Abstract
Background
We sought to determine the association between the presence of a fecalith and acute/nonperforated appendicitis, gangrenous/perforated appendicitis and the healthy appendix.
Methods
We retrospectively analyzed appendectomies performed between October 2003 and February 2012. We collected data on age, sex, appendix histology and the presence of a fecalith.
Results
During the study period, 1357 appendectomies were performed. Fecaliths were present in 186 patients (13.7%). There were 94 male (50.5%) and 92 female patients, and the mean age was 32 (range of 10–76) years. The fecalith rate was 13%–16% and was nonexistant after age 80 years. The main groups with fecaliths were those with acute/nonperforated appendicitis (n = 121, 65.1%, p = 0.041) and those with a healthy appendix (n = 65, 34.9%, p = 0.003). The presence of fecaliths in the gangrenous/perforated appendicitis group was not significant (n = 19, 10.2%, p = 0.93). There were no fecaliths in patients with serositis, carcinoid or carcinoma.
Conclusion
Our data confirm the theory of a statistical association between the presence of a fecalith and acute (nonperforated) appendicitis in adults. There was also a significant association between the healthy appendix and asymptomatic fecaliths. There was no correlation between a gangrenous/perforated appendix and the presence of a fecalith. The fecalith is an incidental finding and not always the primary cause of acute (nonperforated) appendictis or gangrenous (perforated) appendicitis. Further research on the topic is recommended.
Abstract
Contexte
Nous avons voulu examiner le lien entre la présence d’un fécalome et l’appendicite aiguë/non perforée, l’appendicite gangreneuse/perforée et un appendice sain.
Méthodes
Nous avons analysé de manière rétrospective les appendicectomies effectuées entre octobre 2003 et février 2012. Nous avons recueilli des données sur l’âge, le sexe, l’histologie de l’appendice et la présence d’un fécalome.
Résultats
Durant la période de l’étude, 1357 appendicectomies ont été effectuées. Des fécalomes étaient présents chez 186 patients (13,7 %). L’étude regroupait 94 hommes (50,5 %) et 92 femmes; l’âge moyen était de 32 ans (entre 10 et 76 ans). Le taux de fécalome était de 13 % à 16 % et non existant après l’âge de 80 ans. Les principaux groupes porteurs de fécalomes étaient ceux qui présentaient une appendicite aiguë/non perforée (n = 121, 65,1 %, p = 0,041) et ceux dont l’appendice était sain (n = 65, 34,9 %, p = 0,003). La présence de fécalomes dans le groupe souffrant d’appendicite gangreneuse/perforée s’est révélée non significative (n = 19, 10,2 %, p = 0,93). Les patients qui souffraient de sérosite, de carcinoïde ou de carcinome ne présentaient pas de fécalomes.
Conclusion
Nos données confirment la théorie d’un lien statistique entre la présence d’un fécalome et une appendicite aiguë (non perforée) chez l’adulte. On a également observé un lien significatif entre un appendice sain et des fécalomes asymptomatiques. On n’a observé aucune corrélation entre un appendice gangreneux/perforé et la présence de fécalomes. Le fécalome est une observation accessoire qui n’est pas toujours la principale cause de l’appendicite aiguë (non perforée) ou de l’appendicite gangreneuse (perforée). Une recherche plus approfondie à ce sujet est recommandée.
It is generally accepted that the main etiology of appendicitis is obstruction due to fecalith in adults and lymphoid hyperplasia in children. It is also accepted that perforated/gangrenous appendicitis is associated with an obstructed appendix secondary to the presence of a fecalith. A standard PubMed search on the topic reveals a plethora of literature on appendiceal fecaliths or coproliths. There are many associated articles documenting fecalith rates ranging from 1.5% to 51%, but certainly no level I evidence on the topic.
