. 2008;131(9):2464–2478. doi: 10.1093/brain/awn099. doi:10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. doi:10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlamings PHJM, Jonkman LM, Hoeksma MR, van Engeland H, Kemner C. Reduced error monitoring in children with autism spectrum disorder: An ERP study. European Journal of Neuroscience. 2008;28(2):399–406. doi: 10.1111/j.1460-9568.2008.06336.x. doi:10.1111/j.1460-9568.2008.06336.x. [DOI] [PubMed] [Google Scholar]
- Vocat R, Pourtois G, Vuilleumier P. Unavoidable errors: A spatio-temporal analysis of time-course and neural sources of evoked potentials associated with error processing in a speeded task. Neuropsychologia. 2008;46(10):2545–2555. doi: 10.1016/j.neuropsychologia.2008.04.006. doi:10.1016/j.neuropsychologia.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Voracek M, Dressler SG. Lack of correlation between digit ratio (2D:4D) and Baron-Cohen's “reading the mind in the eyes” test, empathy, systemising, and autism-spectrum quotients in a general population sample. Personality and Individual Differences. 2006;41:1481–1491. doi:10.1016/j.paid.2006.06.009. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Fourth Edition Pearson; San Antonio, TX: 2003. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Fourth Edition Pearson; San Antonio, TX: 2008. [Google Scholar]
- Wessel JR. Error awareness and the error-related negativity: Evaluating the first decade of evidence. Frontiers in Human Neuroscience. 2012 doi: 10.3389/fnhum.2012.00088. doi: http://dx.doi.org/10.3389/fnhum.2012.00088. [DOI] [PMC free article] [PubMed]
- Wiersema JR, van der Meere JJ, Roeyers H. Developmental changes in error monitoring: An event-related potential study. Neuropsychologia. 2007;45(8):1649–1657. doi: 10.1016/j.neuropsychologia.2007.01.004. doi:10.1016/j.neuropsychologia.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Williams P, Weiss L, Rolfhus E. Wechsler Intelligence Scale for Children–IV, Technical Report 2, Psychometric Properties. The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Zajdel RT, Bloom JM, Fireman G, Larsen JT. Children's understanding and experience of mixed emotions: The roles of age, gender, and empathy. The Journal of Genetic Psychology: Research and Theory on Human Development. 2013:582–603. doi: 10.1080/00221325.2012.732125. doi: http://dx.doi.org/10.1080/00221325.2012.732125. [DOI] [PubMed]