Trinidad & Tobago is a twin island state located off the northern tip of South America and Venezuela; the islands are the southern-most islands in the Caribbean. The population is diverse owing to a history of invasion by Spain, Portugal, France and Britain and to migration from India, Africa, China, Syria and Lebanon as well as other Arabic nations and Amerindian areas. The composition of the population is estimated as follows: East Indian descent (37%), Afro-Caribbean (36%), mixed races (24%) and white, Arabic, Chinese and Amerindian (3%). We present our data on fecalith rates and acute appendicitis in this population.
Methods
We retrospectively collected data from the electronic records of the Department of Pathology at the General Hospital, Port-of-Spain, Trinidad, for all appendectomies performed between October 2003 and February 2012 in patients aged 5–100 years old. Data included demographic information regarding date of collection, age, sex, hospital of origin; details of the morphologic appearance of the specimen; histology; and the presence or absence of a fecalith. The collection of this information is standard protocol at the Department of Pathology, where only 2 pathologists have been appointed to the department in more than 30 years thereby enabling a level of consistency with the accuracy of the morphologic appearances and histopathology reporting. Data were collected from The General Hospital, Port-of-Spain (POSGH), The Sangre Grande District General Hospital (SGH) and The Scar-borough Regional Hospital (SRH) in Tobago. Most patients who undergo appendectomy at these institutions have a clinical indication for the procedure and the clinical syndrome of appendicitis. Information regarding race was not collected for our analysis and would have to be documented in a further study. Ethics approval was granted from the relevant authorities.
Statistical analysis
We entered the data entered into SPSS software version 20.0 (IBM Statistics). Histologic information was documented and coded based on the presence of acute/nonperforated) appendicitis, gangrenous/perforated appendicitis or a healthy appendix and based on whether the patient had serosal edema/congestion, serositis and/or lymphoid hyperplasia. The presence of a fecalith or other associations, such as carcinoma or carcinoid, was noted for all specimens.
Results
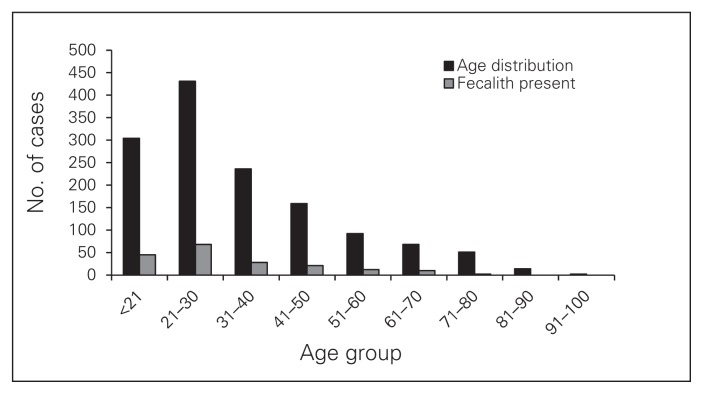
There were 1357 appendectomies performed during the study period in 687 male (50.6%) and 670 female patients (49.4%). The mean age of patients was 34 (range 5–91) years. The most common age group affected was the 21–30 group (n = 431, 31.8%) followed by the under-21 group (n = 304, 22.4%), the 31–40 group (n = 236, 17.4%), the 41–50 group (n = 159, 11.7%), the 51–60 group (n = 92, 6.8%), the 61–70 group (n = 68, 5%), the 71–80 group (n = 51, 3.8%), the 81–90 group (n = 14, 1%) and the older than 90 group (n = 2, 0.1%; Fig. 1).
Fig. 1.
Age distribution of the entire study population compared to those with fecaliths.
Hospital information was available for 1355 patients; most were from the POSGH (n = 1006, 74.2%), followed by the SRH (n = 175, 12.9%), SGH (n = 172, 12.7%) and other (n = 2, 0.1%).
The mean number of cases per year was 136 (n = 29 in the last 3 months of 2003, n = 192 in 2004, n = 171 in 2005, n = 122 in 2006, n = 163 in 2007, n = 118 in 2008, n = 143 in 2009, n = 219 in 2010, n = 185 in 2011 and n = 15 in the first 2 months of 2012). There were 968 cases of appendicitis (71.3%), of which 136 were gangrenous, necrotic or perforated. There were 183 patients with serosal edema, 20 with serositis and 88 with lymphoid hyperplasia. These patients were included in the total sample (1357). Some specimens contained more than 1 factor on histology. Fecaliths were present in 186 patients (13.7%).
In the fecalith subset analysis, there were 94 male (50.5%) and 92 female patients with a mean age of 32 (range 10–76) years. The following are the fecalith rates in each age group: 14.8% (< 21 yr), 15.8% (21–30 yr), 11.9% (31–40 yr), 13.2% (41–50 yr), 13.0% (51–60 yr), 14.7% (61–70 yr), 3.9% (71–80 yr); there were no fecaliths in patients older than 80 years (Fig. 1).
From a histological perspective, we analyzed only the patients with fecaliths (n = 186). A Pearson χ2 test showed a significant association between the presence of fecaliths and acute/nonperforated appendicitis (n = 121, 65.1%, p = 0.041; Table 1) and healthy appendicies (n = 65, 34.9%, p = 0.003; Table 2). There was no significant association between the presence of fecaliths and gangrenous/perforated appendicitis (n = 19, 10.2%, p = 0.93; Table 3).
Table 1.
Significance of having a fecalith and acute (nonperforated) appendicitis*
| Appendicitis | No fecalith | Fecalith | Total |
|---|---|---|---|
| No appendicitis | 324 | 65 | 389 |
| Appendicitis | 847 | 121 | 968 |
| Total | 1171 | 186 | 1357 |
Pearson χ2 (asymp sig 2-sided), p = 0.041.
Table 2.
Significance of having a fecalith and a healthy appendix*
| Appendix | No fecalith | Fecalith | Total |
|---|---|---|---|
| Not healthy | 882 | 121 | 1003 |
| Healthy | 289 | 65 | 354 |
| Total | 1171 | 186 | 1357 |
Pearson χ2 (asymp sig 2-sided), p = 0.003.
Table 3.
Significance of having a fecalith and a gangrenous (perforated) appendicitis*
| Type of appendicitis | No fecalith | Fecalith | Total |
|---|---|---|---|
| Not gangrenous/necrotic | 1054 | 167 | 1221 |
| Gangrenous/necrotic | 117 | 19 | 136 |
| Total | 1171 | 186 | 1357 |
Pearson χ2 (asymp sig 2-sided), p = 0.93.
Subgroup analyses using the Pearson χ2 test under the crosstabs option revealed a significant association between the presence of fecaliths and serosal edema (n = 37, 19.9%, p = 0.006; Table 4) but not lymphoid hyperplasia (n = 11, 5.9%, p = 0.73; Table 5). These were subsets of the overall group of 186 patients and were not additional or discrete cases. Some of these patients had a combination of factors. There were no fecaliths in patients with serositis, carcinoid or carcinoma.
Table 4.
Significance of having a fecalith and serosal edema*
| Edema | No fecalith | Fecalith | Total |
|---|---|---|---|
| No serosal edema | 1025 | 149 | 1174 |
| Serosal edema | 146 | 37 | 183 |
| Total | 1171 | 186 | 1357 |
Pearson χ2 (asymp sig 2-sided), p = 0.006.
Table 5.
Significance of having a fecalith and lymphoid hyperplasia*
| Lymphoid hyperplasia | No fecalith | Fecalith | Total |
|---|---|---|---|
| No lymphoid hyperplasia | 1094 | 175 | 1269 |
| Lymphoid hyperplasia | 77 | 11 | 88 |
| Total | 1171 | 186 | 1357 |
Pearson χ2 (asymp sig 2-sided), p = 0.73.
Discussion
The first description of the vermiform appendix causing a perityphilitic suppuration was reported by Fitz1 in 1886. This was followed by a landmark article by Wangensteen and Bowers2 in 1937, in which the theory of the obstructive component was discussed as a causative factor for acute appendicitis. Subsequently, there were a few articles exploring the association between the appendix and the fecalith written by Durcharme and colleagues3 in 1966 and Gill and Cudmore4 in 1975 explaining the etiology and outcomes.
In 1985, Jones and colleagues5 postulated that appendicitis was more common in developed than in developing regions, and appendiceal fecaliths are thought to have an etiologic role in the disease. The geographic distribution of appendiceal fecaliths was investigated by systematic, intra-operative palpation of the appendix in patients in Toronto, Canada, and Johannesburg, South Africa. The incidence of fecaliths found on pathologic sectioning of the appendix in patients with appendicitis in both cities were compared. In the Canadian population, the prevalence of fecaliths in patients whose appendices were palpated incidentally was 32% versus 52% for those with appendicitis. In the South African population, the prevalence of fecaliths in patients whose appendices were palpated incidentally was 4% versus 23% for those with appendicitis. The difference in prevalence of incidental appendiceal fecaliths in the 2 populations was statistically significant, showing a higher prevalence in developed than in developing countries as well as a higher prevalence in patients with appendicitis. The authors concluded that low-fibre diets consumed in developed countries lead to fecalith formation and predisposes those populations to appendicitis.5
In 1990, Nitecki and colleagues6 conducted a study to determine the association between appendiceal fecaliths or appendiceal calculi and the presence of acute appendicitis. They found that fecaliths were 6 times more common than calculi, but that calculi were more often associated with perforated appendicitis or periappendiceal abscesses (45%) than fecaliths (19%).6
Concensus dictates that the main etiology of appendicitis is obstruction secondary to fecalith formation within the lumen of the appendix in adults. Other uncommon causes may include parasites, undigested plant or fruit residues, trauma and foreign bodies.7 Appendicitis in children is closely associated with lymphoid hyperplasia and may be often due to viral causes.7 It is also assumed that perforated, gangrenous or necrotic appendicitis is associated with an obstructed appendix secondary to the presence of a fecalith, as shown by Alaedeen and colleagues8 in 2008; they assessed 388 patients and found a fecalith rate of 31%. The appendix was perforated in 57% of patients who had a fecalith versus 36% of patients without a fecalith.8 However, there are differing opinions on the topic, bringing into question the theory of the appendiceal fecalith (for an example, see the study by Maenza and colleagues9 on “the myth of a fecalith.”). A PubMed search reveals a plethora of literature on the topic “appendicitis and fecolith” or “appendicitis and coprolith.”
In 2008, Sgourakis and colleagues10 examined the role of coprostasis and coproliths in recurrent appendicitis. Of 427 histology reports, 294 showed acute appendicitis, 56 showed acute recurrent appendicitis, 34 showed subacute recurrent appendicitis, 28 showed chronic appendicitis and 15 showed noninflamed appendicitis. Coprostasis was observed in 58 patients (13.58%), and the presence of coprolith was observed in 6 (1.4%). The authors concluded that coprostasis, but not coproliths, is a contributing factor to acute exacerbations of chronic appendicitis.10
In addition, Makaju and colleagues11 provided data from Kathmandu in 2010 on 518 appendectomy specimens. They found a fecalith rate of 1.54%. Histology revealed that 180 (34.75%) cases were early acute appendicitis, 250 (48.26%) were acute suppurative appendicitis and 88 (16.99%) cases acute gangrenous appendicitis. Their study did not confirm the existing popular notion that luminal obstruction is the pathogenetic hallmark for acute appendicitis.11 Another supporting 10-year study by Chandrasegaram and colleagues12 in Australia on appendectomies that were positive for fecaliths, worms, endometriosis or appendiceal tumours showed the fecalith rate to be 3.6% of 4670 specimens, with 39.5% of patients having appendicitis.12 The findings of these studies did not support the fecalith/coprolith theory.
A recent study by Singh and Mariadason13 showed that of 1014 emergency appendectomy specimens the fecalith rate was 18.1% in appendicitis specimens and 28.6% in negative specimens, a rate similar to that found in the present study. Fecalith prevalence for positive cases was 29.9% (79 of 264) in pediatric patients and 13.7% (99 of 722) in adults. Furthermore, fecalith prevalence was 39.4% in perforated appendicitis but only 14.6% in nonperforated appendicitis (27.5% v. 12.0%, respectively, in adults and 56.1% v. 22.7%, respectively, in children). The authors concluded that fecalith prevalence was too low to consider it the most common cause of nonperforated appendicitis and that fecaliths are more prevalent in pediatric than in adult appendicitis.13
Regarding the use of computed tomography in patients with appendicitis and fecaliths, Huwart and colleagues14 reported that the appendix was visualized in 82% of cases and a fecalith found in 13%. They concluded that the fecalith was found in a significant number of healthy patients and that the presence of a fecalith did not represent a specific sign for appendicitis.14
These more recent studies13,14 support the theory that the fecalith is merely an incidental finding and that it is not always causative for appendicitis.
We found that the male:female fecalith ratio was 1:1 in our population, which had a mean age of 32 years. The fecalith prevalence rate ranged from 11.9% to 15.8% in patients aged 10–76 years (14.8% in the under-21 group, 15.8% in the 21–30 group, 11.9% in the 31–40 group, 13.2% in the 41–50 group, 13.0% in the 51–60 group and 14.7% in the 61–70 group), dropped in patients aged 71–80 years (3.9%) and was nonexistent in patients older than 80 years (Fig. 1).
From a histological perspective, considering only the patients with fecaliths (n = 186), we found that the presence of a fecalith was significant in patients with acute/nonperforated appendicitis (n = 121, 65.1%, p = 0.041) and, quite interestingly, in patients with healthy appendices (n = 65, 34.9%, p = 0.003). We performed subgroup analyses involving overlapping factors in this group of 186 patients: gangrenous/perforated appendix, serosal edema and lymphoid hyperplasia. There was no statistical correlation between the presence of a fecalith and having a gangrenous/perforated appendix (n = 19, 10.2%, p = 0.93; Tables 1–3). In addition, there were no fecaliths in patients with serositis, carcinoid or carcinoma. We do expect some degree of error in reporting the fecaliths over the study period; however, because there have only been 2 senior pathologists in the department of pathology in the last 30 years, we expected an adequate level of consistency in reporting. Moreover, patients would undergo surgery only once indicated by a clinical picture of appendicitis. Therefore, although the negative appendectomy rate was estimated to be 28%, appendices were still indicated to be removed at the time of surgery and had nothing to do with the palpation of a fecalith, as is done in many centres worldwide. Of note, most of the appendectomies at our hospital are performed by residents in training and senior house officer–level staff, who have usually been in practice for fewer than 5 years.
Conclusion
The data we presented confirm the theory of a statistical association between the presence of a fecalith and acute appendicitis, but also show contradictory information whereby having a healthy, asymptomatic appendix was also strongly associated with the presence of a fecalith. Interestingly, there was no significant correlation between gangrenous/perforated appendicitis and the presence of a fecalith. We conclude that the fecalith is merely an incidental finding and is not the primary cause of acute (nonperforated) or gangrenous (perforated) appendicitis, but merely an association. We postulate that the underlying cause is most often related to some other factor when fecaliths are found in patients with perforated or gangrenous appendices. This study is relevant to current surgical practice in the United Kingdom, North America and Europe, where there are increasing migrant West Indian, East Indian and African populations, and is useful for clinical and radiologic decision-making since our populace is a multicultural racial composition. With so many differing views the only way forward is to encourage further research on the topic to bring firm conclusions to the table.
Acknowledgements
We acknowledge the tireless work of the late Dr. Neville Jankey, Consultant Pathologist, General Hospital, Port-of-Spain, who was one of the main contributors to the database.
Footnotes
Competing interests: None declared.
Contributors: All authors designed the study, acquired the data and wrote the article. M. Ramdass, Q. Young Sing, D. Milne and J. Mooteeram analyzed the data. S. Barrow reviewed the article. All authors approved the final version for publication.
References
- 1.Fitz RH. Perforating inflammation of the vermiform appendix with special reference to its early diagnosis and treatment. Am J Med Sci. 1886;92:321–46. [Google Scholar]
- 2.Wangensteen OH, Bowers WF. Significance of the obstructive factor in the genesis of acute appendicitis. Arch Surg. 1937;34:496–526. [Google Scholar]
- 3.Ducharme JC, Hurtubise M, Anouty I. Calcified appendiceal fecalith in children: incidence and significance. J Can Assoc Radiol. 1966;17:155–7. [PubMed] [Google Scholar]
- 4.Gill B, Cudmore RE. Significance of fecaliths in the diagnosis of acute appendicitis. Br J Surg. 1975;62:535–6. doi: 10.1002/bjs.1800620708. [DOI] [PubMed] [Google Scholar]
- 5.Jones BA, Demetriades D, Segal I, et al. The prevalence of appendiceal fecaliths in patients with and without appendicitis. A comparative study from Canada and South Africa. Ann Surg. 1985;202:80–2. doi: 10.1097/00000658-198507000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nitecki S, Karmeli R, Sarr MG. Appendiceal calculi and fecaliths as indications for appendectomy. Surg Gynecol Obstet. 1990;171:185–8. [PubMed] [Google Scholar]
- 7.Engin O, Muratli A, Ucar AD, et al. The importance of fecaliths in the aetiology of acute appendicitis. Chirurgia (Bucur) 2012;107:756–60. [PubMed] [Google Scholar]
- 8.Alaedeen DI, Cook M, Chwals WJ. Appendiceal fecalith is associated with early perforation in pediatric patients. J Pediatr Surg. 2008;43:889–92. doi: 10.1016/j.jpedsurg.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 9.Maenza RL, Smith L, Wolfson AB. The myth of the fecalith. Am J Emerg Med. 1996;14:394–7. doi: 10.1016/S0735-6757(96)90058-3. [DOI] [PubMed] [Google Scholar]
- 10.Sgourakis G, Sotiropoulos GC, Molmenti EP, et al. Are acute exacerbations of chronic inflammatory appendicitis triggered by coprostasis and/or coproliths? World J Gastroenterol. 2008;14:3179–82. doi: 10.3748/wjg.14.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makaju R, Mohammad A, Shakya A. Acute appendicitis: analysis of 518 histopathologically diagnosed cases at the Kathmandu University Hospital, Nepal. Kathmandu Univ Med J (KUMJ) 2010;8:227–30. doi: 10.3126/kumj.v8i2.3564. [DOI] [PubMed] [Google Scholar]
- 12.Chandrasegaram MD, Rothwell LA, An EI, et al. Pathologies of the appendix: a 10-year review of 4670 appendicectomy specimens. ANZ J Surg. 2012;82:844–7. doi: 10.1111/j.1445-2197.2012.06185.x. [DOI] [PubMed] [Google Scholar]
- 13.Singh JP, Mariadason JG. Role of the fecalith in modern-day appendicitis. Ann R Coll Surg Engl. 2013;95:48–51. doi: 10.1308/003588413X13511609954851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huwart L, El Khoury M, Lesavre A, et al. Is appendicolith a reliable sign for acute appendicitis at MDCT? [article in French] J Radiol. 2006;87:383–7. doi: 10.1016/s0221-0363(06)74017-3. [DOI] [PubMed] [Google Scholar]